Abstract
Objectives: Improvements in human stem cell-derived cardiomyocyte (hSC-CM) technology have promoted their use for drug testing and disease investigations. Several in silico hSC-CM models have been proposed to augment interpretation of experimental findings through simulations. This work aims to assess the response of three hSC-CM in silico models (Koivumäki2018, Kernik2019, and Paci2020) to simulated drug action, and compare simulation results against in vitro data for 15 drugs.
Methods: First, simulations were conducted considering 15 drugs, using a simple pore-block model and experimental data for seven ion channels. Similarities and differences were analyzed in the in silico responses of the three models to drugs, in terms of Ca2+ transient duration (CTD90) and occurrence of arrhythmic events. Then, the sensitivity of each model to different degrees of blockage of Na+ (INa), L-type Ca2+ (ICaL), and rapid delayed rectifying K+ (IKr) currents was quantified. Finally, we compared the drug-induced effects on CTD90 against the corresponding in vitro experiments.
Results: The observed CTD90 changes were overall consistent among the in silico models, all three showing changes of smaller magnitudes compared to the ones measured in vitro. For example, sparfloxacin 10 µM induced +42% CTD90 prolongation in vitro, and +17% (Koivumäki2018), +6% (Kernik2019), and +9% (Paci2020) in silico. Different arrhythmic events were observed following drug application, mainly for drugs affecting IKr. Paci2020 and Kernik2019 showed only repolarization failure, while Koivumäki2018 also displayed early and delayed afterdepolarizations. The spontaneous activity was suppressed by Na+ blockers and by drugs with similar effects on ICaL and IKr in Koivumäki2018 and Paci2020, while only by strong ICaL blockers, e.g. nisoldipine, in Kernik2019. These results were confirmed by the sensitivity analysis.
Conclusion: To conclude, The CTD90 changes observed in silico are qualitatively consistent with our in vitro data, although our simulations show differences in drug responses across the hSC-CM models, which could stem from variability in the experimental data used in their construction.
Keywords: human stem cell-derived cardiomyocyte, action potential, calcium transient, in silico modeling, drug test, sensitivity analysis
Introduction
Human stem cell-derived cardiomyocytes (hSC-CMs) have emerged as a promising tool for translational research, cardiac repair and drug development (Bezzerides et al., 2017; Miyagawa and Sawa, 2018; de Korte et al., 2020). After the initial hype, it has, however, become widely acknowledged that with current methods and technology hSC-CMs have a fundamental limitation: the cells cannot be fully matured into human ventricular cardiomyocytes (hV-CMs). Instead, hSC-CMs have a distinct phenotype of their own that shares features with partially differentiated developing cells (Khan et al., 2013). Furthermore, hSC-CMs show less robustness and greater tendency for arrhythmic events than native hV-CMs; a phenomenon that appears to be linked to a weaker repolarization reserve (Paci et al., 2015; Koivumäki et al., 2018; Lemoine et al., 2018; Treat et al., 2019). Thus, although hSC-CMs do resolve the problem of species-dependent differences related to use of animal models, the translation of data obtained from hSC-CMs to humans is not straightforward.
Computational modeling and simulation offer tools to augment experimental investigations by aiding their interpretation, and identifying possible sources and modulators of hSC-CM electrophysiology. For example, in silico approaches have already been successfully employed to investigate the translatability of drug responses from immature hSC-CMs to mature hV-CMs (Gong and Sobie, 2018; Tveito et al., 2018), creating a math-based and mechanistic virtual link to the phenotype of hV-CMs. In silico hSC-CM models also helped to gain insight on specific features of hSC-CMs, e.g. automaticity and its causes (Koivumäki et al., 2018; Paci et al., 2020), and to tackle the huge electrophysiological variability observed in action potentials (AP) and Ca2+ transients (CaT) recorded in vitro (Paci et al., 2017; Kernik et al., 2019), e.g. showing potential combination of ion currents that could make cardiac cells more prone to develop arrhythmic behaviors in response to drugs or in presence of disease phenotypes (Paci et al., 2017; Paci et al., 2018a; Kernik et al., 2020). Currently, there are three pedigrees of hSC-CM in silico models, all parametrized on hSC-CM data, developed and published by 1) Paci et al., 2) Koivumäki et al., and 2) Kernik et al. (Koivumäki et al., 2018; Kernik et al., 2019; Paci et al., 2020).
The goal of this work is to investigate differences and similarities in the response of the three latest hSC-CM models to 15 different drugs, and their comparison against an in vitro hSC-CM experimental dataset. We expect the insights from our study will enable other researchers to select the most appropriate model for their specific investigations.
Methods
Model Components and Structures
Table 1 shows the number of compartments and the ion currents, pumps and exchangers included in the three considered computational (in silico) cellular models: Kernik2019 (Kernik et al., 2019), Koivumäki2018 (Koivumäki et al., 2018), and Paci2020 (Paci et al., 2019; Paci et al., 2020). They are all single cell models, with a similar formulation for certain ionic currents, as they are all based on the original hSC-CM model from Paci et al. (Paci et al., 2013). The following currents/fluxes are modeled using the Hodgkin-Huxley paradigm, even if with different formulations: fast Na+ current (INa), funny current (If), L-type Ca2+ current (ICaL), rapid and slow delayed rectifier K+ currents (IKr and IKs), inward rectifying K+ current (IK1), transient outward K+ current (Ito). Of note, ICaL driving force is formulated according to the Goldman-Hodgkin-Katz flux equation in Paci2020 and Kernik2019. Na+ and Ca2+ background currents were represented as resistive fluxes. The Na+/K+ pump (INaK), the Na+/Ca2+ exchanger (INCX) and the Ca2+ sarcolemmal pump (IpCa) follow the formulation used in (ten Tusscher et al., 2004). Kernik2019 and Koivumäki2018 use two different Markov formulations for the Ca2+ release from the sarcoplasmic reticulum (SR) (Shannon et al., 2004) and (Keizer and Levine, 1996), respectively. Conversely, Paci2020 uses a Hodgkin-Huxley formulation. The Kernik2019 model is the only one including the T-type Ca2+ current (ICaT), as well as the Na+ and K+ fluxes via ICaL channels. Paci2020 is the only model including the late Na+ (INaL) current (Ma et al., 2013). Koivumäki2018 is the only model including the inositol 1,4,5 triphosphate (IP3) receptor-mediated Ca2+ release (JIP3).
TABLE 1.
Summary of the compartments and the ion currents and fluxes included in the three models.
| Paci2020 | Koivumäki2018 | Kernik2019 | |
|---|---|---|---|
| Compartments | 2 | 61 | 2 |
| INa | HH | HH | HH |
| INaL | HH | — | — |
| If | HH | HH | HH |
| ICaL Ca2+ | HH, GHK | HH | HH, GHK |
| ICaL Na+ | — | — | HH, GHK |
| ICaL K+ | — | — | HH, GHK |
| ICaT | — | — | HH |
| IKr | HH | HH | HH |
| IKs | HH | HH | HH |
| IK1 | HH | HH | HH |
| Ito | HH | HH | HH |
| INCX | TT | TT | TT |
| INaK | TT | TT | TT |
| IpCa | TT | TT | TT |
| IbNa | HH, R | HH, R | HH, R |
| IbCa | HH, R | HH, R | HH, R |
| JRyR | HH, R | M 2 | M 1 |
| JSERCA | TT | TT | TT |
| Jleak | HH, R | HH, R | HH, R |
| JIP3 | — | M 3 | — |
We reported the modeling paradigm for each ion current/flux: Hodgkin and Huxley (HH), Hodgkin and Huxley as resistive current/flux (HH,R), Hodgkin and Huxley gates with Goldman-Hodgkin-Katz driving force (HH,GHK), Markov (M), based on the TenTusscher2004 model (ten Tusscher et al., 2004) (TT).
The three models show differences in their compartmental structure and description of Ca2+ dynamics. Paci2020 and Kernik2019 share the same cylindrical structure and compartmentalization: one compartment for cytosol and one for SR. Koivumäki2018 includes an additional Ca2+ outward flux from the SR, transmitted by IP3, and formulated as a Markov model. Moreover, Koivumäki2018 has a more complex layered “onion-like” compartmentalization for Ca2+. In order to describe the Ca2+ spatial distribution and diffusion, the CM is approximated as a sphere whose radius from SR towards the sarcolemma is divided in 61 sub-compartments. This enables a more refined description of the cytosolic Ca2+ dynamics, with no need for partial differential equations, at the price of longer computing time, as shown in the Results section.
In vitro Drug Data
A total of 15 drugs were tested in vitro at multiple concentrations: antiarrhythmic drugs (bepridil, dofetilide, flecainide, lidocaine, mexiletine, procainamide and verapamil), an antiepileptic drug (phenytoin), antibiotics (moxifloxacin and sparfloxacin), hypertension drugs (nimodipine and nisoldipine), anticonvulsants (primidone), antianginals (ranolazine) and Ba2+ salts (BaCl2). Data were the same used in (Passini et al., 2017).
