Abstract
J Clin Hypertens (Greenwich). 2013; 15:435–442 ©2012 Wiley Periodicals, Inc.
Allopurinol is a potent xanthine oxidase inhibitor that is used in hyperuricemic patients to prevent gout. It has also been shown to decrease cardiovascular complications in a myriad of cardiovascular conditions. However, studies have reported conflicting evidence on its effects on blood pressure (BP). A systematic review was conducted using Medline, PubMed, Embase, and the Cochrane Library for all the longitudinal studies that assessed the efficacy of allopurinol on systolic and diastolic BP. A total of 10 clinical studies with 738 participants were included in the analysis. Compared with the control group, systolic BP decreased by 3.3 mm Hg (95% confidence interval [CI], 1.4–5.3 mm Hg; P=.001) and diastolic BP decreased by 1.3 mm Hg (95% CI, 0.1–2.5 mm Hg; P=.03) in patients treated with allopurinol. When analysis was restricted to the higher‐quality randomized controlled trials, similar changes in systolic and diastolic BPs were found: 3.3 mm Hg (95% CI, 0.8–5.8 mm Hg; P<.001) and 1.4 mm Hg (95% CI, 0.1–2.7 mm Hg; P=.04), respectively. Allopurinol is associated with a small but significant reduction in BP. This effect can be potentially exploited to aid in controlling BP in hypertensive patients with hyperuricemia.
Uric acid (UA) is the end product of purine metabolism catalyzed by the enzyme xanthine oxidoreductase, which is frequently elevated in patients with gout. Hyperuricemia is commonly associated with hypertension 1 , 2 and is present in 25% of untreated patients with hypertension, in 50% of patients taking diuretics, and in >75% of patients with malignant hypertension. 1 Hypertensive patients with hyperuricemia have a 3‐ to 5‐fold increased risk of coronary or cerebrovascular disease compared with hypertensive patients with normal UA levels. 3 Allopurinol is a potential inhibitor of the xanthine oxidoreductase enzyme, a key component in the production of UA pathway. Multiple studies examining the effects of allopurinol treatment on UA levels have documented conflicting results on its effects on blood pressure (BP). 4 , 5 , 6 , 7 The quandary regarding the use of allopurinol was pointed out in an editorial by Michael Alderman 8 in which he noted that while the present evidence does not justify the use of hypouricemic therapy for cardioprotection, there is also no evidence to show that lowering UA levels may be harmful. However, Feig and colleagues 9 found that allopurinol significantly lowered BP in adolescents with newly diagnosed hypertension. In view of these findings, we decided to conduct a systematic review of the existing literature to examine the effect of allopurinol on BP.
Materials and Methods
Search Strategy
We systematically searched the electronic databases, Medline, PubMed, EMBASE, and the Cochrane Library for Central Register of Clinical Trials, using the MESH terms “allopurinol,”“blood pressure,”“blood pressure monitoring, ambulatory,” and “hypertension,” with the key words “xanthine oxidase inhibitor,”“uric acid,” and “oxypurinol.” We limited our search to studies in human patients and English language in peer‐reviewed journals from 1966 to February 2012. The reference lists of identified articles and bibliographies of original articles were also reviewed.
Study Selection
Eligible studies included (1) prospective (randomized or nonrandomized) or retrospective study designs assessing the effect of allopurinol on BP, (2) parallel or cross‐over study design, (3) availability of data for mean and standard deviation of baseline and follow‐up BP (systolic and diastolic), (4) trial duration of at least 4 weeks in each study arm, and (5) clear documentation of change or no change in other antihypertensive agents. Exclusion criteria included (1) no quantitative description of end points, (2) lack of clear and reproducible results, (3) trials reported in abstract form only, and (4) studies with duplicated data, including same group of patients or for whom there were updated results available. Since 24‐hour ambulatory BP monitoring is better than casual BP reading, we included the 24‐hour ambulatory BP reading in our analysis when available. 5 , 9
Data Extraction and Quality
The data were independently extracted by two authors (V.A. and N.H.) using standardized protocol and reporting form. Disagreements were resolved by arbitration (F.M.), and consensus was reached after discussion. We extracted characteristics of each study including baseline and follow‐up BP; baseline demographic patterns; known diagnosis of hypertension, hyperuricemia, or chronic kidney disease; type of study design; use of other antihypertensive agents; and total duration of follow‐up, among others. Authors of the papers were individually contacted in case the data were unclear.
