Abstract
The immune system depends on two major paths—the innate and the adaptive immunity. Macrophage, with its unique features as the first line of immune defense to engulf and digest invaders, serves as the key effector cells integrating those two paths. The dynamic plasticity of macrophage activation during wound repair, inflammation resolution, and tissue remodeling are emerging biomedical and bioengineering hot topics in immune function studies such as the various secretions of cytokines and chemokines and the signaling pathways with ligands and their cognate receptors. Better knowledge on how physical/mechanical and multicellular microenvironment on the modulation of macrophage functions will create innovative therapies to boost host defense mechanism and assist wound healing. In this, we describe an easy method to measure functions (gene expressions) of human and mouse macrophages in response to mechanical microenvironment changes by embedding isolated macrophages in polymerized hyaluronan gel with different wound matrix stiffness.
Keywords: Hyaluronan, Extracellular matrix, Matrix stiffness, Cytokines, Chemokines, Gene expression, PBMC
1. Introduction
Macrophages contribute to diverse biology including tissue development, angiogenesis, inflammation, organ injury, and repair [1, 2]. These multifunctional macrophages phenotypes are due to their dynamic adoption/polarization in responding to signals from their microenvironments [3]. Macrophages play essential roles in all phases of wound healing process, such as boosting host immune defense from microbes via promoting and resolving inflammation through inflammatory cytokines secretions, dead cells removal, supporting epithelial cell proliferation, and tissue remodeling (scar formation) after a wound occurs [4]. Macrophages respond to chemical and physical cues upon wound healing. As such, dysregulated macrophages-mediated inflammation could also lead to tissue destruction. The tissue microenvironments such as matrix property of wound stiffness have recently opened up new research avenues and therapeutic opportunities in wound healings.
Hyaluronan (HA, hyaluronic acid) is a polyanionic, nonsulfated glycosaminoglycan composed of long chain disaccharide units of GlcNAc and D-glucuronic acid polymer that repeat up to 20,000 or more times to form a linear cable with length > 20 μm and high molecular mass (>8 million Da) [5, 6]. In fact, the wide range of molecular sizes of HA dictates its different biological functions and cellular signaling [7]. HA is a major extracellular matrix (ECM) carbohydrate polymer widely distributed throughout human body and enriched in many tissues including connective, epithelial, and neural tissues, joints, and skin. In normal human body, HA constitutes 0.02% body weight (15 g in 70 kg body weight) with rapid turnover as approximately a third of the body’s HA turns over daily [5]. HA is involved in many physiological and pathobiological processes such as fertilization, cell proliferation, tissue development, immune response, wound healing, atherosclerosis, angiogenesis, tissue fibrosis, and cancers [8, 9]. These versatile biological functions are related to its unique biosysnthesis process. HA is formed directly within the pericellular undersurface of the plasma membrane and extruded into extracellular space by active plasma membrane-localized hyaluronan synthase (HAS) which is transported as inactive enzyme within vesicles through the Golgi [10].
HA greatly affects tissue mechanics during wound healing as its production could escalate to 80 fold of basal level during injury and inflammation, and decline to normal level after wound remodeling [5]. It is widely accepted that cellular actions of HA are mediated by specific HA surface receptors expressed on immune cells such as CD44 [11]. Exogenous low molecular weight HA (defined as <300 kDa) was shown to enhance wound repair and increases collagen III synthesis in aged murine dermal wounds model [12]. Both matrix-bound HA content and hydrogel stiffness affect mechanical environment [13]. Moreover, high and low molecular weight HA differentially influence macrophage activation [14]. In vitro gel systems were thus designed to assess activation of macrophages infiltrating in wound with different stiffness. For example, HA polymer mixed with formulated supplemental elements to polymerize into gels with defined stiffness was used to simulate mechanical environment of stiffed wound [15].
In this protocol, we have outlined a standard protocol applying HA gel to study macrophages activation over wounds mechanical environment, such as stiffness. We demonstrated how physical changes in the stiffness of extracellular matrix (ECM) alter macrophages functions via changes in expression of inflammatory mediators. Culture of macrophages isolated from peripheral blood mononuclear cells (PBMC) on crosslinked hyalruonan hydrogels with different HA contents (stiffness) posed different inflammatory activation. Our studies confirmed that physical tension independently contributes toward macrophage activation with subsequent differential induction of gene expressions between wildtype and a mutant mouse strain. This study thus provides insight on the role of HA on macrophage function during wound healing or tissue remodeling during disease.
