Abstract
The CaSR is a class C G protein-coupled receptor (GPCR) that acts as a multimodal chemosensor to maintain diverse homeostatic functions. The CaSR is a clinical therapeutic target in hyperparathyroidism and has emerged as a putative target in several other diseases. These include hyper- and hypocalcaemia caused either by mutations in the CASR gene or in genes that regulate CaSR signaling and expression, and more recently in asthma. The development of CaSR-targeting drugs is complicated by the fact that the CaSR possesses many different binding sites for endogenous and exogenous agonists and allosteric modulators. Binding sites for endogenous and exogenous ligands are located throughout the large CaSR protein and are interconnected in ways that we do not yet fully understand. This review summarizes our current understanding of CaSR physiology, signaling, and structure and how the many different binding sites of the CaSR may be targeted to treat disease.
Keywords: CaSR, hyperparathyroidism, asthma, CASR gene, FHH, ADH, osteoporosis, allosteric modulator
The CaSR is ubiquitously expressed in the human body but is found abundantly in the parathyroid glands and kidney. In these organs, the CaSR is responsible for exquisite control of extracellular Ca2+ (Ca2+o) to maintain systemic ionized Ca2+o concentrations within 1.2–1.4 mM (reviewed in ref (1)). The CaSR negatively regulates parathyroid hormone (PTH) secretion in response to elevated Ca2+o. When Ca2+o concentrations rise, CaSR-mediated suppression of PTH synthesis and secretion decreases Ca2+ resorption from bone and Ca2+ reabsorption in the renal thick ascending limb of the loop of Henle. Within the kidneys, the CaSR responds to elevated Ca2+o independently of PTH to further decrease Ca2+ reabsorption.2 Elevated Ca2+o concentrations are thus reduced. The CaSR is expressed in additional tissues involved in Ca2+o homeostasis. In the thyroid, elevated Ca2+o stimulates calcitonin release via the CaSR,2 leading to Ca2+ uptake into bone. The CaSR also promotes differentiation and proliferation of bone-forming osteoblasts,3 inhibits osteoclast-mediated bone resorption,4 and facilitates chondrocyte-mediated skeletal growth and development.5 In mammary epithelial cells, the CaSR mediates Ca2+ transport into milk6 and suppresses mammary gland release of parathyroid hormone-related protein to reduce osteoclast-mediated release of Ca2+ from bone.7
The pivotal role of the CaSR in Ca2+o homeostasis is well-established; however, the CaSR also responds to additional stimuli to mediate a number of noncalciotropic functions. In taste buds, the CaSR responds to food-derived γ-glutamyl peptides to enhance certain tastes.8 The CaSR is expressed along the entire gastrointestinal (GI) tract, where it senses amino acids to regulate inflammatory responses9 as well as nutrient intake and digestion via the release of satiety and pancreatic hormones.10,11 CaSRs in the pancreas also contribute to glucose-mediated insulin secretion, thus helping to maintain blood glucose levels.12 In the vasculature, CaSR activation on vascular smooth muscle leads to vasodilatation, thus contributing to blood pressure control.13 In the skin, the CaSR promotes keratinocyte differentiation, barrier function, and wound healing.14 CaSRs located in the lungs detect fluctuations in local polyamines to mediate airway defense mechanisms such as airway contraction and inflammation.15 In addition to responding to different stimuli in a tissue specific manner, CaSR expression in both calciotropic and noncalciotropic tissues is controlled in a tissue- and environment-specific manner. CASR gene promoters contain response elements for 1,25-dihydroxyvitamin D, pro-inflammatory cytokines, and the parathyroid cell-specific transcription factor, glial cells missing-2. Consequently, CaSR expression may be increased in inflammation and decreased in states of vitamin D deficiency.16,17 The many diverse CaSR functions and changes in CaSR expression during pathophysiological situations highlight that the CaSR is a multifunctional chemosensor in human (patho)physiology. This review will focus on how its many allosteric ligands bind to the CaSR and how allosteric binding sites have been targeted to manipulate CaSR activity in disease.
CaSR Signaling
The CaSR couples primarily to Gi/o and Gq/11 G proteins to reduce cyclic adenosine monophosphate (cAMP) levels18 and trigger the release of intracellular Ca2+ (Ca2+i) from stores, respectively (Figure 1). The CaSR also increases Ca2+i via influx through L-type voltage-gated and transient receptor potential ion channels on the plasma membrane,19−21 in part via a PKC-dependent mechanism.20
Figure 1.
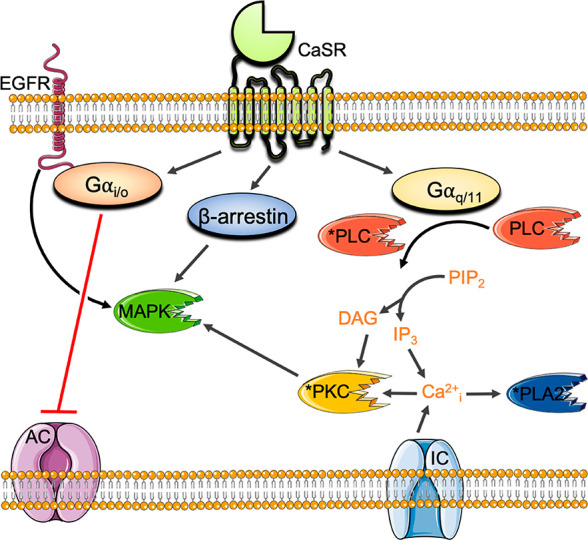
Principal CaSR signaling pathways. CaSR activation of Gi/o inhibits adenylyl cyclase (AC) to reduce cAMP levels. CaSR coupling to Gq/11 activates phosphatidylinositol-specific phospholipase C (PI–PLC) to increase inositol triphosphate (IP3) and diacyl glycerol (DAG) and trigger the release of Ca2+i from stores. Ca2+i activates phospholipase A2 (PLA2) and PKC. The CaSR also increases Ca2+i via influx through L-type voltage-gated and transient receptor potential ion channels (IC), in part via PKC. The CaSR activates MAPK signaling cascades via Gq/11-mediated PKC, Gi/o-mediated activation of epidermal growth factor receptor (EGFR), and β arrestin.
In addition to canonical G protein coupling, the CaSR stimulates mitogen activated protein kinases (MAPK) downstream from Gq/11, Gi/o, and β arrestin.22−25 In some cell types, the CaSR couples to G12/13,26 although the (patho)physiological relevance is unknown. The CaSR also activates Gs proteins in immortalized or malignant breast cells27 and in murine pituitary corticotroph-derived AtT-20 cells,28 resulting in increased cAMP production in these cell types. Thus, the CaSR is promiscuously coupled to several different G protein families, adding even greater pharmacological complexity.
Due to its promiscuous G protein coupling preferences, the CaSR is subject to biased agonism whereby distinct ligands activate or inhibit a subset of possible signaling pathways linked to the CaSR to the relative exclusion of others.25,29,30 While the physiological relevance of biased CaSR agonism is not known, it likely plays an important role in regulating CaSR activation by its many varied endogenous ligands. Small molecule allosteric ligands also engender biased modulation at the CaSR (discussed in more detail below), demonstrating that CaSR function may be fine-tuned with allosteric drugs.
Structure
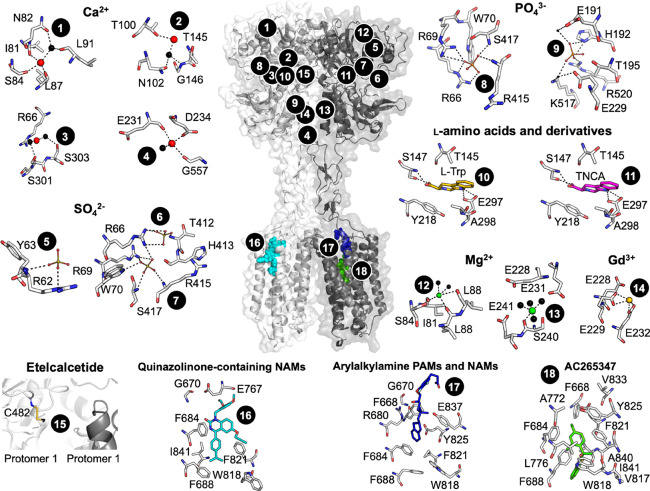
The CaSR protein is encoded by 7 exons and is expressed as a 1078 amino acid polypeptide. Exons 2–6 encode the large extracellular domain (ECD), while exon 7 encodes the 7 transmembrane (7TM)-spanning region and C-terminal tail. The CaSR forms a homodimer mediated by covalent and noncovalent interactions.31,32 The ECD contains a bilobed “venus flytrap” (VFT) domain, so-called because the two lobes (named LB1 and LB2) open and close around a ligand-binding cleft31,33 much like the VFT plant closes around its prey (Figure 2).
Figure 2.
CaSR model and predicted ligand binding sites. The published crystal structure of the CaSR ECD (PDB 5K5S) was superimposed onto a published model of the CaSR 7TM, ECLs, and ICLs71 based on homology with the mGlu5 crystal structure (PDB 6N51). Numbers correspond to ligand binding sites predicted as follows: Ca2+ (sites 1–4) by anomalous scattering analysis and SO42– (5–7), PO43– (8–9), and l-Trp (10) by electron density distribution analysis of the crystallized ECD (PDBs 5K5S and 5K5T);31 TNCA (11), Mg2+ (12–13), and Gd3+ (13) by electron density distribution analysis of the crystallized VFT (PDBs 5FBK and 5FBH);33 etelcalcetide (15) from mutagenesis and mass spectrometry, where the yellow stick represents a putative disulfide bond as a rough depiction of where etelcalcetide is predicted to bind;66 quinazolinone-containing NAMs (16), arylalkylamine PAMs and NAMs (17), and AC265347 (18) from mutagenesis combined with homology modeling and computational docking.41,71
The VFT is linked via a cysteine-rich domain (CRD) to the 7TM and its connecting intracellular and extracellular loops (ICL and ECL, respectively). The structural integrity of the 7TM is in part maintained by a disulfide bond between C677 in ECL1 and C765 in ECL2.34 The 7TM is proceeded by a large C-terminal tail that contributes to cell surface expression, signaling, and binding to accessory proteins.35−37 Further, C-terminal tail residues (S875 and T888) are predicted to be key protein kinase C (PKC) phosphorylation sites, which serve to negatively regulate CaSR activity.38,39
Endogenous CaSR Ligands and Their Binding Sites
The primary physiological ligand of the CaSR is Ca2+. X-ray crystallography combined with anomalous scattering analysis suggest Ca2+ binds to four sites within the VFT domain (Figure 2)31 in a cooperative manner, such that binding to one site positively modulates Ca2+ binding to the other sites.40 A loss in Ca2+o agonism was observed upon mutation of residues located in the four Ca2+ sites, although the effects of mutations on receptor expression, Ca2+o affinity, and Ca2+o efficacy were not delineated. Consequently, it is unclear to what extent these mutations alter Ca2+o binding to the CaSR and therefore whether these sites are physiologically relevant or simply an artifact of the crystallization conditions. Ca2+ also binds to at least one site in the 7TM or ECLs, evidenced by the fact that Ca2+o retains agonist activity at a CaSR lacking its entire ECD.41 Thus, while Ca2+o is considered the orthosteric agonist, strictly speaking Ca2+o is an allosteric modulator of itself. In addition to Ca2+o, the CaSR responds to additional divalent cations, including Mg2+, as well as trivalent cations such as Gd3+.42,43 Structural studies suggest a Mg2+ binding site that overlaps with a Ca2+ binding site in the VFT, as well as other distinct binding sites for Mg2+ and Gd3+33 (Figure 2). However, anomalous scattering analysis was not used to assign Mg2+ or Gd3+ in the CaSR VFT crystal structure and can therefore be made with less confidence. Importantly, all six cation binding sites are topographically distinct from the VFT cleft between LB1 and LB2, which is the orthosteric agonist binding site in all other class C GPCRs.
