Abstract
Nebivolol, a vasodilatory β1‐blocker, may be well suited for the hemodynamics of the younger hypertensive patient. In this 8‐week trial, 18‐ to 54‐year‐olds with a diastolic blood pressure (DBP) of 95 mm Hg to 109 mm Hg who completed a 4‐week placebo‐only phase were randomized to receive nebivolol (5 mg/d, titrated to 10–20 mg/d based on achievement of blood pressure <140/90 mm Hg [n=427]) or placebo (n=214). Primary and secondary efficacy parameters were changes in trough seated DBP and systolic blood pressure (SBP), respectively. Safety parameters included adverse events (AEs). The baseline mean age was 45.3 years; SBP/DBP, 154/100 mm Hg; and heart rate, 78 beats per minute. Completion rates were 91.3% (nebivolol) and 88.3% (placebo). At endpoint, there was a significant effect of nebivolol over placebo for DBP (−11.8 mm Hg vs −5.5 mm Hg, P<.001) and SBP (−13.7 mm Hg vs −5.5 mm Hg, P<.001). Total AE rates were 34.7% (nebivolol) and 32.2% (placebo). Nebivolol monotherapy is efficacious and well tolerated in adults younger than 55 years of age with increased DBP.
Studies have demonstrated a positive correlation between elevated blood pressure (BP; ie, prehypertension or hypertension) at a younger age and rates of cardiovascular morbidity and mortality later in life.1, 2, 3, 4 These findings are supported by observations that elevated BP among young individuals is associated with pathological changes in target organs, such as coronary artery calcification,5 atherosclerosis,6 increased carotid intima‐media thickness,7, 8 endothelial dysfunction,9 and increased coronary calcium.10
Probably because the prevalence of hypertension among individuals 60 years and older is approximately twice the one observed among individuals aged 40 to 59 years,11 clinical trials have largely neglected to specifically address treatment issues among younger patients. A post hoc analysis conducted by E. Freis on behalf of the Veterans Administration Cooperative Study Group on Antihypertensive Agents suggested that the effect of thiazide diuretics on BP is larger in older (55–69 years) compared with younger patients (21–55 years), a difference that could not be observed with β‐blockers.12 In addition, a meta‐analysis by F. Turnbull and colleagues, done on behalf of the Blood Pressure Lowering Treatment Trialists' Collaboration, found that age (younger than 65 vs 65 years and older) is not a factor that significantly determines a drug's BP‐lowering effects or protection against major cardiovascular events, regardless of drug class.13 However, the hemodynamic profile of hypertension in a younger individual—increased sympathetic tone, pulse rate, and left ventricular contractility (dP/dt), as well as decreased proximal arterial compliance14, 15—is distinct from the hemodynamic profile of the elderly, which includes lower cardiac output, pulse rate, stroke volume, and plasma renin activity.16 As such, hypertension in younger patients deserves a separate assessment in prospective, randomized clinical trials.
Nebivolol is a β1‐selective blocker with nitric oxide–dependent vasodilatory properties.17 In clinical trials, it has been shown to have an adverse event profile similar to that of placebo,18, 19 making it attractive for use in younger individuals. The purpose of this trial was to assess the efficacy and tolerability of nebivolol as first‐line therapy in adults 18 to 54 years old with stage 1 or 2 hypertension. To our knowledge, this is the first prospective, randomized, placebo‐controlled trial of an antihypertensive agent in this age group.
Methods
Ethical Conduct
This study was conducted in compliance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) General Considerations for Clinical Trials and the US Food and Drug Administration guidelines for good clinical practice and in accordance with the ethical principles that originate from the Declaration of Helsinki and the US Food and Drug Administration Code of Federal Regulations Title 21, section 312.120. All enrolled patients provided voluntary, written informed consent prior to participating in any study procedures. The study protocol, informed consent form, and information sheet advertisements were approved by the centralized institutional review board (Quorum IRB, Seattle, WA).
