Abstract

People with advanced cancer are at heightened risk of desire for hastened death (DHD), suicidal ideation (SI), and completed suicide. Loss of Meaning (LoM), a component of demoralization, can be elevated by a cancer diagnosis and predicts DHD and SI in this population. We completed a randomized controlled trial in which psilocybin-assisted psychotherapy (PAP) produced rapid and sustained improvements in depression, demoralization, and hopelessness in people with cancer. Converging epidemiologic and clinical trial findings suggests a potential antisuicidal effect of this treatment. To probe our hypothesis that PAP relieves SI through its beneficial impacts on depression and demoralization (LoM in particular), we performed secondary analyses assessing within- and between-group differences with regard to LoM and an SI composite score. Among participants with elevated SI at baseline, PAP was associated with within-group reductions in SI that were apparent as early as 8 h and persisted for 6.5 months postdosing. PAP also produced large reductions in LoM from baseline that were apparent 2 weeks after treatment and remained significant and robust at the 6.5 month and 3.2 and 4.5 year follow-ups. Exploratory analyses support our hypothesis and suggest that PAP may be an effective antisuicidal intervention following a cancer diagnosis due to its positive impact on hopelessness and demoralization and its effects on meaning-making in particular. These preliminary results implicate psilocybin treatment as a potentially effective alternative to existing antidepressant medications in patients with cancer that are also suicidal, and warrant further investigation in participants with elevated levels of depression and suicidality.
Keywords: psilocybin, psychedelic, cancer, depression, suicidal ideation, demoralization, loss of meaning
Introduction
There are approximately 48 000 and 804 000 deaths by suicide each year in the United States1 and worldwide,2 respectively, making suicide a leading cause of death. A diagnosis of cancer is a known risk factor for increased suicidal ideation (SI) and behavior and studies of completed suicide in cancer patients have reported prevalence rates up to 4 times greater than that in the general population.3−10 A systematic review and meta-analysis (k = 22 studies) of cancer diagnosis and suicide mortality reported a pooled standardized mortality ratio (SMR) of 1.55 (95% CI = 1.37–1.74).10 However, a retrospective, population-based study of a nationally representative sample including 8 651 569 patients with cancer reported a SMR = 4.44, indicating that risk of completed suicide might increase 4-fold following a cancer diagnosis.9 The association between cancer and increased suicide risk is predominantly mediated by advanced illness (i.e., poor prognosis and disease progression), uncontrolled pain, psychiatric distress (major depression in particular), and existential distress (hopelessness and helplessness).11−18 Importantly, within the context of cancer-related psychiatric and existential distress, there is little evidence for current pharmacologic interventions effectively targeting SI [for a review, see ref (19)]. There is, therefore, a need for exploring innovative treatment strategies for reducing SI among individuals with life-threatening cancer, especially interventions that can work rapidly.
A more subtle manifestation of SI in patients with advanced cancer is the desire for hastened death (DHD), that is, the wish for a more rapid death than would naturally occur. DHD has been reported in 9–22% of patients with advanced or terminal cancer, is more common in palliative care settings,20−24 and has similarly been shown to be associated with physical distress, low social support, depression, demoralization, hopelessness, and lack of spiritual well-being.25,26 Among these, depression and hopelessness appear to predominantly mediate DHD in advanced cancer,21,27−31 and improving spiritual well-being in advanced cancer is associated with decreased hopelessness, depression, and desired for hastened death.32 In a study of HIV/AIDS patients, a population with similar rates of existential distress,33 DHD was significantly elevated among patients with past histories of suicide attempts,34 suggesting a bridge between DHD and SI. Demoralization syndrome, a manifestation of existential distress in patients with advanced cancer, consists of hopelessness and helplessness caused by lack of purpose and meaning related to confronting death, and occurs in up to one-third of patients with advanced cancer.35 Loss of meaning (LoM) associated with demoralization syndrome has been shown to be positively correlated with DHD.36
Psilocybin-Assisted Psychotherapy for Cancer-Related Anxiety, Depression, and Existential Distress
Among the numerous clinical indications for which treatment with classic hallucinogens, such as psilocybin, have been investigated, psychiatric and existential disorders related to life-threatening cancer emerge as front-runners in terms of the published evidence from modern RCTs.37,38 Two recently published review articles39,40 identified several published clinical trials in which subjects with advanced- or terminal-cancer-related psychiatric and existential distress were treated with a serotonergic psychedelic [i.e., psilocybin, LSD, and dipropyltryptamine (DPT)], including four open-label trials of LSD therapy (N = 244) published between 1964 and 198041−44 and four RCTs (N = 104) published between 2011 and 2016.45−48 The historical open-label trials suggested that LSD-assisted psychotherapy was associated with rapid analgesic effects that endured for several weeks, improvements in psychiatric distress (depression, anxiety, insomnia) and existential distress (death anxiety), and improved psychiatric outcomes associated with mystical experiences.41−44 Three RCTs completed within the past decade (UCLA-Harbor, NYU Langone Health (NYULH)/Bellevue Hospital, and Johns Hopkins) assessed the efficacy of single-dose psilocybin-assisted psychotherapy in patients with advanced-cancer-related psychiatric and existential distress (N = 92).45,47−49 Results from these trials indicate that a single moderate to high dose of psilocybin, delivered in conjunction with psychotherapy, produces rapid, substantial (i.e., large effect sizes and high rates of psychiatric illness remission), and sustained (i.e., months to years) reductions in anxiety and depressive symptoms, as well as sustained reductions in existential distress, and improved quality-of-life. In addition, psilocybin occasioned mystical-type experiences, experienced as highly meaningful and spiritual, partially mediated anxiolytic and antidepressant effects assessed longitudinally (i.e., 5–7 weeks) postadministration of psilocybin.47,48
Psilocybin-Assisted Psychotherapy for Cancer-Related SI: Converging evidence
There is indirect evidence from epidemiologic studies that psilocybin may also have antisuicidal effects. Population-level analysis of data from the National Survey on Drug Use and Health found that lifetime classic psychedelic use was associated with 14% reduced likelihood of past-year suicidal thinking, 29% reduced likelihood of past year suicidal planning, and 36% reduced likelihood of past year suicide attempts.50 An epidemiologic study of marginalized women (i.e., street-involved sex workers, unhoused, and drug users) in Canada found that lifetime psychedelic use was associated with a 60% reduced suicidality hazard (adjusted hazard ratio: 0.40; 95% CI 0.17–0.94).51 An open-label trial in which individuals with treatment-resistant major depressive disorder received two doses of psilocybin along with supportive psychotherapy reported decreases in SI up to 2 weeks after the intervention.52 Additional evidence from an open-label53 and a RCT54 suggest that single-dose ayahuasca administration is associated with rapid (i.e., within 40 min) and sustained (i.e., 1–3 weeks) decreases in suicidality in patients with major depressive disorder. Interestingly, among the classic psychedelics, psilocybin in particular may be protective against SI and behaviors.55
The NYULH/Bellevue Hospital trial and its ancillary long-term follow-up study did not explore suicidality as primary or secondary outcomes, and excluded participants with active SI or suicidal behaviors at screening. However, findings indicate that psilocybin-assisted psychotherapy improves several constructs related to SI and DHD, including depression, cancer-related hopelessness and demoralization, and spiritual well-being.47,49 Meaning-making was a key existentially oriented component of the psychotherapeutic platform within which psilocybin treatment was delivered,47 and several themes have emerged from qualitative analyses that implicate LoM specifically as a likely symptom of demoralization syndrome that psilocybin-assisted psychotherapy might target reduction of SI.56 Suicidality and symptoms of depression often change independently of one another,57 and not all interventions for depression are effective for relieving suicidality. For instance, SSRIs, a first-line intervention for depression, show limited efficacy for suicidality in particular,58 and may even increase suicidality within certain populations.59 Thus, determining whether and how psilocybin-assisted psychotherapy reduces SI in addition to its established antidepressive effects in people with cancer has emerged as an important question.