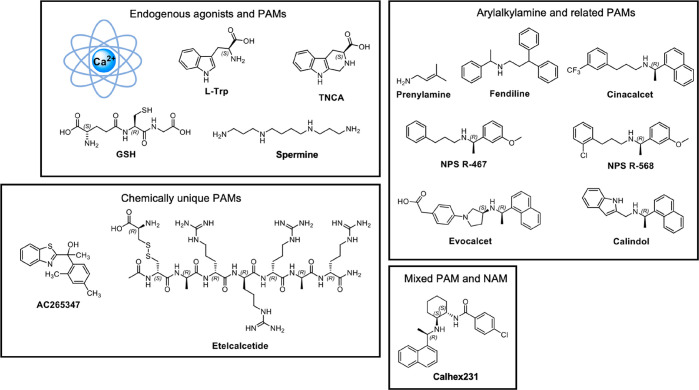
The CaSR VFT cleft is the binding site for endogenous positive allosteric modulators (PAMs), which include aromatic L-amino acids44 and γ-glutamyl peptides.45 Larger aliphatic and aromatic L-amino acids, in particular l-Phe and l-Trp, are the most potent PAMs at the CaSR.44 CaSR VFT domain structures solved by X-ray crystallography suggest the fully active (closed) VFT conformation only exists when the cleft is occupied by an l-amino acid or similar entity.31,33 The CaSR also responds to other positively charged endogenous ligands that interact with allosteric sites. These include polyamines such as spermine, spermidine, and putrescine.46 Polyamines activate the CaSR in the absence of Ca2+o and are therefore agonists, but they may also act as PAMs.46 The binding site for polyamines has not been elucidated, but it is located somewhere in the 7TM, ECLs, or ICLs.47
In addition to numerous endogenous activators, anions such as phosphate and sulfate, protons, or elevated osmolarity all serve to inhibit CaSR activity.31,48−50 While anions bind in the CaSR VFT,31 the site of action of protons is unknown.49 These findings suggest potential clinical implications for changes in the CaSR’s environment, which may occur in pathophysiological states such as alkalosis, elevated serum phosphate observed in chronic kidney disease, or increased osmolarity as occurs during dehydration. Altered levels of endogenous CaSR activators or inhibitors in different physiological or disease states may also impact discovery and validation of CaSR small molecule allosteric ligands.
Exogenous Allosteric Ligands and Their Binding Sites
Small Molecule PAMs
Given that the endogenous ligands of the CaSR bind to a number of distinct sites in a cooperative manner, it is unsurprising that the CaSR possesses several distinct allosteric sites for exogenous molecules. The first CaSR-targeting small molecules discovered were the calcium channel blockers fendiline and prenylamine (Figure 3), which gave rise to the arylalkylamine CaSR PAMs, NPS R-467, NPS R-568, and cinacalcet (Figure 3, reviewed in ref (51)). Several structurally related CaSR PAMs have since been identified, including evocalcet, calindol, and variants thereof (Figure 3). Arylalkylamine PAMs potentiate CaSR-mediated Ca2+i mobilization in recombinant cells or suppress PTH secretion from parathyroid cells in culture in the presence of physiological Ca2+o concentrations with reduced potency or affinity in the absence of Ca2+o.52−54 However, high arylalkylamine concentrations (>1 μM) activate the CaSR in the absence of cations,51 indicating arylalkylamines are most accurately referred to as PAM agonists. The fact that the CaSR functions in the absence of Ca2+o raises the question of whether endogenous agonists can activate the CaSR by binding to the small molecule allosteric binding site.
Figure 3.
CaSR agonists and PAMs and their structures.
In the absence of a CaSR structure with a bound allosteric modulator, mutagenesis studies combined with computational docking have predicted the binding site of arylalkylamine PAMs.41,53,55−58 Due to a lack of radiolabeled ligands, early drug discovery campaigns did not determine the affinity of CaSR PAMs; therefore, initial binding site predictions were based on the effect of mutations on PAM potency for potentiation of a single Ca2+o concentration.55−58 However, potency changes do not discern individual effects of mutations on affinity, cooperativity or efficacy. More recent studies have quantified the effect of amino acid substitutions using an operational model of allosterism59,60 (Figure 4). Key amino acid residues that contribute to the affinity of arylalkylamine PAMs are located in TMs 2, 3, 5, 6, and 7 as well as ECLs 2 and 3, where numbering in superscript throughout this manuscript denotes residue positions relative to the most highly conserved residue in each TM domain across the class C GPCRs;61 F6682.56, F6843.36, F6883.40, A7725.39, W8186.50, F8216.53, Y8256.57, E8377.32, A8407.35, I8417.36, E767ECL2, and V833ECL3.41,53 Mapping these residues onto a homology model of the CaSR based on the metabotropic glutamate receptor subtypes 1 and 5 (mGlu1 and mGlu5) crystal structures revealed a large cavity that spans from the top to the middle of the 7TMs (Figure 2).41 The arylalkylamine PAM secondary amine is protonated at physiological pH to form an ammonium salt that facilitates PAM binding by hydrogen bonding with E8377.32 in addition to forming a strong electrostatic interaction.41,53,55−58 The predicted 7TM binding pocket of CaSR arylalkylamine PAMs is commensurate with that observed for the binding site of a NAM cocrystallized with mGlu162 and common across the class C GPCR family.
Figure 4.
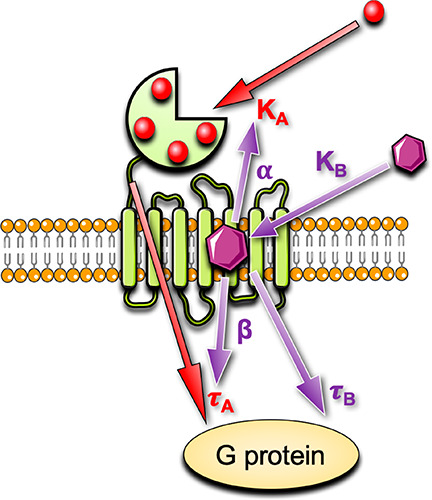
An operational model of allosterism to quantify CaSR agonist, PAM, and NAM actions. Endogenous CaSR agonists such as Ca2+o bind with a mean equilibrium dissociation constant, KA, to multiple sites. When the receptor is occupied by an agonist, the agonist stimulates a response, depicted by an operational measure of efficacy, τA. Allosteric modulators may alter the equilibrium dissociation constant of the agonist via a cooperativity factor, α, or alter the efficacy of the orthosteric agonist via a scaling factor, β. Allosteric modulators may also have their own efficacy, τB.
In 2010, Acadia Pharmaceuticals discovered benzothiazole-containing CaSR PAMs that were structurally and chemically distinct from the arylalkylamine PAMs, leading to the identification of AC265347 (Figure 3).63,64 Like the arylalkylamines, AC265347 demonstrates agonist activity in the absence of cations, but it is a more potent agonist when compared to arylalkylamine PAMs.54 AC265347 is also a biased CaSR allosteric modulator that preferentially enhances CaSR-mediated phosphorylation of ERK1/2 (pERK1/2) versus Ca2+i mobilization.54 In contrast, phenylalkylamine PAMs show the reverse biased modulatory profile.54 While AC265347 is predicted to bind within the 7TM cavity, it is unaffected by many of the mutations that reduce arylalkylamine affinity.41,53 Importantly, AC265347 lacks an ionizable nitrogen and is therefore not predicted to form an ionic interaction with E8377.32. Computational docking studies supported by mutagenesis suggest that AC265347 sits deeper in the 7TM bundle in comparison to the arylalkylamine PAMs41 (Figure 2). By binding deeper within the 7TM bundle, AC265347 may stabilize distinct receptor states relative to arylalkylamine PAMs, engendering biased CaSR signaling.
The Peptide PAM, Etelcalcetide
In addition to small molecule PAMs, etelcalcetide was identified as a unique CaSR PAM, being an octapeptide comprising a linear chain of seven d-amino acids linked to a l-cysteine via a disulfide bond.65 Both the C- and N-terminus are capped and the d-amino acid backbone is attached to four d-Arg residues. Etelcalcetide is predicted to bind to the CaSR VFT by forming a disulfide bond with C482 located near a “hinge” region in VFT LB1 that mediates VFT closure.66 In the absence of Ca2+o, etelcalcetide activity in HEK293 cells is significantly decreased.65 Nonetheless, etelcalcetide retains agonist activity in the absence of Ca2+o; therefore, it is a PAM agonist.65 Given that etelcalcetide interacts with a unique site relative to the small molecule PAMs, etelcalcetide is likely to stabilize a distinct receptor conformation and therefore has the potential to engender biased CaSR agonism or modulation, although this remains to be determined.
CaSR NAMs
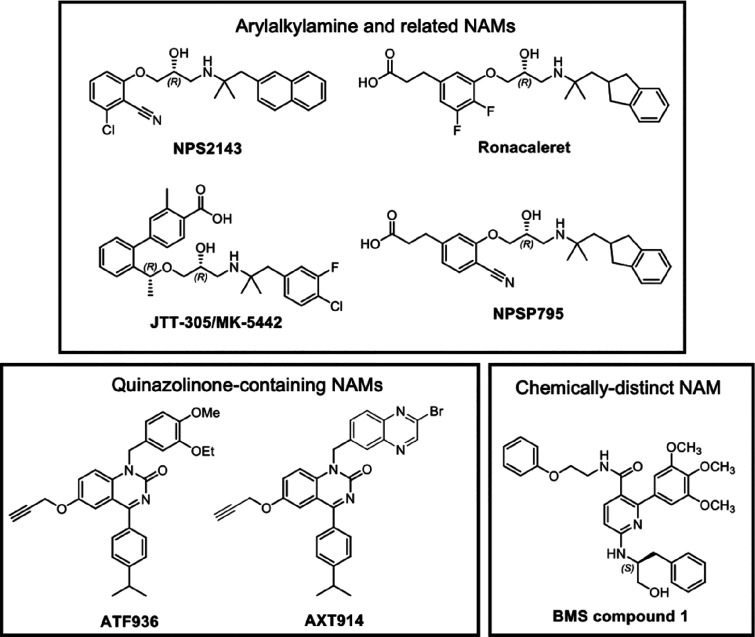
A high throughput screen and subsequent medicinal chemistry effort at NPS Pharmaceuticals and SmithKline Beecham led to the discovery of the first CaSR NAM, NPS2143 (Figure 5), which has an arylalkylamine scaffold.67 Subsequent efforts to progress arylalkylamine NAMs clinically led to the development of several NAMs with structural and chemical similarity to NPS2143, including ronacaleret,67,68 JTT305 (otherwise known as MK3552),69 and NPSP79570 (Figure 5). Arylalkylamine NAMs are predicted to bind within the same 7TM cavity as arylalkylamine PAMs, with the NAM secondary amine predicted to interact with E8377.32 in a manner akin to the arylalkylamine PAMs.41,71 In fact, the predicted binding pose for the arylalkylamine NAMs is very similar to the predicted pose for arylalkylamine PAMs, making it difficult to discern from computational modeling how these structurally similar PAMs and NAMs could have opposing effects on CaSR signaling. Future structural elucidation of the CaSR 7TM bound to PAMs and NAMs is needed to fully appreciate how these small molecules differentially alter CaSR structure and function.
Figure 5.
CaSR NAMs and their structures.
In addition to the arylalkylamine NAMs, a screening program at Novartis identified quinazolinone-containing compounds as CaSR NAMs, which were advanced to yield ATF936 and AXT914 (Figure 5).72,73 ATF936 has greater negative cooperativity in comparison to NPS2143, meaning that it is better at blocking CaSR activity.71 Mutagenesis and docking studies predict the quinazolinone-containing NAMs bind in the 7TM allosteric cavity but in a distinct manner to the arylalkylamine PAMs and NAMs.71 For instance, some mutations that reduce NPS2143 affinity have no effect on the affinity of ATF936 affinity.71 A more detailed structural understanding of the binding of quinazolinone-containing NAMs may afford the opportunity to design NAMs with even greater affinity or cooperativity.
Intriguingly, a structurally and chemically distinct CaSR NAM, known as BMS compound 1, is predicted to bind to a second as yet unidentified allosteric site in the 7TM, ECLs, or ICLs of the CaSR.71 Multiple allosteric binding sites within the 7TM of class C GPCRs is not unprecedented. For mGlu5, several PAM chemotypes are thought to bind outside the common allosteric pocket.74,75 Structural resolution of the BMS compound 1 binding site could provide opportunities to identify novel allosteric modulators that target this site and that may possess biased modulatory properties.
Calhex231: A Mode-Switching Allosteric Modulator
Calhex231 is structurally and chemically related to cinacalcet and the other arylalkylamine PAMs, and indeed it was discovered from an SAR study based on the PAM calindol.76 Surprisingly, calhex231 was reported to be a NAM because it inhibited a maximally effective concentration of Ca2+o in an IP accumulation assay.76 Recent work, however, has revealed that calhex231 is both a PAM and a NAM depending on whether it occupies a single protomer in the CaSR dimer or both protomers.77 The binding of calhex231 to one protomer inhibits the binding of calhex231 to the second protomer. Using an allosteric quaternary complex model, it was shown that calhex231 switches to a NAM because its negative cooperativity with itself is greater in the presence of an agonist.77 The calhex231 binding site overlaps with the binding site for cinacalcet, NPS2143 and other arylalkylamine PAMs and NAMs.77 The ability of calhex231, but not other PAMs and NAMs, to mode-switch is predicted to be due to a disubstituted cyclohexane ring in calhex231, which may offer more flexibility when bound to the CaSR and thus allow calhex231 to adopt at least two distinct binding poses.77
Clinical Utility of CaSR Allosteric Modulators
CaSR PAMs for Hyperparathyroidism
Given the pivotal role of the CaSR in negatively regulating PTH secretion, three CaSR PAMs are currently on the market to treat hyperparathyroidism. Hyperparathyroidism is typically caused by parathyroid adenoma or carcinoma, resulting in primary hyperparathyroidism (PHPT). Alternatively, it is secondary to chronic kidney disease, where impaired phosphate excretion and renal 1,25-dihydroxyvitamin D3 synthesis leads to decreased Ca2+o and a consequent increase in PTH synthesis and secretion as well as parathyroid hyperplasia. Cinacalcet (Sensipar) was the first CaSR-targeting drug to gain FDA approval in 2004 for hemodialysis patients with secondary hyperparathyroidism (SHPT) caused by chronic kidney disease. Cinacalcet was also the first FDA-approved GPCR allosteric modulator to reach the market. Cinacalcet has since been approved to treat hypercalcemia in adults with parathyroid carcinoma or who cannot undergo parathyroidectomy.