Briefly, CaT recordings from hSC-CMs (Cor.4U) were acquired at 37°C and 5% CO2 on pre-plated preparations from Axiogenesis (Cologne, Germany). Cells were seeded in fibronectin-coated 96-well plates at a density suited to form a monolayer and maintained in culture in a stage incubator. The tested 15 drugs were dissolved in DMSO to obtain a stock solution of 1,000x the highest test concentration. Further dilutions were made with DMSO to obtain concentrations of 1,000x intended concentration. On the experiment day these solutions were diluted with Tyrode (Sigma), supplemented with 10 mM HEPES to solutions containing twice the intended concentration (compound plate: 2x intended concentration). Final DMSO concentration in test solutions and vehicle control was 0.1%. Full method details are reported earlier in (Lu et al., 2019) and (Kopljar et al., 2018). Ca2+ transients (CaT) recorded as the calcium dye-fluorescence signal integrated over the whole well. CaT duration at 90% of the initial base value (CTD90) was quantified, as it is known to be correlated with APD (Gauthier et al., 2012; Spencer et al., 2014), similarly to other studies (Lu et al., 2015; Zeng et al., 2016). The CTD90 values used in this study were measured from 24 to 28 min after compound addition. Therefore, an excessive increment of CTD90, as well as an anomalous CaT time-course, can be considered as markers of APD or QT prolongation and pro-arrhythmic risk - early afterdepolarization (EAD) or torsades de pointes (TdP).
In silico Drug Trials
All the drugs and concentrations tested in vitro were simulated in silico using the three hSC-CM models. Drug effects on seven ion currents (INa, IKr, ICaL, INaL, IKs, Ito and IK1) were simulated using the single pore block model (Brennan et al., 2009; Paci et al., 2015; Williams and Mirams, 2015), formulated as
where Ii is the ion current corresponding to the drug concentration [D], Ii′ the ion current in control conditions, IC50 is the half-maximal dose response and nH is the Hill coefficient. The used IC50 and Hill coefficients are reported in Supplementary Table S1 (Passini et al., 2017). For seven drugs, multiple (IC50, Hill coefficient) pairs were considered, as in (Passini et al., 2017). Each model was run for 800 s to reach the steady state. For each drug, concentration and model, simulations were run for 200 s starting from steady state, and then CTD90 was computed on the last eight spontaneous CaTs, and the occurrence of proarrhythmic events was assessed. No external pacing was applied to the three models. Since each of the models was tuned and validated on a specific working point, we used the baseline models as originally published by the authors.
Arrhythmic Event Classification
Abnormal rhythms in vitro were visually assessed from CaT traces. In silico, they were defined based on the AP time-course, as in (Paci et al., 2020). Five types of arrhythmic events were considered. Early after-depolarizations (EADs) were defined as aberrant and transient reversals of the membrane potential repolarization (during phase two or three of the AP) or of the CaT decay. Delayed afterdepolarizations (DADs, observed only in silico) were defined as transient depolarizations of the membrane potential or increments of the cytosolic Ca2+ from its basal value during their diastolic phases. EADs and DADs were visually assessed. Repolarization failure (RF), i.e. when the membrane potential or the cytosolic Ca2+ do not return to their diastolic values for several seconds, was identified when a stable (maximum upstroke velocity smaller than 0.2 V/s) membrane potential >−40 mV was observed during the last 15 s of simulation. Irregular rhythm (IRR) was identified if the difference in cycle length between two consecutive APs or CaTs was greater than 150%. Spontaneous rate greater than 2 Hz was labeled as ventricular tachycardia-like rhythm (VT, observed only in vitro).
We also identified two patterns related to the cessation of the spontaneous electrical activity of hSC-CMs, that we did not considered pro-arrhythmic: quiescence (Q) and residual activity (RESAC). If the membrane potential showed small peaks, greater than −40 mV but smaller than 0 mV, not developing APs, the model was labeled as RESAC. Conversely, we considered the model quiescent (Q), i.e. not producing spontaneous AP and CaTs, if during the last 15 s the average membrane potential was smaller than −40 mV or the residual activity’s peak potentials were all the smaller than −40 mV.
Sensitivity to Simultaneous Current Blocks
A systematic sensitivity analysis was also conducted by considering simultaneous blocks of IKr vs ICaL and INa vs ICaL by blocking (0, 20, 40, 60, 80, 100)% of each current (thirty six tests for each couple of currents). For each test we assessed if the model 1) produced spontaneous APs, 2) triggered repolarization abnormalities or irregular rhythms, 3) showed quiescence or residual activity without or 4) with irregular rhythm after drug administration. Simulations with Paci2020 and Kernik2019 models were run for 500 s, while simulations with Koivumäki2018 were run for 200 s only, due to the long computing time required by this model (only the tests showing irregular rhythm were extended until 500 s to check if it persisted or turned into quiescence or residual activity).
Results
Simulations With the Three Models Yield Different Action Potentials and Ca2+ Transients Shapes
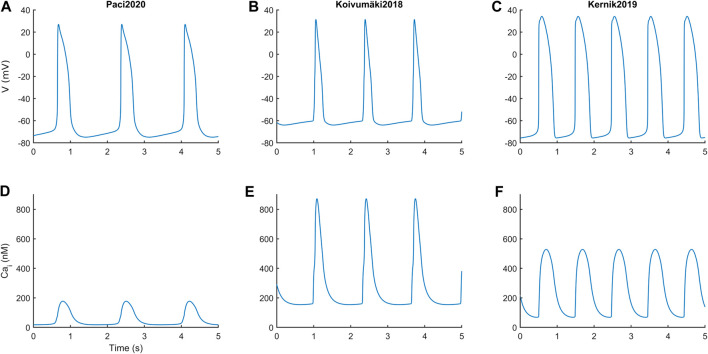
Figure 1 reports the spontaneous APs and CaTs produced by the three models in steady state, and Table 2 summarizes the main AP and CaT morphological features. The AP and CaT traces are quite different, reflecting the fact that hSC-CMs can exhibit remarkable differences, in silico as well as in vitro, e.g. different AP morphologies (Paci et al., 2017; Kernik et al., 2019). In terms of APs, Koivumäki2018 shows the most triangular ones, with depolarized maximum diastolic potential (MDP) and the shortest AP duration at 90% (APD90). Paci2020 and Kernik2019 show quite similar AP features, in agreement with (Ma et al., 2011), except for the rate of the spontaneous APs, which is almost double in the latter.
FIGURE 1.
Action potentials (A and B, C) and Ca2+ transients (D and E, F) in steady state for the three in silico hSC-CM models.
TABLE 2.
Morphological features from spontaneous action potentials (APs) and Ca2+ transients (CaTs).
| Morphological feature | Paci2020 | Kernik2019 | Koivumäki2018 |
|---|---|---|---|
| APA (mV) | 102 | 110 | 95 |
| MDP (mV) | −75 | −76 | −64 |
| APD90 (ms) | 390 | 414 | 247 |
| AP_Tri (-) | 2.8 | 2.6 | 1.7 |
| Rate (bpm) | 35 | 61 | 45 |
| Diastolic Cai (nM) | 18 | 68 | 154 |
| Systolic Cai (nM) | 177 | 529 | 873 |
| CTD90 (ms) | 550 | 601 | 407 |
APA: AP amplitude. MDP: maximum diastolic potential. APD90: AP duration at 90% of repolarization. AP_Tri: AP triangulation ratio, defined as (APD40-APD30)/(APD80-APD70). Rate: rate of the spontaneous APs/CaTs. CTD90: CaT duration at 90% decay.
In terms of CaT, Paci2020 shows the lowest diastolic and systolic cytosolic Ca2+ levels, and CaT amplitude is in agreement with the measurements by Rast et al. (Rast et al., 2015). The Kernik2019 CaT was calibrated on the data from Garg et al. (Garg et al., 2018), after their conversion from fluorescence level to actual concentrations.
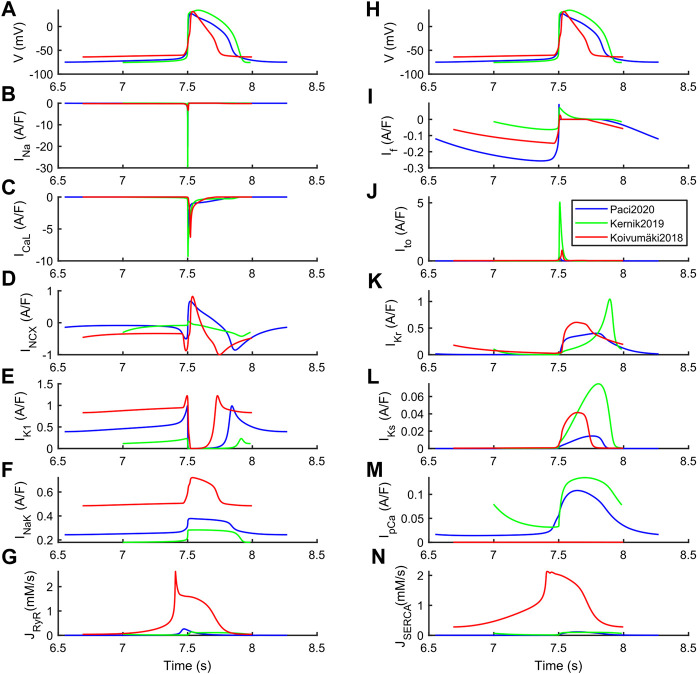
The differences among the three models can be explained based on their steady state ion currents and fluxes, reported in Figure 2 and further expanded in Supplementary Figures S1–S3. Kernik2019 has the greatest INa, ICaL, Ito, IKr and IKs. Conversely, it has the smallest IK1, and the highest rate of spontaneous APs (a 20% IK1 increment would reduce the spontaneous rate to 38 bpm, similar to the other two models). Paci2020 and Koivumäki2018 show a very similar INCX shape, coming from the fact that in both models the automaticity is sustained by the pre-upstroke inward component of INCX (only partly for Paci2020). This pre-upstroke INCX inward component is missing in Kernik2019. Paci2020 and Koivumäki2018 also have a similar ICaL peak current, and Koivumäki2018 shows the smallest INa across the three models.