Rationale for Present Analyses
To assess whether a single dose of psilocybin, administered in conjunction with psychotherapy, produces acute and sustained antisuicidal effects in patients with advanced cancer, we performed post hoc analyses of relevant data from our completed NYULH/Bellevue Hospital trial.47 Our aims were to (1) determine whether single-dose psilocybin-assisted psychotherapy acutely relieves SI and LoM; (2) evaluate the longevity of reductions in SI and LoM; and (3) examine relationships between SI, other (non-SI-related) depressive symptoms, and potential mediators of psilocybin’s antisuicidal effects, including demoralization, hopelessness, and spiritual well-being within people that have been given a cancer diagnoses. We hypothesized that psilocybin treatment would be associated with significant acute and sustained reductions in SI and LoM and that reductions in SI would be positively associated with improvements in other depressive symptoms, LoM, demoralization, hopelessness, and spiritual well-being.
Methods
Study Design and Interventions
Our previously completed double-blind, randomized, crossover, controlled trial was designed to assess the efficacy of a single, moderate-to-high dose of oral psilocybin per session (0.3 mg/kg) versus a single-dose session of an orally administered active control (niacin 250 mg). Both treatment conditions were administered in conjunction with dyadic psychotherapy to treat clinically significant anxiety and/or depression as well as existential distress in patients with life-threatening cancer [see ref (47) for more details]. A subsample of participants from the parent trial participated in two additional long-term follow-up assessments that occurred on average at 3.2 years (range = 2.3–4.5 years) and 4.5 years (range = 3.5–5.5 years) following the participants’ psilocybin dosing date.49 The parent trial and long-term follow-up were approved by the NYULH Perlmutter Cancer Protocol Review and Monitoring Committee and the Institutional Review Board (IRB) of the New York University (NYU) School of Medicine (SOM). Figure 1 presents an overview of the relevant aspects of the parent trial and ancillary follow-up study.
Figure 1.
Temporal relationships between drug administration, psychosocial interventions, and assessments in the main trial and ancillary long-term follow-up trial. Prep PT = preparatory psychotherapy; D1 = double-blind dosing 1; Post-Integrative PT = post-integrative psychotherapy.
Sample
Major qualifying criteria for the parent trial include a DSM-IV Axis I Disorders-Patient Version diagnosis of adjustment disorder with anxiety ± depression, acute stress disorder, generalized anxiety disorder (GAD), or anxiety disorder due to cancer; a projected life expectancy of at least 1 year; and no psychiatric risk factors such as family history of schizophrenia. Of 108 prescreened participants, 42 provided informed consent, 31 were randomized into the experimental or control group, 29 received study medication, and 11 were included in this secondary analysis due to detectable baseline levels of SI (see Figure 2 for the CONSORT flow diagram).
Figure 2.

CONSORT flow diagram depicting number of participants from parent trial and ancillary long-term follow-up trial that completed assessment time points included in the current analyses.
Assessments
Suicidal Ideation (SI)
As our primary outcome, we created a composite score representing SI from scores on two items from the Beck Depression Inventory-II (BDI-II)60 and the Brief Symptom Inventory (BSI):61 item 9 on the BSI (“thoughts of ending your life”) and item 9 on the BDI, which queries SI with response options of “I do not have any thoughts of killing myself” (0), “I have thoughts of killing myself, but I would not carry them out” (1), “I would like to kill myself” (2), and “I would kill myself if I had the chance” (3). There is ample precedent for calculating a composite SI score using all available items (or measures) that examine the effects of a pharmacological agent on suicidality, both in clinical trials that have included stand-alone SI measures and those that have not.62−66 Price et al.64 used a similar methodology including items from the BSI and the Montgomery–Asberg Depression Rating Scale (MADRS), and Ballard et al.63 used items from the BDI and Hamilton Depression Rating Scale (HAM-D) to create composite SI scores. Because the BDI-II and BSI utilize disparate Likert scales, we computed Z-scores for each item, summed them, and transformed the summed Z-scores into standardized T-scores with a range of 0–100,67 with higher scores indicating a higher SI. SI was assessed at baseline, 8 h after dose 1, 2 weeks after dose 1, 7 weeks after dose 1 (corresponding to 1 day prior to dose 2), and 6.5 months after dose 2. Because only the BDI-II was administered at the 3.2 and 4.5 year assessments, a composite SI score was not generated at these time points. The Supporting Information shows the relationship between responses to the two items that contributed to the SI composite score.
Loss of Meaning (LoM)
The Demoralization Scale (DS), a 24 item questionnaire with Likert scale response categories ranging from 0 to 4 developed to measure existential distress in advanced cancer, has shown good reliability (Cronbach’s α ranging from 0.71 to 0.89) and concurrent validity with related scales.36 The scale generates five factors: LoM, despair, disheartened feelings, helpless feelings, and sense of failure. Because the LoM factor from the DS has been shown to positively correlate with scores on a validated measure of DHD (the Schedule of Attitudes toward Hastened Death, SAHD; r = 0.67),36 we chose scores on this factor as our secondary outcome. Scores on the following five items from the Demoralization Scale contributed to LoM factor scores: “Life is no longer worth living”, “I would rather not be alive”, “My life seems to be pointless”, “My role in life has been lost”, and “There is no purpose to the activities in my life”. LoM was assessed at baseline, 2 weeks post dose 1, 6.5 months post dose 2, and at the 3.2 and 4.5 year assessments.
Other Depressive Symptoms
The remaining items from the BDI-II (SI items removed) were scored to represent other (non-SI) depressive symptoms. The BDI-II60 is a self-report measure of depression comprising 21 questions related to depressive symptoms experienced over the previous 2 weeks rated on a 3-point scale (total score ranges from 0 to 63). Scores greater than 12 indicate evidence of clinical depression. This measure demonstrates good reliability (internal consistency 0.90) and factorial validity.68 Change in other depressive symptoms was assessed from baseline to 2 weeks after dose 1.
Hopelessness
The Hopelessness Assessment in Illness (HAI)69 is an 8 item existential distress scale that assesses hopelessness in advanced cancer. Total scores range from 0 to 16, with higher scores indicating greater levels of hopelessness. This measure has shown adequate internal consistency (Cronbach’s α = 0.87) and concurrent validity (r = 0.70–0.78).69 The HAI was assessed at baseline, 2 weeks post dose 1, 6.5 months post dose 2, and at the 3.2 year and 4.5 year follow-up assessments.
Spiritual Well-Being
The Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being (FACIT-Sp-12)70 is a 12 item measure of spiritual well-being in patients with cancer and other forms of chronic illness. The items are rated on a five-point Likert scale yielding three subscales: a sense of meaning, inner peace and purpose in life; a sense of comfort and strength derived from one’s faith; and a total spiritual well-being score. The FACIT-Sp-12 shows strong internal consistency (Cronbach’s α ranging from 0.81 to 0.88) and is a well-validated scale.70 The FACIT-Sp-12 was assessed at baseline, 2 weeks post dose 1, 6.5 months post dose 2, and at the 3.2 and 4.5 year follow-up assessments.
