Abstract
A number of conditions have been associated with functional changes of large arteries. The aim of this study was to evaluate the factors associated with aortic stiffness in patients with peripheral arterial disease (PAD). The authors studied 86 patients with PAD (ankle‐brachial pressure index [ABPI] ≤0.9) and 86 controls. Aortic stiffness was determined by pulse wave velocity (aPWV) using applanation tonometry. In PAD patients, aPWV was higher compared with controls (11±3 vs 9.8±1.8; P=.002). In multiple regression analysis, aPWV was independently associated with pulse pressure (β=0.05, P=.01) in the PAD patients and with age in the control group (β=0.08, P=.0005). The results of this study confirm an aPWV increase in patients with PAD and emphasize the association between blood pressure and aPWV. Further studies are necessary to assess whether higher aortic stiffening adds prognostic value to ABPI, which is the most powerful prognostic indicator in PAD.
Patients with peripheral arterial disease (PAD) often have cardiovascular risk factors such as diabetes mellitus, smoking, and systemic hypertension, and these conditions are associated with increased aortic stiffness.1, 2, 3 However, aortic stiffness has not received much attention in patients with PAD4 and the relative importance of classical risk factors and nonpathological factors5, 6, 7 on arterial stiffness in these patients has not been well studied. This is of clinical relevance because aortic stiffening (increased aortic pulse wave velocity [aPWV]) is considered a new cardiovascular risk factor.8 Arterial stiffening reduces the buffering capacity of the main elastic arteries, which leads to increased systolic and pulse pressure, promotes left ventricular hypertrophy and dysfunction, and impairs capacity for myocardial perfusion.9 It is an independent predictor of all‐cause and cardiovascular death in high‐risk patients10 and, in PAD patients, an association between arterial stiffness and exercise performance has also been noted.11, 12 The aim of our study was to compare aPWV in PAD patients and controls and investigate the predictors of aPWV.
Methods
Study Population
The study group included 172 patients (age range, 40–89 years) recruited from the angiology unit of the Research Center on Vascular Diseases at the University of Milan L. Sacco Hospital after receiving informed consent from each participant.
Risk Factors Assessment
Cardiovascular risk factors were ascertained through direct examination and interview by trained research assistants. Hypertension was defined as systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg at the time of the visit (mean of two readings) or history of hypertension or use of antihypertensive medications (diuretics, β‐blockers, angiotensin‐converting enzyme inhibitors, AT2‐blockers or Ca‐antagonists). Type 2 diabetes mellitus was defined as a fasting blood glucose ≥126 mg/dL or history of diabetes or of use of diabetes medications (insulin or oral hypoglycemic agents). Hypercholesterolemia was defined as total serum cholesterol >200 mg/dL or of the use of lipid‐lowering treatment. A history of angina or myocardial infarction (coronary artery disease [CAD]), stroke or transient ischemic attack (cerebrovascular disease [CVD]), and heart failure were also noted. The patients' exclusion criteria were the following: coronary revascularization or cerebrovascular events during the past 6 months, previous revascularization procedures of the lower limb, cardiac arrhythmias. All women were in the postmenopausal stage and none were taking hormonal medication. Height and weight were measured and body mass index was calculated as weight to height squared (kg/h²).
Assessment of Hemodynamics and Arterial Stiffness
Blood Pressure
Patients rested in a supine position for 5 minutes in a quiet room. Brachial blood pressure (BP) was measured in the dominant arm using a common sphygmomanometer. Three readings separated by 1‐minute intervals were taken, and the mean was used for analysis. Peripheral pulse pressure (PP) was calculated as the difference between brachial systolic BP (SBP) and diastolic BP (DBP). Mean BP (MBP) was calculated from the formula (1/3 PP+DBP).
Aortic Pulse Wave Velocity
aPWV was measured by sequentially recording electrocardiography (ECG)‐gated carotid and femoral artery waveforms. Wave transit time was calculated by software using the R wave of a simultaneously recorded ECG as a reference frame (SphygmoCor; AtCor Medical, Sydney, Australia). The distance between the carotid and the femoral sampling sites was measured above the surface of the body with a tape. aPWV was determined by dividing the distance between the two recording sites by the wave transit time.13 All measurements were made in duplicate and mean values were used for analysis. Pharmacologic treatment was suspended (when possible) 12 hours before the measurements, which took place in a comfortable environment at a temperature of 22±1°C.