2. Materials
Peripheral blood mononuclear cells (PBMC) derived from animals (wildtype or mutant mouse strain) or human origin (see Note 1).
Ficoll Histopaque-1077 (colloidal silica particles with density 1.077 g/mL, Sigma 10771) for PBMC isolation. Allow to come to room temperature before use.
Cell counting (hematocytometer and 0.4% trypan blue).
HyStem®-C Hydrogel Kits (from Advanced BioMatrix) (see Note 2).
RNA extraction with TRIzol (Life Technologies) and RNeasy Mini Kit (Qiagen).
c-DNA synthesis with reverse transcription and gene expression polymerase chain reaction (PCR) arrays (various vendors).
Standard tissue culture media RPMI 1640, fetal bovine serum (FBS), and penicillin and streptomycin.
Confocal microscope LSM 780 (Zeiss).
3. Methods
3.1. PBMC and Macrophage Isolation
Collect whole blood, roughly 1 mL per mouse, into a heparinized tube (see Note 3).
In a biosafety hood, add three parts (by volume) sterile RPMI 1640 medium to dilute the whole blood in a 15-mL Falcon conical tube.
Pour 4 mL Ficoll Histopaque-1077 into a 15-mL conical tube for each blood sample isolation.
Carefully layer 4 mL diluted blood on top of each Ficoll layer, taking care not to mix blood and Ficoll (lay Falcon conical tube almost horizontal and slowly layer the diluted blood over Ficoll).
Perform density gradient centrifugation with a horizontal rotor. Centrifuge at 400 × g for 30 min at room temperature (15–20 °C) with deceleration and acceleration both set at 1, brake off.
Following centrifugation, carefully remove the top plasma layer using a Pasteur pipette and discard.
Gently aspirate the middle PBMC layer using a Pasteur pipette and add to a new Falcon tube.
Wash PBMCs by adding 10 mL sterile RPMI 1640 and centrifuging at 300 × g for 10 min at RT.
Carefully remove supernatant with a pipette to avoid disturbing the loose pellet.
Repeat washing step once.
Resuspend cell pellet in 10 mL of complete culture media (RPMI 1640–10% FBS).
Enrich macrophages from PBMC preparation by adherence method (steps 13 and 14) (see Note 4).
Resuspend the PBMCs (roughly10–15 × 106 cells) and seed cells into T-75 flask.
Allow to adhere in a 5% CO2 incubator at 37 °C for 2 h in 10 mL of complete culture media (RPMI 1640–10% FBS).
Remove nonadherent cells and carefully wash the adherent cells twice with RPMI 1640–10% FBS.
Verify cell viability and numbers (by 0.4% trypan blue exclusion method) under microscopy with hemocytometer or alternative cell counter.
3.2. Preparation of Hyaluronan Hydrogel Components with Optimal Stiffness
The desired stiffness of hydrogel is achieved by crosslinking various HA contents. In the current study, we focused on two HA stiffness groups, low (equivalent to Young’s modulus 70 Pa) and high (110 Pa) [15]. A blank control was included with macrophages cultured in growth medium only (zero stiffness). Table 1 serves as an example of 24-well tissue culture format. Meanwhile, HA hydrogel preparation should be coordinated with “macrophage plating” (Subheading 3.3) to maximize cell viability and wellness in casted gel.
Table 1.
Formulations of blank, low, and high stiffness HA gel
| Macrophage in RPM11640–10% FBS | 100 μL | 100 μL | 100 μL |
|---|---|---|---|
| Glycosil (100-kDa hyaluronate) | 0 | 50 μL (1%) |
50 μL (2%) |
| Gelin-S (thiolated collagen) | 0 | 50 μL (1%) |
50 μL (2%) |
| Extralink (aPEGDA-crosslinker) | 0 | 400 μL (1%) |
400 μL (2%) |
| RPMI 1640-10 FBS | 750 μL | 250 μL | 250 μL |
| Stiffnessb (pa) | Blank (0 pa) |
Low (70 pa) |
High (110 pa) |
PEGDA-Polyethylene Glycol Diacrylate Acrylate
Stiffness is a mechanical property that a solid material preserves its shape resisting to stress applied on it. It is measured according to Young’s modulus, derived as the reciprocal of the stress, presented in unit of Pa. In SI base units, Pa = kg m−1 s−2
-
Prepare the working solutions for each of the three components from HyStem®-C Hydrogel Kits (see Note 5).
