Abstract
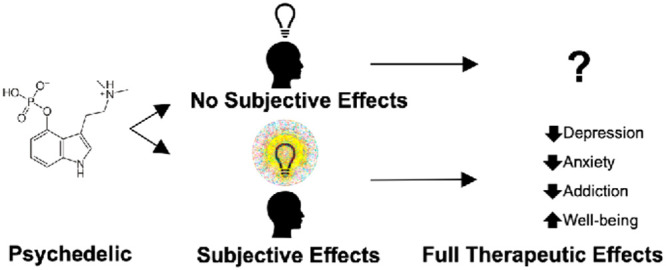
Classic psychedelics produce altered states of consciousness that individuals often interpret as meaningful experiences. Across a number of human studies, when the participant-rated intensity of the overall drug effects are statistically controlled for, certain subjective effects predict therapeutic and other desirable outcomes. Underlying neurobiological mechanisms are likely necessary but not sufficient to confer full and enduring beneficial effects. We propose that the subjective effects of psychedelics are necessary for their enduring beneficial effects and that these subjective effects account for the majority of their benefit.
Introduction
The classic psychedelics are a structurally diverse group of compounds that are partial agonists at 5-HT2A serotonin receptors and produce a unique profile of subjective effects.2 These compounds include tryptamines such as psilocybin (the main psychoactive constituent in psychedelic mushrooms), N,N-dimethyltryptamine (DMT, an ingredient of the plant admixture ayahuasca), phenethlyamines such as mescaline (from peyote and other cacti), and the ergotamines (such as lysergic acid diethylamide, LSD). Pharmacological blocking of the 5-HT2A receptor blocks many subjective and other major effects of psychedelics in humans and infrahuman animals.3
These classic psychedelics have low toxicity and limited abuse liability,4 and several recent studies have investigated their treatment potential for mood and substance use disorders.5−10 While favorable outcomes have been observed when psychedelics are taken under supportive conditions, questions remain regarding their mechanisms of action. Here we argue that some subjective effects occasioned by moderate to high doses of psychedelics in humans are necessary for their full and enduring therapeutic and otherwise beneficial outcomes. In this article, subjective effects refer to first-person experience, which is empirically measured by self-report data. Our view is neatly captured by the thought experiment (which we elaborate on later in this piece): would psychedelics confer their therapeutic benefits if they were administrated to someone who was under heavy sedation? We suspect the answer is no.
Our position contrasts the idea that subjective effects of psychedelics may be irrelevant to their therapeutic effects. The position that subjective effects are irrelevant to therapeutic effects is probably true of many pharmacological treatments. Suggestive evidence supporting this position include studies in which psychedelics have been shown to produce positive effects in a rodent model of depression.11 Although we cannot completely discount the possibility of subjective drug effects in rodents, it seems unlikely that rodents would have experiences similar to those to which humans attribute deep personal meaning and positive, therapeutically relevant mood and behavioral change after taking a psychedelic. From this perspective, the subjective experiences elicited by psychedelic substances are merely epiphenomena of the underlying neurobiological mechanisms which convey the beneficial effects. For example, psychedelics promote structural and functional neural plasticity in the prefrontal cortex through 5-HT2A receptor-mediated mechanisms,12 or, to cite another example of a neurobiological model that may not require subjective experience, it is observed that the antidepressant effects of psychedelics are associated with brain network reorganization.13 While these and other neurobiological mechanisms could plausibly account for some of the therapeutic actions of psychedelics, none rule out an essential mediating role of subjective effects in humans.
Subjective Effects of Psychedelics
Naturally occurring psychedelics have been used for millennia in some cultures in religious and healing rituals, with an emphasis on the subjective experiences that they produce.14 The importance of acute subjective effects in therapeutic outcomes has been also been documented in qualitative interview studies of patients treated with psychedelics in contemporary settings.15,16 There is a great deal of historical, anecdotal, and qualitative data supporting the value of the subjective effects of psychedelics.
The meaning and significance attributed to psychedelic experiences has been well established in laboratory settings. Psilocybin administration studies have repeatedly shown that participants frequently rate their psychedelic experiences as among the most meaningful of their entire lives6,7,9,17−20 and they are sometimes compared to the birth of a first-born child or death of a parent. Due to their salience, such experiences may serve as narrative “inflection points” in one’s life that could provide an impetus for changing one’s identification with certain patterns of thoughts, feelings, and behaviors.
