Abstract
The beneficial effects of nebivolol on arterial stiffness and endothelial dysfunction are well documented in untreated hypertensive patients and differ from nonvasodilatory β‐blockers. This study tests the hypothesis that the addition of nebivolol in predominantly African American patients with type 2 diabetes already receiving maximally tolerated doses of renin‐angiotensin system (RAS) blockers will further improve large artery compliance. Patients with type 2 diabetes and hypertension on maximal RAS blockade (n=70) were randomized to nebivolol or metoprolol succinate daily. Doses were titrated until systolic blood pressure (SBP) was <130 mm Hg. Radial artery applanation tonometry and pulse wave velocity (PWV) analysis were used to derive central aortic pressures and hemodynamic indices at repeated visits at intervals during a 6‐month period. Both metoprolol succinate and nebivolol groups demonstrated reductions in brachial SBP (−8.2±4.3 mm Hg [P=.01] and −7.8±3.7 [P=.002], respectively) and aortic DBP (−2.4±1.8 [P=.039] and −4.0±2.9 mm Hg [P=.013], respectively). Aortic SBP decreased in the nebivolol group only (125.3±8 to 121.6±8.2, P=.025). There were no between group differences in aortic SBP, DBP, augmentation index, or PWV reduction. A significant increase in hemoglobin A1c was observed only in the metoprolol group. In patients with well‐controlled type 2 diabetes and hypertension treated with maximally tolerated RAS blockade, nebivolol does not offer significant reductions in aortic BP over metoprolol succinate but maintains a stable metabolic profile.
Nebivolol, a highly selective β1‐antagonist, has different chemical and mechanistic properties than previously developed β‐blockers. Compared with conventional β1‐selective adrenergic receptor antagonists such as metoprolol succinate, nebivolol exerts additional vasodilatory properties by stimulating endothelial cell nitric oxide (NO) production1, 2 mediated by β3‐receptor activation3 and interaction with the estrogen receptor.4 Nebivolol decreases oxidative stress in primary hypertension and increases NO bioavailability through upregulation of endothelial NO synthase (eNOS) and reduction of circulating asymmetric dimethylarginine (ADMA).5, 6 Nebivolol administration also restores NO bioavailability in endothelial cells obtained from African Americans who have an impaired release of NO to conventional stimuli.7
Central pressure is also more closely associated with cardiovascular (CV) outcomes relative to brachial blood pressure (BP).8 Increases in pulse wave velocity (PWV) indicating stiffer vessels are associated with increases in CV risk and chronic kidney disease (CKD) progression.9, 10, 11 Data from the Conduit Artery Function Evaluation (CAFE) trial12 show that agents that reduce central aortic as well as peripheral pressures are associated with a lower CV risk profile compared with agents that lower only peripheral pressures. Traditional β‐blockers, eg, atenolol or metoprolol, while reducing CV risk in people with coronary disease do not affect aortic stiffness compared with blockers of the renin‐angiotensin system (RAS). Vascular compliance studies comparing traditional β‐blockers with newer vasodilating β‐blockers in patients with type 2 diabetes on maximally dosed background RAS blockade have not been performed. Given that patients with diabetes have increased pulse wave velocities compared with age‐matched patients without diabetes13 and that African Americans have about a 50% lower response to stimuli of NO, the present study was designed to examine the effect of nebivolol therapy compared with metoprolol succinate therapy on peripheral and aortic arterial pressure as well as aortic compliance. The null hypothesis tested states that there will be no benefit on aortic compliance with nebivolol compared with metoprolol in the presence of maximally tolerated RAS therapy in a predominantly African American population with type 2 diabetes.
Methods
Study design
The EFFORT trial is assessing the impact of treatment with nebivolol vs metoprolol succinate on central aortic pressures and hemodynamics in patients with type 2 diabetes already taking maximally tolerated RAS therapy. The study utilized a randomized, parallel‐group blinded endpoint (PROBE) design (Figure 1.