Outcome Assessed
The main outcome of the present analysis was reduction of BP (systolic/diastolic) from baseline to follow‐up.
Quality Assessment
The study quality was evaluated according to the Jadad composite score, 10 which is a 5‐point quality scale, with low‐quality studies having a score of ≤2 and high‐quality studies a score of ≥3. 11
Data Analysis and Synthesis
An intention‐to‐treat traditional meta‐analysis was performed in accordance with the recommendations from the Cochrane Collaboration, the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) Statement for randomized controlled trials, and Meta‐Analysis of Observational Studies in Epidemiology (MOOSE) statement for others. All analyses were performed by metan command of Stata 10.1 (Stata Corporation, College Station, TX). A priori, we assumed that substantial clinical heterogeneity would be present in the included trials. We therefore planned to apply a random‐effects model (DerSimonian–Laird approach) 12 to pool the BP changes. Heterogeneity was assessed with the I 2 statistic proposed by Higgins and Thompson, 13 with I 2<25% considered low and I 2>75% considered high. Reported values are two‐tailed, and hypothesis testing results were considered statistically significant at P>.05. Small study effect, including publication bias, was tested using funnel plot and the regression intercept of Egger and colleagues 14 and corrected by the nonparametric trim‐and‐fill method of Duvall and Tweedie. We separately examined, if there were any differences in the outcomes between randomized vs nonrandomized studies, short duration of follow‐up (≤4 months) vs longer duration of follow‐up (>4 months), studies with and without chronic kidney disease patients, low‐dose allopurinol (≤300 mg) vs higher doses of allopurinol (>300 mg) in patients with normal kidney functions, and studies comparing ambulatory BP measurement vs office BP measurement. To determine whether individual studies had an undue influence on the overall results (because of size or magnitude of effect), we conducted a post hoc influence analysis using the METANINF command in STATA, which estimates the impact of single studies on the overall pooled estimates, where influential studies are identified by a large magnitude of change in BP after the exclusion of the study.
Results
Study Selection
We identified 10 clinical studies, with 10 control arms and 11 intervention arms, which fulfilled our inclusion criteria and were included in the final analysis (Figure 1). These include 9 randomized control trials 5 , 6 , 7 , 9 , 15 , 16 , 17 , 18 , 19 and 1 prospective study. 4 Of the 9 randomized studies, 3 studies with 4 comparison arms were cross‐over studies. 7 , 9 , 17 The duration of follow‐up of the studies varied from 4 weeks to 2 years.
Figure 1.
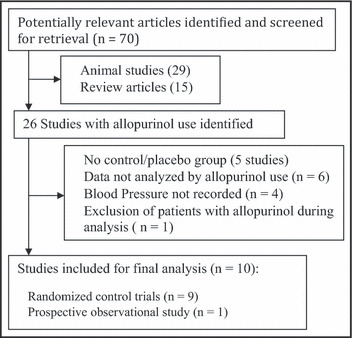
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram of study selection.
