Abstract
The aim of this study was to explore the therapeutic effects of fat grafting on radiation-induced hind limb contracture. Radiation therapy (RT) is used to palliate and/or cure a range of malignancies but causes inevitable and progressive fibrosis of surrounding soft tissue. Pathological fibrosis may lead to painful contractures which limit movement and negatively impact quality of life. Fat grafting is able to reduce and/or reverse radiation-induced soft tissue fibrosis. We explored whether fat grafting could improve extensibility in irradiated and contracted hind limbs of mice. Right hind limbs of female 60-day-old CD-1 nude mice were irradiated. Chronic skin fibrosis and limb contracture developed. After 4 weeks, irradiated hind limbs were then injected with (a) fat enriched with stromal vascular cells (SVCs), (b) fat only, (c) saline, or (d) nothing (n = 10/group). Limb extension was measured at baseline and every 2 weeks for 12 weeks. Hind limb skin then underwent histological analysis and biomechanical strength testing. Irradiation significantly reduced limb extension but was progressively rescued by fat grafting. Fat grafting also reduced skin stiffness and reversed the radiation-induced histological changes in the skin. The greatest benefits were found in mice injected with fat enriched with SVCs. Hind limb radiation induces contracture in our mouse model which can be improved with fat grafting. Enriching fat with SVCs enhances these beneficial effects. These results underscore an attractive approach to address challenging soft tissue fibrosis in patients following RT.
Keywords: contracture, fat grafting, hind limb contracture, irradiation, radiation fibrosis
1 ∣. INTRODUCTION
Radiation therapy (RT) is extremely effective at shrinking tumor size and reducing local recurrence1 but causes substantial collateral damage to healthy tissue. The hallmark of long-term radiation-induced damage is progressive and pathological fibrosis,2 including formation of contractures.3 Contractures are especially common following RT to the extremities or head and neck.4 Treatment options for radiation-induced contracture are limited; however, fat grafting is increasingly recognized for its ability to reverse soft tissue fibrosis following RT in other clinical situations.5,6 Superior outcomes are found when grafted fat is enriched with adipose-derived stromal cells (ASCs) (“cell-assisted lipotransfer”)7 implicating ASCs as the key drivers of tissue regeneration.8 Few reports have described the beneficial effects of fat grafting in irradiated tissue on function: fat grafts injected into the base of the tongue may ameliorate severe dysphagia in patients irradiated for nasopharyngeal carcinoma9,10; and fat injections into the irradiated subcutaneous tissue of patients with head and neck cancer can improve phonation and swallowing up to 2 years post-RT.11,12 We therefore adapted a mouse model for radiation-induced soft tissue fibrosis to induce limb contracture13,14 and explored whether fat grafting may have a therapeutic effect in this setting.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Animal hind limb irradiation
The ventral right hind limbs of adult female CD-1 nude mice (Charles River) (total n = 40, mean weight 24.40 ± 0.23 g) were irradiated with 30 Gy delivered in six 5 Gy doses across 12 days. A 1-month recovery period followed to allow contracture to develop. Dosing and fractionation protocols were selected based on previous protocols generating radiation-induced fibrosis in the calvarial region for studying fat grafts.6,7 All experiments were performed under approved APLAC protocols (APLAC #31212).
2.2 ∣. Stromal vascular cell isolation
Lipoaspirate was obtained from healthy donors (n = 3 female, mean age: 48.33 ± 1.76 years) under an approved protocol (IRB #2188). The fat was washed, allowed to settle for 30 minutes at 4°C, and the layer of fat was retrieved; 4 mL was set aside for grafting, and the remainder was digested with collagenase (0.75 mg/mL, Sigma-Aldrich, St. Louis, MO #C6685 in Medium 199 [HyClone, Logan, UT #SH30223.02], 5% fetal bovine serum [FBS, Gibco, #10082147], 100 U/mL DNase I [Worthington], 0.1% Poloxamer 188 [Sigma-Aldrich, #P5556-100ML], 20 mM HEPES [Sigma-Aldrich, #15630080], 1 mM CaCl2) for 30 minutes at 37°C in a shaking water bath (150 rpm). The enzyme was quenched with FACS buffer (phosphate-buffered saline [PBS], 2% FBS, 1% penicillin-streptomycin (#15140122, ThermoFisher, Waltham, MA), and the digest was filtered (100 μm nylon strainer), pelleted (450 g, 5 minutes, 4°C), resuspended in FACS buffer, placed onto Histopaque (1.077 g/mL, #10771, Sigma Aldrich), and centrifuged (1500 rpm 27°C, 30 minutes, without deceleration) to isolate the stromal vascular cell (SVC) layer.