Statistical Analysis
Data were combined from the original parent investigation47 and the ancillary long-term follow-up study49 for posthoc analyses. Participants who met criteria for having a baseline level of suicidality (defined as a composite SI score > 0 on BDI item 9 + BSI item 9) were included; this approach was chosen to mitigate floor effects and optimally address the current aims. We performed post hoc power analyses to determine how much power we had to detect small, medium, and large effect sizes with the sample size and measures available to us from the parent trial. Results indicated that analyses of outcomes with four repeated measures were sufficiently powered (>0.80) to detect large effect sizes (f > 0.4), and analyses with two repeated measures were only sufficiently powered to detect very large effect sizes (f > 0.5).
Mixed effect models with repeated measures (MMRMs) were constructed in SPSS (version 25) using an AR(1) covariance structure and fixed effects of group and time. Several planned within-group (change from baseline) and between-group (psilocybin-first vs niacin-first) comparisons of interest were specified a priori to assess the impact of psilocybin treatment on SI and LoM (Tukey’s posthoc). Scores on SI and LoM within each treatment group were compared at each available follow-up assessment, and scores at each available follow-up assessment time point were contrasted with baseline scores within each treatment group. After the crossover, both the psilocybin-first and niacin-first dose-sequence groups were collapsed and combined into one group. This approach was fchosen to increase power given the modest sample size, since the crossover design prevented further meaningful between-group comparisons. The long-term effects of psilocybin on SI and LoM were assessed within the MMRM framework using planned within-subject comparisons assessing change from baseline to each available postcrossover follow-up time point. Effect sizes were tentatively estimated as Cohen’s d, although deviations from normality and small sample size were expected to introduce bias into these estimates.
Finally, we assessed correlations between composite SI scores and LoM scores, and between change in SI from baseline to 2 weeks after dose 1 and change in other depressive symptoms, demoralization (DS), hopelessness (HAI), and spiritual well-being (FACIT-Sp-12) from baseline to 2 weeks after dose 1.
Results
Demographics
Demographic information for the subsample of participants included in the current analyses is presented in Table 1. The mean age of participants was 60.3 years old (standard deviation (SD) = 7.1 years), and they were predominately female (63.6%). The majority of participants were non-Hispanic White (90.9%), followed by Multiracial (9.1%). Approximately 46% of participants identified as Atheist/Agnostic versus some other organized religious affiliation. Gynecological cancers (54.6%) comprised most disease types. Nearly three-quarters of participants (72.8%) were diagnosed with advanced [III–IV] versus early [I–II] stage (27.2%) cancers. Approximately one-third (36.4%) of all participants reported one or more occasions of prior psychedelic use. The majority of participants met Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV)71 criteria for cancer-related adjustment disorder with anxious and depressed features (45.5%) or adjustment disorder with anxious (only) features (45.5%), followed by generalized anxiety disorder (9.1%). Compared to the parent study sample, the proportions of current study participants were roughly equivalent in all demographic variables and did not statistically differ, nor did the two dose-sequence groups.
Table 1. Demographic and Clinical Characteristics of the Study Samplea,b.
| characteristic | categories | psilocybin-first N = 6a | niacin-first N = 5a | total N = 11 | |||
|---|---|---|---|---|---|---|---|
| sex | female | 3 | 50% | 4 | 80% | 7 | 63.6% |
| male | 3 | 50% | 1 | 20% | 4 | 36.4% | |
| age at follow-up; mean (SD) | range 48–71 | 57.5 (7.5) | 63.6 (5.3) | 60.3 (7.1) | |||
| race | White/Caucasian | 5 | 100% | 4 | 80% | 10 | 90.9% |
| multiracial | 0 | 0% | 1 | 20% | 1 | 9.1% | |
| religious/spiritual beliefs | atheist/agnostic | 1 | 16.7% | 4 | 80% | 5 | 45.5% |
| Jewish | 1 | 16.7% | 0 | 0% | 1 | 9.1% | |
| Catholic | 2 | 33.3% | 0 | 0% | 2 | 18.2% | |
| Unitarian | 1 | 16.7% | 0 | 0% | 1 | 9.1% | |
| other faith/tradition | 1 | 16.7% | 1 | 20% | 2 | 18.2% | |
| site of cancer | breast | 0 | 0% | 1 | 9.1% | 1 | 9.1% |
| reproductive | 3 | 50% | 18.2% | 5 | 45.5% | ||
| lymphoma/leukemia | 0 | 0% | 1 | 9.1% | 1 | 9.1% | |
| colon | 0 | 0% | 1 | 9.1% | 1 | 9.1% | |
| other types | 3 | 50% | 0% | 3 | 27.3% | ||
| stage of cancer | stage IV | 2 | 40% | 1 | 16.7% | 3 | 27.3% |
| stage III | 2 | 40% | 5 | 45.5% | 5 | 45.5% | |
| stage II | 1 | 20% | 0 | 0% | 1 | 9.1% | |
| stage I | 0 | 0% | 2 | 33.3% | 2 | 18.2% | |
| SCID (DSM-IV) diagnosisb | adjustment disorder w/anxiety and depressed mood, chronic | 2 | 33.3% | 3 | 60% | 5 | 45.5% |
| adjustment disorder w/anxiety, chronic | 3 | 50% | 2 | 40% | 5 | 45.5% | |
| generalized anxiety disorder | 1 | 16.7% | 0 | 0% | 1 | 9.1% | |
| previous psychedelic use | no | 4 | 66.7% | 3 | 60% | 7 | 63.6% |
| yes | 2 | 33.3% | 2 | 40% | 4 | 36.4% | |
| education | grade 7–12 without graduating high school | 1 | 16.7% | 0 | 0% | 1 | 9.1% |
| part-college | 1 | 16.7% | 1 | 20% | 2 | 18.2% | |
| graduated 4 year college | 2 | 33.3% | 2 | 40% | 4 | 36.4% | |
| completed grad/professional school | 2 | 33.3% | 2 | 40% | 4 | 36.4% | |
The two dose-sequence groups did not differ on any demographic measures at baseline.
Psychiatric classification was based on the Structured Interview for the DSM-IV (SCID-IV).
Acute Effects of Psilocybin-Assisted Psychotherapy on SI and LoM
Within the psilocybin-first group, significant main effects of time were detected in analyses of SI [F(4,23) = 9.55, p < 0.001] and LoM [F(4,18) = 5.60, p = 0.004]. Within-group comparisons specified a priori indicated that scores on SI and LoM differed significantly at all post dose 1 administration session time points relative to baseline (LoM: 2 weeks post dose 1, p = 0.005; SI: 8 h post dose 1, p < 0.001; 2 weeks, post dose 1 p < 0.001; and 7 weeks post dose 1, p < 0.001). Prior to the crossover in the niacin-first group, the effect of time approached, but did not reach significance for SI [F(4,19) = 2.64, p = 0.065] or LoM [F(4,10) = 9.64, p = 0.079]. We did not detect a main effect of group on SI (p = 0.614), and the interaction between group and time approached, but did not reach, significance (p = 0.065; Figure 3a). LoM analyses revealed a main effect of group [F(1,11) = 6.49, p = 0.027] with no interaction between group and time [F(4,27) = 0.67 p = 0.429; Figure 3a]. Pairwise comparisons specified a priori indicated that mean scores on LoM differed substantially between the psilocybin-first and niacin-first groups at 2 weeks after dose 1 (p = 0.021). Effect sizes across statistically significant comparisons were large (Cohen’s d = 1.20–3.50).
