Abstract
Oral diseases are among the most common encountered health issues worldwide, which are usually associated with anomalies of the oral cavity, jaws, and salivary glands. Despite the availability of numerous treatment modalities for oral disorders, a limited clinical response has been observed because of the inefficacy of the drugs and countless adverse side effects. Therefore, the development of safe, efficacious, and wide-spectrum therapeutics is imperative in the battle against oral diseases. Curcumin, extracted from the golden spice turmeric, is a well-known natural polyphenol that has been extensively studied for its broad pleiotropic attributes and its ability to modulate multiple biological processes. It is well-documented to target pro-inflammatory mediators like NF-κB, ROS, COX-2, IL-1, IL-2, TGF-β, growth factors, apoptotic proteins, receptors, and various kinases. These properties make curcumin a promising nutraceutical in the treatment of many oral diseases like oral submucous fibrosis, oral mucositis, oral leukoplakia, oral erythroplakia, oral candidiasis, aphthous stomatitis, oral lichen planus, dental caries, periodontitis, and gingivitis. Numerous in vitro and in vivo studies have shown that curcumin alleviates the symptoms of most of the oral complications, including the inhibition of the progression of oral cancer. In this regard, many clinical trials have been completed, and many are ongoing to investigate the “curcumin effect” in oral maladies. Therefore, the current review delineates the mechanistic framework of curcumin’s propensity in curbing oral diseases and present outcomes of the clinical trials of curcumin-based therapeutics that can provide a breakthrough in the clinical management of these diseases.
Keywords: oral diseases, Curcuma longa, curcumin, clinical trials
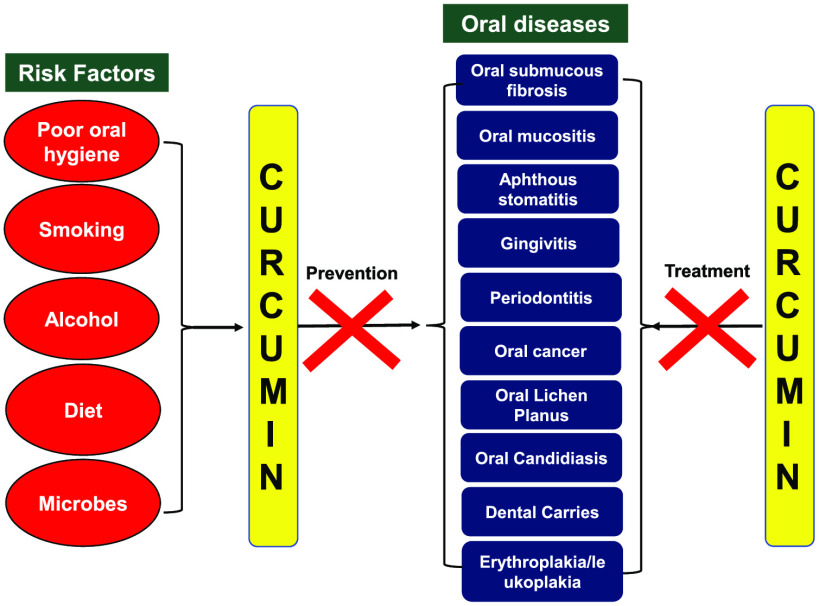
From simple dental cavities to complex oral cancers, oral diseases are the most common health problem faced by individuals worldwide.1−6 According to the Global Disease Burden 2017, oral ailments afflict almost 3.5 billion people worldwide annually.7 The oral cavity is the body’s crucial contributor in maintaining overall health, and detrimental lifestyle factors lead to the development of several oral diseases such as dental caries, periodontal diseases, preoral lesions, oral cancer, fluorosis of teeth, and other oral manifestations.8 Despite the progressive nature and high prevalence of these multigenic diseases, no effective clinical treatment modalities exist for many disorders that ensure a complete cure without a relapse. Though most of the oral diseases could be easily prevented, there exists a high incidence rate that could be due to differences in socioeconomic status and low health literacy awareness especially in middle to low-income countries.1,9−13 Most of the complications involved in oral ailments are mostly infectious yet preventable and are related to the risk factors which can be classified as modifiable and nonmodifiable. The modifiable risk factors include smoking, tobacco chewing, poor oral hygiene, unhealthy diet, hormonal changes in females, medications, and stress; the nonmodifiable factors include diabetes, aging, and heredity.14 These factors are linked with the variations caused in the oral microenvironment and decreased immunosurveillance that results in the shift of the oral microbiome and an increase in inflammation which can lead to severe complications, if left unchecked.15 Apart from the accumulation of plaque-causing bacteria (Streptococcus mutans, Fusobacterium, and Actinobacteria), poor oral hygiene can lead to tooth decay which causes severe discomfort, pain, and social isolation.16 Increased sugar in the diet can shift the microbiome influx which helps the bacterium to degrade sugars into acids that start to dissolve tooth enamel. Tobacco chewing is also implicated in suppressing the immune response to infections, reducing healing capabilities in accidental wounds, and promoting gingivitis and periodontitis in other noncommunicable diseases.17,18 Moreover, tobacco chewing in conjugation with alcohol or areca nut is a major risk factor for oral cancer.19,20
Innovations in the pathophysiological understanding of oral biology have augmented the range of treatment regimens for local and advanced diseases leading to individual treatment plans. Still, these therapeutics suffer from limited clinical success because of the monotargeted approach which in turn produce adverse side effects like vomiting, diarrhea, inflammation, tooth staining, and so on.11,21 Moreover, prolonged treatment can increase the resistance to antibiotics and chemotherapeutics and make patients susceptible to opportunistic infections.22−25 Therefore, the exploration of various alternative natural products and phytochemicals from plants could be a promising alternative for safe, wide spectrum, and efficacious therapeutic intervention for oral diseases.
The idea to use natural compounds to treat various human diseases has existed since time immemorial, and studies over the decades have proved that these compounds show promising effects against various chronic diseases.26−45 Curcumin, a natural polyphenol derived from the plant Curcuma longa, has gained immense attention in clinics because of its medicinal and wide pharmacological activities.46−50 The principal pigment in turmeric, that is, curcuminoids, consists of curcumin and its derivatives demethoxycurcumin (DMC) and bisdemethoxycurcumin (BDMC). Accumulating evidence over the past several decades has established curcumin’s anti-inflammatory, antimicrobial, antiproliferative, antioxidant, anticancer, antiaging, antiarthritic, antiatherosclerotic, antidepressant, hypoglycemic, wound healing, and chemosensitization properties.51−59 Curcumin with its wide pleiotropic nature can target intricate biological processes and diverse inflammatory factors like cytokines, interleukins (ILs), nuclear factor kappa B (NF-κB), reactive oxygen species (ROS), cyclooxygenase-2 (COX-2), C-reactive proteins, transforming growth factor-β (TGF-β), and other enzymes involved in inflammation. Curcumin also potentially inhibits protein kinase C (PKC), epidermal growth factor–receptor tyrosine kinase (EGF-RTKs), and expression of proteins such as c-jun, c-fos, c-myc, NF-κB-inducing kinase (NIK), mitogen-activated protein kinases (MAPKs), extracellular signal-regulating kinase (ERK), phosphoinositide 3-kinase (PI3K), Akt, cyclin-dependent kinases (CDKs), vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), and inducible nitric oxide synthase (iNOS).60−67 These attributes make curcumin an interesting and promising candidate to combat numerous oral disorders like oral submucous fibrosis, oral mucositis, oral leukoplakia, oral erythroplakia, oral candidiasis, aphthous stomatitis, oral lichen planus, dental caries, periodontitis, and gingivitis (Figure 1). Although turmeric comprises extensive diversity of noncurcuminoid phytochemicals such as zingiberene, curcumenol, curcumol, eugenol, turmerin, turmerones, bisacurone, calebin A, etc., still curcuminoids remain the best-researched active constituent among them.35,37,68−70 Numerous in vitro and in vivo studies have advocated curcumin to be safe, well-tolerated, and highly efficacious in the treatment and clinical management of oral diseases. Because of these promising features, curcumin has entered clinical trials for various oral diseases and is also promoted as a nutraceutical or supplement with conventional therapeutics across the globe.71−74 Despite all these alluring attributes, curcumin has met limited therapeutic response because of its poor bioavailability and unsuitable pharmacodynamics for in vivo systems. When administered orally, 40–85% of the curcumin passes and remains unaffected through the gastrointestinal tract, where the majority of its flavonoids are metabolized in the intestine and liver. Furthermore, it shows lower tissue accumulation and rapid systematic elimination from the system.75−77 Therefore, substantial efforts have been made at the research front to improve curcumin bioavailability in the human system using adjuncts, nanoparticle formulations, conjugating phospholipid complexes, using synthetic analog, and reformulations using plant oils.51,78,79 It can be acknowledged that curcumin’s biological and medicinal value far overshadows its lower bioavailability.80,81 Nonetheless, several formulations of curcumin have already been investigated for increasing the pharmacokinetics of curcumin-based therapeutics. Piperine, an alkaloid well-known for inhibiting hepatic and intestinal glucuronidation has been extensively used in combination with curcumin in the prevention of many chronic diseases. It has been shown that concomitant treatment of 20 mg piperine with curcumin can increase its bioavailability 2000% with no adverse effects.82 Therefore, numerous clinical trials are ongoing to explore curcumin’s potential against oral diseases, and results from early studies are quite promising (Clinical Trials.gov Identifier: NCT03790605, NCT03877679, NCT04355416). In this review, we present the comprehensive utility of curcumin-based therapeutics in combating oral diseases. We have highlighted the insights gained from current research that are entering the preclinical evaluation and information about clinical developments, which could help shape the future of curcuminoids in oral maladies.
Figure 1.
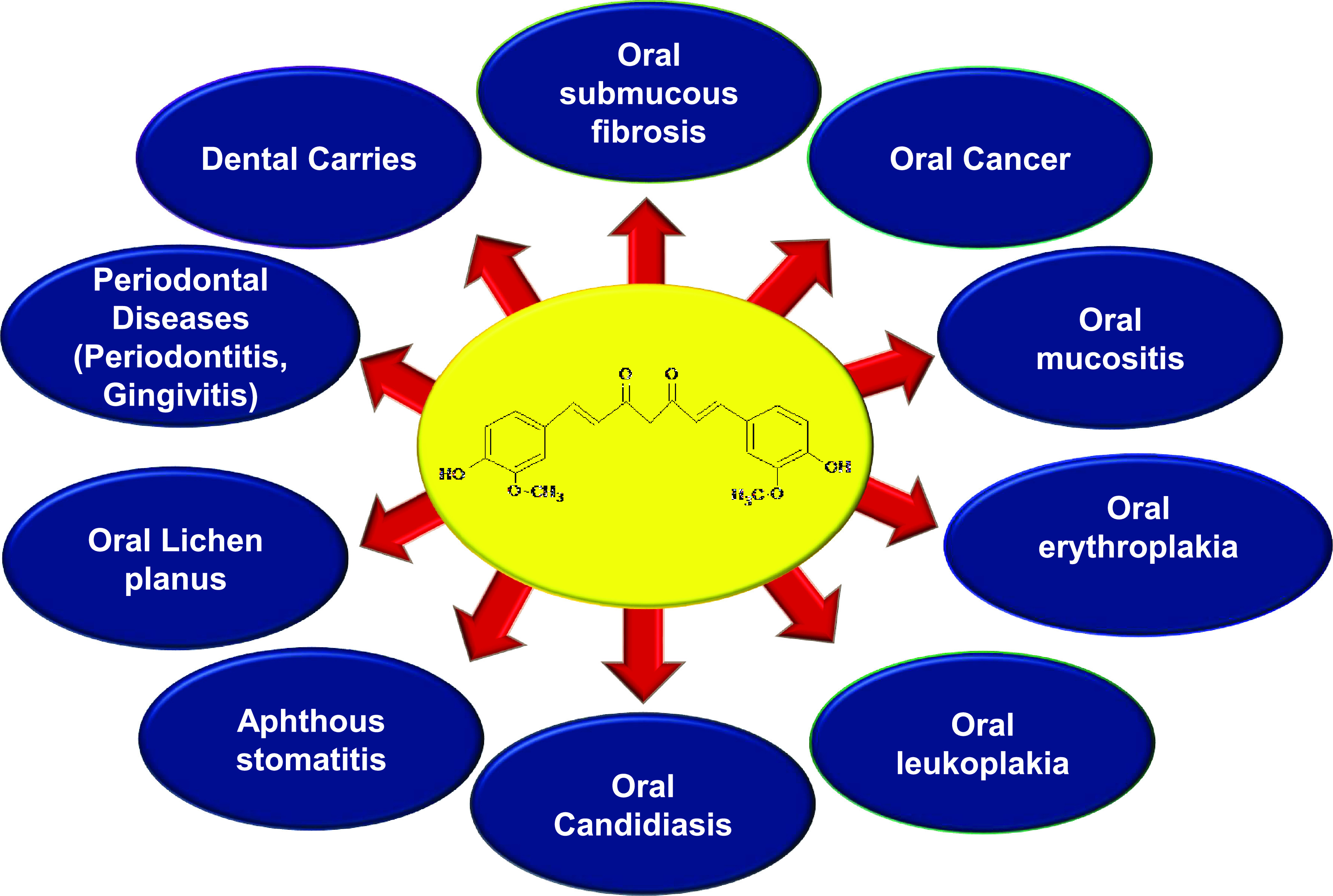
Role of curcumin in the prevention and treatment of different oral diseases.
Mechanisms of Action
Curcumin is well-documented to modulate multiple signaling pathways and target diverse protein families like transcription factors, growth factors, apoptotic proteins, inflammatory mediators, receptors, and various other kinases10,63 (Figure 2). Most of the evidence links inflammation as a major causative factor in the initiation of a variety of oral disorders.83−85 As the oral cavity is a huge reservoir for microbes making it an ecologically rich microenvironment, the intricate cross-talk between the microbes and host defines the overall health of an individual.86 The perturbations in the host cellular machinery initiate the production of several cytokines like tumor necrosis factor-alpha (TNF-α), IL-1β, IL-6, IL-7, and so on, at the site of inflammation, which, if left unchecked, leads to the development of various oral diseases like oral submucous fibrosis, oral mucositis, periodontitis, and gingivitis.87,88 In response to the signal transduction by T-cell receptors, NF-κB is also activated, which further modulates the inflammatory lesions.89,90 Curcumin is known to target NF-κB, signal transducer and activator of transcription 3 (STAT3), cytokines molecules, COX-2, ROS, C-reactive proteins, TGF-β, and so on, making it a promising molecule against these diseases.10,61,65 Curcumin showed an anticandida effect with improved ulcer healing efficacy in buccal mucosal ulcers induced in hamsters.91,92 Curcumin’s anti-inflammatory effects were investigated on lipopolysaccharides (LPS)-induced response in gingival fibroblast in vitro. In that study, curcumin abrogated the production of IL-1β and TNF-α and inhibited the osteoprotegerin (OPG) and soluble receptor activator of nuclear factor kappa-B ligand (sRANKL) and NF-κB activation.93 Another study explored the efficacy of a curcumin-based mucoadhesive formulation on 5-fluorouracil-induced oral mucositis (5-FU-OM) hamster models. The findings suggested curcumin to have a therapeutic response on OM models by increasing wound healing and modulation of angiogenesis and TGF-β1 expression.94 Numerous in vivo studies on periodontal rat models has enumerated the immense potential of curcumin in ameliorating the indices of periodontitis by downregulating the expression levels of TNF-α, IL-1β, IL-6, interferon (IFN)-γ, IL-17, IL-23, MMP-9, p38 MAPK, prostaglandin E2 (PGE2), and NF-κB activity.95−101 Furthermore, curcumin was also shown to decrease alveolar bone resorption and osteoclastogenesis linked with the experimental periodontal disease models.102,103 These findings corroborate curcumin to be a robust anti-inflammatory molecule by modulating various signaling pathways and cytokines in suppressing the progression of several oral diseases.
Figure 2.
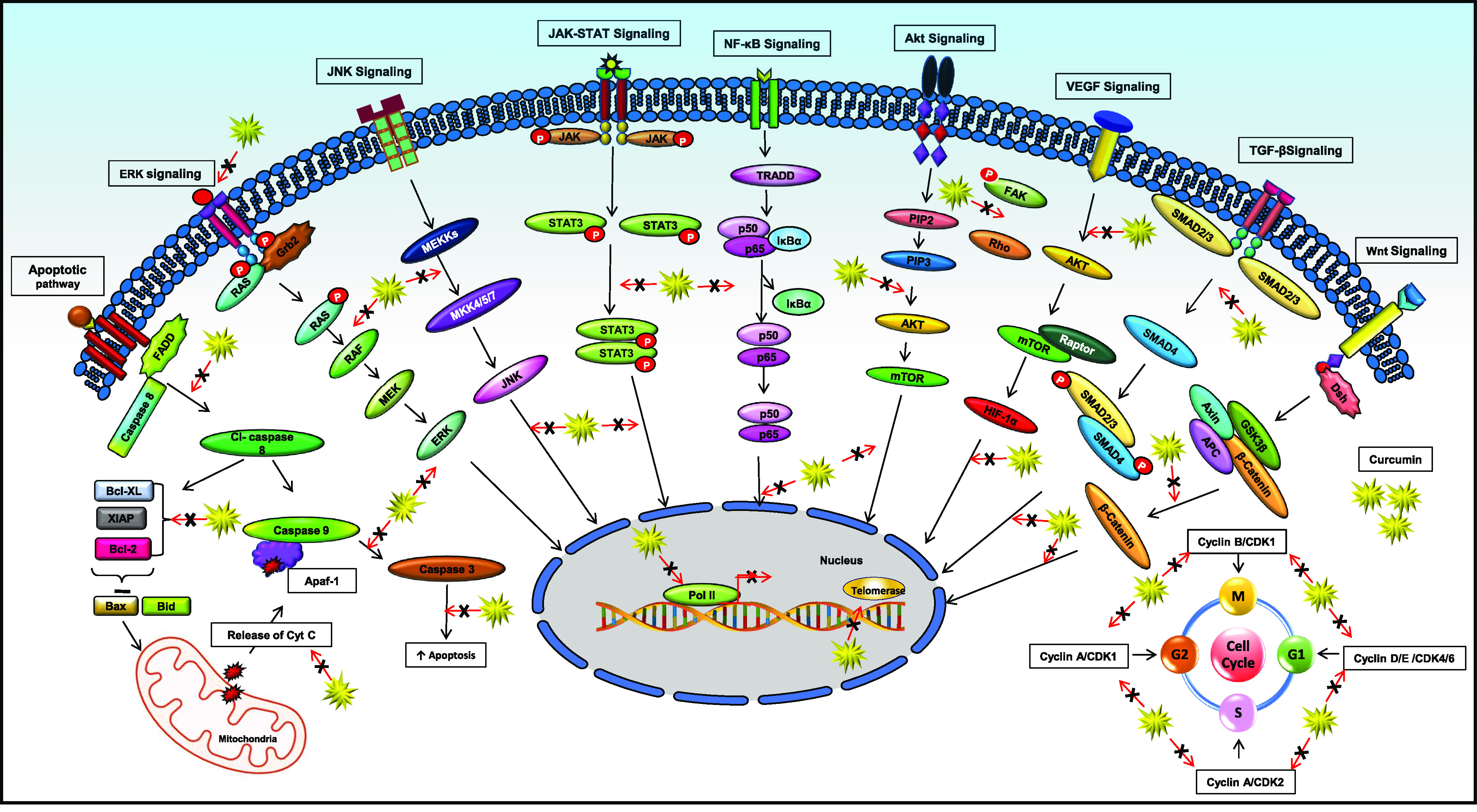
Curcumin modulates multiple signaling pathways and transcription factors involved in the initiation and progression of various oral disorders.
