Abstract
Although many studies focus on patients with resistant hypertension, general practitioners (GPs) are more likely to face patients in clinical practice with not‐at‐goal hypertension, whose antihypertensive treatment needs to be modified. However, information regarding such patients is limited. In the present study, 710 GPs in France each included their first 10 not‐at‐goal hypertensive patients, ie, the patients for whom they decided to modify antihypertensive treatment. The study population was composed of 7032 patients (58% men, mean age 62.4±11.5 years). Anthropometric and biologic measurements and clinical data were collected, and vascular age and 10‐year cardiovascular risk were estimated by standard formula. Of 7032 participants, cardiovascular risk factors were prevalent, with 15.1% current smokers, 26.1% obese, 22.8% with diabetes mellitus, 35.1% with dyslipidemia, 12.0% with left ventricular hypertrophy, and 4.9% with renal insufficiency. In the subgroup (n=4697) of patients aged between 30 and 74 years and undergoing primary cardiovascular prevention, vascular age was superior (13 to 28 years) when compared with chronological age in different subgroups. The patients' estimated 10‐year cardiovascular global risk was 25.3±13.6%, with 16.0±10.5% for coronary heart disease, 8.7±6.8% for myocardial infarction, 5.8±4.5% for stroke, and 6.8±6.6% for cardiovascular mortality. Patients with not‐at‐goal hypertension in primary care bear a heavy burden of cardiovascular diseases.
Arterial hypertension is a prevalent condition and the leading cause of various cerebrovascular and cardiovascular (CV) events and mortality.1, 2, 3 In a recent report on worldwide blood pressure (BP) control, with 786 country‐years and 5.4 million participants, Danaei and colleagues4 documented that from 1980 to 2008, the global mean reductions in systolic BP (SBP) were only 0.8 mm Hg and 1.0 mm Hg per decade for men and women, respectively. The situation is partly attributable to resistant and not‐at‐goal hypertension. In routine clinical practice, it is common for a general practitioner (GP) to have difficulty in controlling patients' BP with antihypertensive agents. In this case, a decision on the modification of chronic antihypertensive treatments needs to be made. The key for the GP to make an effective decision is to know the characteristics of their patients, as well as their future CV risk. Many studies have focused on patients with resistant hypertension, but few studies have focused on patients with not‐at‐goal hypertension, especially in a nationwide scan in general practice. We therefore conducted a cross‐sectional study of 7032 patients with not‐at‐goal hypertension, for whom their GPs decided to modify their chronic antihypertensive treatments. Our goal was to investigate the charac‐teristics and future CV risk of these patients.
Methods
Study Design
The Age Vasculaire et Risqué Résiduel Chez I'Hypertendu Traité vu en Médicine Générale (AVANT'AGE) study was an epidemiological observational study that focused on patients with not‐at‐goal hypertension in general practice. A total of 710 GPs, representative of currently active GPs in France, each included the first 10 not‐at‐goal hypertensives, ie, the hypertensive patients for whom they decided to modify the chronic antihypertensive treatment. The decision of treatment modification was based on uncontrolled BP (91%) and/or poor compliance/tolerance (46%). A BP goal was set at 130/80 mm Hg for patients with diabetes, renal dysfunction, or established CV diseases, and at 140/90 mm Hg for those without these conditions. The definition of resistant hypertension and not‐at‐goal hypertension are summarized in Table 1. In total, 7032 patients (58% men) were included in the present study, with a mean age±standard deviation (SD) of 62.4±11.5 years (range, 21 to 98 years). Written informed consent was obtained from each study participant.
Table 1.
Definition of Resistant Hypertension and Not‐At‐Goal Hypertension
| Resistant Hypertension | Not‐At‐Goal Hypertension |
|---|---|
| BP remains above goal in spite of the concurrent use of ≥3 antihypertensive agents of different classes at optimal dosages, with one of the agents a diuretic | Patients' chronic antihypertensive treatment needs to be modified by their GPs because of uncontrolled BP and/or poor compliance/tolerance |
Abbreviations: BP, blood pressure; GP, general practitioner.