In studies performed on two separate days in 19 PAD patients by a single operator, the within‐patient coefficient of variation (CV) of aPWV was 5.05%.
Ankle‐Brachial Pressure Index
BP measurements for calculation of the ankle‐brachial pressure index (ABPI) were obtained using a 8‐mHz Doppler probe and a BP cuff after 10 minutes of rest with the patient in a supine position. The systolic pressure was measured from either the posterior tibial and dorsalis pedis artery (in each leg) and was compared with the higher brachial artery pressure taken from either arm. PAD was defined as the presence of an ABPI ≤0.9.14 The participants of the study were divided into two groups: 86 patients with PAD (ABPI ≤0.9) and 86 controls (ABPI ≥0.91).
Statistical Analysis
Values are expressed as mean±standard deviation, and were compared with categorical variables using chi‐square test. Differences in the mean values were compared with the two groups using t test. A P value of <.05 was considered significant. Univariate linear regression analysis and multivariate regression models and estimating coefficient β were first built to identify variables and independent association among aPWV. R² values were reported for model with a significance level of <.05.
Results
The clinical characteristics of the groups are summarized in Table 1. The study group consisted of 86 patients with PAD (71 men, 15 women), aged 66±8 years. Among PAD patients, 23% with intermittent claudication (stage II, as defined by Fontaine), 30% had a history of smoking, 30% had a history of smoking, 77% had arterial hypertension, 43% had type 2 diabetes mellitus, 26% had CAD, and 3% had CVD. Of these, 58% of the patients were taking antihypertensive treatment, 28% were taking antidiabetic medication, 64% were taking antiplatelet therapy, and 44% were taking statin therapy. The control group consisted of 86 patients (71 men, 15 women), aged 65±9 years. Among these, 19% had a history of smoking, 44% had arterial hypertension, 33% had type 2 diabetes mellitus, and 7% had CAD. In this group, 35% were taking antihypertensive therapy, 15% were taking antidiabetic treatment, 19% were taking antiplatelet therapy, and 21% were taking statin therapy. There was no significant difference between the patients and the controls in age, sex, height, body mass index, glucose, high‐density lipoprotein, low‐density lipoprotein cholesterol, triglycerides, uric acid, creatinine, smoking and diabetes history, DBP, MBP, and heart rate. Total cholesterol and ABPI were lower in patients with PAD (P=.02; P=.0001). Glycate hemoglobin A1c (HbA1c) (P<.0001) and history of CAD (P=.0002) and hypertension (P<.0001) were higher in the PAD group. Differences between the groups also occurred in SBP (P<.001), PP (P<.0001), and aPWV (P<.002). Differences were also found in antihypertensive therapy (P=.0001), antidiabetic therapy (P=.03), and antiplatelet medication (P=.0001).
Table 1.
Clinical Characteristics of PAD and Controls
| PAD (n=86) | Controls (n=86) | P Value | |
|---|---|---|---|
| Age, y | 66±8 | 65±9 | ns |
| Men/women, No. | 71/15 | 71/15 | ns |
| Body height, cm | 165±8 | 166±9 | ns |
| Body mass index, kg/m² | 27±4 | 28±4 | ns |
| Smoking history, % | 30 | 19 | ns |
| Hypertension, % | 77 | 44 | .0001 |
| Diabetes, type 2, % | 43 | 33 | ns |
| CVD history, % | 3 | 0 | ns |
| CAD history, % | 26 | 7 | .0002 |
| Glycemia, mg/dL | 112±38 | 106±24 | ns |
| Hemoglobin A1c, % | 7±1 | 6±0.7 | .0001 |
| Total cholesterol, mg/dL | 193±44 | 209±37 | .02 |
| LDL, mg/dL | 124±39 | 133±38 | ns |
| HDL, mg/dL | 51±16 | 52±14 | ns |
| Triglycerides, mg/dL | 124±51 | 127±78 | ns |
| Uric acid, mg/dL | 5.6±1 | 5.6±1 | ns |
| Creatinine | 0.8±0.1 | 0.9±0.1 | ns |
| Systolic blood pressure, mm Hg | 142±21 | 132±20 | .001 |
| Diastolic blood pressure, mm Hg | 79±10 | 80±10 | ns |
| Pulse pressure, mm Hg | 62±20 | 51±16 | .0001 |
| Mean blood pressure, mm Hg | 101±12 | 98±13 | ns |
| Heart rate, beats per min | 68±11 | 71±12 | ns |
| Pulse wave velocity, m/s | 11±3 | 9.8±1.8 | .002 |
| ABPI | 0.7±0.1 | 1.1±0.1 | .0001 |
| Antihypertensives, % | 57 | 35 | .0001 |
| Antidiabetics, % | 28 | 15 | .03 |
| Antiplatelets, % | 64 | 19 | .0001 |
| Statins, % | 44 | 21 | ns |
Abbreviations: ABPI, ankle‐brachial pressure index; CAD, coronary artery disease; CVD, cerebrovascular disease; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; ns, not significant; PAD, peripheral arterial disease. A P value of <.05 was considered significant. Continuous variables are presented as mean±standard deviation and categorical variables are presented as percentages.
