Abstract
The authors investigated whether erectile dysfunction (ED) in the early stages of hypertension is associated with heightened end‐organ damage. A total of 174 consecutive men with untreated, newly diagnosed essential hypertension (aged 50.3 years, office blood pressure [BP] 150/98 mm Hg) were studied. All participants underwent 24‐hour ambulatory BP monitoring, blood examination, albumin‐creatinine ratio, carotid‐femoral pulse‐wave velocity assessment, and echocardiography for estimation of left ventricular mass index and diastolic function. Hypertensive men with ED (n=43, 24.7%) compared with those without ED were older (by 6.4 years, P<.05), had greater 24‐hour pulse pressure (by 4.3 mm Hg, P=.011) and a greater prevalence of nondipping status (72.2% vs 46.7%, P=.008), while the two groups did not differ in plasma glucose, lipid, creatinine, and albumin/creatinine ratio levels. Regarding cardiac adaptations, hypertensive men with ED exhibited only significantly lower tissue Doppler imaging–derived Em (by 1.6 cm/s, adjusted P=.035), while no difference in left ventricular mass index or pulse wave velocity were detected. ED in the setting of untreated newly diagnosed essential hypertension does not have an unfavorable impact on traditional markers of target organ damage. This finding suggests that ED assessment might not refine the traditional risk stratification procedure at least in the early stages of hypertensive disease.
Erectile dysfunction (ED) is the most common sexual dysfunction, with a high prevalence rate in middle‐aged and older men.1, 2 ED is almost twice as frequent in hypertensive patients than in normotensive individuals and appears to be of higher severity.3, 4 Traditionally, in hypertension the negative influence on erectile function is accomplished through administrated antihypertensive medication.4 Real or perceived impairment of sexual function attributable to antihypertensive agents is one of the predominant causes of nonadherence and discontinuation of antihypertensive therapy.4
It has recently been reported that ED of vascular origin significantly increases the risk of coronary heart disease, stroke, and all‐cause mortality independently of conventional cardiovascular risk factors.5, 6 Although ED was considered another manifestation of widespread atherosclerosis, which usually anticipates overt coronary artery disease,7, 8, 9, 10 data regarding the extent of hypertension related target organ damage in hypertensive men with ED are scarce. Therefore, the aim of our study was to evaluate whether the combination of hypertension with ED contributes to cardiovascular and renal damage to a higher extent compared with hypertension alone in untreated newly diagnosed essential hypertensive patients.
Methods
Study Population
This cross‐sectional study was performed in the outpatient hypertension unit of our institute. The study population consisted of Caucasian men aged 30 to 75 years with untreated, newly diagnosed essential hypertension. All included patients had normal blood pressure (BP) during different time periods preceding a short period (<6 months in all cases) of high BP readings. Patients with long‐standing diagnosed but untreated hypertension were not included in the study population. All patients underwent the usual clinical and laboratory workup in order to rule out secondary hypertension.
Exclusion criteria included history of coronary artery disease, heart failure, moderate or severe valvular heart disease, severe arrhythmias, stroke, peripheral artery disease, diabetes mellitus, familial dyslipidemia, severe renal or hepatic dysfunction (n=27), and high clinical suspicion of sleep apnea syndrome (n=21). To study only vasculogenic ED, patients with suspected ED according to the 5‐item form of the International Index of Erectile Function (IIEF) also underwent urologic and psychiatric examination. Accordingly, those with sexual dysfunction of different origin (eg, psychogenic ED, n=6); prostate disease or lower urinary tract symptoms (n=4); pelvic surgery or trauma (n=1); penile curvature (n=1); and endocrinologic (n=1), neurologic (n=1), and psychiatric disease (n=2) were excluded.
Based on the above criteria, 174 consecutive hypertensive men (mean age 50.3 years, mean office BP 150/98 mm Hg) were included in the study (Figure). The study protocol was approved by the ethics committee of our institution and all patients gave written informed consent.