2.3 ∣. Fat grafting
Mice were anesthetized (1%-4% isoflurane), skin was disinfected with 70% ethanol, and a 0.5 cm oblique groin incision was made using sterile scissors. The subcutaneous space overlying the muscle was injected in a retrograde fashion with (a) 200 μL of fat with SVCs (10 000 cells/200 μL (“fat + SVC”); (b) 200 μL of fat (“fat”); or (c) 200 μL PBS (“saline”) using 1-cc syringes and a 14-gauge needle.15 A fourth control group of mice underwent the same surgery but did not receive an injection (“sham”) (n = 10/group) (Figure 1A).
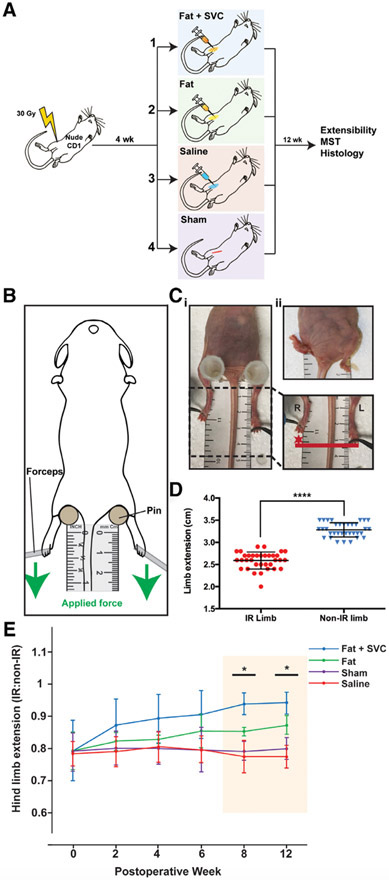
FIGURE 1.
A, The experimental design and four designated animal groups. Total n = 40 (n = 10 mice/group). B, Assessment of hind limb extension. To measure limb extension, mice were stabilized supine using pins to support the hips, with the tail equidistant between the two hind limbs. Equal force was applied to the lower limbs in the caudal direction (green arrow) until resistance was felt, at which point maximum extension was measured (in millimeters) from the middle digit. C, Radiation-induced pronounced hind limb contracture. Representative photographs (i and ii) showing shortening and contracture of the IR (right) hind limb compared with the non-IR (left) hind limb. D, Hind limb extension was significantly reduced on the IR (right) compared with the non-IR (left) side upon assessment of extensibility (****P < .0001). E, Hind limb extension improved over 12 weeks in mice receiving fat grafts. The mice receiving fat + SVCs or fat alone had progressively improved hind limb extension compared with mice in the two control groups. This trend reached significance at weeks 8 and 12 post-grafting (*P < .05). Hind limb extension was not improved beyond baseline in mice receiving saline or sham treatment. IR, irradiated; MST, mechanical strength testing; SVC, stromal vascular cells
2.4 ∣. Assessment of limb extension
Limb extension was measured at baseline and every 2 weeks for 12 weeks (Figure 1B). Mice were anesthetized (1%-4% isoflurane) supine, and the hips were stabilized with the tail equidistant between the limbs. The irradiated (right) and non-irradiated (left) limbs were extended against a millimeter ruler with equal and minimal force until considerable resistance, at which point readings were made from the tip of the central digit (3/mouse). The extension of the irradiated side was calculated as a ratio to extension of the non-irradiated side, with a value of one representing equivalent extension.