Study Design
This was a phase IV, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group dose‐titration trial (NEB‐MD‐28; NCT01415531). Following a 1‐week screening, participants entered a 4‐week single‐blind placebo run‐in phase, followed by a double‐blind treatment period in which participants were randomized (2:1) to treatment with nebivolol 5 mg/d or placebo. Randomization was stratified by body mass index (BMI). After their BMI was calculated at randomization, participants at each center were divided according to BMI values (<30 or ≥30 kg/m2) before being assigned their randomization codes. Dosages could be uptitrated to 10 mg/d or 20 mg/d nebivolol (or the placebo equivalent) every 2 weeks as necessary to achieve BP control, defined as <140/90 mm Hg for individuals without diabetes or <130/80 mm Hg for those with diabetes. After 8 weeks of double‐blind treatment, the study drug could be tapered off in a 1‐week double‐blind down‐titration phase at the principal investigators' discretion.
Participants
Men and women 18 to 54 years of age were eligible to participate if they had a heart rate of ≥55 beats per minute, a normal physical examination, and stage 1 or 2 hypertension with a recent diastolic BP (DBP) measurement of ≥90 mm Hg and <110 mm Hg if currently receiving hypertension treatment or DBP ≥95 mm Hg and <110 mm Hg at screening if currently untreated. Participants were randomized if they demonstrated ≥80% and ≤120% adherence with single‐blind study medication, DBP measurements ≥95 mm Hg and <110 mm Hg, and a seated pulse rate of ≥55 beats per minute. Major reasons for exclusion were secondary hypertension, severe hypertension (systolic BP [SBP] ≥180 mm Hg or DBP ≥110 mm Hg), current treatment with >2 antihypertensive medications (including components of fixed‐dose combinations), contraindication to discontinuing current antihypertensive treatment, upper arm circumference >42 cm, the presence of coronary artery disease, reactive airway disease, chronic obstructive pulmonary disease, second‐ or third‐degree heart block or sick sinus syndrome, or type 1 diabetes, poorly controlled type 2 diabetes (hemoglobin A1C >8%), or uncontrolled thyroid disease within 3 months of screening.
Outcome Parameters
The primary and secondary efficacy parameters were the changes in trough seated DBP and SBP, respectively (calculated as the mean of 3 recordings made using a BP monitor), from randomization (baseline) to week 8. Additional efficacy parameters included change from baseline in mean trough seated DBP and SBP at each visit, proportion of patients achieving treatment goal (SBP/DBP <140/90 mm Hg without diabetes or <130/80 mm Hg with diabetes) at week 8, and proportion of responders (patients achieving treatment goal or ≥10 mm Hg reduction in SBP or ≥8 mm Hg reduction in DBP) at week 8. Protocol‐specified subgroup analyses for the primary and secondary efficacy parameters were performed according to participants' sex, BMI category, and ethnicity (Hispanic/Latino or not). Post hoc efficacy assessments included an analysis of pulse rate and subgroup analyses by hypertension stage, metabolic syndrome status, and race (black vs non‐black).
Safety and tolerability were assessed by recording adverse events (AEs) and monitoring vital signs at each visit, by performing physical examinations at screening and week 8, by performing electrocardiogram measurements at screening, and by determining clinical laboratory parameters from blood and urine taken at screening, baseline, and at week 8.
Sample Size Determination
Assuming a DBP mean±standard deviation (SD) between‐group treatment difference of 3±10 mm Hg and a 2:1 randomization ratio, it was determined that 414 patients randomized to nebivolol and 207 randomized to placebo would provide approximately 90% overall power to detect that difference (or greater) at the 2‐sided 5% significance level.
Data Analysis
Efficacy parameters were analyzed based on the intent‐to‐treat population, defined as all participants who had at least one postbaseline DBP measurement, using the last‐observation‐carried‐forward approach to impute missing data; the observed‐cases approach was used for sensitivity analyses. Continuous efficacy parameters were analyzed using an analysis of covariance model, with treatment group and BMI category (<30 kg/m2 or ≥30 kg/m2) as factors and baseline value as a covariate. Subgroup analyses were based on an analysis of covariance model with treatment group, BMI category (except for the BMI subgroup analysis), subgroup factor, and treatment group–by–subgroup factor interaction as factors and baseline value as a covariate. Binary parameters were analyzed by means of a logistic regression model, with treatment group and BMI category as factors and baseline BP values as explanatory variables. Analyses of safety measures were based on the safety population, defined as all randomized participants who took at least one dose of double‐blind study medication, and are presented using descriptive statistics. No interim analyses were planned or performed.
