Abstract
Left ventricular (LV) hypertrophy (LVH) is classified according to geometric pattern into 4 types: concentric hypertrophy, eccentric hypertrophy, concentric remodeling, and normal geometry. Prevalence of death and cardiovascular complications associated with hypertension depend on the geometric pattern. Although soluble ST2 levels, a novel cardiac biomarker of mechanical strain is increased in hypertension, the relationship with hypertensive LV geometric patterns has not been studied. The authors investigated the relationship between soluble ST2 levels and LV geometric patterns in a cohort of hypertensive patients. LVH was considered present when echocardiographic LV mass index exceeded 49.2 g/m2.7 in men and 46.2 g/m2.7 in women. Patients with concentric hypertrophy had higher soluble ST2 levels compared with patients with normal geometry (20.4±8.4 ng/mL vs 14.3±5.4 ng/mL, P<.002). Therefore, soluble ST2 level is not only affected by hypertensive LV, but may be a future biomarker in differentiating concentric hypertrophy from normal geometry in hypertension.
Left ventricular (LV) hypertrophy (LVH) is an independent risk factor for all cardiovascular complications of hypertension,1 and therefore the early detection and management of LVH is important in order to reduce complications. LVH is often classified according to geometric pattern into 4 types: concentric hypertrophy, eccentric hypertrophy, concentric remodeling, and normal geometry.2 Patients with concentric LVH have a higher prevalence of associated cardiovascular complications or death than patients with other geometric patterns.3 Such patients also have the most advanced extracardiac target‐organ damage compared with other groups.4 Thus, there is a need for early diagnosis and therapy for LVH, especially concentric hypertrophy.
Current studies show that the electrocardiographic diagnosis of LVH has a low sensitivity but high specificity in the general population and in African Americans with hypertensive kidney disease.5, 6 Echocardiography is not generally accessible especially in resource‐poor settings, and there may be problems of interpretation in patients with obesity or pulmonary disease.7 There is therefore a need for cardiovascular scientists to look for easier and accurate methods, particularly at the bedside, for assessing the cardiac structural changes in hypertension. Another biomarker that is currently of interest to cardiovascular scientists is soluble ST2, which is a member of the interleukin 1 receptor host defense and inflammation family.8, 9 Soluble ST2 is induced in conditions of myocardial overload such as acute myocardial infarction when the remaining viable myocardium must bear more mechanical stress9, 10 and has also recently been reported to be increased in patients with hypertension.11 In spite of the usefulness of serum‐soluble ST2 in the field of cardiovascular medicine, there are no published data on the relationship between soluble ST2 and LV geometric pattern in hypertension. We therefore studied this relationship in a cohort of hypertensive patients.
Methods
A total of 133 consecutive patients with a diagnosis of hypertension presenting for the first time to the cardiology clinic of the Department of Medicine at the University of Abuja Teaching Hospital were studied after informed consent was obtained. Ethics approval was obtained from the University of Abuja Teaching Hospital Ethical committee. The study conforms to the principles outlined in the Declaration of Helsinki12 and wherever possible it also adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for an observational study of this kind.13 Hypertension was defined according the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) guidelines.14 Patients with diabetes mellitus, history suggestive of myocardial infarction, regional wall motion abnormality on transthoracic echocardiography, and plasma creatinine levels >170 μmol/L were excluded from the study. Each patient had fasting blood sugar, fasting lipid profile, electrolyte, urea and creatinine, and full blood cell count assessed after an 8‐ to 12‐hour fast. Each patient also had blood collected, processed, and plasma stored at –80°C until assay serum‐soluble ST2. They also had a transthoracic echocardiography performed the same day the sample was collected for soluble ST2.
Soluble ST2 Analysis
Blood samples were centrifuged and stored at −80°C and the frozen samples were shipped on dry ice to the laboratory at Hatter Institute of Cardiovascular Research in Africa, Cape Town, South Africa, within 3 months of sample collection. Soluble ST2 was measured by a sandwich double monoclonal antibody enzyme‐linked immunosorbent assay method, according to manufacturer's instructions (Presage ST2 assay, Critical Diagnostics, New York, NY).15 Even though most reported studies refer to soluble ST2 levels in serum, plasma ST2 was also increased in patients with acute heart failure.16 Furthermore, using the Presage ST2 assay as we did, soluble ST2 in frozen plasma samples has long‐term stability up to 18 months.17
NT‐proBNP Assay
Plasma N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) was measured by a standard electrochemiluminescence immunoassay (BNP Fragment EIA, BIOMEDICA GRUPPE gmbHa Co, Vienna, Austria) according to manufacturer's specification.18 NT‐proBNP concentrations were also determined by experts blinded to the clinical details of the patients.