Formulate HA gels accordingly (see Table 1).
Low stiffness group: 1% Glycosil: 1% Gelin-S: 1% Extralink in volume ratio of 1:1:8.
High stiffness group: 2% Glycosil: 2% Gelin-S: 2% Extralink in volume ratio of 1:1:8.
Reconstitute and prepare 1% and 2% Thiolated collagen (Gelin-S) solution in sterile ddH2O following manufacturer’s protocol. Reconstitute each vacuumed vial with lyophilized Gelin-S powder with 0.5- or 1- volume/mL ddH2O which would give rise to either 2% or 1%, respectively.
Reconstitute and prepare 1% and 2% Sodium HA solution (Glycosil) in sterile ddH2O by reconstituting each vacuumed vial with lyophilized Glycosil powder with 0.5- or 1- volume/mL ddH2O which would give rise to either 2% or 1%, respectively.
Prepare the two “Glycosil: Gelin-S mixtures” (Table 1) and set aside. First, Mix 1% Gelin-S (from Subheading 3.2, step 2) with 1% glycosil (from Subheading 3.2, step 3) at 1:1 ratio by volume. Second, mix 2% Gelin-S with 2% glycosil at 1:1 ratio by volume.
Reconstitute and prepare 1% and 2% polyethylene glycol diacrylate (PEGDA) and set aside. Reconstitute PEGDA powder in vial with ddH2O to generate 1% or 2% solutions. It will require four parts (by volume) of PEGDA for each one part (volume) of “Glycosil:Gelin-S mixture.”
3.3. Macrophage Plating and HA Hydrogel Polymerization
Prepare macrophage at a cell density of 1–5 × 105 cells per well (24-well culture plate) (see Note 6).
The hydrogel which will crosslink its constituents must be in a complete fluidity form when macrophages are mixed in.
Gently mix macrophages with the “Glycosil: Gelin-S mixture” (from Subheading 3.2, step 4) according to Table 1; ensure well mixing. This final mixture, in volume, contains macrophages, Glycosil, and Gelin-S at a ratio of 2:1:1.
Initiate polymerization by adding crosslinker PEGDA (from Subheading 3.2, step 5) into above mixture (from Subheading 3.3, step 3) with gentle pipetting (see Note 7).