Results
Study Conduct, Patient Disposition, and Baseline Characteristics
The study was conducted between September 2011 and May 2012 at 76 sites in the United States. A total of 641 individuals were randomized to treatment with nebivolol (n=427) or placebo (n=214), with completion rates of 91.3% and 88.3%, respectively (Figure S1). The most common reasons for early discontinuation in the overall study population were loss to follow‐up (3.1%) and adverse events (2.7%).
The groups were well matched at baseline (Table 1). Participants in the safety population had a mean age of 45.3 years, 356 (55.5%) were men, 245 (38.2%) were black, and 202 (31.5%) self‐identified as Hispanic or Latino. A total of 388 (60.5%) were obese (BMI ≥30 kg/m2), 75 (11.7%) had diabetes, and 376 (58.7%) had metabolic syndrome. Among the patients who formed the intent‐to‐treat population, mean baseline SBP and DBP were 154 mm Hg and 100 mm Hg, respectively, and mean pulse rate was 78 beats per mintute. During the double‐blind phase (safety population), mean±SD adherence in both groups (defined as [total number of tablets taken/total number of tablets expected to be taken]*100) was 99%±4%. The mean±SD final nebivolol dose was 16.0±5.9 mg/d. At the end of the double‐blind treatment phase, 14.5%, 18.5%, and 67.0% of participants in the nebivolol group were treated with the 5 mg, 10 mg, and 20 mg daily dose, respectively.
Table 1.
Baseline Demographic and Clinical Characteristics (Safety Population)
| Characteristic | Placebo (n=214) | Nebivolol (n=427) |
|---|---|---|
| Demographic | ||
| Age, y | 46.0±6.9 | 44.9±6.9 |
| Age, y (median) | 48.0 | 46.0 |
| Age, y (range) | 18–54 | 21–54 |
| Men, No. (%) | 119 (55.6) | 237 (55.5) |
| Race, No. (%) | ||
| White | 115 (53.7) | 246 (57.6) |
| Black | 87 (40.7) | 158 (37.0) |
| Asian | 10 (4.7) | 16 (3.7) |
| Ethnicity, No. (%) | ||
| Hispanic | 72 (33.6) | 130 (30.4) |
| Clinical | ||
| Trough seated SBP, mm Hga | 153.4±11.1 | 153.7±11.2 |
| Trough seated DBP, mm Hga | 99.9±3.7 | 99.9±3.8 |
| Trough seated pulse rate, beats per mina | 78.4±11.5 | 77.7±10.8 |
| Stage 1 hypertension, No. (%) | 136 (63.6) | 266 (62.3) |
| Stage 2 hypertension, No. (%) | 78 (36.4) | 161 (37.7) |
| Weight, kg | 94.2±21.7 | 95.2±22.6 |
| BMI ≥30 kg/m2, No. (%) | 129 (60.3) | 259 (60.7) |
| Diabetes, No. (%) | 21 (9.8) | 54 (12.6) |
| Metabolic syndrome, No. (%) | 127 (59.4) | 249 (58.3) |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Data are expressed as mean±standard deviation unless otherwise indicated.
Intent‐to‐ treat population: n=211 (placebo) and n=423 (nebivolol).
Efficacy
At the end of the 8‐week, double‐blind treatment phase, nebivolol significantly reduced DBP and SBP, compared with placebo. The effects of nebivolol on DBP and SBP were significant at each visit, beginning at week 2 (Table 2). Nebivolol was also associated with a reduction in pulse rate (initially designated as a safety parameter; statistical analysis performed post hoc) throughout the double‐blind treatment period (Table 2).
Table 2.