Anthropometric, Clinical, and Biological Parameters
Body height, body weight, and waist circumference were measured and body mass index (BMI) was calculated as body weight in kilogram divided by the square of body height in meters. Clinical information was collected from the patient's medical document by the GPs for each participant, including smoking habit and the presence of diabetes mellitus, dyslipidemia, coronary heart disease, microalbuminuria, or renal insufficiency, as well as the use of medications. Left ventricular hypertrophy (LVH) was previously defined by echocardiography with the criteria of left ventricular mass index >125 g/m2 (men) and >110 g/m2 (women), and reviewed by the GPs in patients' medical records. Biochemical tests were not performed in the context of our study, but previously obtained biological data were used for characterizing each patient.
Antihypertensive Treatment
Use of antihypertensive agents was recorded for each patient by his or her GP, including number of antihypertensive agents and their categories. Antihypertensive agents were then categorized into 4 classes, namely diuretics, anti–renin‐angiotensin‐aldosteronism (RAS) system agents (including angiotensin‐converting enzyme inhibitors, angiotensin receptor inhibitors, and renin inhibitors), calcium channel blockers (CCBs), and antiadrenergic agents (including β‐blockers and central‐acting agents).
BP Measurement
Each participant's BP was measured with the electronic device currently used by his or her GP after at least 5 minutes of rest in the sitting position.
Estimation of 10‐Year CV Risk by the Framingham Formula and Vascular Age
Patients' absolute risk for 10‐year CV diseases were estimated by the Framingham formula proposed by Anderson and colleagues5 based on conventional CV risk factors (age, total and high‐density lipoprotein [HDL] cholesterol, BP, diabetes, and smoking status). The relative risk was estimated as the calculated risk of the present population divided by the risk of a similar population in terms of nonmodifiable risk factors (age and sex) but optimal modifiable risk factors (SBP=120 mm Hg, no diabetes, no LVH, no smoking, total/HDL cholesterol=4). Risk excess was estimated as the calculated risk of the present population minus the risk of the above‐mentioned optimal population. Vascular age was estimated based on lipoprotein, according to the formula proposed by D'Agostino and colleagues.6
Statistics
Anthropometric, clinical, and biological parameters were compared between men and women by Student's t test and Fisher's exact test for quantitative and qualitative variables, respectively. Percentages of the use of each antihypertensive agent were calculated in patients with 1, 2, 3, and ≥4 antihypertensive therapies, and were compared by chi‐square test. Differences between chronological age and vascular age were compared with 0 by the Student's t test. Analysis of variance was applied to test the associations of estimated 10‐year CV diseases and mortality with age. Statistical analysis was performed using SAS software, version 9.1 (SAS Institute, Cary, NC). P<.05 was considered statistically significant.
Results
In the 7032 participants, mean SBP and diastolic BP (DBP) were 154.2±12.5/89.6±9.1 mm Hg; mean total and low‐density lipoprotein (LDL) cholesterol were 5.38±1.04 and 3.31±0.91 mmol/L, respectively; and mean plasma glucose was 6.98±1.57 mmol/L. In addition, other CV risk factors were also prevalent in this population, with 15.1% current smokers, 26.1% obese, 22.8% with diabetes mellitus, 35.1% with dyslipidemia, 12.0% with LVH, and 4.9% with renal insufficiency. Table 2 shows characteristics of participants by sex. Specifically, men, compared with women, had significantly higher BMI (27.9±4.2 vs 27.0±5.4 kg/m2, P<.001) and waist circumference values (100.4±11.9 vs 91.2±13.7 cm, P<.001); higher DBP (89.8±9.0 vs 89.3±9.1 mm Hg, P=.03), plasma glucose (7.05±1.56 vs 6.85±1.59 mmol/L, P<.001), and triglycerides (1.22±0.27 vs 1.18±0.27 mmol/L, P<.001); more frequently reported smoking (18.9% vs 10.3%, P<.001); and a higher prevalence of related disorders (P≤.048) and corresponding treatments (P≤.004). Women, on the contrary, were significantly older (age, 63.8±12.0 vs 61.4±11.0 years, P<.001) and had significantly higher SBP (154.6±12.7 vs 154.0±12.4 mm Hg, P=.04), pulse pressure (65.3±12.3 vs 64.2±11.8 mm Hg, P<.001), and total (5.44±1.00 vs 5.35±1.06 mmol/L, P<.001) and high‐density lipoprotein (HDL) cholesterol (1.46±0.43 vs 1.33±0.41 mmol/L, P<.001).