Gel formation with embedded macrophages will proceed in sterile condition in cell culture incubator at 37 °C, 5% carbon dioxide with saturated humidity for 30 min.
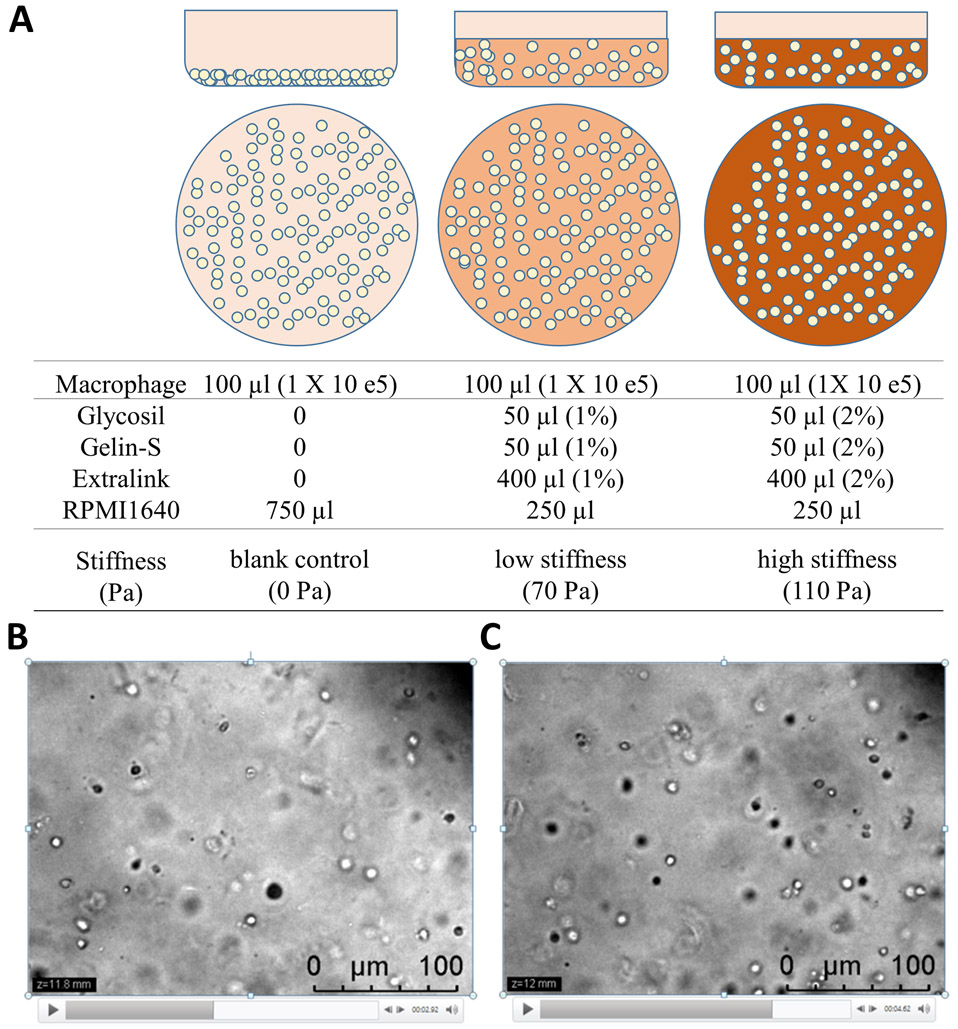
Replenish wells with 250 μL fresh culture medium (RPMI 1640, FBS10%) and culture for 72 h (see Note 8 and Fig. 1a).
Image macrophages in HA hydrogels with confocal microscope (Fig. 1b, c).
Fig. 1.
3D z-stack images of plated macrophages embedded in hyaluronan gel. HA gel could be formulated with various stiffness. (a) Schematic diagram depicts three various formulations to prepare three HA hydrogels (blank, low, or high stiffness), respectively. Diagrams shown side (top panels) and top (middle panels) views of cultured macrophages with different HA formulations (bottom panels). (b and c) Two representative z-stack microscopic images captured from a high-stiffness gel at different z-levels (11.8-mm and 12-mm for b and c, respectively). Macrophages were shown evenly embedded in HA gels with nearly identical cell density at different z-levels. Scale bars, 100 μm
3.4. Gene Expression Analysis with RT-PCR Arrays
Remove top layer of culture media.
Retrieve gel. Cells retrieving and lysis are done by applying 120 μL of TRIzol to gently crush the HA hydrogel by applying gentle force and pipetting (see Note 9).
Proceed with total RNA extraction and purification with TRIzol reagent and RNeasy Mini Kit according to manufacturer’s protocols.
Measure RNA concentration using a NanoDrop spectrophotometer and use 500–1000 ng RNA to synthesize cDNA using high-capacity RNA to cDNA kit according to manufacturer’s protocols. The cDNA can be stored at —20 °C until PCR reaction (see Note 10).
Dilute cDNA 10× with PCR-grade water.
Set up PCR reaction with RT2 PCR array analysis according to manufacturer’s protocols (see Note 11).
Alternatively, PCR could be performed with designed target primers (see Note 12).
Run RT-PCR reaction with target-specific PCR program.
