Abstract
Hypertension is highly prevalent and remains poorly controlled. The purpose of this study was to evaluate blood pressure (BP) control in patients with uncontrolled hypertension 1 year after referral to a hypertension specialist. A retrospective chart review was performed on 158 patients evaluated by a single hypertension specialist between 2005 and 2010 at the Penn Hypertension Program. Patients were included if they had at least 1 year of follow‐up and had baseline plasma renin activity and plasma aldosterone concentration measured. Drug regimens were adjusted with particular attention to results of renin‐aldosterone profiling. Mean BP of the entire cohort decreased from 149/87 mm Hg to 129/78 mm Hg at 1 year (P<.0001), without a significant change in the number of antihypertensive medications. The authors observed that referral to a hypertension specialist was worthwhile and associated with a significant reduction in BP without an increase in the number of BP medications used at 1 year.
Hypertension is a common condition, affecting approximately one third of the US adult population.1 Hypertension is an important risk factor for cardiovascular events, including myocardial infarction and stroke, and uncontrolled blood pressure (BP) is associated with worse outcomes.1 Despite these risks and the availability of numerous effective antihypertensive medications, fewer than half of patients with hypertension have controlled BP.2 Among patients with hypertension, the prevalence of resistant hypertension (defined as a BP above goal despite use of at least 3 antihypertensive agents, one of which is a diuretic) is estimated to be 13% but may be even higher.3 , 4 It is recommended that physicians refer patients to a hypertension specialist if after use of maximal therapy, often with 3 drugs, they still fail to achieve BP control. In patients with resistant or uncontrolled hypertension it is often helpful to ensure that combinations of therapy are being utilized that work on different pathophysiological pathways in order to optimize BP control. BP is regulated by a variety of mechanisms, which include (1) increase in sodium‐related volume expansion, for which diuretics are particularly effective; (2) autoregulation, for which direct vasodilators and calcium channel blockers are effective; (3) activation of hormonal systems, for which angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, mineralocorticoid receptor blockers, and direct renin inhibitors are effective; and (4) sympathetic nervous system activity, for which centrally acting sympatholytics, α‐blockers, β‐blockers, and a combination of these are effective. Effective combination therapy remains largely empirical and some patients are not controlled despite blockade of these systems of BP control. We reasoned that a hypertension specialist may be able to improve BP control in drug‐resistant hypertensive patients, despite adherence to a 2‐ or 3‐drug regimen, and we conducted this study to evaluate BP control 1 year after referral to a hypertension specialist.
Methods
Study Population
We conducted a retrospective chart review of adult patients who underwent a new patient evaluation by a single physician (D.C.) at the Hypertension Program at the University of Pennsylvania between 2005 and 2010. Patients who had at least 1 year of follow‐up were eligible to be included in our study. Patients were excluded if they had not had a plasma renin activity (PRA) and plasma aldosterone concentration (PAC) measured at baseline, as these tests are part of our initial evaluation of new patients with resistant hypertension and also helped determine the treatment protocol. Patients were also excluded if they had a known cause of secondary hypertension such as pheochromocytoma present at initial evaluation. Study participants were 18 years or older except for one patient who was 15 years old at the time of the initial hypertension evaluation. The study protocol was approved by the University of Pennsylvania's institutional review board with waiver of written informed consent.
BP Measurement
All BPs were measured by auscultation using a mercury sphygmomanometer and appropriately sized cuff in the outpatient office. Patients maintained an upright seated position with feet flat on the floor for at least 5 minutes prior to measurements with the patient's arm supported at the approximate level of the heart. All study participants had BP measured at baseline and at 1 year. At each visit, BP was measured twice in the same arm, with at least 1 minute between readings; the average of the two readings was used for data analysis. At the baseline visit, 13 patients had BP measured only once, and 19 patients had BP measured only once at 1 year. For these patients, the single BP reading was used to represent the mean BP for the respective visit.