Figure 3.
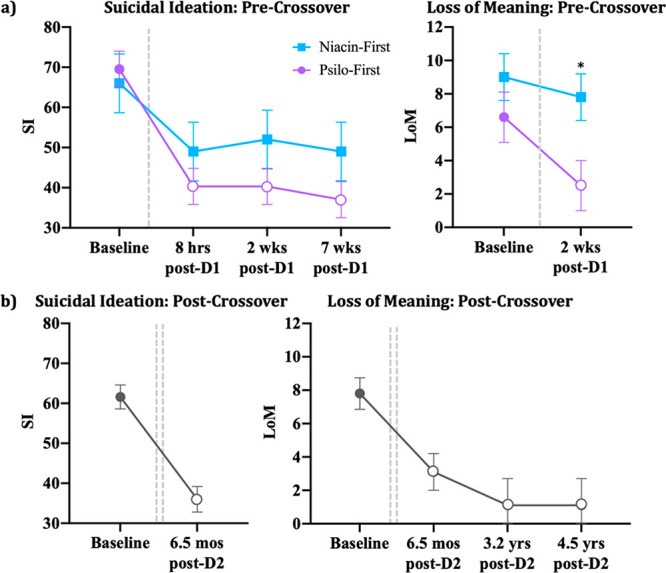
Effects of treatment on SI and LoM. (a) Mean (±SE) scores on SI and LoM at baseline and each precrossover assessment; (b) mean (±SE) scores on SI and LoM at Baseline and each postcrossover assessment. Dotted lines indicate medication sessions. Open shapes indicate a within-group difference from baseline score at p < 0.05, and * denotes a between-group difference at p < 0.05.
Long-Term Effects of Psilocybin-Assisted Psychotherapy on SI and LoM
After the crossover, within-subjects reductions, relative to baseline, remained significant at the 6.5 month follow-up for SI (p < 0.001) and LoM (p < 0.001; Figure 3b). Reductions in LoM continued to be significant at the 3.2 year (p < 0.001) and 4.5 year (p < 0.001) follow-ups. Effect sizes for significant effects were again large (Cohen’s d = 1.51–2.56).
Relationships between SI, LoM, and Other Depressive Symptoms
There was a moderate positive correlation between LoM scores and SI scores collapsed across all assessment time points (r = 0.41, p = 0.02; Figure 4a). Reductions from baseline to 2 weeks post dose 1 in SI were positively and strongly correlated with reductions from baseline to 2 weeks post dose 1 in other depressive symptoms (r = 0.75, p = 0.008; Figure 4b). Correlations between reductions in SI and hopelessness and demoralization were large, offering support to our hypothesis even though they did not reach significance with the current (underpowered) approach (HAI: r = 0.62, p = 0.059; DS: r = 0.57, p = 0.067), but the correlation between reductions in spiritual well-being (FACIT-Sp-12) did not approach significance (r = 0.015, p = 0.966).
Figure 4.

Relationships between SI, LoM, and other depressive symptoms: (a) Correlation between LoM and SI scores collapsed across assessment time point; (b) correlation between change from baseline to 2 weeks post dose 1 in SI.
Discussion
Despite several limitations precluding optimal assessment of the effects of psilocybin on suicidality in this patient population, our results suggest that a single moderate-to-high dose of psilocybin, in conjunction with psychotherapy, is associated with acute and sustained reductions in SI and LoM in patients with life-threatening cancer. We detected substantial within-group improvements in SI and LoM from baseline to all precrossover follow-up time points in the psilocybin-first, but not in the niacin-first, condition, as well as a between-group difference in LoM 2 weeks after double-blind treatment with psilocybin versus niacin control. Our statistical approach was underpowered to detect small to moderate effects, and our participants started with low levels of SI, suggesting that the size of psilocybin’s antisuicidal effect might be quite large, even in those currently at low risk of suicide. Whether this represents a true clinical benefit in individuals with such low levels of SI is unclear, but it is possible that reducing low levels of passive SI in patients with cancer could be protective against progression to more serious forms of SI (i.e., active intent, preparation, suicidal acts, and completed suicide). As expected, we found a positive relationship between a decrease in LoM and a decrease in SI. This is consistent with a growing literature on the efficacy of existential psychotherapies, specifically targeting LoM in advanced cancer, in decreasing DHD.72,73 We also found support for relationships between reductions in SI and reductions in demoralization in general, as well as with decreases in hopelessness (another component of the demoralization syndrome). Surprisingly, we found no support for a relationship between reduced SI and enhanced spiritual well-being. While we cannot make causal attributions based on our design, it is possible that LoM represents a targetable mediator between receipt of a cancer diagnosis and risk of suicide. A model in which psilocybin-assisted psychotherapy therapy reduces SI through enhancing meaning-making would make sense, especially if the psychotherapeutic platform includes existentially oriented components that target LoM in advanced cancer. Augmented inclusion of such existential psychotherapy ingredients for patients with cancer and elevated baseline SI might represent a feasible way to further bolster antisuicidal effects of psilocybin-assisted psychotherapy, although adequately powered trials designed prospectively to assess these relationships will be necessary to confirm.
Interestingly, we found a positive relationship between decreases in SI and decreases in other depressive symptoms across double-blind treatment with psilocybin versus niacin control. This was somewhat surprising because findings from the clinical depression literature indicate that front-line antidepressant medications are only very modestly effective at reducing suicidality58 and might actually increase suicidality.59 The current results are consistent with epidemiological data suggesting that psilocybin use is associated with antisuicidal effects50,55 and with an open-label study of oral psilocybin treatment in patients with treatment-resistant depression.52 The risks of psilocybin-assisted psychotherapy have been described in detail within the primary publication,47 but are expected to be minimal as long as it is delivered in carefully controlled clinical settings.
Clinical Implications in Psychiatry and Psycho-Oncology
If psilocybin’s antisuicidal effects are confirmed in people with higher baseline SI levels, then it could represent a novel, and much-needed, avenue for treatment of major depressive disorder (MDD) in people that are suicidal. There are some biological interventions within psychiatry that have an evidence base for reducing SI, including electroconvulsive therapy (ECT), clozapine, lithium, and ketamine, although all present significant limitations. ECT takes 1–2 weeks for SI to be reduced,74 and transient and sometimes enduring cognitive side effects have been documented.75 Lithium has been shown to reduce suicide risk in patients with bipolar disorder in the long term, although it has not been shown to work acutely.76 Furthermore, lithium’s narrow therapeutic index requires close monitoring to prevent toxicity and risk of death. Clozapine has been shown to have antisuicidal effects in schizophrenia spectrum disorders77 and some evidence for efficacy in bipolar disorder,78 although it carries a significant and potentially fatal side effect profile79 and has not been shown to decrease SI in the acute setting either.80
It especially makes sense to study psilocybin as a novel treatment for depression and suicidality in MDD given recent findings of ketamine treatment in this population. Despite differing primary receptor mechanisms of action, ketamine (a N-methyl-d-aspartate receptor antagonist and dissociative anesthetic) and psilocybin (a serotonin-2a receptor agonist and serotonergic hallucinogen) possess similarities in terms of modulating glutamatergic transmission and functional connectivity in prefrontal-limbic circuits, subjective effects (i.e., mystical-type experiences), and neuroplastic potential (i.e., increased brain-derived neurotrophic factor (BDNF) expression and increased synaptic plasticity).81,82 This is relevant given that ketamine, the first pharmacologic agent shown to possess rapid and short-term (e.g., up to 1–2 weeks) improvements in depressive symptoms, has been shown to rapidly (i.e., within 1 day) reduce suicidal thoughts for up to 1 week in patients with MDD and SI.83 If psilocybin were found to also have rapid, as well as sustained, antisuicidal effects (i.e., weeks to months to years) after a single-dose, then it would be of considerable clinical benefit for suicide prevention in mood disorders therapeutics.