Apart from having antifungal, anti-inflammatory, and antimicrobial attributes, curcumin has also been investigated for its antineoplastic and antiangiogenic effect in oral cancer. Curcumin has shown promising results for the inhibition of oral cancer in experimental models. Curcumin was reported to abrogate Notch-1 levels, which resulted in reduced NF-κB expression, induction of apoptosis, and inhibition of cell growth and invasion in Cal-27 cell lines.104 Another study demonstrated curcumin to inhibit proliferation in human squamous cancer cell lines (SCC-4) via the modulation of the cell division cycle protein 27 (cdc27), peroxisome proliferator-activated receptor (PPAR)-α, EGFR substrate 15, and H2A histones.105 The combinatorial approach of curcumin with gefitinib decreased the total viable cell number by inducing apoptosis and autophagy. Furthermore, downregulation of B-cell lymphoma 2 (Bcl-2) and MMP-9 with upregulation of caspase-3, antihuman light chain 3 (LC3), p62/Sequestosome-1 (SQSTM1), autophagy-related 5 (ATG5), and Beclin-1 expression was observed in human oral cancer SAS cell lines. Besides, the treatment resulted in reduced tumor volume and weight in SAS xenograft nude mice models.106 Curcumin was observed to promote antitumor response by inhibition of programmed death-ligand 1 (PD-L1) and p-STAT3Y705 in Cal 27 and FaDu cell lines. Also, curcumin was found to attenuate tumor growth and increase antitumor response in the microenvironment by activation of CD8 positive T cells with a concomitant decrease in Tregs and myeloid-derived suppressor cells (MDSCs) in a 4-nitroquinolone-1-oxide (4NQO) in vivo murine model.107 A combinatorial approach of curcumin with tea decreased the tumor volume and number of visible tumors by 69.8% and 52.4%, respectively, in 7, 12-dimethylbenz (a)anthracene (DMBA)-induced oral carcinogenesis hamster models. Also, curcumin alone was able to significantly reduce the squamous cancer cell incidence and the proliferation index in hyperplasia, dysplasia, and papilloma during the postinitiation stage.108 Curcumin inhibited the nicotine-derived nitrosamine ketone (NNK)-induced activation of NF-κB and COX-2 expression in smokeless tobacco extract (STE) exposed oral premalignant and cancer cells.109 Curcumin exhibited strong antimotility and antiproliferation effects against invasive YD-10B oral cancer cells by downregulating ERK/MAP kinases, NF-κB, urokinase-type plasminogen activator (uPA), and MMP-2/9 expression.110 The anticancer activity of curcumin was also explored in 7,12-dimethylbenz(a)anthracene-induced oral cancer rat models. It was concluded that the treated group showed a reduction in NF-κB and COX-2 expression as compared with the control.111 SCC-25 cells, when treated with curcumin, showed decreased cellular proliferation, invasion, and expression of epithelial mesenchymal transition (EMT) markers like, Twist, Snail, and E-cadherin with p53-induced suppression. This study suggested curcumin to be an adjunctive regimen in the treatment and prevention of oral cancer metastasis.112 Administration of curcumin activated the autophagic pathways by formation of autophagic vacuoles and autophagosomes and an increase in expression of LC-II markers leading to the induction of apoptosis in YD10B oral cell lines.113 Another study explored the efficacy of copper supplementation with curcumin on oral squamous cancer cell lines. The treatment induced intracellular ROS production, increased the level of nuclear factor erythroid 2-related factor 2 (Nrf2) and inhibited EMT markers with increased apoptosis in cancer cells.114 These findings suggested that curcumin can be a potent anticancer agent and can help in augmenting the existing therapies. Thus, curcumin-based therapeutics can induce various signaling cascades; target diverse proteins responsible for proliferation, inflammation; induce apoptosis; and inhibit invasion and angiogenesis, making it one of the most promising natural candidates in the therapeutic intervention of various oral diseases.
Effect of Curcumin against Various Oral Diseases
Numerous preclinical studies have dictated the immense potential of curcumin against a wide variety of oral ailments (Table 1). Also, this golden molecule is deemed safe, efficacious, and well-tolerated in preclinical trials. These promising attributes have invited numerous clinical trials that have expanded their investigations against several oral diseases (Table 2). Though more than 120 clinical trials were completed with curcuminoids, systematic Phase III randomized trials are still needed to validate and translate these findings to establish curcumin as a marketable drug.115
Table 1. In Vitro/In Vivo Studies of Curcumin in Oral Disordersa.
| Oral diseases | Combination | In vitro/in vivo | Model | Mechanism | Reference |
|---|---|---|---|---|---|
| Gingivitis | indocyanine green | in vitro | HuGu | ↓proliferation | (204) |
| - | in vitro | HGEPs | ↓TNF-α, ↓IL-1β, ↓IL-6, and ↓MMP-9, ↓NF-κB, ↓TIMP | (208) | |
| -Phenytoin | in vivo | Wistar rats | ↓inflammation, ↓Ki67, ↓α-SMA | (218) | |
| - | in vivo | HGF/HSG | ↑apoptosis, ↑ROS | (199) | |
| - | in vitro | Gingival fibroblast | ↓toxicity, ↑wound healing | (207) | |
| - | in vitro | Gingival fibroblast | ↓TGFβ1, ↓thrombin-induced CCN2 | (210) | |
| - | in vitro | Gingival fibroblast | ↓TGFβ1, ↓Smad2, ↓proliferation | (200) | |
| - | in vitro | Gingival fibroblast | ↓TGFβ1, ↓JNK, ↓Smad3, ↓Src, ↓α-SMA | (211) | |
| genistein | in vitro | Gingival fibroblast | ↓uPA, ↓EGF, ↓JNK | (206) | |
| - | in vitro | Gingival fibroblast | ↓TGFβ1, ↓NOX4, ↓JNK, ↓Smad3 | (209) | |
| - | in vitro | Gingival fibroblast | ↓CTGF/CCN2, ↓JNK | (201) | |
| - | in vitro | Gingival fibroblast | ↓NF-κB, ↓COX-2 | (203) | |
| insulin | in vitro | Gingival fibroblast | ↑wound healing, ↓toxicity | (205) | |
| insulin | in vitro | Gingival fibroblast | ↑apoptosis | (202) | |
| Oral cancer | cetuximab | in vitro | CAL 27 (CAR) | ↑caspase-3 and -9, ↓EGFR, ↓ERK, ↓JNK, ↓p38 | (230) |
| gefitinib | in vitro | SAS | ↓MMP, ↑caspase-3, ↓Bcl-2, ↑ATG5,↑LC3, ↑p62/SQSTM, ↑ULK1, ↑VPS34 | (106) | |
| gefitinib | in vivo | SAS cell xenograft nude mice | ↓tumor weight, ↓tumor volume | (106) | |
| green tea | in vivo | Syrian hamsters | ↑apoptosis, ↓proliferation, ↓angiogenesis | (108) | |
| paclitaxel | in vitro | CAL-27 | ↑apoptosis, ↓Bcl-2, ↓Bcl-2/Bax, ↑Bax, ↑caspase-3 | (233) | |
| - | in vivo | Sprague-Dawley rats | ↓NFκB, ↓COX-2 | (111) | |
| - | in vitro | 93VU147T | ↓Bcl-2, ↓cIAP, ↑Bax, ↓c-Jun, ↓JunB, ↓JunD, ↓p50, ↓p65 | (234) | |
| - | in vitro | . . . . . ... | ↓NF-κB, ↓COX-2 | (109) | |
| - | in vitro | SCC25 | ↓MMP-9, ↓MMP-2, ↓Snail, ↓Twist, ↑E-cadherin, ↑p53 | (112) | |
| - | in vitro | YD10B | ↑cleaved PARP, ↑caspase-3, ↑ROS | (113) | |
| - | in vitro | SCC-25 | G2/M phase arrest, ↓MMP-9, ↓MMP-2, ↓uPA, ↓uPAR, ↓p-EGFR, ↓p-Akt, ↓p-ERK1/2, ↓p-STAT3 | (240) | |
| - | in vitro | CAL-27 | G2/M phase arrest, ↓Notch-1, ↓Hes-1, ↓Hes-5, ↓Hey-1 ↓Bcl-2, ↓cyclin D1, ↓MMP-9, ↓VEGF | (104) | |
| - | in vitro | YD-10B | ↓MMP-2/9, ↓uPA, ↓uPAR, ↓NF-κB, ↓ERK/MAPK | (110) | |
| - | in vitro | CAL 27 (CAR) | ↑cytochrome c, ↑ APAF-1, ↑AIF, ↑Bax | (229) | |
| metformin | in vivo | 4NQO models | ↓Notch-1, ↓STAT 3, ↓CD44, ↓CD133 | (235) | |
| - | in vitro | HPV16+ve/-ve | ↑apoptosis, ↓proliferation, ↓miR-21 | (239) | |
| gefitinib | in vitro | SAS | ↓proliferation, ↑cytochrome c, ↑caspase-3, ↑PARP, ↑p53 | (232) | |
| - | in vivo | 4NQO models | ↓PCNA, ↓Bcl-2, ↓SOCS1 e -3, ↓STAT3 | (231) | |
| Oral candidiasis | - | in vivo | Hamsters | effective, safe | (92) |
| - | in vivo | Mice | ↓colony counts, effective | (147) | |
| - | in vivo | BALB/c mice | ↓oral fungal burden | (91) | |
| Oral erythroplakia | - | ex vivo | Chicken buccal mucosa | ↑mucoadhesion activity | (146) |
| Oral mucositis | - | in vivo | Syrian hamsters | ↓angiogenesis, ↓TGF-β1, ↑ROS | (94) |
| Periodontitis | - | in vitro | Gingival fibroblasts | ↓TNF-α, ↓IL-1β, ↓NF-κB activation, ↓OPG/sRANKL | (93) |
| - | in vitro | Periodontal stem cell | ↑TIMP-1, ↓MMP-2 | (186) | |
| - | in vivo | Holtzman rats | ↓NF-κB activation, ↓IL-6, ↓TNF-α, ↓PGE2-s mRNA expression | (98) | |
| - | ex vitro | Human gingival tissue | ↓MMP-9 | (188) | |
| - | in vivo | Holtzman rats | ↓apoptosis, ↓NF-κB activity | (177) | |
| - | in vivo | Wistar rats | ↓RANKL, ↓RANK, ↓OPG, ↓TNF-α, ↓IL-6 | (217) | |
| - | in vivo | Holtzman rats | ↓inflammation, ↓osteoclast counts | (103) | |
| resveratrol | in vivo | Wistar rats | ↓TNF-α, ↓IFN-γ, ↓IL-1β | (96) | |
| - | in vivo | Sprague-Dawley rats | ↓inflammation, ↓MMP-9, ↓TNF-α, ↓IL-1β, ↓IL-6 | (180) | |
| piperine | in vivo | Holtzman rats | ↓NF-κB activity, ↑TGF-β1 | (97) | |
| - | in vivo | Holtzman rats | ↓MMP-9, ↓TNF-α, ↓IL-1β, ↓IL-6, ↓NF-κB activation, ↓p38 MAPK | (179) | |
| - | in vivo | Wistar rats | ↓IL-17, ↓RORγt, ↓IL-23, ↓IL-1β, ↓IL-6 | (176) | |
| - | in vivo | Holtzman rats | ↓TNF-α, ↓osteoclastogenesis | (102) | |
| - | in vivo | Wistar rats | ↓IL-1β, ↓IL-10, ↓alveolar bone loss | (95) | |
| - | in vivo | Holtzman rats | ↓NF-κB activity, ↓p38 MAPK, ↓ PGE2 synthase, ↓IL-6 | (182) | |
| insulin | in vivo | Wistar rats | ↓TNF-α, ↓IL-1β, ↓IL-6, ↓IFN-γ, ↓IL-17 | (99) | |
| - | in vivo | Sprague-Dawley rats | ↓MMP-9, ↓TNF-α, ↓IL-1β | (100) | |
| - | in vivo | Wistar rats | ↓IL-1β, ↓IL-6, ↓IL-17, ↓IL-23 | (101) | |
| - | in vivo | Wistar albino rats | ↓edema, ↓inflammation | (183) | |
| - | in vivo | Holtzman rats | ↓MMP-8, ↓IL-6, ↓IL-1β | (181) | |
| - | in vivo | Dogs | ↓MMP-9, ↓IL-1β, ↓IL-6, ↓p38 MAPK | (178) |
Abbreviations: AIF: apoptosis inducing factor, APAF1: apoptotic protease activating factor 1, ATG5: autophagy related 5, Bax: B-cell lymphoma 2-associated X protein, Bcl-xL: B-cell lymphoma-extra-large, Bcl-2: B-cell lymphoma 2, CCN2: cellular communication network factor 2, cIAP: cellular inhibitor of apoptosis protein, COX-2: cyclooxygenase-2, CTGF: connective tissue growth factor, EGFR: epidermal growth factor receptor, ERK: extracellular signal regulating kinase, Hes: hairy/enhancer of split, Hey-1: Hes related with YRPW motif protein 1, HGEPs: human gingival epithelium progenitors, IFN: interferon, IL: interleukin, JNK: Jun N-terminal kinase, LC3: antihuman light chain 3, MAPK: mitogen-activated protein kinase, MMP: matrix metalloproteinase, NF-κB: nuclear factor kappa B, NOX: NADPH oxidase; OPG: osteoprotegerin, PARP: poly (ADP-ribose) polymerase, PCNA: proliferating cell nuclear antigen, PGE2-s: prostaglandin E2 synthase, RANK: receptor activator of nuclear factor kappa-B, RANKL: receptor activator of nuclear factor kappa-B ligand, RORγt: retinoic-acid-receptor-related orphan nuclear receptor gamma, ROS: reactive oxygen species, SMA:smooth muscle actin, SOCS: suppressor of cytokine signaling, STAT3: signal transducer and activator of transcription 3, SQSTM1: sequestosome-1, TGF-β1: transforming growth factor-β1, TIMP: tissue inhibitor of metalloproteinases, TNF-α: tumor necrosis factor-α, uPA: urokinase-type plasminogen activator, ULK: Unc-51 like autophagy activating kinase, uPA: urokinase-type plasminogen activator, uPAR: urokinase-type plasminogen activator receptor, VEGF: vascular endothelial growth factor, VPS 34: vacuolar protein sorting 34, 4-NQO: 4-nitroquinolone-1-oxide.
