Abstract
Sterol biosynthesis is a critical homeostatic mechanism of the body. Sterol biosynthesis begins during early embryonic life and continues throughout life. Many commonly used medications, prescribed >200 million times in the United States annually, have a sterol biosynthesis inhibition side effect. Using our high-throughput LC-MS/MS method, we assessed the levels of post-lanosterol sterol intermediates (lanosterol, desmosterol, and 7-dehydrocholesterol (7-DHC)) and cholesterol in 1312 deidentified serum samples from pregnant women. 302 samples showing elevated 7-DHC were analyzed for the presence of 14 medications known to inhibit the 7-dehydrocholesterol reductase enzyme (DHCR7) and increase 7-DHC. Of the 302 samples showing 7-DHC elevation, 43 had detectable levels of prescription medications with a DHCR7-inhibiting side effect. Taking more than one 7-DHC-elevating medication in specific combinations (polypharmacy) might exacerbate the effect on 7-DHC levels in pregnant women, suggesting a potentially additive or synergistic effect. As 7-DHC and 7-DHC-derived oxysterols are toxic, and as DHCR7-inhibiting medications are considered teratogens, our findings raise potential concerns regarding the use of prescription medication with a DHCR7-inhibiting side effect during pregnancy. The use of prescription medications during pregnancy is sometimes unavoidable, but choosing a medication without a DHCR7-inhibiting side effect might lead to a heathier pregnancy and prevent putatively adverse outcomes for the developing offspring.
Keywords: cholesterol, 7-dehydrocholesterol reductase enzyme, 7-dehydrocholesterol, pregnancy, neurodevelopment
Sterol biosynthesis is a critical homeostatic mechanism of the body.1 Cholesterol synthesis involves dozens of complex enzymatic reactions, using acetate as the starting precursor.2−4 Intact sterol biosynthesis is critical for embryonic development, as cholesterol is an essential component of cell membranes.5 This biosynthetic pathway also generates many critical molecules, including hormones.6 Cholesterol synthesis takes place in the smooth endoplasmic reticulum, and cholesterol is essential for efficient endoplasmic reticulum-to-Golgi transport of secretory membrane proteins.7,8 Finally, cholesterol plays a critical role in vesicle fusion and motion.9
Sterol biosynthesis begins during early embryonic life and continues throughout life.10,11 A complete absence of cholesterol biosynthesis is incompatible with life. Mutations in the post-squalene pathway can be viable if partial cholesterol synthesis is preserved, but result in severe intellectual and developmental disorders.10,12 Mutations in the enzymes in the post-lanosterol biosynthesis pathway lead to Smith–Lemli–Opitz syndrome (SLOS) (mutations in DHCR7),13−15 desmosterolosis (mutations in DHCR24),16,17 chondrodysplasia punctata (mutations in EBP),18,19 lathosterolosis (mutations in SC5D),10 and CHILD syndrome (mutations in NSDHL).20,21
In addition to genetic disruptions of cholesterol’s biosynthesis, sterol biosynthesis enzymes can be chemically inhibited.22−28 This appears to most severely impact the child during intrauterine development: When women are exposed to 7-dehydrocholesterol reductase enzyme (DHCR7) inhibitors during pregnancy, these chemicals act as teratogens for a developing child.29 A 2016 review by Boland and Tatonetti suggests that first-trimester exposure to DHCR7-inhibiting medications results in outcomes similar to those of known teratogens and that DHCR7 activity should be considered during drug development and prenatal toxicity assessment.29 However, this effect is not a simple result of insufficient levels of cholesterol. DHCR7 inhibition strongly elevates levels of 7-dehydrocholesterol (7-DHC), a highly unstable and reactive molecule that is toxic for cells.30−34
Seven years ago, Hall et al. made a critical observation of cholesterol biosynthesis interference by medications, noting that some patients with developmental disabilities, who used aripiprazole (ARI) and trazodone (TRZ), were misidentified as SLOS patients based on their blood 7-DHC levels.23 Follow-up studies validated that haloperidol (HAL), ARI, TRZ, and cariprazine (CAR) are all strong inhibitors of sterol biosynthesis and that they have profound biochemical effects on the fetal development in rodent models.25,35,36 In these studies, the offspring of female mice exposed to ARI, TRZ, and CAR had elevated 7-DHC levels in multiple tissues, including the brain and liver. The increase in 7-DHC led to a strong elevation of 7-DHC-derived oxysterols. Alarmingly, oxysterol levels in the brain of pups whose mothers were exposed to CAR reached levels comparable to those detected in transgenic mice models of SLOS.36
Importantly, recent studies suggest that the list of DHCR7-inhibiting prescription medications is quite extensive, and might encompass ARI, TRZ, HAL, CAR, buspirone (BUS), sertraline (SER), fluoxetine (FLX), metoprolol (MTP), bupropion (BUP), fluphenazine, formoterol, nebivolol, oxybutynin, and propranolol.24,27,28,35−37 We estimate that the combined volume of prescription medications with a sterol-inhibiting side effect, in the United States alone, exceeded 200 million prescriptions in 2018 (Table 1).
Table 1. Action, Indication and Prescription Information of 7-DHC Elevating Medications Detected in Pregnant Human Sera.
| assessed DHCR7- inhibiting medications | brand name | 2018 yearly prescriptions in the US (rank)d | action | primary indicationi | number of samples with medicationb (% of total) | 7-DHCc (FOC) |
|---|---|---|---|---|---|---|
| metoprolol (MTP) | lopressor | 71,581,961 (6) | beta-blocker | hypertension, angina | 3 (1.0%) | 2.5 ± 1.3f |
| sertraline (SER) | zoloft | 38,383,042 (14) | antidepressant | MDD, OCD, PTSD | 24 (7.9%) | 2.7 ± 0.7 |
| fluoxetine (FLX) | prozac | 25,619,277 (23) | antidepressant | MDD. multiple | 3 (1.0%) | 2.2 ± 0.5f |
| bupropion (BUP) | wellbutrin | 24,488,843 (27) | antidepressant | MDD, smoking cessation | 4 (1.7%) | 2.0 ± 0.2 |
| trazodone (TRZ) | oleptro | 23,889,624 (31) | antidepressant | MDD, multiple | 4 (1.3%) | 3.7 ± 1.7f |
| propranolola | inderal | 14,571,767 (53) | antihypertensive | hypertension, angina | not detected | N/A |
| formoterola | symbicort | 12,339,319 (62) | bronchodilator | asthma | not detected | N/A |
| buspirone (BUS) | buspar | 8,149,119 (92) | anxiolytic | anxiety disorders | 1 (0.3%) | e |
| aripiprazole (ARI) | abilify | 7,406,248 (101) | antipsychotic | schizophrenia, BPD | 4 (1.3%) | 7.5 ± 3.2 |
| oxybutynina | ditropan | 7,339,185 (102) | antimuscarinic | overactive bladder | not detected | N/A |
| nebivolola | bystolic | 2,955,158 (197) | beta-blocker | hypertension | not detected | N/A |
| haloperidol (HAL) | haldol | 1,241,881 (296)g | antipsychotic | schizophrenia, BPD | 4 (1.3%) | 1.8 ± 0.3 |
| cariprazinea | vraylar | unavailable | antipsychotic | schizophrenia, BPD | not detected | N/A |
| fluphenazine | prolixin | unavailable | antipsychotic | schizophrenia, psychosis | not detected | N/A |
| total | 236,723,543 | 47 (15.9%)h |
DHCR7-inhibiting medications assayed by LC-MS/MS but not detected in any samples in our study.