Transthoracic Echocardiography
Echocardiography was performed by experienced echocardiographers using a commercially available ultrasound system (IVIS‐60). Patients were examined in the left lateral decubitus position using standard parasternal, short‐axis, and apical views. Studies were performed according to the recommendations of the American Society of Echocardiography.19 Measurements were averaged over 3 cardiac cycles. The LV measurements taken include interventricular septal thickness at end‐diastole (IVSd), the posterior wall thickness at end‐diastole (PWTd), and the LV internal dimensions at end‐diastole (LVIDd) and at end‐systole (LVIDs). LV systolic function was calculated by Teichholz's formula.20 LV mass (LVM) was calculated using the formula21: LVM = 0.8 (1.04 [IVSTd + LVIDd + PWT d] 3 + 0.6 g). This has been shown to yield values closely related to necropsy LV weight and that has good interstudy reproducibility (r=0.90). Relative wall thickness (RWT) was calculated as 2 × posterior wall thickness/LV internal dimension in diastole. LVH was considered when LVMI exceeded 49.2 g/m2.7 in men and 46.7 g/m2.7 in women.22 Patients were divided into 4 geometric patterns23 after transthoracic echocardiography. Patients with normal LVMI and relative wall thickness <0.44 were considered to have normal geometry while those with increased LVMI and relative wall thickness >0.44 were considered to have concentric hypertrophy. On the other hand, patients had eccentric hypertrophy when there was increased LVMI but the relative wall thickness was <0.44, while patients had concentric remodeling when there was normal LVMI but relative wall thickness >0.44. Diastolic function was categorized using mitral inflow and tissue Doppler imaging parameters.
Statistical Analysis
SPSS software version 16.0 (SPSS Inc, Chicago, IL) was used for statistical analysis. Continuous variables were expressed as mean±standard deviation. Comparison of demographic, clinical, laboratory, and echocardiographic parameters among the geometric patterns was performed by one‐way analysis of variance with Sheffe's post hoc test. Correlation coefficients were calculated by linear regression analysis, while multiple regression analysis was applied for analysis of the dependency between variables. P<.05 was considered statistically significant. Analysis of variation was performed to determine the sensitivity and specificity of soluble ST2 in differentiating concentric hypertrophy from normal geometry.
Results
One hundred and thirty‐three patients with an average age of 48.8±10.7 years were studied; 50.4% were men with an average age of 50.3±10.2 years, while women had an average age of 47.3±11.1 years. Average duration of hypertension was 3.8±1.1 years.
Comparison of Laboratory and Echocardiographic Parameters Between Hypertensive Patients With and Without LVH
Patients with hypertension and LVH (HTLVH) had higher concentrations of soluble ST2 when compared with patients with hypertension without LVH (23.0±8.3 ng/mL vs 14.5±4.9 ng/mL) (Table 1). There was no statistical difference in the NT‐proBNP levels between patients with hypertension and those with HTLVH, however, even though patients with HTLVH had higher levels (P=.68). Patients with HTLVH also had significantly higher interventricular and LV posterior wall hypertrophy thickness when compared with patients with hypertension and hypertensive heart failure (HHF) (P<.001 and .001, respectively) and also had higher LVM and LVMI when compared with patients with hypertension.
Table 1.