FIGURE 2.
Ion currents underlying the steady-state action potentials in the Paci2020 (blue), Kernik2019 (green) and Koivumäki2018 (red) models. (A and H) Membrane potential. (B) Fast Na+ current (INa). (C) L-type Ca2+ current (ICaL). (D) Na+-Ca2+ exchanger (INCX). (E) Inward rectifying K+ current (IK1). (F) Na+-K+ pump (INaK). (G) Ca2+ release from sarcoplasmic reticulum (JRyR). (I) Funny current (If). (J) Transient outward K+ current (Ito). (K) Rapid delayed rectifying K+ current (IKr). (L) Slow delayed rectifying K+ current (IKs). (M) Sarcolemma Ca2+ pump (IpCa). (N) SERCA pump (JSERCA).
These differences are particularly relevant to understand the different responses to some of the drugs, reported in the next section. The structural differences in the three models are also reflected in their running time. Being characterized by simpler structure and compartmentalization, Paci2020 and Kernik2019 are faster (210 s simulations take 22 s). Conversely, due to its higher complexity, Koivumäki2018 is more than 100 times slower (210 s simulations take 2,331 s). These benchmark simulations were run in Matlab 2017b on a laptop computer (i7 @2.80 GHz and 32 GB memory).
In silico drug tests: characterization of diverse responses in the three models
Before proceeding with an in vitro–in silico comparison, we aim to show in detail how the three models react to a specific set of three drugs, namely dofetilide (Figure 3), flecainide (Figure 4) and nisoldipine (Figure 5), each one highlighting a specific mechanism of action.
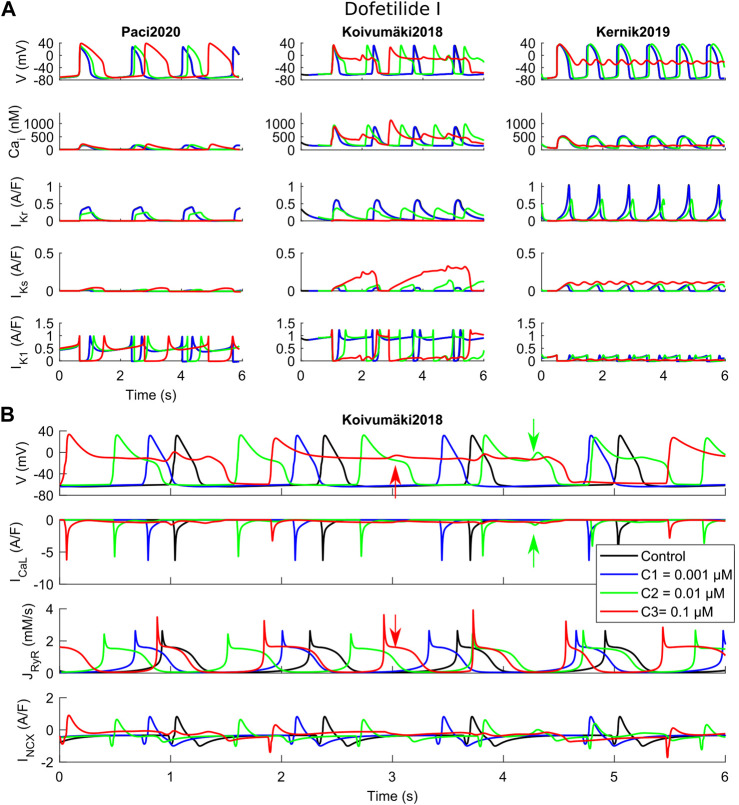
FIGURE 3.
Effects of dofetilide I on the three in silico models for the three tested concentrations (C1, C2 and C3). (A) Comparison of action potentials, Ca2+ transients and the main K+ currents. (B) Detail of the Koivumäki2018 traces, showing the development of EADs following reactivation of ICaL (green arrows) and spontaneous Ca2+ releases from the sarcoplasmic reticulum (red arrows).
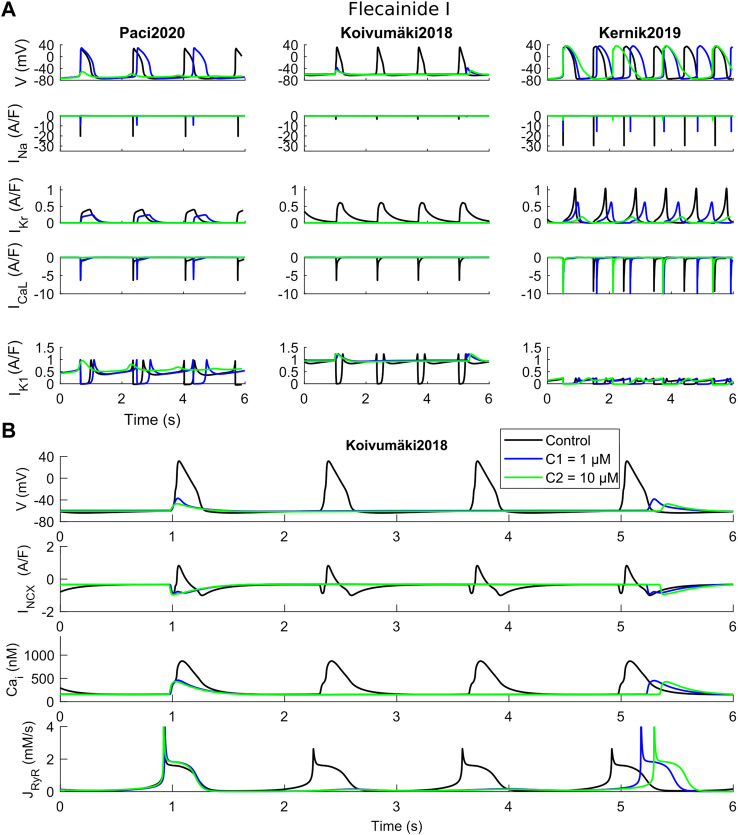
FIGURE 4.
Effects of flecainide I on the three in silico models for the two tested concentrations (C1 and C2). (A) Comparison of action potentials, Ca2+ transients and ion currents. (B) Detail of the Koivumäki2018 traces, showing a residual activity due to spontaneous Ca2+ releases from the sarcoplasmic reticulum that activate the inward component of INCX.
FIGURE 5.
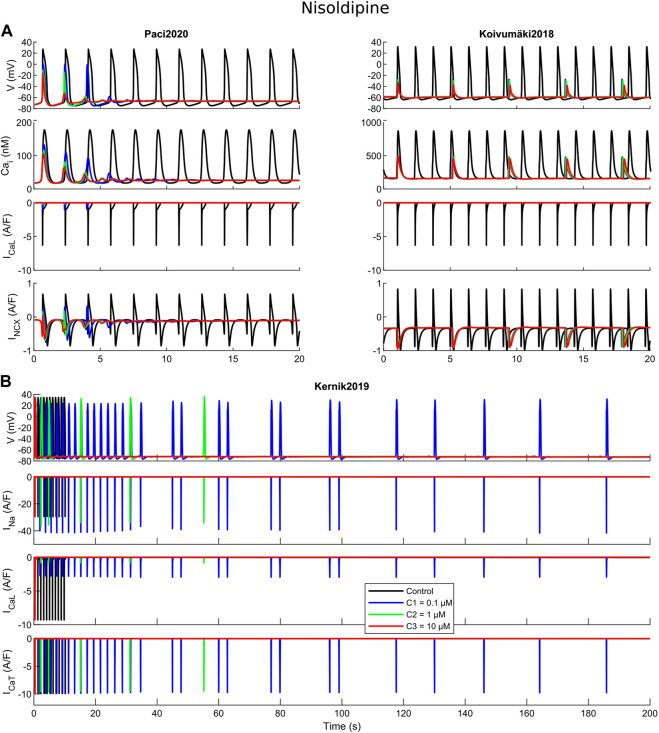
Cessation of the spontaneous electrical activity upon administration of nisoldipine. For all the three tested concentrations (C1, C2 and C3), Paci2020 becomes quiescent and Koivumäki2018 maintains a residual electrical activity not sufficient to trigger action potentials. For concentrations one and two, Kernik2019 shows an irregular rhythm before quiescence, while concentration three terminates immediately the automaticity.