Table 2.
Characteristics of Participants by Sex
| Total (N=7032) | Men (n=4073) | Women (n=2959) | P Value | |
|---|---|---|---|---|
| Age, y | 62.4±11.5 | 61.4±11.0 | 63.8±12.0 | <.001 |
| Body mass index, kg/m2 | 27.6±4.8 | 27.9±4.2 | 27.0±5.4 | <.001 |
| Waist circumference, cm | 96.5±13.5 | 100.4±11.9 | 91.2±13.7 | <.001 |
| Current smoke, No. (%) | 1058 (15.3) | 760 (18.9) | 298 (10.3) | <.001 |
| Systolic BP, mm Hg | 154.2±12.5 | 154.0±12.4 | 154.6±12.7 | .04 |
| Diastolic BP, mm Hg | 89.6±9.1 | 89.8±9.0 | 89.3±9.1 | .03 |
| Mean BP, mm Hg | 111.1±8.6 | 111.2±8.6 | 111.1±8.7 | .58 |
| Pulse pressure, mm Hg | 64.6±12.0 | 64.2±11.8 | 65.3±12.3 | <.001 |
| Heart rate, beats per s | 75.6±8.8 | 75.5±8.9 | 75.8±8.7 | .16 |
| Plasma glucose, mmol/L | 6.98±1.57 | 7.08±1.56 | 6.85±1.59 | <.001 |
| Hemoglobin A1c, % | 6.74±1.08 | 6.71±1.04 | 6.77±1.13 | .29 |
| Total cholesterol, mmol/L | 5.38±1.04 | 5.35±1.06 | 5.44±1.00 | <.001 |
| LDL cholesterol, mmol/L | 3.31±0.94 | 3.29±0.97 | 3.33±0.91 | .11 |
| HDL cholesterol, mmol/L | 1.39±0.42 | 1.33±0.41 | 1.46±0.43 | <.001 |
| Triglyceride, mmol/L | 1.20±0.27 | 1.22±0.27 | 1.18±0.27 | <.001 |
| Diabetes mellitus, No. (%) | 1572 (22.8) | 955 (23.9) | 617 (21.2) | .008 |
| Dyslipidemia, No. (%) | 2007 (35.1) | 1246 (37.8) | 761 (31.4) | <.001 |
| Left ventricular hypertrophy, No. (%) | 753 (12.0) | 289 (12.7) | 298 (11.1) | .048 |
| Coronary heart disease, No. (%) | 519 (7.8) | 387 (10.1) | 132 (4.7) | <.001 |
| Microalbuminuria, No. (%) | 399 (7.1) | 257 (7.8) | 142 (6.1) | .01 |
| Renal insufficiency, No. (%) | 328 (4.9) | 166 (4.3) | 162 (5.8) | .004 |
| Antidiabetic therapy, No. (%) | 1537 (22.1) | 938 (23.3) | 599 (20.5) | .004 |
| Antihyperlipidemic therapy, No. (%) | 3110 (44.7) | 1932 (47.9) | 1178 (40.3) | <.001 |
| Antiplatelet therapy, No. (%) | 1947 (28.1) | 1247 (31.0) | 700 (24.0) | <.001 |
Abbreviations: BP, blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein. Values are presented as mean±standard deviation or numbers (percentages). Disorders and therapy were defined by reading patients' medical document.