Baseline‐to‐Endpoint Changes in SBP, DBP, and Pulse Rate (Intent‐to‐Treat Population, Last Observation Carried Forward)
| Outcome Measure | Placebo (n=211) | Nebivolol (n=423) |
|---|---|---|
| DBP (primary) | ||
| Baseline, mm Hg | 99.9±3.7 | 99.9±3.8 |
| Endpoint, mm Hg | 94.3±9.9 | 88.1±8.9 |
| Baseline‐to‐endpoint change, mm Hg | −5.5±9.5 | −11.8±8.8 |
| LSMD (95% CI), mm Hg | −6.3 (−7.7 to −4.8) | |
| P value | <.001 | |
| SBP (secondary) | ||
| Baseline, mm Hg | 153.4±11.1 | 153.7±11.2 |
| Endpoint, mm Hg | 147.9±15.8 | 140.0±15.7 |
| Baseline‐to‐endpoint change, mm Hg | −5.5±13.9 | −13.7±14.5 |
| LSMD (95% CI), mm Hg | −8.1 (−10.4 to −5.8) | |
| P value | <.001 | |
| Pulse rate (post hoc) | ||
| Baseline, beats per min | 78.3±11.8 | 77.4±10.6 |
| Endpoint, beats per min | 75.9±10.1 | 66.7±10.4 |
| Baseline‐to‐endpoint change, beats per min | −2.4±10.7 | −10.7±10.1 |
| LSMD (95% CI), beats per min | −8.8 [−10.3, −7.2] | |
| P value | <.001 | |
Abbreviations: CI, confidence interval; DBP, diastolic blood pressure; LSMD, least‐squares mean difference; SBP, systolic blood pressure. Data are expressed as mean±standard deviation unless otherwise indicated.
Subgroup analyses revealed a significant nebivolol effect for all categories examined and no significant differences in treatment response between patients with stage 1 and stage 2 hypertension, men and women, blacks and non‐blacks, obese (≥30 kg/m2) and not obese (<30 kg/m2), and those with and without metabolic syndrome. There was, however, a significant difference in treatment effect between Hispanics and non‐Hispanics, as well as a potential trend toward significant difference in treatment effect in blacks vs non‐blacks that may have not reached a P value <.05 due to an insufficient sample size (Figure 1). This difference between Hispanics and non‐Hispanics appeared to be driven by a greater placebo effect in the Hispanic subgroup: there was a 2‐ to 4‐fold greater placebo response in Hispanics compared with non‐Hispanics (DBP: −9.4 mm Hg vs −3.7 mm Hg; SBP: −11.5 mm Hg vs −2.6 mm Hg), which was stronger than the 20% to 30% higher response in Hispanics to nebivolol (DBP: −13.4 mm Hg vs −11.2 mm Hg; SBP: −16.2 mm Hg vs −12.6 mm Hg). In blacks and non‐blacks, however, the difference in treatment effect appeared to be driven by the response to nebivolol (DBP, blacks vs non‐blacks: −9.8 mm Hg vs −13.0 mm Hg; SBP: −10.7 mm Hg vs −15.4 mm Hg), whereas the response to placebo was similar (DBP, blacks vs non‐blacks: −5.2 mm Hg vs −5.8 mm Hg; SBP: −5.0 mm Hg vs −5.8 mm Hg).
Figure 1.
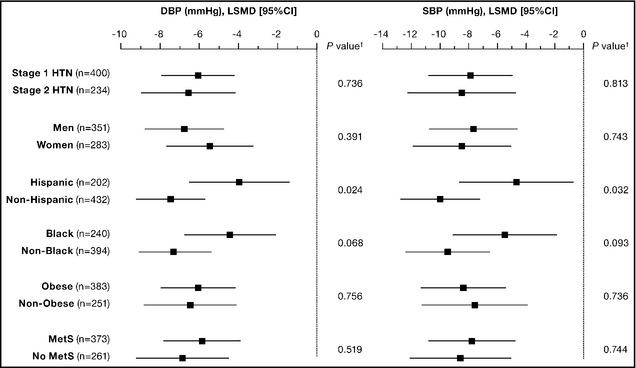
Blood pressure reduction at week 8 (nebivolol minus placebo) by subgroup (intent to treat population, last observation carried forward). †Analysis of covariance model, treatment‐by‐subgroup category interaction. CI indicates confidence interval; DBP, diastolic blood pressure; LSMD, least‐squares mean difference; HTN, hypertension; SBP, systolic blood pressure; MetS, metabolic syndrome.
Finally, at week 8, a significantly higher proportion of patients treated with nebivolol achieved their BP treatment goal or treatment response (see Methods) compared with those treated with placebo (treatment goal: 38.3% vs 25.1%; treatment response: 72.8% vs 47.9%; P<.001 for both) (Figure 2).
Figure 2.