Table 2. Clinical Trials of Curcumin/Curcuminoids/Turmeric Used in Different Oral Diseases.
| Oral diseases | Combination | Phase | Patients | Outcome | Reference |
|---|---|---|---|---|---|
| Aphthous stomatitis | - | - | 60 | effective | (151) |
| - | - | 16 | effective | (153) | |
| - | - | 40 | effective | (155) | |
| - | - | 105 | effective | (156) | |
| - | - | 58 | effective | (154) | |
| - | - | 57 | effective; ↓pain, ↓ulcer size | (152) | |
| Gingivitis | - | - | 30 | effective | (214) |
| - | - | 60 | effective | (198) | |
| - | - | 30 | effective | (213) | |
| - | - | 150 | effective | (215) | |
| - | - | 40 | effective | (220) | |
| lycopene and piperine | - | 60 | effective | (219) | |
| Oral erythroplakia | - | - | 10 | effective; ↓pain, ↑healing | (146) |
| Oral leukoplakia | - | II B | 223 | well-tolerated | (143) |
| Oral mucositis | - | - | 20 | effective | (129) |
| - | - | 32 | effective | (131) | |
| - | - | 40 | safe and effective | (136) | |
| honey | - | 60 | effective | (138) | |
| - | - | 7 | safe and well-tolerated | (137) | |
| Oral submucous fibrosis | - | - | 48 | beneficial and effective | (125) |
| - | - | 40 | beneficial and effective | (124) | |
| - | - | 30 | effective | (128) | |
| - | 30 | effective | (116) | ||
| tulsi | - | 41 | safe and effective | (123) | |
| - | - | 30 | significant improvement | (121) | |
| - | - | 28 | efficient | (126) | |
| - | - | 60 | effective | (127) | |
| - | - | 90 | significant improvement | (118) | |
| - | - | 119 | effective | (73) | |
| lycopene and piperine | - | 40 | significant improvement | (122) | |
| triamcinolone-hyaluronidase gel | - | 120 | significant improvement | (71) | |
| Oral lichen planus | - | - | 40 | effective | (163) |
| - | - | 50 | complete remission | (168) | |
| - | - | 20 | no detectable effects | (161) | |
| - | - | 75 | improvement | (164) | |
| - | II | 20 | well-tolerated | (162) | |
| - | - | 27 | effective | (166) | |
| Periodontitis | - | - | 30 | effective | (191) |
| - | - | 20 | significant improvement | (193) | |
| - | - | 23 | beneficial and effective | (195) | |
| - | - | 30 | significant improvement | (216) | |
| photodynamic therapy | - | 25 | short-term clinical benefits | (190) | |
| - | - | 10 | significant improvement | (192) | |
| - | - | 30 | mild/moderate improvement | (189) | |
| - | - | 30 | significant improvement | (184) | |
| - | - | 20 | Effective | (187) | |
| - | - | 30 | significant improvement | (185) | |
| - | - | 23 | mild improvement | (195) |
Oral Submucous Fibrosis (OSMF)
Oral submucous fibrosis (OSMF) is a chronic, fibrotic disorder of the mouth, oropharynx, and upper part of the esophagus that advances with time and is generally associated with major functional morbidity and an increased risk in malignancy.116−118 It generally afflicts the oral mucosa of males in the age group of the 20s to 40s.119 Most of the cases of OSMF are exorbitant in South Asian populations majorly because of their habit of areca nut chewing. Different kinds of treatment regimens are available which focus on palliative care and not the complete cure. Curcumin is a well-known anti-inflammatory mediator and is known to improve the symptoms of OSMF significantly. Various clinical investigations are ongoing to explore the curcumin potential as a drug or adjunct in the treatment of OSMF patients.71,120−124
A study was carried out to investigate the effectiveness of curcumin dispensed in two forms, mainly curcumin capsules and turmeric oil in 48 OSMF patients. Patients were clinically and histopathologically evaluated for OSMF and were classified into three groups: Group I was administered with curcumin capsules; Group II with turmeric oil; and Group III as control were delivered muttinal tablets for 3 months and 6 months follow up. The combinatorial group (curcumin and turmeric oil) resulted in the improvement of clinical signs and symptoms and also showed histopathological reversal as compared with other control groups.125 Another study compared the efficacy of curcumin with the existing Tenovate ointment for 30 OSMF patients. Two different cohorts containing 15 patients in each group of control and treated were topically administrated Tenovate ointment (clobetasol propionate (0.05%) and Longvida (curcumin) lozenges on control and treated group respectively for 3 months duration and 6 months follow-up study. Treatment of curcumin resulted in the overall improvement of mouth opening and visual analogue scale (VAS) for regular food and VAS for spicy food status.116 A pilot study investigated the expression level of different proteins such as p53, TGF-β, and iNOS in 28 OSMF patients during pre and postcurcumin administration. These proteins are known mediators of OSMF pathophysiology and inhibiting them might be a novel approach for the clinical management of OSMF. It was observed that curcumin intake could downregulate the expression of proteins which suggested curcumin’s chemopreventive attribute in the management of OSF.126 Another study assessed the potential of curcumin and lycopene in patients suffering from OSMF. The study group consisted of 60 patients who were equally divided into Group A and B and were administrated with lycopene (4 mg) and curcumin (300 mg), respectively, thrice a day for 3 months. Both the groups showed a decrease in burning sensation in OSMF patients; however, contrastingly, lycopene was more effective for improvement in mouth opening.127
In a recent study, curcumin administration led to a significant overall improvement in the symptoms of OSMF, such as mouth opening, burning sensation, tongue protrusion, and cheek flexibility.118 Similarly, six other clinical trials explored curcumin’s potency to curb OSMF in 298 patients. Three studies showed marked amelioration in burning sensation as compared with the other control patients.119 A similar improvement in burning sensation was observed with commercial turmeric treatment in 30 patients.128 In another study, 119 patients were categorized into three groups, where the patients in group I received antioxidants, group II received systemic curcumin, and group III received both systemic and topical curcumin. All the groups showed improvement in the symptoms such as opening of the mouth, burning sensation, and tongue protrusion after the 12th week; however, group III patients showed substantial improvements as compared with the other two groups corroborating the necessity of a combinatorial approach for curbing OSMF.73 These findings suggested curcumin to be safe and efficacious in improving the deleterious symptoms of OSMF patients.
Oral Mucositis (OM)
Oral mucositis (OM) is a complex and unique pathological condition that arises because of mucosal injuries which are generally outcomes of the patient’s treatment regimens under conventional chemotherapy and radiation therapy.129 The symptoms usually represent erythema and burning sensation and may evolve to noticeable painful ulcerative lesions affecting the patient’s ability to eat and speak, thus affecting overall health.130 Curcumin, being an anti-inflammatory molecule, could play a vital role in palliative care and management of this disorder.131
In the oral mucositis in vitro model, naturally purified curcumin was compared with synthetic curcumin to find the bioequivalence of both formulations. It was observed that both forms of curcumin are equally effective in inhibiting pro-inflammatory cytokines such as IL-8 and IL-6.132 A study presented the antifibrotic potential of curcumin through the inhibition of proliferation in fibroblasts and myofibroblasts from the human oral mucosa, downregulation of type I and III collagen in myofibroblasts, and deregulation of the cell cycle. It was also found to induce apoptosis in the myofibroblasts cells through the downregulation of the B-cell lymphoma 2(Bcl-2)/B-cell lymphoma 2-associated X protein (Bax) ratio.133 Interestingly, an in vivo study investigated the effects of curcumin and capsaicin with or without visible light irradiation on the oral mucous membrane. Both the compounds were observed to induce apoptosis and could act as photosensitizers when exposed to visible light in the presence of oxygen; therefore, these compounds could be used as photodynamic therapy in this oral ailment.134 Another in vivo study on 72 hamsters determined the effect of a mucoadhesive formulation containing curcuminoid (MFC) from C. longa extract on 5-FU-OM models divided into four groups (i.e., control, placebo, chamomilla, and MFC). Clinical and histopathological investigations revealed that MFC and chamomilla groups exhibited better efficiency of wound healing. Furthermore, MFC cohorts showed reduced angiogenesis and TGF-β1 expression, suggesting the therapeutic potential of MFC in curbing OM.94 These preclinical studies advocated the use of curcuminoids either as drug or adjunct in treatment and prevention of oral mucositis.
A nontoxic formulation containing curcumin, α-tocopherol, and sunflower oil resulted in reduced occurrence of radiation-induced mucositis, which validated the effectiveness of the combinations against the disease.135 Curcumin gel, when topically administered, was found to be safe, effective, and a promising alternative in treating oral mucositis.136 A pilot study was conducted to determine the tolerance of curcumin mouthwash with pediatric OM patients undergoing current doxorubicin-comprising chemotherapy. The curcumin containing mouthwash was found to be safe and well-tolerated in OM patients.137 Another study evaluated the effectiveness and safety profile of curcumin in combating OM. Twenty cancer patients undergoing radio-chemotherapy were segregated into two groups, in which group I was administered with regular chlorhexidine mouthwash 0.2% and group II with fresh curcumin mouthwash thrice a day. The follow-up was monitored at day 0, 10, and 20, in which curcumin administration was found to be more effective in terms of NRS (numerical rating scale), erythema, ulceration, and WHO scores. It further showed better wound healing and patient compliance in managing radio-chemotherapy-induced OM.129 Another study investigated the effect of turmeric powder in combination with honey on 60 OM patients. Patients were selected on the basis of the nonprobability purposive sampling method and were divided into an experimental and control group with 30 patients each. It was found that the applied turmeric with honey was effective in improving the symptoms of patients with OM conditions.138 These findings indicated curcumin to be efficacious in reducing the chemo/radiotherapy-induced inflammations in patients, and their association can be an indicator of improved quality of life in OM patients
Oral Leukoplakia
Oral leukoplakia clinically present as white lesions in the oral mucosa, some of which can lead to malignant transformation.139,140 It is considered as the most common oral precancerous lesion that can progress to invasive oral cancer ranging from 0% to 36% if left untreated.141,142 A randomized study was undertaken to investigate the safety and efficacy of curcumin in 223 oral leukoplakia patients, out of which 112 patients were grouped in the placebo and 111 patients were administered curcumin (3.6 g/day) orally for 6 months. The treatment was well-tolerated with a significant and durable clinical response for 6 months in 75 (67.5%) patients.143
Oral Erythroplakia
As stated by the WHO in 1978, oral erythroplakia present as bright red color velvety plaques having excluded other red conditions that can be defined clinically or histopathologically. It is also recognized with higher rates of malignant transformation.144,145 The evaluation of curcumin solid-lipid nanoparticle (CurSLN)-loaded with mucoadhesive gel was tested in in vitro drug dialysis and 10 patients suffering from oral erythroplakia. In the same study, the buccal mucosa of the chicken showed that CurSLN had remarkable muco-adhesion activity, and histological examination showed a major amount of curcumin retained in the chicken oral mucosal tissue when monitored for ex vivo muco-adhesion test and ex vivo permeation study. Furthermore, short-term evaluation of CurSLN efficacy on 10 erythroplakia patients resulted in reduced pain and complete healing after 6 weeks of treatment.146 Though curcumin has been found to exhibit strong pharmacological activity against oral erythroplakia, very limited clinical trials have investigated its existing potential against these disorders.
Oral Candidiasis
The wide variety of human oral infections, from localized mucocutaneous lesions to serious invasive processes, usually arises due to the invasion of harmful pathogens. Some of these infections with clinical significance include oropharyngeal candidiasis and Candida-related denture stomatitis.147 Oral candidiasis is one of the common oral infections that are usually caused by an overgrowth of Candida species, most commonly the Candida albicans.148,149 The oral administration of curcumin with the dosage of 20, 40, and 80 μM in the immunosuppressed mice caused a significant decrease in C. albicans growth after photodynamic therapy in all doses plus LED exposures. However, the highest reduction of log10 in colony counts (4 logs) was observed for the 80 μM dose of curcumin, which indicates that curcumin acted as an effective photosensitizer against C. albicans to inactivate it without destroying the healthy tissue of the host mice.147 Similar treatment with curcumin at 40 μM in the presence of light imparted a major antifungal effect against the yeast populations of C. albicans, C. glabrata, and C. tropicalis and also decreased the metabolic activity and biofilm biomass of all the species.150
Aphthous Stomatitis
Aphthous ulcer, also referred to as recurrent aphthous stomatitis (RAS), is one of the most common ailments characterized by the development of painful, recurring solitary or multiple ulcers in the oral cavity.151 Accumulating evidence has implicated the usefulness of curcumin-based therapeutics in various in vitro and in vivo models of RAS. A randomized clinical trial was investigated to assess the safety and efficacy of curcumin in 60 patients diagnosed with RAS. Patients were divided into two treatment groups: Group I and Group II treated with curcumin gel and triamcinolone acetonide gel, respectively, for 3 times/day. The findings reported a significant difference in size, pain, number, and duration of ulcers in both groups within a 7-day period.151 A similar study performed with 28 patients treated with curcumin gel (containing 2% curcumin), and 29 patients in placebo gel treatment for 2 weeks resulted in a reduction of pain intensity and size of the ulcer, which suggested that curcumin is effective against minor aphthous stomatitis.152 A comparative study involving 16 minor RAS patients with the application of 2% turmeric extract gel reduced the erythematous halo, ulcer size, and pain intensity in patients.153 Besides, the treatment with curcumin orabase in 29 patients was reported to be effective in reducing the size of oral lesions, which is similar to the effect of standard control in patients (n = 29) with 0.1% of triamcinolone acetonide treatment.154 Moreover, in a study comparing the effect of curcumin with the triamcinolone acetonide treatment in 20 patients, it was found that both treatments were equally effective and safe in RAS patients.155 In a recent study, curcumin treatment yielded significant results in terms of improvement in size, VAS score, erythema, and exudations.156 These findings suggested curcumin to be efficacious in the treatment and palliative care of recurrent aphthous stomatitis patients.
Oral Lichen Planus (OLP)
Oral lichen planus is a comparatively common disorder, estimated to affect 0.5% to 2.0% of the general population.157,158 It is due to abnormal T cell immune response where the epithelial cell’s surface antigenicity is recognized as foreign.158 Lichen planus is a mucocutaneous disease that affects buccal mucosa, gingiva, and tongue, with sites of palate lesions being rare.159 This disease can be clinically classified into different forms, such as reticular, papular, plaque-like, atrophic, erosive, and bullous.160 Most of it is nonsymptomatic, where the atrophic erosive can produce symptoms that range from burning sensation to severe pain, causing interference in speaking, eating, and swallowing.158 Curcumin has been found to be well-tolerated and effective in ameliorating the symptoms of OLP, while in some studies it leads to complete remission.161−164
In one of the clinical studies, curcumin was found to be safe and well-tolerated when the initial dose started at 1 g for 2 weeks, followed by a reduced dose of 500 mg for the next 2 weeks and then to 250 mg for the next 2 weeks, followed by 1 month of local application. There was significant amelioration in symptoms with no change in normal mucosa appearance, and recurrence was also not observed in the patients after curcumin treatment.165 Another study explored the efficiency of curcumin in comparison with triamcinolone acetonide for 27 OLP patients. The subjects were divided into two groups: Group I with 12 patients treated with 0.1% triamcinolone acetonide and Group II having 15 patients treated with curcumin ointment, thrice a day for a 2-week period, where it was observed that curcumin cohort improved in relation to pain, erythema, and ulceration. These results indicated that curcumin could be an alternative to steroid treatment of OLP.166 Curcumin treatment also mediated the increase in vitamins C and E levels that helped in the prevention of lipid peroxidation and DNA damage.167 In a study comparing the efficacy of triamcinolone and curcumin, 50 OLP patients in the age range of 38–73 years were divided into groups, each of which received either 0.1% triamcinolone or 5% curcumin oral paste thrice a day for 4 weeks. At the end of the study, the complaints of burning sensation, itching, mild swelling, and xerostomia had disappeared during the first week of treatment.168
Dental Caries
Dental caries may be defined as an infectious microbiological disorder of the teeth that leads to local dissolution and destruction of the calcified tissues. It is the second leading cause of tooth loss globally irrespective of age, sex, caste, creed, or geographic location.169 The formation of the microbial biofilm leads to an acidic and anaerobic state that results in the progression of dental caries because of adherence and colonization of Streptococcus mutans (S. mutans).170 Other factors that are responsible for the initiation of this disease include cariogenic bacteria, fermentable carbohydrates, a susceptible tooth, host, and time; however, the risk factors in infants and young children may vary because their bacterial flora and host defense mechanism are in the process of development and the surface of the tooth that are at new eruption might show hypoplastic defects which may require diet negotiation.
Curcumin treatment was reported to exert antibiofilm activity from the 5th minute to the 24th hour, and the sessile minimum inhibitory concentration (SMIC 50%) against the biofilm of S. mutans was reported to be 500 μM. Moreover, curcumin treatment could also abate live bacterial count and decrease short-term production of extracellular polysaccharide and genes related to polysaccharide synthesis, carbohydrate metabolism, adherence, and the two-component transduction system.171 Another study evaluated the effect of curcumin on inhibition of S. mutans’ adherence to collagen and fibronectin-coated glass surfaces and in vitro inoculated human teeth surfaces (related to oral conditions in vivo). It was observed that curcumin inhibited bacterial growth completely at a minimum inhibitory concentration (MIC) of 128 μg/mL, and the concentration below MIC inhibited bacterial adherence to the glass and tooth surfaces, suggesting the antiadhesive activity mediated through collagen and fibronectin. This property also suggested the use of curcumin as a food-based antimicrobial agent.172 Further, the antibacterial activity of the novel nanocomposite of carboxymethyl starch (CMS)-chitosan (CS)-montmorillonite (MMT) for the delivery of curcumin was investigated against S. mutans, which showed effective inhibition on the biofilm formation on dental models.173
Periodontitis
Periodontitis is a disease with chronic inflammation of supporting structures of teeth because of the formation of bacterial biofilm near the tooth surfaces. The pathogenic microbes activate the progression of the disease, yet most damage to periodontium is due to the host’s immune response against the bacterial pathogens.174 The extent of inflammation or the swelling caused at surgical sites following periodontal therapy might drive one of the particular sensations, such as postoperative pain, which could degrade the quality of life.175 Curcumin has been demonstrated to decrease the expression of various inflammatory markers and angiogenic factors in different preclinical models of dogs and rats making it a potent candidate for human clinical trials.101,176−183 Thus, numerous clinical studies of curcumin (supplement or gel-based form) have been undertaken to tackle and prevent chronic periodontitis.184−193 The mucoadhesive film of curcumin had shown its analgesic attributes, leading to reduced postoperative pain and swelling over a week after periodontal surgery.175 Also, the local administration of curcumin-loaded nanoparticles in 16 rats divided into two groups (LPS-injected group and vehicle control group) showed marked inhibition of inflammation and bone resorption that are associated with periodontal symptoms.103
In a retroprospective study, with a nonsurgical approach, the effect of curcumin collagen gel was compared with the conventional chlorohexidine (CHX) chips as adjuncts to mitigate scaling and root planning for chronic periodontitis patients. After 6 months, patients were monitored on the basis of pocket depth and clinical attachment levels to access the efficacy of the treatment. The findings reported a significant decrease in gingival and plaque index scores with ameliorated microbial parameters, indicating curcumin’s efficiency in curbing the symptoms for chronic periodontitis patients.194 Similar investigations have been carried out by other groups where 1% of curcumin irrigation was used as a supplement before scaling and root planning.195,196 Hence, observations from the earlier studies led to the use of 2% whole turmeric gel, which was reported to have higher pharmacological activity and can be used as a supplement in the treatment and palliative care of periodontal pockets.197 Treatment indicated relief in inflammatory symptoms with a mild to moderate beneficiary effect for cases of chronic periodontitis. A randomized, double-blinded Phase III clinical trial (ClinicalTrials.gov Identifier: NCT03790605) is ongoing to investigate the administration of 1% curcumin chips locally in a nonsurgical isolated periodontal pocket.