Relationship Between aPWV and Other Variables
In both groups, a significant relationship was found in the univariate analysis between aPWV and age (β=0.11, P=.0001), SBP (β=0.04, P=.0001), PP (β=0.05, P=.0001), MBP (β=0.04, P=.01), hypertension (β=1.36, P=.003), type 2 diabetes mellitus (β=1.02, P=.02), HbA1c (β=0.73, P=.0009), and antihypertension (r=0.98, P=.04), and a negative correlation was found with ABPI (β=−3.9, P=.0001) (Table 2). In the PAD group, aPWV was correlated with PP (β=0.05, P=.01) and marginally with age (β=0.13, P=.06). In the control group, aPWV was correlated with age (β=0.07, P=.0004), SBP (β=0.03, P=.0002), PP (β=0.04, P=.0004), MBP (β=0.04, P=.001), and arterial hypertension (β=0.86, P=.03).
Table 2.
Results of Univariate Regression Analysis for Anthropometric, Hemodynamic, Clinical, and Biochemical Parameters Using Pulse Wave Velocity as the Dependent Variable for the Total Population
| Parameters | Total (β) | PAD (β) | Controls (β) |
|---|---|---|---|
| PP | 0.05 (P=.0001) | 0.05 (P=.01) | 0.04 (P=.0004) |
| Age | 0.11 (P=.0001) | 0.13 (P=.06) | 0.07 (P=.0004) |
| SBP | 0.04 (P=.0001) | 0.03 (P=.10) | 0.03 (P=.0002) |
| MBP | 0.04 (P=.01) | 0.003 (P=.92) | 0.04 (P=.001) |
| ABPI | −3.9 (P=.0001) | −3.88 (P=.17) | 0.20 (P=.90) |
| Hypertension | 1.36 (P=.003) | 0.33 (P=.77) | 0.86 (P=.03) |
| Diabetes mellitus, type 2 | 1.02 (P=.02) | 1.19 (P=.23) | −0.06 (P=.87) |
| Hemoglobin A1c | 0.73 (P=.0009) | 0.63 (P=.07) | 0.16 (P=.55) |
| Antihypertension | 0.98 (P=.04) | −0.03 (P=.97) | 0.43 (P=.40) |
| Heart rate | 0.007 (P=.71) | 0.05 (P=.22) | 0.004 (P=.82) |
| DBP | −0.005 (P=.80) | 0.06 (P=.14) | 0.03 (P=.09) |
| BMI | 0.10 (P=.08) | 0.17 (P=.19) | 0.05 (P=.28) |
Abbreviations: ABPI, ankle‐brachial pressure index; BMI, body mass index; DBP, diastolic blood pressure; MBP, mean blood pressure; PAD, peripheral arterial disease; PP, pulse pressure; SBP, systolic blood pressure.
In the multiple regression model, aPWV was independently associated with PP only (β=0.05, P=.01) and with age for the patient group (β=0.13, P=.06), whereas a significant independent association of PWV occurred with age in the control group (β=0.08, P=.0005) (Table 3).
Table 3.