Renin‐Aldosterone Profiling and Medication Use
At the baseline visit, PRA and PAC were measured with the patient in a seated upright posture. All medications, except for aldosterone antagonists, were continued prior to testing, and patients did not receive sodium loading. Patients in our program were assigned into 1 of 4 categories based on the results of their baseline renin‐aldosterone profile. Results of the renin‐aldosterone profiling were then used to tailor BP management. The patients were divided into the following groups depending on their renin‐aldosterone profile: normal (1 ng/mL/h < PRA <15 ng/mL/h, PAC/PRA ratio <20), salt‐sensitive (PRA ≤1 ng/mL/h, PAC < 15 ng/dL), hyperaldosteronism (PAC ≥15 ng/dL, PAC/PRA ratio ≥20), and high renin (PRA ≥15 ng/mL/h).
Approach to Treatment
Our general approach to treatment was to evaluate and maximize all patients' drug regimens to include at least one drug where appropriate from 1 of the 4 categories described above (diuretic, vasodilator, sympatholytic, and renin‐angiotensin‐aldosterone system [RAAS] blocker). Renin‐aldosterone profiling was also considered with regard to choice of drug therapy. If a patient fell into the normal group, a drug such as a β‐blocker or RAAS blockade was added to therapy, as renin was not suppressed. If a patient was salt‐sensitive, a diuretic was initiated or maximized and often a potassium‐sparing diuretic was added in addition to a thiazide‐type or loop diuretic. If a patient's renin‐aldosterone profiling was consistent with hyperadosteronism, a potassium‐sparing diuretic (ie, spironolactone, eplerenone, or amiloride) was added with consideration for adrenal vein sampling and possible adrenalectomy. If a patient fell into the high renin category, consideration for renal artery stenosis was given; however, this decision was individualized on a case to case basis. All changes to medications were made by a single physician (D.C.) during the course of the study. Adherence to medications was assessed at each visit and was based on patient self‐report, but this variable was not recorded in the data. Antihypertensive medication use was recorded at baseline and at 1 year for each patient. Medications were counted and categorized by class. Diuretics were further subcategorized as potassium‐sparing or not. All patients, regardless of profile results, received counseling to limit dietary sodium and alcohol intake and to increase exercise and weight loss. Patients were also encouraged to record their home BP readings twice daily and to fax in their readings every 2 to 3 weeks. Changes were made to medication regimens at office visits and according to home readings between office visits. Other baseline laboratory data, such as serum creatinine and potassium, were also measured.
Outcome
The primary outcome of interest was the change in BP from baseline to 1 year after referral to the program. Systolic BP (SBP), diastolic BP (DBP), and mean arterial pressure (MAP) readings were collected during the year. Secondary outcomes were comparisons of the number and class of medications used at baseline and at 1 year.
Data Management
All demographic and clinical data for study participants were recorded in the outpatient electronic medical record at the time of the visit. For the purposes of the study, data were later extracted from the medical record, entered into a spreadsheet, and then imported into statistical software.
Statistical Analysis
Descriptive statistics are presented as means or medians for continuous variables that are normally distributed or non‐normally distributed, respectively. Categoric variables are presented as percentages. Student paired t tests were used to compare continuous data at baseline with data at 1 year. Chi‐square and McNemar's tests were used for the comparison of categoric variables. One‐way analysis of variance (ANOVA) tests with Bonferroni adjustments were used for comparisons of normally distributed data among categories of renin‐aldosterone profiles. The assumptions of ANOVA, homogeneity of variance, and normality were verified for each variable with Bartlett's test. When the null hypothesis was rejected for ANOVA tests, t tests for independent samples were used for pairwise comparisons. For comparison of non‐normally distributed data among profile categories, Kruskal‐Wallis test was performed.
A P value of <.05 was determined, a priori, to represent the nominal threshold for statistical significance. Analyses were performed using STATA version 12.0 (StataCorp LP, College Station, TX).
Results
A total of 158 patients were included in the study. Baseline characteristics are provided in Table 1. The study cohort was predominantly (74%) white and had a slightly higher proportion of female patients. The changes in BP and number of medications from baseline to 1 year are shown in Table 2. At 1 year, SBP, DBP, and MAP decreased significantly from baseline for the entire study group from baseline (P<.0001), without a statistically significant difference in the number of medications required (P=.77).