Several subjective features of psychedelic experiences are measurable through psychometric survey instruments. Building on the foundational scholarship of William James, Walter Stace, Walter Pahnke, and others,21 the Mystical Experience Questionnaire (MEQ) was developed and subsequently revised and psychometrically validated to provide a self-report measure of the acute effects of psilocybin.22 This scale includes four subscales: 1, an authoritative sense of unity or connectedness accompanied by feelings of reverence; 2, positively valenced feelings such as love or peace; 3, alterations to the sense of both time and space; and 4, difficulty with putting the experience into words. The MEQ likely taps several different cognitive and affective processes that ongoing psychometric studies are further delineating.
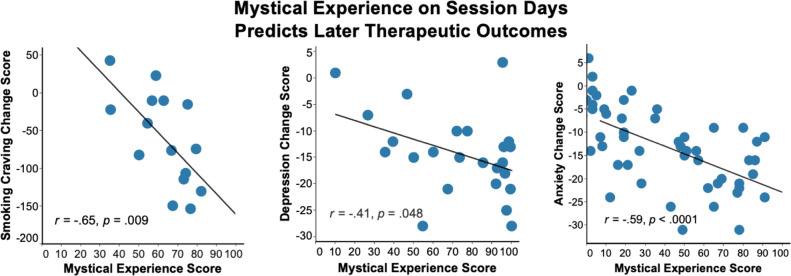
Scores on questionnaires assessing mystical-type experiences are predictive of beneficial outcomes from psychedelics administered in experimental contexts (Figure 1). An initial double-blind study from Johns Hopkins showed that 61% of 36 psychedelic naïve participants met a priori criteria for having a “complete” mystical experience at the end of the psilocybin session day compared to 11% after methylphenidate.17 Two months after sessions, participants attributed significantly greater positive changes in attitudes about life and self, positive mood, positive behaviors, and positive social effects to experiences during the psilocybin than methylphenidate sessions. Importantly, correlation and regression analyses indicated a central role of the mystical experience assessed on the session day, but not the intensity of the psilocybin experience, in predicting the high ratings of personal meaning assessed at 14 months.18 For instance, r-values of 0.61 were found between mystical experience scores immediately after psilocybin sessions and the follow-up ratings of the personal meaning of the experience after controlling for three different measures of the intensity of the drug effect. A systematic replication of the first study in 18 healthy participants showed that mystical experience on session days and positive ratings on follow-up increased as an orderly function of psilocybin dose.19 A further extension of this research explored the role of psilocybin-occasioned mystical experience in combination with meditation on enduring changes in trait measures of prosocial attitudes and behaviors.20 In that randomized double-blind study, 50 participants received moderate-high doses of psilocybin on each of two sessions while 25 received a low placebo-like dose on both sessions. Overall, 61% of those receiving moderate-high doses of psilocybin had complete mystical experiences in contrast to 4% for those receiving the low placebo-like dose with the same levels of psychological support. Hierarchical regression analysis showed that mystical experience (MEQ scores) on session days contributed significantly (improving the r-square of a model that included only spiritual practices by 0.54) to predicting a composite measure of positive outcomes such as positive attitudes about life, self, mood, and behavior at 6 months.
Figure 1.
Left panel shows data from a study (N = 15) of psilocybin on cigarette smoking cessation (replotted from Garcia et al.23). Smoking craving data are change scores from pretreatment to the 6-month follow-up. Mystical experience data for each participant are the mean total score on the 43-item version of the Mystical Experience Questionnaire (expressed as a percentage of the maximum possible score) assessed at the end of each of 2 or 3 psilocybin sessions. The middle panel shows data from a study (N = 24) of psilocybin on depression (adapted from Davis et al.10). Depression was measured with GRID-Hamilton Depression Rating Scale and expressed as change scores from pretreatment to 4 weeks after the second psilocybin session. Mystical experience data for each participant are the highest of two total scores on the 30-item Mystical Experience Questionnaire (expressed as a percentage of the maximum possible score) assessed at the end of each of two psilocybin sessions. The right panel shows data from a study of (N = 50) of individuals with a life-threatening cancer diagnosis who received either a very low dose or a moderately high dose of psilocybin (Griffiths et al.6). Mystical experience data for each participant are the total score on the 30-item Mystical Experience Questionnaire (expressed as a percentage of the maximum possible score) assessed at the end of the first psilocybin session. Anxiety was measured with the Hamilton Anxiety Rating Scale and expressed as a change score from baseline to 5 weeks postsession. More details regarding these images can be found in the citations above describing the original studies.