Figure 1.
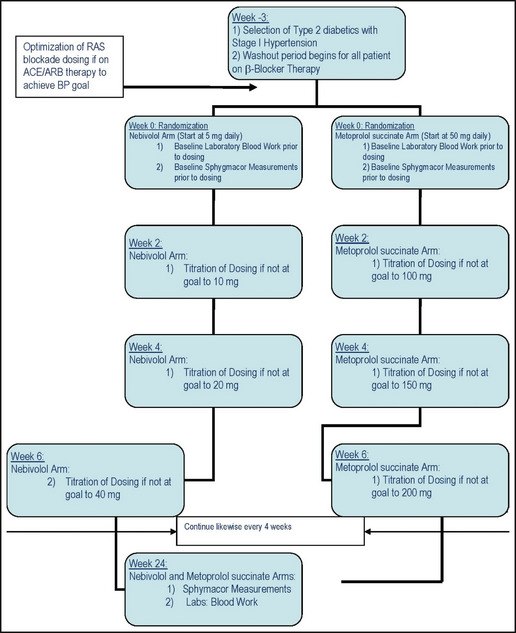
Study design. RAS indicates renin‐angiotensin system; ACE, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure.
Inclusion criteria for the trial included age older than 50 years, established type 2 diabetes for at least 5 years not treated with insulin (hemoglobin A1c [HbA1c] <8.5%), and elevated BP (>130/80 mm Hg and <160/100 mm Hg) on a maximally tolerated dose of a RAS blocker before randomization. Exclusion criteria included presence of an acute myocardial infarction, unstable angina, stroke, heart failure, or transient ischemic attack within the past year, atrial fibrillation, an estimated glomerular filtration rate (eGFR) <45 mL/min/1.73 m2, and history of asthma.
Patients taking pre‐existing β‐blockade had a washout period of 3 weeks. Randomization occurred concurrently and patients were randomized to either nebivolol or metoprolol succinate arms. Every 2 weeks thereafter a visit occurred for titration of study medication followed by once a month for BP monitoring and measurements of PWV, augmentation index (AI), and aortic compliance using a Sphygmacor device (AtCor Medical, Sydney, New South Wales, Australia). At each titration visit, if the target BP of 130/80 mm Hg was not reached, up‐titration of the study drugs took place. The last study visit took place at month 6 and all final laboratory measurements were performed at that time. For patients randomized to nebivolol, the initial dose was 5 mg, titrated to a maximum of 40 mg if needed, to achieve the BP goal at 2‐week intervals if the BP was not at goal. For patients randomized to metoprolol succinate, the initial dose was 50 mg daily, with titration up to 200 mg every 2 weeks if BP goal was not attained.
Laboratory measurements
Laboratory measurements were assessed at week 0, 12, and 24 (all in fasting state) and included serum lipids and subfractions, glucose, HbA1c, serum electrolytes (sodium, potassium, chloride, bicarbonate), and assessment of eGFR as well as urine for albumin: creatinine. All samples went through a central laboratory, Quest Labs (Chicago, IL).
Brachial artery BP measurement and radial artery pulse‐wave analysis
BP measurements, using American Heart Association guidelines, were obtained at each visit using automated machines that were validated in previous clinical trials or manual sphygmomanometer‐derived values taken by a qualified technician or physician. In all cases, the mean of 3 separate BP measurements over a 5‐minute period was used. Heart rate was also assessed in the same manner each time.
Pulse wave velocity measurements were obtained using the Sphygmacor device (AtCor) with applanation tonometry. Change was assessed from the radial artery. A physician with formal training of the device performed the procedure. The device automates the assessment of the AI as the ratio of the difference between the pressure at the first systolic shoulder and diastolic blood pressure (DBP) to that between DBP and the pressure at the second inflection point (AI=100×(P2−DBP)/(P1−DBP). The AI and pressure reported were obtained from the transformed and scaled waveforms that are taken as representing the central aortic BP. AI and pulse pressure were expressed as a percentage. The pulse pressure (PP) amplification between aortic PP and brachial PP (mm Hg) was calculated as the A/B ratio.