Number of samples containing the medications listed and the percent of samples with that drug in over the total number of samples analyzed.
Levels of 7-DHC shown as fold increase over control (mean ± SEM). These values correspond to the average of 7-DHC with only a single medication detected.
Available at www.clincalc.com.
Buspirone was only detected in one sample with more than one medication, and the 7-DHC values are depicted in Table 2.
Values correspond to the mean ± SEM of two measurements.
2017 yearly prescriptions (2018 data unavailable).
MDD: major depressive disorder; BPD: bipolar disorder; OCD: obsessive-compulsive disorder; and PTSD: post-traumatic stress disorder.
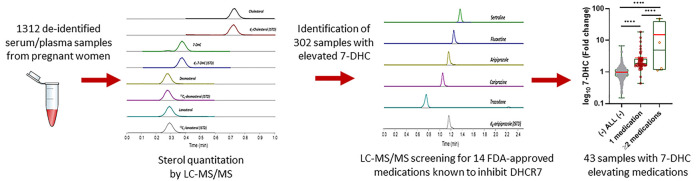
Medication use in pregnancy is often unavoidable.29,38−40 Pregnant women take many different medications, including antidepressants, antipsychotics, and antihypertensives.38−52 These medications can alter sterol biosynthesis and might represent a long-term health risk for the developing child. In our study, we were interested in the biochemical consequences of 7-DHC-elevating medications during pregnancy. We obtained 1312 deidentified serum/plasma samples from pregnant women from the Nebraska Biobank. Using our high-throughput LC-MS/MS method,53 we assessed the levels of post-lanosterol sterol intermediates (lanosterol (LAN), desmosterol (DES), and 7-DHC) and cholesterol (CHOL) in all 1312 samples. The sera were analyzed without basic hydrolysis, and the levels reported are due to free sterols only and do not include sterol esters. 7-DHC and its fatty acid esters are particularly sensitive to oxidation. The vigorous conditions of basic hydrolysis were avoided in order to minimize loss of these sensitive sterols during sample workup. The 302 samples showing 7-DHC levels above the 99% mean confidence of interval were further analyzed for the presence of medications known to inhibit DHCR7 and increase 7-DHC.
Results and Discussion
Age and Race Effects on 7-DHC Levels during Pregnancy
Chemicals and/or medications that inhibit cholesterol biosynthesis have been associated with fetal malformation and negative pregnancy outcomes.29,39,51,54,55 Inhibition of DHCR7 is of concern, as it leads to the elevation of 7-DHC, a highly oxidizable compound with documented toxicity to multiple cell types.30,31,56 A classic example is compound AY9944, a potent DHCR7 inhibitor that has been extensively utilized for generating pharmacological models of SLOS.57−59 There are many medications regularly taken by adults that elevate 7-DHC levels.24,37 Although their use in the general population leads to beneficial outcomes for the patients, their use during pregnancy may lead to deleterious consequences to the offspring. Previous studies from our group described a significant increase in 7-DHC in multiple tissues of mice who have been maternally exposed to either ARI, CAR, or TRZ.25,35,36 Elevated 7-DHC levels resulted in an increase of toxic 7-DHC-derived oxysterols, including 3β,5α-dihydroxycholest-7-en-6-one (DHCEO), a compound considered to be a biomarker of oxidative stress associated with SLOS.31−33,36,60−62 Such changes are likely to affect the adult brain differently than a developing one. Therefore, maternal elevation of 7-DHC blood levels can potentially translate into elevated 7-DHC in the developing fetus. This maternal–fetus 7-DHC transfer has been only documented in animal models to date, yet the highly conserved sterol biosynthesis across species suggests that this mechanism is likely to also occur in humans.
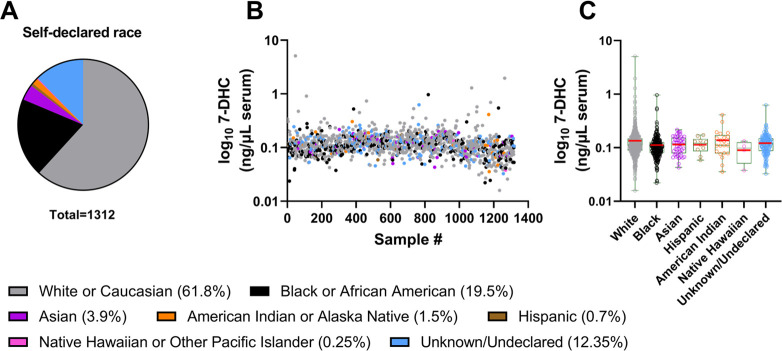
To determine if prescription medications known to inhibit DHCR7 can alter 7-DHC in maternal serum during pregnancy, we obtained 1312 deidentified serum/plasma samples from pregnant women from the Nebraska Biobank. Samples were assigned to one of seven groups according to the individuals’ self-declared race. The majority of the samples originated from White/Caucasian (61.8%) and Black/African American (19.5%) women (Figure 1A). The other five groups corresponded to <20% of the total number of samples. Figure 1B depicts free 7-DHC levels for each sample, denoting the race of each individual. Figure 1C depicts free 7-DHC levels averaged according to the self-declared race. The results suggest that 7-DHC levels do not depend on self-declared race. The White/Caucasian group presented the highest variability in free 7-DHC levels, followed by the Black/African American and Unknown/Undeclared groups. This is, perhaps, due to the sample size, as these three groups contain most of the samples, making up >90% of the participating samples.
Figure 1.
7-DHC level distribution by self-declared race. (A) Samples were grouped into seven categories based on self-declared race depicted in the figure legend. The group labeled “unknown/undeclared” contained samples from individuals who provided no answer or identified themselves outside the main categories. The proportion of each group is depicted in parentheses. (B) Free 7-DHC levels of all studied samples. X-axis denotes the unique identifier of each sample, and free 7-DHC (ng/μL of serum) is presented using a log10 representation on the Y-axis. Each marker represents a single 7-DHC measurement in a single sample, color-coded for self-declared race. (C) Free 7-DHC (ng/μL of serum) averaged across the samples according to self-declared race. Samples were graphed using a box plot. The red bars correspond to the mean values, and each symbol corresponds to an individual sample. We found that although free 7-DHC levels showed almost a 100-fold variability, mean levels of 7-DHC were comparable across the race categories.
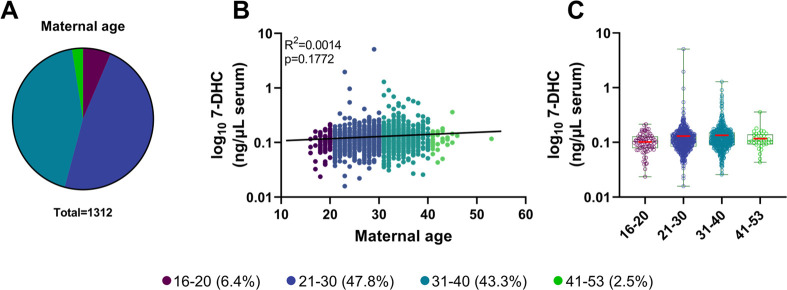
In addition to assessing the self-declared race as a variable that could influence circulating free 7-DHC levels, we also investigated the association between free 7-DHC and age. The individuals’ ages were assembled into four age groups, 16–20, 21–30, 31–40, and 41–53 years old (Figure 2A). The vast majority of the participants fell between ages of 21 and 40, with <10% of the samples obtained from individuals outside this age range. No significant correlation was observed between free 7-DHC levels and maternal age (R2 = 0.0014, p = 0.1772) (Figure 2B). Figure 2C depicts mean distribution of 7-DHC across the age groups. The highest variability in 7-DHC was observed in individuals within the 21–30 and 31–40 year age groups. Notably, these groups contained the highest number of samples with detectable levels of 7-DHC-elevating medications.