As shown in Table 3, 1 antihypertensive treatment was used in 74.4% of patients, 2 treatments in 19.9%, 3 treatments in 4.8%, and ≥4 in 0.9% of participants. The trend of antihypertensive agent use was investigated in patients with increasing number of antihypertensive therapy. Specifically, the use of diuretics and CCBs were similar in patients with different numbers of antihypertensive agents, but a progressively decreasing use of anti‐RAS system agents, as well as a progressively increasing use of antiadrenergic agents, were detected in patients with increasing number of antihypertensive therapy (P<.001).
Table 3.
Use of Antihypertensive Agents in Patients With Not‐At‐Goal Hypertension
| Overall (N=7032) | One Agent (n=5232) | Two Agents (n=1397) | Three Agents (n=340) | Four or More Agents (n=63) | P Value | |
|---|---|---|---|---|---|---|
| Diuretics, No. (%) | 2048 (29.1) | 1134 (21.7) | 624 (22.3) | 233 (23.8) | 57 (22.6) | .81 |
| Anti‐RAS system agents, No. (%) | 4232 (60.2) | 3013 (57.6) | 979 (70.1) | 277 (81.5) | 53 (84.1) | <.001 |
| Calcium channelblocker, No. (%) | 2120 (30.2) | 1102 (21.1) | 724 (25.9) | 243 (23.8) | 51 (20.3) | <.001 |
| Antiadrenergic agents, No. (%) | 1385 (19.7) | 441 (8.4) | 627 (44.9) | 254 (74.7) | 63 (100.0) | <.001 |
Values are presented as numbers (percentage). Anti–renin‐angiotensin‐aldosteronism (RAS) system agents include angiotensin‐converting enzyme inhibitors, angiotensin receptor inhibitors, and renin inhibitors. Antiadrenergic agents include β‐blockers and central‐acting agents.
In the subgroup (n=4697) aged between 30 and 74 years and in primary CV prevention, patients' vascular age estimated by lipoprotein was compared with patents' chronological age. Vascular age was significantly greater (13 to 28 years) than the chronological age in the different age subgroups (Table 4, P<.001).
Table 4.
Comparison of Patients' Chronological Age and Vascular Age Estimated by Lipoprotein
| Age Groups | Chronological Age | Vascular Age by Lipoprotein | Difference | P Value |
|---|---|---|---|---|
| Overall (N=4697) | 59.0±9.1 | 81.2±8.0 | 22.2±8.1 | <.001 |
| 30–39 y (n=124) | 36.5±2.5 | 61.3±13.4 | 24.9±12.9 | <.001 |
| 40–49 y (n=619) | 43.6±2.8 | 73.9±11.4 | 28.3±10.8 | <.001 |
| 50–59 y (n=1491) | 54.8±2.9 | 81.1±6.8 | 26.3±6.6 | <.001 |
| 60–69 y (n=1808) | 64.0±2.8 | 84.0±3.5 | 20.0±4.1 | <.001 |
| 70–74 y (n=655) | 71.8±1.4 | 84.6±2.0 | 12.8±2.4 | <.001 |
Vascular age was estimated based on lipoprotein according to the formula proposed by D'Agostino and colleagues. Values are presented as means±standard deviation of Patients' age.