Figure 1.
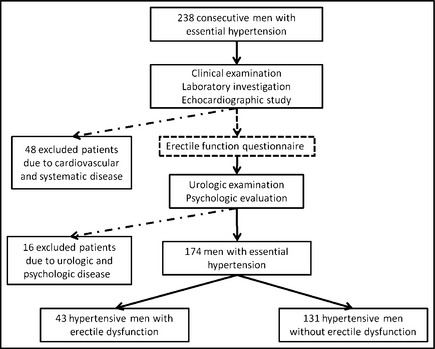
Flow chart of the study population.
Office and Ambulatory BP Measurement
BP measurement was performed at two different visits in our outpatient clinic using a mercury sphygmomanometer with the patient in the sitting position after at least 5 minutes rest according to current guidelines.11 We performed 3 measurements at 1‐minute intervals, and the average values of systolic and diastolic BP were obtained. In case of large differences in systolic BP between the first and the second measurement (>20 mm Hg) the average of the last two readings was used. In all other cases we took into consideration the average of all 3 readings.
Ambulatory BP was recorded over a working day using the automatic Spacelabs units 90207 (Redmond, WA). The cuff was fixed to the nondominant arm and the device was set to obtain automatic BP readings at 15‐minute intervals during the daytime and at 30‐minute intervals during the nighttime. Daytime and nighttime were defined using short fixed‐clock time intervals, which ranged from 10 am to 8 pm and from midnight to 6 am, respectively. Twenty‐four–hour systolic and diastolic BP values were the mean of the overall 24‐hour recordings after artifact editing. We also defined patients with a nocturnal reduction of both systolic and diastolic BP of <10% of the corresponding daytime values as nondippers and the remaining patients as dippers.
Laboratory Determinations
Venous blood sampling was performed between 8 am and 9 am after an overnight fasting and abstention from smoking, alcohol, and caffeinated beverages. Total cholesterol, high‐ and low‐density lipoprotein cholesterol, triglycerides, plasma glucose, and serum creatinine were measured in all participants using colorimetric enzymatic method in a Technicon automatic analyzer RA‐1000 (Dade‐Behring Marburg GmbH, Marburg, Germany). The abbreviated Modification of Diet in Renal Disease (MDRD) study group equation was used to estimate glomerular filtration rate (mL/min/1.73 m2).
Subclinical Target Organ Damage Assessment
Renal Damage
Urinary albumin excretion was expressed as the albumin‐to‐creatinine ratio (ACR). In all patients, ACR was determined as the average of two nonconsecutive morning spot urine samples at least 2 weeks apart, by using a quantitative assay (DCA 2000; Bayer Diagnostics Europe, Dublin, Ireland) with a coefficient of variation of 2.8%.
Cardiac Damage
Each participant underwent a complete echocardiographic study performed by an experienced senior operator who was blinded to the clinical status of the examinees using a Vivid 3 (General Electric, Milwaukee, WI) ultrasound imager equipped with a 2.5/5 MHz (harmonics) phased‐array transducer according to established methods. Left ventricular (LV) mass was calculated by the Devereaux formula normalized for body surface area to obtain LV mass index (LVMI).12 LV diastolic function was determined using conventional Doppler parameters (peak velocities of E/A waves of transmitral flow) and tissue Doppler imaging–derived indexes (ratio of peak early diastolic velocity to peak atrial systolic velocity [Em/Am]) and averaging mean values obtained from measurements at the basal site of the lateral, septal, anterior, and inferior walls in 5 consecutive cardiac cycles as previously described.13
Arterial Stiffness
Aortic stiffness was evaluated with carotid‐femoral pulse wave velocity (PWV) measurements using a validated noninvasive device (Complior SP; Artech Medical, Pantin, France).14 Measurements of PWV were performed with the patient in the supine position with their head in a slight extension and their right lower limb in external rotation. Two transducers were implemented to obtain two simultaneous recordings of pulse wave tracings at the base of the neck for the common carotid artery and over the right femoral artery, respectively. Five consecutive measurements were taken in each patient and the mean PWV value was calculated. All of the measurements were taken by the same observer, who was unaware of the patient's clinical data, just after the completion of 24‐hour ambulatory BP monitoring.