Gingivitis
Gingivitis is a common periodontal disorder that afflicts more than 80% of the population worldwide.198 It is an inflammation of the gums due to the accumulation of plaque or bacteria. Curcumin-based therapeutics hold great potential in the treatment of gingivitis because of their anti-inflammatory and antioxidant activity. Numerous in vitro studies of curcumin treatment on gingival fibroblasts have shown inhibition of proliferation and angiogenesis with downregulation of several inflammatory markers like TNF-α, TGFβ1, NF-κB, IL-1β, and IL-6.199−211 In a study, whole turmeric formulation exhibited a similar response as that of curcumin extracts in the prevention and treatment of plaque and gingivitis.212 As curcumin posesses anti-inflammatory attributes, its mouth wash formulations were observed to be equally efficacious as CHX and can be used as a potential supplement in mechanical periodontal therapy.213 Similarly, another study compared curcumin’s efficacy with the CHX-metronidazole (MTZ) combination. Administration of curcumin was found to be as effective as CHX-MTZ, and it reduced the CCL28 and IL-1β level better than the combinatorial formulation.198 Similar studies involving curcumin mouth rinse had been shown to reduce reactive oxygen metabolites (ROM) levels at the end of 4 weeks, which suggest the alternative approach to gingivitis treatment using curcumin-based therapeutics.214 Another study exhibited curcumin mouthwash activity against plaque and gingivitis by reducing the plaque index (PI), gingival index (GI), and sulcus bleeding index (SBI) scores.215 Further, the curcumin gel application combined with scaling and root planning had ameliorated the periodontal parameters such as PI, GI, probing depth (PD), clinical attachment level (CAL), and microbiologic parameters in test groups as compared with the control (without curcumin gel and only SRP).216 Another study evaluated the efficiency of intragastric administration of curcumin where it was observed to reduce the alveolar bone loss through the abridged expression of inflammatory mediators like receptor activator of nuclear factor-κB (RANK), RANKL, and OPG.217 Furthermore, curcumin could decrease phenytoin-induced gingival expansion by decreasing the expression of Ki67 and alpha-smooth muscle actin (α-SMA), inflammation, epithelial thickness, and number of blood vessels with an increase in cross-sectional area.218 The treatment of curcumin could be effectively used to control the plaque spread which might be due to its anti-inflammatory action in the gingivitis.219,220
Oral Cancer
Oral squamous cell carcinoma (OSCC) is one of the most frequent malignant tumors of the oral cavity associated with high incidence and mortality rates worldwide.5,221,222 It has become a major public health problem in Southeast Asia because of the habit of chewing tobacco, smoking, and the use of alcohol.223−225 The oral squamous cell carcinomas (OSCC) constitute more than 90% of oral cancers that originate from the squamous cell lining of the lip or oral cavity.226,227 Though any abnormality in the oral cavity is easy to monitor, still most of the OSCC cases are diagnosed at advanced stages, which results in reduced overall and progression-free survival rates.226,228 Accumulating evidence has implicated the usefulness of curcumin-based therapeutics in various in vitro and in vivo models of oral cancers.106,111,229−235
The dose-dependent curcumin treatment was reported to inhibit PD-L1 and p-STAT3Y705 expression and also reduced the tumor growth in oral cancer cell lines.107 Besides, the administration of curcumin downregulated the expression of Notch1, which further lead to reduced expression of NF-κB that cause inhibition of cell growth and invasion.104 The combinatorial approach of tea and curcumin in DMBA-induced oral cancer hamsters’ models exhibited reduced tumor volume and incidence, which were correlated with decreased cellular proliferation, induction of apoptosis, and inhibition of angiogenesis.108 Similarly, the combination of curcumin and lycopene administered at different doses of 3, 4.25, 5.50, and 6.75 μM resulted in increased cell cytotoxicity and decreased migration in oral cancer cell lines. Moreover, this combination, along with irradiation, exhibited favorable synergistic activity against oral cancer.236 Further, curcumin treatment could dose-dependently inhibit cell proliferation and invasion and also influence the cell cycle of the SS4 cells dose-dependently.105 In another study, curcumin was reported to possess anticancer attributes against OSCC via induction of autophagy and apoptosis through the production of ROS and autophagic vacuoles formation.113 Curcumin could also increase the expression of CCAAT/enhancer-binding protein alpha (C/EBP α) through the activation of p38 and its interaction to binding elements of insulin-like growth factor binding protein 5 (IGFBP-5) promoter region, which induced the level of IGFBP-5 that further complemented the reduced xenograft tumorigenesis in mice.237 Further reduction of luciferase activity and base excision repair (BER) expression and PARylation suggest the promising efficiency of curcumin and olaparib in combination to inhibit BER activity in the oral cells. In vivo study of curcumin with olaparib showed a similar outcome with the decreased tumor growth and induction of apoptosis and improvement in body weight of tumor mice.238
Curcumin treatment in the range of 0–50 μM dose dependently inhibited the cancer cell proliferation, stemness, and expression of miRNA-21 in human papillomavirus (HPV)+ve/HPV-ve oral cancer cells. Furthermore, the effect was more prominent in the case of HPV-positive cancer stem cells (CSCs) as compared with the other cancer cells.239 Administration of curcumin with copper adjunct enhanced the suppression of proliferation and migration by upregulating the E-cadherin expression with a simultaneous decrease in Vimentin levels in oral cancer cells, which led to the suppression of EMT. Moreover, the combinatorial strategy also induced early apoptosis in the cancer cells as compared with single curcumin or copper treatment.114 Further, treatment of curcumin in 4NQO-induced oral carcinogenesis model for 12 weeks at 100 mg/kg significantly reduced the expression levels of proliferating cell nuclear antigen (PCNA), Bcl-2, suppressor of cytokine signaling (SOCS)-1 e -3, and STAT3 and also eliminated the cellular atypia and minimized genes associated with EMT.231 Though curcumin has shown the potential to inhibit proliferation, migration, and invasion with increased apoptosis, more randomized clinical trials are paramount for establishing curcumin as an alternative approach in the clinical management of oral cancer.
Conclusions
As well-documented, oral diseases afflict millions worldwide and intense research is going on globally to find efficient, specific, and targeted natural compounds that can replace the nonspecific, nontargeted drugs and their associated debilitating side effects. For 200 years, curcumin, the golden nutraceutical has been researched for its wide pleiotropic activities and multitargeted approach against different chronic diseases. The current review mainly highlights the therapeutic potential of curcumin in treating several oral diseases like oral cancer, oral submucous fibrosis, oral mucositis, oral leukoplakia, oral erythroplakia, oral candidiasis, aphthous stomatitis, oral lichen planus, dental caries, periodontitis, and gingivitis. The focus of this review is to elucidate the effects of curcumin on the inhibition of various proteins and signaling pathways associated with the development and progression of oral diseases.
Turmeric or Curcuma longa is a perennial herb belonging to the family of Zingiberaceae, which is mostly used in South Asian countries for decades as a spice, food preservative, and coloring agent. Although turmeric has more than 300 active compounds, curcumin has been extensively studied and researched with over 16 000 citations in PubMed. Curcumin has been well-documented to induce anti-inflammatory, antiangiogenic, antioxidant, anticancer, antimicrobial, and wound healing attributes against various diseases including oral disorders. These traits make curcumin a promising nutraceutical in the treatment and palliative care of several oral pathological diseases such as oral mucositis, oral cancer, gingivitis, oral lichen planus, etc. Curcumin has been reported to ameliorate the overall status of oral mucositis patients. Further, curcumin could result in overall improvements in the symptoms such as mouth opening, burning sensation, tongue protrusion, and cheek flexibility in OSMF patients. Curcumin also exhibited strong anticancer and antiangiogenic traits against oral cancer by modulating signaling pathways and inflammatory mediators. Curcumin could also decrease the plaque, inflammation, and gingival index in gingivitis and periodontitis patients. Moreover, toothpaste formulations containing turmeric are also reported for its efficacies in preventing dental plaques and gingivitis by inhibiting various microbes.241,242
Thus, from the above-mentioned studies, the effectiveness of turmeric and its golden compound curcumin should be considered in the prevention and treatment of several oral diseases. Still, systematic randomized placebo-controlled clinical trials are needed with a large sample size and participants from different ethnic backgrounds to corroborate these results and aid in the clinical paradigm for establishing curcumin as a next-generation smart drug.
Acknowledgments
This work was supported by BT/556/NE/U-Excel/2016 grant awarded to Ajaikumar B. Kunnumakkara by Department of Biotechnology (DBT), Government of India. The work was supported by a grant from the Singapore Ministry of Education Tier 2 (MOE-T2EP30120-0016) awarded to A.P.K. A.P.K. is also supported by the National Medical Research Council of Singapore and the Singapore Ministry of Education under its Research Centres of Excellence initiative to Cancer Science Institute of Singapore, National University of Singapore.
Glossary
Abbreviations
- ATG5
autophagy related 5
- Bax
B-cell lymphoma 2-associated X protein
- Bcl-2
B-cell lymphoma 2
- BER
base excision repair
- CAL
clinical attachment level
- C/EBP α
CCAAT/enhancer-binding protein alpha
- Cdc 27
cell division cycle protein 27
- CDKs
cyclin-dependent kinases
- CHX
chlorohexidine
- CMS
carboxymethyl starch
- COX-2
cyclooxygenase-2
- CS
chitosan
- CSCs
cancer stem cells
- Cur-SLN
curcumin solid-lipid nanoparticle
- DMBA
7,12-dimethylbenz(a)anthracene
- EGFR
epidermal growth factor receptor
- EMT
epithelial mesenchymal transition
- ERK
extracellular signal regulating kinase
- GI
gingival index
- HPV
human papillomavirus
- iNOS
inducible nitric oxide synthase
- IFN
interferon
- IGFBP5
insulin like growth factor binding protein 5
- IL
interleukin
- LC3
antihuman light chain 3
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MDSCs
myeloid-derived suppressor cells
- MFC
mucoadhesive formulation containing curcumin
- MIC
minimum inhibitory concentration
- MMP
matrix metalloproteinase
- MMT
montmorillonite
- MTZ
metronidazole
- NF-κB
nuclear factor kappa B
- NIK
NF-κB-inducing kinase
- NNK
nicotine-derived nitrosamine ketone
- Nrf2
nuclear factor erythroid 2-related factor 2
- OLP
oral lichen planus
- OM
oral mucositis
- OPG
osteoprotegerin
- OSCC
oral squamous cell carcinoma
- OSMF
oral submucous fibrosis
- PCNA
proliferating cell nuclear antigen
- PD
probing depth
- PDL1
programmed death-ligand 1
- PGE2-s
prostaglandin E2 synthase
- PI
plaque index
- PI3K
phosphoinositide 3-kinase
- PK
protein kinase
- PPAR
peroxisome proliferator-activated receptor
- RANK
receptor activator of nuclear factor kappa-B
- RANKL
receptor activator of nuclear factor kappa-B ligand
- RAS
recurrent aphthous stomatitis
- ROM
reactive oxygen metabolites
- ROS
reactive oxygen species
- RTK
receptor tyrosine kinases
- SBI
sulcus bleeding index
- SD
Spargue-Dawley
- SMA
smooth muscle actin
- SOCS
suppressor of cytokine signaling
- STAT3
signal transducer and activator of transcription 3
- STE
smokeless tobacco extract
- SQSTM1
sequestosome-1
- TGF-β1
transforming growth factor-β1
- TNF-α
tumor necrosis factor-α
- VAS
visual analogue scale
- VEGF
vascular endothelial growth factor
- VL
visible light
- 4-NQO
4-nitroquinolone-1-oxide
- 5-FU-OM
5-fluorouracil induced oral mucositis
Author Contributions
# (S.G., A.K.) These authors contributed equally.
The authors declare no competing financial interest.
References
- Bordoloi D.; Monisha J.; Roy N. K.; Padmavathi G.; Banik K.; Harsha C.; Wang H.; Kumar A. P.; Arfuso F.; Kunnumakkara A. B. (2019) An Investigation on the Therapeutic Potential of Butein, A Tretrahydroxychalcone Against Human Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 20, 3437–3446. 10.31557/APJCP.2019.20.11.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwairakpam A. D., Monisha J., Roy N. K., Bordoloi D., Padmavathi G., Banik K., Khatoon E., and Kunnumakkara A. B. (2019) Vietnamese coriander inhibits cell proliferation, survival and migration via suppression of Akt/mTOR pathway in oral squamous cell carcinoma. J. Basic Clin. Physiol. Pharmacol., 31, 10.1515/jbcpp-2019-0162 [DOI] [PubMed] [Google Scholar]
- Harsha C.; Banik K.; Ang H. L.; Girisa S.; Vikkurthi R.; Parama D.; Rana V.; Shabnam B.; Khatoon E.; Kumar A. P.; Kunnumakkara A. B. (2020) Targeting AKT/mTOR in Oral Cancer: Mechanisms and Advances in Clinical Trials. Int. J. Mol. Sci. 21, 3285. 10.3390/ijms21093285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L. J.; Lamster I. B.; Greenspan J. S.; Pitts N. B.; Scully C.; Warnakulasuriya S. (2016) Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Dis. 22, 609–619. 10.1111/odi.12428. [DOI] [PubMed] [Google Scholar]
- Monisha J.; Roy N. K.; Padmavathi G.; Banik K.; Bordoloi D.; Khwairakpam A. D.; Arfuso F.; Chinnathambi A.; Alahmadi T. A.; Alharbi S. A.; et al. (2018) NGAL is downregulated in oral squamous cell carcinoma and leads to increased survival, proliferation, migration and chemoresistance. Cancers 10, 228. 10.3390/cancers10070228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres M. A.; Macpherson L. M. D.; Weyant R. J.; Daly B.; Venturelli R.; Mathur M. R.; Listl S.; Celeste R. K.; Guarnizo-Herreño C. C.; Kearns C.; et al. (2019) Oral diseases: a global public health challenge. Lancet 394, 249–260. 10.1016/S0140-6736(19)31146-8. [DOI] [PubMed] [Google Scholar]
- Mathers C., Stevens G., Hogan D., Mahanani W. R., and Ho J. (2017) Global and regional causes of death: patterns and trends, 2000–15. In Disease Control Priorities: Improving Health and Reducing Poverty (Jamison D. T., Gelband H., Horton S., Jha P., Laxminarayan R., Mock C. N., and Nugent R., Eds.) The International Bank for Reconstruction and Development/The World Bank, Washington (DC), 10.1596/978-1-4648-0527-1_ch4. [DOI] [PubMed] [Google Scholar]
- Bratthall D., Petersen P. E., Ramanathan J., and Brown L. J. (2006) Chapter 38. Oral and Craniofacial Diseases and Disorders. In Disease Control Priorities in Developing Countries (Jamison D. T., Breman J. G., Measham A. R., Alleyne G., Claeson M., Evans D. B., Jha P., Mills A., and Musgrove P., Eds.), The International Bank for Reconstruction and Development/The World Bank, Oxford University Press, Washington (DC), NY. [Google Scholar]
- Chandra Shekar B. R.; Nagarajappa R.; Suma S.; Thakur R. (2015) Herbal extracts in oral health care - A review of the current scenario and its future needs. Pharmacogn. Rev. 9, 87–92. 10.4103/0973-7847.162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara A. B.; Bordoloi D.; Padmavathi G.; Monisha J.; Roy N. K.; Prasad S.; Aggarwal B. B. (2017) Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br. J. Pharmacol. 174, 1325–1348. 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo E. A. (2011) Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evidence-based Complement. Altern. Med. 2011, 680354. 10.1093/ecam/nep067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. K.; Roy N. K.; Bordoloi D.; Padmavathi G.; Banik K.; Khwairakpam A. D.; Kunnumakkara A. B.; Sukumar P. (2020) Orai-1 and Orai-2 regulate oral cancer cell migration and colonisation by suppressing Akt/mTOR/NF-κB signalling. Life Sci. 261, 118372. 10.1016/j.lfs.2020.118372. [DOI] [PubMed] [Google Scholar]