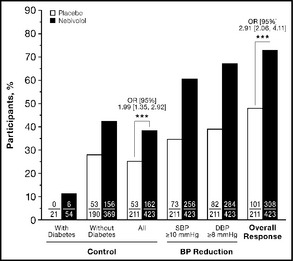
Blood pressure (BP) control and response rates (intent‐ to‐treat population, last observation carried forward). BP control (treatment goal) was defined as systolic BP/diastolic BP (SBP/DBP) <140/90 mm Hg for participants without diabetes or <130/80 mm Hg for those with diabetes. Treatment response was defined as achieving treatment goal or a decrease of ≥10 mm Hg in SBP or ≥8 mm Hg in DBP. Overall response indicates achievement of either BP control or treatment response. Data above the columns represent odds ratios (ORs) with 95% confidence intervals (CIs), calculated using a logistic regression model with treatment group and body mass index category (<30 kg/m2 or ≥30 kg/m2) as factors and baseline SBP and DBP values as explanatory variables. ***P<.001.
Safety and Tolerability
During the double‐blind treatment phase, 10 (2.3%) nebivolol‐treated patients and 7 (3.3%) placebo‐treated patients discontinued the study due to an AE, including 1 patient in the nebivolol group and 2 patients in the placebo group who discontinued due to a serious AE. In that phase, treatment‐emergent AEs were reported in 148 (34.7%) patients in the nebivolol group and 69 (32.2%) patients in the placebo group. The most frequent treatment‐emergent AEs experienced in the nebivolol group were upper respiratory tract infection (nebivolol, 5.4%; placebo, 1.9%), headache (2.6% vs 7.0%), peripheral edema (1.9% vs 0.5%), increase in alanine aminotransferase (1.6% vs 2.3%), and cough (1.6% vs 1.4%). Most treatment‐emergent AEs were mild in severity and were deemed not related to study medication by the investigator. Similar rates of distribution of treatment‐emergent AEs by severity and relationship to study medication were observed between treatment groups. The rates of AEs considered typical of β‐blocker treatment were as follows: bradycardia, 1.2% (nebivolol) vs 0 (placebo); fatigue, 0.9% vs 0.5%; erectile dysfunction (men only): 0.4% vs 0.
A total of 10 serious AEs were reported in 9 patients during the study. Serious AEs were experienced by 3 (0.7%) patients in the nebivolol group and 4 (1.9%) in the placebo group during the double‐blind treatment phase. All serious AEs were considered unrelated to treatment, with the exception of transient ischemic attack in the placebo group, which was considered possibly related to treatment. No participants died during the study.
At each postbaseline visit, the mean pulse rate (assessed as a safety parameter using descriptive statistics only) was lower among nebivolol‐treated patients compared with placebo‐treated patients; the difference in change from baseline steadily increased over time from 6.1 beats per minute (week 2) to 8.7 beats per minute (end of double‐blind treatment phase). In terms of key metabolic parameters, nebivolol treatment was associated with a higher rate of patients who shifted from normal baseline levels to low endpoint levels of high‐density lipoprotein (HDL) cholesterol (nebivolol, 17.5%; placebo, 9.8%), but also with higher rates of patients whose low‐density lipoprotein cholesterol levels shifted from high to normal (10.9% vs 5.5%, respectively). Normal‐to‐high shift rates were 20.8% (nebivolol) and 20.5% (placebo) for glucose, and 15.0% and 16.8%, respectively, for triglycerides. In addition, the overall incidence of potentially clinically significant laboratory values was low and similar between groups, with the most notable difference being observed for endpoint HDL cholesterol levels <35 mg/dL (nebivolol, 10.5%; placebo, 2.7%). (For an overview of changes in key metabolic parameters, see Table S1.)
Discussion
Nebivolol treatment resulted in significant DBP and SBP reductions (observed as early as 2 weeks after treatment initiation) and improvement in BP control among relatively young adult patients (mean age: 45.3 years) with increased DBP. A significant endpoint effect on DBP and SBP was observed regardless of sex, race (black vs non‐black), ethnicity (Hispanic vs non‐Hispanic), hypertension stage, obesity status, and metabolic syndrome status. Overall, the safety and tolerability profile of nebivolol was similar to that of placebo, with a relatively low incidence of AEs seen with traditional β‐blockers (eg, bradycardia, fatigue, erectile dysfunction20).