Figure 2.
7-DHC level distribution by age. (A) Samples were grouped into four age groups, 16–20, 21–30, 31–40, and 41–53 years. The proportion of each group is depicted in parentheses. (B) Distribution of individual 7-DHC levels. Free 7-DHC (ng/μL of serum) levels are presented as log10 scales on the Y-axis, and X-axis denotes the age of pregnant women. Each marker represents a single 7-DHC measurement in a single sample, color-coded for age. The solid black line represents a simple linear regression. (C) Free 7-DHC (ng/μL of serum) averaged across the samples belonging to the four age categories. Samples were graphed using a box plot. The red bars correspond to the mean values; each symbol corresponds to an individual sample. Note that there was no correlation between 7-DHC and maternal age (R2 = 0.0014, p = 0.1772).
Serum Sterol Profile of Pregnant Women Taking Prescription Medication with a DHCR7-Inhibiting Side Effect
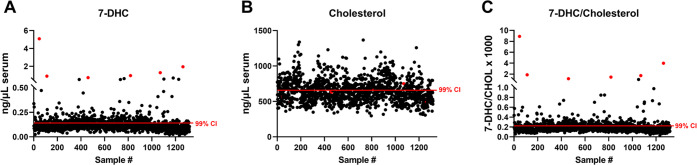
The sterol profile was determined in all pregnant women serum samples, and their levels were plotted against their individual deidentified sample ID (Figure 3 and Supporting Information 1). The 7-DHC/cholesterol ratio (Figure 3C) was determined by dividing 7-DHC by cholesterol levels from each sample. Analysis of the 7-DHC and 7-DHC/CHOL ratio revealed a wide range of 7-DHC in the sera of pregnant women, with values ranging from 0.015 to 5.1 ng/μL. Figure 3B denotes cholesterol levels in all pregnant women sera. Furthermore, the six samples with the highest 7-DHC values are denoted in red across the three panels. Note that although these samples stand out by their elevated 7-DHC and 7-DHC/CHOL levels in Figure 3A,B, they cannot be distinguished from other samples based on their CHOL levels (Figure 3C).
Figure 3.
Distribution of 7-DHC and CHOL levels in the serum of pregnant women and outlier identification. Levels of free (A) 7-DHC, (B) free CHOL, and (C) 7-DHC/CHOL in pregnant women. Sterol levels are reported as ng/μL of serum in panels (A and B). Each symbol corresponds to an individual sample. Red lines denote the upper 99% mean confidence of interval (CI), which identified 302 samples with elevated 7-DHC levels in LC-MS/MS assessment for the presence of 14 7-DHC-elevating medications. Red-colored symbols correspond to the same six samples in all three panels and denote samples with the highest 7-DHC and 7-DHC/cholesterol levels (A and B). DES and LAN measures are presented in Supporting Information 1. Note that these samples were unremarkable based on their CHOL levels (C), suggesting that CHOL levels are not the best readout of sterol biosynthesis health.
Previous studies have shown that different sterol biosynthesis enzymes (CYP51, EBP, SC5D, DHCR14, and DHCR24) can also be inhibited by prescription medications, at least under in vitro conditions.24,27,28,63 We show plots for DES and LAN (Supplemental Figure 1A,B, respectively) suggesting that altered sterol biosynthesis might occur at multiple points in the post-lanosterol biosynthesis pathway. Such increases in LAN and DES can occur due to either genetic mutations in CYP51 or DHCR24, enzymes that convert lanosterol and desmosterol, or medications that inhibit these enzymes. However, since CYP51 and DHCR24 mutations have severe consequences,16,64 we favor the interpretation that these alterations are an effect of lifestyle or the use of other prescription medications.
Due to the known toxicity of 7-DHC and its negative consequences to embryonic development, we focused our follow-up assessment on the samples with elevated 7-DHC. 302 samples showing 7-DHC elevation above the 99% mean CI (Figure 3A, CI denoted by a red line) were assessed for the presence of medications with a known DHCR7-inhibiting side effect. The list of medications analyzed and information about action, indication, and prescription information are shown in Table 1. All medications in our LC-MS/MS drug panel had been extensively assessed for side effects of elevated 7-DHC in either cell culture or animal models.22,24,26−28,35−37,63 Our previous high-throughput screening identified BUP, a highly prescribed antidepressant, with almost 25 million prescriptions in 2018, which is often prescribed to pregnant women (Table 1). BUP was added to our prescription medication panel based on our initial findings.24 Here in our cell culture experiments, we confirmed that BUP induced a dose-dependent increase in 7-DHC in both HEPG2 and Neuro2a cell lines (Supporting Information 2 and 3), thus warranting inclusion in our LC-MS/MS drug panel of putative DHCR7 inhibitors.
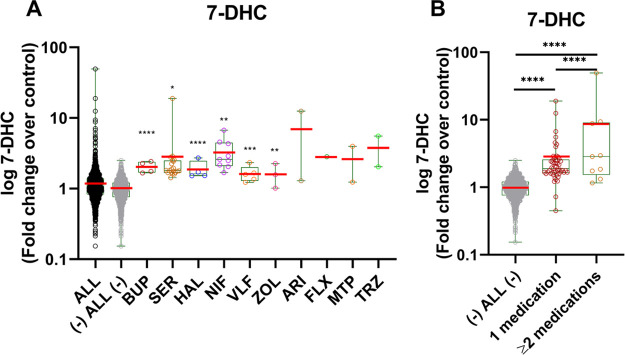
Our prescription medication assessment of pregnant women sera with elevated 7-DHC levels revealed the presence of eight DHCR7-inhibiting medications in 43 of the 302 assessed samples. Figure 4A depicts the levels of 7-DHC in samples with only one DHCR7 inhibiting medication detected. The group labeled “ALL” included all samples analyzed, the group labeled “(−) ALL (−)” denotes all samples with the 43 samples with detectable levels of 7-DHC-elevating medications removed. 7-DHC values are presented as fold change over control using the “(−) ALL (−)” group as the baseline. Levels of 7-DHC were significantly elevated in samples containing BUP, SER, HAL, and ARI. Samples with FLX, MTP, or TRZ also contained elevated 7-DHC levels, but due to the small sample size (two samples per group), no assessment for significance was possible. Figure 4B denotes the comparison of 7-DHC levels in samples with one, two, or more 7-DHC-elevating medications and baseline. Samples containing two or more 7-DHC-elevating medications are shown in Table 2. 7-DHC levels in samples containing one 7-DHC-elevating medications were significantly higher than the baseline (p < 0.0001). Interestingly, in sera samples containing two or more DHCR7-inhibiting medications, the levels of 7-DHC are on average significantly higher than those of samples with only one medication (p < 0.0001), raising the possibility of an additive or synergistic 7-DHC-elevating effect with specific combinations of polypharmacy (e.g., BUP + BUSP).