Some Laboratory and Echocardiographic Characteristics in Patients With HTN and LVH and Those Without LVH (HTN)
| Variable | HTN (83) | HTN+LVH (50) | P Value |
|---|---|---|---|
| RVD, cm | 3.15 (0.42) | 3.10 (0.43) | .48 |
| IVSD, cm | 0.92 (0.15) | 1.30 (0.16) | <.0002 |
| PWD, cm | 0.91 (0.13) | 1.26 (0.20) | <.0004 |
| EDD, cm | 4.38 (0.49) | 4.37 (0.95) | .95 |
| ESD, cm | 2.51 (0.45) | 2.72 (0.95) | .10 |
| LVM, g | 202.51 (62.2) | 296.1 (151.8) | <.000 |
| Soluble ST2, ng/mL | 14.5 (4.9) | 23.0 (8.33) | <.001 |
| NT‐proBNP, pg/mL | 341.0 (95.8) | 353.2 (161.4) | .68 |
Abbreviations: EDD, end‐diastolic diameter; ESD, end‐systolic diameter; IVSD, interventricular diameter in diastole; LVM, left ventricular mass; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PWD, posterior wall diameter in diastole; RVD, right ventricular diameter in diastole. P<.05 was considered significant.
Pattern of LV Geometry in Patients
Concentric hypertrophy was the commonest form of geometry in 41.4% of cases, followed by eccentric hypertrophy in 30.8% of cases, concentric remodeling in 14.3% of cases, and normal geometry in 13.5% of cases.
Pattern of LV Geometry by Sex
A total of 42.4% of the women in the study had concentric hypertrophy compared with 40.4% of the men. The male population, however, had higher percentages of patients with eccentric and concentric remodeling (32.8% and 14.9%, respectively) compared with those in the female population (28.8% and 13.6%, respectively).
Clinical Features of the 4 LV Geometric Patterns
Patients with concentric remodeling were the oldest (average age, 56.1±8.4 years) compared with patients with normal geometry (average age, 46.3±10.8 years; P<.004) (Table 2). Patients with eccentric hypertrophy weighed most (mean body mass index [BMI] 29.6±5.0 kg/m2) compared with patients with normal geometry (BMI 25.4±4.1 kg/m2; P<.003). Patients with concentric hypertrophy had the second largest BMI (28.7±5.4 kg/m2) compared with patients with normal geometry (P=.011). There was no significant difference in the blood pressure profiles of the 4 groups. Patients with concentric hypertrophy had the highest concentrations of soluble ST2 (mean 20.4±8.4 pg/mL) vs patients with normal geometry (14.3±5.4 pg/mL; P=.002).
Table 2.
Clinical Features of the 4 LV Geometric Patterns
| Parameter | Normal Geometry | Concentric Hypertrophy | Eccentric Hypertrophy | Concentric Remodeling |
|---|---|---|---|---|
| Age, y | 46.3±10.8 | 49.3±11.0 | 45.9±9.8 | 56.1±8.4a |
| Women/men, % | 15/12 | 42/40 | 28/33 | 13/15 |
| BMI, kg/m2 | 25.4±4.1 | 28.7±5.4a | 29.6±5.0a | 25.4±5.3 |
| SBP, mm Hg | 151.1±20.0 | 158.5±27.2 | 148.0±22.4 | 145.3±19.0 |
| DBP, mm Hg | 94.4±11.0 | 99.2±15.8 | 94.8±15.0 | 92.9±13.0 |
| PP, mm Hg | 56.7±14.1 | 59.3±20.9 | 53.2±14.7 | 52.3±12.9 |
| MAP, mm Hg | 118.1±15.5 | 122.4±21.9 | 128.8±16.2 | 123.3±13.9 |
| Soluble ST2, ng/mL | 14.3±5.4 | 20.4±8.4b | 17.8±8.3 | 16.2±2.9 |
| Serum creatinine | 86.1±20.9 | 98.7±36.1 | 93.0±24.0 | 93.0±18.9 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; LV, left ventricular; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure. aSignificantly higher compared with normal geometry. b P<.002.
The sensitivity of soluble ST2 in differentiating concentric hypertrophy from normal geometry was 68.2% and the specificity was 88.2% at a cut‐off mark of 17.4 ng/mL (Figure 1). The area under the curve (AUC) was 0.76 and positive predictive value (PPV) was 68% (P<.001). Figure 2 on the other hand shows that the sensitivity and specificty of soluble ST2 in differentiating hypertension without LVH from hypertension with LVH are 87.0% and 56.7% respectively.
Figure 1.
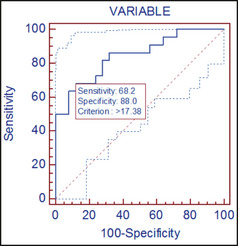
Receiver operator curve showing the sensitivity and specificity of soluble ST2 in differentiating concentric hypertrophy from normal geometry.