The placebo‐subtracted DBP and SBP reductions in our trial were similar to those seen in pivotal, fixed‐dose trials of nebivolol, in which participants' mean age was 54 years.19 Our efficacy results are generally in agreement with findings of the age‐stratified pooled analysis18 of 3 pivotal nebivolol trials21, 22, 23 (N=1585) and a German observational study (N=5031),24 which both showed efficacy of nebivolol across the entire age spectrum of adulthood. In the German study, a stronger effect for both DBP and SBP was observed among younger patients (younger than 59 years) vs older patients,24 and, in the pooled analysis, a significant effect on SBP in the oldest group (63–84 years) was observed only with the dosage of 20 mg/d.18 However, the extent to which our trial can be compared with those two studies is limited: The German study and the 3 pooled pivotal studies were all fixed‐dose trials,18, 24 plus the German study was open‐label, with a majority of patients taking other antihypertensives.24 In addition, a high percentage of patients who experienced DBP reduction ≥8 mm Hg (Figure 2) is encouraging, considering the fact that in individuals younger than 50 years, DBP is a stronger predictor of coronary heart disease than SBP or pulse pressure.25
The pharmacologic profile of nebivolol, which includes high β1 selectivity26 and nitric oxide–dependent vasodilatory properties triggered via β3 receptor activation in the endothelium,17 may well be suited for the etiology of hypertension in younger patients. In younger patients, elevated BP appears to be associated with a slightly increased cardiac output, consistent with elevated sympathetic tone and inappropriately increased peripheral vascular resistance.14, 27 For example, an analysis of Strong Heart Study participants younger than 40 years (N=1940) demonstrated a significant association between elevated BP and mean pulse pressure/stroke volume index and total peripheral resistance index.15 A highly cardioselective agent with vasodilatory properties, such as nebivolol, is positioned to interrupt this pathophysiologic sequence. Indeed, nebivolol treatment resulted in a notable reduction of both pulse rate and BP (Figure 1), ie, it elicited reduction of the rate‐pressure product, also a cardiovascular risk factor.28, 29
Study Limitations
There were several limitations to our study. First, it should be pointed out that the responder analysis demonstrated a notable proportion of placebo‐treated patients who achieved BP control (25.1%) or response (47.9%) (Figure 2). The use of ambulatory BP monitoring (ABPM) devices likely would have resulted in a lower placebo response30, 31, 32; however, it is not certain that the use of ABPM would have improved the response rates in the nebivolol group. Second, our data suggest that the overall metabolic effect of nebivolol was relatively neutral, with the exception of a decrease in HDL cholesterol levels in some patients, which is in agreement with observations from previous short‐term studies.33, 34 However, data from an 8‐week trial are insufficient for safety assessment of a long‐term treatment, and long‐term safety effects of nebivolol, including metabolic changes, remain to be elucidated. Third, medication adherence was assessed using pill counts, a method that has been associated in some studies with significant variability.35, 36, 37 However, it can be argued that the treatment regimen applied in our trial—once‐daily administration and labeled blister packs instead of bottles—favored adherence, and that because of randomization, any variability due to nonadherence would be equally distributed between the groups and actually bias the outcome against a significant therapeutic effect. Finally, high rates of obesity and metabolic syndrome in our trial (Table 1) exceeded those observed in the general population38 and are a consequence of our selection criteria (a younger person with hypertension is expected to be burdened by other cardiovascular risk factors as well).
Conclusions
Our study showed that nebivolol monotherapy is an efficacious and well‐tolerated treatment option for the phenotype of a younger adult with diastolic hypertension, a relatively neglected patient population. Future trials should take into account the interaction between relevant phenotypic characteristics and clinical outcomes.
Supporting information
Figure S1. Study flow.
Table S1. Baseline‐to‐Endpoint Changes in Key Metabolic Parameters.
Authors' Contributions and the Role of Medical Writers
T. Giles and B. Khan were involved in the study concept and design, acquisition of data, data interpretation, and drafting and critical revision of the manuscript. J. Lato, L. Brener, and T. Lukic were involved in the study concept and design, data interpretation, and drafting and critical revision of the manuscript. Y. Ma was involved in data analysis, data interpretation, and drafting and critical revision of the manuscript. All authors approved the final version of the manuscript. A. Kelly and V. Pejović assisted with draft coordination, creation of figures and tables, literature searches, and writing based on authors' input and guidance.