2.5 ∣. Tensile strength testing
Twelve weeks post-surgery the ventral skin overlying the hind limbs was harvested for mechanical strength testing using a microtester (model 5848) as previously described.16
2.6 ∣. Histology
Twelve weeks post-surgery, skin was also fixed in 4% paraformaldehyde (Electron Microscopy Sciences, #15710) (24 hours, 4°C) and prepared for histological analysis. Samples were dehydrated, embedded in paraffin, cut using a microtome (8 μm), and mounted on glass. Samples were stained with Hematoxylin and Eosin (H&E, Vector Laboratories, Burlingame, CA, #H-3502), Masson's Trichrome (Abcam, #ab150686), and Picrosirius Red (Abcam, #ab150681, Cambridge, England). Bright-field and polarized light images were taken with a Leica DM5000 B light microscope (Leica Microsystems, Wetzlar, Germany) at ×20 and ×40. Sections were also immunostained. Samples were blocked (×1 Power Block Universal Solution, BioGenex, Fremont, CA, #HK083-5 K) (1 hour room temperature), then incubated with anti-CD31 (Abcam, #ab28364, 1:100), anti-CD26 (Abcam, #ab28340), anti-Dlk-1 (Abcam, #ab119930), and/or anti-vimentin (Abcam, #ab24525) (16 hours, 4°C). Secondary Alexa Flour (AF)-conjugated antibodies were AF488 (ThermoFisher Scientific, #A-11008), AF647 (Abcam, #ab27040), and/or AF594 (ThermoFisher Scientific, #R37121). Samples mounted in DAPI Fluoromount-G (ThermoFisher Scientific, #00-4959-52), and fluorescent images were captured by confocal microscopy (Leica TCS SP8) with a uniform frame size of 1024 × 1024.
2.7 ∣. Statistical analysis
Image analysis was performed with ImageJ (NIH; http://rsbweb.nih.gov/ij). Dermal thickness was determined from H&E-stained sections (×20), and collagen density determined from the mean blue area in pixels per high-power field of Trichrome-stained slides (×40). Ten randomly selected images were used per sample. Collagen fiber characteristics were calculated using MATLAB 2017a with Image Processing Toolbox on images of Picrosirius Red-stained specimens (×40). Images were color deconvoluted, binarized, and skeletonized to trace collagen fibers. Collagen fiber length, width, persistence, and branch points were measured using the regionprops command. Positive immunostaining was measured as mean pixel density. Data are presented as means and standard error of the mean when parametric, and with the median and range when nonparametric. The irradiated and non-irradiated sides were compared using the nonparametric paired Wilcoxon signed-rank test. Group comparisons were made using the Kruskal-Wallis test. Statistical analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, California). A P value of <.05 was considered significant.
3 ∣. RESULTS
3.1 ∣. Radiation-induced significant hind limb contracture and was reversed by fat grafting
Right hind limb irradiation resulted in pronounced contracture and shortening on gross inspection (Figure 1Ci-ii) and significantly reduced extension compared to the non-irradiated (left) side (****P < .0001) (Figure 1C-D). Limb extension progressively improved in mice injected with fat, with the greatest benefit seen in mice injected with fat + SVCs. This trend reached significance at 8 and 12 weeks post-surgery (both *P < .05) (Figure 1E).
3.2 ∣. Fat grafting increased skin elasticity and reduced skin stiffness
Irradiated skin was stiffer than non-irradiated skin (saline vs non-irradiated side). Fat grafting reduced skin stiffness, with the greatest improvement found in hind limbs grafted with fat enriched with SVCs (both *P < .05). Skin from animals grafted with fat + SVCs was comparable in elasticity to the non-irradiated side (Figure 2Ai and Aii).