Dofetilide (Figure 3) is a well-known hERG blocker that triggers AP prolongation and repolarization abnormalities, such as EAD and RF. The rationale behind this test is to show how these phenomena are recapitulated by the three models in response to a strong hERG block. These phenomena are recapitulated, as shown in Figure 3A, when considering dofetilide I IC50 as in Supplementary Table S1. For the two lowest doses, 0.001 and 0.01 µM, dofetilide prolongs the APs and CaTs in all three models. However, at the highest concentration (0.1 µM, corresponding to an IKr 96% block), dofetilide triggers three different behaviors. It prolongs the AP and CaT durations in Paci2020, but it does not trigger any repolarization abnormality. In this model, IKs and the strong IK1 can support the late repolarization phase, despite the strong AP prolongation. Conversely, Kernik2019 has a small IK1 compared to Paci2020 and Koivumäki2018, which makes it more difficult to stabilize the membrane potential to its diastolic value. In fact, at the highest dofetilide concentration, Kernik2019 fails to repolarize in the late repolarization phase and it sets around −20 mV. Particular attention is paid to Koivumäki2018, which triggers EADs at both intermediate and high concentrations, showing two different mechanisms. At the intermediate concentration, ICaL reactivation occurs, due to the prolonged AP (Figure 3B, green arrows). This influx of positive charges depolarizes the membrane potential and generates the EAD. In this example, all the Ca2+ releases from the SR are synchronized with the AP upstrokes. At the highest concentration, spontaneous releases of Ca2+ from the SR occur (Figure 3B, red arrows). They are not synchronized with the AP upstrokes, and instead they happen during the prolonged repolarization phase. Such releases induce an increment in the cytosolic Ca2+ concentration, which enhances the inward INCX component, and then triggers the EAD.
Flecainide blocks primarily IKr and INa, but also ICaL to a minor extent, as shown in Supplementary Table S1. Figure 4 shows APs and CaTs when considering flecainide I (as defined in Supplementary Table S1). The lowest concentration (1 µM) blocks INa, IKr and ICaL by 30%, 40% and 4%, while the intermediate concentration (10 µM) blocks the same currents by 69%, 81% and 28%, respectively. The results illustrate how flecainide stops spontaneous electrical activity, or allows only a residual activity, in Paci2020 and Koivumäki2018, whereas with the Kernik2019 model, beating still occurs spontaneously, showing stronger automaticity. Indeed, in agreement with our in vitro data (Supplementary Figure S7), both Paci2020 and Kernik2019 show AP/CaT prolongation at low dose. However, with Paci2020 spontaneous activity stops, not sustained anymore by INa which is blocked by 69%, while Kernik2019 still produces APs. This phenomenon is even clearer in Koivumäki2018, where INa is extremely small also in control conditions. Already at the low concentration, APs do not develop anymore, but there is a residual activity, only due to spontaneous Ca2+ releases from SR (about every 4 s). These releases trigger the inward INCX component that slightly depolarizes the membrane potential. Conversely, Kernik2019 maintains its automaticity, despite the strong INa block: indeed, the authors of the Kernik2019 model reported that the model spontaneously beats also in case of full INa block. However, flecainide at the intermediate concentration does induce a prolongation of APs and CaTs in the Kernik2019 model.
Nisoldipine is a powerful ICaL blocker, with minor effects also on INa, INaL, IKr and IKs. The three concentrations considered here, 0.1, 1 and 10 μM, induced ICaL blocks of 85%, 97% and 99%. The rationale behind this test is showing how a strong ICaL blocker can affect the automaticity in the three models. Few seconds after drug administration, Paci2020 and Koivumäki2018 show complete quiescence and a residual activity, respectively, at all three nisoldipine concentrations. The importance of ICaL in the first two models is related to the role of Ca2+ handling and INCX in sustaining the automaticity. As reported in (Koivumäki et al., 2018; Paci et al., 2020), one mechanism sustaining the automaticity is a small inward component of INCX inducing the depolarization of the membrane potential until it reaches the activation threshold for INa. After ICaL block, less Ca2+ enters the cytosol and is available for the Ca2+-induced Ca2+ release, and the Ca2+ transient pre-upstroke component becomes smaller and smaller and this reduces the activity of INCX that cannot support anymore proper automaticity. This phenomenon is particularly clear in the Paci2020 model (Figure 5A). Koivumäki2018 shows a residual activity since it has a greater spontaneous Ca2+ release from SR (Figure 2) that can still trigger the membrane potential depolarization via INCX up to about -40 or -30 mV, but not enough to trigger a full AP. ICaL is also fundamental in Kernik2019. Actually, spontaneous APs in Kernik2019 rely more on ICaL than INa, even in their upstroke phase. This model continues to beat spontaneously with INa full block (tested up to 1,000 s, not shown): ICaL can compensate the lack of INa and sustain the upstroke. On the other hand, when ICaL is blocked (e.g. by concentration one), the spontaneous activity slows down and becomes irregular, until it stops. Kernik2019 can also count on an additional depolarizing current, not present in the other two models: ICaT. Combining nisoldpine at concentration two with the full ICaT block, the automaticity terminates upon in silico drug administration.
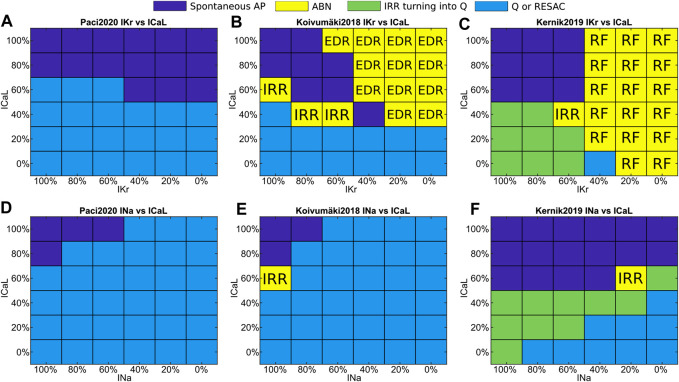
In order to generalize the results obtained for dofetilide, flecainide and nisoldipine, we ran a sensitivity analysis by testing the simultaneous current blocks showed in Figure 6: IKr vs ICaL (Figures 6A–C) and INa vs ICaL (Figures 6D–F). Firstly, the Paci2020 model does not develop repolarization abnormalities in response to IKr block, Kernik2019 responds to high IKr block only with repolarization failures, while Koivumäki2018 develops multiple repolarization abnormalities (EADs, DADs and RF). Secondly, Koivumäki2018 and Paci2020 are more sensitive to INa block than Kernik2019 (Figures 6D–F). They show much smaller INa peaks (Figure 2), and this INa cannot sustain the upstroke in case of ∼50% block for Paci2020 and ∼30% block for Koivumäki2018. This is clear in case of blockade of Na+ currents (e.g., mexiletine I, II, III at concentration three and lidocaine II at concentration two), which impairs automaticity in all models but Kernik2019 (that shows spontaneous APs also with INa fully blocked) (Figure 6F). Thirdly, Koivumäki2018 and Paci2020 are the most sensitive to ICaL block. Finally, Kernik2019 has the strongest automaticity. This has been confirmed also by additional tests (not shown), e.g. Kernik2019 spontaneous activity lasts about 400 s in conditions of 90% If block, and a 90% INCX block does not stop the spontaneous APs and CaTs as observed in Paci2020 and Koivumäki2018. For some of the drugs blocking at the same time ICaL and IKr (e.g. moxifloxacin I, II at concentration two or bepridil I, II, III at concentration two), this enables Kernik2019 to trigger repolarization abnormalities typical of IKr block, when the other models show residual or cessation of spontaneous activity.
FIGURE 6.
Sensitivity maps to simultaneous IKr and ICaL blocks (A and B, C) or INa and ICaL blocks (D and E, F). Color code: Spontaneous APs, blue; quiescence or residual activity, cyan; transient irregular rhythm turning into quiescence, green; arrhythmic events, yellow. The yellow squares include also the event type: irregular rhythm (IRR), repolarization failure (RF), and simultaneous occurrence of EAD, DAD and repolarization failure (EDR).
In silico Drug Tests: Comparison With the in vitro Results
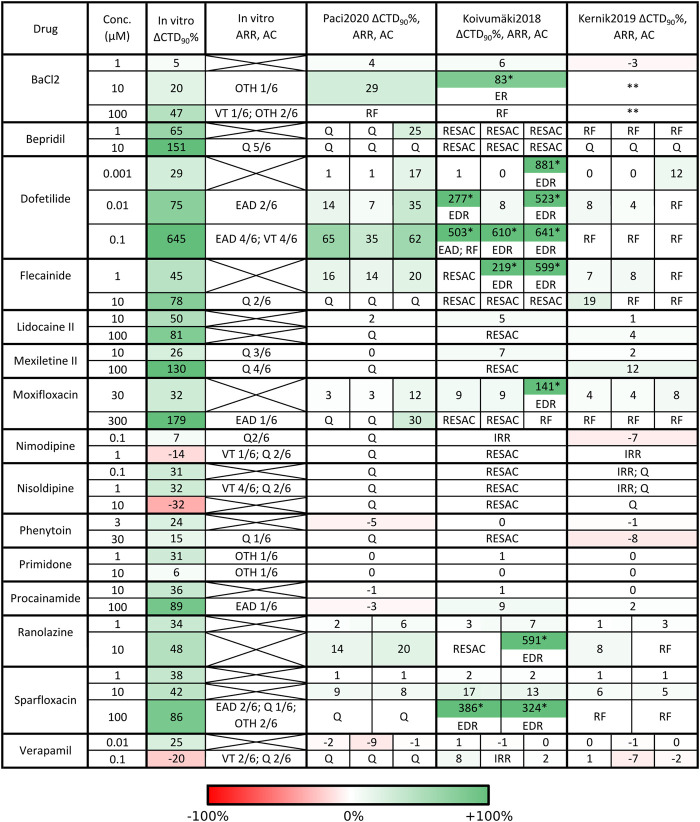
A detailed in vitro–in silico comparison is presented in Figure 7, Supplementary Figures S5–S7 and Supplementary Table S2. Figure 7 and Supplementary Table S2 report the in silico drug trial results and the comparison with the in vitro experiments in terms of CTD90 percent variations, occurrence of arrhythmic events, and cessation of automaticity, also for the alternative drug formulations not shown in Supplementary Figures S5–S7. In these three supplementary figures, we graphically report the percent in vitro and in silico CTD90 variations, arrhythmic events or the cessation of the spontaneous activity, and the main mechanisms of action, for the 15 drugs in the panel. If an in vitro or in silico CTD90 data is missing, it means that the spontaneous activity stopped, or an arrhythmic event prevented measuring CTD90. In vitro, most of the drugs induced prolongation of CTD90, except nimodipine, nisoldipine and verapamil. Bepridil, flecainide, lidocaine, mexiletine, phenytoin and ranolazine did not induce arrhythmic events, despite all but ranolazine stopping the spontaneous activity at the highest doses in at least a few cells.