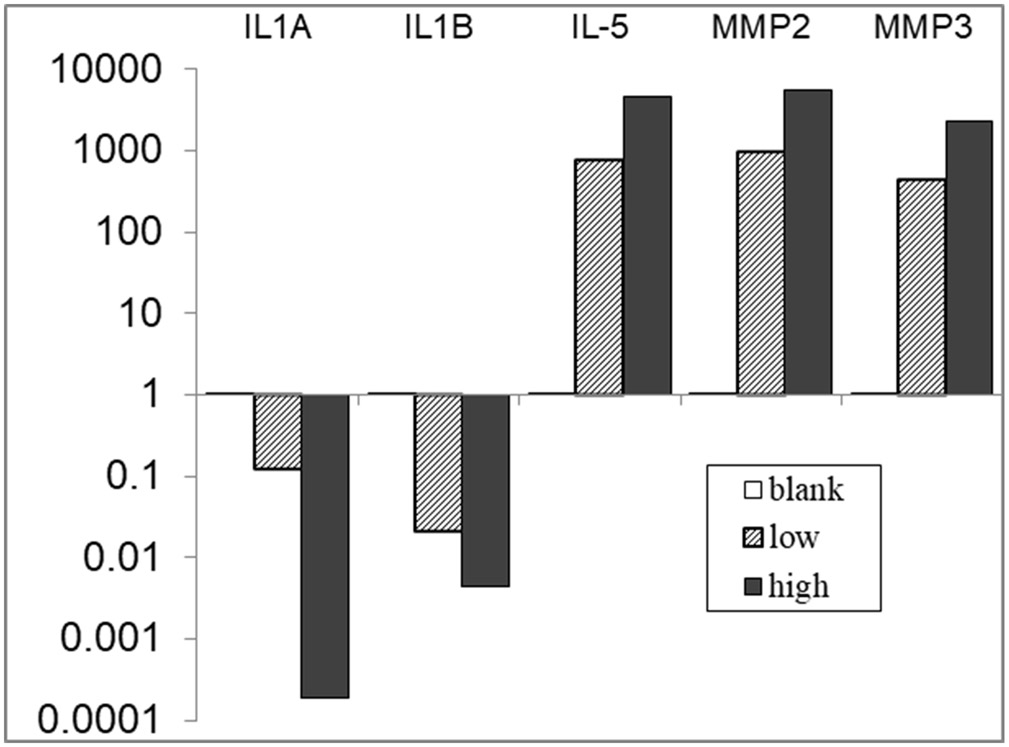
Calculate expression of IL-1α, IL-1β, IL-5, MMP2 and MMP3 using the ΔΔCT method. Use average expression of the three housekeeping genes (GAPDH, actin, and HSP90) to calculate the relative expression (Fig. 2).
Fig. 2.
Stiffness of HA hydrogel affects gene expressions of isolated macrophages derived from wildtype and mutant mice. Differential gene expressions (RT2 PCR array) after 72-h culture under different HA gel stiffness were detected from macrophages of wildtype and mutant strain, e.g., wound-prone (IL10−/−) mice. Changes of gene expression (relative to wildtype) were indicated by folds (2−ΔΔCt, in logarithm). Compared to wildtype macrophages, mutant macrophages displayed aberrant cytokines and MMP gene expressions in response to different microenvironmental HA stiffness
Acknowledgments
This work was supported by an Ohio State University intramural Lockwood Research grant (to P.H.L).
Footnotes
The PBMC could be obtained from either mouse or human origins. PBMCs contain enriched leukocytes and monocytes. This method could be applied to isolate macrophages from wildtype or mutant murine strains with immune dysfunctions [16]. Alternatively, heparinized human whole blood (65 mL) could be obtained from normal healthy donors (sourced from blood bank) or specific patient targets [17].
HyStem®-C Hydrogel Kits contains three biocompatible components: thiol-modified hyaluronan (Glycosil) (100 kDa), thiol-modified denatured collagen (Gelin-S®), and thiol-reactive crosslinker, PEGDA (Extralink). Each is packaged as sterile lyophilized powder.
We demonstrated mouse whole blood PBMC isolation here. For human PBMC isolation, scale up reagents accordingly. In general, routinely 1–2 × 106 PBMC will be obtained per mL blood. Assuming 10% monocyte content, 2.5–3 × 107 monocytes is expected to be isolated from 250 mL whole blood. Compared with normal subjects, abnormal macrophages behaviors might be detected from patients with remarkable keloid scar condition.
A typical yield would be achieved for monocyte (assuming 10–25% in content) by seeding 1.0 × 106 PBMC per cm2 of adhesive area. An alternative to isolate monocytes is affinity column purification where monocyte/macrophage will be retained in affinity column, while the rest of the cells elute off [16]. Follow manufacturer’s protocol accordingly.
We followed gel preparation method according to Nagy et al. ([15]) to achieve three different (blank control, low and high) gel stiffness by mixing sodium hyaluronate (HA) in thiolated collagen (Gelin-S) and crosslinker PEGDA.
One mL of mouse peripheral blood would yield 1–7 × 106 PBMC and 25% of them are macrophage/monocytes. A practical preparation is to pool total recovery macrophages from plastic adherence of mouse PBMC, make a master mixing, and reconstitute to 0.5 mL with culture media (RPMI 1640–10% FBS). Divide those concentrated cells to 6–8 aliquots. Each aliquot, containing approximately 1–5 × 105 monocyte/macrophages, is subjected to each experimental group analysis.
Gently pipette-mixing the cells in all three hydrogel components would ensure even distribution of cells embedded in gel from bottom to top. This would ensure that macrophages respond to only calculated gel stiffness in all three experimental groups. This step is critical and might require several practices to achieve perfection.