Baseline Characteristics
The overall characteristics of the included studies are listed in the Table. In total, these studies examined data of 738 patients, with a mean age ranging from 15.1 years to 71.8 years, with a total follow‐up duration of approximately 379 person‐years. The allopurinol dose was 300 mg in 4 comparison arms, 4 , 5 , 6 , 7 100 mg in 2 comparison arms, 15 , 16 400 mg in 1 comparison arm, 9 600 mg in 2 comparison arms 7 , 17 and 900 mg in 1 comparison arm. 19 While 5 studies specifically recruited patients with hyperuricemia (>6 to >7.6 mg/dL), 4 , 5 , 6 , 9 , 18 the mean UA levels in the other studies was also elevated, varying between 5 mg/dL and 7.6 mg/dL. The mean systolic BP of most of the studies was between 120 mm Hg and 140 mm Hg, with 2 studies with mean systolic BP >140 mm Hg 15 , 16 and 1 study with systolic BP <120 mm Hg. 19 While the majority of the studies had diastolic BP between 70 mm Hg and 80 mm Hg, 3 studies had diastolic BP >80 mm Hg. 4 , 9 , 16 While the majority of the studies had a short follow‐up duration (≤4 months), 2 studies had a long follow‐up duration of 12 months and 24 months. 15 , 18 While 3 studies specifically looked at patients with underlying kidney dysfunction, including chronic kidney disease 15 , 18 and diabetic nephropathy, 16 3 studies allowed the uptitration of other BP medications during the follow‐up period. 15 , 16 , 18
Table TABLE.
Baseline Characteristics of Studies Included in the Meta‐Analysis
| Author (Y) | Design | Duration of Follow‐Up | Daily Allopurinol Dose, mg | Jadad Score | Relevant Features of Study |
|---|---|---|---|---|---|
| George et al. (2006) 7 | RCT, cross‐over | 4 wk | 300 and 600 | 5 | Included: documented LV systolic dysfunction with NYHA class III and IV symptoms, mean serum UA=7.1 mg/dL; excluded: serum Cr >2.03 mg/dL or uncontrolled hypertension (>160/90 mm Hg) |
| Siu et al. (2006) 18 | RCT | 12 mo | 100–300 | 3 | Included: CKD (proteinuria >0.5 g/d or serum Cr >1.35 mg/dL), serum UA >7.6 mg/dL; excluded: advanced CKD (>4.5 mg/dL); other: change in dosage of other medications during trial period allowed |
| Feig et al. (2008) 9 | RCT, cross‐over | 4 wk | 400 | 5 | Included: adolescents (11–17 y), new diagnosis of stage I essential HTN, serum UA >6 mg/dL; excluded: pre‐HTN/stage II HTN, or taking other antihypertensive medications |
| Khan et al. (2008) 6 | RCT | 8 wk | 300 | 5 | Included: stroke survivors, serum UA ≥6.4 mg/dL |
| Noman et al. (2010) 17 | RCT, cross‐over | 6 wk | 600 | 5 | Included: angiographically documented CAD, positive exercise tolerance test, stable chronic angina (≥2 mo), mean serum UA=6.1 mg/dL; excluded: GFR <45 mL/min or serum Cr >2.36 mg/dL |
| Goicoechea et al. (2010) 15 | RCT | 24 mo | 100 | 3 | Included: CKD (eGFR <60 mL/min), stable kidney function, and no recent hospitalization or cardiac events, mean serum UA=7.6 mg/dL; excluded: active infection; other: change in dosage of other medications during trial period; |
| Momeni et al. (2010) 16 | RCT | 4 mo | 100 | 5 | included: diabetic nephropathy (serum Cr <3 mg/dL and proteinuria >500 mg/d), mean serum UA=6.2 mg/dL; excluded: advanced CKD (serum Cr >3 mg/dL); other: additional antihypertensive drugs such as BB could be added |
| Kanbay et al. (2011) 5 | RCT | 4 mo | 300 | 3 | Included: asymptomatic hyperuricemic patients (serum UA >7 mg/dL), normal kidney function; excluded: use of ACE inhibitor/ARB/statin/supplemental vitamins or history of CAD or DM |
| Dogan et al. (2011) 19 | RCT | 12 wk | 900 | 3 | Included: diabetic normotensive patients, mean serum UA=5 mg/dL; excluded: patients taking ACE inhibitor/BB/CCB or with known CAD/CHF |
| Kanbay et al. (2007) 4 | Prospective | 3 mo | 300 | 1 | Included: only eGFR >60 mL/min; intervention group=serum UA >7 mg/dL and controls=normouricemic; excluded: uncontrolled HTN or DM or CAD/CHF/PAD |
Abbreviations: BB, β‐blocker; CAD, coronary artery disease; CCB, calcium channel blocker; CHF, congestive heart failure; CKD, chronic kidney disease; Cr, creatinine; eGFR, estimated glomerular filtration rate; LV, left ventricular; HTN, hypertension; PAD, peripheral arterial disease; RCT, randomized control trial; UA, uric acid.