Erectile Function's Evaluation
A full sexual history was obtained in all participants. ED was diagnosed according to the score of the 5‐item form of the IIEF, the Sexual Health Inventory for Men (SHIM score, ≤21 indicates ED).15 All patients with SHIM score suggestive of ED underwent urologic and psychiatric examination in order to exclude other causes of ED. In the first 20 participants, the IIEF was administered 2 times, with an approximate elapsing time of 4 weeks, indicating a reassuring reproducibility of the achieved score.
Statistical Analysis
The SPSS statistical package 15.0 (SPSS Inc, Chicago, IL) was used for all statistical analysis. Differences between groups were evaluated using the independent samples Student t test for continuous variables (given as mean±standard deviation) and the chi‐square for categorical variables. Linear regression analysis was performed in order to assess the independent determinants of continuous variables. Analysis of covariance was performed in order to eliminate any influence of age on detected differences between hypertensive patients with ED and those without ED. All tests were considered to be significant at the level of P<.05.
Results
ED was diagnosed in 43 patients (24.7%). In the whole study population, LV hypertrophy was detected in 5.2% and microalbuminuria in 18.2% of the study population, whereas mean PWV was 8.43 m/s. No difference was observed in the prevalence of LV hypertrophy or microalbuminuria between hypertensive men with and without ED (Tables 1, 2, 3).
Table 1.
Clinical Characteristics of the Study Population
| Hypertensives With ED (n=43) | Hypertensives Without ED (n=131) | P Value | |
|---|---|---|---|
| Age, y | 55.2±10 | 48.8±7.1 | <.001 |
| Waist circumference, cm | 102.4±9 | 102.8±9.4 | .81 |
| Body mass index, kg/m2 | 28.4±3.4 | 29.1±3.6 | .32 |
| Smoking | 35.7 | 42.7 | .29 |
| Office systolic BP, mm Hg | 150.3±18.4 | 149.9±16.7 | .89 |
| Office diastolic BP, mm Hg | 95.2±13.1 | 98.9±9.8 | .10 |
| Office pulse pressure, mm Hg | 55.1±12.5 | 51±13.8 | .09 |
| 24‐h systolic BP, mm Hg | 134.8±17.5 | 132.9±1 1.1 | .52 |
| 24‐h diastolic BP, mm Hg | 83±12.6 | 85.4±8.4 | .28 |
| 24‐h pulse pressure, mm Hg | 51.8±9.3 | 47.5±6.6 | .011 |
| Daytime systolic BP, mm Hg | 137.7±17 | 137.8±11.9 | .96 |
| Daytime diastolic BP, mm Hg | 85.4±12.4 | 89.2±9 | .09 |
| Daytime pulse pressure, mm Hg | 52.2±9.4 | 48.6±7.2 | .036 |
| Nighttime systolic BP, mm Hg | 127.7±21 | 121.8±12.2 | .038 |
| Nighttime diastolic BP, mm Hg | 76.1±14.4 | 76.8±9.5 | .72 |
| Nighttime pulse pressure, mm Hg | 51.6±12.2 | 45±7.3 | .004 |
| Nondippers, % | 72.2 | 46.7 | .008 |
Abbreviations: BP, blood pressure; ED, erectile dysfunction.
Table 2.