Given the limited evidence that pharmacologic interventions effectively target SI among individuals with cancer,19 if the above findings can be replicated in future trials, then it could represent a potentially novel paradigm in psychiatry and psycho-oncology in terms of a single dose of a medication leading to rapid or acute reductions in suicidality with enduring (i.e., months to years) and clinically meaningful effects. Given the major risk factors for suicide in cancer (i.e., male, middle age, advanced cancer illness, clinically significant depression, hopelessness, and pain), psilocybin-assisted psychotherapy could target patients with advanced or terminal forms of cancer who are experiencing elevated rates of depression, existential distress, or pain, and associated DHD, SI, and suicidal behaviors. The therapy could be applied in inpatient or outpatient settings and could be especially helpful to patients at the end-of-life in hospice settings. More broadly, psilocybin could be used at any stage of cancer, including early diagnosis or chronic forms of cancer where there are relevant target risk factors such as depression, pain, and existential distress. Such preventive interventions could potentially serve to alter or arrest the process by which LoM and DHD could lead to more overt SI and suicidal behaviors. It is also possible that psilocybin-assisted psychotherapy may have efficacy in treating suicidal ideation in life-threatening medical conditions beyond cancer, with associated depression and existential distress.
Putative Mechanisms of the Antisuicidal Effects of Psilocybin Treatment
Our findings are consistent with prior research suggesting central roles for depression and demoralization (i.e., LoM, hopelessness) in mediating DHD and SI in advanced cancer.21,27−31 If psilocybin-assisted psychotherapy is found to produce acute/rapid and long-term sustained reductions in suicidality in cancer patients, then it would be important to explore how this happens, keeping in mind the main drivers of DHD and SI in cancer patients: depression, demoralization, hopelessness, and chronic pain. A conceptual model of causality, supported by evidence in published literature, could be broken down into neurobiological and psycho-spiritual pathways leading to persisting effects and change mechanisms, further narrowing to final common change mechanisms, and ultimately reduction of DHD and SI (see Figure 5). To assess potential causal mechanisms of action, an optimally designed trial would be necessary to interrogate each potential mechanism of action and could include the following elements: parallel design without crossover; specifically recruit participants with clinically relevant suicidality (i.e., passive and active SI, intent, planning, and parasuicidal behaviors) and DHD in cancer patients; DHD and SI as the primary outcome measures; large sample size (e.g., N = 100 or more) to provide sufficient power for mediation analysis of target psychological change mechanisms in accounting for improvement in long-term clinical outcomes; inclusion of measures to assess for the various psychological change mechanisms (i.e., mystical experience, cognitive flexibility, structural personality assessment, challenging experiences, and emotional breakthrough); inclusion of a sufficient sample with cancer pain to examine pain as a causal mechanism; and inclusion of techniques to assess potential neurobiological mechanisms of action (i.e., neuroimaging to assess sustained changes in brain network connectivity, neuro-inflammatory biomarkers such as tumor necrosis factor α (TNF-α), and neuroplasticity biomarkers such as BDNF.
Figure 5.
Proposed theoretical causal model. Therapeutic effects of psilocybin therapy in the treatment of DHD and SI following a cancer diagnosis. 5HT2A: serotonin 2A receptor; CSTC: cortico-striato-thalamo-cortical; DMN: default mode network.
Limitations
The primary limitation of our approach is that the parent trial was not designed to assess the antisuicidal effects of psilocybin in patients with advanced cancer. The trial specifically excluded more serious forms of suicidality and did not specifically target patients with suicidality. Because of this, our analyses were substantially underpowered to detect small-to-moderate effect sizes, but they were sufficiently powered to detect the substantial differences reported here. The crossover design of the parent trial limits our ability to assess long-term clinical benefits relative to a control condition, and the relatively homogeneous sample (e.g., 64% female, 91% Caucasian) diminishes the generalizability of the current findings. While post hoc construction of a composite score for SI has sufficient precedent, inclusion of a dedicated measure to assess SI would clearly benefit interpretability of future trials assessing it. Other statistical approaches may also offer additional clarity to the current results, such as those that take the ordinal structure of the response items for the BDI-II and BSI into account.
In conclusion, in conjunction with psychotherapy, a single moderate-to-high dose of psilocybin was associated with acute and enduring anti-SI and anti-LoM effects in patients with psychiatric and existential distress related to life-threatening cancer. Improvements in other depressive symptoms, as well as in LoM and hopelessness related to demoralization, could represent psychological mechanisms that mediate psilocybin’s effects in reducing SI in patients with cancer. These preliminary results suggest that psilocybin-assisted psychotherapy could be a novel pharmacological–psychosocial treatment modality for advanced-cancer-related suicidality. Further empirical research is needed to definitively establish its efficacy and potential mechanisms of action.
Acknowledgments
The authors acknowledge the contributions of the Center for Psychedelic Medicine Publications Review Committee and Lindsey Owens for their valuable manuscript review.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.1c00020.
Responses to individual suicidal ideation items (PDF)
The parent study was supported by grants from the Heffter Research Institute, the RiverStyx Foundation, and the New York University-Health and Hospitals Corporation (NYU-HHC) Clinical and Translational Science Institute (CTSI) [NYU CTSA grant UL1 TR000038 from the National Center for Advancing Translational Sciences, National Institutes of Health]. Funding for the parent trial was also provided by Carey and Claudia Turnbull, William Linton, Robert Barnhart, Efrem Nulman, Arthur Altschul, Kelly Fitzsimmons, George Goldsmith, and Ekaterina Malievskaia. Funding for G.A.L. was provided by NIH grant T32DA007250.
The authors declare no competing financial interest.
Supplementary Material
References
- Centers for Disease Control and Prevention . (2018) CDC WISQARS: Ten Leading Causes of Death by Age Group, 1981–2018, https://webappa.cdc.gov/sasweb/ncipc/leadcause.html.