Cinacalcet is generally safe and well tolerated, although GI adverse events including nausea, vomiting, or loss of appetite occur in approximately 30% of patients.78 Cinacalcet can also cause transient episodes of hypocalcaemia in some patients. Further, there is some variability in the degree to which patients respond to cinacalcet.79 While cinacalcet responsiveness can depend on the severity of SHPT, CaSR single nucleotide polymorphisms (SNP) may influence cinacalcet efficacy. For instance, SHPT patients with an R990G SNP demonstrate higher sensitivity to cinacalcet, with a larger proportion of G990 carriers experiencing a cinacalcet-mediated suppression in PTH compared to patients with the predominant R990 allele.80 These findings suggest that a personalized medicines approach may need to be considered when treating patients with CaSR-targeting therapies.
In 2017, etelcalcetide (Parsabiv) was approved by the FDA as an intravenous CaSR PAM for the treatment of SHPT in adults. The intravenous administration of etelcalcetide is advantageous because it can be delivered at the end of a hemodialysis session, thus ensuring patient compliance. While intravenous etelcalcetide was expected to induce fewer GI adverse events compared to oral cinacalcet, self-reported symptoms of nausea and vomiting were not significantly different between SHPT patients given etelcalcetide or cinacalcet.81 Nonetheless, a one-year safety and efficacy trial of intravenous etelcalcetide administration revealed no major safety concerns,82 although, like cinacalcet, etelcalcetide can cause hypocalcaemia.83
Recently, evocalcet (alternative names MT-4580 and KHK7580) was approved for the management of SHPT in Japanese patients that remain refractory to cinacalcet treatment because adverse GI events prevent cinacalcet dose escalation. Evocalcet has higher bioavailability in comparison to cinacalcet, and lower doses are therefore required to suppress serum PTH levels.84 In rats, evocalcet suppresses PTH secretion while having no significant effect on gastric emptying, which is delayed in cinacalcet-treated rats and patients.84 Evocalcet also had a reduced incidence of vomiting in marmosets.84,85 In humans, evocalcet offers good short-term tolerability in terms of upper GI symptoms while still providing therapeutic efficacy similarly to cinacalcet.86 However, while the severity of GI side effects is reduced compared to cinacalcet,83 approximately 19% of evocalcet-treated patients still experience nausea and vomiting compared to 33% of patients treated with cinacalcet.78 Evocalcet also causes hypocalcaemia in some patients.83 Thus, while all three clinically approved CaSR PAMs are effective at reducing PTH levels in hyperparathyroidism, the risk of hypocalcaemia and incidence of GI side effects limits their use in the clinic.83 There is still therefore a need for novel PAMs with reduced adverse effects.
PAMs for Hypercalcaemia
The importance of the CaSR in Ca2+o homeostasis is highlighted by the many naturally occurring mutations in the CASR gene or in genes encoding Gα11 (GNA11), which mediates CaSR signal transduction, or adapter protein 2 sigma subunit 1 (AP2S1), which regulates CaSR cell surface expression. Inactivating mutations in these proteins cause familial hypocalciuric hypercalcaemia types 1 to 3 (FHH1–3) or neonatal severe primary hyperparathyroidism (NSHPT).
FHH1 (the most common form of FHH at 1 per 1350 people87) and NSHPT are caused by inactivating CASR mutations. These mutations reduce CaSR sensitivity to Ca2+o or impair the biosynthesis and post-translational processing of the CaSR within the endoplasmic reticulum or Golgi apparatus, leading to CaSR misfolding and impaired cell surface expression.88,89 Furthermore, some mutations alter CaSR coupling to signaling pathways to the relative exclusion of others.90 FHH1 is characterized by mild or moderate elevations of serum calcium and magnesium with mildly elevated or normal PTH levels. While FHH1 patients are often asymptomatic, up to 30% of patients experience symptomatic hypercalcaemia, whereas others develop chondrocalcinosis, acute pancreatitis, and gallstones.91 Importantly, FHH1-causing mutations increase the risk of numerous diseases, most notably cardiovascular, neurodegenerative, and psychiatric diseases.87 These findings suggest that it may be appropriate to treat FHH1 even in asymptomatic patients. Further, the much rarer but more severe disorder, NSHPT, is characterized by life-threatening hypercalcaemia, skeletal under-mineralization and deformities, and death if left untreated.91 It is therefore essential that infants diagnosed with NSHPT are treated. Increasingly, cinacalcet has shown some success in treating NHSPT in addition to complications related to FHH1 in patients harboring loss-of-function or loss-of-expression CaSR mutations.92−98 There are, however, increasing reports of NSHPT patients who do not respond adequately to cinacalcet, in some cases due to homozygous mutations that result in truncation of the CaSR before the 7TM cinacalcet binding site.99,100 Cinacalcet-unresponsive patients may also harbor missense mutations or in-frame deletions that result in expression of a full-length CaSR with single amino acid mutations or a shortened CaSR in which exon 5, encoding amino acids 476–536 in the ECD, is deleted.101−103 In these instances, cinacalcet may be ineffective because the mutation may reduce cinacalcet affinity or its ability to potentiate Ca2+o by decreasing allosteric cooperativity. In cases of severe mutation-induced receptor impairment, the mutation may render cinacalcet unable to sufficiently restore receptor function even if affinity or cooperativity are unaffected.104 Interestingly, compared to cinacalcet, AC265347 was more effective at potentiating Ca2+o-mediated signaling responses at some FHH1/NSHPT-causing CaSR mutants, suggesting that alternative PAMs may be better than cinacalcet at rescuing inactivating CaSR mutants.64 However, as AC265347 is not approved clinically, total parathyroidectomy is currently required to normalize serum PTH levels in patients who do not respond to current pharmacological interventions.
Four GNA11 mutations have been identified in FHH2-affected individuals (FHH2 is the least common form of FHH), which are predicted to impair guanine nucleotide binding or disrupt G protein activation of intracellular signaling proteins such as PLC.91,105 FHH2 patients typically have mild hypercalcaemia and normal serum concentrations of PTH.105 In recombinant cells expressing FHH2-causing GNA11 mutations, cinacalcet restored impaired CaSR signaling.106 Similarly, in mice with a germline loss-of-function GNA11 mutation, cinacalcet corrected hypercalcaemia and reduced elevated serum PTH concentrations.107 Cinacalcet also normalized serum calcium concentrations in a FHH2 patient with hypercalcaemia.108 These studies suggest that cinacalcet stabilizes a CaSR conformation that couples more favorably to G11, thus overcoming mutations that impair G11 function. However, given the typically asymptomatic nature of FHH2, there is no clear benefit in treating most FHH2 patients with pharmacological interventions.
Four missense AP2S1 mutations that cause FHH3 have been identified.109−111 FHH3-causing mutations disrupt AP2σ-mediated CaSR endocytosis and consequently impair CaSR signaling from endosomes.105,112 FHH3 is the most severe form of FHH and is more commonly characterized by symptomatic hypercalcaemia.109,113 FHH3 may also be associated with recurrent pancreatitis and cognitive dysfunction.114 Cinacalcet corrected impaired CaSR signaling resulting from FHH3-causing AP2S1 mutations and rectified symptomatic hypercalcaemia in three FHH3 patients.115 The molecular mechanisms by which cinacalcet corrects mutation-induced impairments in AP2σ-mediated CaSR internalization are not known, but there are several possibilities. Cinacalcet may simply stabilize a CaSR conformation that interacts more favorably with AP2σ, thus restoring CaSR internalization and trafficking to endosomes. Alternatively, by crossing the cell membrane, cinacalcet may potentiate the activity of CaSRs already localized to endosomes. Regardless, these findings demonstrate that CaSR PAMs such as cinacalcet may be useful in the management of FHH3.
NAMs for Osteoporosis
The first manifestation of osteoporosis is typically a fracture.116 Therefore, treatments are aimed at preventing further bone loss, such as with the use of bisphosphonates (e.g., alendronate), or restoring bone mass and density with recombinant human PTH(1–34) (rhPTH(1–34) or rhPTH(1–84)), which have anabolic actions by increasing the number of bone-forming osteoblasts.117 However, rhPTH(1–34) has received a black box warning label in the United States because high doses induced osteosarcoma in long-term carcinogenicity studies in rats.118 Further, rhPTH requires daily subcutaneous administration, and an orally active anabolic compound therefore continues to be of interest. Small molecule CaSR NAMs were consequently developed as potential orally available therapeutics for osteoporosis because they stimulate the release of endogenous PTH by mimicking a drop in Ca2+o levels.
NPS2143 was the first CaSR NAM to be evaluated in an ovariectomized rat model of postmenopausal osteoporosis. However, following 5 weeks of daily NPS2143 administration, no net increase in bone mass and density was observed.67,119,120 The high volume of NPS2143 distribution resulted in prolonged NPS2143 exposure and sustained elevations in PTH levels, in contrast to plasma levels of rhPTH(1–34), which reached a comparable maximum concentration but returned to baseline much more rapidly. It was soon realized that CaSR NAMs would best exert an anabolic effect if they had a short half-life to ensure transient stimulation of PTH release that promptly returned to basal levels. This is because prolonged exposure to PTH mimics hyperparathyroidism, thus stimulating bone resorption at the expense of bone formation.121
Ronacaleret was the second CaSR NAM to be evaluated in osteoporosis. While ronacaleret is structurally similar to NPS2143, it is more metabolically labile.122,123 However, a clinical trial in postmenopausal women given ronacaleret for 12 months demonstrated only a modest increase in bone mass and density of the lumbar spine compared to large increases seen in patients receiving rhPTH(1–34) or alendronate, while hip, femoral neck, and trochanter bone mass and density was decreased in the ronacaleret-treated group.124 Similarly, in a phase 2 clinical trial in postmenopausal women treated with JTT305 for 6 months, no significant increase in bone mass and density was observed over placebo, despite evidence of an increase in markers of bone formation.125 A clinical trial of AXT914 was also terminated early due to a lack of effect of AXT914 on bone formation markers and a dose-limiting increase in serum calcium after four weeks of treatment.126
The reasons why CaSR NAMs do not stimulate bone formation are not fully understood but may be linked to on-target CaSR effects in cells and tissues outside the parathyroid gland. For instance, CaSR NAMs may inhibit the important function of the CaSR in bone-forming osteoblasts, thus counteracting the effects of transient PTH release. Further, while the pharmacokinetic profiles of ronacaleret, JTT305, and AXT914 were more favorable than NPS2143, NAM-mediated elevations in serum PTH concentrations remained above baseline in humans for more than 3.5 h,124−126 whereas levels of rhPTH return to baseline rapidly following rhPTH injection.127 Prolonged PTH release was more apparent at higher NAM doses that were cleared less rapidly. The design of NAMs that can be administered at lower doses, such as those with greater affinity or cooperativity, could help to overcome this issue. Regardless of the reasons for CaSR NAM failures in the clinic, the development of NAMs for osteoporosis has been discontinued, and efforts have instead focused on repurposing CaSR NAMs for alternative disorders.
NAMs for ADH and Bartter Syndrome V
Recent interest has been garnered in repurposing CaSR NAMs for heterozygous activating mutations in the CASR or GNA11 genes, which cause autosomal dominant hypocalcaemia type 1 (ADH1; caused by CASR mutations), Bartter syndrome V (CASR mutations), or ADH2 (GNA11 mutations). ADH is characterized by a mild or moderate decrease in serum calcium and PTH concentrations.91 Many ADH sufferers experience symptomatic hypocalcaemia, which may include tingling and painful muscular spasms in the hands and feet as well as seizures. Some ADH1 patients also suffer from calcifications in the kidneys and basal ganglia or elevated bone mineral density.91 In more severe cases, gain-of-function CASR mutations promote renal loss of sodium, potassium, magnesium, and chloride ions and consequent hypokalaemic alkalosis and hyperreninaemic hyperaldosteronism, a condition called Bartter syndrome V.91 The prevalence of ADH1 is approximately 1 per 25 000.87 Currently, over 90 different CaSR mutations have been linked to ADH1. Among them, over 95% are missense mutations, with the remaining 5% represented by frameshift or in-frame insertion and deletion mutations.128 ADH2 is rarer and has been associated with six different activating missense GNA11 mutations. ADH2-causing mutations are located at the interface between the helical and GTPase domains of the Gα11 protein and involved in GDP-GTP exchange or located at the Gα11 carboxyl terminal, which is involved in receptor coupling.129,130 CaSR NAMs are a viable therapeutic strategy for reducing hyper-function caused by gain-of-function mutations in both the CaSR and Gα11.