Acknowledgments and Disclosures
Thomas Giles has received research support from Laboratories, Inc, has acted as their consultant, and is a member of Forest's speakers' bureau. Bobby Khan is a recipient of Forest research grants for investigator‐initiated studies and has served as investigator for Forest‐sponsored studies. June Lato, Lillian Brener, Yimin Ma, and Tatjana Lukic are employees of Forest Research Institute, a subsidiary of Forest Laboratories. Editorial assistance was provided by Autumn Kelly, MA, and Vojislav Pejović, PhD, of Prescott Medical Communications Group, Chicago, IL. Editorial assistance was funded by Forest Research Institute. Funding for this study (design, conduct, data collection, statistical analysis) and its publication was provided by Forest Laboratories, Inc, the US marketer of nebivolol.
J Clin Hypertens (Greenwich). 2013;15:687–693. ©2013 Wiley Periodicals, Inc.24034663
References
- 1. Falkstedt D, Koupil I, Hemmingsson T. Blood pressure in late adolescence and early incidence of coronary heart disease and stroke in the Swedish 1969 conscription cohort. J Hypertens. 2008;26:1313–1320. [DOI] [PubMed] [Google Scholar]
- 2. Gray L, Lee IM, Sesso HD, Batty GD. Blood pressure in early adulthood, hypertension in middle age, and future cardiovascular disease mortality: HAHS (Harvard Alumni Health Study). J Am Coll Cardiol. 2011;58:2396–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCarron P, Okasha M, McEwen J, Davey Smith G. Blood pressure in early life and cardiovascular disease mortality. Arch Intern Med. 2002;162:610–611. [DOI] [PubMed] [Google Scholar]
- 4. Miura K, Daviglus ML, Dyer AR, et al. Relationship of blood pressure to 25‐year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago Heart Association Detection Project in Industry. Arch Intern Med. 2001;161:1501–1508. [DOI] [PubMed] [Google Scholar]
- 5. Mahoney LT, Burns TL, Stanford W, et al. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine Study. J Am Coll Cardiol. 1996;27:277–284. [DOI] [PubMed] [Google Scholar]
- 6. Berenson GS, Srinivasan SR, Bao W, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. [DOI] [PubMed] [Google Scholar]
- 7. Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal‐medial thickness is related to cardiovascular risk factors measured from childhood through middle age: the Muscatine Study. Circulation. 2001;104:2815–2819. [DOI] [PubMed] [Google Scholar]
- 8. Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–2276. [DOI] [PubMed] [Google Scholar]
- 9. Juonala M, Viikari JS, Ronnemaa T, et al. Elevated blood pressure in adolescent boys predicts endothelial dysfunction: the cardiovascular risk in young Finns study. Hypertension. 2006;48:424–430. [DOI] [PubMed] [Google Scholar]
- 10. Pletcher MJ, Bibbins‐Domingo K, Lewis CE, et al. Prehypertension during young adulthood and coronary calcium later in life. Ann Intern Med. 2008;149:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 12. Freis ED. Age and antihypertensive drugs (hydrochlorothiazide, bendroflumethiazide, nadolol and captopril). Am J Cardiol. 1988;61:117–121. [DOI] [PubMed] [Google Scholar]
- 13. Turnbull F, Neal B, Ninomiya T, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta‐analysis of randomised trials. BMJ. 2008;336:1121–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davis JT, Rao F, Naqshbandi D, et al. Autonomic and hemodynamic origins of pre‐hypertension: central role of heredity. J Am Coll Cardiol. 2012;59:2206–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drukteinis JS, Roman MJ, Fabsitz RR, et al. Cardiac and systemic hemodynamic characteristics of hypertension and prehypertension in adolescents and young adults: the Strong Heart Study. Circulation. 2007;115:221–227. [DOI] [PubMed] [Google Scholar]
- 16. Messerli FH, Sundgaard‐Riise K, Ventura HO, et al. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet. 1983;2:983–986. [DOI] [PubMed] [Google Scholar]
- 17. Münzel T, Gori T. Nebivolol: the somewhat‐different beta‐adrenergic receptor blocker. J Am Coll Cardiol. 2009;54:1491–1499. [DOI] [PubMed] [Google Scholar]