Quality Assessment
Since we included both randomized and observational studies in our analyses, the included studies were of variable quality. There were 5 studies of good quality (Jadad score ≥3) with low risk of bias and 7 studies of low quality (Jadad score <3) with high risk of bias.
Antihypertensive Efficacy
When all the studies were combined, irrespective of the study design, the mean baseline BP in these studies was 133.5±15.7/78.3±10 mm Hg. After treatment with allopurinol over a mean follow‐up period of 6.2 months, the systolic BP decreased by 3.3 mm Hg (95% confidence interval [CI]: 1.4–5.3 mm Hg; P= .001) (Figure 2) and the diastolic BP by 1.3 mm Hg (95% CI: 0.1–2.5 mm Hg; P=.03) (Figure 3). As expected with the variable nature and quality of studies, there was significant heterogeneity among the observational studies (I 2=87.3%, P<.001). When we restricted the analysis to only the higher‐quality randomized control studies, the changes in systolic and diastolic BP were 3.3 mm Hg (95% CI: 0.8–5.8 mm Hg; P<.001) and 1.4 mm Hg (95% CI: 0.1–2.7 mm Hg; P=.04). No one study was found to be influential on the overall changes in the systolic and diastolic BP (4, 5, respectively).
Figure 2.
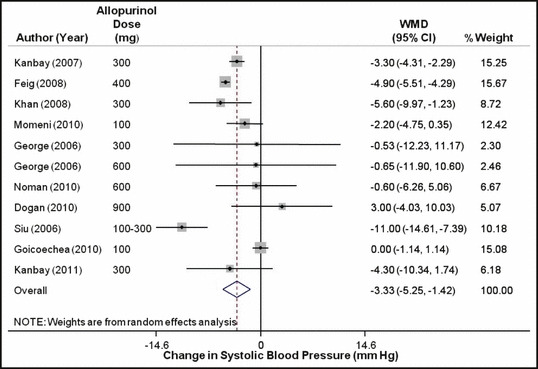
Forest plot showing the effect of allopurinol on systolic blood pressure. WMD indicates weighted mean difference; CI, confidence interval.
Figure 3.
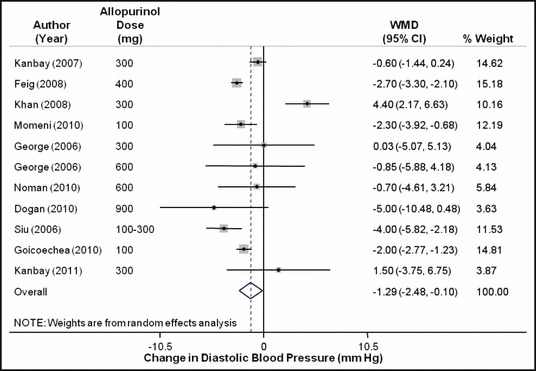
Forest plot showing the effect of allopurinol on diastolic blood pressure. WMD indicates weighted mean difference; CI, confidence interval.
Figure 4.
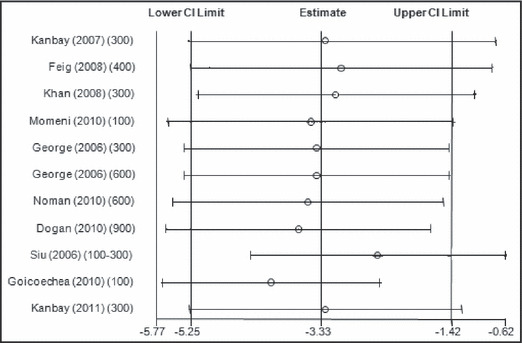
Metaninf analysis for changes in systolic blood pressure with allopurinol use. CI indicates confidence interval.