Figure 4.
7-DHC levels in the serum of pregnant women taking medications with the side effects of DHCR7 enzyme inhibition. 7-DHC levels are presented on Y-axis as a log10-fold change over control using “(−) ALL (−)” group as the baseline. The “(−) ALL (−)” group includes all samples without detectable levels of the selected medications. The red bars correspond to the mean values; each symbol corresponds to an individual sample. (A) Comparison of free 7-DHC levels in samples with one detectable DHCR7-inhibiting medication. Groups and medications are denoted on the X-axis (BUP: bupropion; SER: sertraline, HAL: haloperidol; ARI: aripiprazole; FLX: fluoxetine; MTP: metoprolol; and TRZ: trazodone). The 14 medications tested are denoted in Table 1. Samples were graphed using a box plot. Significance was established utilizing an unpaired two-tailed t test in comparison to “(−) ALL (−)” group. (B) Free 7-DHC levels in samples with one or multiple detected DHCR7-inhibiting medications. The multiple medications detected in samples are listed in Table 2. One-way ANOVA and Tukey’s multiple comparisons tests were performed to compare the difference between the three groups; statistical significance: ****p < 0.0001.
Table 2. Polypharmacy List and Combination of Drugs Detected in Pregnant Human Sera.
| polypharmacy
effect on 7-DHC | ||
|---|---|---|
| two or more DHCR7-inhibiting drugs | nb | 7-DHCa (FOC) |
| ARI + SER | 1 | 1.2 |
| TRZ + SER | 1 | 1.3 |
| BUP + BUS | 1 | 48.1 |
| FLX + MTP + TRZ | 1 | 8.6 |
Levels of 7-DHC shown as fold over control (FOC) with a baseline of all samples without 43 samples containing 7-DHC-elevating medications.
Number of samples with the corresponding combination of medications (n).
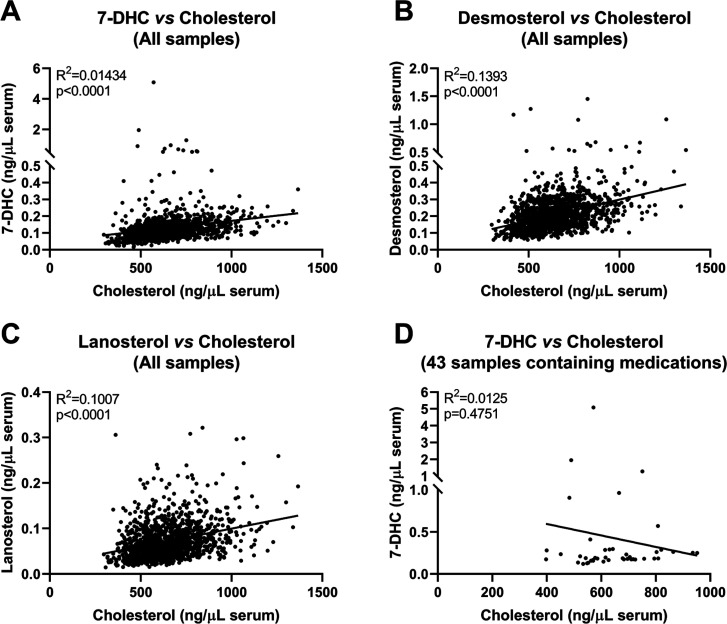
Next, we correlated levels of each sterol intermediate with cholesterol levels in individual samples (Figure 5). Across all samples, we observed a positive correlation between cholesterol and sterol intermediate levels (7-DHC, desmosterol, and lanosterol on Figure 5A–C, respectively). In contrast, no correlation was observed between 7-DHC and cholesterol levels in samples containing 7-DHC-elevating medications (Figure 5D), confirming the primary effect of the medications on the DHCR7 enzyme and that cholesterol levels are an imprecise readout of sterol biosynthesis inhibition.
Figure 5.
Correlations between sterol intermediate and CHOL levels in pregnant women sera. (A) 7-DHC vs CHOL (B) DES vs CHOL, and (C) LAN vs CHOL. (D) Correlation between 7-DHC and CHOL in 43 samples where 7-DHC-elevating medications were detected. All sterol levels are reported as ng/μL of serum. Each symbol denotes an individual sample. Pearson coefficient (R2) and two-tailed p values were determined using GraphPad Prism 9. Note that all three sterol intermediates significantly correlated with CHOL across all studied samples (A–C). However, for the 43 samples containing 7-DHC-elevating medications, we found no correlation between 7-DHC and CHOL levels, suggesting that CHOL levels are not the ideal readout of sterol biosynthesis disruptions.
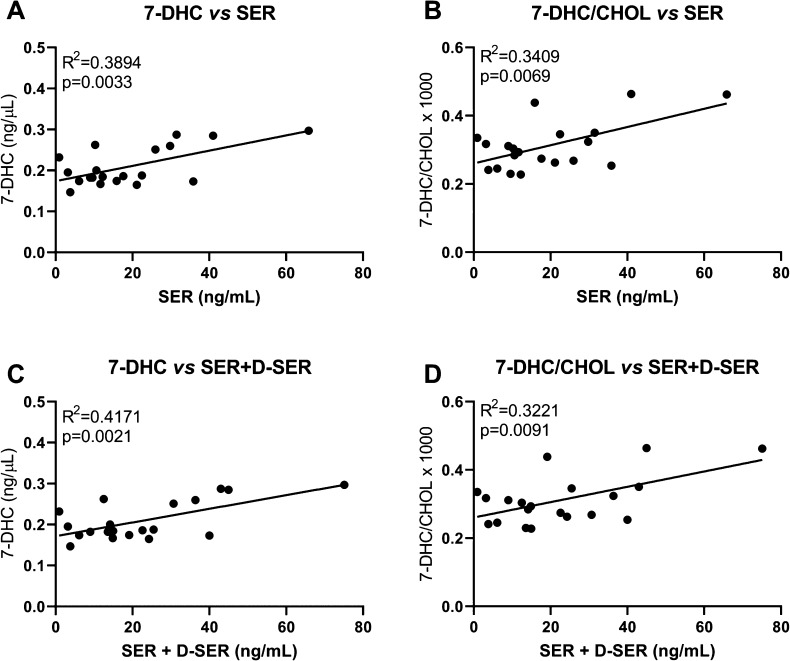
Out of all eight medications detected in the sera of pregnant women, SER was the medication detected in most of the samples (n = 20), which allowed us to correlate SER and 7-DHC levels. Correlations between 7-DHC and 7-DHC/cholesterol with SER or SER combined with its active metabolite desmethyl-sertraline (D-SER) are presented in Figure 6. Significant positive correlations were observed between 7-DHC and SER (Figure 6A), 7-DHC/CHOL and SER (Figure 6B), 7-DHC and SER + D-SER (Figure 6C), and 7-DHC/CHOL and SER + D-SER (Figure 6D). These results further underscore the causality of our findings. Correlations of SER and SER + D-SER with other sterols are presented in Supplemental Figure 4.
Figure 6.