Promisingly, NPS2143 can normalize signaling responses associated with ADH-causing CASR and GNA11 mutations in vitro(104,106,131,132) as well as increase Ca2+o and PTH concentrations in ADH1 and ADH2 mouse models131,133,134 and prevent nephrocalcinosis in an ADH1 mouse model.135 However, NPS2143 is less effective, at least in vitro, at rectifying gain-of-function CASR mutations that cause Bartter syndrome V.104,132 In contrast, quinazolinone-derived NAMs (e.g., AXT914 and ATF936) can better rectify Bartter syndrome V mutations in vitro(136) and may represent a class of NAMs with lower propensity to be affected by pharmacogenetic effects compared to arylalkylamine-derived NAMs like NPS2143.
Although originally developed for osteoporosis, the arylalkylamine-derived NAM, NPSP795, entered phase II clinical trials for the treatment of ADH1. NPSP795 robustly increased PTH in 3 out of 5 ADH1 trial patients and caused a small reduction in renal Ca2+ excretion. However, NPSP795 had no significant effect on serum Ca2+o levels and had variable effects on PTH.137 The high variability in NPSP795 efficacy may in part be attributable to the underlying disease-causing mutations. Some ADH1 mutations are in close proximity to the common 7TM allosteric binding site, or NPSP795 may not have sufficient affinity or cooperativity to overcome some mutation-induced enhancement in CaSR signaling.41,104 However, pharmacogenetic effects do not completely explain interpatient variation in the efficacy of NPSP795. For instance, two patients in the study carried the same mutation (A840 V). A840 V faces into the 7TM binding cavity and contributes to the binding of the arylalkylamine NAM, NPS2143.41 However, while serum PTH levels were robustly increased in one A840 V-harboring patient, NPSP795 had only a modest effect on PTH levels in the other, despite similar NPSP795 concentrations being reached in both patients. It therefore remains to be determined why some ADH1 patients may respond to CaSR NAMs, while others do not.
NAMs for Asthma
Asthma affects ∼340 million people worldwide, posing significant health risks particularly to approximately 10% of asthmatics whose asthma is poorly controlled with current drugs. The efficacy of asthma medications, which include β2 adrenergic receptor agonists and corticosteroids, is further limited by acute exacerbations typically caused by respiratory virus infections or environmental pollutants.138 Identifying novel treatments for poorly controlled asthma is therefore a key health priority.
Recently, the CaSR was identified as a putative therapeutic target in asthma. The CaSR is expressed in bronchial smooth muscle and the epithelium.15 CaSR expression is upregulated in human bronchial biopsies from asthmatics, in murine asthma models, and in human airway smooth muscle cells exposed to asthma-associated cytokines,15 presumably via STAT and κB response elements in the CASR gene promoters. Further, CaSR agonists such as polyamines are established mediators of airway inflammation, remodelling, and constriction;139−146 polyamine concentrations are elevated in the sputum or blood of human asthmatics and in murine models of allergic airways disease.141,143,147 While spermine potentiated airway smooth muscle contraction induced by acetylcholine, this effect was diminished in mice with selective CaSR ablation in airway smooth muscle cells.15 These findings were consistent with observations that the CaSR NAM, NPS2143, attenuated Ca2+i release in human airway smooth muscle cells in response to acetylcholine or histamine,15 suggesting potential benefit in opposing CaSR signaling in asthma.
In murine models of allergic airways disease, chronic treatment with CaSR NAMs attenuated airway inflammation, fibrosis, and airway hyper-responsiveness (AHR) to the muscarinic acetylcholine receptor agonist, methacholine.15,148,149 The CaSR NAM, NPS2143, also decreased immune cell counts in mouse bronchoalveolar lavage fluid (BALF) following allergen challenge15 or lipopolysaccharide (LPS)-induced lung injury,148 and suppressed serum and BALF cytokine levels.148 The latter effects of in vivo treatment with NPS2143 are consistent with the established role of the CaSR in promoting pro-inflammatory cytokine release from T cells,150 macrophages,151,152 and airway epithelial cells in vitro.148 More recent findings demonstrated that the NAMs ronacaleret, JTT-305, NPSP795, and AXT914 all reduced airway inflammation and prevented goblet cell hyperplasia in a chronic airway inflammation model.153 Taken together, the potential benefits of CaSR NAMs in directly reducing aberrant airway smooth muscle Ca2+i signaling and contraction as well as attenuating airway inflammation, remodelling, and inhibiting AHR in chronic disease models suggest the CaSR may be a novel therapeutic target in asthma.
Conclusions
The CaSR is a multimodal chemosensor that responds to diverse exogenous and endogenous stimuli via multiple allosteric binding sites. While great efforts have been made to therapeutically target the CaSR, to date, only CaSR PAMs have reached the clinic. Despite CaSR NAMs demonstrating promise as treatments for ADH, the reasons for the potential interpatient variability in responsiveness to NAMs need to be established to progress development of such compounds. Thus, much can still be learned about how CaSR NAMs bind to the receptor and how naturally occurring mutations alter the binding and function of the NAMs. A better identification of the CaSR’s many allosteric binding sites may enable drug discovery efforts that target novel CaSR binding sites with potential to identify distinct chemotypes of allosteric modulators with unique pharmacological properties.
The authors declare no competing financial interest.
References
- Leach K.; Hannan F. M.; Josephs T. M.; Keller A. N.; Møller T. C.; Ward D. T.; Kallay E.; Mason R. S.; Thakker R. V.; Riccardi D.; Conigrave A. D.; Bräuner-Osborne H. (2020) International Union of Basic and Clinical Pharmacology. CVIII. Calcium-sensing receptor nomenclature, pharmacology, and function. Pharmacol. Rev. 72, 558–604. 10.1124/pr.119.018531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantham L.; Quinn S. J.; Egbuna O. I.; Baxi K.; Butters R.; Pang J. L.; Pollak M. R.; Goltzman D.; Brown E. M. (2009) The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am. J. Physiol. 297, E915–923. 10.1152/ajpendo.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak M. M.; Siddiqua A.; Ward D. T.; Carter D. H.; Dallas S. L.; Nemeth E. F.; Riccardi D. (2004) Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc. Natl. Acad. Sci. U. S. A. 101, 5140–5145. 10.1073/pnas.0306141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepenhorst N. A.; Leach K.; Keller A. N.; Rueda P.; Cook A. E.; Pierce T. L.; Nowell C.; Pastoureau P.; Sabatini M.; Summers R. J.; Charman W. N.; Sexton P. M.; Christopoulos A.; Langmead C. J. (2018) Divergent effects of strontium and calcium-sensing receptor positive allosteric modulators (calcimimetics) on human osteoclast activity. Br. J. Pharmacol. 175, 4095–4108. 10.1111/bph.14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W.; Tu C.; Chen T. H.; Bikle D.; Shoback D. (2008) The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci. Signal 1, ra1. 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanHouten J.; Dann P.; McGeoch G.; Brown E. M.; Krapcho K.; Neville M.; Wysolmerski J. J. (2004) The calcium-sensing receptor regulates mammary gland parathyroid hormone-related protein production and calcium transport. J. Clin. Invest. 113, 598–608. 10.1172/JCI200418776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshirpour L.; Dann P.; Pollak M.; Wysolmerski J.; VanHouten J. (2006) The calcium-sensing receptor regulates PTHrP production and calcium transport in the lactating mammary gland. Bone 38, 787–793. 10.1016/j.bone.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Ohsu T.; Amino Y.; Nagasaki H.; Yamanaka T.; Takeshita S.; Hatanaka T.; Maruyama Y.; Miyamura N.; Eto Y. (2010) Involvement of the calcium-sensing receptor in human taste perception. J. Biol. Chem. 285, 1016–1022. 10.1074/jbc.M109.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. X.; Lightfoot Y. L.; Yang T.; Zadeh M.; Tang L.; Sahay B.; Wang G. P.; Owen J. L.; Mohamadzadeh M. (2014) Epithelial CaSR deficiency alters intestinal integrity and promotes proinflammatory immune responses. FEBS Lett. 588, 4158–4166. 10.1016/j.febslet.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamshah A.; Spreckley E.; Norton M.; Kinsey-Jones J. S.; Amin A.; Ramgulam A.; Cao Y.; Johnson R.; Saleh K.; Akalestou E.; Malik Z.; Gonzalez-Abuin N.; Jomard A.; Amarsi R.; Moolla A.; Sargent P. R.; Gray G. W.; Bloom S. R.; Murphy K. G. (2017) l-phenylalanine modulates gut hormone release and glucose tolerance, and suppresses food intake through the calcium-sensing receptor in rodents. Int. J. Obes. 41, 1693–1701. 10.1038/ijo.2017.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelstoft M. S.; Park W. M.; Sakata I.; Kristensen L. V.; Husted A. S.; Osborne-Lawrence S.; Piper P. K.; Walker A. K.; Pedersen M. H.; Nohr M. K.; Pan J.; Sinz C. J.; Carrington P. E.; Akiyama T. E.; Jones R. M.; Tang C.; Ahmed K.; Offermanns S.; Egerod K. L.; Zigman J. M.; Schwartz T. W. (2013) Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol. Metab. 2, 376–392. 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinsky V. N.; Hannan F. M.; Ramracheya R. D.; Zhang Q.; Nesbit M. A.; Hugill A.; Bentley L.; Hough T. A.; Joynson E.; Stewart M.; Aggarwal A.; Prinz-Wohlgenannt M.; Gorvin C. M.; Kallay E.; Wells S.; Cox R. D.; Richards D.; Rorsman P.; Thakker R. V. (2017) Mutant mice with calcium-sensing receptor activation have hyperglycemia that is rectified by calcilytic therapy. Endocrinology 158, 2486–2502. 10.1210/en.2017-00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepelmann M.; Yarova P. L.; Lopez-Fernandez I.; Davies T. S.; Brennan S. C.; Edwards P. J.; Aggarwal A.; Graca J.; Rietdorf K.; Matchkov V.; Fenton R. A.; Chang W.; Krssak M.; Stewart A.; Broadley K. J.; Ward D. T.; Price S. A.; Edwards D. H.; Kemp P. J.; Riccardi D. (2016) The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure. Am. J. Physiol. 310, C193–204. 10.1152/ajpcell.00248.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. L.; Crumrine D. A.; Man M. Q.; Chang W.; Elalieh H.; You M.; Elias P. M.; Bikle D. D. (2012) Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. J. Invest. Dermatol. 132, 2350–2359. 10.1038/jid.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarova P. L.; Stewart A. L.; Sathish V.; Britt R. D. J.; Thompson M. A. P.; Lowe A.; Freeman M.; Aravamudan B.; Kita H.; Brennan S. C.; Schepelmann M.; Davies T.; Yung S.; Cholisoh Z.; Kidd E. J.; Ford W. R.; Broadley K. J.; Rietdorf K.; Chang W.; Bin Khayat M. E.; Ward D. T.; Corrigan C. J. T.; Ward J.; Kemp P. J.; Pabelick C. M.; Prakash Y. S.; Riccardi D. (2015) Calcium-sensing receptor antagonists abrogate airway hyperresponsiveness and inflammation in allergic asthma. Sci. Transl Med. 7, 284ra260. 10.1126/scitranslmed.aaa0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendy G. N.; Canaff L. (2016) Calcium-sensing receptor, proinflammatory cytokines and calcium homeostasis. Semin. Cell Dev. Biol. 49, 37–43. 10.1016/j.semcdb.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Hendy G. N.; Canaff L. (2016) Calcium-sensing receptor gene: regulation of expression. Front Physiol 7, 394. 10.3389/fphys.2016.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W.; Pratt S.; Chen T. H.; Nemeth E.; Huang Z.; Shoback D. (1998) Coupling of calcium receptors to inositol phosphate and cyclic AMP generation in mammalian cells and Xenopus laevis oocytes and immunodetection of receptor protein by region-specific antipeptide antisera. J. Bone Miner. Res. 13, 570–580. 10.1359/jbmr.1998.13.4.570. [DOI] [PubMed] [Google Scholar]