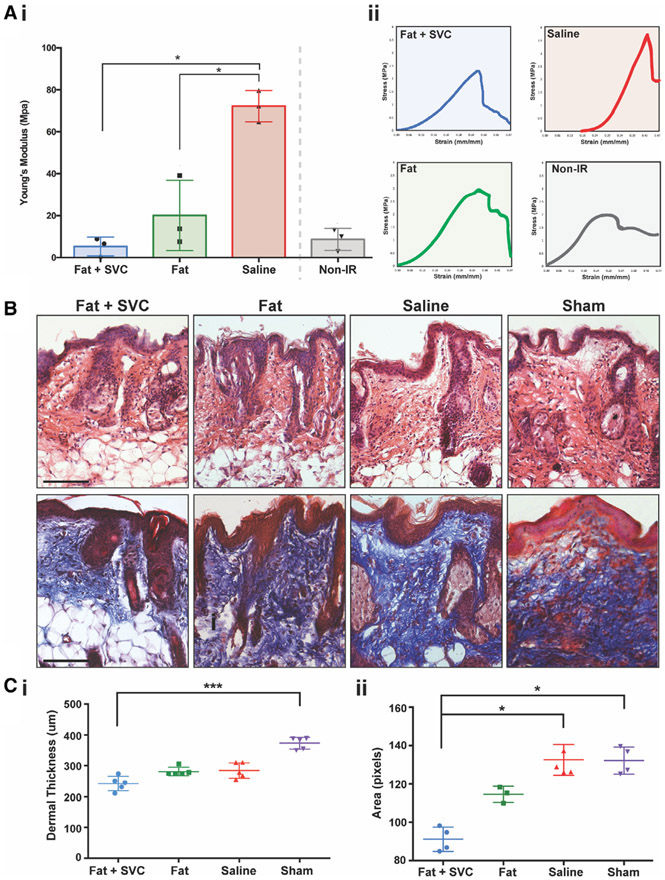
FIGURE 2.
A, Tensile testing of irradiated (IR) and non-IR hind limb skin. Ai, Comparison of the Young's modulus determined from the stress-strain curves and plotted by the treatment group, showing greatest stiffness in mice receiving injections of saline, and least stiffness in mice receiving fat grafts or non-IR mice. Aii, Stress-strain curves derived from tensile testing of skin overlying the irradiated hind limbs of mice who received injections of fat + stromal vascular cells (SVCs), fat alone, and saline. B, Histological assessment of hind limb skin shows an anti-fibrotic effect of fat grafts. Representative images of hematoxylin and eosin (H&E, top row) and Trichrome-stained hind limb skin (bottom row) in all four experimental groups of mice. Ci, Dermal thickness was greatest in mice receiving sham treatment and thinnest in mice grafted with fat + SVCs (***P < .001). Cii, Collagen density was reduced in mice receiving fat + SVCs compared with mice receiving saline or sham treatment (both *P < .05). Scale bar = 100 μm
3.3 ∣. Functional differences were associated with reduced fibrosis of the overlying skin
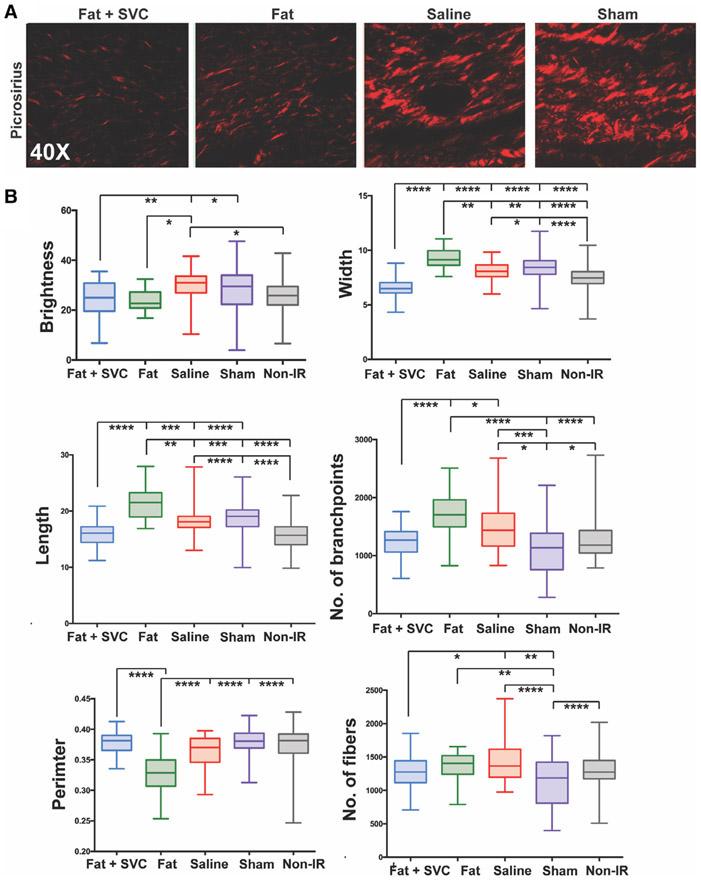
Fat grafts also exerted antifibrotic effects on the irradiated hind limb skin. Twelve weeks post-grafting, dermal thickness was decreased in the skin of irradiated hind limbs that received fat, and this reached significance when comparing mice receiving fat + SVCs to mice undergoing sham treatment (***P < .001) (Figure 2B [top row] and Figure 2Ci). The skin of mice grafted with fat + SVCs had less dense collagen staining than mice receiving saline or sham treatment (both *P < .05) (Figure 2B [bottom row] and Cii). The collagen networks in mice receiving fat + SVCs were similar to those in non-irradiated skin in terms of collagen fiber brightness, length, perimeter, number of branch points, and number of fibers suggesting that the irradiated skin overlying fat grafts underwent significantly more regenerative remodeling (Figure 3A,B).