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | Data |
|---|---|
| Number of subjects | 158 |
| Male, n (%) | 73 (46.2%) |
| Female, n (%) | 85 (53.8%) |
| Age, y | 54 [43‐65] |
| Race, n (%) | |
| White | 117 (74) |
| African American | 33 (21) |
| Other | 6 (3.8) |
| Missing | 2 (1.3) |
| BMI, kg/m2 | 29.7 [26.4–33.8] |
| eGFR, mL/min/1.73 m2 | 78.4 ± 25.5 |
| Diabetes mellitus, n (%) | 18 (11.4%) |
Values are mean ± SD or median [IQR]. Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate. eGFR calculated using the four‐variable MDRD equation. For the two subjects with missing race data, eGFR was calculated assuming non‐black race.
Table 2.
Change in BP and Number of Antihypertensive Medications Among All Study Participants
| Characteristic | Baseline | 1 y |
|---|---|---|
| SBP, mm Hg | 149 ± 23 | 129 ± 19* |
| DBP, mm Hg | 87 ± 15 | 78 ± 14* |
| MAP, mm Hg | 108 ± 15 | 95 ± 14* |
| Number of BP meds | 3.2 ± 1.9 | 3.3 ± 1.8 |
Values are mean ± SD. Abbreviation: MAP, mean arterial pressure. *P<.0001.
We also analyzed results according to renin‐aldosterone profiling. Table 3 shows the baseline characteristics as well as changes in BP and number of medications, stratified by profile category. Patients were categorized as normal, salt‐sensitive, hyperaldosteronism, or high renin, which comprised 36.1%, 27.2%, 25.3%, and 11.4% of the full cohort, respectively. Age, body mass index, and estimated glomerular filtration rate did not statistically differ among patients in the 4 categories. Mean serum potassium in the hyperaldosteronism group was 3.7 mmol/L at baseline, which was lower than that of the other 3 groups (P<.0001). Baseline SBPs were not different among the 4 groups (P=.1). The high renin group had lower baseline DBP than the other 3 groups (P=.002). At 1 year, SBPs and DBPs were not different when compared across the 4 categories (P=.19 and P=.74, respectively). At baseline, patients in the normal group were taking fewer medications compared with those in the hyperaldosteronism group (P=.02) but not compared with the other two groups. The number of medications were not different among the 4 groups at 1 year (P=.2).
Table 3.
Baseline Demographic and Clinical Characteristics As Well As Change in BP and Number of Antihypertensive Medications From Baseline to 1 Year by Renin‐Aldosterone Profile Category
| Characteristic | Normal | Salt‐sensitive | Hyper‐aldosteronism | High renin |
|---|---|---|---|---|
| n (%) | 57 (36.1) | 43 (27.2) | 40 (25.3) | 18 (11.4) |
| Age, y | 49.8 ± 17.2 | 54.8 ± 13.8 | 55.1 ± 13.5 | 52.8 ± 19.6 |
| Male, n (%) | 20 (35.1) | 17 (39.5) | 28 (70) | 7 (38.9) |
| eGFR, mL/min/1.73 m2 | 83.4 ± 25.9 | 80.2 ± 24.3 | 73.3 ± 23.3 | 69.7 ± 29.5 |
| Baseline serum potassium, mmol/L | 4.1 ± .5 | 4.1 ± .5 | 3.7 ± .4¶ | 4.5 ± .6 |
| SBP baseline, mm Hg | 149 ± 25 | 154 ± 24 | 149 ± 22 | 138 ± 18 |
| DBP baseline, mm Hg | 88 ± 16 | 87 ± 13 | 90 ± 14 | 75 ± 15** |
| SBP 1 y, mm Hg | 131 ± 19* | 130 ± 19* | 123 ± 17* | 132 ± 24 |
| DBP 1 y, mm Hg | 80 ± 16* | 78 ± 12† | 77 ± 10* | 77 ± 16 |
| Number of BP meds baseline | 2.8 ± 2.0∥ | 3.1 ± 1.9 | 3.9 ± 1.7 | 3.5 ± 1.4 |
| Number of BP meds 1 y | 2.9 ± 1.8 | 3.6 ± 1.8‡ | 3.4 ± 1.8§ | 3.5 ± 1.4 |
Values are mean ± SD or median [IQR]. Within group comparisons from baseline to 1 year: *P<.0001, † P=.0006, ‡ P=.004, § P=.03. Between group comparisons: normal compared to hyperaldosteronism ∥ P=.02, hyperaldosteronism compared to other three groups ¶ P<.0001, high renin compared to other three groups **P=.002.