Mystical-type experience scores on psilocybin session days are also predictive of treatment success at long-term follow-up in clinical studies (see Figure 1). Two double-blind crossover studies showed that psilocybin produced substantial and enduring decreases in symptoms of anxiety and depression among patients with a life-threatening cancer diagnosis.6,7 In the first of these studies,6 mean percentage of maximum total possible score on the MEQ was significantly higher immediately after a moderate psilocybin dose (64%) than after a low placebo-like dose (27%). These scores after the first session were significantly correlated with most of the enduring changes in therapeutic outcome measured 5 weeks later. For most measures, this relationship continued to be significant when the intensity of overall psilocybin effect was controlled for in a partial correlation analysis, suggesting that mystical-type experience per se has an important role apart from overall intensity of drug effect. Furthermore, a statistical mediation analysis suggested that mystical-type experience was a mediator in positive therapeutic response. The results of the second of these studies7 were very similar, with correlation analysis controlling for the intensity of drug effect and a mediation analysis suggesting that mystical experience was a mediator of therapeutic effects. Open-label pilot studies of psilocybin in the treatment of substance dependence and depression have reported data consistent with these findings. In a smoking cessation study, 9 of 15 participants (60%) had a “complete” mystical experience during one or more psilocybin session(s).23 Results showed significant correlations between mean MEQ total scores assessed on session days and change from baseline in smoking craving scores (r = −0.65) and urine cotinine (r = −0.56) at the 6-month follow-up. Further, those participants who showed stronger mystical experiences on psilocybin sessions were more likely to be successful in biologically assessed smoking abstinence. In a psilocybin study in 20 patients with treatment-resistant depression, a measure assessing oceanic boundlessness (a construct related to mystical experience) on session days correlated with reductions in depression and was a significantly better predictor than subjective measures assessing visual or auditory alterations.24 Finally, in a psilocybin study in 24 patients with major depressive disorder there was a moderate correlation (r = −0.41) between peak postsession mystical experience ratings and decreases in depression, but no such correlation with postsession challenging experience ratings, thus again suggesting some specificity to mystical-type experiences.10
In addition to mystical-type experiences, meaningful insights and belief changes are also frequently cited as fundamentally important to enduring positive outcomes in anecdotal descriptions of psychedelic treatments. For example, in a study of successful smoking cessation after psilocybin treatment, participants reported gaining vivid insights into self-identity and reasons for smoking along with strengthened belief that they had the ability remain abstinent.9,16 In a double-blind study comparing psilocybin and dextromethorphan, psychological or personal insight rated after sessions increased as a function of psilocybin dose and was identified as an important domain associated with motivation to use psilocybin.25 Although experiences of insight may sometimes overlap with mystical-type experience, a statistical path analysis of cross-sectional survey data suggests that insightful and mystical experiences independently mediate positive therapeutic outcomes on depression, anxiety, and substance use after psychedelics.26−28 A prospective survey study that assessed respondents before and after taking a psychedelic in a noncontrolled, naturalistic manner showed that a measure of “emotional breakthrough,” likely related to psychological insight, predicted well-being 2 weeks later after controlling for mystical and challenging types of experiences.24 A recent open label study of psilocybin in depression (N = 24) showed a strong correlation (r = 0.60) between ratings of psychological insight the day after the session and decreases in depression 4 weeks later.10
Proposal for a Critical Test of the Relevance of Subjective Effects
Although preliminary, the foregoing experimental observations make a case that some subjective effects occasioned by moderate to high doses of psychedelics play a key role in their enduring beneficial effects. It is our contention that the only definitive study to disprove the importance of such subjective effects would be one in which a psychedelic was administered to individuals who were rendered fully unconscious (e.g., via deep anesthesia) and who subsequently reported no memory for a psychedelic-like experience. Although we think it to be highly unlikely, if full and lasting therapeutic efficacy remained under these conditions, we would concede that the subjective effects are irrelevant.
