Abstract
Background
Nonsense mutation or inactivation of SMARCA4 (BRG1) is associated with a monomorphic undifferentiated histological appearance in tumors at different sites. The association between SMARCA4 alteration and undifferentiated colonic carcinoma needs to be further elucidated.
Methods
A 61-year-old male patient presented to the hospital with intermittent epigastric pain in the right upper abdomen and abdominal distension. The enhanced computed tomography detected a mass in the hepatic flexure of the colon and multiple liver metastases.
Results
The right hemicolectomy contained a 4.5-cm undifferentiated malignancy with cells arranged in sheets, abundant necrosis, and areas showing rhabdoid morphology. The immunohistochemistry result showed that these tumor cells were focally positive for cytokeratin (CK), CK8, and CK18; however, diffusely positive for vimentin, P53, Fli-1, and SALL-4. Notably, tumor cells showed a heterogeneous loss of SMARCA4 expression pattern and intact SMARCB1 expression. Next-generation sequencing showed a germline SMARCA4 c.3277C>T(p.R1093*)mutation, somatic APC mutation, and no abnormal SMARCB1 gene. The tumor exhibited microsatellite stability, negative PD-L1 expression, and few infiltrating CD8 + T cells. The patient died a month later after surgery.
Conclusions
We presented a rare case of undifferentiated colonic neoplasm with loss of SMARCA4 protein expression and germline SMARCA4 mutation. Moreover, the role of SMARCA4 alterations in tumor diagnosis and treatment was also summarized.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13000-021-01091-6.
Keywords: Undifferentiated colonic carcinoma, SMARCA4, Germline mutation
Background
The switch/sucrose non-fermenting (SWI/SNF) complex was first discovered in S. cerevisiae and revealed evolutionary conservation from yeast to mammals. It contains approximately 10–12 subunits. The SWI/SNF interacts with histones and transcription factors to modulate chromatin structure and control gene expression. The ATP-dependent chromatin remodeling complex is involved in regulating diverse biological functions, including differentiation and cell proliferation. Tumor suppressor SMARCA4 is a catalytic ATPase subunit of the SWI/SNF complex. Inactivation of SMARCA4 and other subunits of the SWI/SNF complex have been associated with a monomorphic undifferentiated histological appearance in tumors at different sites. SMARCA4 is frequently inactivated by mutations or other mechanisms in malignancies, such as non-small cell lung cancer, thoracic sarcoma, and malignant rhabdoid tumors [1–3]. SMARCA4 mutations (both somatic and germline mutations) are currently recognized as genetic driver events in almost all small cell carcinomas of the ovary, hypercalcemic type (SCCOHT), which is the most common undifferentiated ovarian malignancy in women under 40 years of age [4, 5]. Potential targeted therapies, such as anti-PD-1 antibodies and CDK4/6 inhibitors, have been reported for treating SMARCA4-deficient non-small cell lung cancer (SMARCA4-deficient NSCLC) [1, 6]. In the gastrointestinal tract, undifferentiated carcinomas are a rare highly aggressive malignancy with frequent rhabdoid features [5]. The association between undifferentiated carcinoma and SMARCA4 alterations, especially the genetic alterations, has not been elucidated. We reported a rare case of a 61-year-old man diagnosed with undifferentiated colonic neoplasm with a heterogeneous pattern of loss expression of SMARCA4 and germline SMARCA4 mutation, as well as predictive markers for potential immunotherapy or targeted therapy. Moreover, the role of SMARCA4 alterations in diagnosing and treating other tumors was also discussed.
Case presentation
A 61-year-old man presented to the Xuanwu Hospital with intermittent epigastric pain in the right upper abdomen and abdominal distension. He had a history of gastric ulcer. The patient’s aunt previously died of colorectal cancer when she was 50 years old. An abdominal computed tomography (CT) scan revealed a mass in the right colon and multiple liver metastases (Fig. 1a, b). The patient subsequently underwent a partial colectomy. The right hemicolectomy revealed a 4.5-cm tumor invading through the muscularis propria into the subserosa (Fig. 1c). The cut surface showed a white to a reddish-brown tumor with central hemorrhage and necrosis. Histologically, the tumor exhibited a sheet-like structure composed of large cells with nucleoli. We also observed abundant necrosis and areas showing rhabdoid morphology (Fig. 2a-d). The immunohistochemistry result showed that these tumor cells were focally positive for cytokeratin (CK), CK8, and CK18; however, diffusely positive for vimentin, P53, Fli-1, and SALL-4. They were negative for CK20, CDX-2, Desmin, Myoglobin, MyoD1, Myogenin, CD56, Syn, and S-100. Notably, tumor cells showed loss of SMARCA4 protein and intact SMARCB1 protein expression (Fig. 2e-h). Next-generation sequencing (NGS) was performed, and the details are available in the Supplementary Material. NGS showed a germline SMARCA4 c.3277C>T(p.R1093*)mutation and no abnormal SMARCB1 gene. The tumor exhibited microsatellite stability (tumor mutation burden (TMB) of 1.68muts/Mb) and negative PD-L1 expression (tumor proportion score less than 1% and combined positive score less than 1) (Table 1).