Safety assessment
Safety and tolerability were assessed by monitoring treatment‐emergent adverse events at each postrandomization clinic visit. Each side effect was noted and characterized.
Endpoints (primary and secondary)
The primary endpoint was the change from baseline to end‐of‐treatment central aortic BP between groups. Secondary endpoints were the changes in AI, PWV, brachial systolic and diastolic blood pressures, and PP from baseline to the end of treatment between the treatment groups.
Statistical analysis
The sample size of 60 patients assumed a 10% dropout rate and was computed to provide statistical power of 90% (P<.05) to detect a 3‐mm Hg difference in central aortic systolic pressure at 26 weeks. All statistical analyses were performed with SPSS (version 19.0; SPSS, Chicago, IL). All variables were tested for normal data distribution. The analyses were performed on an intention‐to‐treat as well as an on‐treatment basis. Normally distributed data were expressed as means±standard deviations. Non‐normally distributed data were presented as the median and interquartile range. For categorical variables, the chi‐square or Fisher exact tests were used to compare the distributions for the two randomized groups. Nonpaired Student t tests were used for between‐treatment comparisons of continuous variables. Post hoc mixed‐model repeated‐measures analysis was used to evaluate the endpoints of treatment over time with nebivolol and metoprolol succinate as main effects and brachial and central pressures, AI, and PWV, as well as metabolic parameters as dependent variables. Mixed models were performed using direct likelihood estimation with fixed effects of treatment, follow‐up, and interaction of treatment by follow‐up. An unstructured covariance matrix was used to model within‐patient error.
All significance tests were two‐tailed and conducted at the 5% significance level.
The protocol was approved by the institutional review board and listed on ClinicalTrials.gov (NCT00829296).
Results
Seventy patients were randomized into EFFORT: 34 to nebivolol and 36 to metoprolol succinate. The demographic, biochemical, and hemodynamic data for both treatment groups is shown in Table 1. There was no difference between the two groups in the on‐treatment demographic and clinical characteristics with the exception of statin use, which was higher at baseline in the nebivolol group. Nine patients (4 in the metoprolol and 5 in the nebivolol group) discontinued the study medications early in the 26‐week follow‐up and did not undergo final assessment; thus, they were excluded from the on‐treatment analysis. The reasons for discontinuation are discussed in the adverse events section. Thus, 61 patients (32 in the metoprolol and 29 in the nebivolol group) completed the study protocol. An intention‐to‐treat analysis was also performed as a secondary analysis and revealed similar baseline and endpoint results to the on‐treatment analysis, due to the small size of the cohort. Given that our interest was on efficacy in this small cohort, we thus reported only on those who completed the study.
Table 1.