Conclusion
Based on the results from experimental studies of moderate to high dose psychedelics we believe that the case for subjective effects playing a major role in enduring beneficial effects is compelling. Across a number of studies, when the intensity of the subjective psychedelic effect is controlled, certain subjective effects predict desirable outcomes. Underlying neurobiological-based mechanisms are undoubtedly necessary but likely not sufficient to confer full beneficial effects. In the nonsubjective anesthesia test that we describe, we would not be surprised to see some therapeutic effects but that they would be of lower magnitude and/or more transient. We suspect that the proportion of the long-term beneficial outcomes that are mediated through subjective effects is substantial, accounting for the majority of the lasting beneficial effects of psychedelics. For an alternative perspective, please see a companion Viewpoint in this issue.1
Acknowledgments
We thank Chris Letheby (University of Western Australia), Albert Garcia-Romeu (Johns Hopkins University School of Medicine), Brian D. Earp (Yale University), Derek E. Anderson (Boston University), and Chaz Firestone (Johns Hopkins University) for their helpful comments and suggestions. Support for Drs. D. Yaden and Griffiths through the Johns Hopkins Center for Psychedelic and Consciousness Research was provided by Tim Ferriss, Matt Mullenweg, Blake Mycoskie, Craig Nerenberg, and the Steven and Alexandra Cohen Foundation.
Dr. Griffiths reports grants from the Riverstyx Foundation, a crowdsourced funding campaign organized by Tim Ferris, and National Institute on Drug Abuse (grant R01DA03889) for research support outside of submitted the work; personal fees from the Heffter Research Institute (HRI) to cover travel costs as member of the board of directors of HRI outside the submitted work; and is site principal investigator for a multisite trial of psilocybin-facilitated treatment of major depressive disorder, which is sponsored by the Usona Institute.
The authors declare no competing financial interest(s).
References
- Nichols D. E. (2018) (2016). Psychedelics. Pharmacol. Rev. 68 (2), 264–355. 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider F. X.; Vollenweider-Scherpenhuyzen M. F.; Bäbler A.; Vogel H.; Hell D. (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport 9 (17), 3897–3902. 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Johnson M. W.; Hendricks P. S.; Barrett F. S.; Griffiths R. R. (2019) Classic psychedelics: An integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol. Ther. 197, 83–102. 10.1016/j.pharmthera.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Bogenschutz M. P.; Forcehimes A. A.; Pommy J. A.; Wilcox C. E.; Barbosa P. C. R.; Strassman R. J. (2015) Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J. Psychopharmacol. 29 (3), 289–299. 10.1177/0269881114565144. [DOI] [PubMed] [Google Scholar]
- Griffiths R. R.; Johnson M. W.; Carducci M. A.; Umbricht A.; Richards W. A.; Richards B. D.; Cosimano M. P.; Klinedinst M. A. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 30 (12), 1181–1197. 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S.; Bossis A.; Guss J.; Agin-Liebes G.; Malone T.; Cohen B.; Mennenga S. E; Belser A.; Kalliontzi K.; Babb J.; Su Z.; Corby P.; Schmidt B. L (2016) Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J. Psychopharmacol. 30 (12), 1165–1180. 10.1177/0269881116675512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R. L; Bolstridge M.; Rucker J.; Day C. M J; Erritzoe D.; Kaelen M.; Bloomfield M.; Rickard J. A; Forbes B.; Feilding A.; Taylor D.; Pilling S.; Curran V. H; Nutt D. J (2016) Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3 (7), 619–627. 10.1016/S2215-0366(16)30065-7. [DOI] [PubMed] [Google Scholar]
- Johnson M. W.; Garcia-Romeu A.; Cosimano M. P.; Griffiths R. R. (2014) Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J. Psychopharmacol. 28 (11), 983–992. 10.1177/0269881114548296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. K., Barrett F. S., May D. G., Cosimano M. P., Sepeda N. D., Johnson M. W., Finan P. H., and Griffiths R. R.. Effects of psilocybin-assisted therapy for major depressive disorder: A randomized clinical trial. JAMA Psychiatry, 2020, online. 10.1001/jamapsychiatry.2020.3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibicke M.; Landry A. N.; Kramer H. M.; Talman Z. K.; Nichols C. D. (2020) Psychedelics, but not ketamine, produce persistent antidepressant-like effects in a rodent experimental system for the study of depression. ACS Chem. Neurosci. 11 (6), 864–871. 10.1021/acschemneuro.9b00493. [DOI] [PubMed] [Google Scholar]