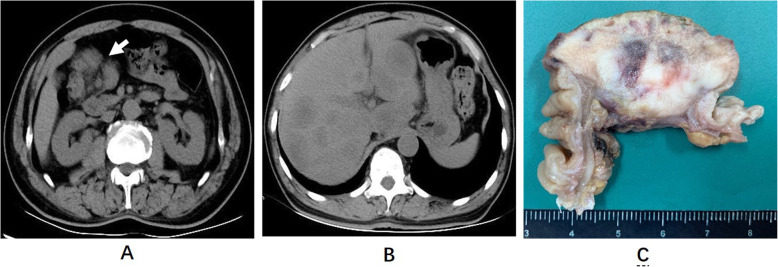
Fig. 1.
Axial CT scan at the level of the kidneys (a) and liver (b) demonstrates a mass (white arrow) within the hepatic flexure of the colon and multiple liver masses, respectively. The cut surface (c) shows the tumor has penetrated through the muscle wall of the colon, exhibiting fine texture with central hemorrhagic necrosis
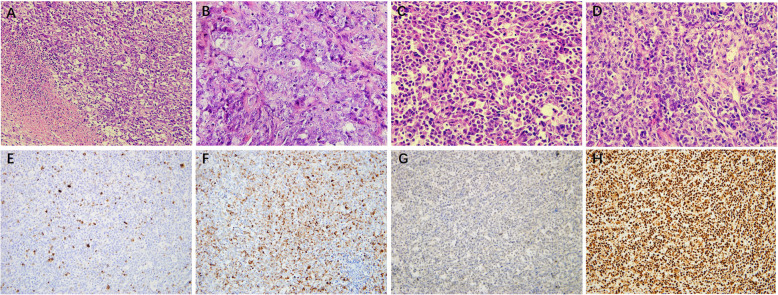
Fig. 2.
Hematoxylin and eosin staining show the tumor exhibited a sheet-like structure with necrosis (a), vesicular nuclei and prominent nucleoli (b) and areas with rhabdoid morphology (c). The tumor cells show a high mitotic rate (d). Tumor cells are positive for (e) CK and (f) vimentin. SMARCA4 (BRG1) (g) was lost, while SMARCA2 (INI-1) (h) was retained in the tumor cell nuclei
Table 1.
Next-generation sequencing analysis
| Gene/Biomarker | Alteration (frequency) |
|---|---|
| SMARCA4 | Germline mutation p.R1093* |
| APC | Somatic mutation p.R216*(31.46%) |
| JUN | Somatic mutation p.K311Sfs*6 (20.77%) & pN85Pfs*21 15.93%) |
| NRAS | Wild-type |
| KRAS | Wild-type |
| BRAF | Wild-type |
| PD-L1 | Negative expression |
| TNM | 1.68Muts/Mb |
| MSA | MSS |
| HLA-I | Heterozygous type |
TNM tumor mutation burden, MSA microsatellite analysis, MSS microsatellite stability
The patient’s physical condition was poor, with multiple liver metastases and several serious complications, such as obstructive jaundice, ascites, anemia, and incomplete intestinal obstruction during hospitalization. The patient died a month later after surgery.
Published literature describes the clinicopathological and molecular features of SMARCA4-deficient tumors, which are summarized in Supplementary Table 1.
Discussion and conclusions
SMARCA4 gene encodes a protein called BRG1, which is suggested to have a tumor suppressor role [5]. SMARCA4 inactivation has been identified as the main genetic driver event in several malignancies, such as SMARCA4-deficient undifferentiated uterine sarcoma, SCCOHT, SMARCA4-deficient thoracic sarcoma (SMARCA4-DTS), and SMARCA4-deficient NSCLC [4, 7–9]. Patients with SMARCA4-deficient neoplasms refer to high-grade malignancies associated with a dismal prognosis. Therefore, a diagnosis based on morphology and immunohistochemistry is particularly important.