- Turecki G.; Brent D. A. (2016) Suicide and suicidal behaviour. Lancet 387 (10024), 1227–1239. 10.1016/S0140-6736(15)00234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocetti E.; Arniani S.; Acciai S.; Barchielli A.; Buiatti E. (1998) High suicide mortality soon after diagnosis among cancer patients in central Italy. Br. J. Cancer 77 (7), 1194–1196. 10.1038/bjc.1998.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J. J. (2002) A current perspective of suicide and attempted suicide. Ann. Intern. Med. 136 (4), 302–311. 10.7326/0003-4819-136-4-200202190-00010. [DOI] [PubMed] [Google Scholar]
- Hem E.; Loge J. H.; Haldorsen T.; Ekeberg Ø. (2004) Suicide risk in cancer patients from 1960 to 1999. J. Clin. Oncol. 22 (20), 4209–4216. 10.1200/JCO.2004.02.052. [DOI] [PubMed] [Google Scholar]
- Misono S.; Weiss N. S.; Fann J. R.; Redman M.; Yueh B. (2008) Incidence of suicide in persons with cancer. J. Clin. Oncol. 26 (29), 4731–4738. 10.1200/JCO.2007.13.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn E.; Shin D. W.; Cho S. I.; Park S.; Won Y. J.; Yun Y. H. (2010) Suicide rates and risk factors among Korean cancer patients, 1993–2005. Cancer Epidemiol., Biomarkers Prev. 19 (8), 2097–2105. 10.1158/1055-9965.EPI-10-0261. [DOI] [PubMed] [Google Scholar]
- Nasseri K.; Mills P. K.; Mirshahidi H. R.; Moulton L. H. (2012) Suicide in cancer patients in California, 1997–2006. Arch. Suicid.e Res. 16 (4), 324–333. 10.1080/13811118.2013.722056. [DOI] [PubMed] [Google Scholar]
- Zaorsky N. G.; Zhang Y.; Tuanquin L.; Bluethmann S. M.; Park H. S.; Chinchilli V. (2019) Suicide among cancer patients. Nat. Commun. 10 (1), 207. 10.1038/s41467-018-08170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri S.; Behnezhad S. (2020) Cancer diagnosis and suicide mortality: a systematic review and meta-analysis. Arch. Suicide. Res. 24 (sup2), S94–S112. 10.1080/13811118.2019.1596182. [DOI] [PubMed] [Google Scholar]
- Chochinov H. M.; Wilson K. G.; Enns M.; Lander S. (1998) Depression, hopelessness, and suicidal ideation in the terminally ill. Psychosomatics 39 (4), 366–370. 10.1016/S0033-3182(98)71325-8. [DOI] [PubMed] [Google Scholar]
- Chochinov H. M. (2001) Depression in cancer patients. Lancet Oncol. 2 (8), 499–505. 10.1016/S1470-2045(01)00456-9. [DOI] [PubMed] [Google Scholar]
- Madeira N.; Albuquerque E.; Santos T.; Mendes A.; Roque M. (2011) Death ideation in cancer patients: contributing factors. J. Psychosoc. Oncol. 29 (6), 636–642. 10.1080/07347332.2011.615381. [DOI] [PubMed] [Google Scholar]
- Shim E. J.; Park J. H. (2012) Suicidality and its associated factors in cancer patients: results of a multi-center study in Korea. Int. J. Psychiatry Med. 43 (4), 381–403. 10.2190/PM.43.4.g. [DOI] [PubMed] [Google Scholar]
- Kim J. M.; Jang J. E.; Stewart R.; Kim S. Y.; Kim S. W.; Kang H. J.; Shin I. S.; Park M. H.; Yoon J. H.; Yoon J. S. (2013) Determinants of suicidal ideation in patients with breast cancer. Psychooncology 22 (12), 2848–2856. 10.1002/pon.3367. [DOI] [PubMed] [Google Scholar]
- Recklitis C. J.; Zhou E. S.; Zwemer E. K.; Hu J. C.; Kantoff P. W. (2014) Suicidal ideation in prostate cancer survivors: understanding the role of physical and psychological health outcomes. Cancer 120 (21), 3393–3400. 10.1002/cncr.28880. [DOI] [PubMed] [Google Scholar]
- Costantini A.; Pompili M.; Innamorati M.; Zezza M. C.; Di Carlo A.; Sher L.; Girardi P. (2014) Psychiatric pathology and suicide risk in patients with cancer. J. Psychosoc. Oncol. 32 (4), 383–395. 10.1080/07347332.2014.917136. [DOI] [PubMed] [Google Scholar]
- Vyssoki B.; Gleiss A.; Rockett I. R.; Hackl M.; Leitner B.; Sonneck G.; Kapusta N. D. (2015) Suicide among 915,303 Austrian cancer patients: who is at risk?. J. Affective Disord. 175, 287–291. 10.1016/j.jad.2015.01.028. [DOI] [PubMed] [Google Scholar]
- Kawashima Y.; Yonemoto N.; Inagaki M.; Inoue K.; Kawanishi C.; Yamada M. (2019) Interventions to prevent suicidal behavior and ideation for patients with cancer: A systematic review. Gen. Hosp. Psychiatry 60, 98–110. 10.1016/j.genhosppsych.2019.07.003. [DOI] [PubMed] [Google Scholar]
- Rosenfeld B.; Breitbart W.; Galietta M.; Kaim M.; Funesti-Esch J.; Pessin H.; Nelson C. J.; Brescia R. (2000) The schedule of attitudes toward hastened death: Measuring desire for death in terminally ill cancer patients. Cancer 88 (12), 2868–2875. . [DOI] [PubMed] [Google Scholar]
- Breitbart W.; Rosenfeld B.; Pessin H.; Kaim M.; Funesti-Esch J.; Galietta M.; Nelson C. J.; Brescia R. (2000) Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA 284 (22), 2907–2911. 10.1001/jama.284.22.2907. [DOI] [PubMed] [Google Scholar]
- Kelly B.; Burnett P.; Pelusi D.; Badger S.; Varghese F.; Robertson M. (2002) Terminally ill cancer patients’ wish to hasten death. Palliat. Med. 16 (4), 339–345. 10.1191/0269216302pm538oa. [DOI] [PubMed] [Google Scholar]
- Kelly B.; Burnett P. C.; Pelusi D.; Badger S.; Varghese F.; Robertson M. M. (2003) Factors associated with the wish to hasten death: a study of patients with terminal illness. Psychol. Med. 33 (1), 75–81. 10.1017/S0033291702006827. [DOI] [PubMed] [Google Scholar]
- McClain C. S.; Rosenfeld B.; Breitbart W. (2003) Effect of spiritual well-being on end-of-life despair in terminally-ill cancer patients. Lancet 361 (9369), 1603–1607. 10.1016/S0140-6736(03)13310-7. [DOI] [PubMed] [Google Scholar]
- Rodin G.; Zimmermann C.; Rydall A.; Jones J.; Shepherd F. A.; Moore M.; Fruh M.; Donner A.; Gagliese L. (2007) The desire for hastened death in patients with metastatic cancer. J. Pain Symptom Manage. 33 (6), 661–675. 10.1016/j.jpainsymman.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Rodin G.; Lo C.; Mikulincer M.; Donner A.; Gagliese L.; Zimmermann C. (2009) Pathways to distress: the multiple determinants of depression, hopelessness, and the desire for hastened death in metastatic cancer patients. Soc. Sci. Med. 68 (3), 562–569. 10.1016/j.socscimed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Akechi T.; Okamura H.; Yamawaki S.; Uchitomi Y. (2001) Why do some cancer patients with depression desire an early death and others do not?. Psychosomatics 42 (2), 141–145. 10.1176/appi.psy.42.2.141. [DOI] [PubMed] [Google Scholar]
- Jones J. M.; Huggins M. A.; Rydall A. C.; Rodin G. M. (2003) Symptomatic distress, hopelessness, and the desire for hastened death in hospitalized cancer patients. J. Psychosom. Res. 55 (5), 411–418. 10.1016/S0022-3999(03)00526-9. [DOI] [PubMed] [Google Scholar]
- Parpa E.; Tsilika E.; Galanos A.; Nikoloudi M.; Mystakidou K. (2019) Depression as mediator and or moderator on the relationship between hopelessness and patients’ desire for hastened death. Support. Care Cancer 27 (11), 4353–4358. 10.1007/s00520-019-04715-2. [DOI] [PubMed] [Google Scholar]