FIGURE 3.
Collagen fiber network analysis. A, Representative images of Picrosirius-stained irradiated hind limb of the mice in the four experimental groups. B, Quantification of collagen fiber network characteristics between the four groups along six parameters: brightness, width, length, number of branchpoints, perimeter, and number of fibers. Irradiated hind limbs grafted with fat + stromal vascular cells (SVCs) had collagen fibers of similar brightness, length, branchpoints, perimeter, and number of fibers to the collagen fibers in non-irradiated mouse hind limb skin, suggesting more regeneration in mice grafted with fat + SVCs compared with control groups of mice receiving saline or undergoing sham treatment. *P < .05, **P < .01, ***P < .001, ****P < .0001
3.4 ∣. Fat grafting improved vascularity of the overlying skin
CD31 staining indicated significantly increased vascularity in the irradiated hind limb skin overlying areas grafted with fat + SVCs compared to the irradiated hind limbs of mice receiving sham (**P < .01) or saline treatment (****P < .0001; Figure 4A,B).
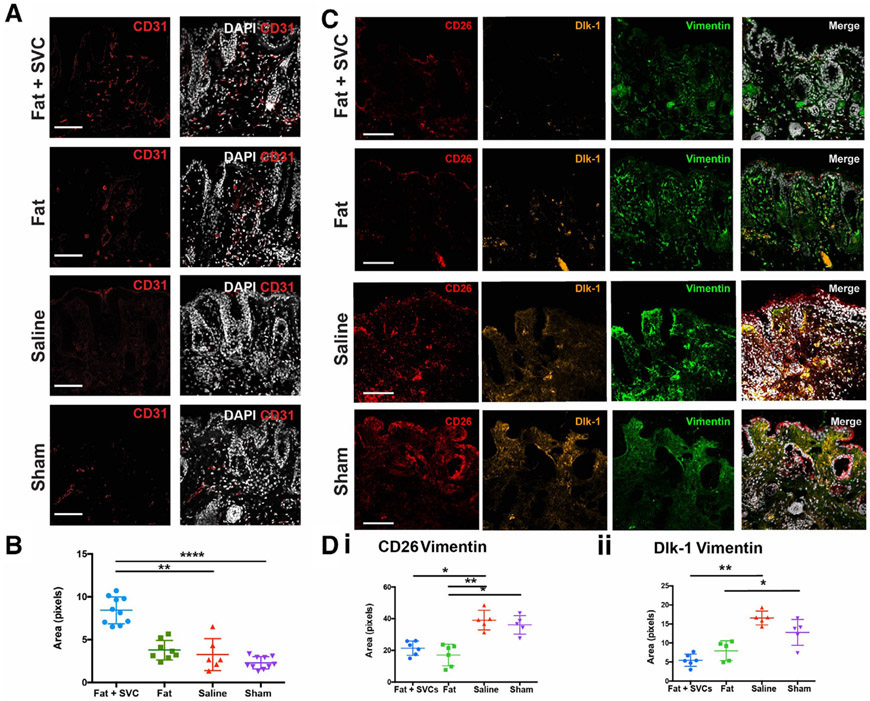
FIGURE 4.