Laboratory Data of the Study Population
| Hypertensives With ED (n=43) | Hypertensives Without ED (n=131) | P Value | |
|---|---|---|---|
| Glucose, mg/dL | 97.5±17.6 | 99.2±11.4 | .46 |
| Serum creatinine, mg/dL | 1.02±.18 | .99±.15 | .30 |
| Glomerular filtration rate, mL/min/m1.73 | 83.6±17.1 | 88.5±18.2 | .14 |
| Albumin to creatinine ratio, mg/g | 7.5 (5–16) | 7 (4.7–15) | .57 |
| Brain natriuretic peptide | 22.0±13.2 | 12.2±8.2 | .004 |
| Total cholesterol, mg/dL | 211.6±39 | 219.8±39 | .25 |
| HDL cholesterol, mg/dL | 49.9±10.2 | 47.9±9.9 | .28 |
| LDL cholesterol, mg/dL | 137±33 | 145.5±33 | .16 |
| Triglycerides, mg/dL | 127.3±57 | 141.8±81 | .31 |
Abbreviations: ED, erectile dysfunction; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Table 3.
Echocardiographic and Arterial Stiffness Data of the Study Population
| Hypertensives With ED (n=43) | Hypertensives Without ED (n=131) | P Value | |
|---|---|---|---|
| LV mass index, g/m2 | 90.5±22.7 | 88.5±19.1 | .58 |
| Left atrial diameter, mm | 40.1±6 | 39.4±4.1 | .50 |
| E, m/s | .72±.16 | .72±.14 | .86 |
| A, m/s | .76±.16 | .73±.15 | .34 |
| E/A | .97±.22 | 1.02±.24 | .27 |
| Isovolumic relaxation time, ms | 96.4±21 | 93±18.3 | .32 |
| Deceleration time, ms | 238.1±46 | 234.8±43 | .68 |
| Em, cm/s | 7.1±1.8 | 8.7±2.0 | <.001 |
| Am, cm/s | 10.8±2.0 | 10.8±1.7 | .93 |
| Em/Am | .68±.23 | .82±.22 | .001 |
| E/Em | 10.8±3.3 | 8.7±2.7 | <.001 |
| Pulse wave velocity, m/s | 8.58±1.82 | 8.39±1.65 | .54 |
Abbreviations: ED, erectile dysfunction; E/A, peak early and late transmitral flow velocity; Em/Am, peak early and late myocardial velocity; LV, left ventricular.
Patients were classified according to the presence or absence of ED. Hypertensive men with ED were older compared with those with normal erectile function (by 6.4 years, P<.05). The two groups did not differ significantly regarding body mass index, waist circumference, smoking status, office systolic and diastolic BP, and office pulse pressure (PP). Regarding ambulatory BP measurements, hypertensive men with ED compared with men without ED had higher 24‐hour PP (by 4.3 mm Hg, P=.011), as well as both daytime (by 3.6 mm Hg, P=.036) and nighttime PP values (by 6.6 mm Hg, P=.004). Moreover, hypertensives with ED also had greater nighttime systolic BP levels (by 5.9 mm Hg, P=.038). Nondipping status was more prevalent in hypertensive patients with ED compared with those without ED (72.2% vs 46.7%, P=.008); however, after analysis of covariance, difference in daytime PP levels lost significance.
The two groups did not differ in plasma glucose, lipid levels, and renal function parameters (P=not significant for all comparisons). With respect to echocardiographic data, the two groups did not differ in either LVMI or left atrial diameter; however, hypertensive men with ED compared with those without ED had evidence of more impaired LV diastolic function by means of tissue Doppler imaging–derived indexes. Specifically, Em and the Em/Am ratio were significantly lower in the former group compared with the latter (by 1.6 cm/s [P<.001] and .14 [P=.001], respectively). Additionally, hypertensive men with ED exhibited increased E/Em ratio (by 2.1, P<.001). Only the difference in Em between groups remained significant after adjustment for age (adjusted P=.035).
Linear regression analyses were performed to assess the independent determinants of LVMI, PWV, and renal function parameters. An absence of any association of ED status with all these parameters was observed. Moreover, the presence of ED (β=−.177, P=.047) was independently associated only with early diastolic‐myocardial velocity Em.