Results of the Multiple Regression Analysis Using Pulse Wave Velocity as the Dependent Variable for Each Group (PAD and Controls)
| Parameters | Total (β) | PAD (β) | Controls (β) |
|---|---|---|---|
| PP | 0.06 (P=.23) | 0.05 (P=.01) | 0.02 (P=.61) |
| Age | 0.06 (P=.02) | 0.13 (P=.07) | 0.08 (P=.0005) |
| MBP | −0.007 (P=.91) | 0.003 (P=.92) | 0.02 (P=.63) |
Abbreviations: MBP, mean blood pressure; PAD, peripheral arterial disease; PP, pulse pressure.
Furthermore, PP explained (R²=11.8%, P=.01) the variability in aPWV for PAD patients and age explained (R²=15.8%, P=.0004) the variability in aPWV for the control group.
Discussion
This study examined arterial stiffness and related factors in PAD patients. The results revealed that aortic stiffness as assessed by aPWV was higher in PAD patients as compared with control patients (P=.002) and that aPWV was independently associated with PP (P=.01).
Zagura and colleagues15 and Kals and colleagues16 recently reported a significant association between PAD and aPWV using the same carotid‐femoral artery waveforms method with the SphygmoCor (AtCor Medical) device. In our study, in addition to a higher aPWV, patients with PAD also exhibited a higher PP (P=.0001). Although PP and aPWV are correlated, they represent two aspects of hemodynamics.17
aPWV is an integrative marker of arterial function, whereas PP is viewed as a surrogate marker of stiffness potentially confounded by factors related to cardiac function such as heart rate, stroke volume, pattern of ventricular ejection, and timing and intensity of wave reflections.18
Increased aortic stiffness is an intermediate endpoint for cardiovascular events, independently of and beyond peripheral PP. aPWV has been associated with various factors such as age, BP, and heart rate.
In our study, the data from all 172 patients were used to construct a linear and multiple regression model with aPWV as the dependent variable. Known or likely determinants of aPWV were added to the models.5, 6, 7 The results of multiple regression analysis (Table 3) indicated that PP only was significantly correlated with aPWV (P=.01), which reflects the sclerotic changes of the aortic vessel wall. The results also indicate that the changes in aPWV in PAD patients could be explained in terms of PP. One possible explanation of this relationship is that arterial stiffening may be caused by the fracture of load‐bearing elastic lamellae and degeneration of the arterial wall as a result of cyclic stress19 or permanent BP elevation, independently of MBP.20 These findings are consistent with data on type 2 diabetes by Smith and colleagues21 and with the idea that more pronounced aortic stiffening is responsible for the different BP pattern observed in PAD patients compared with control patients.22
We also found no significant relationship of aPWV with other cardiovascular risk factors (diabetes mellitus, smoking), which is consistent with a recent systematic review concerning aPWV and cardiovascular risk factors.23 In PAD patients, aortic stiffness may be locally exacerbated by atherosclerosis, which is a systemic chronic inflammatory disease.24 Biomarkers of inflammation are positively associated with PAD and with the parameters of arterial stiffness (aPWV, PP).25, 26, 27 In other words, inflammatory, high‐grade oxidative stress and calcification processes within the vessel wall (as part of vascular remodeling) can modulate arterial stiffness (Figure1).28 Kals and colleagues demonstrated that elevated plasma β2‐microglobulin (β2μ) levels were associated with higher aortic stiffness irrespective of cardiovascular disease risk factors, suggesting that β2μ may influence the pathogenesis of aortic stiffness in atherosclerosis. Clancy and colleagues29 recently reported an association between serum levels of osteoprotegerin (OPG) and infrarenal abdominal aorta calcification in patients with PAD. In the article by Zagura and colleagues, OPG levels were independently associated with increased aPWV in PAD patients and controls. This association remained significant after the correction of confounding factors (BP levels, pharmacologic therapy, and cardiovascular risk factors).
Figure 1.
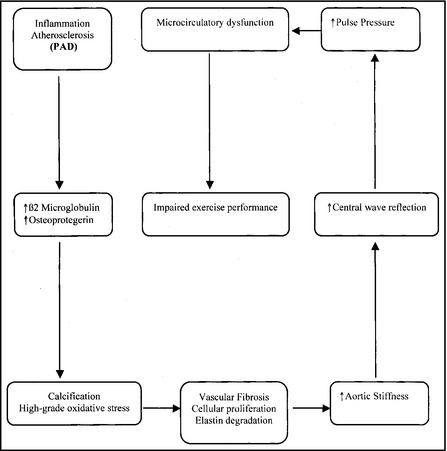
Potential pathophysiologic mechanisms and microcirculation implications for aortic stiffness in patients with peripheral arterial disease (PAD).