FIGURE 7.
Qualitative and quantitative comparison of in silico drug tests in the three hSC-CM models against our in vitro data, for the 15 reference compounds. For each drug we report i) the tested concentrations, ii) the percent CaT duration variation with respect to baseline (ΔCTD90%), arrhythmic events (ARR) and automaticity cessation (AC) recorded in vitro and in silico. Arrhythmic events: early and delayed after-depolarization (EAD, DAD), repolarization failure (RF), irregular rhythm (IRR), ventricular tachycardia-like rhythm (VT) and other arrhythmic events (OTH). Automaticity cessation (AC): quiescence (Q) and residual activity (RESAC). Q and RESAC are not considered pro-arrhythmic. For Koivumäki2018, the ΔCTD90% marked with a star were computed on EADs and the model showed arrhythmic patterns including the combination of EADs, DADs and RF (shortened as EDR) or EADs and RF (shortened as ER). For Kernik2019, two stars in BaCl2 mean high rate Ca2+ oscillations and depolarized maximum diastolic potential, but no RF. When multiple combinations of IC50 and Hill coefficient were tested in simulation for the same compound, the corresponding in silico results consist of multiple sub-columns. In case of no in vitro arrhythmic events, the corresponding cell contains an X. An expanded version of this table is presented in the Supplementary material as Supplementary Table S2.
The in silico results with the three models are in agreement (all showing consistent CTD90 prolongation or shortening). In absence of arrhythmic events, all the three models showed smaller CTD90 changes than in vitro experiments, e.g. sparfloxacin at concentration two prolonged by 42% in vitro CaTs, while in silico sparfloxacin I induced 17% prolongation in Koivumäki2018 and less than 10% prolongation in Paci2020 and Kernik2019. The extreme prolongations for Koivumäki2018 for dofetilide, flecainide II and III (concentration one), and moxifloxacin III (concentration one) are due to computing CTD90 manually on EADs. We did not compute the CTD90 for BaCl2 concentrations two and three for Kernik2019. In fact, IK1 block slightly depolarizes the maximum diastolic potential in Kernik2019 but not to the extent to trigger a repolarization failure (Supplementary Figure S4). However, the membrane potential and cytosolic Ca2+ start oscillating at a high rate and these oscillations do not have the morphology of CaTs.
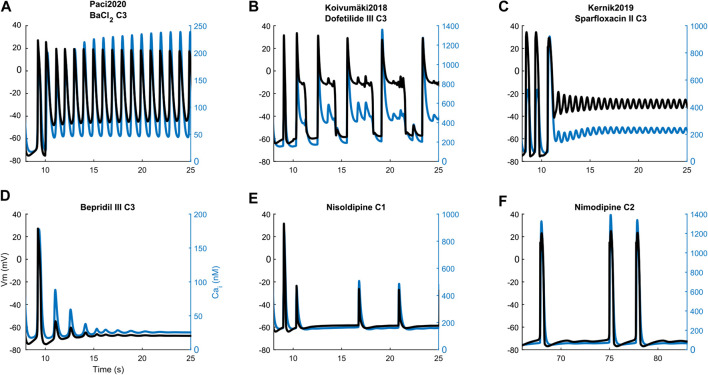
Illustrative arrhythmic events and abnormal rhythms are presented in Figure 8. Paci2020 did not show arrhythmic events for any drug but for the maximum concentration of BaCl2 where it failed to repolarize (Figure 8A). It also showed the greatest CTD90 prolongations with no EADs among the three models (e.g. dofetilide III +62%). Furthermore, it simulated well the cessation of the spontaneous activity (Q) for drugs mainly blocking Na+ currents (e.g., mexiletine and lidocaine), ICaL (e.g., nimodipine, nisoldipine and phenytoin), or a mixed combination of IC50 (e.g. bepridil, flecainide and sparfloxacin) (Figure 8D).
FIGURE 8.
Illustrative action potential (black) and Ca2+ transient (blue) arrhythmic rhythms, abnormalities and the cessation of spontaneous activity produced by the three models. Paci2020: repolarization failure (A), quiescence (D). Koivumäki2018: early after-depolarizations (B) and residual activity (E). Kernik2019: repolarization failure (C) and irregular rhythm (F). The drug concentrations are reported as C1, C2 and C3, consistently with Supplementary Table S2.
Conversely, Koivumäki2018 was the most sensitive one, triggering arrhythmic events, e.g. EAD and RF, for most of the drugs affecting the K+ currents (BaCl2, dofetilide and sparfloxacin) (Figure 8B). It never showed a complete stop of the spontaneous activity, but responded with a residual activity (RESAC), i.e. small depolarization of the membrane potential with peaks reaching about −30 mV, that cannot be considered APs (Figure 8E). This happened consistently with the quiescence of Paci2020 and the in vitro data, in case of drugs affecting mainly INa or ICaL.
Kernik2019 never produced EADs, but it responded with RF in most of the cases when the Koivumäki2018 model showed arrhythmogenic events (Figure 8C). Kernik2019 maintained also its automaticity in all the cases the Paci2020 and the Koivumäki2018 models showed quiescence or residual activity, except for nimodipine (Figure 8F) and nisoldipine, where it also showed cases of irregular rhythm (IRR). Nonetheless, these irregular rhythms lasted only 320 s (nimodipine concentration one) and 250 s (nisoldipine concentration one), then the automaticity stopped consistently with the other two models. None of the models showed ventricular tachycardia-like events that were on the contrary observed in vitro.
In silico Drug Tests: Influence of Different IC50 and Ion Channel Characterizations
The same drug can have different effects in the same model (Figure 7), according to the drug characterization in terms of IC50 magnitude and how many ion channels were considered in the specific characterization. In fact, we used multiple characterizations for seven drugs, as in (Passini et al., 2017). For example, moxifloxacin I and II show consistent results in each of the three models. On the contrary, moxifloxacin III triggers different responses in Paci2020 (concentration two does not stop automaticity as it happens for moxifloxacin I and II) and Koivumäki2018 (concentration one triggers EADs and DADs instead of CTD90 prolongation and concentration two fails the repolarization). This is explained by the fact that characterizations moxifloxacin I and II show very similar IC50 and act on the same ion currents (INa, IKr and ICaL), while moxifloxacin III does not block INa and ICaL (that the sensitivity analysis demonstrated fundamental to keep the automaticity in Paci 2019 and Koivumäki2018), acting only on IKr, IKs and INaL (which is present only in Paci2020 as individual ion current). Therefore, the results for moxifloxacin I and II are typical of an INa and/or ICaL blocker, while moxifloxacin III behaves mainly as K+ current blocker. The same considerations can be also done for ranolazine I and II, which have different effects at concentration two in Koivumäki2018 (residual activity vs EADs and DADs).
Discussion
In this work, we analyze and compare the simulated response of three recent in silico models of hSC-CM in control and under 15 drugs action, in terms of CaT duration, pro-arrhythmic behavior and automaticity. A comparison is also conducted between simulations with the three models and corresponding in vitro experiments on hSC-CMs. The generated insights provide a characterization and comparison of the three models that could inform selecting the model best suited for specific applications. The main contributions of our work are:
• The three models show qualitatively consistent in silico results in terms of CTD90 prolongation and shortening for specific drugs.
• The three models show different susceptibility to drug-induced pro-arrhythmic events. Paci2020 is the least prone to develop them, whereas Kernik2019 produces repolarization failures and Koivumäki2018 exhibits the highest variety of events (including EADs and DADs) and triggering mechanisms (ICaL reactivation and spontaneous Ca2+ releases from SR).
• The strength of the automaticity is different across the three models. Kernik2019 shows the strongest spontaneous activity, supported by a combination of inward currents where ICaL plays a fundamental role. Paci2020 and Koivumäki2018 are more sensitive to drug-induced impairment of automaticity, being mainly supported by Ca2+ handling and INCX.
• The three models simulate CTD90 percent variations qualitatively in agreement, even though smaller, than the specific in vitro data considered here.
• For compounds with multiple experimental ion channel data, the sets of ion channels taken into account (e.g. INa, IKr, ICaL vs IKr only) and the variations in IC50 and Hill values can lead to divergent simulation results, e.g. the AP/CaT prolongation and the occurrence of pro-arrhythmic events (typical of K+ current blockers), or the depolarization impairment and suppression of hSC-CM automaticity (typical of Na+ and Ca2+ blockers). The input ion channel data are therefore essential in determining the results of in silico drug tests.