- Robinson S.; Kissane D. W.; Brooker J.; Burney S. (2015) A systematic review of the demoralization syndrome in individuals with progressive disease and cancer: a decade of research. J. Pain Symptom Manage. 49 (3), 595–610. 10.1016/j.jpainsymman.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Prat A.; Balaguer A.; Booth A.; Monforte-Royo C. (2017) Understanding patients’ experiences of the wish to hasten death: an updated and expanded systematic review and meta-ethnography. BMJ. Open 7 (9), e016659 10.1136/bmjopen-2017-016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. J.; Rosenfeld B.; Breitbart W.; Galietta M. (2002) Spirituality, religion, and depression in the terminally ill. Psychosomatics 43 (3), 213–220. 10.1176/appi.psy.43.3.213. [DOI] [PubMed] [Google Scholar]
- Anderson B. T.; Danforth A.; Daroff R.; Stauffer C.; Ekman E.; Agin-Liebes G.; Trope A.; Boden M. T.; Dilley J.; Mitchell J.; Woolley J. (2020) Psilocybin-assisted group therapy for demoralized older long-term AIDS survivor men: An open-label safety and feasibility pilot study. EClinical Medicine 27, 100538–100538. 10.1016/j.eclinm.2020.100538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld B.; Breitbart W.; Stein K.; Funesti-Esch J.; Kaim M.; Krivo S.; Galietta M. (1999) Measuring desire for death among patients with HIV/AIDS: the schedule of attitudes toward hastened death. Am. J. Psychiatry 156 (1), 94–100. 10.1176/ajp.156.1.94. [DOI] [PubMed] [Google Scholar]
- Robinson S.; Kissane D. W.; Brooker J.; Burney S. (2015) A systematic review of the demoralization syndrome in individuals with progressive disease and cancer: a decade of research. J. Pain Symptom Manage. 49 (3), 595–610. 10.1016/j.jpainsymman.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Kissane D. W.; Wein S.; Love A.; Lee X. Q.; Kee P. L.; Clarke D. M. (2004) The Demoralization Scale: a report of its development and preliminary validation. J. Palliat. Care 20 (4), 269–276. 10.1177/082585970402000402. [DOI] [PubMed] [Google Scholar]
- Bogenschutz M. P.; Ross S. (2016) Therapeutic applications of classic hallucinogens. Curr. Top. Behav. Neurosci. 36, 361–391. 10.1007/7854_2016_464. [DOI] [PubMed] [Google Scholar]
- Ross S, Franco S., Reiff C., and Agin-Liebes G. (2021) Psilocybin, in Handbook of Medical Hallucinogens (Grob C. S., and Grigsby J., Eds), Guilford Press. [Google Scholar]
- Ross S. (2018) Therapeutic use of classic psychedelics to treat cancer-related psychiatric distress. Int. Rev. Psychiatry 30 (4), 317–330. 10.1080/09540261.2018.1482261. [DOI] [PubMed] [Google Scholar]
- Reiche S.; Hermle L.; Gutwinski S.; Jungaberle H.; Gasser P.; Majić T. (2018) Serotonergic hallucinogens in the treatment of anxiety and depression in patients suffering from a life-threatening disease: A systematic review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 81, 1–10. 10.1016/j.pnpbp.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Kast E. C.; Collins V. J. (1964) Study of lysergic acid diethylamide as an analgesic agent. Anesth. Analg. (Philadelphia, PA, U. S.) 43, 285–291. [PubMed] [Google Scholar]
- Kast E. (1967) Attenuation of anticipation: a therapeutic use of lysergic acid diethylamide. Psychiatr. Q. 41 (4), 646–657. 10.1007/BF01575629. [DOI] [PubMed] [Google Scholar]
- Pahnke W. N.; Kurland A. A.; Goodman L. E.; Richards W. A. (1969) LSD-assisted psychotherapy with terminal cancer patients. Curr. Psychiatr. Ther. 9, 144–152. [PubMed] [Google Scholar]
- Grof S.; Goodman L. E.; Richards W. A.; Kurland A. A. (2017) LSD-assisted psychotherapy in patients with terminal cancer. Int. Pharmacopsychiatry 8 (3), 129–144. 10.1159/000467984. [DOI] [PubMed] [Google Scholar]
- Grob C. S.; Danforth A. L.; Chopra G. S.; Hagerty M.; McKay C. R.; Halberstadt A. L.; Greer G. R. (2011) Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch. Gen. Psychiatry 68 (1), 71–78. 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- Gasser P.; Holstein D.; Michel Y.; Doblin R.; Yazar-Klosinski B.; Passie T.; Brenneisen R. (2014) Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J. Nerv. Ment. Dis. 202 (7), 513–520. 10.1097/NMD.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S.; Bossis A.; Guss J.; Agin-Liebes G.; Malone T.; Cohen B.; Mennenga S. E.; Belser A.; Kalliontzi K.; Babb J.; Su Z.; et al. (2016) Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J. Psychopharmacol. 30 (12), 1165–1180. 10.1177/0269881116675512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. R.; Johnson M. W.; Carducci M. A.; Umbricht A.; Richards W. A.; Richards B. D.; Cosimano M. P.; Klinedinst M. A. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 30 (12), 1181–1197. 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agin-Liebes G. I.; Malone T.; Yalch M. M.; Mennenga S. E.; Ponté K. L.; Guss J.; Bossis A. P.; Grigsby J.; Fischer S.; Ross S. (2020) Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. J. Psychopharmacol. 34 (2), 155–166. 10.1177/0269881119897615. [DOI] [PubMed] [Google Scholar]
- Hendricks P. S.; Thorne C. B.; Clark C. B.; Coombs D. W.; Johnson M. W. (2015) Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J. Psychopharmacol. 29 (3), 280–288. 10.1177/0269881114565653. [DOI] [PubMed] [Google Scholar]
- Argento E.; Strathdee S. A.; Tupper K.; Braschel M.; Wood E.; Shannon K. (2017) Does psychedelic drug use reduce risk of suicidality? Evidence from a longitudinal community-based cohort of marginalised women in a Canadian setting. BMJ Open 7 (9), e016025 10.1136/bmjopen-2017-016025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R. L.; Bolstridge M.; Day C. M. J.; Rucker J.; Watts R.; Erritzoe D. E.; Kaelen M.; Giribaldi B.; Bloomfield M.; Pilling S.; Rickard J. A.; et al. (2018) Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology 235 (2), 399–408. 10.1007/s00213-017-4771-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeifman R. J.; Singhal N.; dos Santos R. G.; Sanches R. F.; de Lima Osório F.; Hallak J. E.; Weissman C. R. (2020) Rapid and sustained decreases in suicidality following a single dose of ayahuasca among individuals with recurrent major depressive disorder: results from an open-label trial. Psychopharmacology 238, 453–459. 10.1007/s00213-020-05692-9. [DOI] [PubMed] [Google Scholar]
- Zeifman R. J.; Palhano-Fontes F.; Hallak J.; Arcoverde E.; Maia-Oliveira J. P.; Araujo D. B. (2019) The impact of ayahuasca on suicidality: Results from a randomized controlled trial. Front. Pharmacol. 10, 1325–1325. 10.3389/fphar.2019.01325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks P. S.; Johnson M. W.; Griffiths R. R. (2015) Psilocybin, psychological distress, and suicidality. J. Psychopharmacol. 29 (9), 1041–1043. 10.1177/0269881115598338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift T. C.; Belser A. B.; Agin-Liebes G.; Devenot N.; Terrana S.; Friedman H. L.; Guss J.; Bossis A.; Ross S. (2017) Cancer at the Dinner Table: Experiences of Psilocybin-Assisted Psychotherapy for the Treatment of Cancer-Related Distress. J. Humanistic Psychol. 57, 488–519. 10.1177/0022167817715966. [DOI] [Google Scholar]