Potential Pathophysiologic Implications
In PAD patients, a less distensible aorta cannot efficiently accommodate the blood volume ejected by the left ventricle, which results in high systolic pressure and PP. These hemodynamic modifications may influence ventricular afterload and microcirculation function.30 Among PAD patients, increased PP and higher aortic augmentation index, a measure of arterial wave reflection that is affected by arterial stiffness, are associated with impaired walking ability and vascular bed reserve.31
A reduction of aPWV by an angiotensin‐converting enzyme inhibitor has been shown to improve performance, suggesting aortic stiffness as a potential target of intervention.32 However, further studies are needed to understand the relationship between arterial stiffness and functional performance in PAD patients.
Study Limitations
The present study has some limitations. First, this was an observational study that could not reveal causal relationships between PAD and aortic stiffening and between PP and aPWV. Future long‐term longitudinal studies, preferably starting in young and normotensive patients, will be needed to elucidate these issues. Second, the regression model could only predict a part of the variability of aPWV (R²=11.8%, P=.01), indicating that other factors (such as inflammation and calcification) not currently studied may play an important role in aortic stiffness in PAD patients.
Conclusions
This study demonstrates that PAD is characterized by an increase in aortic stiffness at the same age, sex, MBP, and heart rate in respect to controls. In these patients, the contribution of cardiovascular risk factors on aortic stiffening appears to be insignificant. Our results focus attention on the major role of PP in determining aortic stiffening in PAD patients. Thus, excessive aortic stiffness and increased PP contribute to damage of the arterial wall and may represent both a cause and a consequence of atherogenesis. In PAD patients, the prognostic usefulness of ABPI measurement is well defined but the exact role of aPWV in this regard is not clear. The independence of aPWV from cardiovascular risk factors and lower ABPI increases the potential for aortic stiffness measurements to contribute to cardiovascular risk stratification in PAD.
J Clin Hypertens (Greenwich). 2013;15:712–716. ©2013 Wiley Periodicals, Inc.
References
- 1. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the national health and nutrition survey, 1999–2000. Circulation. 2004;110:738–743. [DOI] [PubMed] [Google Scholar]
- 2. Jatoi NA, Jerrard‐Dunne P, Feely J, Mahmud A. Impact of smoking and smoking cessation on arterial stiffness and aortic wave reflection in hypertension. Hypertension. 2007;49:981–985. [DOI] [PubMed] [Google Scholar]
- 3. Lacy PS, O'Brien DG, Stanley AG, et al. Increased pulse wave velocity is not associated with elevated augmentation index in patients with diabetes. J Hypertens. 2004;22:1937–1944. [DOI] [PubMed] [Google Scholar]
- 4. van Popele NM, Grobbee DE, Bots ML, et al. Association between arterial stiffness and atherosclerosis. The Rotterdam study. Stroke. 2001;32:454–460. [DOI] [PubMed] [Google Scholar]
- 5. Nürnberger J, Dammer S, Saez AO, et al. Diastolic blood pressure is an important determinant of augmentation index and pulse wave velocity in young, healthy males. J Hum Hypertens. 2003;17:153–158. [DOI] [PubMed] [Google Scholar]
- 6. Alecu C, Gueguen R, Salvi P, et al. Determinants of arterial stiffness in an apparently health population over 60 years. J Hum Hypertens. 2006;20:749–756. [DOI] [PubMed] [Google Scholar]
- 7. Lantelme P, Mestre C, Lievre M, et al. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39:1083–1087. [DOI] [PubMed] [Google Scholar]
- 8. Arnett DK, Evens GW, Riley WA. Arterial stiffness: a new cardiovascular risk factors? Am J Epidemiol. 1994;140:669–682. [DOI] [PubMed] [Google Scholar]
- 9. Nichols WW, O'Rourke MF. McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 5th ed. London: Hodder Arnold; 2005. [Google Scholar]