SMARCA4-deficient neoplasms typically show an undifferentiated, often rhabdoid morphology and demonstrate a loss of nuclear staining for BRG1 by immunohistochemistry [10]. However, poorly differentiated and at least focal differentiated (glandular or squamous) phenotypes are also observed in SMARCA4-DTS and SMARCA4-deficient NSCLC, respectively [9, 11]. Currently, whether it is called SMARCA4-deficient sarcoma or SMARCA-deficient carcinoma remains controversial. Witkowski et al. questioned the current classification of SCCOHT. Despite the name of ‘carcinoma’, SCCOHT is classified as ‘miscellaneous ovarian tumors’ according to the 4th edition World Health Organization classification of ovary tumors [4]. Moreover, previous studies reported that loss of SMARCA4 expression caused tumor cells to undergo an epithelial-to-mesenchymal transition in lung adenocarcinoma and human mammary epithelial cells [12, 13]. Given the molecular and genetic features of SMARCA4-deficient neoplasms, the name ‘SMARCA4-deficient sarcoma’ might be more suitable. We present an undifferentiated colonic neoplasm with loss of SMARCA4 expression and germline SMARCA4 nonsense mutation where tumor cells were focally positive for CK, CK8, and CK18, but diffusely positive for vimentin.
Twenty-six percent of undifferentiated carcinomas showed loss of core SWI/SNF proteins in the digestive system, including SMARCA4, SMARCA2, SMARCB1, ARID1A, and ARID1B proteins. However, none of the poorly differentiated gastrointestinal carcinomas had a loss of core SWI/SNF detected proteins. SWI/SNF-deficient undifferentiated carcinomas exhibited a sheet-like growth pattern, with cellular decohesion and a rhabdoid appearance. The frequency of loss of SMARCA4 expression was lower than that of SMARCA2, which was the most common non-expressed protein among the five core SWI/SNF proteins. SMARCA4-deficient undifferentiated carcinoma was not common, often with an intact expression of SMARCB1 [5, 14].
Four types of altered SMARCA4 expression were identified in gastric cancer, exhibiting lost, reduced, heterogeneous pattern of lost, and heterogeneous pattern of reduced. These patients were mainly male (male: female = 2.1:1), and the median age was 70 years old (range 43–87). Inactivating mutations in the SMARCA4 gene led to the loss of the SMARCA4 protein. SMARCA4 mutations were detected mainly in SMARCA4-lost and heterogeneous pattern of SMARCA4-lost gastric cancer, while ARIDIA mutations mainly in SMARCA4-reduced and heterogenous pattern of SMARCA4-reduced gastric cancer [15], which is consistent with the finding report in the current study case. We showed that germline SMARCA4 mutations were detected in an undifferentiated colonic neoplasm with a heterogeneous pattern of SMARCA4 loss expression. Moreover, the patient’s aunt previously died of colorectal cancer when she was 50 years old. Ulrika A. Hänninen et al. reported that SMARCA4 mutations were detected by NGS in 6% of small bowel adenocarcinoma. However, SMARCA4 immunohistochemical stains were not performed, and data about the SMARCA4 protein expression is not available [16].
Somatic SMARCA4 mutations occur in lung adenocarcinomas, lymphomas, and medulloblastomas. They are associated with a poor prognosis [17]. In NSCLC, somatic SMARCA4 mutations were detected in 10% NSCLC. Forty-five percent of SMARCA4 mutant NSCLC reported the loss of SMARCA4 expression, and 90% had truncating SMARCA4 mutation. In other words, truncating SMARCA4 mutations are the key genetic event accounting for the SMARCA4-deficient NSCLC. Moreover, clinical outcomes are poor in this molecular subgroup [18, 19].
Rhabdoid Tumor Predisposition Syndrome (RTPS) are due to pathogenic variant in genes of SMARCB1 (PTRS1, commonly,) or SMARCA4(PTRS2, rarely), which are inherited in an autosomal dominant fashion. Patients with RTPS, especially RTPS1, are at increased risk to grow rare and highly aggressive rhabdoid tumor, predominantly in infants and children younger than 3 years old. RTPS2 exposes female carriers to an ill-defined risk of SCCOHT, which may appear in prepubertal females. The penetrance of RTPS1 may be over 90% by 5 years. However, the penetrance of RTPS2 was not well known. Similar to somatic SMARCA4 mutation, RTPS1 and RTPS2 are characterized by a predominance of truncating mutation [20]. The germline SMRACA4 mutation in the present study was also a truncating mutation. In SCCOHT, at least one germline or somatic deleterious SMARCA4 mutation was detected in 94% of cases. Although germline and somatic SMRACA4 mutations were detected in one familial case, germline SMRACA4 mutations were the key genetic event [4].