- Warnakulasuriya S. (2009) Significant oral cancer risk associated with low socioeconomic status. Evid. Based. Dent. 10, 4–5. 10.1038/sj.ebd.6400623. [DOI] [PubMed] [Google Scholar]
- Petersen P. E.; Bourgeois D.; Ogawa H.; Estupinan-Day S.; Ndiaye C. (2005) The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 83, 661–669. [PMC free article] [PubMed] [Google Scholar]
- Bowen W. H.; Burne R. A.; Wu H.; Koo H. (2018) Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 26, 229–242. 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron R.; Grenier D.; Maheu-Robert L.-F. (2000) The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2, 897–906. 10.1016/S1286-4579(00)00391-9. [DOI] [PubMed] [Google Scholar]
- Winn D. M. (2001) Tobacco use and oral disease. J. Dent Educ. 65 (4), 306–12. 10.1002/j.0022-0337.2001.65.4.tb03400.x. [DOI] [PubMed] [Google Scholar]
- Warnakulasuriya S.; Dietrich T.; Bornstein M. M.; Peidró E. C.; Preshaw P. M.; Walter C.; Wennström J. L.; Bergström J. (2010) Oral health risks of tobacco use and effects of cessation. Int. Dent. J. 60, 7–30. [PubMed] [Google Scholar]
- Kumar M.; Nanavati R.; Modi T. G.; Dobariya C. (2016) Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 12, 458. 10.4103/0973-1482.186696. [DOI] [PubMed] [Google Scholar]
- Llewellyn C. D.; Johnson N. W.; Warnakulasuriya K. (2001) Risk factors for squamous cell carcinoma of the oral cavity in young people—a comprehensive literature review. Oral Oncol. 37, 401–418. 10.1016/S1368-8375(00)00135-4. [DOI] [PubMed] [Google Scholar]
- Nammour S.; Zeinoun T.; Yoshida K.; Brugnera Junior A. (2016) Oral Biology, Oral Pathology, and Oral Treatments. BioMed Res. Int. 2016, 1. 10.1155/2016/2849795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatoon E., Banik K., Harsha C., Sailo B. L., Thakur K. K., Khwairakpam A. D., Vikkurthi R., Devi T. B., Gupta S. C., and Kunnumakkara A. B. (2020) Phytochemicals in cancer cell chemosensitization: Current knowledge and future perspectives. Semin. Cancer Biol. 10.1016/j.semcancer.2020.06.014. [DOI] [PubMed] [Google Scholar]
- Sedghizadeh P. P.; Mahabady S.; Allen C. M. (2017) Opportunistic Oral Infections. Dent. Clin. North Am. 61, 389–400. 10.1016/j.cden.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar S.; Roberts A. P.; Martin F. E.; Adler C. J. (2016) Metagenomic insights into transferable antibiotic resistance in oral bacteria. J. Dent. Res. 95, 969–976. 10.1177/0022034516648944. [DOI] [PubMed] [Google Scholar]
- Thakur K. K., Bordoloi D., Prakash J., Javadi M., Roy N. K., and Kunnumakkara A. B. (2018) Different Chemosensitization Approaches for the Effective Management of HNSCC. Cancer Cell Chemoresistance Chemosensitization, pp 399–423, World Scientific, Singapore, 10.1142/9789813208575_0014. [DOI] [Google Scholar]
- Dai X.; Zhang J.; Arfuso F.; Chinnathambi A.; Zayed M. E.; Alharbi S. A.; Kumar A. P.; Ahn K. S.; Sethi G. (2015) Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp. Biol. Med. 240, 760–773. 10.1177/1535370215579167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daimary U. D.; Parama D.; Rana V.; Banik K.; Kumar A.; Harsha C.; Kunnumakkara A. B. (2021) Emerging roles of cardamonin, a multitargeted nutraceutical in the prevention and treatment of chronic diseases. Curr. Res. Pharmacol. Drug Discovery 2, 100008. 10.1016/j.crphar.2020.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girisa S.; Shabnam B.; Monisha J.; Fan L.; Halim C. E.; Arfuso F.; Ahn K. S.; Sethi G.; Kunnumakkara A. B. (2019) Potential of Zerumbone as an Anti-Cancer Agent. Molecules 24, 734. 10.3390/molecules24040734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henamayee S.; Banik K.; Sailo B. L.; Shabnam B.; Harsha C.; Srilakshmi S.; Vgm N.; Baek S. H.; Ahn K. S.; Kunnumakkara A. B. (2020) Therapeutic Emergence of Rhein as a Potential Anticancer Drug: A Review of Its Molecular Targets and Anticancer Properties. Molecules 25, 2278. 10.3390/molecules25102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh Y.-S.; Yang S.-F.; Sethi G.; Hu D.-N. (2015) Natural bioactives in cancer treatment and prevention. BioMed Res. Int. 2015, 1. 10.1155/2015/182835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna D.; Sethi G.; Ahn K. S.; Pandey M. K.; Kunnumakkara A. B.; Sung B.; Aggarwal A.; Aggarwal B. B. (2007) Natural products as a gold mine for arthritis treatment. Curr. Opin. Pharmacol. 7, 344–351. 10.1016/j.coph.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Khwairakpam A. D.; Damayenti Y. D.; Deka A.; Monisha J.; Roy N. K.; Padmavathi G.; Kunnumakkara A. B. (2018) Acorus calamus: a bio-reserve of medicinal values. J. Basic Clin. Physiol. Pharmacol. 29, 107–122. 10.1515/jbcpp-2016-0132. [DOI] [PubMed] [Google Scholar]
- Khwairakpam A. D.; Bordoloi D.; Thakur K. K.; Monisha J.; Arfuso F.; Sethi G.; Mishra S.; Kumar A. P.; Kunnumakkara A. B. (2018) Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol. Res. 133, 53–64. 10.1016/j.phrs.2018.04.021. [DOI] [PubMed] [Google Scholar]
- Kim C.; Cho S. K.; Kapoor S.; Kumar A.; Vali S.; Abbasi T.; Kim S.; Sethi G.; Ahn K. S. (2014) β-caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol. Carcinog. 53, 793–806. 10.1002/mc.22035. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara A. B.; Banik K.; Bordoloi D.; Harsha C.; Sailo B. L.; Padmavathi G.; Roy N. K.; Gupta S. C.; Aggarwal B. B. (2018) Googling the Guggul (Commiphora and Boswellia) for Prevention of Chronic Diseases. Front. Pharmacol. 9, 686. 10.3389/fphar.2018.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara A. B., Koca C., Dey S., Gehlot P., Yodkeeree S., Danda D., Sung B., and Aggarwal B. B. (2009) Traditional uses of spices: an overview. Molecular targets and therapeutic uses of spices: modern uses for ancient medicine, pp 1–24, World Scientific, Singapore, 10.1142/9789812837912_0001. [DOI] [Google Scholar]
- Kunnumakkara A. B.; Sailo B. L.; Banik K.; Harsha C.; Prasad S.; Gupta S. C.; Bharti A. C.; Aggarwal B. B. (2018) Chronic diseases, inflammation, and spices: how are they linked?. J. Transl. Med. 16, 14. 10.1186/s12967-018-1381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H.; Kim C.; Sethi G.; Ahn K. S. (2015) Brassinin inhibits STAT3 signaling pathway through modulation of PIAS-3 and SOCS-3 expression and sensitizes human lung cancer xenograft in nude mice to paclitaxel. Oncotarget 6, 6386. 10.18632/oncotarget.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T.; Warnakulasuriya S.; Nakamura T.; Kato S.; Yamamoto K.; Fukano H.; Suzuki K.; Shimozato K.; Hashimoto S. (2015) Treatment of oral leukoplakia with a low-dose of beta-carotene and vitamin C supplements: A randomized controlled trial. Int. J. Cancer 136, 1708–1717. 10.1002/ijc.29156. [DOI] [PubMed] [Google Scholar]
- Padmavathi G.; Roy N. K.; Bordoloi D.; Arfuso F.; Mishra S.; Sethi G.; Bishayee A.; Kunnumakkara A. B. (2017) Butein in health and disease: A comprehensive review. Phytomedicine 25, 118–127. 10.1016/j.phymed.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Parama D.; Boruah M.; Yachna K.; Rana V.; Banik K.; Harsha C.; Thakur K. K.; Dutta U.; Arya A.; Mao X.; et al. (2020) Diosgenin, a steroidal saponin, and its analogues: Effective therapies against different chronic diseases. Life Sci. 260, 118182. 10.1016/j.lfs.2020.118182. [DOI] [PubMed] [Google Scholar]
- Ranaware A. M.; Banik K.; Deshpande V.; Padmavathi G.; Roy N. K.; Sethi G.; Fan L.; Kumar A. P.; Kunnumakkara A. B. (2018) Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 19, 2362. 10.3390/ijms19082362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N. K.; Parama D.; Banik K.; Bordoloi D.; Devi A. K.; Thakur K. K.; Padmavathi G.; Shakibaei M.; Fan L.; Sethi G.; Kunnumakkara A. B. (2019) An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. Int. J. Mol. Sci. 20, 4101. 10.3390/ijms20174101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam M. K.; Ong T. H.; Kumar A. P.; Lun C. K.; Ho P. C.; Wong P. T. H.; Hui K. M.; Sethi G. (2012) Ursolic acid inhibits the initiation, progression of prostate cancer and prolongs the survival of TRAMP mice by modulating pro-inflammatory pathways. PLoS One 7, e32476. 10.1371/journal.pone.0032476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-F.; Weng C.-J.; Sethi G.; Hu D.-N. (2013) Natural bioactives and phytochemicals serve in cancer treatment and prevention. Evidence-based complementary and alternative medicine. 2013, 698190. 10.1155/2013/698190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara A. B.; Bordoloi D.; Harsha C.; Banik K.; Gupta S. C.; Aggarwal B. B. (2017) Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin. Sci. 131, 1781–1799. 10.1042/CS20160935. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara A. B.; Guha S.; Krishnan S.; Diagaradjane P.; Gelovani J.; Aggarwal B. B. (2007) Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 67, 3853–3861. 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- Moballegh Nasery M.; Abadi B.; Poormoghadam D.; Zarrabi A.; Keyhanvar P.; Khanbabaei H.; Ashrafizadeh M.; Mohammadinejad R.; Tavakol S.; Sethi G. (2020) Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 25, 689. 10.3390/molecules25030689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandur S. K.; Ichikawa H.; Pandey M. K.; Kunnumakkara A. B.; Sung B.; Sethi G.; Aggarwal B. B. (2007) Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radical Biol. Med. 43, 568–580. 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam M. K.; Warrier S.; Kumar A. P.; Sethi G.; Arfuso F. (2017) Potential role of natural compounds as anti-angiogenic agents in cancer. Curr. Vasc. Pharmacol. 15, 503–519. 10.2174/1570161115666170713094319. [DOI] [PubMed] [Google Scholar]
- Amalraj A.; Varma K.; Jacob J.; Divya C.; Kunnumakkara A. B.; Stohs S. J.; Gopi S. (2017) A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study. J. Med. Food 20, 1022–1030. 10.1089/jmf.2017.3930. [DOI] [PubMed] [Google Scholar]
- Bordoloi D.; Roy N. K.; Monisha J.; Padmavathi G.; Kunnumakkara A. B. (2016) Multi-Targeted Agents in Cancer Cell Chemosensitization: What We Learnt from Curcumin Thus Far. Recent Pat. Anti-Cancer Drug Discovery 11, 67–97. 10.2174/1574892810666151020101706. [DOI] [PubMed] [Google Scholar]
- Chaushu L., Rahmanov Gavrielov M., Chaushu G., Keidar Z., and Vered M. (2020) Curcumin Promotes Primary Oral Wound Healing in a Rat Model. J. Med. Food. 10.1089/jmf.2020.0093. [DOI] [PubMed] [Google Scholar]
- Goel A.; Kunnumakkara A. B.; Aggarwal B. B. (2008) Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 75, 787–809. 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Guerrero-Romero F.; Simental-Mendía L. E.; Martínez-Aguilar G.; Sánchez-Meraz M. A.; Gamboa-Gómez C. I. (2020) Hypoglycemic and antioxidant effects of five commercial turmeric (Curcuma longa) supplements. J. Food Biochem. 44, e13389 10.1111/jfbc.13389. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara A. B.; Diagaradjane P.; Guha S.; Deorukhkar A.; Shentu S.; Aggarwal B. B.; Krishnan S. (2008) Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin. Cancer Res. 14, 2128–2136. 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- Singh M.; Sharma D.; Kumar D.; Singh G.; Swami G.; Rathore M. S. (2020) Formulation, Development, and Evaluation of Herbal Effervescent Mouthwash Tablet Containing Azadirachta Indica (Neem) and Curcumin for the Maintenance of Oral Hygiene. Recent Pat. Drug Delivery Formulation 14, 145–161. 10.2174/1872211314666200820142509. [DOI] [PubMed] [Google Scholar]
- Sung B.; Kunnumakkara A. B.; Sethi G.; Anand P.; Guha S.; Aggarwal B. B. (2009) Curcumin circumvents chemoresistance in vitro and potentiates the effect of thalidomide and bortezomib against human multiple myeloma in nude mice model. Mol. Cancer Ther. 8, 959–970. 10.1158/1535-7163.MCT-08-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Li L.; Zhang J. (2020) Curcumin in antidepressant treatments: An overview of potential mechanisms, pre-clinical/clinical trials and ongoing challenges. Basic Clin. Pharmacol. Toxicol. 127, 243–253. 10.1111/bcpt.13455. [DOI] [PubMed] [Google Scholar]
- Aggarwal B. B.; Kunnumakkara A. B.; Harikumar K. B.; Tharakan S. T.; Sung B.; Anand P. (2008) Potential of spice-derived phytochemicals for cancer prevention. Planta Med. 74, 1560–1569. 10.1055/s-2008-1074578. [DOI] [PubMed] [Google Scholar]
- Anand P.; Sundaram C.; Jhurani S.; Kunnumakkara A. B.; Aggarwal B. B. (2008) Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 267, 133–164. 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Deng S.; Shanmugam M. K.; Kumar A. P.; Yap C. T.; Sethi G.; Bishayee A. (2019) Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 125, 1228–1246. 10.1002/cncr.31978. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara A. B.; Anand P.; Aggarwal B. B. (2008) Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 269, 199–225. 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara A. B.; Diagaradjane P.; Anand P.; Kuzhuvelil H. B.; Deorukhkar A.; Gelovani J.; Guha S.; Krishnan S.; Aggarwal B. B. (2009) Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int. J. Cancer 125, 2187–2197. 10.1002/ijc.24593. [DOI] [PubMed] [Google Scholar]
- Monisha J.; Padmavathi G.; Roy N. K.; Deka A.; Bordoloi D.; Anip A.; Kunnumakkara A. B. (2016) NF-κB Blockers Gifted by Mother Nature: Prospectives in Cancer Cell Chemosensitization. Curr. Pharm. Des. 22, 4173–4200. 10.2174/1381612822666160609110231. [DOI] [PubMed] [Google Scholar]
- Sethi G., Sung B., Kunnumakkara A. B., and Aggarwal B. B. (2009) Targeting TNF for treatment of cancer and autoimmunity. Therapeutic Targets of the TNF Superfamily, pp 37–51, Springer, 10.1007/978-0-387-89520-8_3. [DOI] [PubMed] [Google Scholar]
- Tewari D.; Nabavi S. F.; Nabavi S. M.; Sureda A.; Farooqi A. A.; Atanasov A. G.; Vacca R. A.; Sethi G.; Bishayee A. (2018) Targeting activator protein 1 signaling pathway by bioactive natural agents: Possible therapeutic strategy for cancer prevention and intervention. Pharmacol. Res. 128, 366–375. 10.1016/j.phrs.2017.09.014. [DOI] [PubMed] [Google Scholar]
- Aggarwal B. B.; Yuan W.; Li S.; Gupta S. C. (2013) Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 57, 1529–1542. 10.1002/mnfr.201200838. [DOI] [PubMed] [Google Scholar]
- Gupta S. C.; Patchva S.; Aggarwal B. B. (2013) Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 15, 195–218. 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A.; Amalraj A.; Jacob J.; Kunnumakkara A. B.; Gopi S. (2019) Non-Curcuminoids from Turmeric and Their Potential in Cancer Therapy and Anticancer Drug Delivery Formulations. Biomolecules 9, 13. 10.3390/biom9010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanjekar A. B.; Bhowate R. R.; Bakhle S.; Narayane A.; Pawar V.; Gandagule R. (2020) Comparison of Efficacy of Topical Curcumin Gel with Triamcinolone-hyaluronidase Gel Individually and in Combination in the Treatment of Oral Submucous Fibrosis. J. Contemp. Dent. Pract. 21, 83–90. 10.5005/jp-journals-10024-2726. [DOI] [PubMed] [Google Scholar]
- Neetha M. C.; Panchaksharappa M. G.; Pattabhiramasastry S.; Shivaprasad N. V.; Venkatesh U. G. (2020) Chemopreventive Synergism between Green Tea Extract and Curcumin in Patients with Potentially Malignant Oral Disorders: A Double-blind, Randomized Preliminary Study. J. Contemp. Dent. Pract. 21, 521–531. 10.5005/jp-journals-10024-2823. [DOI] [PubMed] [Google Scholar]