Finally, in the subgroup aged between 30 and 74 years and in primary CV prevention (n=4697), Patients' estimated 10‐year CV global risk was 25.3%±13.6%. Specifically, the 10‐year risk for coronary heart disease, myocardial infarction, stroke, and CV mortality were 16.0%±10.5%, 8.7%±6.8%, 5.8%±4.5%, and 6.8%±6.6%, respectively. In comparison with patients with optimal modifiable CV risk factors, relative risk was 2.2±1.4 for coronary heart disease, 3.9±4.2 for myocardial infarction, 3.8±2.2 for stroke, and 4.1±4.5 for CV mortality, and the corresponding risk excess were 8.7%±6.8%, 6.0%±4.5%, 4.3%±4.1%, and 4.6%±5.5%, respectively. Furthermore, as shown in the Figure, with advancing age, the estimated 10‐year CV risk and risk excess as compared with optimal group increased progressively and significantly (P<.001), whereas relative risk as compared with optimal group decreased (P<.001), except for the relative risk for stroke (P=.34).
Figure .
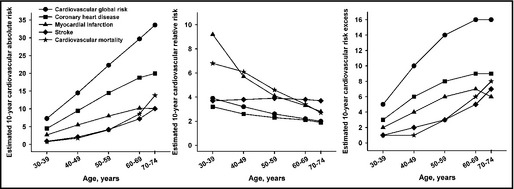
Estimated 10‐year cardiovascular absolute risk, relative risk, and risk excess in patients with not‐at‐goal hypertension by age. All trends of estimated 10‐year cardiovascular risk with age were statistically significant (P<.001) except for the relative risk for stroke (P=.34). Ten‐year cardiovascular absolute risk was estimated by the Framingham formula; relative risk was estimated as the calculated risk of the present population divided by the risk of a similar populations in terms of nonmodifiable risk factors (age and sex) but optimal modifiable risk factors (systolic blood pressure=120 mm Hg, no diabetes, no left ventricular hypertrophy, no smoking, total/high‐density lipoprotein cholesterol=4). Risk excess was estimated as the calculated risk of the present population minus the risk of the above‐mentioned optimal population.
Discussions
The present study contains two major findings: (1) patients with not‐at‐goal hypertension in primary care were characterized with aggregation of CV risk factors, as well as high estimated 10‐year CV risk; and (2) the absolute and excess risk of estimated 10‐year CV diseases increased with advancing age, but the relative risk decreased.
Patients with not‐at‐goal hypertension, as shown in the present study, had a high prevalence of many CV risk factors, namely 15.1% were current smokers, 26.1% were obese, 39.3% of men and 53.5% of women had abdominal obesity, 22.8% were diabetic, 35.1% had dyslipidemia, 12.0% had LVH, and 4.9% had renal insufficiency. Cuspidi and colleagues7 also documented that, compared with patients with controlled BP, patients with resistant hypertension had a significant higher prevalence of LVH, increased carotid intima–media thickness, and microalbuminuria. Similar findings could also be observed in other studies.8, 9 Those findings and ours showed that in patients with not‐at‐goal or resistant hypertension, the aggregation of CV risk factors was frequently reported, indicating that the resistant condition of BP control in these patients was partly attributable to the observed risk aggregation. In other words, the key to successful BP control in these patients probably lies in the effective countermeasures to those risk factors.
In the present study, we also noted that use of diuretics and CCBs were similar in patients with not‐at‐goal hypertension, with almost 30% for each, whereas patients taking more antihypertensive agents were less likely to take anti‐RAS system agents but more antiadrenergic agents. This trend in antihypertensive therapy of not‐at‐goal hypertensive patients was in accordance with current guideline.10
It is not surprising that in patients with not‐at‐goal hypertension, vascular age was superior of 13 to 28 years than the chronological age in the different age subgroups. Moreover, Patients' estimated 10‐year CV risk increased with advancing age and reached about 1 out of 3 for CV global risk, about 1 out of 5 for CHD, and about 1 out of 10 for myocardial infarction and stroke. Compared with a similar population with optimal modifiable risk factors, the excess risk in patients with not‐at‐goal hypertension also increased with age, but the relative risk decreased. In other words, the young patients with not‐at‐goal hypertension, compared with older patients, would have higher relative CV risk than their healthy counterparts. This finding was likely attributed to the fact that younger patients have fewer CV risk factors than older patients, so uncontrolled hypertension would be the only abnormality, with fewer other competing risk factors in term of risk assessment. Other reports also mentioned a decreasing relative risk of cardiovascular disease with increasing age in hypertensive patients. Antihypertensive treatment benefit was less efficient in the elderly in terms of relative risk reduction but more efficient in terms of absolute risk reduction.11
It is also interesting to note that, in the present study, these patients were recruited mainly because of their high BP, but high BP is obviously not the only uncontrolled risk factor. For instance, mean plasma glucose and waist circumference in the present population were 7.08±1.56 mmol/L and 100.4±11.9 mm in men and 6.85±1.59 mmol/L and 91.2±13.7 mm in women, respectively, which were all higher or around the normal range. This finding indicated that in patients with not‐at‐goal hypertension, although high BP was apparently the major problem, other risk factors, especially obesity and high plasma glucose, also need to be considered.