- Fajtova V. T.; Quinn S. J.; Brown E. M. (1991) Cytosolic calcium responses of single rMTC 44-2 cells to stimulation with external calcium and potassium. Am. J. Physiol. 261, E151–158. 10.1152/ajpendo.1991.261.1.E151. [DOI] [PubMed] [Google Scholar]
- McGehee D. S.; Aldersberg M.; Liu K.; Hsuing S.; Heath M. J. S.; Tamir H. (1997) Mechanism of extracellular Ca2+ receptor-stimulated hormone release from sheep thyroid parafollicular cells. J. Physiol. 502, 31–44. 10.1111/j.1469-7793.1997.031bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muff R.; Nemeth E. F.; Haller-Brem S.; Fischer J. A. (1988) Regulation of hormone secretion and cytosolic Ca2+ by extracellular Ca2+ in parathyroid cells and C-cells: role of voltage-sensitive Ca2+ channels. Arch. Biochem. Biophys. 265, 128–135. 10.1016/0003-9861(88)90378-5. [DOI] [PubMed] [Google Scholar]
- Ogata S.; Kubota Y.; Satoh S.; Ito S.; Takeuchi H.; Ashizuka M.; Shirasuna K. (2006) Ca2+ stimulates COX-2 expression through calcium-sensing receptor in fibroblasts. Biochem. Biophys. Res. Commun. 351, 808–814. 10.1016/j.bbrc.2006.10.098. [DOI] [PubMed] [Google Scholar]
- Kifor O.; Macleod R. J.; Diaz R.; Bai M.; Yamanguchi T.; Yao T.; Kifor I.; Brown E. M. (2001) Regulation of MAP kinase by calcium-sensing receptor in bovine parathyroid and CaR-transfected HEK293 cells. Am. J. Physiol. 280, F291–F302. 10.1152/ajprenal.2001.280.2.F291. [DOI] [PubMed] [Google Scholar]
- MacLeod R. J.; Yano S.; Chattopadhyay N.; Brown E. M. (2004) Extracellular calcium-sensing receptor transactivates the epidermal growth factor receptor by a triple-membrane-spanning signaling mechanism. Biochem. Biophys. Res. Commun. 320, 455–460. 10.1016/j.bbrc.2004.05.198. [DOI] [PubMed] [Google Scholar]
- Thomsen A. R.; Hvidtfeldt M.; Brauner-Osborne H. (2012) Biased agonism of the calcium-sensing receptor. Cell Calcium 51, 107–116. 10.1016/j.ceca.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Huang C.; Hujer K. M.; Wu Z.; Miller R. T. (2004) The Ca2+-sensing receptor couples to Gα12/13 to activate phospholipas D in Madin-Darby canine kidney cells. Am. J. Physiol. 286, C22–C30. 10.1152/ajpcell.00229.2003. [DOI] [PubMed] [Google Scholar]
- Mamillapalli R.; VanHouten J.; Zawalich W.; Wysolmerski J. (2008) Switching of G-protein Usage by the Calcium-sensing Receptor Reverses Its Effect on Parathyroid Hormone-related Protein Secretion in Normal Versus Malignant Breast Cells. J. Biol. Chem. 283, 24435–24447. 10.1074/jbc.M801738200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamillapalli R.; Wysolmerski J. (2010) The calcium-sensing receptor couples to Galpha(s) and regulates PTHrP and ACTH secretion in pituitary cells. J. Endocrinol. 204, 287–297. 10.1677/JOE-09-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienitz M. C.; Niemeyer A.; Konig G. M.; Kostenis E.; Pott L.; Rinne A. (2019) Biased signaling of Ca(2+)-sensing receptors in cardiac myocytes regulates GIRK channel activity. J. Mol. Cell. Cardiol. 130, 107–121. 10.1016/j.yjmcc.2019.03.022. [DOI] [PubMed] [Google Scholar]
- Thomsen A. R.; Worm J.; Jacobsen S. E.; Stahlhut M.; Latta M.; Brauner-Osborne H. (2012) Strontium is a biased agonist of the calcium-sensing receptor in rat medullary thyroid carcinoma 6–23 cells. J. Pharmacol. Exp. Ther. 343, 638–649. 10.1124/jpet.112.197210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y.; Mosyak L.; Kurinov I.; Zuo H.; Sturchler E.; Cheung C. T.; Subramanyam P.; Brown A. P.; Brennan S. C.; Mun H. C.; Bush M.; Chen Y.; Nguyen T. X.; Cao B.; Chang D. D.; Quick M.; Conigrave A. D.; Colecraft H. M.; McDonald P.; Fan Q. R. (2016) Structural mechanism of ligand activation in human calcium-sensing receptor. eLife 5. 10.7554/eLife.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.; Minet E.; Zhang Z.; Silver P. A.; Bai M. (2004) Modulation of interprotomer relationships is important for activation of dimeric calcium-sensing receptor. J. Biol. Chem. 279, 14147–14156. 10.1074/jbc.M307422200. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Zhang T.; Zou J.; Miller C. L.; Gorkhali R.; Yang J.; Schilmiller A.; Wang S.; Huang K.; Brown E. M.; Moremen K. W.; Hu J.; Yang J. J. (2016) Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Sci. Adv. 2, e1600241. 10.1126/sciadv.1600241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray K.; Ghosh S. P.; Northup J. K. (2004) The Role of Cysteines and Charged Amino Acids in Extracellular Loops of the Human Ca2+ Receptor in Cell Surface Expression and Receptor Activation Processes. Endocrinology 145, 3892–3903. 10.1210/en.2003-1653. [DOI] [PubMed] [Google Scholar]
- Ray K.; Fan G.; Goldsmith P. K.; Spiegel A. M. (1997) The carboxyl terminus of the human calcium receptor. Requirements for cell-surface expression and signal transduction. J. Biol. Chem. 272, 31355–31361. 10.1074/jbc.272.50.31355. [DOI] [PubMed] [Google Scholar]
- Chang W.; Pratt S.; Chen T.; Bourguignon L.; Shoback D. (2001) Amino acids in the cytoplasmic C terminus of the parathyroid Ca2+-sensing receptor mediate efficient cell-surface expression and phospholipase c activation. J. Biol. Chem. 276, 44129–44136. 10.1074/jbc.M104834200. [DOI] [PubMed] [Google Scholar]
- Hjälm G.; MacLeod R. J.; Kifor O.; Chattopadhyay N.; Brown E. M. (2001) Filamin-A binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase. J. Biol. Chem. 276, 34880–34887. 10.1074/jbc.M100784200. [DOI] [PubMed] [Google Scholar]
- Bai M.; Trivedi S.; Lane C. R.; Yang Y.; Quinn S.; Brown E. M. (1998) Protein kinase C phosphorylation of threonine at position 888 in Ca2+o-sensing receptor stably expressed in HEK293 cells. Am. J. Physiol. 292, 1895–1905. 10.1074/jbc.273.33.21267. [DOI] [PubMed] [Google Scholar]
- Binmahfouz L. S.; Centeno P. P.; Conigrave A. D.; Ward D. T. (2019) Identification of serine-875 as an inhibitory phosphorylation site in the calcium-sensing receptor. Mol. Pharmacol. 96, 204–211. 10.1124/mol.119.116178. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Zhou Y.; Castiblanco A.; Yang W.; Brown E. M.; Yang J. J. (2009) Multiple Ca(2+)-binding sites in the extracellular domain of the Ca(2+)-sensing receptor corresponding to cooperative Ca(2+) response. Biochemistry 48, 388–398. 10.1021/bi8014604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K.; Gregory K. J.; Kufareva I.; Khajehali E.; Cook A. E.; Abagyan R.; Conigrave A. D.; Sexton P. M.; Christopoulos A. (2016) Towards a structural understanding of allosteric drugs at the human calcium-sensing receptor. Cell Res. 26, 574–592. 10.1038/cr.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handlogten M. E.; Shiraishi N.; Awata H.; Huang C.; Miller R. T. (2000) Extracellular Ca(2+)-sensing receptor is a promiscuous divalent cation sensor that responds to lead. Am. J. Physiol. 279, F1083–1091. 10.1152/ajprenal.2000.279.6.F1083. [DOI] [PubMed] [Google Scholar]
- Brown E. M.; Fuleihan G. e.-H.; Chen C. J.; Kifor O. (1990) A comparison of the effects of divalent and trivalent cations on parathyroid hormone release, 3′,5′-cyclic-adenosine monophosphate accumulation, and the levels of inositol phosphates in bovine parathyroid cells. Endocrinology 127, 1064–1071. 10.1210/endo-127-3-1064. [DOI] [PubMed] [Google Scholar]
- Conigrave A. D.; Quinn S. J.; Brown E. M. (2000) L-Amino acid sensing by the extracellular Ca2+-sensing receptor. Proc. Natl. Acad. Sci. U. S. A. 97, 4814–4819. 10.1073/pnas.97.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhead G. K.; Mun H. C.; Avlani V. A.; Jourdon O.; Church W. B.; Christopoulos A.; Delbridge L.; Conigrave A. D. (2011) Allosteric modulation of the calcium-sensing receptor by gamma-glutamyl peptides: inhibition of PTH secretion, suppression of intracellular cAMP levels, and a common mechanism of action with L-amino acids. J. Biol. Chem. 286, 8786–8797. 10.1074/jbc.M110.149724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn S. J.; Ye C. P.; Diaz R.; Kifor O.; Bai M.; Vassilev P.; Brown E. (1997) The Ca2+-sensing receptor: a target for polyamines. Am. J. Physiol. 273, C1315–1323. 10.1152/ajpcell.1997.273.4.C1315. [DOI] [PubMed] [Google Scholar]
- Ray K.; Northup J. (2002) Evidence for distinct cation and calcimimetic compound (NPS 568) recognition domains in the transmembrane regions of the human Ca2+ receptor. J. Biol. Chem. 277, 18908–18913. 10.1074/jbc.M202113200. [DOI] [PubMed] [Google Scholar]
- Quinn S. J.; Bai M.; Brown E. M. (2004) pH Sensing by the calcium-sensing receptor. J. Biol. Chem. 279, 37241–37249. 10.1074/jbc.M404520200. [DOI] [PubMed] [Google Scholar]
- Campion K. L.; McCormick W. D.; Warwicker J.; Khayat M. E.; Atkinson-Dell R.; Steward M. C.; Delbridge L. W.; Mun H. C.; Conigrave A. D.; Ward D. T. (2015) Pathophysiologic changes in extracellular pH modulate parathyroid calcium-sensing receptor activity and secretion via a histidine-independent mechanism. J. Am. Soc. Nephrol. 26, 2163–2171. 10.1681/ASN.2014070653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno P. P.; Herberger A.; Mun H. C.; Tu C.; Nemeth E. F.; Chang W.; Conigrave A. D.; Ward D. T. (2019) Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat. Commun. 10, 4693. 10.1038/s41467-019-12399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E. F.; Van Wagenen B. C.; Balandrin M. F. (2018) Discovery and development of calcimimetic and calcilytic compounds. Prog. Med. Chem. 57, 1–86. 10.1016/bs.pmch.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Nemeth E. F.; Heaton W. H.; Miller M.; Fox J.; Balandrin M. F.; Van Wagenen B. C.; Colloton M.; Karbon W.; Scherrer J.; Shatzen E.; Rishton G.; Scully S.; Qi M.; Harris R.; Lacey D.; Martin D. (2004) Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J. Pharmacol. Exp. Ther. 308, 627. 10.1124/jpet.103.057273. [DOI] [PubMed] [Google Scholar]