- Rai A.; Kaur M.; Gombra V.; Hasan S.; Kumar N. (2019) Comparative evaluation of curcumin and antioxidants in the management of oral submucous fibrosis. J. Investig. Clin. Dent. 10, e12464 10.1111/jicd.12464. [DOI] [PubMed] [Google Scholar]
- Siddharth M.; Singh P.; Gupta R.; Sinha A.; Shree S.; Sharma K. (2020) A Comparative Evaluation of Subgingivally Delivered 2% Curcumin and 0.2% Chlorhexidine Gel Adjunctive to Scaling and Root Planing in Chronic Periodontitis. J. Contemp. Dent. Pract. 21, 494–499. 10.5005/jp-journals-10024-2828. [DOI] [PubMed] [Google Scholar]
- Dei Cas M.; Ghidoni R. (2019) Dietary curcumin: Correlation between bioavailability and health potential. Nutrients 11, 2147. 10.3390/nu11092147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labban L. (2014) Medicinal and pharmacological properties of Turmeric (Curcuma longa): A review. Int. J. Pharm. Biomed Sci. 5, 17–23. [Google Scholar]
- Lopresti A. L. (2018) The problem of curcumin and its bioavailability: could its gastrointestinal influence contribute to its overall health-enhancing effects?. Adv. Nutr. 9, 41–50. 10.1093/advances/nmx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P.; Kunnumakkara A. B.; Newman R. A.; Aggarwal B. B. (2007) Bioavailability of Curcumin: Problems and Promises. Mol. Pharmaceutics 4, 807–818. 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Nair H. B.; Sung B.; Yadav V. R.; Kannappan R.; Chaturvedi M. M.; Aggarwal B. B. (2010) Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem. Pharmacol. 80, 1833–1843. 10.1016/j.bcp.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopi S.; Jacob J.; Varma K.; Jude S.; Amalraj A.; Arundhathy C. A.; George R.; Sreeraj T. R.; Divya C.; Kunnumakkara A. B.; Stohs S. J. (2017) Comparative Oral Absorption of Curcumin in a Natural Turmeric Matrix with Two Other Curcumin Formulations: An Open-label Parallel-arm Study. Phytother. Res. 31, 1883–1891. 10.1002/ptr.5931. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara A. B.; Harsha C.; Banik K.; Vikkurthi R.; Sailo B. L.; Bordoloi D.; Gupta S. C.; Aggarwal B. B. (2019) Is curcumin bioavailability a problem in humans: lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 15, 705–733. 10.1080/17425255.2019.1650914. [DOI] [PubMed] [Google Scholar]
- Shoba G.; Joy D.; Joseph T.; Majeed M.; Rajendran R.; Srinivas P. (1998) Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 64, 353–356. 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- Gupta S. C.; Kunnumakkara A. B.; Aggarwal S.; Aggarwal B. B. (2018) Inflammation, a double-edge sword for cancer and other age-related diseases. Front. Immunol. 9, 2160. 10.3389/fimmu.2018.02160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Sun L.; Byrd K. M.; Ko C.-C.; Zhao Z.; Fang J. (2020) AIM2 Inflammasome’s First Decade of Discovery: Focus on Oral Diseases. Front. Immunol. 11, 1487. 10.3389/fimmu.2020.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Wan P.; Wang H.; Zhang S.; Liu J.; Chang C.; Yang K.; Pan Y. (2020) An Antibacterial Strategy of Mg-Cu Bone Grafting in Infection-Mediated Periodontics. Biomed Res. Int. 2020, 7289208. 10.1155/2020/7289208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont R. J.; Koo H.; Hajishengallis G. (2018) The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 16, 745–759. 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk H.; Kantarci A.; Van Dyke T. E. (2012) Oral inflammatory diseases and systemic inflammation: role of the macrophage. Front. Immunol. 3, 118. 10.3389/fimmu.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel J. E.; O’Boyle C.; Krishnan S. (2019) Distal consequences of oral inflammation. Front. Immunol. 10, 1403. 10.3389/fimmu.2019.01403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambili R.; Janam P. (2017) A critique on nuclear factor-kappa B and signal transducer and activator of transcription 3: The key transcription factors in periodontal pathogenesis. J. Indian Soc. Periodontol. 21, 350. 10.4103/jisp.jisp_301_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.; Zhang L.; Joo D.; Sun S. C. (2017) NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2, 17023. 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman M.; Ayyıldız Z. A.; Fırıncı F.; Kiray M.; Bağrıyanık A.; Yilmaz O.; Uzuner N.; Karaman Ö. (2011) Effects of curcumin on lung histopathology and fungal burden in a mouse model of chronic asthma and oropharyngeal candidiasis. Arch. Med. Res. 42, 79–87. 10.1016/j.arcmed.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Mahattanadul S.; Mustafa M. W.; Kuadkaew S.; Pattharachayakul S.; Ungphaiboon S.; Sawanyawisuth K. (2018) Oral ulcer healing and anti-candida efficacy of an alcohol-free chitosan-curcumin mouthwash. Eur. Rev. Med. Pharmacol. Sci. 22, 7020–7023. 10.26355/eurrev_201810_16173. [DOI] [PubMed] [Google Scholar]
- Xiao C.-J.; Yu X.-J.; Xie J.-L.; Liu S.; Li S. (2018) Protective effect and related mechanisms of curcumin in rat experimental periodontitis. Head Face Med. 14, 1. 10.1186/s13005-017-0158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T. R.; Curra M.; Wagner V. P.; Martins M. A. T.; de Oliveira A. C.; Batista A. C.; Valadares M. C.; Marreto R. N.; Martins M. D. (2019) Mucoadhesive formulation containing Curcuma longa L. reduces oral mucositis induced by 5-fluorouracil in hamsters. Phytother. Res. 33, 881–890. 10.1002/ptr.6279. [DOI] [PubMed] [Google Scholar]
- Akpinar A.; Calisir M.; Cansın Karakan N.; Lektemur Alpan A.; Goze F.; Poyraz O. (2017) Effects of Curcumin on Alveolar Bone Loss in Experimental Periodontitis in Rats: A Morphometric and Histopathologic Study. Int. J. Vitam. Nutr. Res. 87, 262–270. 10.1024/0300-9831/a000243. [DOI] [PubMed] [Google Scholar]
- Corrêa M. G.; Pires P. R.; Ribeiro F. V.; Pimentel S. Z.; Casarin R. C. V; Cirano F. R.; Tenenbaum H. T.; Casati M. Z. (2017) Systemic treatment with resveratrol and/or curcumin reduces the progression of experimental periodontitis in rats. J. Periodontal Res. 52, 201–209. 10.1111/jre.12382. [DOI] [PubMed] [Google Scholar]
- Guimaraes-Stabili M. R.; de Aquino S. G.; de Almeida Curylofo F.; Tasso C. O.; Rocha F. R. G.; de Medeiros M. C.; de Pizzol J. P.; Cerri P. S.; Romito G. A.; Rossa C. (2019) Systemic administration of curcumin or piperine enhances the periodontal repair: a preliminary study in rats. Clin. Oral Investig. 23, 3297–3306. 10.1007/s00784-018-2755-9. [DOI] [PubMed] [Google Scholar]
- Guimarães M. R.; Coimbra L. S.; de Aquino S. G.; Spolidorio L. C.; Kirkwood K. L.; Rossa C. Jr (2011) Potent anti-inflammatory effects of systemically administered curcumin modulate periodontal disease in vivo. J. Periodontal Res. 46, 269–279. 10.1111/j.1600-0765.2010.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel S. P.; Casati M. Z.; Ribeiro F. V.; Corrêa M. G.; Franck F. C.; Benatti B. B.; Cirano F. R. (2020) Impact of natural curcumin on the progression of experimental periodontitis in diabetic rats. J. Periodontal Res. 55, 41–50. 10.1111/jre.12683. [DOI] [PubMed] [Google Scholar]
- Wang H. H.; Lee H.-M.; Raja V.; Hou W.; Iacono V. J.; Scaduto J.; Johnson F.; Golub L. M.; Gu Y. (2019) Enhanced efficacy of chemically modified curcumin in experimental periodontitis: Systemic implications. J. Exp. Pharmacol. 11, 1. 10.2147/JEP.S171119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetkin Ay Z.; Bakır B.; Bozkurt S. B.; Kayis S. A.; Hakki S. S. (2020) Positive effect of curcumin on experimental peridontitis via suppression of IL-1-beta and IL-6 expression level. Int. J. Vitam. Nutr. Res. Int. Zeitschrift fur Vitamin- und Ernahrungsforschung. J. Int. Vitaminol. Nutr. 1–9. 10.1024/0300-9831/a000672. [DOI] [PubMed] [Google Scholar]
- de Almeida Brandão D.; Spolidorio L. C.; Johnson F.; Golub L. M.; Guimarães-Stabili M. R.; Rossa C. Jr (2019) Dose-response assessment of chemically modified curcumin in experimental periodontitis. J. Periodontol. 90, 535–545. 10.1002/JPER.18-0392. [DOI] [PubMed] [Google Scholar]
- Zambrano L. M. G.; Brandao D. A.; Rocha F. R. G.; Marsiglio R. P.; Longo I. B.; Primo F. L.; Tedesco A. C.; Guimaraes-Stabili M. R.; Junior C. R. (2018) Local administration of curcumin-loaded nanoparticles effectively inhibits inflammation and bone resorption associated with experimental periodontal disease. Sci. Rep. 8, 6652. 10.1038/s41598-018-24866-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S.; Xia J.; Chen Z.; Zhang S.; Ahmad A.; Miele L.; Sarkar F. H.; Wang Z. (2011) Inhibitory effect of curcumin on oral carcinoma CAL-27 cells via suppression of Notch-1 and NF-κB signaling pathways. J. Cell. Biochem. 112, 1055–1065. 10.1002/jcb.23019. [DOI] [PubMed] [Google Scholar]
- Jiao-Wen C.; Ya-Ling T.; Hong L.; Zhi-Yu Z.; Di L.; Ning G.; Yu C. (2011) Anti-proliferative and anti-metastatic effects of curcumin on oral cancer cells. West China J. Stomatol. 29 (1), 83–86. [PubMed] [Google Scholar]
- Hsiao Y.; Kuo C.; Lin J.; Huang W.; Peng S.; Chueh F.; Bau D.; Chung J. (2018) Curcuminoids combined with gefitinib mediated apoptosis and autophagy of human oral cancer SAS cells in vitro and reduced tumor of SAS cell xenograft mice in vivo. Environ. Toxicol. 33, 821–832. 10.1002/tox.22568. [DOI] [PubMed] [Google Scholar]
- Liao F.; Liu L.; Luo E.; Hu J. (2018) Curcumin enhances anti-tumor immune response in tongue squamous cell carcinoma. Arch. Oral Biol. 92, 32–37. 10.1016/j.archoralbio.2018.04.015. [DOI] [PubMed] [Google Scholar]
- Li N.; Chen X.; Liao J.; Yang G.; Wang S.; Josephson Y.; Han C.; Chen J.; Huang M.-T.; Yang C. S. (2002) Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis 23, 1307–1313. 10.1093/carcin/23.8.1307. [DOI] [PubMed] [Google Scholar]
- Sharma C.; Kaur J.; Shishodia S.; Aggarwal B. B.; Ralhan R. (2006) Curcumin down regulates smokeless tobacco-induced NF-κB activation and COX-2 expression in human oral premalignant and cancer cells. Toxicology 228, 1–15. 10.1016/j.tox.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Shin H. K.; Kim J.; Lee E. J.; Kim S. H. (2010) Inhibitory effect of curcumin on motility of human oral squamous carcinoma YD-10B cells via suppression of ERK and NF-κB activations. Phyther. Res. An Int. J. Devoted to Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 24, 577–582. 10.1002/ptr.2989. [DOI] [PubMed] [Google Scholar]
- Maulina T.; Hadikrishna I.; Hardianto A.; Sjamsudin E.; Pontjo B.; Yusuf H. Y. (2019) The therapeutic activity of curcumin through its anti-cancer potential on oral squamous cell carcinoma: A study on Sprague Dawley rat. SAGE Open Med. 7, 1–10. 10.1177/2050312119875982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. Y.-L.; Fan C.-C.; Chen Y.-A.; Cheng C.-W.; Sung Y.-J.; Hsu C.-P.; Kao T.-Y. (2015) Curcumin inhibits invasiveness and epithelial-mesenchymal transition in oral squamous cell carcinoma through reducing matrix metalloproteinase 2, 9 and modulating p53-E-cadherin pathway. Integr. Cancer Ther. 14, 484–490. 10.1177/1534735415588930. [DOI] [PubMed] [Google Scholar]
- Kim J. Y.; Cho T. J.; Woo B. H.; Choi K. U.; Lee C. H.; Ryu M. H.; Park H. R. (2012) Curcumin-induced autophagy contributes to the decreased survival of oral cancer cells. Arch. Oral Biol. 57, 1018–1025. 10.1016/j.archoralbio.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Lee H.-M.; Patel V.; Shyur L.-F.; Lee W.-L. (2016) Copper supplementation amplifies the anti-tumor effect of curcumin in oral cancer cells. Phytomedicine 23, 1535–1544. 10.1016/j.phymed.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Nagpal M.; Sood S. (2013) Role of curcumin in systemic and oral health: An overview. J. Nat. Sci. Biol. Med. 4, 3–7. 10.4103/0976-9668.107253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazarey V. K.; Sakrikar A. R.; Ganvir S. M. (2015) Efficacy of curcumin in the treatment for oral submucous fibrosis-A randomized clinical trial. J. oral Maxillofac. Pathol. JOMFP 19, 145. 10.4103/0973-029X.164524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr A. R.; Warnakulasuriya S.; Mighell A. J.; Dietrich T.; Nasser M.; Rimal J.; Jalil A.; Bornstein M. M.; Nagao T.; Fortune F.; et al. (2011) A systematic review of medical interventions for oral submucous fibrosis and future research opportunities. Oral Dis. 17, 42–57. 10.1111/j.1601-0825.2011.01791.x. [DOI] [PubMed] [Google Scholar]
- Piyush P.; Mahajan A.; Singh K.; Ghosh S.; Gupta S. (2019) Comparison of therapeutic response of lycopene and curcumin in oral submucous fibrosis: A randomized controlled trial. Oral Dis. 25, 73–79. 10.1111/odi.12947. [DOI] [PubMed] [Google Scholar]
- Al-Maweri S. A. (2019) Efficacy of curcumin for management of oral submucous fibrosis: a systematic review of randomized clinical trials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 127, 300–308. 10.1016/j.oooo.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Alok A.; Singh I. D.; Singh S.; Kishore M.; Jha P. C. (2015) Curcumin-pharmacological actions and its role in oral submucous fibrosis: a review. J. Clin. Diagn. Res. 9, ZE01. 10.7860/jcdr/2015/13857.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopuri R. K. C.; Chakravarthy C.; Sunder S.; Patil R. S.; Shivaraj W.; Arakeri G. (2016) A comparative study of the clinical efficacy of lycopene and curcumin in the treatment of oral submucous fibrosis using ultrasonography. J. Int. Oral Heal. 8, 687. 10.2047/jioh-08-06-09. [DOI] [Google Scholar]
- Mahato B.; Prodhan C.; Mandal S.; Dutta A.; Kumar P.; Deb T.; Jha T.; Chaudhuri K. (2019) Evaluation of efficacy of curcumin along with lycopene and piperine in the management of oral submucous fibrosis. Contemp. Clin. Dent. 10, 531–541. 10.4103/ccd.ccd_937_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A.; Agarwal R.; Chaturvedi T. P.; Chandra A.; Singh O. P. (2015) Clinical evaluation of the role of tulsi and turmeric in the management of oral submucous fibrosis: A pilot, prospective observational study. J. Ayurveda Integr. Med. 6, 45. 10.4103/0975-9476.146563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M.; Aravinda K.; Saxena V. S.; Srinivas K.; Ratnakar P.; Gupta J.; Sachdev A. S.; Shivhare P. (2014) Comparison of curcumin with intralesional steroid injections in Oral Submucous Fibrosis-A randomized, open-label interventional study. J. oral Biol. craniofacial Res. 4, 169–173. 10.1016/j.jobcr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A.; Balan A.; Sreelatha K. (2010) Comparative Study of the Efficacy of Curcumin and Turmeric Oil as Chemopreventive Agents in Oral Submucous Fibrosis: A Clinical and Histopathological Evaluation. J. Indian Acad. Oral Med. Radiol. 22, 88–92. 10.5005/jp-journals-10011-1021. [DOI] [Google Scholar]
- Gupta S.; Ghosh S.; Gupta S.; Sakhuja P. (2017) Effect of curcumin on the expression of p53, transforming growth factor-β, and inducible nitric oxide synthase in oral submucous fibrosis: A pilot study. J. Investig. Clin. Dent. 8, e12252 10.1111/jicd.12252. [DOI] [PubMed] [Google Scholar]
- Saran G.; Umapathy D.; Misra N.; Channaiah S.; Singh P.; Srivastava S.; Shivakumar S. (2018) A comparative study to evaluate the efficacy of lycopene and curcumin in oral submucous fibrosis patients: A randomized clinical trial. Indian J. Dent. Res. 29, 303–312. 10.4103/ijdr.IJDR_551_16. [DOI] [PubMed] [Google Scholar]
- Agarwal N.; Singh D.; Sinha A.; Srivastava S.; Prasad R. K.; Singh G. (2014) Evaluation of efficacy of turmeric in management of oral submucous fibrosis. J. Indian Acad. Oral Med. Radiol. 26, 260. 10.4103/0972-1363.144998. [DOI] [Google Scholar]
- Patil K.; Guledgud M. V.; Kulkarni P. K.; KeShari D.; Tayal S. (2015) Use of curcumin mouthrinse in radio-chemotherapy induced oral mucositis patients: a pilot study. J. Clin. diagnostic Res. JCDR 9, ZC59. 10.7860/jcdr/2015/13034.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normando A. G. C.; de Menêses A. G.; de Toledo I. P.; Borges G. Á.; de Lima C. L.; Dos Reis P. E. D.; Guerra E. N. S. (2019) Effects of turmeric and curcumin on oral mucositis: A systematic review. Phytother. Res. 33, 1318–1329. 10.1002/ptr.6326. [DOI] [PubMed] [Google Scholar]