Vascularity of the irradiated hind limb. A, Immunofluorescent staining using CD31 (red) to label endothelial cells and DAPI (white) to label nuclei the four treatment groups. B, Quantification of CD31 staining density revealed significantly increased vascularity in the irradiated hind limb skin of mice grafted with fat + SVCs compared with irradiated hind limbs of mice receiving sham (**P < .01) or saline (****P < .0001) treatment. C, Immunofluorescent staining showing fewer profibrotic CD26+ and Dlk-1+ fibroblast subpopulations in hind limb skin of mice receiving fat grafts. CD26 (red), Dlk-1 (yellow), vimentin (green, to label fibroblasts), and DAPI (white). D, Quantification of the total pixels co-staining for CD26 and vimentin (i) or Dlk-1 and vimentin (ii) between treatment groups. There were significantly fewer CD26+ fibroblasts in the irradiated hind limb skin of mice grafted with fat + SVCs vs mice grafted with saline (*P < .05) and in mice grafted with fat vs mice receiving either saline (**P < .01) or sham (*P < .05) treatment. There were also significantly fewer Dlk-1+ fibroblasts in the irradiated hind limb skin of mice grafted with fat + SVCs vs mice receiving saline (**P < .01) and in mice receiving fat vs sham treatment (*P < .05). Scale bar = 100 μm
3.5 ∣. Fat grafting decreases the proportions of profibrotic fibroblast subpopulations
Immunostaining indicated significantly fewer profibrotic CD26- and Dlk-1-positive fibroblasts in the irradiated skin overlying fat grafts enriched with SVCs than control mice (*P < .05, **P < .01; Figure 4C and D).
4 ∣. DISCUSSION
RT can result in progressive soft tissue fibrosis and contractures which cause pain, spasms, and reduce range-of-motion.3 Although treatment options for radiation-induced contracture are limited, fat grafting is reported to improve the biomechanical and histological quality of irradiated skin.6 We show for the first time that fat grafting increased the extension of irradiated and contracted hind limbs of mice. The grafted fat also improved the quality and vascularity of overlying skin and reduced the dermal thickness, with histological evidence of regenerative tissue remodeling. The greatest functional and histological benefits were evident in mice receiving fat enriched with SVCs. ASCs, found within the SVC fraction, secrete numerous cytokines and growth factors with potent proangiogenic, antifibrotic, and adipogenic effects,7,8 which may promote tissue regeneration and collagen remodeling. Interestingly, the mice receiving fat grafts also had fewer CD26- and Dlk-1-positive fibroblasts, which are known to be profibrotic16-18 suggesting that the interaction between cells within grafted fat and the recipient soft tissue may mediate the antifibrotic effects and functional improvements observed. The ability of fat to improve mobility as well as vascularity and cosmesis holds enormous clinical potential, given adipose tissue is abundant, easily harvested, and autologous.
Significance statement.
Radiation therapy stimulates progressive and pathological soft tissue fibrosis, which distorts tissue form and impacts its function. Fat grafting has regenerative effects and has been found to reduce and even reverse radiation-induced fibrosis of the skin. The study describes a mouse model of radiation-induced hind limb contracture and shows that fat grafting can improve skin vascularity, reduce skin fibrosis, and improve extensibility of the affected limb.
ACKNOWLEDGMENTS
This work was supported by a generous gift from Carmelita Ko and Keith Tsu. Additional grants supporting this work were received from the Oak Foundation, the Hagey Laboratory for Pediatric Regenerative Medicine, the California Institute for Regenerative Medicine, the Plastic Surgery Research Foundation, the Gunn/Olivier Research Fund, and the Sarnoff Cardiovascular Foundation. We would also like to acknowledge Patty Lovelace in the Shared FACS Facility in the Lokey Stem Cell Institute and the Dauskardt laboratory at Stanford University for use of the microtester.