PD-1 inhibitors and CDK4/6 inhibitors were reported to be effective for treating certain SMARCA4-deficient neoplasms. Specifically, a patient diagnosed with SMARCA4-DTC showed a partial response with only one dose of Pembrolizumab. Sixty percent of the tumor cells expressed programmed cell death ligand-1 protein [21]. A patient with SMARCA4-deficient lung adenocarcinoma exhibiting a high tumor mutation burden was successfully treated with nivolumab [6]. In SMARCA4-deficient NSCLC, SMARCA4 loss results in reduced cyclin D1 expression and selective sensitivity to cyclin-dependent kinase 4/6(CDK4/6) inhibitors in vitro and in vivo, suggesting that FDA-approved CDK4/6 inhibitors could be effective for treating neoplasm [1]. Similarly, the immunochemical analysis of the present case indicated that the tumor cells were negative for CyclinD1. Furthermore, the tumor was PD-L1 negative and showed few infiltrating CD8+ T cells and a low tumor burden. Therefore, the patient may not benefit from the immune checkpoint inhibitors [22]. However, wild type of NRAS, KRAS, and BRAF genes suggest the tumor may be sensitive to endothelial growth factor receptor targeting antibody.
Somatic APC mutation p.R216* was also detected in 31% of tumor cells in this case. APC gene is widely accepted as a colorectal cancer tumor suppressor gene. Somatic APC mutations are detected in at least 80% of sporadic colorectal tumors [23].
We reported a rare case of a 61-year-old man who was diagnosed with undifferentiated colonic neoplasm with a heterogeneous pattern of loss expression of SMARCA4 and germline SMARCA4 mutation, as well as predictive markers for potential immunotherapy or targeted therapy. Moreover, the role of SMARCA4 alterations in diagnosing and treating other tumors was also summarized.
Supplementary Information
Additional file 1: Supplementary Table 1. The clinicopathological and molecular features of SMARCA4-deficient tumors were summarized.
Acknowledgements
Not applicable.
Abbreviations
- CK
Cytokeratin
- SWI/SNF
Switch/sucrose non-fermenting
- CT
Computed tomography
- TMB
Tumor mutation burden
- MSS
Microsatellite stability
- MSA
Microsatellite analysis
- SCCOHT
SMARCA4-deficient undifferentiated uterine sarcoma, small cell carcinoma of the ovary, hypercalcemic type
- SMARCA4-DTS
SMARCA4-deficient thoracic sarcoma
- SMARCA4-deficient NSCLC
SMARCA4-deficient non-small cell lung cancer
- CDK4/6
Cyclin-dependent kinase 4/6
- RTPS
Rhabdoid Tumor Predisposition Syndrome
Authors’ contributions
HLD, WG, LMW and FC: figures and tables collection/analysis and all cowrite the manuscript. HLD and LHT: reviewing and editing of the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included in this article and its supplementary information files.
Declarations
Ethics approval and consent to participate
The institutional review board of Xuanwu Hospital, Capital Medical University approved the study.