Correlation between sertraline (and its active metabolite desmethyl-sertraline (D-SER)) and 7-DHC levels in the sera of pregnant women. Significant correlations were observed between the detected levels of (A) SER and 7-DHC, (B) SER and 7-DHC/CHOL, (C) SER + D-SER and 7-DHC, and (D) SER + D-SER and 7-DHC/CHOL ratio in pregnant women with confirmed SER in their sera. Each symbol denotes an individual sample. Two samples that contained SER were removed from the correlations because either 7-DHC or SER levels were 1 order of magnitude higher than seen in other samples (extreme outliers). Lines denote simple linear regressions.
Our studies give rise to multiple conclusions: (1) Postlanosterol sterol levels are steady and do not depend on maternal age or self-declared race. (2) Commonly used DHCR7-inhibiting prescription medications are widely used in pregnancy. (3) There is a significant positive correlation between sterol intermediates and cholesterol in pregnant women sera. However, this correlation disappears with the use of medications with DHCR7-inhibiting side effects, thus making 7-DHC a better readout of sterol biosynthesis health than cholesterol. (4) Commonly used prescription medications disrupt sterol biosynthesis and increase 7-DHC in the sera of pregnant mothers. (5) Taking two or more specific combinations of 7-DHC-elevating medications (polypharmacy) might further exacerbate the rise of 7-DHC in the sera of pregnant mothers, suggesting an additive or synergistic effect of specific combinations of the drugs.
Of the 302 samples showing 7-DHC elevation, 43 had detectable levels of prescription medications with a DHCR7-inhibiting side effect. 7-DHC elevation in the remaining 259 samples might be due to multiple factors. The majority of samples in this group are likely to represent normal human variability, which are only slightly above the 99% CI. In addition, elevation of 7-DHC in a few samples is likely due to polymorphisms in sterol biosynthesis enzymes. Furthermore, some patients might have pathophysiological conditions where 7-DHC elevation is part of the disease process. Finally, we believe that there are many more medications (for which we do not test currently) that interfere with sterol biosynthesis and cause elevated 7-DHC levels.
We have previously shown that a maternal exposure to ARI, TRZ, and CAR lead to a significant increase in 7-DHC in the brain of newborn pups. Maternal exposure to CAR leads to elevated 7-DHC in other organs as well, including the liver, heart, kidneys, lungs, and spleen of the offspring.36 Each tissue may be differently affected by 7-DHC, and both the immediate and long-term consequences are still unknown. It is known, however, that the embryonic exposure to CAR translates into higher 7-DHC up until postnatal day 21 (P21), a period of neurodevelopment where proper cholesterol biosynthesis and sterol homeostasis are also essential.2,5 Yet, it is likely that the brain is not the only highly sensitive tissue to DHCR7 inhibition: Genetic DHCR7 inhibition in SLOS patients presents with malformations in several organ systems, including particular facial features, cleft palate, heart defects, fused second and third toes, extra fingers and toes, and underdeveloped external genitals.10,13−15 It is believed that DHCR7 inhibition, and subsequently elevated 7-DHC and 7-DHC-derived oxysterol levels, plays a critical role in these negative outcomes, as 7-DHC-derived oxysterols are known to compromise neuronal viability and differentiation.30,31 Furthermore, DHCR7 inhibition, elevation of 7-DHC, and a sharp rise of 7-DHC-derived oxysterols have been observed in human dermal fibroblast cultures when exposed to CAR, TRZ, or ARI.22,26 In addition to a particular stage of life when 7-DHC may have more severe consequences, each specific tissue may exhibit a differential resistance or vulnerability to 7-DHC and 7-DHC-derived oxysterols.
Our studies should also be considered in the context of precision medicine. We have previously shown that human dermal fibroblasts and animal models carrying single-allele DHCR7 (DHCR7+/−) mutations are more vulnerable to 7-DHC-elevating medications than their wild-type (WT) counterparts.26,35,36 When subjected to the same chemical DHCR7 inhibition, human dermal fibroblasts and animals with a Dhcr7+/−genotype elevate 7-DHC and its oxysterol levels more robustly than DHCR7+/+ fibroblasts and Dhcr7+/+ offspring. In some cases, the effect of the combined genotype–chemical inhibition resulted in sterol biosynthesis disruptions that were comparable in magnitude to those seen in in vitro and transgenic SLOS mouse models.36 These gene–medication interactions should be a concern, particularly to the 1–3% of the human population carrying single-allele DHCR7 mutations.29,65
Finally, our studies have a potential public health relevance. In this study, we assessed for the presence of 14 medications known to elevate 7-DHC, which accounted for over 200 million prescriptions in the United States in 2018. Our data suggest that pregnant women are also commonly prescribed these medications, with unknown consequences for the long-term health of maternally exposed children. Furthermore, our knowledge of the consequences of DHCR7-inhibiting polypharmacy on child development and health is virtually nonexistent. Our studies suggest that the effects of simultaneous utilization of some combinations of DHCR7-inhibiting medications might potentiate the sterol biosynthesis disruptions, and this should be a subject of future studies. In conclusion, the use of prescription medications during pregnancy is sometimes unavoidable, but choosing a medication without a DHCR7-inhibiting side effect might lead to a healthier pregnancy.
Methods
Chemicals
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich Co (St. Louis, MO). HPLC grade solvents were purchased from Thermo Fisher Scientific Inc. (Waltham, MA). All sterol standards used in this study are available from Kerafast, Inc. (Boston, MA).
Human Serum Analysis
The Office of Regulatory Affairs has determined that this study does not constitute human subject research as defined at 45CFR46.102(f). Serum and plasma samples were obtained from the Nebraska Biobank that is part of the Center for Clinical and Translational Research. The Biobank contains deidentified residual samples from patients who consent to donate any left-over after the laboratory testing. 1312 human serum and plasma samples from pregnant women were obtained from the Nebraska Biobank. The deidentified samples contained information on race and age, which were used to group samples and ascertain potential differences in sterol levels. LC-MS/MS analyses were performed to determine the sterol profile in all samples and assess for the presence of 7-DHC-elevating medications in those with elevated 7-DHC. The details for the LC-MS/MS analyses are described below. Noteworthy, previous studies show that there are no significant differences in the sterol profile between serum and plasma.36
LC-MS/MS (SRM) Analyses
Lipids from 10 μL serum or plasma aliquots were extracted without hydrolysis, derivatized with PTAD as described previously53 and placed in an Acquity UPLC system equipped with an ANSI-compliant well plate holder coupled to a Thermo Scientific TSQ Quantis mass spectrometer equipped with an APCI source. Then 10 μL was injected onto the column (Phenomenex Luna Omega C18, 1.6 μm, 100 Å, 2.1 mm × 50 mm) with 100% MeOH (0.1% v/v acetic acid) mobile phase for 1.0 min runtime at a flow rate of 500 μL/min. Natural sterols were analyzed by selective reaction monitoring (SRM) using the following transitions: Chol 369 → 369, 7-DHC 560 → 365, desmosterol 592 → 560, and lanosterol 634 → 602, with retention times of 0.7, 0.4, 0.3, and 0.3 min, respectively. SRMs for the internal standards were set to d7-Chol 376 → 376, d7-7-DHC 567 → 372, 13C3-desmosterol 595 → 563, and 13C3-lanosterol 637 → 605. Final sterol numbers are reported as ng/μL serum or plasma.