Funding information
NIH R01, Grant/Award Numbers: DE027346, GM116892, U01 HL099776; the NIH S10 Shared Instrumentation Grant, Grant/Award Number: 1S10OD02349701
Footnotes
CONFLICT OF INTEREST
N.M.D.D. declared research funding from the California Institute for Regenerative Medicine. A.M. declared Consultant/Advisory role with Allergan, AxoGen, Sientra, Stryker and research funding from AxoGen. The remaining authors declare no potential conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1.Mojica P, Smith D, Ellenhorn JD. Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J Clin Oncol. 2007;25(9):1043–1047. [DOI] [PubMed] [Google Scholar]
- 2.Stubblefield MD. Radiation fibrosis syndrome: neuromuscular and musculoskeletal complications in cancer survivors. PM R. 2011;3(11):1041–1054. [DOI] [PubMed] [Google Scholar]
- 3.Stubblefield MD, Levine A, Custodio CM, Fitzpatrick T. The role of botulinum toxin type A in the radiation fibrosis syndrome: a preliminary report. Arch Phys Med Rehabil. 2008;89(3):417–421. [DOI] [PubMed] [Google Scholar]
- 4.Hutcheson KA, Lewin JS, Barringer DA, et al. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer. 2012;118(23):5793–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno A, delli Santi G, Fasciani L, Cempanari M, Palombo M, Palombo P. Burn scar lipofilling: immunohistochemical and clinical outcomes. J Craniofac Surg. 2013;24(5):1806–1814. [DOI] [PubMed] [Google Scholar]
- 6.Garza RM, Paik KJ, Chung MT, et al. Studies in fat grafting: part III. Fat grafting irradiated tissue: improved skin quality and decreased fat graft retention. Plast Reconstr Surg. 2014;134(2):249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luan A, Duscher D, Whittam AJ, et al. Cell-assisted lipotransfer improves volume retention in irradiated recipient sites and rescues radiation-induced skin changes. STEM CELLS. 2016;34(3):668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrelli MR, Patel RA, Sokol J, et al. Fat chance: the rejuvenation of irradiated skin. Plast Reconstr Surg. 2019;7:e2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigotti G, Marchi A, Galie M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119(5):1409–1422. [DOI] [PubMed] [Google Scholar]
- 10.Navach V, Calabrese LS, Zurlo V, Alterio D, Funicelli L, Giugliano G. Functional base of tongue fat injection in a patient with severe postradiation dysphagia. Dysphagia. 2011;26(2):196–199. [DOI] [PubMed] [Google Scholar]
- 11.Santamaría JG, Gridilla JM, Romero JP, López-de-Sagredo JG, Atín MB. Fat grafting is a feasible technique for the sequelae of head and neck cancer treatment. J Craniomaxillofac Surg. 2017;45(1):93–98. [DOI] [PubMed] [Google Scholar]
- 12.Phulpin B, Gangloff P, Tran N, Bravetti P, Merlin J-L, Dolivet G. Rehabilitation of irradiated head and neck tissues by autologous fat transplantation. Plast Reconstr Surg. 2009;123(4):1187–1197. [DOI] [PubMed] [Google Scholar]
- 13.Stone HB. Leg contracture in mice: an assay of normal tissue response. Int J Radiat Oncol Biol Phys. 1984;10(7):1053–1061. [DOI] [PubMed] [Google Scholar]
- 14.Ejaz A, Epperly MW, Hou W, Greenberger JS, Rubin JP. Adipose-derived stem cell therapy ameliorates ionizing irradiation fibrosis via hepatocyte growth factor-mediated transforming growth factor-β downregulation and recruitment of bone marrow cells. STEM CELLS. 2019;37(6):791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paik KJ, Zielins ER, Atashroo DA, et al. Studies in fat grafting: part V. cell-assisted lipotransfer to enhance fat graft retention is dose dependent. Plast Reconstr Surg. 2015;136(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinkevich Y, Walmsley GG, Hu MS, et al. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science. 2015;348(6232):aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrelli MR, Irizzary D, Patel RA, et al. Pro-fibrotic CD26-positive fibroblasts are present in greater abundance in breast capsule tissue of irradiated breasts. Aesthet Surg J. 2019;sjz109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Driskell RR, Lichtenberger BM, Hoste E, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504(7479):277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.