Consent for publication
The consent for publication of images and information about the deceased patient from his daughter has been obtained.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xue Y, Meehan B, Fu Z, Wang XQD, Fiset PO, Rieker R, Levins C, Kong T, Zhu X, Morin G, Skerritt L, Herpel E, Venneti S, Martinez D, Judkins AR, Jung S, Camilleri-Broet S, Gonzalez AV, Guiot MC, Lockwood WW, Spicer JD, Agaimy A, Pastor WA, Dostie J, Rak J, Foulkes WD, Huang S. SMARCA4 loss is synthetic lethal with CDK4/6 inhibition in non-small cell lung cancer. Nat Commun. 2019;10(1):557. doi: 10.1038/s41467-019-08380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muppala R, Donenberg T, Huang MS, Schlumbrecht MP. SMARCA4 germline gene mutation in a patient with epithelial ovarian: a case report. Gynecol Oncol Rep. 2017;22:45–47. doi: 10.1016/j.gore.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart BD, Kaye F, Machuca T, Mehta HJ, Mohammed TL, Newsom KJ, Starostik P. SMARCA4-deficient thoracic sarcoma: a case report and review of literature. Int J Surg Pathol. 2020;28(1):102–108. doi: 10.1177/1066896919865944. [DOI] [PubMed] [Google Scholar]
- 4.Witkowski L, Carrot-Zhang J, Albrecht S, Fahiminiya S, Hamel N, Tomiak E, Grynspan D, Saloustros E, Nadaf J, Rivera B, Gilpin C, Castellsagué E, Silva-Smith R, Plourde F, Wu M, Saskin A, Arseneault M, Karabakhtsian RG, Reilly EA, Ueland FR, Margiolaki A, Pavlakis K, Castellino SM, Lamovec J, Mackay HJ, Roth LM, Ulbright TM, Bender TA, Georgoulias V, Longy M, Berchuck A, Tischkowitz M, Nagel I, Siebert R, Stewart CJR, Arseneau J, McCluggage WG, Clarke BA, Riazalhosseini Y, Hasselblatt M, Majewski J, Foulkes WD. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat Genet. 2014;46(5):438–443. doi: 10.1038/ng.2931. [DOI] [PubMed] [Google Scholar]
- 5.Agaimy A, Daum O, Markl B, Lichtmannegger I, Michal M, Hartmann A. SWI/SNF complex-deficient undifferentiated/Rhabdoid carcinomas of the gastrointestinal tract: a series of 13 cases highlighting mutually exclusive loss of SMARCA4 and SMARCA2 and frequent co-inactivation of SMARCB1 and SMARCA2. Am J Surg Pathol. 2016;40(4):544–553. doi: 10.1097/PAS.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 6.Naito T, Umemura S, Nakamura H, Zenke Y, Udagawa H, Kirita K, Matsumoto S, Yoh K, Niho S, Motoi N, Aokage K, Tsuboi M, Ishii G, Goto K. Successful treatment with nivolumab for SMARCA4-deficient non-small cell lung carcinoma with a high tumor mutation burden: a case report. Thorac Cancer. 2019;10(5):1285–1288. doi: 10.1111/1759-7714.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin DI, Allen JM, Hecht JL, Killian JK, Ngo NT, Edgerly C, Severson EA, Ali SM, Erlich RL, Ramkissoon SH, Vergilio JA, Ross JS, Elvin JA. SMARCA4 inactivation defines a subset of undifferentiated uterine sarcomas with rhabdoid and small cell features and germline mutation association. Mod Pathol. 2019;32(11):1675–1687. doi: 10.1038/s41379-019-0303-z. [DOI] [PubMed] [Google Scholar]
- 8.Kolin DL, Dong F, Baltay M, Lindeman N, MacConaill L, Nucci MR, Crum CP, Howitt BE. SMARCA4-deficient undifferentiated uterine sarcoma (malignant rhabdoid tumor of the uterus): a clinicopathologic entity distinct from undifferentiated carcinoma. Mod Pathol. 2018;31(9):1442–1456. doi: 10.1038/s41379-018-0049-z. [DOI] [PubMed] [Google Scholar]
- 9.Perret R, Chalabreysse L, Watson S, Serre I, Garcia S, Forest F, Yvorel V, Pissaloux D, Thomas de Montpreville V, Masliah-planchon J, Lantuejoul S, Brevet M, Blay JY, Coindre JM, Tirode F, le Loarer F. SMARCA4-deficient thoracic sarcomas: Clinicopathologic study of 30 cases with an emphasis on their nosology and differential diagnoses. Am J Surg Pathol. 2019;43(4):455–465. doi: 10.1097/PAS.0000000000001188. [DOI] [PubMed] [Google Scholar]