Assessment of medications was performed by LC-MS/MS. Medications were extracted from 75 μL aliquots using methyl tert-butyl ether and ammonium hydroxide as described previously.36 Medication levels were determined in an Acquity UPLC system coupled to a Thermo Scientific TSQ Quantis mass spectrometer using an ESI source in the positive ion mode. Ten μL of each sample was injected onto the column (Phenomenex Luna Omega C18, 1.6 μm, 100 Å, 2.1 mm × 50 mm) using water (0.1% v/v acetic acid) (solvent A) and acetonitrile (0.1% v/v acetic acid) (solvent B) as mobile phase. The gradient was: 10% to 40% B for 0.5 min; 40% to 95% B for 0.4 min; 95% B for 1.5 min; 95% to 10% B for 0.1 min; 10% B for 0.5 min. Medications were analyzed by SRM using the following transitions: ARI 448 → 285, TRZ 372 → 176, BUS 387 → 122, SER 306 → 159, FLX 310 → 44, HAL 376 → 165, MTP 268 → 133, BUP 240 → 184, CAR 427 → 382, fluphenazine 439 → 171, formoterol 345 → 149, nebivolol 406 → 151, oxybutynin 358 → 142, and propranolol 260 → 116. Response factors were calculated to accurately determine the medication levels. Final levels are reported as ng/mL serum or plasma.
Statistical Analyses
Statistical analyses were performed using Graphpad Prism 9 for Windows. Unpaired two-tailed t-tests were performed for individual comparisons between two groups. The Welch’s correction was employed when the variance between the two groups was significantly different. One-way ANOVA analyses were performed for comparisons between three or more groups. Multiple comparisons across samples were performed using Tukey’s correction. The p values for statistically significant differences are highlighted in the figure legends.
Acknowledgments
This work was supported by The National Institutes of Health NIMH MH110636 (K.M./N.A.P.), MH067234 (K.M.), and NICHD HD064727 (N.A.P.). The query of the Nebraska Biobank of deidentified electronic health records has been subsidized by the Great Plains IDeA-CTR grant NIH U54GM115458/NIGMS NIH. Special thanks to the participants who opted to donate their left-over serum samples to Nebraska Biobank and to Dr. Guda Purnima and Neeharica Kodali for searching the electronic health records.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.1c00012.
Supplemental Figure 1: Distribution of desmosterol and lanosterol levels in serum of pregnant women and outlier identification Supplemental Figure 2: Sterol levels in cultured HEPG2 cells treated with bupropion in a 96-well plate. Supplemental Figure 3: Sterol levels in cultured Neuro2a cells treated with bupropion in a 96-well plate. Supplemental Figure 4: Correlation between sertraline (and its active metabolite desmethyl-sertraline) and sterol intermediates in sera of pregnant women. Supplemental methods include cell cultures (PDF)
Author Contributions
K.M. and N.A.P. were responsible for conceptualization, funding acquisition, and supervision. T.G.-M., Z.K., K.A.T., A.A., K.K., and L.B.A. performed the methodology, investigation, and visualization. All authors wrote, reviewed, and edited the article.
The authors declare no competing financial interest.
Supplementary Material
References
- Porter F. D. (2002) Malformation syndromes due to inborn errors of cholesterol synthesis. J. Clin. Invest. 110, 715–724. 10.1172/JCI0216386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M.; Turley S. D. (2001) Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 12, 105–112. 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M.; Turley S. D. (2004) Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 45, 1375–1397. 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Nes W. D. (2011) Biosynthesis of cholesterol and other sterols. Chem. Rev. 111, 6423–6451. 10.1021/cr200021m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z.; Kenworthy A. K. (2008) Lipid rafts, cholesterol, and the brain. Neuropharmacology 55, 1265–1273. 10.1016/j.neuropharm.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne A. H.; Hales D. B. (2004) Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 25, 947–970. 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Koczok K.; Gurumurthy C. B.; Balogh I.; Korade Z.; Mirnics K. (2019) Subcellular localization of sterol biosynthesis enzymes. J. Mol. Histol. 50, 63–73. 10.1007/s10735-018-9807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridsdale A.; Denis M.; Gougeon P. Y.; Ngsee J. K.; Presley J. F.; Zha X. (2006) Cholesterol is required for efficient endoplasmic reticulum-to-Golgi transport of secretory membrane proteins. Mol. Biol. Cell 17, 1593–1605. 10.1091/mbc.e05-02-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. T.; Kreutzberger A. J. B.; Lee J.; Kiessling V.; Tamm L. K. (2016) The role of cholesterol in membrane fusion. Chem. Phys. Lipids 199, 136–143. 10.1016/j.chemphyslip.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter F. D.; Herman G. E. (2011) Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 52, 6–34. 10.1194/jlr.R009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tint G. S.; Yu H.; Shang Q.; Xu G.; Patel S. B. (2006) The use of the Dhcr7 knockout mouse to accurately determine the origin of fetal sterols. J. Lipid Res. 47, 1535–1541. 10.1194/jlr.M600141-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman G. E. (2003) Disorders of cholesterol biosynthesis: prototypic metabolic malformation syndromes. Hum. Mol. Genet. 12, R75–R88. 10.1093/hmg/ddg072. [DOI] [PubMed] [Google Scholar]
- Porter F. D. (2008) Smith-Lemli-Opitz syndrome: pathogenesis, diagnosis and management. Eur. J. Hum. Genet. 16, 535–541. 10.1038/ejhg.2008.10. [DOI] [PubMed] [Google Scholar]
- Porter F. D. (2000) RSH/Smith-Lemli-Opitz syndrome: a multiple congenital anomaly/mental retardation syndrome due to an inborn error of cholesterol biosynthesis. Mol. Genet. Metab. 71, 163–174. 10.1006/mgme.2000.3069. [DOI] [PubMed] [Google Scholar]
- Smith D. W.; Lemli L.; Opitz J. M. (1964) A Newly Recognized Syndrome of Multiple Congenital Anomalies. J. Pediatr. 64, 210–217. 10.1016/S0022-3476(64)80264-X. [DOI] [PubMed] [Google Scholar]
- Allen L. B.; Genaro-Mattos T. C.; Porter N. A.; Mirnics K.; Korade Z. (2019) Desmosterolosis and desmosterol homeostasis in the developing mouse brain. J. Inherited Metab. Dis. 42, 934–943. 10.1002/jimd.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotushko J.; Flusser H.; Markus B.; Shelef I.; Langer Y.; Heverin M.; Bjorkhem I.; Sivan S.; Birk O. S. (2011) The desmosterolosis phenotype: spasticity, microcephaly and micrognathia with agenesis of corpus callosum and loss of white matter. Eur. J. Hum. Genet. 19, 942–946. 10.1038/ejhg.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman G. E.; Kelley R. I.; Pureza V.; Smith D.; Kopacz K.; Pitt J.; Sutphen R.; Sheffield L. J.; Metzenberg A. B. (2002) Characterization of mutations in 22 females with X-linked dominant chondrodysplasia punctata (Happle syndrome). Genet. Med. 4, 434–438. 10.1097/00125817-200211000-00006. [DOI] [PubMed] [Google Scholar]