Origins and Basic Features of the Three Human Stem Cell-Derived Cardiomyocyte Models
Since the proposal of the Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative (Colatsky et al., 2016; Strauss et al., 2019), the acceptance of hSC-CMs and in silico models in the field of pharmacology and drug safety has increased steadily. As a consequence, the number of hSC-CM in silico models available has also increased. At the time of the publication of the first in silico hSC-CM model (Paci et al., 2013), a limited amount of in vitro data was available in spite of the seminal work by Ma et al. (Ma et al., 2011), that represented the most complete characterization of ion currents and APs from control hSC-CMs. Due to the growing interest in the hSC-CM technology and its applications, additional experimental data were published, enabling the development of more sophisticated hSC-CM models (Paci et al., 2018b; Paci et al., 2020; Koivumäki et al., 2018; Kernik et al., 2019).
The first Paci model, Paci 2013 (Paci et al., 2013), was developed entirely based on the Ma et al. (Ma et al., 2011) dataset (Ma 2011) and additional in vitro data from cardiomyocytes derived from human embryonic stem cells for the ion currents missing in the Ma et al. dataset. Its structure closely resembles the structure of the TenTusscher hV-CM model (ten Tusscher et al., 2004). Over the years, many new Paci models were released (Paci et al., 2015; Paci et al., 2017; Paci et al., 2018b; Paci et al., 2020), integrating more mechanisms based on new in vitro data, e.g. INaL (Ma et al., 2013) or a more accurate Ca2+ handling calibrated on in-house measurements, described in (Paci et al., 2018b). The Paci2020 model used in this paper is the latest one and, in addition to being able simulate all the mechanisms successfully reproduced by its predecessors, it also encapsulates a more physiological description of the mechanisms underlying the hSC-CM automaticity, i.e. the joint role of If and INCX in sustaining the hSC-CM spontaneous electrical activity.
The Koivumäki2018 model was mainly based as well on the Ma2011 dataset, but it included also new in-house measurements of ICaL (Koivumäki et al., 2018). Many currents (e.g. Ito, IKr, IKs, IK1) in Koivumäki2018 shared the same structure (with conveniently tuned parameters) observed in the Paci models. A core difference with respect to the Paci models, is a more detailed description of the cell topology. Without need of partial differential equations, Koivumäki2018 can accurately simulate the spatial diffusion of Ca2+ in the cytosol, showing good agreement with the in vitro data. The formulation was inherited from a previous model of cardiomyocyte derived from mouse embryonic stem cells (Korhonen et al., 2008). However, such refined description of Ca2+ diffusion comes at the cost of 100 times longer simulation time.
The Kernik2019 model (Kernik et al., 2019) adopts the same “simple” structure of the Paci models, while integrating a plethora of additional new in vitro data (Bellin et al., 2013; Cordeiro et al., 2013; Ma et al., 2015; Es-Salah-Lamoureux et al., 2016; Herron et al., 2016; Veerman et al., 2016; Li et al., 2017; Garg et al., 2018), in addition to the well-known Ma2011 dataset; see Table 1 in the original Kernik et al. publication. However, the authors reformulated seven key ion currents (INa, ICaL, IKr, IKs, IK1, Ito and If). Their motivation was to compensate the lack of time constant information in several published datasets of hSC-CM voltage-clamp data: remarkably, they fit the normalized current recordings to single exponential functions in order to estimate activation/inactivation time constants, to be used then to optimize model parameters. The remaining ion currents followed canonical formulations from literature, e.g. (Shannon et al., 2004; ten Tusscher et al., 2004). Given the shared model structure, Kernik2019 and Paci2020 have similar computing time.
At the time of this paper, two more hSC-like CM models were published, based on reparameterization of hV-CMs models: Zhang et al. (Zhang et al., 2012) and Lemoine et al. (Lemoine et al., 2018). However, their inclusion was out of the scope of this study.
Drug Responses in vitro and in silico
The three models simulated consistently drug-induced changes in CTD90, all showing CaT prolongation or shortening for the same drug. Although qualitatively in agreement with the in vitro experiments, all three models showed smaller percentage variations.
The models demonstrated different susceptibility to generating repolarization abnormalities, summarized in Figure 7, Supplementary Figures S5–S7 and Supplementary Table S2. Paci2020 showed the lowest number of arrhythmic events: the repolarization failure only for the highest concentration of IK1 blocker BaCl2. In case of drugs blocking IKr, this model consistently prolonged the duration of the CaT, but no EAD or DAD appeared. Kernik2019 failed to repolarize in response to six drugs (for at least one formulation in case of multiple drug formulations) blocking prevalently IKr, although it never developed EADs or DADs. Finally, Koivumäki2018 showed the highest variety of arrhythmic events, including EADs, DADs, repolarization failure and irregular rhythm for nine drugs. The drugs that triggered arrhythmic events in Koivumäki2018 and Kernik2019 were all classified as “Known Risk of TdP” by CredibleMeds (Woosley et al., 2020), except for Ranolazine (“Conditional Risk of TdP”), BaCl2 and Nimodipine (not reviewed) and Verapamil (not classified).
Automaticity is one key-feature of hSC-CMs, being a macromarker of the successful differentiation into CMs, but also a sign of hSC-CM immaturity when compared to adult cardiac cells. Automaticity in Paci2020 and Koivumäki2018 stopped (or produced minor residual electrical activity) for the drugs strongly blocking INa and/or ICaL (e.g. bepridil, ranolazine I, flecainide, lidocaine, mexiletine, nimodipine, nisoldipine and phenytoin). Conversely, only bepridil and nisoldipine, Ca2+ current blockers, terminated the spontaneous electrical activity in Kernik2019: this model exhibited the stronger automaticity, as we report in our sensitivity analysis (Figure 6).
It must be noted that the in vitro data included observations of six recordings, and only a few of them showed arrhythmic events or quiescence following drug administration, thus highlighting variability in the in vitro responses. This is not different from the behavior of the three in silico models.
Since in silico drug tests are based on simulations, not only the biophysical accuracy of the models, but also the precision of the characterization of drugs is critical (in terms of what ion channels are affected and to what extent). Our tests showed that the effect of a certain drug concentration can be very different depending on the in vitro characterization, e.g. moxifloxacin I and II vs moxifloxacin III. Recently, Zhou et al. (Zhou et al., 2020) demonstrated how reducing the amount of ion currents considered in drug characterizations (e.g. INa, IKr and ICaL vs IKr only) reduces the specificity of the in silico predictions, marking safe drugs as at risk. The same authors examined also how moderate changes in IC50 and variable Hill coefficients (representing the steepness of the drug dose-response curves) lead to divergent predictions using the same population of hV-CM models, e.g. for sotalol and verapamil.
Accounting for Variability
High phenotypical and electrophysiological variability is one key feature of hSC-CMs and one of the reason of skepticism (together with their structural immaturity (Knollmann, 2013; Koivumäki et al., 2018)) when using them in real-world applications, where results should be then translated to the human. However, it is important to acknowledge that, despite the limitations, in vitro drug tests on hSC-CMs showed already their capability to meet and surpass traditional preclinical tests on animal models, considering as metric the CaT prolongation (Pfeiffer et al., 2016).
A recent work by Biendarra-Tiegs et al. (Biendarra-Tiegs et al., 2019) identifies many potential sources of variability in producing induced pluripotent stem cells (abnormalities at genetic/epigenetic level, differences among donors, endogenous signaling and extrinsic factors such as culture conditions, media, substrate, pH) and differentiating them into cardiomyocytes (differentiation methods, maturation protocols and level of maturation of hSC-CMs when in vitro measurements are done). By deep-diving in the eleven in vitro datasets used to build the three in silico models here studied, we observe already many potential sources of variability:
• Four datasets used only iCell pluripotent stem cells (including the Ma2011 dataset, used for all the three models), six datasets used only in-house cells from donors, in most cases healthy, but e.g. Bellin et al. report fibroblasts from a LQT2 patient (hSC-CMs were corrected later) (Bellin et al., 2013). For the dataset from (Ma et al., 2015), the authors used both iCell hSCs (for control) and in-house hSC-CMs produced from a LQT1 patient.
• Combinations of different reprogramming factors were used to induce the pluripotency in the in-house cells from donors, e.g. combinations from (Takahashi et al., 2007; Yu et al., 2007).
• Different methods were used to differentiate hSCs into CMs: embryoid bodies (e.g. (Garg et al., 2018)), coculturing with mouse visceral endodermal-like cells (END-2) (e.g. in-house cells for the Paci2020 model), modulation of the WNT signaling (e.g. in-house cells for ICaL measures for the Koivumäki2018 model and (Ma et al., 2015)).
There is clear need for standardization for in vitro hSC-CM drug assays. For example, Blinova et al. (Blinova et al., 2018) demonstrated that, despite hSC-CM variability, good consensus can be achieved across multiple laboratories on drug test results, by means of a scrupulously planned multi-site study. In addition to the differences in the equations and structures of the Paci2020, Koivumäki2018 and Kernik2019 models, the aforementioned sources of variability identified in the in vitro datasets could play a role in the differences in the APs and CaTs simulated by the three in silico models, also in basal conditions.