On‐Treatment Baseline Demographic and Laboratory Characteristics
| Variables | Metoprolol (n=32) | Nebivolol (n=29) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD), y | 64.8 (5.6) | 65.5 (7.1) | .69 |
| Female, % | 23 (72) | 20 (69) | .8 |
| Race | |||
| African American, No. (%) | 25 (78.1) | 23 (79.3) | .704 |
| Caucasian, No. | 5 | 4 | – |
| Hispanic, No. | 1 | 0 | – |
| Asian, No. | 1 | 2 | – |
| Smoking | |||
| No. (%) | 19 (59.3) | 12 (41.4) | .074 |
| Former, No. | 4 | 1 | – |
| Yes, No. | 9 | 16 | – |
| Statin use, % | 19 (59.3) | 25 (86.2) | .02 |
| Body mass index | 33.4±6.1 | 34.2±6.5 | .6 |
| Renal‐metabolic | |||
| Hemoglobin A1c, mg/dL | 6.6±0.8 | 6.7±0.8 | .41 |
| eGFR, mL/min/1.73 m2 | 80.4±23.5 | 73.8±20 | .24 |
| Triglycerides, mg/dL | 127.1±77.5 | 107.9±43.8 | .25 |
| LDL, mg/dL | 87.1±29.5 | 92.7±34.8 | .5 |
| Hemodynamics | |||
| Augmentation index at 75 beats per min | 26.2±11.3 | 22.1±9.1 | .09 |
| Pulse wave velocity, m/s | 6.52±1.2 | 6.48±0.8 | .88 |
| Brachial SBP, mm Hg | 139.4±9.1 | 139.6±9.1 | .94 |
| Brachial DBP, mm Hg | 78.9±8.4 | 78.7±7.6 | .89 |
| Aortic SBP, mm Hg | 127.8±8.7 | 125.3±8 | .26 |
| Aortic DBP, mm Hg | 79.8±8.4 | 79±6.9 | .66 |
| PP amplification (central PP/brachial PP) | 0.79±0.09 | 0.77±0.08 | .14 |
Abbreviations: DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; LDL, low‐density lipoprotein; PP, pulse pressure; SBP, systolic blood pressure; SD, standard deviation.
RAS blockade
The most commonly taken angiotensin‐converting enzyme (ACE) inhibitor at baseline was lisinopril 40 mg daily: 53% of the metoprolol succinate group and 55% of the nebivolol group. The most commonly taken angiotensin receptor blocker (ARB) at baseline was losartan 100 mg daily: 46% in the metoprolol succinate group and 43% in the nebivolol group. No significant differences were present between the proportions of patients taking the different classes of agents.
Heart rate and brachial BPs
A significant reduction in heart rate was found in both treatment groups (P<.001) (Table 2). Office sitting brachial SBP decreased significantly after treatment with metoprolol succinate and nebivolol (P=.010 and P=.002, respectively) (Table 2). Brachial DBP also decreased in both groups (metoprolol group P=.031, nebivolol P=.007) (Table 2). However, no significant differences in BP decrease were noted between the two experimental groups at any time point throughout the study.
Table 2.
Changes in Hemodynamic and Metabolic Parameters at 6 Months
| Metoprolol (n=32) | Nebivolol (n=29) | Between‐Group P Value | |
|---|---|---|---|
| Heart rate, beats per min | |||
| Baseline | 74.5±12.9 | 76.5±10.1 | .63 |
| 26 weeks | 66.3±12.8 | 65.1±10.4 | |
| Within‐group P value | <.001 | <.001 | |
| Brachial SBP, mm Hg | |||
| Baseline | 139.4±9.1 | 139.6±9.1 | .67 |
| 26 weeks | 131.8±12 | 132.8±10.7 | |
| Within‐group P value | .010 | .002 | |
| Brachial DBP, mm Hg | |||
| Baseline | 78.9±8.4 | 78.7±7.6 | .33 |
| 26 weeks | 76.2±8 | 74.4±7.9 | |
| Within‐group P value | .031 | .007 | |
| Aortic SBP, mm Hg | |||
| Baseline | 127.8±8.8 | 125.3±8.0 | .34 |
| 26 weeks | 123.8±11.1 | 121.6±8.2 | |
| Within‐group P value | .1 | .025 | |
| Aortic DBP, mm Hg | |||
| Baseline | 79.8±8.4 | 78.9±6.9 | .3 |
| 26 weeks | 77.4±8.4 | 74.9±8.1 | |
| Within‐group P value | .039 | .013 | |
| Pulse pressure amplification | |||
| Baseline | 0.79±0.09 | 0.77±0.08 | .1 |
| 26 weeks | 0.85±0.12 | 0.83±0.09 | |
| Within‐group P value | .02 | .003 | |