Discussion
As a general comment, ED was highly prevalent in untreated newly diagnosed essential hypertensive men and did not determine increased cardiovascular and renal damage surrogates, as compared with patients without ED, in the early stages of hypertension. More specifically, ED was not associated with LVMI, aortic stiffness, and microalbuminuria, but was associated with only LV diastolic dysfunction.
Hypertension is one of the more hazardous cardiovascular risk factors and it is frequently accompanied by the presence of ED.4 The Treatment of Mild Hypertension Study (TOMHS) was a pivotal investigation that examined sexual function in hypertensive patients and reported a low prevalence of ED (12.2%), while older patients, those with diabetes or hyperlipidemia, and those with moderate to severe hypertension were preliminarily excluded.16 Using the SHIM questionnaire, Giuliano and colleagues reported a 67% prevalence of ED (defined as a SHIM score of 21) among 3906 nondiabetic hypertensive men.17 Doumas and associates18 studied hypertensive patients of Hellenic origin and identified ED in 35.2% of the examinees, with age, duration of hypertension, and use of antihypertensive drugs to qualify as independent determinants of ED prevalence. The lower prevalence of ED in our study (24.7%) could be attributed to the fact that we examined newly diagnosed, untreated hypertensive men. However, the magnitude of ED prevalence in our population was higher than the prevalence of other traditional surrogates of cardiac and renal damage.
We also assessed the hemodynamic load in our hypertensive cohort by means of ambulatory BP monitoring. Accordingly, hypertensive patients with ED demonstrated increased ambulatory PP, in line with previous findings in a large cohort of ED patients without hypertension.19 Likewise, although 24‐hour systolic and diastolic BP levels did not differ between study groups, nondipping status was more prevalent and nighttime systolic BP was more increased in those with ED compared with those without, in accordance with previous findings.20 This blunted reduction in nocturnal BP is potentially related to endothelial dysfunction and may facilitate subsequent target organ damage, including penile vascular dysfunction.21
In hypertensive patients, it is currently unknown whether ED is related to any additional risk on top of hypertension. It has been shown that ED is associated with a high prevalence of subclinical coronary artery disease, frequently precedes the clinical onset of coronary artery disease when both conditions coexist, and also resembles an independent predictor of cardiovascular outcomes.5, 6, 7, 8 Consequently, vasculogenic ED is frequently but nonunanimously considered a manifestation of generalized arterial disease.4 Also, data regarding the impact of ED on hypertension‐related organ damage in the preclinical era are rather scarce. In our study, untreated newly diagnosed hypertensive men with ED exhibited only a more deteriorated LV diastolic function accompanied by relatively higher BNP levels compared with those without ED, while no difference was observed in LV structure. From a pathophysiological point of view, impaired coronary flow reserve may promote mild cardiovascular adaptations in patients with ED such as LV diastolic dysfunction.4, 22, 23 However, our findings suggest that this mechanism could not determine more pronounced cardiac damage (ie, structural LV adaptations), at least in the early stages of hypertension.
Focusing on the relation of ED with arterial stiffness, it is still debated whether the presence of ED in hypertension determines a stiffer aorta beyond increased PP levels. Vlachopoulos and colleagues reported that hypertensive patients with ED had deteriorated structural and functional markers of vascular performance as depicted by higher common carotid intima‐media thickness and PWV, lower flow‐mediated dilation of the brachial artery, and by higher levels of asymmetric dimethylarginine in the context of enhanced low‐grade systemic inflammation.24 However, in a previous study9 and in accordance with our findings, ED was not accompanied by either higher intima‐media thickness or higher PWV, possibly because of the different hypertension investigated in studies.9, 24 Beyond PWV, the impact of ED on glomerular filtration rate and ACR, was not evident in the early stages of hypertension. Although albuminuria qualified as an independent risk factor for ED in men with type 2 diabetes, data on the impact of ED in treated and untreated essential hypertensive patients are not yet available.25
Our findings suggest that established indexes of cardiovascular and renal damage are not influenced by the presence of ED and that evidence of impaired LV diastolic function per se cannot justify the use of a more aggressive antihypertensive therapy, even because there was no significant difference in 24‐hour systolic BP levels between hypertensive patients with and without ED. On the other hand, older patients with ED compared with their younger counterparts without ED, demonstrated increased PP, higher prevalence of nondipping, and more impaired diastolic function. Although, the effect of different age burden between groups could explain these latter findings, we should acknowledge that they are also associated with increased risk of future cardiovascular events.