- Batterham P. J.; Calear A. L.; O’Dea B.; Larsen M. E.; Kavanagh D. J.; Titov N.; March S.; Hickie I.; Teesson M.; Dear B. F.; et al. (2019) Stakeholder perspectives on evidence for digital mental health interventions: Implications for accreditation systems. Digit. Health 5, 2055207619878069. 10.1177/2055207619878069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näslund J.; Hieronymus F.; Lisinski A.; Nilsson S.; Eriksson E. (2018) Effects of selective serotonin reuptake inhibitors on rating-scale-assessed suicidality in adults with depression. Brit. J. Psychiatry 212, 148–154. 10.1192/bjp.2017.24. [DOI] [PubMed] [Google Scholar]
- Hengartner M. P.; Plöderl M. (2019) Newer-Generation Antidepressants and Suicide Risk in Randomized Controlled Trials: A Re-Analysis of the FDA Database. Psychotherapy and Psychosomatics 88, 247–248. 10.1159/000501215. [DOI] [PubMed] [Google Scholar]
- Beck A. T.; Steer R. A.; Carbin M. G. (1988) Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 8 (1), 77–100. 10.1016/0272-7358(88)90050-5. [DOI] [Google Scholar]
- Derogatis L. R. (1993) BSI: Brief Symptom Inventory. Administration, Scoring, And Procedures Manual, National Computer Systems. [Google Scholar]
- Ballard E. D.; Ionescu D. F.; Vande Voort J. L.; Niciu M. J.; Richards E. M.; Luckenbaugh D. A.; Brutsche N. E.; Ameli R.; Furey M. L.; Zarate C. A. Jr. (2014) Improvement in suicidal ideation after ketamine infusion: Relationship to reductions in depression and anxiety. J. Psychiatry Res. 58, 161–166. 10.1016/j.jpsychires.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard E. D.; Yarrington J. S.; Farmer C. A.; Richards E.; Machado-Vieira R.; Kadriu B.; et al. (2018) Characterizing the course of suicidal ideation response to ketamine. J. Affective Disord. 241, 86–93. 10.1016/j.jad.2018.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. B.; Iosifescu D. V.; Murrough J. W.; et al. (2014) Effects of ketamine on explicit and implicit suicidal cognition: A randomized controlled trial in treatment-resistant depression. Depression Anxiety 31 (4), 335–343. 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. B.; Nock M. K.; Charney D. S.; Mathew S. J. (2009) Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry 66 (5), 522–526. 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeifman R. J.; Wagner A. C.; Watts R.; Kettner H.; Mertens L. J.; Carhart-Harris R. L. (2020) Post-psychedelic reductions in experiential avoidance are associated with decreases in depression severity and suicidal ideation. Front. Psychiatry 11, 782. 10.3389/fpsyt.2020.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M. K.; Lin F. C.; Ward S. E.; Fine J. P. (2013) Composite variables: when and how. Nursing Research 62 (1), 45–49. 10.1097/NNR.0b013e3182741948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch E. A.; Roberti J. W.; Roth D. A. (2004) Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory-Second Edition in a sample of college students. Depression Anxiety 19 (3), 187–189. 10.1002/da.20002. [DOI] [PubMed] [Google Scholar]
- Rosenfeld B.; Pessin H.; Lewis C.; Abbey J.; Olden M.; Sachs E.; Amakawa L.; Kolva E.; Brescia R.; Breitbart W. (2011) Assessing hopelessness in terminally ill cancer patients: development of the Hopelessness Assessment in Illness Questionnaire. Psychol. Assess. 23 (2), 325–336. 10.1037/a0021767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredle J. M.; Salsman J. M.; Debb S. M.; Arnold B. J.; Cella D. (2011) Spiritual well-being as a component of health-related quality of life: the functional assessment of chronic illness therapy—spiritual well-being scale (FACIT-Sp). Religions 2 (1), 77–94. 10.3390/rel2010077. [DOI] [Google Scholar]
- Task Force on DSM-IV and other committees and work groups of the American Psychiatric Association . (2000) Diagnostic and Statistical Manual of Mental Disorders, 4th ed., American Psychiatric Association. [Google Scholar]
- Breitbart W.; Rosenfeld B.; Pessin H.; Applebaum A.; Kulikowski J.; Lichtenthal W. G. (2015) Meaning-Centered Group Psychotherapy: An Effective Intervention for Improving Psychological Well-Being in Patients With Advanced Cancer. J. Clin. Oncol. 33, 749. 10.1200/JCO.2014.57.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart W.; Pessin H.; Rosenfeld B.; Applebaum A.; Lichtenthal W. G.; Li Y.; Saracino R. M.; Marziliano A. M.; Masterson M.; Tobias K.; Fenn N. (2018) Individual Meaning-Centered Psychotherapy for the Treatment of Psychological and Existential Distress: A Randomized Controlled Trial in Patients With Advanced Cancer. Cancer 124, 3231–3239. 10.1002/cncr.31539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner C. H.; Fink M.; Knapp R.; Petrides G.; Husain M.; Rummans T.; Mueller M.; Bernstein H.; Rasmussen K.; O’Connor K.; et al. (2005) Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am. J. Psychiatry 162 (5), 977–982. 10.1176/appi.ajp.162.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semkovska M.; McLoughlin D. M. (2010) Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol. Psychiatry 68 (6), 568–577. 10.1016/j.biopsych.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Cipriani A.; Hawton K.; Stockton S.; Geddes J. R. (2013) Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. Bmj 346, f3646 10.1136/bmj.f3646. [DOI] [PubMed] [Google Scholar]
- Ernst C. L.; Goldberg J. F. (2004) Antisuicide properties of psychotropic drugs: a critical review. Harv. Rev. Psychiatry 12 (1), 14–41. 10.1080/10673220490425924. [DOI] [PubMed] [Google Scholar]
- Wilkowska A.; Wiglusz M. S.; Cubała W. J. (2019) Clozapine: promising treatment for suicidality in bipolar disorder. Psychiatry Danub 31, 574–578. [PubMed] [Google Scholar]
- Lally J.; MacCabe J. H. (2015) Antipsychotic medication in schizophrenia: a review. Br. Med. Bull. 114 (1), 169–179. 10.1093/bmb/ldv017. [DOI] [PubMed] [Google Scholar]
- Hill M.; Freudenreich O. (2013) Clozapine: key discussion points for prescribers. Clin. Schizophr. Relat. Psychoses 6 (4), 177–185. 10.3371/CSRP.HIFR.01062013. [DOI] [PubMed] [Google Scholar]
- Vollenweider F. X.; Kometer M. (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat. Rev. Neurosci. 11 (9), 642–651. 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- Ly C.; Greb A. C.; Cameron L. P.; Wong J. M.; Barragan E. V.; Wilson P. C.; Burbach K. F.; Soltanzadeh Zarandi S.; Sood A.; Paddy M. R.; et al. (2018) Psychedelics promote structural and functional neural plasticity. Cell Rep. 23 (11), 3170–3182. 10.1016/j.celrep.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. T.; Ballard E. D.; Bloch M. H.; Mathew S. J.; Murrough J. W.; Feder A.; Sos P.; Wang G.; Zarate C. A. Jr; Sanacora G. (2018) The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am. J. Psychiatry 175 (2), 150–158. 10.1176/appi.ajp.2017.17040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