| Augmentation index at 75 beats per min | |||
| Baseline | 26.2±8.3 | 22.0±7.1 | .19 |
| 26 weeks | 24.6±6.7 | 22.1±7.4 | |
| Within‐group P value | .280 | .9 | |
| Pulse wave velocity | |||
| Baseline | 6.52±1.2 | 6.48±0.8 | .86 |
| 26 weeks | 6.4±1.3 | 6.3±0.9 | |
| Within‐group P value | .55 | .55 | |
| Hemoglobin A1c | |||
| Baseline | 6.55±0.81 | 6.72±0.81 | .9 |
| 26 weeks | 7.0±1.6 | 6.72±0.84 | |
| Within‐group P value | .007 | .39 | |
| Total cholesterol | |||
| Baseline | 165.34±31.3 | 169.8±40.8 | .894 |
| 26 weeks | 166.65±33.2 | 167±30.9 | |
| Within‐group P value | .62 | .83 | |
| Estimated GFR | |||
| Baseline | 80.4±23.5 | 73.8±18.9 | .55 |
| 26 weeks | 74.1±22.3 | 75.0±21.3 | |
| Within‐group P value | .008 | .63 | |
Abbreviations: DBP, diastolic blood pressure; GFR, glomerular filtration rate; SBP, systolic blood pressure. Data are expressed as means and percentage±standard deviation.
Aortic BPs
As shown in Table 2, similar changes in aortic DBPs were detected in both groups after 26 weeks of treatment. Both metoprolol and nebivolol significantly decreased aortic DBP (P=.039 and P=.013, respectively). Nebivolol significantly decreased aortic SBP (P=.025), whereas the administration of metoprolol did not affect aortic SBP significantly. However, throughout the study the aortic SBP and DBP did not differ between the treatment groups (Figure 2).
Figure 2.
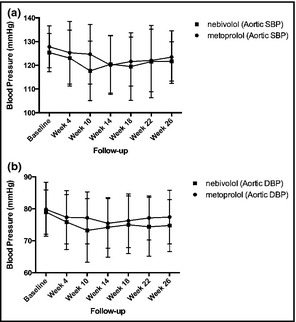
Effects of treatment on aortic (a) systolic blood pressure and (b) diastolic blood pressure.
Neither nebivolol nor metoprolol significantly decreased aortic pulse pressures at 26 weeks (nebivolol: baseline 46.75±8.3 to 49.89±12.28 mm Hg [P=.27] and metoprolol baseline 48.31±10.23 to 47±11.76 mm Hg [P=.42]). Both treatments increased pulse pressure amplification, however (metoprolol P=.02 and nebivolol .003) (Table 2). No significant correlations between heart rate differences and aortic SBP or DBP in the metoprolol or nebivolol groups were found.
AI and PWV
No significant differences in the adjusted AI for heart rate (AI75) between the metoprolol or nebivolol groups was noted (P=.85) (Table 2). Moreover, a plot of PWV and heart rate did not reveal any significant differences in PWV between the metoprolol and the nebivolol groups (P=.5 for both groups) (Table 2).
Biochemical parameters
In the metoprolol succinate group, HbA1c was increased after 26 weeks of treatment (P=.007), whereas nebivolol did not affect HbA1c (P=.39) at 26 weeks (Table 2. Total cholesterol was not affected by either metoprolol (P=.62) or nebivolol (P=.83) after 26 weeks of follow‐up (Table 2). Neither low‐density lipoprotein (LDL) nor high‐density lipoprotein (HDL) was significantly affected by either agent.
Treatment with metoprolol led to an acute and sustained 10% decrease in eGFR (P=.008), whereas nebivolol did not affect kidney function (P=.63) (Table 2).
Adverse effects
The study had relatively few adverse effects. Among the 9 patients who discontinued β‐blockers, 3 reported fatigue and dizziness and refused to undergo further follow‐up assessment. Among patients treated with metoprolol, 6 developed fatigue while 2 patients had fatigue in the nebivolol group. Four patients in the nebivolol group and 2 in the metoprolol succinate group reported diarrhea. No episodes of symptomatic bradycardia leading to discontinuation were reported. The frequency of fatigue and diarrhea did not differ statistically between treatment groups.