- 10.Early CA, Wangsiricharoen S, Jones RM, VandenBussche CJ. Review of SMARCA4 (BRG1)-deficient carcinomas following a malignant pleural effusion specimen confounded by reduced claudin-4 expression. J Am Soc Cytopathol. 2020;10(2):197–207. doi: 10.1016/j.jasc.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Herpel E, Rieker RJ, Dienemann H, Muley T, Meister M, Hartmann A, Warth A, Agaimy A. SMARCA4 and SMARCA2 deficiency in non-small cell lung cancer: immunohistochemical survey of 316 consecutive specimens. Ann Diagn Pathol. 2017;26:47–51. doi: 10.1016/j.anndiagpath.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Matsubara D, Kishaba Y, Ishikawa S, Sakatani T, Oguni S, Tamura T, Hoshino H, Sugiyama Y, Endo S, Murakami Y, Aburatani H, Fukayama M, Niki T. Lung cancer with loss of BRG1/BRM, shows epithelial mesenchymal transition phenotype and distinct histologic and genetic features. Cancer Sci. 2013;104(2):266–273. doi: 10.1111/cas.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Bierie B, Li AG, Pathania S, Toomire K, Dimitrov SD, Liu B, Gelman R, Giobbie-Hurder A, Feunteun J, Polyak K, Livingston DM. BRCA1/FANCD2/BRG1-driven DNA repair stabilizes the differentiation state of human mammary epithelial cells. Mol Cell. 2016;63(2):277–292. doi: 10.1016/j.molcel.2016.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tessier-Cloutier B, Schaeffer DF, Bacani J, Marginean CE, Kalloger S, Köbel M, Lee CH. Loss of switch/sucrose non-fermenting complex protein expression in undifferentiated gastrointestinal and pancreatic carcinomas. Histopathology. 2020;77(1):46–54. doi: 10.1111/his.14096. [DOI] [PubMed] [Google Scholar]
- 15.Huang SC, Ng KF, Yeh TS, Cheng CT, Chen MC, Chao YC, Chuang HC, Liu YJ, Chen TC. The clinicopathological and molecular analysis of gastric cancer with altered SMARCA4 expression. Histopathology. 2020;77(2):250–261. doi: 10.1111/his.14117. [DOI] [PubMed] [Google Scholar]
- 16.Hanninen UA, Katainen R, Tanskanen T, et al. Exome-wide somatic mutation characterization of small bowel adenocarcinoma. PLoS Genet. 2018;14(3):e1007200. doi: 10.1371/journal.pgen.1007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connor YD, Miao D, Lin DI, Hayne C, Howitt BE, Dalrymple JL, DeLeonardis KR, Hacker MR, Esselen KM, Shea M. Germline mutations of SMARCA4 in small cell carcinoma of the ovary, hypercalcemic type and in SMARCA4-deficient undifferentiated uterine sarcoma: clinical features of a single family and comparison of large cohorts. Gynecol Oncol. 2020;157(1):106–114. doi: 10.1016/j.ygyno.2019.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagogo-Jack I, Schrock AB, Kem M, Jessop N, Lee J, Ali SM, Ross JS, Lennerz JK, Shaw AT, Mino-Kenudson M. Clinicopathologic characteristics of BRG1-deficient NSCLC. J Thorac Oncol. 2020;15(5):766–776. doi: 10.1016/j.jtho.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Chatzopoulos K, Boland JM. Update on genetically defined lung neoplasms: NUT carcinoma and thoracic SMARCA4-deficient undifferentiated tumors. Virchows Arch. 2021;478(1):21–30. doi: 10.1007/s00428-020-03011-3. [DOI] [PubMed] [Google Scholar]
- 20.Fruhwald MC, Nemes K, Boztug H, et al. Current recommendations for clinical surveillance and genetic testing in rhabdoid tumor predisposition: a report from the SIOPE host genome working group. Familial Cancer. 2021. 10.1007/s10689-021-00229-1. [DOI] [PMC free article] [PubMed]
- 21.Takada K, Sugita S, Murase K, Kikuchi T, Oomori G, Ito R, Hayasaka N, Miyanishi K, Iyama S, Ikeda H, Kobune M, Emori M, Kato J, Hasegawa T. Exceptionally rapid response to pembrolizumab in a SMARCA4-deficient thoracic sarcoma overexpressing PD-L1: a case report. Thorac Cancer. 2019;10(12):2312–2315. doi: 10.1111/1759-7714.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurkmans DP, Kuipers ME, Smit J, van Marion R, Mathijssen RHJ, Postmus PE, Hiemstra PS, Aerts JGJV, von der Thüsen JH, van der Burg SH. Tumor mutational load, CD8(+) T cells, expression of PD-L1 and HLA class I to guide immunotherapy decisions in NSCLC patients. Cancer Immunol Immunother. 2020;69(5):771–777. doi: 10.1007/s00262-020-02506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Shay JW. Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer. J Natl Cancer Inst. 2017;109(8):djw332. doi: 10.1093/jnci/djw332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. The clinicopathological and molecular features of SMARCA4-deficient tumors were summarized.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary information files.