Limitations
It is important to note that our study has some limitations. First, the 710 GPs were not randomly chosen from all French GPs; nevertheless, they were representative of the French GP population in terms of age, sex, and geographic location. Second, the concept of “not‐at‐goal hypertension” is not a well‐accepted concept, such as resistant hypertension or severe hypertension, but we wanted to assess a “real hypertensive” population in which the GP decided to modify the antihypertensive treatment. Third, no specific complementary examinations were performed in the setting of our study, and therefore the prevalence of some complications such as LVH or coronary heart disease was probably underestimated. Finally, antihypertensive drugs were not randomly allocated and the cause‐and‐effect relationship cannot be assessed, but again, our goal was to be in the real hypertensive life in the setting of primary care.
Conclusions
In summary, patients with not‐at‐goal hypertension in primary care bear a heavy burden of CV diseases, with the aggregation of many CV risk factors and high estimated 10‐year CV risk. Moreover, CV burden grows with advancing age, but the relative risk decreases. Medical attention is needed in order to decrease the elevated CV risk in this specific population.
Acknowledgments
SERVIER was the sole sponsor of the AVANT'AGE study. Jacques Blacher, principal investigator of the AVANT'AGE study, received honoraria from SERVIER. The authors would like to acknowledge the contribution of each general practitioner and each patient who accepted to enter the AVANT'AGE study. The authors thank Jean‐Charles Kerihuel for statistical analysis performance. The authors would like to thank the SERVIER Company who made this study possible, namely their representatives Stéphane Curti, Thibault Lefebvre, and Caroline Crison.
J Clin Hypertenas (Greenwich). 2013;00:00–00. ©2013 Wiley Periodicals, Inc.
References
- 1. Staessen JA, Wang J, Bianchi G, Birkenhäger WH. Essential hypertension. Lancet. 2003;361:1629–1641. [DOI] [PubMed] [Google Scholar]
- 2. Kearney PM, Whelton M, Reynolds K, et al. Worldwide prevalence of hypertension: a systematic review. J Hypertens. 2004;22:11–19. [DOI] [PubMed] [Google Scholar]
- 3. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 4. Danaei G, Finucane MM, Lin JK, et al; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Pressure) . National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country‐years and 5.4 million participants. Lancet. 2011;377:568–577. [DOI] [PubMed] [Google Scholar]
- 5. Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–298. [DOI] [PubMed] [Google Scholar]
- 6. D'Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 7. Cuspidi C, Macca G, Sampieri L, et al. High prevalence of cardiac and extracardiac target organ damage in refractory hypertension. J Hypertens. 2001;19:2063–2070. [DOI] [PubMed] [Google Scholar]
- 8. Pisoni R, Ahmed MI, Calhoun DA. Characterization and treatment of resistant hypertension. Curr Cardiol Rep. 2009;11:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee . Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. [DOI] [PubMed] [Google Scholar]
- 10. Mancia G, De Backer G, Dominiczak A, et al; Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology . 2007 Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 11. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]