Nevertheless, it is not common considering sources of variability such as differences among donors or maturation level when modeling the electrophysiology of hSC-CMs. What has been already done with the three in silico models we considered is to account for the in vitro variability observed in AP features, responses to drugs, and sensitivity to channelopathies by developing in silico hSC-CM populations, following different approaches. In (Paci et al., 2020), Paci et al. randomly sampled 22 models parameters (maximum conductances and dynamic parameters, covering all the main ion currents/exchangers/pumps in the model) using the Latin Hypercube sampling, thus obtaining a random population. The random population was then experimentally calibrated using AP and CaT features. Kernik et al. followed a different approach, sampling the model parameters (five dynamic parameters and maximum conductance for five ion currents) from independent distributions, each one centered on the baseline value of each of the chosen parameters. Also, Koivumäki2018 was used as baseline for a population where seven maximum conductances/currents were sampled according to literature data, but only two AP features (maximum diastolic potential and AP peak) were used as exclusion criteria.
Conclusion
In this paper, we investigate the simulated response of three hSC-CM models to drug action in order to 1) highlight the differences in their principal functional features, and 2) compare their drug responses, based on a panel of in vitro data. We extract the following recommendations.
• In order to assess the potential arrhythmogenic activity of a compound on a single simulation, Koivumäki2018 might be the best option because of its high sensitivity to INa, ICaL and IKr blocks. However, Koivumäki2018 could predict an excess of false positives. Also, for a population-based study, the Koivumäki2018 model requires an excessively long running time.
• To account for hSC-CM variability (in terms of responses to drugs and sensitivity to channelopathies), the Paci2020 and Kernik2019 models are more suitable to be baselines for populations of in silico models. Furthermore, because of the very similar structure and running time of Paci2020 and Kernik2019, variability could also be simulated in population-based studies not only by sampling parameters (according to the techniques presented in this section), but also by using both models as baselines for a hybrid population.
Our simulation study shows differences in the drug responses across the different hSC-CM models, which could reflect the variability in the in vitro data used to design them and, more generally, of the results of in vitro drug tests done on hSC-CMs.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available on request by contacting the authors, without undue reservation.
Author Contributions
MP, EP and BR conceived and designed the study. HRL and DJG performed the experimental drug assays. MP and EP performed the in silico drug assays. MP and JTK analyzed the in silico data and prepared the figures. MP, JTK, EP and BR drafted the manuscript. All the authors interpreted the results and revised the manuscript.
Funding
MP was supported by the Academy of Finland (decision number 307967) and by the Finnish Cultural Foundation (Central Fund, grant number 00160735). JTK was supported by the Academy of Finland Center of Excellence in Body-on-Chip Research and the Pirkanmaa regional fund of the Finnish Cultural Foundation (grant numbers 50171514 and 50201322). EP and BR were supported by an NC3Rs Infrastructure for Impact Award (NC/P001076/1), a Wellcome Trust Senior Research Fellowship in Basic Biomedical Sciences (214290/Z/18/Z), the CompBioMed project (European Commission grant agreement No. 823712), the Oxford BHF Center of Research Excellence (RE/13/1/30181), and the TransQST project (Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No. 116030, receiving support from the European Union’s Horizon 2020 research and innovation program and EFPIA).
Conflict of Interest
MP and Tampere University collaborated with InSilicoTrials Technologies SRL for developing a software for predicting drug cardiac safety risk based on an in silico model of human induced pluripotent stem cell-derived cardiomyocyte. HRL, and DJG are employees of Janssen Pharmaceutica NV.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.604713/full#supplementary-material.
References
- Bellin M., Casini S., Davis R. P., D’Aniello C., Haas J., Ward-van Oostwaard D., et al. (2013). Isogenic human pluripotent stem cell pairs reveal the role of a KCNH2 mutation in long-QT syndrome. EMBO J. 32, 3161–3175. 10.1038/emboj.2013.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzerides V. J., Zhang D., Pu W. T. (2017). Modeling inherited arrhythmia disorders using induced pluripotent stem cell-derived cardiomyocytes. Circ. J. 81, 12–21. 10.1253/circj.CJ-16-1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biendarra-Tiegs S. M., Secreto F. J., Nelson T. J. (2019). Addressing variability and heterogeneity of induced pluripotent stem cell-derived cardiomyocytes. Adv. Exp. Med. Biol. 6, 1–29. 10.1007/5584_2019_350 [DOI] [PubMed] [Google Scholar]
- Blinova K., Dang Q., Millard D., Smith G., Pierson J., Guo L., et al. (2018). International multisite study of human-induced pluripotent stem cell-derived cardiomyocytes for drug proarrhythmic potential assessment. Cell Rep. 24, 3582–3592. 10.1016/j.celrep.2018.08.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan T., Fink M., Rodriguez B. (2009). Multiscale modelling of drug-induced effects on cardiac electrophysiological activity. Eur. J. Pharm. Sci. 36, 62–77. 10.1016/j.ejps.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Colatsky T., Fermini B., Gintant G., Pierson J. B., Sager P., Sekino Y., et al. (2016). The comprehensive in Vitro Proarrhythmia assay (CiPA) initiative — update on progress. J. Pharmacol. Toxicol. Methods 81, 15–20. 10.1016/j.vascn.2016.06.002 [DOI] [PubMed] [Google Scholar]
- Cordeiro J. M., Nesterenko V. V., Sicouri S., Goodrow R. J., Treat J. A., Desai M., et al. (2013). Identification and characterization of a transient outward K+ current in human induced pluripotent stem cell-derived cardiomyocytes. J. Mol. Cell. Cardiol. 60, 36–46. 10.1016/j.yjmcc.2013.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Korte T., Katili P. A., Mohd Yusof N. A. N., van Meer B. J., Saleem U., Burton F. L., et al. (2020). Unlocking personalized biomedicine and drug discovery with human induced pluripotent stem cell–derived cardiomyocytes: fit for purpose or forever elusive?. Annu. Rev. Pharmacol. Toxicol. 60, 529–551. 10.1146/annurev-pharmtox-010919-023309 [DOI] [PubMed] [Google Scholar]
- Es-Salah-Lamoureux Z., Jouni M., Malak O. A., Belbachir N., Al Sayed Z. R., Gandon-Renard M., et al. (2016). HIV-Tat induces a decrease in I Kr and I Ks via reduction in phosphatidylinositol-(4,5)-bisphosphate availability. J. Mol. Cell. Cardiol. 99, 1–13. 10.1016/j.yjmcc.2016.08.022 [DOI] [PubMed] [Google Scholar]
- Garg P., Oikonomopoulos A., Chen H., Li Y., Lam C. K., Sallam K., et al. (2018). Genome editing of induced pluripotent stem cells to decipher cardiac channelopathy variant. J. Am. Coll. Cardiol. 72, 62–75. 10.1016/j.jacc.2018.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier L. D., Greenstein J. L., Winslow R. L. (2012). Toward an integrative computational model of the Guinea pig cardiac myocyte. Front. Physiol. 3, 1–19. 10.3389/fphys.2012.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J. Q. X., Sobie E. A. (2018). Population-based mechanistic modeling allows for quantitative predictions of drug responses across cell types. Npj Syst. Biol. Appl. 4, 11. 10.1038/s41540-018-0047-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron T. J., Rocha A. M. Da., Campbell K. F., Ponce-Balbuena D., Willis B. C., Guerrero-Serna G., et al. (2016). Extracellular matrix–mediated maturation of human pluripotent stem cell–derived cardiac monolayer structure and electrophysiological function. Circ. Arrhythmia Electrophysiol. 9, 139–148. 10.1161/CIRCEP.113.003638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keizer J., Levine L. (1996). Ryanodine receptor adaptation and Ca2+-lnduced Ca2+ release- dependent Ca2+ oscillations. Biophys. J. 71, 3477–3487. 10.1016/S0006-3495(96)79543-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernik D. C., Morotti S., Wu H., Garg P., Duff H. J., Kurokawa J., et al. (2019). A computational model of induced pluripotent stem‐cell derived cardiomyocytes incorporating experimental variability from multiple data sources. J. Physiol. 597, 4533–4564. 10.1113/jp277724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernik D. C., Yang P.-C., Kurokawa J., Wu J. C., Clancy C. E. (2020). A computational model of induced pluripotent stem-cell derived cardiomyocytes for high throughput risk stratification of KCNQ1 genetic variants. PLoS Comput. Biol. 16, e1008109. 10.1371/journal.pcbi.1008109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan J. M., Lyon A. R., Harding S. E. (2013). The case for induced pluripotent stem cell-derived cardiomyocytes in pharmacological screening. Br. J. Pharmacol. 169, 304–317. 10.1111/j.1476-5381.2012.02118.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knollmann B. C. (2013). Induced pluripotent stem cell-derived cardiomyocytes: boutique science or valuable arrhythmia model?. Circ. Res. 112, 969–976. 10.1161/CIRCRESAHA.112.300567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivumäki J. T., Naumenko N., Tuomainen T., Takalo J., Oksanen M., Puttonen K. A., et al. (2018). Structural immaturity of human iPSC-derived cardiomyocytes: in silico investigation of effects on function and disease modeling. Front. Physiol. 9, 1–17. 10.3389/fphys.2018.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopljar I., Lu H. R., Van Ammel K., Otava M., Tekle F., Teisman A., et al. (2018). Development of a human iPSC cardiomyocyte-based scoring system for cardiac hazard identification in early drug safety de-risking. Stem Cell Rep. 11, 1365–1377. 10.1016/j.stemcr.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T., Rapila R., Tavi P. (2008). Mathematical model of mouse embryonic cardiomyocyte excitation-contraction coupling. J. Gen. Physiol. 132, 407–419. 10.1085/jgp.200809961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine M. D., Krause T., Koivumäki J. T., Prondzynski M., Schulze M. L., Girdauskas E., et al. (2018). Human induced pluripotent stem cell–derived engineered heart tissue as a sensitive test system for QT prolongation and arrhythmic triggers. Circ. Arrhythmia Electrophysiol. 11, e006035. 10.1161/CIRCEP.117.006035 [DOI] [PubMed] [Google Scholar]