Figure 2.
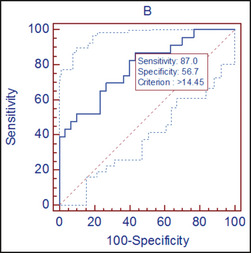
Receiver operator curve showing the sensitivity and specificity of soluble ST2 in differentiating hypertension without left ventricular hypertrophy (LVH) from hypertension with LVH.
There were no significant differences in the soluble ST2 levels among the other geometric patterns. Patients with concentric hypertrophy had the highest concentration of serum creatinine while patients with normal geometry had the lowest levels, but these differences were not statistically significant.
Echocardiographic Characteristics of the 4 Geometric Patterns
Patients with concentric LVH, eccentric LVH, and concentric remodeling had higher IVSd and PWTd compared with patients with normal geometry, while patients with concentric LVH and concentric remodeling had higher RWT compared with patients with normal geometry (Table 3). LVMI was highest in concentric LVH followed by eccentric LVH. LVIDD and lVIDS were higher in patients with all other geometric patterns compared with those with normal geometry. Patients with concentric hypertrophy had the lowest LV ejection fraction vs those with normal geometry (70.7%±18.6% vs 76.7%±18.6%; P=.02).
Table 3.
Echocardiographic Characteristics of the 4 Geometric Patterns
| Parameters | Normal Geometry | Concentric Hypertrophy | Eccentric Hypertrophy | Concentric Remodeling |
|---|---|---|---|---|
| RVD, cm | 3.14±0.35 | 3.19±0.47 | 3.20±0.34 | 3.20±0.44 |
| IVSD, cm | 0.84±0.14 | 1.21±0.22a | 0.97±0.18a | 1.10±0.24a |
| PWD, cm | 0.78±0.08 | 1.20±0.18a | 0.91±0.13a | 1.00±0.15a |
| EDD, cm | 4.18±0.26 | 4.40±0.64a | 4.80±0.71a | 3.61±0.31a |
| ESD, cm | 2.33±0.34 | 2.69±0.70a | 2.80±0.77a | 2.07±0.30a |
| RWT | 0.37±0.04 | 0.57±0.10a | 0.38±0.04 | 0.57±0.12a |
| LVM/HT2.7, g/m2 | 40.70±5.20 | 71.80±21.60a | 65.40±32.30a | 41.60±5.20 |
| LAA, cm2 | 19.30±4.83 | 20.70±3.80 | 18.70±3.30 | 14.00±3.20 |
| RAA, cm2 | 15.20±2.40 | 15.20±3.60 | 15.20±3.50 | 14.70±2.90 |
| LVEF, % | 76.70±18.60 | 70.70±18.60a | 75.20±15.10 | 80.10±11.40 |
| Mitral E/A | 1.10±0.29 | 1.10±0.40 | 1.20±0.44 | 0.84±0.28a |
| DT, ms | 158.90±22.30 | 191.20±50.0a | 172.0±38.50 | 201.00±45.60a |
| TAPSE, mm | 22.20±3.30 | 21.90±5.40 | 22.60±3.90 | 22.90±3.20 |
Abbreviations: DT, deceleration time; EDD, end‐diastolic diameter; ESD, end‐systolic diameter in systole; HT, height; IVSD, interventricular septal diameter in diastole; LAA, left atrial area; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; MA, atrial filling; ME, early mitral filling; PWD, posterior wall diameter in diastole; RAA, right atrial area; RVD, right ventricular diameter; RWT, relative wall thickness; TAPSE, tricuspid annular pulmonary excursion. aSignificantly higher compared with normal geometry (P<.001). bSignificantly higher compared with concentric remodeling (P<.001).
Univariate Association Between Log‐Transformed Soluble ST2 and Clinical and Echocardiographic Parameters
Table 4 and Table 5 show the univariate association between log‐transformed ST2 and clinical, laboratory, and echocardiographic parameters. There was a significant correlation between serum‐soluble ST2 and IVSD, LVPWD, LVIDD, LVIDS, right atrial size, LVMI, LV ejection fraction, and transmitral E/A ratio.
Table 4.