- Kelley R. I.; Wilcox W. G.; Smith M.; Kratz L. E.; Moser A.; Rimoin D. S. (1999) Abnormal sterol metabolism in patients with Conradi-Hunermann-Happle syndrome and sporadic lethal chondrodysplasia punctata. Am. J. Med. Genet 83, 213–219. . [DOI] [PubMed] [Google Scholar]
- Bornholdt D.; Konig A.; Happle R.; Leveleki L.; Bittar M.; Danarti R.; Vahlquist A.; Tilgen W.; Reinhold U.; Poiares Baptista A.; Grosshans E.; Vabres P.; Niiyama S.; Sasaoka K.; Tanaka T.; Meiss A. L.; Treadwell P. A.; Lambert D.; Camacho F.; Grzeschik K. H. (2005) Mutational spectrum of NSDHL in CHILD syndrome. J. Med. Genet 42, e17 10.1136/jmg.2004.024448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig A.; Happle R.; Bornholdt D.; Engel H.; Grzeschik K. H. (2000) Mutations in the NSDHL gene, encoding a 3beta-hydroxysteroid dehydrogenase, cause CHILD syndrome. Am. J. Med. Genet 90, 339–346. . [DOI] [PubMed] [Google Scholar]
- Genaro-Mattos T. C.; Tallman K. A.; Allen L. B.; Anderson A.; Mirnics K.; Korade Z.; Porter N. A. (2018) Dichlorophenyl piperazines, including a recently-approved atypical antipsychotic, are potent inhibitors of DHCR7, the last enzyme in cholesterol biosynthesis. Toxicol. Appl. Pharmacol. 349, 21–28. 10.1016/j.taap.2018.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P.; Michels V.; Gavrilov D.; Matern D.; Oglesbee D.; Raymond K.; Rinaldo P.; Tortorelli S. (2013) Aripiprazole and trazodone cause elevations of 7-dehydrocholesterol in the absence of Smith-Lemli-Opitz Syndrome. Mol. Genet. Metab. 110, 176–178. 10.1016/j.ymgme.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Kim H. Y.; Korade Z.; Tallman K. A.; Liu W.; Weaver C. D.; Mirnics K.; Porter N. A. (2016) Inhibitors of 7-Dehydrocholesterol Reductase: Screening of a Collection of Pharmacologically Active Compounds in Neuro2a Cells. Chem. Res. Toxicol. 29, 892–900. 10.1021/acs.chemrestox.6b00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z.; Allen L. B.; Anderson A.; Tallman K. A.; Genaro-Mattos T. C.; Porter N. A.; Mirnics K. (2021) Trazodone effects on developing brain. Transl. Psychiatry 11, 85. 10.1038/s41398-021-01217-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z.; Genaro-Mattos T. C.; Tallman K. A.; Liu W.; Garbett K. A.; Koczok K.; Balogh I.; Mirnics K.; Porter N. A. (2017) Vulnerability of DHCR7(±) mutation carriers to aripiprazole and trazodone exposure. J. Lipid Res. 58, 2139–2146. 10.1194/jlr.M079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z.; Kim H. Y.; Tallman K. A.; Liu W.; Koczok K.; Balogh I.; Xu L.; Mirnics K.; Porter N. A. (2016) The Effect of Small Molecules on Sterol Homeostasis: Measuring 7-Dehydrocholesterol in Dhcr7-Deficient Neuro2a Cells and Human Fibroblasts. J. Med. Chem. 59, 1102–1115. 10.1021/acs.jmedchem.5b01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wages P. A.; Kim H. H.; Korade Z.; Porter N. A. (2018) Identification and characterization of prescription drugs that change levels of 7-dehydrocholesterol and desmosterol. J. Lipid Res. 59, 1916–1926. 10.1194/jlr.M086991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland M. R.; Tatonetti N. P. (2016) Investigation of 7-dehydrocholesterol reductase pathway to elucidate off-target prenatal effects of pharmaceuticals: a systematic review. Pharmacogenomics J. 16, 411–429. 10.1038/tpj.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z.; Xu L.; Shelton R.; Porter N. A. (2010) Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome. J. Lipid Res. 51, 3259–3269. 10.1194/jlr.M009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Mirnics K.; Bowman A. B.; Liu W.; Da J.; Porter N. A.; Korade Z. (2012) DHCEO accumulation is a critical mediator of pathophysiology in a Smith-Lemli-Opitz syndrome model. Neurobiol. Dis. 45, 923–929. 10.1016/j.nbd.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Porter N. A. (2015) Free radical oxidation of cholesterol and its precursors: Implications in cholesterol biosynthesis disorders. Free Radical Res. 49, 835–849. 10.3109/10715762.2014.985219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Korade Z.; Porter N. A. (2010) Oxysterols from free radical chain oxidation of 7-dehydrocholesterol: product and mechanistic studies. J. Am. Chem. Soc. 132, 2222–2232. 10.1021/ja9080265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Davis T. A.; Porter N. A. (2009) Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J. Am. Chem. Soc. 131, 13037–13044. 10.1021/ja9029076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genaro-Mattos T. C.; Allen L. B.; Anderson A.; Tallman K. A.; Porter N. A.; Korade Z.; Mirnics K. (2019) Maternal aripiprazole exposure interacts with 7-dehydrocholesterol reductase mutations and alters embryonic neurodevelopment. Mol. Psychiatry 24, 491–500. 10.1038/s41380-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genaro-Mattos T. C.; Anderson A.; Allen L. B.; Tallman K. A.; Porter N. A.; Korade Z.; Mirnics K. (2020) Maternal cariprazine exposure inhibits embryonic and postnatal brain cholesterol biosynthesis. Mol. Psychiatry 25, 2685–2694. 10.1038/s41380-020-0801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z.; Liu W.; Warren E. B.; Armstrong K.; Porter N. A.; Konradi C. (2017) Effect of psychotropic drug treatment on sterol metabolism. Schizophr Res. 187, 74–81. 10.1016/j.schres.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. C.; Druschel C. M.; Romitti P. A.; Bell E. M.; Werler M. M.; Mitchell A. A. (2008) Antifungal drugs and the risk of selected birth defects. Am. J. Obstet. Gynecol. 198, 191. 10.1016/j.ajog.2007.08.044. [DOI] [PubMed] [Google Scholar]
- Mitchell A. A.; Gilboa S. M.; Werler M. M.; Kelley K. E.; Louik C.; Hernandez-Diaz S. (2011) Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am. J. Obstet. Gynecol. 205, 51. 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach H.; Matok I.; Gorodischer R.; Sheiner E.; Daniel S.; Wiznitzer A.; Koren G.; Levy A. (2013) Hypertension and antihypertensive drugs in pregnancy and perinatal outcomes. Am. J. Obstet. Gynecol. 208, 301. 10.1016/j.ajog.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Adam M. P.; Polifka J. E.; Friedman J. M. (2011) Evolving knowledge of the teratogenicity of medications in human pregnancy. Am. J. Med. Genet., Part C 157C, 175–182. 10.1002/ajmg.c.30313. [DOI] [PubMed] [Google Scholar]