When assessing the longitudinal change in BP, there was a statistically significant decrease in SBP from baseline to 1 year in the normal, salt‐sensitive, and hyperaldosteronism groups (P<.0001). There was also a statistically significant decrease in DBP in these groups. There was no change in either SBP or DBP in the high renin group. However, there was a statistically significant decrease in SBP in men in the high renin group (P=.013). We also explored racial differences in BP change. Within the hyperaldosteronism group, there was a clinically significant decrease in SBP and DBP in African Americans (151/90–135/84 mm Hg). Although this change was not statistically significant, there were only 8 African American patients in this group. There were no other race or sex differences in BP change.
Figure 1 demonstrates that BP improvement in the salt‐sensitive group was likely related to the increased use of diuretics (P=.0015). Similarly, as shown in Figure 2, there was a statistically significant increase in the use of potassium‐sparing diuretics in the hyperaldosteronism group at 1 year (P=.0009). During the 1 year of follow‐up, 17 (42.5%) patients in the hyperaldosteronism group underwent adrenal vein sampling (AVS), of which 10 patients proceeded to adrenalectomy because of lateralization on AVS. One additional patient in this group did not have AVS but underwent adrenalectomy as a result of the large size of an adrenal mass found on imaging.
Figure 1.
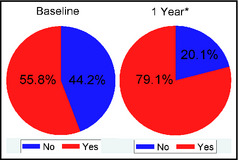
Diuretic use in the salt‐sensitive group at baseline and 1 year. Percentage of subjects in the salt‐sensitive group who were being treated with a diuretic at baseline and 1 year. *P=.0015.
Figure 2.
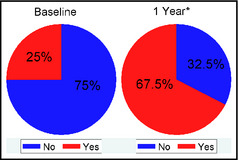
Use of a potassium‐sparing diuretic in the hyperaldosteronism group at baseline and 1 year. Percentage of subjects in the hyperaldosteronism group who were being treated with a potassium‐sparing diuretic at baseline and 1 year. *P=.0009.
At 1 year, the number of antihypertensive medications did not change from baseline in the normal or high renin groups. The number of medications in the salt‐sensitive group increased at 1 year (3.1–3.6, P=.004), while patients in the hyperaldosteronism group were taking fewer medications at 1 year (3.9–3.4, P=.03). When the latter analysis was restricted to the 29 patients who did not undergo adrenalectomy, there was still a trend toward use of fewer medications at 1 year (4.2–3.7), which was not statistically significant (data not shown, P=.09).
Discussion
In this retrospective chart review of patients with uncontrolled hypertension, we observed a significant improvement in BP control at 1 year after referral to a hypertension specialist. We also observed that BP was significantly improved without an increase in the number of medications used to improve BP control. By individualizing therapy for patients with uncontrolled hypertension referred to a hypertension center, BP control can be achieved in most patients. This was accomplished by ensuring that patients who require multiple medications are receiving medications that work on the different pathophysiological pathways involved in BP regulation. In addition, by adding renin‐aldosterone profiling, this helped the physician tailor treatment further. Communicating with patients every 2 to 3 weeks and adjusting medications in response to home BP readings likely also contributed to improved BP control. The average number of antihypertensive medications needed to achieve BP control was 3 or 4 medications. It has been shown in multiple studies that for stage 2 hypertension, most patients will require 3 or 4 medications to achieve BP control.5, 6 We also encouraged the use of combination therapies to improve adherence of patients and to simplify the treatment regimens.
