Background: In December 2020, the U.S. Food and Drug Administration issued an emergency use authorization for the Pfizer-BioNTech and Moderna messenger RNA vaccines for the prevention of COVID-19. This authorization was a significant step toward mitigating the burden of the disease. Nevertheless, hypersensitivity reactions to these vaccines are being reported (5.0 and 2.8 cases per million doses for the Pfizer-BioNTech and Moderna vaccines, respectively) (1), and these rates are higher than those for other similar vaccines (1.3 cases per million doses) (2). No deaths from these COVID-19 vaccines have been reported. However, guidelines from the Centers for Disease Control and Prevention recommend avoiding subsequent vaccine doses in persons who have any immediate hypersensitivity reaction with the first dose (3). Although others have reported skin testing protocols to assess the risk of these patients receiving a second dose (4), there are conflicting opinions about how this information should be used. For other vaccines, if the skin test results are positive and the patient requires subsequent doses, the vaccine is administered in graded doses under observation (5).
Objective: To provide what we believe to be the first report using graded doses to successfully administer a second dose of the Moderna vaccine to 2 patients who had an immediate hypersensitivity reaction with the first dose.
Case Reports: Patient 1 is a 64-year-old woman with a history of shellfish allergy who received dose 1 of the Moderna vaccine on 23 December 2020. Within 10 minutes, she had generalized pruritus, urticaria, and self-reported tachycardia but no angioedema, respiratory or gastrointestinal symptoms, or hypotension. Medical personnel at the vaccination site evaluated her and treated her with 50 mg of oral diphenhydramine. Her symptoms resolved within 90 minutes.
Patient 2 is a 39-year-old woman with a history of allergic rhinitis who received dose 1 of the Moderna vaccine on 5 January 2021 and developed chest and neck urticaria within 15 minutes. Medical personnel treated her with 25 mg of oral diphenhydramine at the vaccination site, but she went on to develop mild facial angioedema within 30 minutes of vaccination. She was transported by ambulance to the emergency department, where she received 20 mg of intravenous famotidine and 125 mg of methylprednisolone without further progression of symptoms during 2 hours of observation. She was then discharged to home in stable condition.
Both patients were referred to our allergy practice for further evaluation. We did skin prick and intradermal testing for polyethylene glycol, polysorbate, and the Moderna vaccine (using remaining overfill from previously used vaccine vials), following recommendations from Banerji and colleagues (4). Results from the skin prick were negative for all components in both patients, whereas both patients had positive results on intradermal testing with the Moderna vaccine. Both patients worked in health care settings with repeated exposure to patients with COVID-19 and participated in shared decision making about proceeding with the second dose of the vaccine. As a result, we administered the vaccine without premedication through a graded dosing protocol as previously described for other vaccines (Table) (5). Patient 1 had no symptoms during the protocol. Patient 2 reported pruritus after doses 2 and 5, but it resolved without medical intervention. Both patients reported no additional symptoms over the following 24 hours. In addition, 3 to 4 weeks after receiving the second dose, both patients had IgG antibodies directed against the spike protein of COVID-19, suggesting vaccination was efficacious despite the graded dosing protocol.
Table. Protocol for Graded Administration of Second Dose of Moderna COVID-19 Vaccine*.
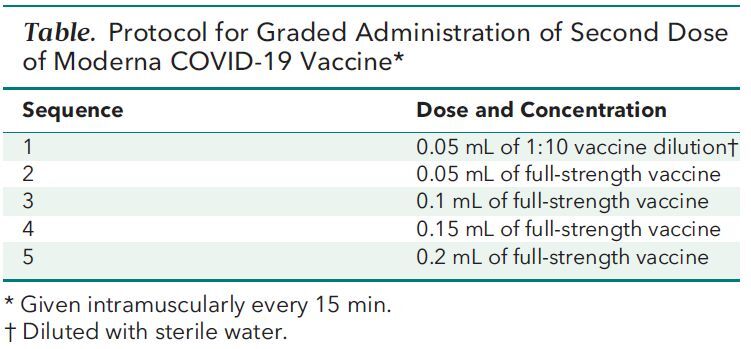
Discussion: Our experience highlights several important points. First, persons who have an immediate hypersensitivity reaction to dose 1 of the Pfizer-BioNTech or Moderna vaccine should not automatically defer dose 2. Instead, referral to an allergy and immunology physician for further evaluation and management should be considered. Second, although the mechanism of hypersensitivity reaction to messenger RNA vaccines is uncertain, skin testing may guide further vaccine administration. Third, we have shown that dose 2 of the Moderna vaccine can be safely administered through a graded dosing protocol to persons who have symptoms consistent with an immediate hypersensitivity reaction to dose 1, resulting in protective antibodies against COVID-19. Limitations of our report include the uncertainty surrounding patients who experience anaphylaxis with the first dose of the Moderna vaccine and whether or not a single dose would be sufficiently protective in these persons. Further study in these rare cases is warranted.
Footnotes
This article was published at Annals.org on 6 April 2021.
References
- 1.Shimabukuro T. COVID-19 vaccine safety update. Advisory Committee on Immunization Practices (ACIP). 27 January 2021. Accessed at www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-01/06-COVID-Shimabukuro.pdf on 20 March 2021.
- 2. McNeil MM , Weintraub ES , Duffy J , et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-78. [PMID: ] doi: 10.1016/j.jaci.2015.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. What to do if you have an allergic reaction after getting a COVID-19 vaccine. Accessed at www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/allergic-reaction.html on 5 February 2021.
- 4. Banerji A , Wickner PG , Saff R , et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2020. [PMID: ] doi: 10.1016/j.jaip.2020.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelso JM , Greenhawt MJ , Li JT , et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130:25-43. [PMID: ] doi: 10.1016/j.jaci.2012.04.003 [DOI] [PubMed] [Google Scholar]