Univariate Association Between Log‐Transformed Soluble ST2 and Clinical Parameters
| Parameters | Coefficient of Association (r) | P Value |
|---|---|---|
| Age | 0.04 | .77 |
| Body mass index | 0.27 | .05 |
| Systolic BP | 0.08 | .59 |
| Diastolic BP | 0.11 | .44 |
| Pulse pressure | 0.02 | .90 |
| Mean arterial pressure | 0.04 | .70 |
| NT‐proBNP | 0.38 | .003 |
Abbreviations: BP, blood pressure; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide
Table 5.
Univariate Association Between Log‐Transformed ST2 and Echocardiographic Parameters
| Parameters | Coefficient of Association | P Value |
|---|---|---|
| RV diameter in diastole | 0.11 | .45 |
| Interventricular septal diameter in diastole | 0.47 | <.001 |
| Posterior wall diameter in diastole | 0.32 | .02 |
| LV internal diameter in diastole | 0.29 | .03 |
| LV internal diameter in systole | 0.32 | .02 |
| Relative wall thickness | 0.12 | .40 |
| LV mass index for HT2.7 | 0.37 | .006 |
| Left atrial area | 0.19 | .19 |
| Right atrial area | 0.28 | .04 |
| LV ejection fraction | 0.28 | .04 |
| Mitral E/A ratio | 0.26 | .05 |
| Deceleration time | 0.18 | .21 |
| Tricuspid annular plane systolic excursion | 0.054 | .77 |
Abbreviations: HT, height; LV, left ventricular; RV, right ventricular.
Multivariate Analysis of Independent Covariates of Log‐Transformed Soluble ST2
In a multivariate analysis, IVSD, LVPWD, LVIDD, and LVIDS remained significant covariates to serum‐soluble ST2 concentrations (Table 6).
Table 6.
Multivariate Analysis of Independent Covariates of Log‐Transformed Soluble ST2
| Parameters | Standardized Coefficient (β) | P Value |
|---|---|---|
| Interventricular septal diameter in diastole | 0.56 | <.0001 |
| LV posterior diameter in diastole | 0.43 | <.001 |
| LV internal diameter in systole | 0.41 | <.003 |
| LV mass index for HT2.7 | 0.56 | <.0001 |
Abbreviations: HT, height; LV, left ventricular.
Discussion
Concentric hypertrophy was the most common geometric pattern in our study cohort, and slightly more common in the female population compared with the male population (42.4% vs 40.4%), similar to previous findings in Nigerian hypertensive patients.24, 25 The higher prevalence of concentric LVH compared with other geometric patterns is an important finding as the prevalence of death and cardiovascular complications associated with hypertension is higher in hypertensive patients with concentric hypertrophy than in other groups, including patients with eccentric hypertrophy.3 We found that plasma ST2 levels were increased in hypertensive patients with all 3 patterns of abnormal LVH geometry but highest in those with concentric hypertrophy despite comparable blood pressures and LVMI values. These results suggest that there is not only a relationship between hypertensive LVH and soluble ST2, but this relationship is related to the particular geometric pattern, particularly concentric hypertrophy. Therefore, measurement of soluble ST2 could be useful in detecting the particular geometric pattern in hypertension, particularly concentric hypertrophy, thereby facilitating risk stratification. We also found that the diagnostic accuracy of soluble ST2 was greater than that of electrocardiography study, which is the most widely used method for the initial assessment of these patients, as evidenced by higher sensitivity and negative predictive value for this marker (Table 7).
Table 7.
Indexes of Diagnostic Validity for Electrocardiography and ST2 in Left Ventricular Hypertrophy
| ECG% (95% CI) | ST2 (>14.45 ng/mL)% 95% CI | P Value | |
|---|---|---|---|
| Sensitivity | 16.0 (8.62–3.8) | 87.0 (75.8–96.4) | <.001 |
| Specificity | 95.2 (83.8–99.7) | 56.7 (48.7–65.7) | <.001 |
| PPV | 51.6 (19.9–82.4) | 40.1 (28.7–52.3) | <.001 |
| NPV | 77.2 (68.8–84.3) | 92.9 (83.2–97.9) | <.001 |
Abbreviations: CI, confidence interval; ECG, electrocardiographic; NPV, negative predictive value; PPV, positive predictive value.