Figure 5.
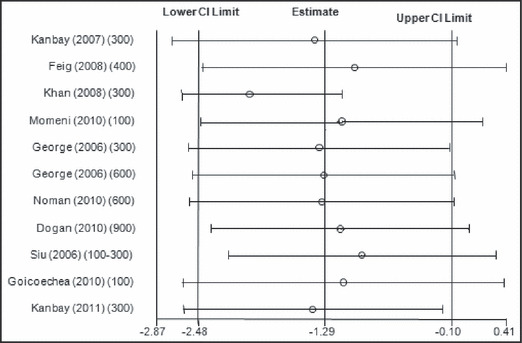
Metaninf analysis for changes in diastolic blood pressure with allopurinol use. CI indicates confidence interval.
The various subgroup analyses for the changes in the systolic and diastolic BP are presented in 6, 7 respectively.
Figure 6.
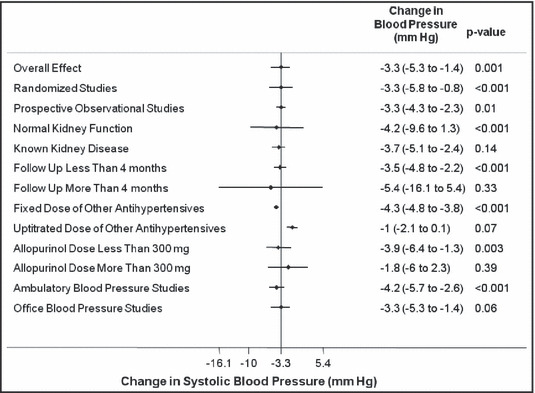
Subgroup analysis for changes in systolic blood pressure with allopurinol use.
Figure 7.
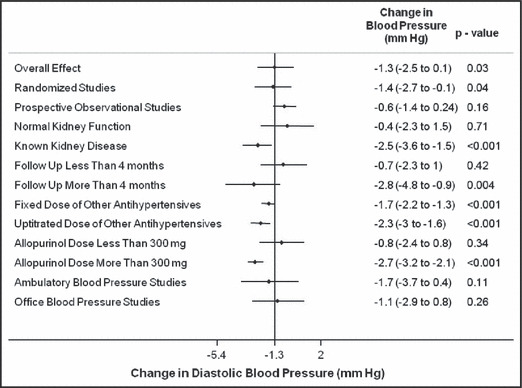
Subgroup analysis for changes in diastolic blood pressure with allopurinol use.
Discussion
In our analysis we found that allopurinol use was associated with a small but significant lowering of systolic BP by 3.3 mm Hg and diastolic BP by 1.3 mm Hg. While there was significant heterogeneity between trials, most of it could be explained by differences in the methodological quality of the trials. Even when the results were restricted to the higher‐quality studies, the differences in systolic and diastolic BP was 3.3 mm Hg and 1.4 mm Hg, respectively.