- 10. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness. A systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 11. Brewer LC, Chai HS, Bailey KR, Kullo IJ. Measures of arterial stiffness and wave reflection are associated with walking distance in patients with peripheral arterial disease. Atherosclerosis. 2007;191:384–390. [DOI] [PubMed] [Google Scholar]
- 12. Amoh‐Tonto CA, Malik AR, Kondragunta V, et al. Brachial‐ankle pulse wave velocity is associated with walking distance in patients referred for peripheral arterial disease evaluation. Atherosclerosis. 2009;206:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laurent S, Cockcroft J, van Bortel L, et al.; on behalf of the European network for Non‐invasive investigation of large Arteries . Expert Consensus on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 14. ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zagura M, Serg M, Kampus P, et al. Association of osteoprotegerin with aortic stiffness in patients with symptomatic peripheral arterial disease and in healthy subjects. Am J Hypertens. 2010;23:586–591. [DOI] [PubMed] [Google Scholar]
- 16. Kals J, Zagura M, Serg M, et al. β2‐microglobulin, a novel biomarker of peripheral arterial disease, independently predicts aortic stiffness in these patients. Scand J Clin Lab Invest. 2011;71:257–263. [DOI] [PubMed] [Google Scholar]
- 17. Laurent S. Surrogate measures of arterial stiffness: do they have additive predictive value or are they only surrogates of a surrogate? Hypertension. 2006;47:325–326. [DOI] [PubMed] [Google Scholar]
- 18. Safar ME, Levy BI, Struijker‐Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. [DOI] [PubMed] [Google Scholar]
- 19. O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–658. [DOI] [PubMed] [Google Scholar]
- 20. Benetos A, Adamopoulos C, Bureau JM, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6‐year period. Circulation. 2002;105:1202–1207. [DOI] [PubMed] [Google Scholar]
- 21. Smith A, Karalliedde J, De Angelis L, et al. Aortic pulse wave velocity and albuminuria in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16:1069–1075. [DOI] [PubMed] [Google Scholar]
- 22. Safar ME, Laurent S, Asmar RG, et al. Systolic hypertension in patients with arteriosclerosis obliterans of the lower limbs. Angiology. 1987;38:287–295. [DOI] [PubMed] [Google Scholar]
- 23. Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54:1328–1336. [DOI] [PubMed] [Google Scholar]
- 24. Ross R. Atherosclerosis an inflammatory disease. N Engl J Med. 1999;340:115–126. [DOI] [PubMed] [Google Scholar]
- 25. Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C‐reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–2485. [DOI] [PubMed] [Google Scholar]
- 26. Yasmin, McEniery CM, Wallace S, et al. C‐reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol. 2004;24:969–974. [DOI] [PubMed] [Google Scholar]
- 27. Engstrom G, Janzon L, Berglund G, et al. Blood pressure increase and incidence of hypertension in relation to inflammation‐sensitive plasma proteins. Arterioscler Thromb Vasc Biol. 2002;22:2054–2058. [DOI] [PubMed] [Google Scholar]
- 28. Maki‐Petaja KM, Wilkinson IB. Inflammation and large arteries: potential mechanisms for inflammation‐induced arterial stiffness. Artery Research. 2012;6:59–64. [Google Scholar]
- 29. Clancy P, Oliver L, Jayalath R, et al. Assessment of a serum assay for quantification of abdominal aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:2574–2576. [DOI] [PubMed] [Google Scholar]
- 30. Mitchell GF, Vita JA, Larson MG, et al. Cross‐sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham heart study. Circulation. 2005;112:3722–3728. [DOI] [PubMed] [Google Scholar]
- 31. Safar ME, Totomoukouo JJ, Asmar RA, Laurent SM. Increased pulse pressure in patients with arteriosclerosis obliterans of the lower limbs. Arteriosclerosis. 1987;7:232–237. [DOI] [PubMed] [Google Scholar]
- 32. Ahimastos AA, Dart AM, Lawler A, et al. Reduced arterial stiffness may contribute to angiotensin‐converting enzyme inhibitor induced improvements in walking time in peripheral arterial disease patients. J Hypertens. 2008;26:1037–1042. [DOI] [PubMed] [Google Scholar]