- Keller A. N.; Kufareva I.; Josephs T. M.; Diao J.; Mai V. T.; Conigrave A. D.; Christopoulos A.; Gregory K. J.; Leach K. (2018) Identification of global and ligand-specific calcium sensing receptor activation mechanisms. Mol. Pharmacol. 93, 619–630. 10.1124/mol.118.112086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. E.; Mistry S. N.; Gregory K. J.; Furness S. G.; Sexton P. M.; Scammells P. J.; Conigrave A. D.; Christopoulos A.; Leach K. (2015) Biased allosteric modulation at the CaS receptor engendered by structurally diverse calcimimetics. Br. J. Pharmacol. 172, 185–200. 10.1111/bph.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedlich S. U.; Gama L.; Seuwen K.; Wolf R. M.; Breitwieser G. E. (2004) Homology modeling of the transmembrane domain of the human calcium sensing receptor and localization of an allosteric binding site. J. Biol. Chem. 279, 7254–7263. 10.1074/jbc.M307191200. [DOI] [PubMed] [Google Scholar]
- Bu L.; Michino M.; Wolf R. M.; Brooks C. L. (2008) Improved model building and assessment of the Calcium-sensing receptor transmembrane domain. Proteins: Struct., Funct., Genet. 71, 215–226. 10.1002/prot.21685. [DOI] [PubMed] [Google Scholar]
- Petrel C.; Kessler A.; Maslah F.; Dauban P.; Dodd R. H.; Rognan D.; Ruat M. (2003) Modeling and mutagenesis of the binding site of Calhex 231, a novel negative allosteric modulator of the extracellular Ca(2+)-sensing receptor. J. Biol. Chem. 278, 49487–49494. 10.1074/jbc.M308010200. [DOI] [PubMed] [Google Scholar]
- Petrel C.; Kessler A.; Dauban P.; Dodd R. H.; Rognan D.; Ruat M. (2004) Positive and negative allosteric modulators of the Ca2+-sensing receptor interact within overlapping but not identical binding sites in the transmembrane domain. J. Biol. Chem. 279, 18990–18997. 10.1074/jbc.M400724200. [DOI] [PubMed] [Google Scholar]
- Gregory K. J.; Giraldo J.; Diao J.; Christopoulos A.; Leach K. (2020) Evaluation of operational models of agonism and allosterism at receptors with multiple orthosteric binding sites. Mol. Pharmacol. 97, 35–45. 10.1124/mol.119.118091. [DOI] [PubMed] [Google Scholar]
- Leach K.; Sexton P. M.; Christopoulos A. (2007) Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol. Sci. 28, 382–389. 10.1016/j.tips.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Pin J. P.; Galvez T.; Prézeau L. (2003) Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol. Ther. 98, 325–354. 10.1016/S0163-7258(03)00038-X. [DOI] [PubMed] [Google Scholar]
- Wu H.; Wang C.; Gregory K. J.; Han G. W.; Cho H. P.; Xia Y.; Niswender C. M.; Katritch V.; Meiler J.; Cherezov V.; Conn P. J.; Stevens R. C. (2014) Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator. Science 344, 58–64. 10.1126/science.1249489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson M.; Jensen J.; Bertozzi S. M.; Currier E. A.; Ma J. N.; Burstein E. S.; Olsson R. (2010) Discovery of a class of calcium sensing receptor positive allosteric modulators; 1-(benzothiazol-2-yl)-1-phenylethanols. Bioorg. Med. Chem. Lett. 20, 5918–5921. 10.1016/j.bmcl.2010.07.077. [DOI] [PubMed] [Google Scholar]
- Ma J.-N.; Owens M.; Gustafsson M.; Jensen J.; Tabatabaei A.; Schmelzer K.; Olsson R.; Burstein E. S. (2011) Characterization of highly efficacious allosteric agonists of the human calcium-sensing receptor. J. Pharmacol. Exp. Ther. 337, 275–284. 10.1124/jpet.110.178194. [DOI] [PubMed] [Google Scholar]
- Walter S.; Baruch A.; Dong J.; Tomlinson J. E.; Alexander S. T.; Janes J.; Hunter T.; Yin Q.; Maclean D.; Bell G.; Mendel D. B.; Johnson R. M.; Karim F. (2013) Pharmacology of AMG 416 (Velcalcetide), a novel peptide agonist of the calcium-sensing receptor, for the treatment of Secondary Hyperparathyroidism in hemodialysis patients. J. Pharmacol. Exp. Ther. 346, 229. 10.1124/jpet.113.204834. [DOI] [PubMed] [Google Scholar]
- Alexander S. T.; Hunter T.; Walter S.; Dong J.; Maclean D.; Baruch A.; Subramanian R.; Tomlinson J. E. (2015) Critical cysteine residues in both the calcium-sensing receptor and the allosteric activator AMG 416 underlie the mechanism of action. Mol. Pharmacol. 88, 853–865. 10.1124/mol.115.098392. [DOI] [PubMed] [Google Scholar]
- Marquis R. W.; Lago A. M.; Callahan J. F.; Rahman A.; Dong X.; Stroup G. B.; Hoffman S.; Gowen M.; DelMar E. G.; Van Wagenen B. C.; Logan S.; Shimizu S.; Fox J.; Nemeth E. F.; Roethke T.; Smith B. R.; Ward K. W.; Bhatnagar P. (2009) Antagonists of the calcium receptor. 2. amino alcohol-based parathyroid hormone secretagogues. J. Med. Chem. 52, 6599–6605. 10.1021/jm900563e. [DOI] [PubMed] [Google Scholar]
- Marquis R. W., Casillas L. N., Ramanjulu J. M., and Callahan J. F. (2010) Calcilytic compounds. US Patent US7829594B2.
- Shinagawa Y.; Inoue T.; Katsushima T.; Kiguchi T.; Ikenogami T.; Ogawa N.; Fukuda K.; Hirata K.; Harada K.; Takagi M.; Nakagawa T.; Kimura S.; Matsuo Y.; Maekawa M.; Hayashi M.; Soejima Y.; Takahashi M.; Shindo M.; Hashimoto H. (2011) Discovery of a potent and short-acting oral calcilytic with a pulsatile secretion of parathyroid hormone. ACS Med. Chem. Lett. 2, 238–242. 10.1021/ml100268k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago M. A., Callahan J. F., Bhatnagar P. K., Del Mar R. G., Bryan W. M., and Burgess J. L. (2006) Calcilytic compounds. US Patent US7109238B2.
- Josephs T. M.; Keller A. N.; Khajehali E.; DeBono A.; Langmead C. J.; Conigrave A. D.; Capuano B.; Kufareva I.; Gregory K. J.; Leach K. (2020) Negative allosteric modulators of the human calcium-sensing receptor bind to overlapping and distinct sites within the 7-transmembrane domain. Br. J. Pharmacol. 177, 1917–1930. 10.1111/bph.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widler L.; Altmann E.; Beerli R.; Breitenstein W.; Bouhelal R.; Buhl T.; Gamse R.; Gerspacher M.; Halleux C.; John M. R.; Lehmann H.; Kalb O.; Kneissel M.; Missbach M.; Müller I. R.; Reidemeister S.; Renaud J.; Taillardat A.; Tommasi R.; Weiler S.; Wolf R. M.; Seuwen K. (2010) 1-Alkyl-4-phenyl-6-alkoxy-1 H-quinazolin-2-ones: a novel series of potent calcium-sensing receptor antagonists. J. Med. Chem. 53, 2250–2263. 10.1021/jm901811v. [DOI] [PubMed] [Google Scholar]
- Widler L. (2011) Calcilytics: antagonists of the calcium-sensing receptor for the treatment of osteoporosis. Future Med. Chem. 3, 535–547. 10.4155/fmc.11.17. [DOI] [PubMed] [Google Scholar]
- Noetzel M. J.; Gregory K. J.; Vinson P. N.; Manka J. T.; Stauffer S. R.; Lindsley C. W.; Niswender C. M.; Xiang Z.; Conn P. J. (2013) A novel metabotropic glutamate receptor 5 positive allosteric modulator acts at a unique site and confers stimulus bias to mGlu5 signaling. Mol. Pharmacol. 83, 835–847. 10.1124/mol.112.082891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond A. S.; Rodriguez A. L.; Townsend S. D.; Niswender C. M.; Gregory K. J.; Lindsley C. W.; Conn P. J. (2010) Discovery of a novel chemical class of mGlu(5) allosteric ligands with distinct modes of pharmacology. ACS Chem. Neurosci. 1, 702–716. 10.1021/cn100051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A.; Faure H.; Petrel C.; Rognan D.; Cesario M.; Ruat M.; Dauban P.; Dodd R. H. (2006) N1-Benzoyl-N2-[1-(1-naphthyl)ethyl]-trans-1,2-diaminocyclohexanes: Development of 4-chlorophenylcarboxamide (calhex 231) as a new calcium sensing receptor ligand demonstrating potent calcilytic activity. J. Med. Chem. 49, 5119–5128. 10.1021/jm051233+. [DOI] [PubMed] [Google Scholar]
- Gregory K. J.; Kufareva I.; Keller A. N.; Khajehali E.; Mun H. C.; Goolam M. A.; Mason R. S.; Capuano B.; Conigrave A. D.; Christopoulos A.; Leach K. (2018) Dual action calcium-sensing receptor modulator unmasks novel mode-switching mechanism. ACS Pharmacol Transl Sci. 1, 96–109. 10.1021/acsptsci.8b00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukagawa M.; Shimazaki R.; Akizawa T. (2018) Head-to-head comparison of the new calcimimetic agent evocalcet with cinacalcet in Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int. 94, 818–825. 10.1016/j.kint.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Rottembourg J.; Urena-Torres P.; Toledano D.; Gueutin V.; Hamani A.; Coldefy O.; Hebibi H.; Guincestre T.; Emery C. (2019) Factors associated with parathyroid hormone control in haemodialysis patients with secondary hyperparathyroidism treated with cinacalcet in real-world clinical practice: Mimosa study. Clin. Kidney J. 12, 871–879. 10.1093/ckj/sfz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe H.; Shapiro W.; Sun W. Y.; Matalon A. (2008) CaSR polymorphism Arg990Gly and response to calcimimetic agents in end-stage kidney disease patients with secondary hyperparathyroidism and in cell culture. Pers. Med. 5, 109–116. 10.2217/17410541.5.2.109. [DOI] [PubMed] [Google Scholar]
- Block G. A.; Bushinsky D. A.; Cheng S.; Cunningham J.; Dehmel B.; Drueke T. B.; Ketteler M.; Kewalramani R.; Martin K. J.; Moe S. M.; Patel U. D.; Silver J.; Sun Y.; Wang H.; Chertow G. M. (2017) Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: A randomized clinical trialetelcalcetide vs cinacalcet in hemodialysis with secondary hyperparathyroidism etelcalcetide vs cinacalcet in hemodialysis with secondary hyperparathyroidism. JAMA 317, 156–164. 10.1001/jama.2016.19468. [DOI] [PubMed] [Google Scholar]
- Bushinsky D. A.; Chertow G. M.; Cheng S.; Deng H.; Kopyt N.; Martin K. J.; Rastogi A.; Urena-Torres P.; Vervloet M.; Block G. A. (2020) One-year safety and efficacy of intravenous etelcalcetide in patients on hemodialysis with secondary hyperparathyroidism. Nephrol., Dial., Transplant. 35, 1769–1778. 10.1093/ndt/gfz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S. C.; Mavridis D.; Johnson D. W.; Tonelli M.; Ruospo M.; Strippoli G. F. M. (2020) Comparative effectiveness of calcimimetic agents for Secondary Hyperparathyroidism in adults: a systematic review and network meta-analysis. Am. J. Kidney Dis. 76, 321–330. 10.1053/j.ajkd.2020.02.439. [DOI] [PubMed] [Google Scholar]
- Kawata T.; Tokunaga S.; Murai M.; Masuda N.; Haruyama W.; Shoukei Y.; Hisada Y.; Yanagida T.; Miyazaki H.; Wada M.; Akizawa T.; Fukagawa M. (2018) A novel calcimimetic agent, evocalcet (MT-4580/KHK7580), suppresses the parathyroid cell function with little effect on the gastrointestinal tract or CYP isozymes in vivo and in vitro. PLoS One 13, e0195316–0195314. 10.1371/journal.pone.0195316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K.; Noguchi T.; Toriie S.; Shimazu E.; Miyake S. (2010) The mechanism of upper-gastrointestinal complication after taking cinacalcet hydrochloride. Nihon Toseki Igakkai Zasshi 43, 309–315. 10.4009/jsdt.43.309. [DOI] [Google Scholar]
- Shigematsu T.; Shimazaki R.; Fukagawa M.; Akizawa T. (2018) Pharmacokinetics of evocalcet in secondary hyperparathyroidism patients receiving hemodialysis: first-in-patient clinical trial in Japan. Clin Pharmacol 10, 101–111. 10.2147/CPAA.S171044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dershem R.; Gorvin C. M.; Metpally R. P.R.; Krishnamurthy S.; Smelser D. T.; Hannan F. M.; Carey D. J.; Thakker R. V.; Breitwieser G. E. (2020) Familial hypocalciuric hypercalcemia type 1 and autosomal-dominant hypocalcemia type 1: Prevalence in a large healthcare population. Am. J. Hum. Genet. 106, 734–747. 10.1016/j.ajhg.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E.; McKenna J.; Cavanaugh A.; Breitwieser G. E. (2009) Pharmacochaperone-mediated rescue of calcium-sensing receptor loss-of-function mutants. Mol. Endocrinol. 23, 1115–1123. 10.1210/me.2009-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Breitwieser G. E. (2007) Rescue of calcium-sensing receptor mutants by allosteric modulators reveals a conformational checkpoint in receptor biogenesis. J. Biol. Chem. 282, 9517–9525. 10.1074/jbc.M609045200. [DOI] [PubMed] [Google Scholar]