Study Limitations and Strengths
The most important limitation of our study is the lack of penile duplex Doppler ultrasonography at least in patients with ED based on IIEF, since IIEF is not completely accurate in differentiating between vasculogenic and psychogenic ED;26 however, all these patients were previously examined by a urologist. It has been also reported that almost a fifth of men with severe ED as assessed by IIEF may have normal erectile hemodynamics. Therefore, it might be suggested that the number of patients with “true” vascular ED was much lower in the ED group and any differences might have been offset by patients with nonvascular ED. Moreover, indices of pulse wave analysis not performed in our patients would have provided a more integrated vision of vascular damage. On the other hand, a strength of our study was the evaluation of untreated hypertension patients, in order to exclude the effects of BP‐lowering or drug‐related treatment on penile cavernous bodies.4
Conclusions
The presence of ED in the setting of untreated newly diagnosed essential hypertension does not demonstrate an unfavorable impact on established subclinical hypertensive sequelae beyond mild deterioration of diastolic function. Despite the absence of significant differences in selected surrogate markers of end‐organ damage in hypertensive men with ED compared with those without ED, the recognition of ED is essential for the quality of life in patients and the possibility to unmask asymptomatic coronary artery disease.
Disclosures
The authors declare no specific funding in relation to this research and no conflicts of interest.
Acknowledgements
None.
J Clin Hypertens (Greenwich). 2013;15:644–649. ©2013 Wiley Periodicals, Inc.24034657
References
- 1. Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. [DOI] [PubMed] [Google Scholar]
- 2. Rosen RC, Fisher WA, Eardley I, et al; Men's Attitudes to Life Events and Sexuality (MALES) Study . The multinational Men's Attitudes to Life Events and Sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin. 2004;20:607–617. [DOI] [PubMed] [Google Scholar]
- 3. Doumas M, Douma S. Sexual dysfunction in essential hypertension: myth or reality? J Clin Hypertens (Greenwich). 2006;8:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Viigimaa M, Doumas M, Vlachopoulos C, et al; European Society of Hypertension Working Group on Sexual Dysfunction . Hypertension and sexual dysfunction: time to act. J Hypertens. 2011;29:403–407. [DOI] [PubMed] [Google Scholar]
- 5. Thompson IM, Tangen CM, Goodman PJ, et al. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996–3002. [DOI] [PubMed] [Google Scholar]
- 6. Dong JY, Zhang YH, Qin LQ. Erectile dysfunction and risk of cardiovascular disease: meta‐analysis of prospective cohort studies. J Am Coll Cardiol. 2011;58:1378–1385. [DOI] [PubMed] [Google Scholar]
- 7. Montorsi F, Briganti A, Salonia A, et al. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol. 2003;44:360–364. [DOI] [PubMed] [Google Scholar]
- 8. Vlachopoulos C, Rokkas K, Ioakeimidis N, et al. Prevalence of asymptomatic coronary artery disease in men with vasculogenic erectile dysfunction: a prospective angiographic study. Eur Urol. 2005;48:996–1002. [DOI] [PubMed] [Google Scholar]
- 9. Kaiser DR, Billups K, Mason C, et al. Impaired brachial artery endothelium‐dependent and ‐independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. J Am Coll Cardiol. 2004;43:179–184. [DOI] [PubMed] [Google Scholar]
- 10. Chiurlia E, D'Amico R, Ratti C, et al. Subclinical coronary artery atherosclerosis in patients with erectile dysfunction. J Am Coll Cardiol. 2005;46:1503–1506. [DOI] [PubMed] [Google Scholar]