UA is produced by the degradation of the purine nucleotides, the last steps of this process being mediated by the enzyme xanthine oxidoreductase. Increased UA levels have been strongly associated with multiple cardiovascular and renal diseases, including insulin resistance, hypertension, dyslipidemia, endothelial dysfunction, and renal impairment. 20 While most studies have identified UA as an independent risk factor for various cardiovascular conditions, including hypertension, the Framingham Heart Study was a major notable exception, which concluded that UA did not have a causal role in the development of coronary heart disease, death from cardiovascular disease, or death from all causes. 21
Although the precise pathophysiologic role of UA in the causation of hypertension remains unclear, multiple hypotheses have been proposed to explain the association between elevated UA levels and hypertension. 22 Recent studies have shown that UA can produce both pro‐oxidant and antioxidant effects in humans. 23 , 24 Under normal conditions, reactive oxygen species are generated because of the activity of xanthine oxidoreductase. These reactive oxygen species levels are also regulated by and broken down by other enzymes. Under conditions of increased xanthine oxidoreductase activity, there is greater production of these reactive oxygen species than can be regulated and broken down by these other regulating enzymes. This leads to accumulation of the reactive oxygen species, which impairs nitric oxide production and consequently produces endothelial dysfunction. 25 In addition to its dichotomous role in oxidation, UA stimulates C‐reactive protein production and inhibits endothelial proliferation, which can also lead to endothelial dysfunction by directly affecting the endothelial and smooth muscle cells of the vascular wall. 26 Endothelial dysfunction not only plays an important role in the pathophysiology of hypertension, 27 but it may also precede the development of hypertension. 28 UA also has been shown to induce smooth muscle proliferation, activate the vascular renin‐angiotensin system, and increase the production of angiotensin II. 29
Similar to the study by Feig and colleagues, 9 which showed a significant reduction in systolic and diastolic BP, our analysis showed that allopurinol was associated with a reduction in systolic and diastolic BP, but the magnitude of the BP change was much smaller. There are many potential explanations for this discrepancy. The most important change is the difference in the patient populations. Feig and colleagues looked at young adolescents (mean age: 15.1 years) with new‐onset hypertension not taking any other hypertensive medication. In contrast, our analysis represents a mixed cohort of patients with mean age ranges from 15.1 to 71.8 years with other comorbidities, including chronic kidney disease, who may have had long‐standing hyperuricemia and hypertension. In addition to being associated with endothelial dysfunction due to impaired nitric oxide production, UA by itself can potentially worsen kidney function by producing additional vasoconstriction by decreasing prostaglandin production and increasing angiotensin II production via the renin‐angiotensin system, with proliferation of vascular smooth muscles. 20 This causes periglomerular vascular injury, glomerular hypertension, and reduced kidney perfusion, which can consequently lead to interstitial fibrosis. 2 , 20 Hence, it is possible that once renal damage has occurred due to a combination of hyperuricemia and hypertension, allopurinol is not as effective in reducing BP. Another possible explanation is associated with the dosage of allopurinol in the included studies. It has been shown that there possibly exists a steep dose‐response relationship between allopurinol and endothelial function, such that 600 mg/d is more effective than 300 mg/d of allopurinol. 7 Hence, while the study by Feig and colleagues used an allopurinol dose of 400 mg/d, the allopurinol dose used in analyzed clinical studies varied from 100 mg/d to 900 mg/d.
Although allopurinol reduced systolic and diastolic BP, the possible beneficial effects of allopurinol need to be weighed against their potential adverse effects. The potential side effects vary from a minor rash to life‐threatening allopurinol hypersensitivity syndrome, reported in 0.38% of inpatients treated with allopurinol, 30 which may include acute renal failure, hepatitis, toxic epidermal necrolysis, fever, leukocytosis, or any combination of these symptoms. These risks increase further in patients with underlying renal insufficiency, as both allopurinol and its major metabolite oxypurinol are excreted by the kidneys.
Limitations
This systematic review has several limitations. Although a few of the studies included in the analysis were double‐blinded randomized controlled trials, other studies were of relatively poor quality, especially with regards to treatment allocation and concealment. It is known that studies with inadequate concealment of treatment allocation may overestimate the actual treatment effect. Most of the studies were not designed to measure the effect of allopurinol on BP and consequently many of the studies had patients with relatively well‐controlled BPs and the dosage of other antihypertensives may have changed during the follow‐up period. Also, the results are subject to limitations inherent to any meta‐analysis based on pooling of data from different trials with different inclusion criteria, different designs, variable follow‐up duration with differing attrition rates, and different patient populations. Another inherent limitation of meta‐analysis is the limitation of data reported in published articles and lack of access to individual patient‐level data. Access to patient‐level data would have been particularly helpful for our subgroup analyses and to explain the heterogeneity among studies. As in other meta‐analyses, given the lack of data in each trial, we did not adjust our analyses for compliance to assigned therapy.