Although we found significant correlations between plasma ST2 interventricular septal wall thickness in diastole, posterior wall thickness in diastole, LV internal diameter in diastole and systole, right atrial size, LVMI, LV ejection fraction and transmitral E/A ratio, and plasma NT‐proBNP, only interventricular septal wall thickness in diastole, posterior wall thickness in diastole, LV internal diameter in systole and diastole, and LVMI were found to be independent predictors of soluble ST2. The correlation of ST2 with LV wall thickness and chamber sizes is a reflection of the mechanism of secretion of ST2, which is related to mechanical stress.10 We also observed that our hypertensive patients with concentric hypertrophy had the lowest LV ejection fractions compared with those with normal geometry, further supporting the finding that concentric hypertrophy carries the worst prognosis of the geometric patterns in hypertension.3 Lower LV ejection fraction is an independent marker of worse prognosis in hypertensive heart disease.25 Takeda and Kohno26 reported higher levels of plasma NT‐proBNP, which is another biomarker that correlates well with serum soluble ST2 in hypertensive patients with concentric LVH compared with other geometric patterns. In our study, NT‐proBNP also correlated with soluble ST2. Soluble ST2 did not correlate well with duration of hypertension. Toda and colleagues27 showed similar findings in patients with essential hypertension in the absence of heart failure using NT‐proBNP.
Study Limitations
Our study is limited by the small number of patients. Therefore, the relationship between plasma ST2 and echocardiographic variables seen in this study should be viewed as hypothesis‐generating for studies in larger patient cohorts. In addition, since the diagnosis of ischemic heart disease was made clinically using history, troponin I levels, and electrocardiography with no myocardial perfusion imaging and coronary angiography performed, it is possible that subtle coronary artery disease might have been missed which is a confounding factor as ischemic heart disease increases the concentration of soluble ST2.9, 10 However, there was careful and extensive phenotyping of our study population with respect to clinical, echocardiographic, and biochemical parameters, which reduced the effect of these limitations in this study.
Conclusions
Our findings suggest that soluble ST2 level is not only affected by hypertensive LVH but may be a future biomarker in differentiating concentric hypertrophy from normal geometry in hypertension.
Funding
The study was partly funded by the Medical Research Council of South Africa, the National Research Foundation, and the University of Cape Town.
Acknowledgments
Our sincere appreciation goes to Mrs Sylvia Dennis for editing this manuscript and all other members of the staff of Hatter Institute for Cardiovascular Research in Africa, Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa.
J Clin Hypertens (Greenwich). 2013;15:899–904. DOI: 10.1111/jch.12205. ©2013 Wiley Periodicals, Inc.
References
- 1. Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med. 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 2. Shigemitsu Y, Hamada M, Mukai M, et al. Clinical evidence for an association between left ventricular geometric adaptation and extracardiac target organ damage in essential hypertension. J Hypertens. 1995;13:155–160. [PubMed] [Google Scholar]
- 3. Shigematsu Y, Hamada M, Ohtsuka T, et al. Left ventricular geometry as an independent predictor for extracardiac target organ damage in essential hypertension. Am J Hypertens. 1998;11:1171–1177. [DOI] [PubMed] [Google Scholar]
- 4. Cuspidi C, Macca G, Michev I, et al. Left ventricular concentric remodeling and extracardiac target organ damage in essential hypertension. J Hum Hypertens. 2002;16:385–390. [DOI] [PubMed] [Google Scholar]
- 5. Esquitin R, Razzouk L, Peterson GE, et al. Left ventricular hypertrophy by electrocardiography and echocardiography in the African American Study of Kidney Disease Cohort Study. J Am Soc Hypertens. 2012;6:193–200. [DOI] [PubMed] [Google Scholar]
- 6. Levy D, Labib SB, Anderson KM, et al. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:815–820. [DOI] [PubMed] [Google Scholar]