Discussion
The results of this study support the null hypothesis that nebivolol does not further improve aortic compliance compared with metoprolol succinate in well‐controlled predominantly African American patients with type 2 diabetes and hypertension receiving maximally tolerated RAS therapy.
The results of our study are distinctive, in that this is the first study to investigate the effects of nebivolol on aortic compliance in a predominantly African American cohort with well‐controlled type 2 diabetes and hypertension taking maximally tolerated RAS therapy. Unlike previous studies, nebivolol was not found to have the same magnitude of benefit on central pressure or PWV when used as monotherapy in people with uncontrolled hypertension and without diabetes.14, 15
There are a number of reasons for the outcomes presented. First, baseline PWV values were substantially lower than in other trials, hence the drug effect expected would be less. Second, BP and blood glucose were well controlled and generally within current guideline values. Prospective data evaluating vasodilatory β‐blockers note improvement in CV risk factors and metabolic control in patients with type 2 diabetes and hypertension.16, 17 Previous studies in small cohorts of untreated hypertensive patients generally demonstrate superior effects of nebivolol on lowering of central BPs and improving arterial stiffness compared with nonvasodilating β‐blockers. In our trial, the brachial BP‐lowering effect of both agents was similar, as was the PWV.
A randomized study of previously untreated patients with hypertension showed a significant reduction in AI and increase in PP amplification with nebivolol compared with atenolol.15 In contrast, a small randomized, double‐blind, crossover study compared atenolol 50 mg with nebivolol 5 mg in 16 patients with previously untreated isolated systolic hypertension.18 No difference was noted in brachial BPs, aortic SBP and DBP, and PWV between the treatment groups. Lastly, in a double‐blind randomized study of 80 previously untreated patients with stage 1 and 2 hypertension, Kampus and colleagues14 compared nebivolol 5 mg or metoprolol succinate 50 or 100 mg daily for 1 year. Both β‐blockers similarly reduced heart rate, brachial BP, AI, and PWV. However, central aortic BP was decreased significantly in the nebivolol group. Our data most closely match the data from Kampus and colleagues except that our patients were maximally controlled for glucose and BP.
The role of maximally tolerated RAS blockers improving vascular compliance must not be overlooked as a confounding factor. ACE inhibitors are known to improve arterial compliance and central pressure in people with diabetes and arterial stiffness.19, 20 In separate randomized, crossover, placebo‐controlled studies, ACE inhibitors and ARBs lowered central aortic BP more than brachial BP and reduced AI in hypertensive patients who were untreated.18, 21 In other clinical trials, RAS blockers and calcium channel blockers had a more pronounced and favorable effect on central arterial pressure compared with β‐blockers. Atenolol, a nonvasodilatory β‐blocker, was the most commonly studied and did not appear to reduce CV outcomes.12, 22, 23, 24
Differences in heart rate reduction between β‐blockers may have influenced the results between RAS blockers and other β‐blockers. However, in our study, all PWV measurements were corrected for a heart rate of 75, regardless of heart rate change from baseline. Thus, the effect of bradycardia did not influence data interpretation.
An additional factor to account for differences may have been baseline statin use, which differed between groups. However, there were no differences in LDL or non‐HDL cholesterol between groups and no interaction in either group with baseline statin use. While we are aware that statin use independent of cholesterol control improves vascular compliance,25 the studies are small and no firm conclusions exist about this relationship.26
The time course of follow‐up could have influenced outcome. Other studies of untreated hypertensive patients, while similar to ours, had more positive outcomes with follow‐up times although some extended to 1 year. Hence, our effect size was likely influenced by presence of maximal RAS therapy over the same time course as other studies. However, this was a main question being addressed by this study.