Conclusions
Allopurinol produced small but significant changes in systolic and diastolic BP. Allopurinol and other xanthine oxidase inhibitors may be utilized as adjunctive antihypertensive agents in select hypertensive patient populations with underlying hyperuricemia while closely monitoring for any adverse effect.
Disclosures: Vikram Agarwal and Nidhi Hans: no relationships to disclose. Franz Messerli has served as an ad hoc consultant for Novartis, Boehringer Ingelheim, Daiichi Sankyo, Sanofi, and Takeda, and has received research funding and grants from Novartis, Boehringer Ingelheim, and Forest. None of the authors received any compensation for their work on this manuscript.
References
- 1. Cannon PJ, Stason WB, Demartini FE, et al. Hyperuricemia in primary and renal hypertension. N Engl J Med. 1966;275:457–464. [DOI] [PubMed] [Google Scholar]
- 2. Nagahama K, Inoue T, Iseki K, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res. 2004;27:835–841. [DOI] [PubMed] [Google Scholar]
- 3. Verdecchia P, Schillaci G, Reboldi G, et al. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension. 2000;36:1072–1078. [DOI] [PubMed] [Google Scholar]
- 4. Kanbay M, Ozkara A, Selcoki Y, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227–1233. [DOI] [PubMed] [Google Scholar]
- 5. Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6:1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khan F, George J, Wong K, et al. Allopurinol treatment reduces arterial wave reflection in stroke survivors. Cardiovasc Ther. 2008;26:247–252. [DOI] [PubMed] [Google Scholar]
- 7. George J, Carr E, Davies J, et al. High‐dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–2516. [DOI] [PubMed] [Google Scholar]
- 8. Alderman MH. Podagra, uric acid, and cardiovascular disease. Circulation. 2007;116:880–883. [DOI] [PubMed] [Google Scholar]
- 9. Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 11. Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Ann Intern Med. 2001;135:982–989. [DOI] [PubMed] [Google Scholar]
- 12. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 14. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Momeni A, Shahidi S, Seirafian S, et al. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010;4:128–132. [PubMed] [Google Scholar]
- 17. Noman A, Ang DS, Ogston S, et al. Effect of high‐dose allopurinol on exercise in patients with chronic stable angina: a randomised, placebo controlled crossover trial. Lancet. 2010;375:2161–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–59. [DOI] [PubMed] [Google Scholar]
- 19. Dogan A, Yarlioglues M, Kaya MG, et al. Effect of long‐term and high‐dose allopurinol therapy on endothelial function in normotensive diabetic patients. Blood Press. 2011;20:182–187. [DOI] [PubMed] [Google Scholar]
- 20. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131:7–13. [DOI] [PubMed] [Google Scholar]
- 22. Kobrin I, Frohlich ED, Ventura HO, Messerli FH. Renal involvement follows cardiac enlargement in essential hypertension. Arch Intern Med. 1986;146:272–276. [PubMed] [Google Scholar]
- 23. Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Santos CX, Anjos EI, Augusto O. Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophys. 1999;372:285–294. [DOI] [PubMed] [Google Scholar]
- 25. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. [DOI] [PubMed] [Google Scholar]
- 26. Khosla UM, Zharikov S, Finch JL, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. [DOI] [PubMed] [Google Scholar]
- 27. Wallace SM, Yasmin , McEniery CM, et al. Isolated systolic hypertension is characterized by increased aortic stiffness and endothelial dysfunction. Hypertension. 2007;50:228–233. [DOI] [PubMed] [Google Scholar]
- 28. Victor VM, Rocha M, Sola E, et al. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des. 2009;15:2988–3002. [DOI] [PubMed] [Google Scholar]
- 29. Corry DB, Eslami P, Yamamoto K, et al. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin‐angiotensin system. J Hypertens. 2008;26:269–275. [DOI] [PubMed] [Google Scholar]
- 30. McInnes GT, Lawson DH, Jick H. Acute adverse reactions attributed to allopurinol in hospitalised patients. Ann Rheum Dis. 1981;40:245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]