- 7. Ogah OS, Adebanjo AT, Otukoya AS, Jagusa TJ. Echocardiography in Nigeria: use, problems, reproducibility and potentials. Cardiovas Ultrasound. 2006;4:13k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Neil L. The Toll/interleukin‐1 receptor domain: a molecular switch for inflammation and host defence. Biochem Soc Trans. 2000;28:557–563. [DOI] [PubMed] [Google Scholar]
- 9. Shimpo M, Morrow DA, Weinberg EO, et al. Serum levels of the interleukin‐1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109(18):2186–2190. [DOI] [PubMed] [Google Scholar]
- 10. Townsend MJ, Fallon PG, Matthews DJ, et al. T1/ST2‐deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 response. J Exp Med. 2000;191:1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coglianese EE, Larson MG, Vasan RS, et al. Distribution and clinical correlates of the interleukin receptor family member soluble ST2 in the Framingham heart study. Clin Chem. 2012;58:1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rickham PP. Declaration of Helsinki. Br Med J. 1964;ii:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. HM K. Outcomes research in the global environment: learning from each other. Circ Cardiovasc Qual Outcomes. 2011;4:489–490. [DOI] [PubMed] [Google Scholar]
- 14. The Seventh Report of the Joint of the Joint National Committee on Prevention, Evaluation and Treatment of High Blood Pressure . U.S Department of Health and Human Services. NIH Publication August 2004;No 04‐5230
- 15. Dieplinger B, Januzzi J, Steinmair M, et al. Analytical and clinical evaluation of novel high sensitivity assay for measurement of soluble ST2 in human plasma‐the Presage ST2 assay. Clin Chem Acta. 2009;409:33–40. [DOI] [PubMed] [Google Scholar]
- 16. Dieplinger B, Egger M, Poelz W, et al. Long‐term stability of soluble ST2 in frozen plasma samples. Clin Biochem. 2010;43:1169–1170. [DOI] [PubMed] [Google Scholar]
- 17. Mueller T, Dieplinger B, Gegenhuber A, et al. Increased plasma concentrations of soluble ST2 are predictive for 1‐year mortality in patients with acute destabilized heart failure. Clin Chem. 2008;54(4):752–756. [DOI] [PubMed] [Google Scholar]
- 18. Prickett TC, Yandle TG, Nicholls MG, et al. Identification of amino‐terminal pro‐C‐type natriuretic peptide in human plasma. Biochem Biophys Res Commun. 2001;286(3):513–517. [DOI] [PubMed] [Google Scholar]
- 19. Devereux RB. Detection of left ventricular hypertrophy by M‐mode echocardiography. Anatomic validation, standardization and comparison to other methods. Hypertension. 1987;9:1119–1126. [DOI] [PubMed] [Google Scholar]
- 20. Ota T, Kisslo J, von Ramm OT, Yoshikawa J. Real‐time, volumetric echocardiography: usefulness of volumetric scanning for the assessment of cardiac volume and function. J Cardiol. 2001;37(Suppl 1):93–101. [PubMed] [Google Scholar]
- 21. Park SH, Shub C, Nobrega TP, et al. Two‐dimensional echocardiographic calculation of left ventricular mass as recommended by the American Society of Echocardiography: correlation with autopsy and M‐mode echocardiography. J Am Soc Echocardiogr. 1996;9:119–128. [DOI] [PubMed] [Google Scholar]
- 22. Devereux RB, Palmieri V, Sharpe N, et al. Effects of once‐daily angiotensin‐converting enzyme inhibition and calcium channel blockade‐based anti‐hypertensive treatment regimens on left ventricular hypertrophy and diastolic filling in hypertension: the Prospective randomized enalapril study evaluating regression of ventricular enlargement (PRESERVE) trial. Circulation. 2001;104:1248–1254. [DOI] [PubMed] [Google Scholar]
- 23. Kolo PM, Omotosho ABO, Katibi IA, et al. Gender differences in left ventricular size and geometric patterns of hypertension subjects. Cardiology. 2008;4:11–15. [Google Scholar]
- 24. Karaye KM, Sani MU. Factors associated with poor prognosis among patients admitted with heart failure in a Nigerian tertiary medical centre: a cross‐sectional study. BMC Cardiovasc Disorders. 2008;22:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weir RAP, Miller AM, Murphy GEJ, et al. Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after myocardial infarction. JACC. 2010;55:243–250. [DOI] [PubMed] [Google Scholar]
- 26. Takeda T, Kohno M. Brain natriuretic peptide in hypertension. Hypertens Res. 1995;18:259–266. [DOI] [PubMed] [Google Scholar]
- 27. Toda K, Sato Y, Hara T, et al. Correlates of NT‐Pro BNP concentration in patients with essential hypertension in absence of congestive heart failure. J Clin Lab Anal. 2010;24(1):12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