Another possible study limitation was use of carotid–radial PWV. Although both carotid–femoral and carotid–radial PWV are known to be accurate surrogate markers of arterial stiffness, the distinction between the two measurements was not available at the time the study started.
Lastly, β‐blockers are associated with worsening glycemic control and lipid abnormalities.27 Vasodilatory β‐blockers do not possess these problems as exemplified by the results of a large clinical trial demonstrating that carvedilol resulted in better glycemic control and increased insulin sensitivity with no change in HbA1c with neutral effects on lipids compared with metoprolol in patients with hypertension and type 2 diabetes.16 Likewise, nebivolol has similar effects on glucose and lipids.17, 28 Our data are consistent with these findings, favoring nebivolol over metoprolol in that the nebivolol group maintained stable glycemic control.
Conclusions
This study is the first to evaluate the effects of different β‐blockers in a cohort of patients with type 2 diabetes and primary hypertension already treated with maximally tolerated doses of ACE inhibitors or ARBs who also had good glycemic control. The results suggest that no specific arterial compliance benefit is conferred by β‐blockers, other than additional BP reduction, when given to already well‐controlled patients.
Conflict of interest
None of the authors has a conflict of interest with this paper except for Dr Bakris who received funding for an investigator‐initiated grant from Forest Laboratories, which generated the data for this paper.
Acknowledgments
We are very appreciative and thank Drs Roberto Lang and Amit Patel for their efforts in assisting with training and compliance measurements.
J Clin Hypertens (Greenwich). 2013;15:473–479. ©2013 Wiley Periodicals, Inc23815535
References
- 1. Ladage D, Brixius K, Hoyer H, et al. Mechanisms underlying nebivolol‐induced endothelial nitric oxide synthase activation in human umbilical vein endothelial cells. Clin Exp Pharmacol Physiol. 2006;33:720–724. [DOI] [PubMed] [Google Scholar]
- 2. Tzemos N, Lim PO, MacDonald TM. Nebivolol reverses endothelial dysfunction in essential hypertension: a randomized, double‐blind, crossover study. Circulation. 2001;104:511–514. [DOI] [PubMed] [Google Scholar]
- 3. Dessy C, Moniotte S, Ghisdal P, et al. Endothelial beta3‐adrenoceptors mediate vasorelaxation of human coronary microarteries through nitric oxide and endothelium‐dependent hyperpolarization. Circulation. 2004;110:948–954. [DOI] [PubMed] [Google Scholar]
- 4. Garban HJ, Buga GM, Ignarro LJ. Estrogen receptor‐mediated vascular responsiveness to nebivolol: a novel endothelium‐related mechanism of therapeutic vasorelaxation. J Cardiovasc Pharmacol. 2004;43:638–644. [DOI] [PubMed] [Google Scholar]
- 5. Oelze M, Daiber A, Brandes RP, et al. Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II‐treated rats. Hypertension. 2006;48:677–684. [DOI] [PubMed] [Google Scholar]
- 6. Pasini AF, Garbin U, Stranieri C, et al. Nebivolol treatment reduces serum levels of asymmetric dimethylarginine and improves endothelial dysfunction in essential hypertensive patients. Am J Hypertens. 2008;21:1251–1257. [DOI] [PubMed] [Google Scholar]
- 7. Mason RP, Kalinowski L, Jacob RF, et al. Nebivolol reduces nitroxidative stress and restores nitric oxide bioavailability in endothelium of black Americans. Circulation. 2005;112:3795–3801. [DOI] [PubMed] [Google Scholar]
- 8. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 9. Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. [DOI] [PubMed] [Google Scholar]