- Ly C.; Greb A. C.; Cameron L. P.; Wong J. M.; Barragan E. V.; Wilson P. C.; Burbach K. F.; Soltanzadeh Zarandi S.; Sood A.; Paddy M. R.; Duim W. C.; Dennis M. Y.; McAllister A. K.; Ori-McKenney K. M.; Gray J. A.; Olson D. E. (2018) Psychedelics promote structural and functional neural plasticity. Cell Rep. 23 (11), 3170–3182. 10.1016/j.celrep.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R. L. (2018) The entropic brain-revisited. Neuropharmacology 142, 167–178. 10.1016/j.neuropharm.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Schultes R. E. (1969) Hallucinogens of plant origin. Science 163, 245. 10.1126/science.163.3864.245. [DOI] [PubMed] [Google Scholar]
- Belser A. B.; Agin-Liebes G.; Swift T. C.; Terrana S.; Devenot N.; Friedman H. L.; Guss J.; Bossis A.; Ross S. (2017) Patient experiences of psilocybin-assisted psychotherapy: an interpretative phenomenological analysis. J. Humanist Psychol 57 (4), 354–388. 10.1177/0022167817706884. [DOI] [Google Scholar]
- Noorani T.; Garcia-Romeu A.; Swift T. C.; Griffiths R. R.; Johnson M. W. (2018) Psychedelic therapy for smoking cessation: qualitative analysis of participant accounts. J. Psychopharmacol. 32 (7), 756–769. 10.1177/0269881118780612. [DOI] [PubMed] [Google Scholar]
- Griffiths R. R.; Richards W. A.; McCann U.; Jesse R. (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology 187 (3), 268–283. 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- Griffiths R. R.; Richards W. A.; Johnson M. W.; McCann U. D.; Jesse R. (2008) Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J. Psychopharmacol. 22 (6), 621–632. 10.1177/0269881108094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. R.; Johnson M. W.; Richards W. A.; Richards B. D.; McCann U.; Jesse R. (2011) Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology 218 (4), 649–665. 10.1007/s00213-011-2358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. R.; Johnson M. W.; Richards W. A.; Richards B. D.; Jesse R.; MacLean K. A.; Barrett F. S.; Cosimano M. P.; Klinedinst M. A. (2018) Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. J. Psychopharmacol. 32 (1), 49–69. 10.1177/0269881117731279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaden D. B.; Haidt J.; Hood R. W. Jr; Vago D. R.; Newberg A. B. (2017) The varieties of self-transcendent experience. Rev. Gen Psychol 21 (2), 143–160. 10.1037/gpr0000102. [DOI] [Google Scholar]
- Barrett F. S.; Johnson M. W.; Griffiths R. R. (2015) Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. J. Psychopharmacol. 29 (11), 1182–1190. 10.1177/0269881115609019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romeu A.; Griffiths R.; Johnson M. (2015) Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr. Drug Abuse Rev. 7 (3), 157–164. 10.2174/1874473708666150107121331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman L.; Nutt D. J.; Carhart-Harris R. L. (2018) Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front. Pharmacol. 8, 974. 10.3389/fphar.2017.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro T. M.; Johnson M. W.; Griffiths R. R. (2020) Subjective features of the psilocybin experience that may account for its self-administration by humans: A double-blind comparison of psilocybin and dextromethorphan. Psychopharmacology 237, 2293–2304. 10.1007/s00213-020-05533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romeu A.; Davis A. K.; Erowid F.; Erowid E.; Griffiths R. R.; Johnson M. W. (2019) Cessation and reduction in alcohol consumption and misuse after psychedelic use. J. Psychopharmacol. 33 (9), 1088–1101. 10.1177/0269881119845793. [DOI] [PubMed] [Google Scholar]
- Garcia-Romeu A.; Davis A. K.; Erowid E.; Erowid F.; Griffiths R. R.; Johnson M. W. (2020) Persisting reductions in cannabis, opioid, and stimulant misuse after naturalistic psychedelic use: An online survey. Front Pharmacol 10, 955. 10.3389/fpsyt.2019.00955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. K.; Barrett F. S.; Griffiths R. R. (2020) Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J. Contextual Behav Sci. 15, 39–45. 10.1016/j.jcbs.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson D.The Subjective Effects of Psychedelics May Not Be Necessary for Their Therapeutic Impact. ACS Pharm. Transl. Sci. 2020 10.1021/acsptsci.0c00192. [DOI] [PMC free article] [PubMed] [Google Scholar]