- Leach K.; Wen A.; Davey A. E.; Sexton P. M.; Conigrave A. D.; Christopoulos A. (2012) Identification of molecular phenotypes and biased signaling induced by naturally occurring mutations of the human calcium-sensing receptor. Endocrinology 153, 4304–4316. 10.1210/en.2012-1449. [DOI] [PubMed] [Google Scholar]
- Hannan F.; Babinsky V.; Thakker R. V. (2016) Disorders of the calcium-sensing receptor and partner proteins: insights into the molecular basis of calcium homeostasis. J. Mol. Endocrinol. 57, R127–R142. 10.1530/JME-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers H. J. L. M.; Karperien M.; Hamdy N. A. T.; De boer H.; Hermus A. R. M. M. (2006) Normalization of serum calcium by cinacalcet in a patient with hypercalcaemia due to a de novo inactivating mutation of the calcium-sensing receptor. J. Intern. Med. 260, 177–182. 10.1111/j.1365-2796.2006.01684.x. [DOI] [PubMed] [Google Scholar]
- Gannon A. W.; Monk H. M.; Levine M. A. (2014) Cinacalcet Monotherapy in Neonatal Severe Hyperparathyroidism: A Case Study and Review. J. Clin. Endocrinol. Metab. 99, 7–11. 10.1210/jc.2013-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm-Bals A.; Parvex P.; Magdelaine C.; Girardin E. (2012) Successful use of bisphosphonate and calcimimetic in neonatal severe Primary Hyperparathyroidism. Pediatrics 129, e812–e816. 10.1542/peds.2011-0128. [DOI] [PubMed] [Google Scholar]
- Fisher M. M.; Cabrera S. M.; Imel E. A. (2015) Successful treatment of neonatal severe hyperparathyroidism with cinacalcet in two patients. Endocrinol Diabetes Metab Case Rep 2015, 102–107. 10.1530/EDM-15-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon U. S.; VanDeVoorde R. G. (2010) Beneficial effect of cinacalcet in a child with familial hypocalciuric hypercalcemia. Pediatr. Nephrol. 25, 1747–1750. 10.1007/s00467-010-1547-5. [DOI] [PubMed] [Google Scholar]
- Gunganah K.; Grossman A.; Druce M. (2014) Recurrent pancreatitis in a patient with familial hypocalciuric hypercalcaemia treated successfully with cinacalcet. Endocrinol Diabetes Metab Case Rep 2014, 675–673. 10.1530/EDM-14-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastromatteo E.; Lamacchia O.; Campo M. R.; Conserva A.; Baorda F.; Cinque L.; Guarnieri V.; Scillitani A.; Cignarelli M. (2014) A novel mutation in calcium-sensing receptor gene associated to hypercalcemia and hypercalciuria. BMC Endocr Disord 14, 239–236. 10.1186/1472-6823-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atay Z.; Bereket A.; Haliloglu B.; Abali S.; Ozdogan T.; Altuncu E.; Canaff L.; Vilaça T.; Wong B. Y. L.; Cole D. E. C.; Hendy G. N.; Turan S. (2014) Novel homozygous inactivating mutation of the calcium-sensing receptor gene (CASR) in neonatal severe hyperparathyroidism—lack of effect of cinacalcet. Bone 64, 102–107. 10.1016/j.bone.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Savas-Erdeve S.; Sagsak E.; Keskin M.; Magdelaine C.; Lienhardt-Roussie A.; Kurnaz E.; Cetinkaya S.; Aycan Z. (2016) Treatment experience and long-term follow-up data in two severe neonatal hyperparathyroidism cases. J. Pediatr Endocrinol Metab 29, 129–129. 10.1515/jpem-2015-0261. [DOI] [PubMed] [Google Scholar]
- Capozza M.; Chinellato I.; Guarnieri V.; Di lorgi N.; Accadia M.; Traggiai C.; Mattioli G.; Di Mauro A.; Laforgia N. (2018) Case report: acute clinical presentation and neonatal management of primary hyperparathyroidism due to a novel CaSR mutation. BMC Pediatr. 18, 340. 10.1186/s12887-018-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Soblechero E.; Ferrer Castillo M. T.; Jiménez Crespo B.; Domínguez Quintero M. L.; González Fuentes C. (2013) Neonatal Hypercalcemia due to a Homozygous Mutation in the Calcium-Sensing Receptor: Failure of Cinacalcet. Neonatology 104, 104–108. 10.1159/000350540. [DOI] [PubMed] [Google Scholar]
- Murphy H.; Patrick J.; Báez-Irizarry E.; Lacassie Y.; Gómez R.; Vargas A.; Barkemeyer B.; Kanotra S.; Zambrano R. M. (2016) Neonatal severe hyperparathyroidism caused by homozygous mutation in CASR: A rare cause of life-threatening hypercalcemia. Eur. J. Med. Genet 1–5. 10.1016/j.ejmg.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Leach K.; Wen A.; Cook A. E.; Sexton P. M.; Conigrave A. D.; Christopoulos A. (2013) Impact of clinically relevant mutations on the pharmacoregulation and signaling bias of the calcium-sensing receptor by positive and negative allosteric modulators. Endocrinology 154, 1105–1116. 10.1210/en.2012-1887. [DOI] [PubMed] [Google Scholar]
- Nesbit M. A.; Hannan F. M.; Howles S. A.; Babinsky V. N.; Head R. A.; Cranston T.; Rust N.; Hobbs M. R.; Heath H.; Thakker R. V. (2013) Mutations affecting G-Protein Subunit α 11 in hypercalcemia and hypocalcemia. N. Engl. J. Med. 368, 2476–2486. 10.1056/NEJMoa1300253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinsky V. N.; Hannan F. M.; Gorvin C. M.; Howles S. A.; Nesbit M. A.; Rust N.; Hanyaloglu A. C.; Hu J.; Spiegel A. M.; Thakker R. V. (2016) Allosteric modulation of the calcium-sensing receptor rectifies signaling abnormalities associated with G-protein alpha-11 mutations causing hypercalcemic and hypocalcemic disorders. J. Biol. Chem. 291, 10876–10885. 10.1074/jbc.M115.696401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howles S. A.; Hannan F. M.; Gorvin C. M.; Piret S. E.; Paudyal A.; Stewart M.; Hough T. A.; Nesbit M. A.; Wells S.; Brown S. D.; Cox R. D.; Thakker R. V. (2017) Cinacalcet corrects hypercalcemia in mice with an inactivating Galpha11 mutation. JCI Insight 2. 10.1172/jci.insight.96540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvin C. M.; Hannan F. M.; Cranston T.; Valta H.; Makitie O.; Schalin-Jantti C.; Thakker R. V. (2018) Cinacalcet rectifies hypercalcemia in a patient with Familial Hypocalciuric Hypercalcemia Type 2 (FHH2) caused by a germline loss-of-function Galpha11 mutation. J. Bone Miner. Res. 33, 32–41. 10.1002/jbmr.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Poussou R. R.; Mansour-Hendili R. L.; Baron R. S.; Bertocchio R. J.-P.; Travers R. C.; Simian R. C.; Treard R. C.; Baudouin R. V.; Beltran R. S.; Broux R. F.; Camard R. O.; Cloarec R. S.; Cormier R. C.; Debussche R. X.; Dubosclard R. E.; Eid R. C.; Haymann R. J.-P.; Kiando R. S.; Kuhn R. J.-M.; Lefort R. G.; Linglart R. A.; Lucas-Pouliquen R. B.; Macher R. M.-A.; Maruani R. G.; Ouzounian R. S.; Polak R. M.; Requeda R. E.; Robier R. D.; Silve R. C.; Souberbielle R. J.-C.; Tack R. I.; Vezzosi R. D.; Jeunemaitre R. X.; Houillier R. P. (2016) Familial Hypocalciuric Hypercalcemia Types 1 and 3 and Primary Hyperparathyroidism: Similarities and Differences. J. Clin. Endocrinol. Metab. 101, 2185–2195. 10.1210/jc.2015-3442. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y.; Yamaguchi R.; Satake E.; Ohtaka K.; Nakanishi T.; Ozono K.; Ogata T. (2013) Identification of AP2S1 mutation and effects of low calcium formula in an infant with hypercalcemia and hypercalciuria. J. Clin. Endocrinol. Metab. 98, E2022–E2027. 10.1210/jc.2013-2571. [DOI] [PubMed] [Google Scholar]
- Hendy N. G.; Canaff S. L.; Newfield L. R.; Tripto-Shkolnik P. L.; Wong C. B. Y.; Lee C. B. S.; Cole C. D. E. (2014) Codon Arg15 mutations of the AP2S1 gene: common occurrence in Familial Hypocalciuric Hypercalcemia cases negative for calcium-sensing receptor (CASR) mutations. J. Clin. Endocrinol. Metab. 99, E1311–E1315. 10.1210/jc.2014-1120. [DOI] [PubMed] [Google Scholar]
- Gorvin C. M.; Metpally R.; Stokes V. J.; Hannan F. M.; Krishnamurthy S. B.; Overton J. D.; Reid J. G.; Breitwieser G. E.; Thakker R. V. (2018) Large-scale exome datasets reveal a new class of adaptor-related protein complex 2 sigma subunit (AP2σ) mutations, located at the interface with the AP2 alpha subunit, that impair calcium-sensing receptor signalling. Hum. Mol. Genet. 27, 901–911. 10.1093/hmg/ddy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan F. M.; Howles S. A.; Rogers A.; Cranston T.; Gorvin C. M.; Babinsky V. N.; Reed A. A.; Thakker C. E.; Bockenhauer D.; Brown R. S.; Connell J. M.; Cook J.; Darzy K.; Ehtisham S.; Graham U.; Hulse T.; Hunter S. J.; Izatt L.; Kumar D.; McKenna M. J.; McKnight J. A.; Morrison P. J.; Mughal M. Z.; O’Halloran D.; Pearce S. H.; Porteous M. E.; Rahman M.; Richardson T.; Robinson R.; Scheers I.; Siddique H.; Van’T Hoff W. G.; Wang T.; Whyte M. P.; Nesbit M. A.; Thakker R. V. (2015) Adaptor protein-2 sigma subunit mutations causing familial hypocalciuric hypercalcaemia type 3 (FHH3) demonstrate genotype-phenotype correlations, codon bias and dominant-negative effects. Hum. Mol. Genet. 24, 5079. 10.1093/hmg/ddv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurtry C. T.; Schranck F. W.; Walkenhorst D. A.; Murphy W. A.; Kocher D. B.; Teitelbaum S. L.; Rupich R. C.; Whyte M. P. (1992) Significant developmental elevation in serum parathyroid hormone levels in a large kindred with familial benign (hypocalciuric) hypercalcemia. Am. J. Med. 93, 247–258. 10.1016/0002-9343(92)90229-5. [DOI] [PubMed] [Google Scholar]
- Howles S. A.; Hannan F. M.; Babinsky V. N.; Rogers A.; Gorvin C. M.; Rust N.; Richardson T.; McKenna M. J.; Nesbit M. A.; Thakker R. V. (2016) Cinacalcet for symptomatic Hypercalcemia caused by AP2S1 mutations. N. Engl. J. Med. 374, 1396–1398. 10.1056/NEJMc1511646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozen T.; Ozisik L.; Calik Basaran N. (2017) An overview and management of osteoporosis. Eur. J. Rheumatol 4, 46–56. 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brixen K. T.; Christensen B.; Ejersted C.; Langdahl B. L. (2004) Teriparatide (biosynthetic human parathyroid hormone 1–34): A new paradigm in the treatment of osteoporosis. Pharmacol. Toxicol. 94, 260–270. 10.1111/j.1742-7843.2004.pto940602.x. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H. J.; Goltzman D. (2008) On the Interpretation of Rat Carcinogenicity Studies for Human PTH(1–34) and Human PTH(1–84). J. Bone Miner. Res. 23, 803–811. 10.1359/jbmr.080208. [DOI] [PubMed] [Google Scholar]
- Gowen M.; Stroup G. B.; Dodds R. A.; James I. E.; Votta B. J.; Smith B. R.; Bhatnagar P. K.; Lago A. M.; Callahan J. F.; DelMar E. G.; Miller M. A.; Nemeth E. F.; Fox J. (2000) Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats. J. Clin. Invest. 105, 1595–1604. 10.1172/JCI9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E. F. (2002) The search for calcium receptor antagonists (calcilytics). J. Mol. Endocrinol. 29, 15–21. 10.1677/jme.0.0290015. [DOI] [PubMed] [Google Scholar]
- Dobnig H.; Turner R. T. (1997) The effects of programmed administration of human parathyroid hormone fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology 138, 4607–4612. 10.1210/endo.138.11.5505. [DOI] [PubMed] [Google Scholar]