- Li M., Kanda Y., Ashihara T., Sasano T., Nakai Y., Kodama M., et al. (2017). Overexpression of KCNJ2 in induced pluripotent stem cell-derived cardiomyocytes for the assessment of QT-prolonging drugs. J. Pharmacol. Sci. 134, 75–85. 10.1016/j.jphs.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Lu H. R., Whittaker R., Price J. H., Vega R., Pfeiffer E. R., Cerignoli F., et al. (2015). High throughput measurement of Ca++dynamics in human stem cell-derived cardiomyocytes by kinetic image cytometery: a cardiac risk assessment characterization using a large panel of cardioactive and inactive compounds. Toxicol. Sci. 148, 503–516. 10.1093/toxsci/kfv201 [DOI] [PubMed] [Google Scholar]
- Lu H. R., Zeng H., Kettenhofen R., Guo L., Kopljar I., van Ammel K., et al. (2019). Assessing drug-induced long QT and proarrhythmic risk using human stem-cell-derived cardiomyocytes in a Ca2+ imaging assay: evaluation of 28 CiPA compounds at three test sites. Toxicol. Sci. 170, 345–356. 10.1093/toxsci/kfz102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Wei H., Lu J., Huang D., Liu Z., Loh L. J., et al. (2015). Characterization of a novel KCNQ1 mutation for type 1 long QT syndrome and assessment of the therapeutic potential of a novel IKs activator using patient-specific induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 6, 39. 10.1186/s13287-015-0027-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Wei H., Zhao Y., Lu J., Li G., Binte N., et al. (2013). Modeling type 3 long QT syndrome with cardiomyocytes derived from patient-specific induced pluripotent stem cells. Int. J. Cardiol. 168, 5277–5286. 10.1016/j.ijcard.2013.08.015 [DOI] [PubMed] [Google Scholar]
- Ma J., Guo L., Fiene S. J., Anson B. D., Thomson J. A., Kamp T. J., et al. (2011). High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. AJP - Hear. Circ. Physiol. 301, H2006–H2017. 10.1152/ajpheart.00694.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa S., Sawa Y. (2018). Building a new strategy for treating heart failure using induced pluripotent stem cells. J. Cardiol. 72, 445–448. 10.1016/j.jjcc.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Paci M., Casini S., Bellin M., Hyttinen J., Severi S. (2018a). Large-scale simulation of the phenotypical variability induced by loss-of-function long QT mutations in human induced pluripotent stem cell cardiomyocytes. Int. J. Mol. Sci. 19, 3583. 10.3390/ijms19113583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paci M., Hyttinen J., Aalto-Setälä K., Severi S. (2013). Computational models of ventricular- and atrial-like human induced pluripotent stem cell derived cardiomyocytes. Ann. Biomed. Eng. 41, 2334–2348. 10.1007/s10439-013-0833-3 [DOI] [PubMed] [Google Scholar]
- Paci M., Hyttinen J., Rodriguez B., Severi S. (2015). Human induced pluripotent stem cell-derived versus adult cardiomyocytes: an in silico electrophysiological study on effects of ionic current block. Br. J. Pharmacol. 172, 5147–5160. 10.1111/bph.13282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paci M., Passini E., Klimas A., Severi S., Hyttinen J., Rodriguez B., et al. (2020). All-optical electrophysiology refines populations of in silico human iPSC-CMs for drug evaluation. Biophys. J. 58, 7250–7257. 10.1016/j.bpj.2020.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paci M., Passini E., Severi S., Hyttinen J., Rodriguez B. (2017). Phenotypic variability in LQT3 human induced pluripotent stem cell-derived cardiomyocytes and their response to antiarrhythmic pharmacologic therapy: an in silico approach. Hear. Rhythm. 14, 1704–1712. 10.1016/j.hrthm.2017.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paci M., Pölönen R.-P., Cori D., Penttinen K., Aalto-Setälä K., Severi S., et al. (2018b). Automatic optimization of an in silico model of human iPSC derived cardiomyocytes recapitulating calcium handling abnormalities. Front. Physiol. 9, 709. 10.3389/FPHYS.2018.00709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paci M., Severi S., Hyttinen J. (2019). “Automaticity in cardiomyocytes derived from human induced pluripotent stem cells as result of different mechanisms,” in Proceedings of the Computing in Cardiology Conference, Singapore, September 8–11, 2019. 10.22489/CinC.2019.101 [DOI] [Google Scholar]
- Passini E., Britton O. J., Lu H. R., Rohrbacher J., Hermans A. N., Gallacher D. J., et al. (2017). Human in silico drug trials demonstrate higher accuracy than animal models in predicting clinical pro-arrhythmic cardiotoxicity. Front. Physiol. 8, 1–15. 10.3389/fphys.2017.00668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer E. R., Vega R., McDonough P. M., Price J. H., Whittaker R. (2016). Specific prediction of clinical QT prolongation by kinetic image cytometry in human stem cell derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 81, 263–273. 10.1016/j.vascn.2016.04.007 [DOI] [PubMed] [Google Scholar]
- Rast G., Weber J., Disch C., Schuck E., Ittrich C., Guth B. D. (2015). An integrated platform for simultaneous multi-well field potential recording and Fura-2-based calcium transient ratiometry in human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 75, 91–100. 10.1016/j.vascn.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Shannon T., Wang F., Puglisi J., Weber C., Bers D. M. (2004). A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys. J. 87, 3351–3371. 10.1529/biophysj.104.047449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneyd J., Dufour J.-F. (2002). A dynamic model of the type-2 inositol trisphosphate receptor. Proc. Natl. Acad. Sci. U S A. 99, 2398–2403. 10.1073/pnas.032281999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer C. I., Baba S., Nakamura K., Hua E. A., Sears M. A. F., Fu C., et al. (2014). Calcium transients closely reflect prolonged action potentials in iPSC models of inherited cardiac arrhythmia. Stem Cell Rep. 3, 269–281. 10.1016/j.stemcr.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss D. G., Gintant G., Li Z., Wu W., Blinova K., Vicente J., et al. (2019). Comprehensive in vitro Proarrhythmia assay (CiPA) update from a cardiac safety research consortium/health and environmental Sciences institute/FDA meeting. Ther. Innov. Regul. Sci. 53, 519–525. 10.1177/2168479018795117 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- ten Tusscher K. H. W. J., Noble D., Noble P. J., Panfilov A. V. (2004). A model for human ventricular tissue. Am. J. Physiol. Hear. Circ. Physiol. 286, H1573–H1589. 10.1152/ajpheart.00794.2003 [DOI] [PubMed] [Google Scholar]
- Treat J. A., Goodrow R. J., Bot C. T., Haedo R. J., Cordeiro J. M. (2019). Pharmacological enhancement of repolarization reserve in human induced pluripotent stem cells derived cardiomyocytes. Biochem. Pharmacol. 169, 113608. 10.1016/j.bcp.2019.08.010 [DOI] [PubMed] [Google Scholar]
- Tveito A., Jæger K. H., Huebsch N., Charrez B., Edwards A. G., Wall S., et al. (2018). Inversion and computational maturation of drug response using human stem cell derived cardiomyocytes in microphysiological systems. Sci. Rep. 8, 17626. 10.1038/s41598-018-35858-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerman C. C., Mengarelli I., Guan K., Stauske M., Barc J., Tan H. L., et al. (2016). hiPSC-derived cardiomyocytes from Brugada Syndrome patients without identified mutations do not exhibit clear cellular electrophysiological abnormalities. Sci. Rep. 6, 30967. 10.1038/srep30967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G., Mirams G. R. (2015). A web portal for in-silico action potential predictions. J. Pharmacol. Toxicol. Methods 75, 10–16. 10.1016/j.vascn.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woosley R., Heise C., Romero K. (2020). QTdrugs list www.CredibleMeds.org. Available at: https://crediblemeds.org/ (Accessed August 31, 2020).
- Yu J., Vodyanik M. A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J. L., Tian S., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920. 10.1126/science.1151526 [DOI] [PubMed] [Google Scholar]
- Zeng H., Roman M. I., Lis E., Lagrutta A., Sannajust F. (2016). Use of FDSS/μCell imaging platform for preclinical cardiac electrophysiology safety screening of compounds in human induced pluripotent stem cell-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 81, 217–222. 10.1016/j.vascn.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Zhang H., Zou B., Yu H., Moretti A., Wang X., Yan W., et al. (2012). Modulation of hERG potassium channel gating normalizes action potential duration prolonged by dysfunctional KCNQ1 potassium channel. Proc. Natl. Acad. Sci. U S A. 109, 11866–11871. 10.1073/pnas.1205266109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Qu Y., Passini E., Bueno-Orovio A., Liu Y., Vargas H. M., et al. (2020). Blinded in silico drug trial reveals the minimum set of ion channels for torsades de Pointes risk assessment . Front. Pharmacol. 10, 1–17. 10.3389/fphar.2019.01643 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available on request by contacting the authors, without undue reservation.