- Anderson K. N.; Ailes E. C.; Danielson M.; Lind J. N.; Farr S. L.; Broussard C. S.; Tinker S. C. (2018) Attention-Deficit/Hyperactivity Disorder Medication Prescription Claims Among Privately Insured Women Aged 15–44 Years - United States, 2003–2015. MMWR Morb Mortal Wkly Rep 67, 66–70. 10.15585/mmwr.mm6702a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. N.; Dutton A. C.; Broussard C. S.; Farr S. L.; Lind J. N.; Visser S. N.; Ailes E. C.; Shapira S. K.; Reefhuis J.; Tinker S. C. (2020) ADHD Medication Use During Pregnancy and Risk for Selected Birth Defects: National Birth Defects Prevention Study, 1998–2011. J. Atten Disord 24, 479–489. 10.1177/1087054718759753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. L.; Ailes E. C.; Gilboa S. M.; Simeone R. M.; Lind J. N.; Farr S. L.; Broussard C. S.; Reefhuis J.; Carrino G.; Biermann J.; Honein M. A. (2016) Antidepressant Prescription Claims Among Reproductive-Aged Women With Private Employer-Sponsored Insurance - United States 2008–2013. MMWR Morb Mortal Wkly Rep 65, 41–46. 10.15585/mmwr.mm6503a1. [DOI] [PubMed] [Google Scholar]
- Gentile S.; Tofani S.; Bellantuono C. (2011) Aripiprazole and pregnancy: a case report and literature review. J. Clin. Psychopharmacol. 31, 531–532. 10.1097/JCP.0b013e318222bc65. [DOI] [PubMed] [Google Scholar]
- Khazaie H.; Ghadami M. R.; Knight D. C.; Emamian F.; Tahmasian M. (2013) Insomnia treatment in the third trimester of pregnancy reduces postpartum depression symptoms: a randomized clinical trial. Psychiatry Res. 210, 901–905. 10.1016/j.psychres.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Klier C. M.; Mossaheb N.; Saria A.; Schloegelhofer M.; Zernig G. (2007) Pharmacokinetics and elimination of quetiapine, venlafaxine, and trazodone during pregnancy and postpartum. J. Clin. Psychopharmacol. 27, 720–722. 10.1097/JCP.0b013e31815a57d8. [DOI] [PubMed] [Google Scholar]
- Lutz U. C.; Hiemke C.; Wiatr G.; Farger G.; Arand J.; Wildgruber D. (2010) Aripiprazole in pregnancy and lactation: a case report. J. Clin. Psychopharmacol. 30, 204–205. 10.1097/JCP.0b013e3181d27c7d. [DOI] [PubMed] [Google Scholar]
- Lynch M. M.; Squiers L. B.; Kosa K. M.; Dolina S.; Read J. G.; Broussard C. S.; Frey M. T.; Polen K. N.; Lind J. N.; Gilboa S. M.; Biermann J. (2018) Making Decisions About Medication Use During Pregnancy: Implications for Communication Strategies. Matern Child Health J. 22, 92–100. 10.1007/s10995-017-2358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis A. V.; Mitchell A. A.; Gilboa S. M.; Werler M. M.; Mittleman M. A.; Glynn R. J.; Hernandez-Diaz S. (2012) Use of topiramate in pregnancy and risk of oral clefts. Am. J. Obstet. Gynecol. 207, 405. 10.1016/j.ajog.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker S. C.; Broussard C. S.; Frey M. T.; Gilboa S. M. (2015) Prevalence of prescription medication use among non-pregnant women of childbearing age and pregnant women in the United States: NHANES, 1999–2006. Matern Child Health J. 19, 1097–1106. 10.1007/s10995-014-1611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werler M. M.; Mitchell A. A.; Hernandez-Diaz S.; Honein M. A. (2005) Use of over-the-counter medications during pregnancy. Am. J. Obstet. Gynecol. 193, 771–777. 10.1016/j.ajog.2005.02.100. [DOI] [PubMed] [Google Scholar]
- Liu W.; Xu L.; Lamberson C.; Haas D.; Korade Z.; Porter N. A. (2014) A highly sensitive method for analysis of 7-dehydrocholesterol for the study of Smith-Lemli-Opitz syndrome. J. Lipid Res. 55, 329–337. 10.1194/jlr.D043877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe P. G.; Gilboa S. M.; Hernandez-Diaz S.; Lind J.; Cragan J. D.; Briggs G.; Kweder S.; Friedman J. M.; Mitchell A. A.; Honein M. A. (2013) Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol. Drug Saf. 22, 1013–1018. 10.1002/pds.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker S. C.; Reefhuis J.; Bitsko R. H.; Gilboa S. M.; Mitchell A. A.; Tran E. L.; Werler M. M.; Broussard C. S. (2019) Use of benzodiazepine medications during pregnancy and potential risk for birth defects, National Birth Defects Prevention Study, 1997–2011. Birth Defects Res. 111, 613–620. 10.1002/bdr2.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallman K. A.; Allen L. B.; Klingelsmith K.; Anderson A.; Genaro-Mattos T. C.; Mirnics K.; Porter N. A.; Korade Z. (2021) Prescription Medications Alter Neuronal and Glial Cholesterol Synthesis. ACS Chem. Neurosci. 12 (4), 735–745. 10.1021/acschemneuro.0c00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler S. J.; Bretillon L. (2010) The ins and outs of cholesterol in the vertebrate retina. J. Lipid Res. 51, 3399–3413. 10.1194/jlr.R010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler S. J.; Peachey N. S.; Richards M. J.; Nagel B. A.; Vaughan D. K. (2004) Retinal degeneration in a rodent model of Smith-Lemli-Opitz syndrome: electrophysiologic, biochemical, and morphologic features. Arch. Ophthalmol. 122, 1190–1200. 10.1001/archopht.122.8.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Liu W.; Sheflin L. G.; Fliesler S. J.; Porter N. A. (2011) Novel oxysterols observed in tissues and fluids of AY9944-treated rats: a model for Smith-Lemli-Opitz syndrome. J. Lipid Res. 52, 1810–1820. 10.1194/jlr.M018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z.; Xu L.; Mirnics K.; Porter N. A. (2013) Lipid biomarkers of oxidative stress in a genetic mouse model of Smith-Lemli-Opitz syndrome. J. Inherited Metab. Dis. 36, 113–122. 10.1007/s10545-012-9504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Korade Z.; Rosado D. A. Jr.; Liu W.; Lamberson C. R.; Porter N. A. (2011) An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J. Lipid Res. 52, 1222–1233. 10.1194/jlr.M014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Korade Z.; Rosado D. A. Jr.; Mirnics K.; Porter N. A. (2013) Metabolism of oxysterols derived from nonenzymatic oxidation of 7-dehydrocholesterol in cells. J. Lipid Res. 54, 1135–1143. 10.1194/jlr.M035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wages P. A.; Joshi P.; Tallman K. A.; Kim H. H.; Bowman A. B.; Porter N. A. (2020) Screening ToxCast for Chemicals That Affect Cholesterol Biosynthesis: Studies in Cell Culture and Human Induced Pluripotent Stem Cell-Derived Neuroprogenitors. Environ. Health Perspect. 128, 017014. 10.1289/EHP5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keber R.; Motaln H.; Wagner K. D.; Debeljak N.; Rassoulzadegan M.; Acimovic J.; Rozman D.; Horvat S. (2011) Mouse knockout of the cholesterogenic cytochrome P450 lanosterol 14alpha-demethylase (Cyp51) resembles Antley-Bixler syndrome. J. Biol. Chem. 286, 29086–29097. 10.1074/jbc.M111.253245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross J. L.; Iben J.; Simpson C. L.; Thurm A.; Swedo S.; Tierney E.; Bailey-Wilson J. E.; Biesecker L. G.; Porter F. D.; Wassif C. A. (2015) Determination of the allelic frequency in Smith-Lemli-Opitz syndrome by analysis of massively parallel sequencing data sets. Clin. Genet. 87, 570–575. 10.1111/cge.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.