- Delavarian Z.; Pakfetrat A.; Ghazi A.; Jaafari M. R.; Homaei Shandiz F.; Dalirsani Z.; Mohammadpour A. H.; Rahimi H. R. (2019) Oral administration of nanomicelle curcumin in the prevention of radiotherapy-induced mucositis in head and neck cancers. Spec. care Dent. Off. Publ. Am. Assoc. Hosp. Dent. Acad. Dent. Handicap. Am. Soc. Geriatr. Dent. 39, 166–172. 10.1111/scd.12358. [DOI] [PubMed] [Google Scholar]
- Lüer S. C.; Goette J.; Troller R.; Aebi C. (2014) Synthetic versus natural curcumin: bioequivalence in an in vitro oral mucositis model. BMC Complement. Altern. Med. 14, 1–7. 10.1186/1472-6882-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.-S.; Gong Z.-J.; Li W.-H.; Wang X.; Ling T.-Y. (2012) Antifibrotic effect of curcumin in TGF-β1-induced myofibroblasts from human oral mucosa. Asian Pac J. Cancer Prev 13, 289–294. 10.7314/APJCP.2012.13.1.289. [DOI] [PubMed] [Google Scholar]
- Okada N.; Muraoka E.; Fujisawa S.; Machino M. (2012) Effects of curcumin and capsaicin irradiated with visible light on murine oral mucosa. In Vivo (Brooklyn). 26, 759–764. [PubMed] [Google Scholar]
- Rezvani M.; Ross G. A. (2004) Modification of radiation-induced acute oral mucositis in the rat. Int. J. Radiat. Biol. 80, 177–182. 10.1080/09553000310001654693. [DOI] [PubMed] [Google Scholar]
- Charantimath S. (2016) Use of curcumin in radiochemotherapy induced oral mucositis patients: A control trial study. Int. J. Med. Heal. Sci. 10, 147–152. 10.5281/zenodo.1124161. [DOI] [Google Scholar]
- Elad S.; Meidan I.; Sellam G.; Simaan S.; Zeevi I.; Waldman E.; Weintraub M.; Revel-Vilk S. (2013) Topical curcumin for the prevention of oral mucositis in pediatric patients: case series. Altern. Ther. Health Med. 19, 21–24. [PubMed] [Google Scholar]
- Francis M.; Williams S. (2014) Effectiveness of Indian Turmeric Powder with Honey as Complementary Therapy on Oral Mucositis: A Nursing Perspective among Cancer Patients in Mysore. Nurs. J. India 105, 258–260. [PubMed] [Google Scholar]
- van der Waal I. (2019) Oral Leukoplakia: Present views on diagnosis, management, communication with patients, and research. Curr. Oral Heal. Reports 6, 9–13. 10.1007/s40496-019-0204-8. [DOI] [Google Scholar]
- Warnakulasuriya S.; Ariyawardana A. (2016) Malignant transformation of oral leukoplakia: a systematic review of observational studies. J. Oral Pathol. Med. 45, 155–166. 10.1111/jop.12339. [DOI] [PubMed] [Google Scholar]
- Lodi G.; Franchini R.; Warnakulasuriya S.; Varoni E. M.; Sardella A.; Kerr A. R.; Carrassi A.; MacDonald L. C. I.; Worthington H. V. (2016) Interventions for treating oral leukoplakia to prevent oral cancer. Cochrane Database Syst. Rev. 7, CD001829. 10.1002/14651858.CD001829.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnakulasuriya S. (2019) White, red, and mixed lesions of oral mucosa: A clinicopathologic approach to diagnosis. Periodontol. 2000 80, 89–104. 10.1111/prd.12276. [DOI] [PubMed] [Google Scholar]
- Kuriakose M. A.; Ramdas K.; Dey B.; Iyer S.; Rajan G.; Elango K. K.; Suresh A.; Ravindran D.; Kumar R. R.; R P.; Ramachandran S.; Kumar N. A.; Thomas G.; Somanathan T.; Ravindran H. K.; Ranganathan K.; Katakam S. B.; Parashuram S.; Jayaprakash V.; et al. (2016) A Randomized Double-Blind Placebo-Controlled Phase IIB Trial of Curcumin in Oral Leukoplakia. Cancer Prev. Res. 9, 683–691. 10.1158/1940-6207.CAPR-15-0390. [DOI] [PubMed] [Google Scholar]
- Patait M.; Nikate U.; Saraf K.; Singh P.; Jadhav V. (2016) Oral erythroplakia-A case report. Int. J. Appl. Dent. Sci. 2, 79–82. [Google Scholar]
- Warnakulasuriya S. (2020) Oral potentially malignant disorders: A comprehensive review on clinical aspects and management. Oral Oncol. 102, 104550. 10.1016/j.oraloncology.2019.104550. [DOI] [PubMed] [Google Scholar]
- Hazzah H. A.; Farid R. M.; Nasra M. M. A.; Zakaria M.; Gawish Y.; El-Massik M. A.; Abdallah O. Y. (2016) A new approach for treatment of precancerous lesions with curcumin solid-lipid nanoparticle-loaded gels: in vitro and clinical evaluation. Drug Delivery 23, 1409–1419. 10.3109/10717544.2015.1065524. [DOI] [PubMed] [Google Scholar]
- Dovigo L. N.; Carmello J. C.; de Souza Costa C. A.; Vergani C. E.; Brunetti I. L.; Bagnato V. S.; Pavarina A. C. (2013) Curcumin-mediated photodynamic inactivation of Candida albicans in a murine model of oral candidiasis. Med. Mycol. 51, 243–251. 10.3109/13693786.2012.714081. [DOI] [PubMed] [Google Scholar]
- Patil S.; Rao R. S.; Majumdar B.; Anil S. (2015) Clinical appearance of oral Candida infection and therapeutic strategies. Front. Microbiol. 6, 1391. 10.3389/fmicb.2015.01391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.; Verma R.; Murari A.; Agrawal A. (2014) Oral candidiasis: An overview. J. Oral Maxillofac. Pathol. 18, S81. 10.4103/0973-029x.141325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovigo L. N.; Pavarina A. C.; Carmello J. C.; Machado A. L.; Brunetti I. L.; Bagnato V. S. (2011) Susceptibility of clinical isolates of Candida to photodynamic effects of curcumin. Lasers Surg. Med. 43, 927–934. 10.1002/lsm.21110. [DOI] [PubMed] [Google Scholar]
- Deshmukh R. A.; Bagewadi A. S. (2014) Comparison of effectiveness of curcumin with triamcinolone acetonide in the gel form in treatment of minor recurrent aphthous stomatitis: A randomized clinical trial. Int. J. Pharm. Investig. 4, 138. 10.4103/2230-973X.138346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manifar S.; Obwaller A.; Gharehgozloo A.; Boorboor Shirazi Kordi H. R.; Akhondzadeh S. (2012) Curcumin Gel in the Treatment of Minor Aphthous Ulcer: a Randomized, Placebo- Controlled Trial. J. Med. Plants 11, 40–45. [Google Scholar]
- Nurdiana N.; Krishnasamy S. (2016) Effect of two percent turmeric extract gel on minor recurrent aphthous stomatitis. Padjadjaran J. Dent. 28, 13503. 10.24198/pjd.vol28no1.13503. [DOI] [Google Scholar]
- Kia S. J.; Mansourian A.; Basirat M.; Akhavan M.; Mohtasham-Amiri Z.; Moosavi M.-S. (2020) New concentration of curcumin Orabase in recurrent aphthous stomatitis: A randomized, controlled clinical trial. J. Herb. Med. 22, 100336. 10.1016/j.hermed.2020.100336. [DOI] [Google Scholar]
- Singh H.; Singh S.; Singh N.; Singh P.; Sharma K. (2018) Comparative analysis of therapeutic efficacy of curcumin & triamcinolone acetonide in recurrent aphthous stomatitis-a clinical study. J. Adv. Med. Dent Scie Res. 6, 136–138. [Google Scholar]
- Pandharipande R.; Chandak R.; Sathawane R.; Lanjekar A.; Gaikwad R.; Khandelwal V.; Kurawar K. (2019) To Evaluate Efficiency of Curcumin and Honey in Patients with Recurrent Aphthous Stomatitis: A Randomized Clinical Controlled Trial. Int. J. Res. Rev. 6, 449–455. [Google Scholar]
- González-Moles M. Á., Warnakulasuriya S., González-Ruiz I., González-Ruiz L., Ayén Á., Lenouvel D., Ruiz-Ávila I., and Ramos-García P. (2020) Worldwide prevalence of oral lichen planus: A systematic review and meta-analysis. Oral Dis. 10.1111/odi.13323. [DOI] [PubMed] [Google Scholar]
- Singh V.; Pal M.; Gupta S.; Tiwari S. K.; Malkunje L.; Das S. (2013) Turmeric-A new treatment option for lichen planus: A pilot study. Natl. J. Maxillofac. Surg. 4, 198. 10.4103/0975-5950.127651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollaoglu N. (2000) Oral lichen planus: a review. Br. J. Oral Maxillofac. Surg. 38, 370–377. 10.1054/bjom.2000.0335. [DOI] [PubMed] [Google Scholar]
- Munde A. D.; Karle R. R.; Wankhede P. K.; Shaikh S. S.; Kulkurni M. (2013) Demographic and clinical profile of oral lichen planus: A retrospective study. Contemp. Clin. Dent. 4, 181. 10.4103/0976-237X.114873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirchaghmaghi M.; Pakfetrat A.; Delavarian Z.; Ghalavani H.; Ghazi A. (2016) Evaluation of the efficacy of curcumin in the treatment of oral lichen planus: a randomized controlled trial. J. Clin. Diagn. Res. 10, ZC134. 10.7860/jcdr/2016/16338.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chainani-Wu N.; Madden E.; Lozada-Nur F.; Silverman S. Jr (2012) High-dose curcuminoids are efficacious in the reduction in symptoms and signs of oral lichen planus. J. Am. Acad. Dermatol. 66, 752–760. 10.1016/j.jaad.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Nosratzehi T.; Arbabi-Kalati F.; Hamishehkar H.; Bagheri S. (2018) Comparison of the effects of curcumin mucoadhesive paste and local corticosteroid on the treatment of erosive oral lichen planus lesions. J. Natl. Med. Assoc. 110, 92–97. 10.1016/j.jnma.2017.01.011. [DOI] [PubMed] [Google Scholar]
- Thomas A. E.; Varma B.; Kurup S.; Jose R.; Chandy M. L.; Kumar S. P.; Aravind M. S.; Ramadas A. A. (2017) Evaluation of efficacy of 1% curcuminoids as local application in management of oral lichen planus-interventional study. J. Clin. Diagn. Res. 11, ZC89. 10.7860/jcdr/2017/20898.9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S.; Solanki S.; Chinmaya B. R.; Tandon S.; Ashwini B. (2014) The magic of herbal curcumin therapy in recurrent oral lichen planus. Am. J. Ethnomedicine 1, 96–101. [Google Scholar]
- Keshari D.; Patil K.; Mahima V. G. (2015) Efficacy of topical curcumin in the management of oral lichen planus: A randomized controlled-trial. J. Adv. Clin. Res. Insights 2, 197–203. 10.15713/ins.jcri.78. [DOI] [Google Scholar]
- Rai B.; Kaur J.; Jacobs R.; Singh J. (2010) Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J. Oral Sci. 52, 251–256. 10.2334/josnusd.52.251. [DOI] [PubMed] [Google Scholar]
- Kia S. J.; Shirazian S.; Mansourian A.; Fard L. K.; Ashnagar S. (2015) Comparative efficacy of topical curcumin and triamcinolone for oral lichen planus: a randomized, controlled clinical trial. J. Dent. (Tehran). 12, 789. [PMC free article] [PubMed] [Google Scholar]
- Haskin C., and Mobley C. (2013) The impact of women’s oral health on systemic health. Women and Health, pp 1473–1488, Elsevier, 10.1016/B978-0-12-384978-6.00100-X. [DOI] [Google Scholar]
- Lemos J. A., Palmer S. R., Zeng L., Wen Z. T., Kajfasz J. K., Freires I. A., Abranches J., and Brady L. J.(2019) The Biology of Streptococcus mutans. Microbiol. Spectr., 7, 10.1128/microbiolspec.GPP3-0051-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.; Li X.; Lin H.; Zhou Y. (2018) Curcumin as a promising antibacterial agent: effects on metabolism and biofilm formation in S. mutans. Biomed Res. Int. 2018, 4508709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.; Choi B.; Jin E.-J.; Yoon Y.; Choi K.-H. (2012) Curcumin suppresses Streptococcus mutans adherence to human tooth surfaces and extracellular matrix proteins. Eur. J. Clin. Microbiol. Infect. Dis. 31, 1347–1352. 10.1007/s10096-011-1448-y. [DOI] [PubMed] [Google Scholar]
- Jahanizadeh S.; Yazdian F.; Marjani A.; Omidi M.; Rashedi H. (2017) Curcumin-loaded chitosan/carboxymethyl starch/montmorillonite bio-nanocomposite for reduction of dental bacterial biofilm formation. Int. J. Biol. Macromol. 105, 757–763. 10.1016/j.ijbiomac.2017.07.101. [DOI] [PubMed] [Google Scholar]
- Livada R.; Shiloah J.; Tipton D. A.; Dabbous M. K. (2017) The Potential Role of Curcumin in Periodontal Therapy: A Review of the Literature. J. Int. Acad. Periodontol. 19, 70–79. [PubMed] [Google Scholar]
- Anil A.; Gujjari S. K.; Venkatesh M. P. (2019) Evaluation of a curcumin-containing mucoadhesive film for periodontal postsurgical pain control. J. Indian Soc. Periodontol. 23, 461. 10.4103/jisp.jisp_700_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakır B.; Yetkin Ay Z.; Büyükbayram H. I.; Kumbul Doğuç D.; Bayram D.; Candan I. A.; Uskun E. (2016) Effect of curcumin on systemic T helper 17 cell response; gingival expressions of interleukin-17 and retinoic acid receptor-related orphan receptor γt; and alveolar bone loss in experimental periodontitis. J. Periodontol. 87, e183-e191 10.1902/jop.2016.150722. [DOI] [PubMed] [Google Scholar]
- Curylofo-Zotti F. A.; Elburki M. S.; Oliveira P. A.; Cerri P. S.; Santos L. A.; Lee H.-M.; Johnson F.; Golub L. M.; Rossa C. Jr.; Guimaraes-Stabili M. R. (2018) Differential effects of natural Curcumin and chemically modified curcumin on inflammation and bone resorption in model of experimental periodontitis. Arch. Oral Biol. 91, 42–50. 10.1016/j.archoralbio.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Deng J.; Golub L. M.; Lee H.-M.; Lin M. C.; Bhatt H. D.; Hong H.-L.; Johnson F.; Scaduto J.; Zimmerman T.; Gu Y. (2020) Chemically-Modified Curcumin 2.24: A Novel Systemic Therapy for Natural Periodontitis in Dogs. J. Exp. Pharmacol. 12, 47. 10.2147/JEP.S236792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elburki M. S.; Rossa C.; Guimarães-Stabili M. R.; Lee H.-M.; Curylofo-Zotti F. A.; Johnson F.; Golub L. M. (2017) A chemically modified curcumin (CMC 2.24) inhibits nuclear factor κB activation and inflammatory bone loss in murine models of LPS-induced experimental periodontitis and diabetes-associated natural periodontitis. Inflammation 40, 1436–1449. 10.1007/s10753-017-0587-4. [DOI] [PubMed] [Google Scholar]
- Elburki M. S.; Moore D. D.; Terezakis N. G.; Zhang Y.; Lee H.; Johnson F.; Golub L. M. (2017) A novel chemically modified curcumin reduces inflammation-mediated connective tissue breakdown in a rat model of diabetes: periodontal and systemic effects. J. Periodontal Res. 52, 186–200. 10.1111/jre.12381. [DOI] [PubMed] [Google Scholar]
- Elburki M. S.; Rossa C.; Guimaraes M. R.; Goodenough M.; Lee H.-M.; Curylofo F. A.; Zhang Y.; Johnson F.; Golub L. M. (2014) A novel chemically modified curcumin reduces severity of experimental periodontal disease in rats: initial observations. Mediators Inflamm. 2014, 959471. 10.1155/2014/959471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães M. R.; de Aquino S. G.; Coimbra L. S.; Spolidorio L. C.; Kirkwood K. L.; Rossa C. Jr (2012) Curcumin modulates the immune response associated with LPS-induced periodontal disease in rats. Innate Immun. 18, 155–163. 10.1177/1753425910392935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosadurga R. R.; Rao S. N.; Jose J.; Rompicharla N. C.; Shakil M.; Shashidhara R. (2014) Evaluation of the efficacy of 2% curcumin gel in the treatment of experimental periodontitis. Pharmacogn. Res. 6, 326. 10.4103/0974-8490.138287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha V.; Rajesh P.; Shanmugam M.; Priya B. M.; Prabhu S.; Shivakumar V. (2015) Comparative evaluation of natural curcumin and synthetic chlorhexidine in the management of chronic periodontitis as a local drug delivery: A clinical and microbiological study. Indian J. Dent. Res. 26, 53. 10.4103/0970-9290.156806. [DOI] [PubMed] [Google Scholar]
- Anuradha B. R.; Bai Y. D.; Sailaja S.; Sudhakar J.; Priyanka M.; Deepika V. (2015) Evaluation of anti-inflammatory effects of curcumin gel as an adjunct to scaling and root planing: a clinical study. J. Int. Oral Health 7, 90. [PMC free article] [PubMed] [Google Scholar]
- Boşca A. B.; Ilea A.; Soriţău O.; Tatomir C.; Miklášová N.; Pârvu A. E.; Mihu C. M.; Melincovici C. S.; Fischer-Fodor E. (2019) Modulatory effect of curcumin analogs on the activation of metalloproteinases in human periodontal stem cells. Eur. J. Oral Sci. 127, 304–312. 10.1111/eos.12625. [DOI] [PubMed] [Google Scholar]
- Elavarasu S.; Suthanthiran T.; Thangavelu A.; Alex S.; Palanisamy V. K.; Kumar T. S. (2016) Evaluation of superoxide dismutase levels in local drug delivery system containing 0.2% curcumin strip as an adjunct to scaling and root planing in chronic periodontitis: A clinical and biochemical study. J. Pharm. Bioallied Sci. 8, S48. 10.4103/0975-7406.191967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guru S. R.; Kothiwale S. V.; Saroch N.; Guru R. C. (2017) Comparative evaluation of inhibitory effect of curcumin and doxycycline on matrix metalloproteinase-9 activity in chronic periodontitis. Indian J. Dent. Res. 28, 560. 10.4103/ijdr.IJDR_461_16. [DOI] [PubMed] [Google Scholar]