Hypertension is a heterogeneous disease in terms of genetic influence, pathophysiology, and phenotype. Patient characteristics (eg, sex, race, comorbidities) can be determinants of response to a certain antihypertensive medication class, but a “one‐size‐fits‐all” approach to treatment may contribute to inadequate BP control. The RAAS plays an integral role in vasoconstriction and in the regulation of sodium and volume homeostasis and thus, is a major determinant of BP. By assessing the level of activity of the RAAS, this information is additive and helps providers gain a better picture of the underlying pathophysiology of this complex disease in an individual patient. PRA and PAC are simple, relatively inexpensive tests to perform that may provide valuable information regarding the pathophysiologic mechanism responsible for maintaining elevated BP. Previous studies have described the utility of renin‐aldosterone profiling in hypertension as a rational approach to medication selection,7, 8, 9 and we feel it is worthwhile to perform renin‐aldosterone profiling in this referred hypertensive population, as the results contribute to decision making and drug choice.
Stratification of patients into categories according to renin‐aldosterone profiling also demonstrated several interesting phenomena. Patients with renin‐aldosterone profiles suggesting aldosterone excess comprised approximately 25% of the cohort, providing further support for how common this diagnosis is among patients with hypertension, especially in a referral population (shown by Calhoun and others).10, 11 We did not routinely perform confirmatory testing with sodium loading in these patients and acknowledge that the prevalence of primary aldosteronism is likely overestimated; however, identifying patients with hyperaldosteronism is crucial, given the detrimental effects of aldosterone on cardiac remodeling (ie, increased left ventricular mass,12 myocardial fibrosis,13 and cardiovascular risk that exceeds that of essential hypertension.14 With treatment of aldosterone excess via medication or adrenalectomy, cardiovascular risk matches that of essential hypertension.14
SBP and DBP decreased during follow‐up in all groups except the high renin group. However, the latter group accounted for only a small percentage (11.4%) of the total number of patients. The renin‐aldosterone profiling was not helpful in guiding therapy in these patients, and BP control was the most difficult to achieve in this subgroup. In patients identified as salt‐sensitive, the decrease in BP was coupled with a small but statistically significant increase in the number of antihypertensive medications, accounting for additional diuretics required in this group. On the other hand, patients identified as having hyperaldosteronism required fewer medications at 1 year. Potassium‐sparing diuretics are the treatment of choice for these patients, and the increased use of potassium‐sparing diuretics likely allowed for other antihypertensive medications to be discontinued. In the patients identified as having normal renin‐aldosterone profiling, they did not require an increase in the number of medications to achieve improved BP control. This suggests that by individualizing and better tailoring therapy, there was no need to increase the overall number of antihypertensive medications overall. Our findings highlight the value of the renin‐aldosterone profile for the identification of important subgroups of hypertension and for providing a physiologic rationale for medication selection.
Study Limitations
There are several limitations of this study. It is a retrospective chart review and therefore presents limitations common to this study design, such as the lack of a comparison group that did not undergo renin‐aldosterone profiling. We did not routinely perform ambulatory BP monitoring to confirm the diagnosis of true hypertension in these patients and, as approximately one third of patients with seemingly resistant hypertension may have white‐coat hypertension,15 we acknowledge that the improvement in BP in up to one third of our cohort may have occurred without intervention. Although we did not specifically record medication adherence, our experience in a referral setting for complex hypertension has been that patients tend to be highly motivated and have high rates of adherence. As nonadherence would tend to bias results toward the null, our results are further strengthened if nonadherence had been a major influence. Although additional interventions (such as increased exercise, dietary sodium restriction, and minimizing alcohol intake) were recommended and likely contributed to improvement in BP, these data did not allow for determining the individual or collective effect of these interventions. The exclusion of patients with <1 year of follow‐up may have introduced a survivorship bias, although we did not collect information regarding the nature of loss to follow‐up. With regards to renin‐aldosterone profiling, we based this on a single measurement of PRA and PAC. Previous studies have demonstrated the variability in PRA and PAC assays depending on time of day,16 posture of the patient,16 or assay method.17 Furthermore, for those in the hyperaldosteronism group, sodium loading was not routinely performed, which could potentially increase the false‐positive rate. The impact of this misclassification is minimized by the finding that patients in this group exhibited an improvement in BP control. Even though only aldosterone antagonists were discontinued prior to renin‐aldosterone testing, we believe that discontinuing all medications that affect the RAAS would be impractical and potentially dangerous for most of these patients with uncontrolled BP. We advocate for interpreting PRA and PAC results in the setting of ongoing treatment as long as mineralocorticoid antagonists have been discontinued. Furthermore, the 2008 Endocrine Society guidelines for diagnosing primary aldosteronism supports this approach.18 Given the predominantly white cohort, our results may not be generalizable to individuals of other races. That a single practitioner made all treatment decisions may limit generalizability, but the findings are also strengthened by the high likelihood of a consistent approach to treatment. In addition, it should be noted that the study cohort was composed of patients specifically referred to our hypertension center, and, thus, the results are not generalizable to all patients with hypertension. The study included a relatively small number of patients but does demonstrate that referral to a hypertension specialist is worthwhile in patients with seemingly resistant hypertension taking multiple drugs in the primary care setting. The approach that includes utilizing medications from the different categories underlying the pathophysiology of hypertension coupled with the results of renin‐aldosterone profiling and providing continuous feedback to the patients was successful in improving BP control without increasing the number of medications required to control BP.