- Balan G.; Bauman J.; Bhattacharya S.; Castrodad M.; Healy D. R.; Herr M.; Humphries P.; Jennings S.; Kalgutkar A. S.; Kapinos B.; Khot V.; Lazarra K.; Li M.; Li Y.; Neagu C.; Oliver R.; Piotrowski D. W.; Price D.; Qi H.; Simmons H. A.; Southers J.; Wei L.; Zhang Y.; Paralkar V. M. (2009) The discovery of novel calcium sensing receptor negative allosteric modulators. Bioorg. Med. Chem. Lett. 19, 3328–3332. 10.1016/j.bmcl.2009.04.044. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Matheny C. J.; Hoffman S. J.; Marquis R. W.; Schultz M.; Liang X.; Vasko J. A.; Stroup G. B.; Vaden V. R.; Haley H.; Fox J.; DelMar E. G.; Nemeth E. F.; Lago A. M.; Callahan J. F.; Bhatnagar P.; Huffman W. F.; Gowen M.; Yi B.; Danoff T. M.; Fitzpatrick L. A. (2010) An orally active calcium-sensing receptor antagonist that transiently increases plasma concentrations of PTH and stimulates bone formation. Bone 46, 534–542. 10.1016/j.bone.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick L. A.; Dabrowski C. E.; Cicconetti G.; Gordon D. N.; Fuerst T.; Engelke K.; Genant H. K. (2012) Ronacaleret, a calcium-sensing receptor antagonist, increases trabecular but not cortical bone in postmenopausal women. J. Bone Miner. Res. 27, 255–262. 10.1002/jbmr.554. [DOI] [PubMed] [Google Scholar]
- Halse J.; Greenspan S.; Cosman F.; Ellis G.; Santora A.; Leung A.; Heyden N.; Samanta S.; Doleckyj S.; Rosenberg E.; Denker A. E. (2014) A phase 2, randomized, placebo-controlled, dose-ranging study of the calcium-sensing receptor antagonist MK-5442 in the treatment of postmenopausal women with osteoporosis. J. Clin. Endocrinol. Metab. 99, E2207–2215. 10.1210/jc.2013-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John M. R.; Harfst E.; Loeffler J.; Belleli R.; Mason J.; Bruin G. J.; Seuwen K.; Klickstein L. B.; Mindeholm L.; Widler L.; Kneissel M. (2014) AXT914 a novel, orally-active parathyroid hormone-releasing drug in two early studies of healthy volunteers and postmenopausal women. Bone 64, 204–210. 10.1016/j.bone.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Caltabiano S.; Dollery C. T.; Hossain M.; Kurtinecz M. T.; Desjardins J. P.; Favus M. J.; Kumar R.; Fitzpatrick L. A. (2013) Characterization of the effect of chronic administration of a calcium-sensing receptor antagonist, ronacaleret, on renal calcium excretion and serum calcium in postmenopausal women. Bone 56, 154–162. 10.1016/j.bone.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Li D.; Opas E. E.; Tuluc F.; Metzger D. L.; Hou C.; Hakonarson H.; Levine M. A. (2014) Autosomal Dominant Hypoparathyroidism caused by germline mutation in GNA11: phenotypic and molecular characterization. J. Clin. Endocrinol. Metab. 99, E1774–E1783. 10.1210/jc.2014-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannstadt M.; Harris M.; Bravenboer B.; Chitturi S.; Dreijerink K. M. A.; Lambright D. G.; Lim E. T.; Daly M. J.; Gabriel S.; Jüppner H. (2013) Germline Mutations Affecting Gα 11 in Hypoparathyroidism. N. Engl. J. Med. 368, 2532–2534. 10.1056/NEJMc1300278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piret S. E.; Gorvin C. M.; Pagnamenta A. T.; Howles S. A.; Cranston T.; Rust N.; Nesbit M. A.; Glaser B.; Taylor J. C.; Buchs A. E.; Hannan F. M.; Thakker R. V. (2016) Identification of a G-Protein subunit-α11 gain-of-function mutation, Val340Met, in a Family With Autosomal Dominant Hypocalcemia Type 2 (ADH2). J. Bone Miner. Res. 31, 1207–1214. 10.1002/jbmr.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan F. M.; Walls G. V.; Babinsky V. N.; Nesbit M. A.; Kallay E.; Hough T. A.; Fraser W. D.; Cox R. D.; Hu J.; Spiegel A. M.; Thakker R. V. (2015) The calcilytic agent NPS 2143 rectifies hypocalcemia in a mouse model with an activating calcium-sensing receptor (CaSR) mutation: Relevance to Autosomal Dominant Hypocalcemia Type 1 (ADH1). Endocrinology 156, 3114–3121. 10.1210/en.2015-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letz S.; Rus R.; Haag C.; Dorr H. G.; Schnabel D.; Mohlig M.; Schulze E.; Frank-Raue K.; Raue F.; Mayr B.; Schofl C. (2010) Novel activating mutations of the calcium-sensing receptor: the calcilytic NPS-2143 mitigates excessive signal transduction of mutant receptors. J. Clin. Endocrinol. Metab. 95, E229–233. 10.1210/jc.2010-0651. [DOI] [PubMed] [Google Scholar]
- Gorvin C. M.; Hannan F. M.; Howles S. A.; Babinsky V. N.; Piret S. E.; Rogers A.; Freidin A. J.; Stewart M.; Paudyal A.; Hough T. A.; Nesbit M. A.; Wells S.; Vincent T. L.; Brown S. D.; Cox R. D.; Thakker R. V. (2017) Gα11 mutation in mice causes hypocalcemia rectifiable by calcilytic therapy. JCI Insight 2, e91103. 10.1172/jci.insight.91103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszko K. L.; Bi R.; Gorvin C. M.; Brauner-Osborne H.; Xiong X. F.; Inoue A.; Thakker R. V.; Stromgaard K.; Gardella T.; Mannstadt M. (2017) Knockin mouse with mutant Galpha11 mimics human inherited hypocalcemia and is rescued by pharmacologic inhibitors. JCI Insight 2, e91079. 10.1172/jci.insight.91079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B.; Endo I.; Ohnishi Y.; Kondo T.; Hasegawa T.; Amizuka N.; Kiyonari H.; Shioi G.; Abe M.; Fukumoto S.; Matsumoto T. (2015) Calcilytic ameliorates abnormalities of mutant calcium-sensing receptor (CaSR) knock-in mice mimicking autosomal dominant hypocalcemia (ADH). J. Bone Miner. Res. 30, 1980–1993. 10.1002/jbmr.2551. [DOI] [PubMed] [Google Scholar]
- Letz S.; Haag C.; Schulze E.; Frank-Raue K.; Raue F.; Hofner B.; Mayr B.; Schofl C. (2014) Amino alcohol- (NPS-2143) and quinazolinone-derived calcilytics (ATF936 and AXT914) differentially mitigate excessive signalling of calcium-sensing receptor mutants causing Bartter syndrome Type 5 and autosomal dominant hypocalcemia. PLoS One 9, e115178. 10.1371/journal.pone.0115178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. S.; Gafni R. I.; Brillante B.; Guthrie L. C.; Streit J.; Gash D.; Gelb J.; Krusinska E.; Brennan S. C.; Schepelmann M.; Riccardi D.; Bin Khayat M. E.; Ward D. T.; Nemeth E. F.; Rosskamp R.; Collins M. T. (2019) Treatment of Autosomal Dominant Hypocalcemia Type 1 with the calcilytic NPSP795 (SHP635). J. Bone Miner. Res. 34, 1609–1618. 10.1002/jbmr.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers Arriagada N.; Horsley J. A.; Palmer A. J.; Morgan G. G.; Tham R.; Johnston F. H. (2019) Association between fire smoke fine particulate matter and asthma-related outcomes: Systematic review and meta-analysis. Environ. Res. 179, 108777. 10.1016/j.envres.2019.108777. [DOI] [PubMed] [Google Scholar]
- Koller D. Y.; Halmerbauer G.; Frischer T.; Roithner B. (1999) Assessment of eosinophil granule proteins in various body fluids: is there a relation to clinical variables in childhood asthma?. Clin. Exp. Allergy 29, 786–793. 10.1046/j.1365-2222.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- Lonnkvist K.; Hellman C.; Lundahl J.; Hallden G.; Hedlin G. (2001) Eosinophil markers in blood, serum, and urine for monitoring the clinical course in childhood asthma: impact of budesonide treatment and withdrawal. J. Allergy Clin. Immunol. 107, 812–817. 10.1067/mai.2001.114246. [DOI] [PubMed] [Google Scholar]
- Kurosawa M.; Shimizu Y.; Tsukagoshi H.; Ueki M. (1992) Elevated levels of peripheral-blood, naturally occurring aliphatic polyamines in bronchial asthmatic patients with active symptoms. Allergy 47, 638–643. 10.1111/j.1398-9995.1992.tb02388.x. [DOI] [PubMed] [Google Scholar]
- North M. L.; Grasemann H.; Khanna N.; Inman M. D.; Gauvreau G. M.; Scott J. A. (2013) Increased ornithine-derived polyamines cause airway hyperresponsiveness in a mouse model of asthma. Am. J. Respir. Cell Mol. Biol. 48, 694–702. 10.1165/rcmb.2012-0323OC. [DOI] [PubMed] [Google Scholar]
- Bartoli M. L.; Bacci E.; Carnevali S.; Cianchetti S.; Dente F. L.; Di Franco A.; Giannini D.; Taccola M.; Vagaggini B.; Paggiaro P. L. (2004) Clinical assessment of asthma severity partially corresponds to sputum eosinophilic airway inflammation. Respir Med. 98, 184–193. 10.1016/j.rmed.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Gibson P. G.; Woolley K. L.; Carty K.; Murree-Allen K.; Saltos N. (1998) Induced sputum eosinophil cationic protein (ECP) measurement in asthma and chronic obstructive airway disease (COAD). Clin. Exp. Allergy 28, 1081–1088. 10.1046/j.1365-2222.1998.00331.x. [DOI] [PubMed] [Google Scholar]
- Coyle A. J.; Ackerman S. J.; Irvin C. G. (1993) Cationic proteins induce airway hyperresponsiveness dependent on charge interactions. Am. Rev. Respir. Dis. 147, 896–900. 10.1164/ajrccm/147.4.896. [DOI] [PubMed] [Google Scholar]
- Homma T.; Bates J. H.; Irvin C. G. (2005) Airway hyperresponsiveness induced by cationic proteins in vivo: site of action. Am. J. Physiol. 289, L413–418. 10.1152/ajplung.00059.2005. [DOI] [PubMed] [Google Scholar]
- Frigas E.; Loegering D. A.; Solley G. O.; Farrow G. M.; Gleich G. J. (1981) Elevated levels of the eosinophil granule major basic protein in the sputum of patients with bronchial asthma. Mayo Clinic Proceedings 56, 345–353. [PubMed] [Google Scholar]
- Lee J. W.; Park H. A.; Kwon O. K.; Park J. W.; Lee G.; Lee H. J.; Lee S. J.; Oh S. R.; Ahn K. S. (2017) NPS 2143, a selective calcium-sensing receptor antagonist inhibits lipopolysaccharide-induced pulmonary inflammation. Mol. Immunol. 90, 150–157. 10.1016/j.molimm.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Thompson M.; Freeman M. R.; Manlove L. J.; Fang Y. H.; Britt R.; Kuipers I.; Riccardi D.; Pabelick C.; Prakash Y. S. (2016) Calcium sensing receptor and airway reactivity in mixed allergen treated mouse model of asthma. Am. J. Respir Crit Care Med. 193, A1266. [Google Scholar]
- Li T.; Sun M.; Yin X.; Wu C.; Wu Q.; Feng S.; Li H.; Luan Y.; Wen J.; Yan L.; Zhao B.; Xu C.; Sun Y. (2013) Expression of the calcium sensing receptor in human peripheral blood T lymphocyte and its contribution to cytokine secretion through MAPKs or NF-kappaB pathways. Mol. Immunol. 53, 414–420. 10.1016/j.molimm.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Lee G. S.; Subramanian N.; Kim A. I.; Aksentijevich I.; Goldbach-Mansky R.; Sacks D. B.; Germain R. N.; Kastner D. L.; Chae J. J. (2012) The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492, 123–127. 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossol M.; Pierer M.; Raulien N.; Quandt D.; Meusch U.; Rothe K.; Schubert K.; Schoneberg T.; Schaefer M.; Krugel U.; Smajilovic S.; Brauner-Osborne H.; Baerwald C.; Wagner U. (2012) Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 3, 1329. 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarova P. L.; Huang P.; Schepelmann M. W.; Bruce R.; Ecker R.; Nica R.; Telezhkin V.; Traini D.; Gomes dos Reis L.; Kidd E. J.; Ford W. R.; Broadley K. J.; Kariuki B. M.; Corrigan C. J.; Ward J. P. T.; Kemp P. J.; Riccardi D. (2021) Characterisation of negative allosteric modulators of the calcium-sensing receptor, CaSR, for repurposing as a treatment for asthma. J. Pharmacol Exp Ther 376 (1), 51–63. 10.1124/jpet.120.000281. [DOI] [PubMed] [Google Scholar]