- 18. Germino FW, Lin Y, Pejovic V, Bowen L. Efficacy and tolerability of nebivolol: does age matter? A retrospective analysis of three randomized, placebo‐controlled trials in stage I‐II hypertension. Ther Adv Cardiovasc Dis. 2012;6:185–199. [DOI] [PubMed] [Google Scholar]
- 19. Weiss RJ, Saunders E, Greathouse M. Efficacy and tolerability of nebivolol in stage I‐II hypertension: a pooled analysis of data from three randomized, placebo‐controlled monotherapy trials. Clin Ther. 2011;33:1150–1161. [DOI] [PubMed] [Google Scholar]
- 20. Ambrosioni E, Borghi C. Tolerability of nebivolol in head‐to‐head clinical trials versus other cardioselective B‐blockers in the treatment of hypertension. High Blood Press Cardiovasc Prev. 2005;12:27–35. [Google Scholar]
- 21. Greathouse M. Nebivolol efficacy and safety in patients with stage I‐II hypertension. Clin Cardiol. 2010;33:E20–E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saunders E, Smith WB, DeSalvo KB, Sullivan WA. The efficacy and tolerability of nebivolol in hypertensive African American patients. J Clin Hypertens (Greenwich). 2007;9:866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weiss RJ, Weber MA, Carr AA, Sullivan WA. A randomized, double‐blind, placebo‐controlled parallel‐group study to assess the efficacy and safety of nebivolol, a novel beta‐blocker, in patients with mild to moderate hypertension. J Clin Hypertens (Greenwich). 2007;9:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ladage D, Reidenbach C, Rieckeheer E, et al. Nebivolol lowers blood pressure and increases weight loss in patients with hypertension and diabetes in regard to age. J Cardiovasc Pharmacol. 2010;56:275–281. [DOI] [PubMed] [Google Scholar]
- 25. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. [DOI] [PubMed] [Google Scholar]
- 26. Bundkirchen A, Brixius K, Bolck B, et al. Beta 1‐adrenoceptor selectivity of nebivolol and bisoprolol. A comparison of [3H]CGP 12.177 and [125I]iodocyanopindolol binding studies. Eur J Pharmacol. 2003;460:19–26. [DOI] [PubMed] [Google Scholar]
- 27. Julius S, Nesbitt S. Sympathetic overactivity in hypertension. A moving target. Am J Hypertens. 1996;9:113S–120S. [DOI] [PubMed] [Google Scholar]
- 28. Lund‐Johansen P. The influence of vasodilating beta‐blockers on cardiac function and vascular resistance in essential hypertension. Clin Nephrol. 1992;38(Suppl 1):S78–S86. [PubMed] [Google Scholar]
- 29. White WB. Heart rate and the rate‐pressure product as determinants of cardiovascular risk in patients with hypertension. Am J Hypertens. 1999;2:50S–55S. [DOI] [PubMed] [Google Scholar]
- 30. Asmar R, Boutelant S, Chaignon M, et al. Repeated measurements of non‐invasive ambulatory blood pressure: distinction between reproducibility and the proper effect of placebo. Blood Press Monit. 1996;1:283–288. [PubMed] [Google Scholar]
- 31. Bakris GL, Lindholm LH, Black HR, et al. Divergent results using clinic and ambulatory blood pressures: report of a darusentan‐resistant hypertension trial. Hypertension. 2010;56:824–830. [DOI] [PubMed] [Google Scholar]
- 32. Mancia G, Omboni S, Parati G, et al. Lack of placebo effect on ambulatory blood pressure. Am J Hypertens. 1995;8:311–315. [DOI] [PubMed] [Google Scholar]
- 33. Forest Laboratories . Bystolic: Full Prescribing Information. St. Louis, MO: Forest Laboratories, Inc; 2011. [Google Scholar]
- 34. Fonseca VA. Effects of beta‐blockers on glucose and lipid metabolism. Curr Med Res Opin. 2010;26:615–629. [DOI] [PubMed] [Google Scholar]
- 35. Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21:1074–1090. [DOI] [PubMed] [Google Scholar]
- 36. Rudd P, Byyny RL, Zachary V, et al. Pill count measures of compliance in a drug trial: variability and suitability. Am J Hypertens. 1988;1:309–312. [DOI] [PubMed] [Google Scholar]
- 37. Rudd P, Byyny RL, Zachary V, et al. The natural history of medication compliance in a drug trial: limitations of pill counts. Clin Pharmacol Ther. 1989;46:169–176. [DOI] [PubMed] [Google Scholar]
- 38. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study flow.
Table S1. Baseline‐to‐Endpoint Changes in Key Metabolic Parameters.