Conclusions
We observed that referral to a hypertension specialist appeared worthwhile and was associated with a significant reduction in BP without an increase in the number of BP medications used at 1 year. This study supports the recommendation that referral to a hypertension specialist or center after failed BP control using 3 medications has a beneficial impact on BP control.
J Clin Hypertens (Greenwich). 2013;15:624–629. ©2013 Wiley Periodicals, Inc.24034654
References
- 1. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294:466–472. [DOI] [PubMed] [Google Scholar]
- 3. Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–1080. [DOI] [PubMed] [Google Scholar]
- 4. Townsend RR. Attending rounds: a patient with drug‐resistant hypertension. Clin J Am Soc Nephrol. 2011;6:2301–2306. [DOI] [PubMed] [Google Scholar]
- 5. Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4:393–404. [DOI] [PubMed] [Google Scholar]
- 6. Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646–661. [DOI] [PubMed] [Google Scholar]
- 7. Blumenfeld JD, Laragh JH. Renin system analysis: a rational method for the diagnosis and treatment of the individual patient with hypertension. Am J Hypertens. 1998;11:894–896. [DOI] [PubMed] [Google Scholar]
- 8. Egan BM, Basile JN, Rehman SU, et al. Plasma renin test‐guided drug treatment algorithm for correcting patients with treated but uncontrolled hypertension: a randomized controlled trial. Am J Hypertens. 2009;22:792–801. [DOI] [PubMed] [Google Scholar]
- 9. Laragh JH. Vasoconstriction‐volume analysis for understanding and treating hypertension: the use of renin and aldosterone profiles. Am J Med. 1973;55:261–274. [DOI] [PubMed] [Google Scholar]
- 10. Calhoun DA, Nishizaka MK, Zaman MA, et al. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–896. [DOI] [PubMed] [Google Scholar]
- 11. Eide IK, Torjesen PA, Drolsum A, et al. Low‐renin status in therapy‐resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22:2217–2226. [DOI] [PubMed] [Google Scholar]
- 12. Rossi GP, Sacchetto A, Visentin P, et al. Changes in left ventricular anatomy and function in hypertension and primary aldosteronism. Hypertension. 1996;27:1039–1045. [DOI] [PubMed] [Google Scholar]
- 13. Rocha R, Funder JW. The pathophysiology of aldosterone in the cardiovascular system. Ann N Y Acad Sci. 2002;970:89–100. [DOI] [PubMed] [Google Scholar]
- 14. Catena C, Colussi G, Nadalini E, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168:80–85. [DOI] [PubMed] [Google Scholar]
- 15. de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902. [DOI] [PubMed] [Google Scholar]
- 16. Tiu SC, Choi CH, Shek CC, et al. The use of aldosterone‐renin ratio as a diagnostic test for primary hyperaldosteronism and its test characteristics under different conditions of blood sampling. J Clin Endocrinol Metab. 2005;90:72–78. [DOI] [PubMed] [Google Scholar]
- 17. Stowasser M, Gordon RD. Aldosterone assays: an urgent need for improvement. Clin Chem. 2006;52:1640–1642. [DOI] [PubMed] [Google Scholar]
- 18. Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. [DOI] [PubMed] [Google Scholar]