- 10. Kocyigit I, Kaya MG, Orscelik O, et al. Early arterial stiffness and inflammatory bio‐markers in normotensive polycystic kidney disease patients. Am J Nephrol. 2012;36:11–18. [DOI] [PubMed] [Google Scholar]
- 11. Peralta CA, Norris KC, Li S, et al. Blood pressure components and end‐stage renal disease in persons with chronic kidney disease: the Kidney Early Evaluation Program (KEEP). Arch Intern Med. 2012;172:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. [DOI] [PubMed] [Google Scholar]
- 13. Schram MT, Henry RM, van Dijk RA, et al. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension. 2004;43:176–181. [DOI] [PubMed] [Google Scholar]
- 14. Kampus P, Serg M, Kals J, et al. Differential effects of nebivolol and metoprolol on central aortic pressure and left ventricular wall thickness. Hypertension. 2011;57:1122–1128. [DOI] [PubMed] [Google Scholar]
- 15. Mahmud A, Feely J. Beta‐blockers reduce aortic stiffness in hypertension but nebivolol, not atenolol, reduces wave reflection. Am J Hypertens. 2008;21:663–667. [DOI] [PubMed] [Google Scholar]
- 16. Bakris GL, Fonseca V, Katholi RE, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292:2227–2236. [DOI] [PubMed] [Google Scholar]
- 17. Ayers K, Byrne LM, DeMatteo A, Brown NJ. Differential effects of nebivolol and metoprolol on insulin sensitivity and plasminogen activator inhibitor in the metabolic syndrome. Hypertension. 2012;59:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dhakam Z, McEniery CM, Yasmin, et al. Atenolol and eprosartan: differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens. 2006;19:214–219. [DOI] [PubMed] [Google Scholar]
- 19. Manolis AJ, Iraklianou S, Pittaras A, et al. Arterial compliance changes in diabetic normotensive patients after angiotensin‐converting enzyme inhibition therapy. Am J Hypertens. 2005;18:18–22. [DOI] [PubMed] [Google Scholar]
- 20. Hirata K, Vlachopoulos C, Adji A, O'Rourke MF. Benefits from angiotensin‐converting enzyme inhibitor ‘beyond blood pressure lowering’: beyond blood pressure or beyond the brachial artery? J Hypertens. 2005;23:551–556. [DOI] [PubMed] [Google Scholar]
- 21. Morgan T, Lauri J, Bertram D, Anderson A. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens. 2004;17:118–123. [DOI] [PubMed] [Google Scholar]
- 22. Warmack TS, Estes MA, Heldenbrand S, Franks AM. Beta‐adrenergic antagonists in hypertension: a review of the evidence. Ann Pharmacother. 2009;43:2031–2043. [DOI] [PubMed] [Google Scholar]
- 23. Davies J, Carr E, Band M, et al. Do losartan and atenolol have differential effects on BNP and central haemodynamic parameters? J Renin Angiotensin Aldosterone Syst. 2005;6:151–153. [DOI] [PubMed] [Google Scholar]
- 24. Khan BV. The effect of amlodipine besylate, losartan potassium, olmesartan medoxomil, and other antihypertensives on central aortic blood pressure and biomarkers of vascular function. Ther Adv Cardiovasc Dis. 2011;5:241–273. [DOI] [PubMed] [Google Scholar]
- 25. Akgullu C, Ozdemir B, Yilmaz Y, et al. Effect of intensive statin therapy on arterial elasticity in patients with coronary artery disease. Acta Cardiol. 2008;63:467–471. [DOI] [PubMed] [Google Scholar]
- 26. Rizos EC, Agouridis AP, Elisaf MS. The effect of statin therapy on arterial stiffness by measuring pulse wave velocity: a systematic review. Curr Vasc Pharmacol. 2010;8:638–644. [DOI] [PubMed] [Google Scholar]
- 27. Sarafidis PA, Bakris GL. Antihypertensive treatment with beta‐blockers and the spectrum of glycaemic control. QJM. 2006;99:431–436. [DOI] [PubMed] [Google Scholar]
- 28. Manrique C, Whaley‐Connell A, Sowers JR. Nebivolol in obese and non‐obese hypertensive patients. J Clin Hypertens (Greenwich). 2009;11:309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]