- Hugar S. S.; Patil S.; Metgud R.; Nanjwade B.; Hugar S. M. (2016) Influence of application of chlorhexidine gel and curcumin gel as an adjunct to scaling and root planing: A interventional study. J. Nat. Sci. Biol. Med. 7, 149. 10.4103/0976-9668.184701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanaga C. A.; Miessi D. M. J.; Nuernberg M. A. A.; Claudio M. M.; Garcia V. G.; Theodoro L. H. (2019) Antimicrobial photodynamic therapy (aPDT) with curcumin and LED, as an enhancement to scaling and root planing in the treatment of residual pockets in diabetic patients: A randomized and controlled split-mouth clinical trial. Photodiagn. Photodyn. Ther. 27, 388–395. 10.1016/j.pdpdt.2019.07.005. [DOI] [PubMed] [Google Scholar]
- Kaur H.; Grover V.; Malhotra R.; Gupta M. (2019) Evaluation of curcumin gel as adjunct to scaling & root planing in management of periodontitis-randomized clinical & biochemical investigation. Infect. Disord. Targets (Formerly Curr. Drug Targets-Infectious Disord. 19, 171–178. 10.2174/1871526518666180601073422. [DOI] [PubMed] [Google Scholar]
- Raghava K. V.; Sistla K. P.; Narayan S. J.; Yadalam U.; Bose A.; Mitra K. (2019) Efficacy of Curcumin as an Adjunct to Scaling and Root Planing in Chronic Periodontitis Patients: A Randomized Controlled Clinical Trial. J. Contemp. Dent. Pract. 20, 842–846. 10.5005/jp-journals-10024-2608. [DOI] [PubMed] [Google Scholar]
- Ravishankar P. L.; Kumar Y. P.; Anila E. N.; Chakraborty P.; Malakar M.; Mahalakshmi R. (2017) Effect of local application of curcumin and ornidazole gel in chronic periodontitis patients. Int. J. Pharm. Investig. 7, 188. 10.4103/jphi.JPHI_82_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottumukkala S.; Sudarshan S.; Mantena S. (2014) Comparative evaluation of the efficacy of two controlled release devices: Chlorhexidine chips and indigenous curcumin based collagen as local drug delivery systems. Contemp. Clin. Dent. 5, 175–181. 10.4103/0976-237X.132310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottumukkala S.; Koneru S.; Mannem S.; Mandalapu N. (2013) Effectiveness of sub gingival irrigation of an indigenous 1% curcumin solution on clinical and microbiological parameters in chronic periodontitis patients: A pilot randomized clinical trial. Contemp. Clin. Dent. 4, 186–191. 10.4103/0976-237X.114874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhag A.; Dixit J.; Dhan P. (2007) Role of curcumin as a subgingival irrigant: a pilot study. PERIO 4, 115. [Google Scholar]
- Behal R.; Mali A. M.; Gilda S. S.; Paradkar A. R. (2011) Evaluation of local drug-delivery system containing 2% whole turmeric gel used as an adjunct to scaling and root planing in chronic periodontitis: A clinical and microbiological study. J. Indian Soc. Periodontol. 15, 35–38. 10.4103/0972-124X.82264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulikkotil S. J.; Nath S. (2015) Effects of curcumin on crevicular levels of IL-1β and CCL28 in experimental gingivitis. Aust. Dent. J. 60, 317–327. 10.1111/adj.12340. [DOI] [PubMed] [Google Scholar]
- Atsumi T.; Tonosaki K.; Fujisawa S. (2006) Induction of early apoptosis and ROS-generation activity in human gingival fibroblasts (HGF) and human submandibular gland carcinoma (HSG) cells treated with curcumin. Arch. Oral Biol. 51, 913–921. 10.1016/j.archoralbio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Chen J.-T.; Wang C.-Y.; Chen M.-H. (2018) Curcumin inhibits TGF-β1-induced connective tissue growth factor expression through the interruption of Smad2 signaling in human gingival fibroblasts. J. Formosan Med. Assoc. 117, 1115–1123. 10.1016/j.jfma.2017.12.014. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Yang W.; Wong M.; Chang H.; Yen-Ping Kuo M. (2012) Curcumin Inhibits Thrombin-Stimulated Connective Tissue Growth Factor (CTGF/CCN2) Production Through c-Jun NH2-Terminal Kinase Suppression in Human Gingival Fibroblasts. J. Periodontol. 83, 1546–1553. 10.1902/jop.2012.110641. [DOI] [PubMed] [Google Scholar]
- Dixit J.; Verma U.; Karamjeet; Sharma R.; Balapure A. (2009) Role of insulin as a growth promoter in regulating the response of curcumin in human primary gingival fibroblasts: An in vitro study. J. Indian Soc. Periodontol. 13, 133. 10.4103/0972-124X.60225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P.; Huang P.; Chen M. W. (2013) Curcumin attenuates cyclooxygenase-2 expression via inhibition of the NF-κB pathway in lipopolysaccharide-stimulated human gingival fibroblasts. Cell Biol. Int. 37, 443–448. 10.1002/cbin.10050. [DOI] [PubMed] [Google Scholar]
- Pourhajibagher M.; Chiniforush N.; Parker S.; Shahabi S.; Ghorbanzadeh R.; Kharazifard M. J.; Bahador A. (2016) Evaluation of antimicrobial photodynamic therapy with indocyanine green and curcumin on human gingival fibroblast cells: an in vitro photocytotoxicity investigation. Photodiagn. Photodyn. Ther. 15, 13–18. 10.1016/j.pdpdt.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Balapure A.; Dixit J.; Lodha D.; Ranjan V.; Sharma R.; Shyam H.; Singh N.; Singh A.; Verma U.; Zaidi D. (2012) Insulin catalyzes the curcumin-induced wound healing: an in vitro model for gingival repair. Indian J. Pharmacol. 44, 458. 10.4103/0253-7613.99304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. C.; Santibañez J. F.; Morales J. P.; Martinez J. (2004) Epidermal growth factor stimulates urokinase-type plasminogen activator expression in human gingival fibroblasts. Possible modulation by genistein and curcumin. J. Periodontal Res. 39, 380–387. 10.1111/j.1600-0765.2004.00753.x. [DOI] [PubMed] [Google Scholar]
- Sukumaran S. K.; Vadakkekuttical R. J.; Kanakath H. (2020) Comparative evaluation of the effect of curcumin and chlorhexidine on human fibroblast viability and migration: An in vitro study. J. Indian Soc. Periodontol. 24, 109. 10.4103/jisp.jisp_173_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraya S.; Uehara O.; Hiraki D.; Harada F.; Neopane P.; Morikawa T.; Takai R.; Yoshida K.; Matsuoka H.; Kitaichi N.; et al. (2020) Curcumin inhibits the expression of proinflammatory mediators and MMP-9 in gingival epithelial cells stimulated for a prolonged period with lipopolysaccharides derived from Porphyromonas gingivalis. Odontology 108, 16–24. 10.1007/s10266-019-00432-8. [DOI] [PubMed] [Google Scholar]
- Yang W. H.; Deng Y. T.; Hsieh Y. P.; Wu K. J.; Kuo M. Y. P. (2015) NADPH oxidase 4 mediates TGFβ1-induced CCN2 in gingival fibroblasts. J. Dent. Res. 94, 976–982. 10.1177/0022034515580986. [DOI] [PubMed] [Google Scholar]
- Yang W. H.; Deng Y. T.; Hsieh Y. P.; Wu K. J.; Kuo M. Y. P. (2016) Thrombin activates latent TGFβ1 via integrin αvβ1 in gingival fibroblasts. J. Dent. Res. 95, 939–945. 10.1177/0022034516634288. [DOI] [PubMed] [Google Scholar]
- Yang W.-H.; Kuo M.-P.; Liu C.-M.; Deng Y.-T.; Chang H.-H.; Chang J.-C. (2013) Curcumin inhibits TGFβ1-induced CCN2 via Src, JNK, and Smad3 in gingiva. J. Dent. Res. 92, 629–634. 10.1177/0022034513488139. [DOI] [PubMed] [Google Scholar]
- Waghmare P. F.; Chaudhari A. U.; Karhadkar V. M.; Jamkhande A. S. (2011) Comparative evaluation of turmeric and chlorhexidine gluconate mouthwash in prevention of plaque formation and gingivitis: a clinical and microbiological study. J. Contemp. Dent. Pract. 12, 221–224. 10.5005/jp-journals-10024-1038. [DOI] [PubMed] [Google Scholar]
- Muglikar S.; Patil K. C.; Shivswami S.; Hegde R. (2013) Efficacy of curcumin in the treatment of chronic gingivitis: a pilot study. Oral Health Prev. Dent. 11, 81–86. 10.3290/j.ohpd.a29379. [DOI] [PubMed] [Google Scholar]
- Arunachalam L. T.; Sudhakar U.; Vasanth J.; Khumukchum S.; Selvam V. V. (2017) Comparison of anti-plaque and anti-gingivitis effect of curcumin and chlorhexidine mouth rinse in the treatment of gingivitis: A clinical and biochemical study. J. Indian Soc. Periodontol. 21, 478. 10.4103/jisp.jisp_116_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A.; Debnath K.; Rao N. K. H. (2017) A comparative evaluation of the efficacy of curcumin and chlorhexidine mouthrinses on clinical inflammatory parameters of gingivitis: A double-blinded randomized controlled clinical study. J. Indian Soc. Periodontol. 21, 132. 10.4103/jisp.jisp_136_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasri M.; Madhulatha M.; Musalaiah S.; Kumar P. A.; Krishna C. H. M.; Kumar P. M. (2015) Efficacy of curcumin as an adjunct to scaling and root planning in chronic periodontitis patients: A clinical and microbiological study. J. Pharm. Bioallied Sci. 7, S554. 10.4103/0975-7406.163537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T.; Chen D.; Li Q.; Sun X.; Song Y.; Wang C. (2013) Curcumin inhibits inflammatory response and bone loss during experimental periodontitis in rats. Acta Odontol. Scand. 71, 349–356. 10.3109/00016357.2012.682092. [DOI] [PubMed] [Google Scholar]
- Eftekharian S.; Seifi S.; Satari F. D.; Moghaddamnia A. A.; Feizi F.; Kazemi S.; Gholinia H. (2019) Curcumin Effect on the Prevention of Gingival Overgrowth Following Phenytoin Consumption in Rats: A Clinicohistological and Immunohistochemical Study. J. Contemp. Dent. Pract. 20, 1146–1150. 10.5005/jp-journals-10024-2653. [DOI] [PubMed] [Google Scholar]
- Kaur S.; Sharma R.; Sarangal V.; Kaur N.; Prashar P. (2017) Evaluation of anti-inflammatory effects of systemically administered curcumin, lycopene and piperine as an adjunct to scaling and root planing: A clinical study. Ayu 38, 117. 10.4103/ayu.AYU_63_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V.; Pathak A. K.; Pal M.; Sareen S.; Goel K. (2015) Comparative evaluation of topical application of turmeric gel and 0.2% chlorhexidine gluconate gel in prevention of gingivitis. Natl. J. Maxillofac. Surg. 6, 67. 10.4103/0975-5950.168238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik K.; Ranaware A. M.; Harsha C.; Nitesh T.; Girisa S.; Deshpande V.; Fan L.; Nalawade S. P.; Sethi G.; Kunnumakkara A. B. (2020) Piceatannol: A natural stilbene for the prevention and treatment of cancer. Pharmacol. Res. 153, 104635. 10.1016/j.phrs.2020.104635. [DOI] [PubMed] [Google Scholar]
- Roy N. K.; Monisha J.; Padmavathi G.; Lalhruaitluanga H.; Kumar N. S.; Singh A. K.; Bordoloi D.; Baruah M. N.; Ahmed G. N.; Longkumar I.; et al. (2019) Isoform-Specific Role of Akt in Oral Squamous Cell Carcinoma. Biomolecules 9, 253. 10.3390/biom9070253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha N.; Warnakulasuriya S.; Vlaanderen J.; Straif K. (2014) Betel quid chewing and the risk of oral and oropharyngeal cancers: a meta-analysis with implications for cancer control. Int. J. Cancer 135, 1433–1443. 10.1002/ijc.28643. [DOI] [PubMed] [Google Scholar]
- Mello F. W.; Melo G.; Pasetto J. J.; Silva C. A. B.; Warnakulasuriya S.; Rivero E. R. C. (2019) The synergistic effect of tobacco and alcohol consumption on oral squamous cell carcinoma: a systematic review and meta-analysis. Clin. Oral Investig. 1–11. 10.1007/s00784-019-02958-1. [DOI] [PubMed] [Google Scholar]
- Warnakulasuriya S.; Sutherland G.; Scully C. (2005) Tobacco, oral cancer, and treatment of dependence. Oral Oncol. 41, 244–260. 10.1016/j.oraloncology.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Cheng Y.-S. L.; Rees T.; Wright J. (2014) A review of research on salivary biomarkers for oral cancer detection. Clin. Transl. Med. 3, 3. 10.1186/2001-1326-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N. W.; Jayasekara P.; Amarasinghe A. A. H. K. (2011) Squamous cell carcinoma and precursor lesions of the oral cavity: epidemiology and aetiology. Periodontol. 2000 57, 19. 10.1111/j.1600-0757.2011.00401.x. [DOI] [PubMed] [Google Scholar]
- Gómez I.; Warnakulasuriya S.; Varela-Centelles P. I.; López-Jornet P.; Suárez-Cunqueiro M.; Diz-Dios P.; Seoane J. (2010) Is early diagnosis of oral cancer a feasible objective? Who is to blame for diagnostic delay?. Oral Dis. 16, 333–342. 10.1111/j.1601-0825.2009.01642.x. [DOI] [PubMed] [Google Scholar]
- Chang P.-Y.; Peng S.-F.; Lee C.-Y.; Lu C.-C.; Tsai S.-C.; Shieh T.-M.; Wu T.-S.; Tu M.-G.; Chen M. Y.; Yang J.-S. (2013) Curcumin-loaded nanoparticles induce apoptotic cell death through regulation of the function of MDR1 and reactive oxygen species in cisplatin-resistant CAR human oral cancer cells. Int. J. Oncol. 43, 1141–1150. 10.3892/ijo.2013.2050. [DOI] [PubMed] [Google Scholar]
- Chen C.; Lu C.; Chiang J.; Chiu H.; Yang J.; Lee C.; Way T.; Huang H. (2018) Synergistic inhibitory effects of cetuximab and curcumin on human cisplatin-resistant oral cancer CAR cells through intrinsic apoptotic process. Oncol. Lett. 16, 6323–6330. 10.3892/ol.2018.9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva Gonçalves V.; Ortega A. A. C.; Guimarães M. R.; Curylofo F. A.; Rossa C. Jr.; Ribeiro D. A.; Spolidorio L. C. (2015) Chemopreventive activity of systemically administered curcumin on oral cancer in the 4-nitroquinoline 1-oxide model. J. Cell. Biochem. 116, 787–796. 10.1002/jcb.25035. [DOI] [PubMed] [Google Scholar]
- Lai K.-C.; Chueh F.-S.; Hsiao Y.-T.; Cheng Z.-Y.; Lien J.-C.; Liu K.-C.; Peng S.-F.; Chung J.-G. (2019) Gefitinib and curcumin-loaded nanoparticles enhance cell apoptosis in human oral cancer SAS cells in vitro and inhibit SAS cell xenografted tumor in vivo. Toxicol. Appl. Pharmacol. 382, 114734. 10.1016/j.taap.2019.114734. [DOI] [PubMed] [Google Scholar]
- Liu M.; Zhang J.; Li J. F.; Wang X. X. (2016) Roles of curcumin combined with paclitaxel on growth inhibition and apoptosis of oral squamous cell carcinoma cell line CAL27 in vitro. Shanghai kou qiang yi xue= Shanghai J. Stomatol. 25, 538–541. [PubMed] [Google Scholar]
- Mishra A.; Kumar R.; Tyagi A.; Kohaar I.; Hedau S.; Bharti A. C.; Sarker S.; Dey D.; Saluja D.; Das B. (2015) Curcumin modulates cellular AP-1, NF-kB, and HPV16 E6 proteins in oral cancer. Ecancermedicalscience 9, 525. 10.3332/ecancer.2015.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddappa G.; Kulsum S.; Ravindra D. R.; Kumar V. V.; Raju N.; Raghavan N.; Sudheendra H. V.; Sharma A.; Sunny S. P.; Jacob T.; et al. (2017) Curcumin and metformin-mediated chemoprevention of oral cancer is associated with inhibition of cancer stem cells. Mol. Carcinog. 56, 2446–2460. 10.1002/mc.22692. [DOI] [PubMed] [Google Scholar]
- Camacho-Alonso F.; López-Jornet P.; Tudela-Mulero M. R. (2013) Synergic effect of curcumin or lycopene with irradiation upon oral squamous cell carcinoma cells. Oral Dis. 19, 465–472. 10.1111/odi.12025. [DOI] [PubMed] [Google Scholar]
- Chang K.; Hung P.; Lin I.; Hou C.; Chen L.; Tsai Y.; Lin S. (2010) Curcumin upregulates insulin-like growth factor binding protein-5 (IGFBP-5) and C/EBPα during oral cancer suppression. Int. J. Cancer 127, 9–20. 10.1002/ijc.25220. [DOI] [PubMed] [Google Scholar]
- Molla S.; Hembram K. C.; Chatterjee S.; Nayak D.; Sethy C.; Pradhan R.; Kundu C. N. (2020) PARP inhibitor olaparib enhances the apoptotic potentiality of curcumin by increasing the DNA damage in oral cancer cells through inhibition of BER cascade. Pathol. Oncol. Res. 26, 2091–2103. 10.1007/s12253-019-00768-0. [DOI] [PubMed] [Google Scholar]
- Bano N.; Yadav M.; Das B. C. (2018) Differential Inhibitory Effects of Curcumin Between HPV+ ve and HPV-ve Oral Cancer Stem Cells. Front. Oncol. 8, 412. 10.3389/fonc.2018.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen L.; Fan D.; Yi X.; Cao X.; Chen D.; Wang L. (2014) Curcumin inhibits oral squamous cell carcinoma proliferation and invasion via EGFR signaling pathways. Int. J. Clin. Exp. Pathol. 7, 6438. [PMC free article] [PubMed] [Google Scholar]
- Singh K.; Singh P.; Oberoi G. (2016) Comparative studies between herbal toothpaste (dantkanti) and nonherbal Toothpaste. Int. J. Dent Res. 4, 53–6. 10.14419/ijdr.v4i2.6633. [DOI] [Google Scholar]
- Chandakavathe B. N.; Deshpande D. K.; Swamy P. V.; Dhadde S. B. (2018) Assessment of Toothpaste Formulations Containing Turmeric and Neem Extract for Prevention of Dental Caries and Periodontal Diseases. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences 88, 1523–9. 10.1007/s40011-017-0897-1. [DOI] [Google Scholar]