Macrophages are cultured in basal medium (RPMI 1640 with 10% FBS, glutamine, and antibiotics penicillin/streptomycin) to ensure no further activation from growth factors such as M-CSF (myeloid-colony stimulating factor (M-CSF) and only respond to physical environmental cue.
TRIzol contains corrosive phenol and should be handled with caution in a chemical hood or a biosafety hood.
Use 500–1000 ng total RNA to synthesize cDNA in a final 20-μL reaction mixture.
Several target RT2PCR arrays are commercially available, such as inflammation, fibrosis cytokines, and chemokines arrays.
Alternatively, PCR gene expression analysis could be performed with self-designed target primers. Mix 5 μL (2×) Fast Sybr Green Master Mix, 0.5 μL forward and reverse primers (from 10 μM stock, the final primer concentration is 500 nM), and 4 μL diluted cDNA (equivalent to original 50–100 ng original RNA) for each 384-well final RT-PCR reaction. Final volume is 10 μL per well.
References
- 1.Hamidzadeh K, Christensen SM, Dalby E et al. (2017) Macrophages and the recovery from acute and chronic inflammation. Annu Rev Physiol 79:567–592. 10.1146/annurev-physiol-022516-034348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vannella KM, Wynn TA (2017) Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol 79:593–617. 10.1146/annurev-physiol-022516-034356 [DOI] [PubMed] [Google Scholar]
- 3.Murray PJ (2018) Immune regulation by monocytes. Semin Immunol 35:12–18. 10.1016/j.smim.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 4.Lin PH, Sermersheim M, Li H et al. (2017) Zinc in wound healing modulation. Nutrients 10(1). 10.3390/nu10010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraser JR, Laurent TC, Laurent UB (1997) Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 242(1):27–33. 10.1046/j.1365-2796.1997.00170.x [DOI] [PubMed] [Google Scholar]
- 6.Hascall VC (2019) The journey of hyaluronan research in the journal of biological chemistry. J Biol Chem 294(5):1690–1696. 10.1074/jbc.TM118.005836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavianatou AG, Caon I, Franchi M et al. (2019) Hyaluronan: molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J 286(15):2883–2908. 10.1111/febs.14777 [DOI] [PubMed] [Google Scholar]
- 8.Jiang D, Liang J, Noble PW (2011) Hyaluronan as an immune regulator in human diseases. Physiol Rev 91(1):221–264. 10.1152/physrev.00052.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spinelli FM, Vitale DL, Demarchi G et al. (2015) The immunological effect of hyaluronan in tumor angiogenesis. Clin Transl Immunology 4(12):e52. 10.1038/cti.2015.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weigel PH, Hascall VC, Tammi M (1997) Hyaluronan synthases. J Biol Chem 272 (22):13997–14000. 10.1074/jbc.272.22.13997 [DOI] [PubMed] [Google Scholar]
- 11.Johnson P, Ruffell B (2009) CD44 and its role in inflammation and inflammatory diseases. Inflamm Allergy Drug Targets 8(3):208–220 [DOI] [PubMed] [Google Scholar]
- 12.Damodarasamy M, Johnson RS, Bentov I et al. (2014) Hyaluronan enhances wound repair and increases collagen III in aged dermal wounds. Wound Repair Regen 22 (4):521–526. 10.1111/wrr.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JE, Pedron S, Harley BAC (2017) The combined influence of hydrogel stiffness and matrix-bound hyaluronic acid content on Glioblastoma invasion. Macromol Biosci 17(8). 10.1002/mabi.201700018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rayahin JE, Buhrman JS, Zhang Y et al. (2015) High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng 1(7):481–493. 10.1021/acsbiomaterials.5b00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagy N, de la Zerda A, Kaber G et al. (2018) Hyaluronan content governs tissue stiffness in pancreatic islet inflammation. J Biol Chem 293 (2):567–578. 10.1074/jbc.RA117.000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Goncalves R, Mosser DM (2008) The isolation and characterization of murine macrophages. Curr Protoc Immunol. Chapter 14:Unit 14.11. 10.1002/0471142735.im1401s83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly A, Grabiec AM, Travis MA (2018) Culture of human monocyte-derived macrophages. Methods Mol Biol 1784:1–11. 10.1007/978-1-4939-7837-3_1 [DOI] [PubMed] [Google Scholar]