- 11. Mancia G, De Backer G, Dominiczak A, et al; Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology . 2007 guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Bierig M, Devereux RB, et al; Chamber Quantification Writing Group, American Society of Echocardiography's Guidelines and Standards Committee, European Association of Echocardiography, American Society of Echocardiography's Guidelines and Standards Committee, European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 13. Tsioufis C, Chatzis D, Tsiachris D, et al. Exaggerated exercise blood pressure response is related to tissue Doppler imaging estimated diastolic dysfunction in the early stages of hypertension. J Am Soc Hypertens. 2008;2:158–164. [DOI] [PubMed] [Google Scholar]
- 14. Laurent S, Cockcroft J, Van Bortel L, et al; European Network for Noninvasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 15. Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5‐item version of the International Index of Erectile Function as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–326. [DOI] [PubMed] [Google Scholar]
- 16. Grimm RH Jr, Grandits GA, Prineas RJ, et al. Longterm effects on sexual function of five antihypertensive drugs and nutritional hygienic treatment in hypertensive men and women: treatment of mild hypertension study (TOMHS). Hypertension. 1997;29:8–14. [DOI] [PubMed] [Google Scholar]
- 17. Giuliano FA, Leriche A, Jaudinot EO, de Gendre AS. Prevalence of erectile dysfunction among 7689 patients with diabetes or hypertension, or both. Urology. 2004;64:1196–1201. [DOI] [PubMed] [Google Scholar]
- 18. Doumas M, Tsakiris A, Douma S, et al. Factors affecting the increased prevalence of erectile dysfunction in Greek hypertensive compared with normotensive subjects. J Androl. 2006;27:469–477. [DOI] [PubMed] [Google Scholar]
- 19. Corona G, Mannucci E, Lotti F, et al. Pulse pressure, an index of arterial stiffness, is associated with androgen deficiency and impaired penile blood flow in men with ED. J Sex Med. 2009;6:285–293. [DOI] [PubMed] [Google Scholar]
- 20. Erden I, Ozhan H, Ordu S, et al. The effect of non‐dipper pattern of hypertension on erectile dysfunction. Blood Press. 2010;19:249–253. [DOI] [PubMed] [Google Scholar]
- 21. Quinaglia T, Martins LC, Figueiredo VN, et al. Non‐dipping pattern relates to endothelial dysfunction in patients with uncontrolled resistant hypertension. J Hum Hypertens. 2011;25:656–664. [DOI] [PubMed] [Google Scholar]
- 22. Tsiachris D, Tsioufis C, Syrseloudis D, et al. Subendocardial viability ratio as an index of impaired coronary flow reserve in hypertensives without significant coronary artery stenoses. J Hum Hypertens. 2012;26:64–70. [DOI] [PubMed] [Google Scholar]
- 23. El‐Sakka AI, Morsy AM, Fagih BI. Severity of erectile dysfunction could predict left ventricular diastolic dysfunction in patients without overt cardiac complaint. J Sex Med. 2011;8:2590–2597. [DOI] [PubMed] [Google Scholar]
- 24. Vlachopoulos C, Aznaouridis K, Ioakeimidis N, et al. Arterial function and intima‐media thickness in hypertensive patients with erectile dysfunction. J Hypertens. 2008;26:1829–1836. [DOI] [PubMed] [Google Scholar]
- 25. Chuang YC, Chung MS, Wang PW, et al. Albuminuria is an independent risk factor of erectile dysfunction in men with type 2 diabetes. J Sex Med. 2012;9:1055–1064. [DOI] [PubMed] [Google Scholar]
- 26. Deveci S, O'Brien K, Ahmed A, et al. Can the International Index of Erectile Function distinguish between organic and psychogenic erectile function? BJU Int. 2008;102:354–356. [DOI] [PubMed] [Google Scholar]


