Abstract
The increased prognostic accuracy of the high‐sensitivity cardiac troponin T (hs‐cTnT) assay vs the conventional cTnT assay has recently been reported in hypertensive patients. The authors aimed to investigate the significance of serum hs‐cTnT marker for prediction of nondipper hypertension (HTN) in hypertensive patients. A total of 317 patients with newly diagnosed HTN were studied. The patients were divided into two groups: 198 dipper hypertensive patients (mean age, 51.7±5.1 years) and 119 nondipper hypertensive patients (mean age, 53.4±7.6 years). Hs‐cTnT and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) were measured in all patients. hs‐cTnT and NT‐proBNP were independent predictors for nondipper HTN (P<.05 for all). The cutoff value of hs‐cTnT obtained by the receiver operator curve analysis was 7.55 ng/L for the prediction of nondipper HTN (sensitivity: 79%, specificity: 70%; 95% confidence interval, 0.769–0.860; P<.001). In patients with HTN, higher serum concentration of hs‐cTnT even within normal range is an independent predictor of nondipper HTN.
Hypertension (HTN) is one of the major risk factors for cardiovascular diseases. Blood pressure (BP) is characterized with alteration of rhythm during 24 hours in hypertensive patients.1 It has been shown that 24‐hour ambulatory BP monitoring (ABPM) is a better predictor of subsequent complications than spot measurements of BP.2 The most recent guidelines recommend ABPM as the primary diagnostic tool for HTN.3
Mean BP values are 10% to 20% lower at night, compared with daytime measurements. This condition is called “dipper” change. However, in some hypertensive patients, contrary to this normal change, nighttime BP lowering does not occur or shows a decrease <10%, which is called “nondipper” change.4 It is known that endothelial dysfunction,5 target organ damage such as left ventricular (LV) hypertrophy,6 arterial stiffness,7 autonomic dysfunction,8 oxidative status, and DNA damage9 are more severe in nondipper hypertension (NDH) than in dipper hypertension (DH). In addition, NDH itself is a risk factor for mortality.10
Most patients with HTN even without myocardial necrosis have cardiac troponin T concentrations that are undetectable with conventional assays.11 Even within the normal range, circulating high‐sensitivity cardiac troponin‐T (hs‐cTnT) strongly correlates with cardiac structure abnormalities such as LV hypertrophy and LV dysfunction in the general population and in hypertensive patients.11, 12 hs‐cTnT predicts worsening of albuminuria in HTN.13
In patients presenting with essential HTN, the serum concentrations of hs‐cTnT are slightly or moderately higher than in normotensive patients.11 However, as far as we know, there have been no studies performed until today on the association of hs‐cTnT with dipper and NDH. We aimed to investigate the significance of serum hs‐cTnT marker for prediction of NDH in newly diagnosed hypertensive patients.
Methods
A total of 374 consecutive patients who were admitted to our outpatient clinic and had office BP ≥140/90 mm Hg were evaluated for the study. All patients underwent ABPM. Previously diagnosed hypertensive patients were not evaluated for this study. Of the 374 patients, 51 were excluded because of normal BP according to ABPM and 6 patients were excluded because of extreme dipper HTN. Therefore, measurements were obtained from 317 (mean age, 52.3±6.2 years; male/female, 142/175) patients with newly diagnosed essential HTN in this study. The study population was divided into two subgroups as dipper vs nondipper with regard to nocturnal decline in mean BP (≥10% vs <10%). Exclusion criteria were previously diagnosed HTN, secondary HTN, heart failure, positive history or clinical signs of ischemic heart disease, positive effort test, positive myocardial perfusion scintigraphy, cerebrovascular disease, severe valve disease, atrial fibrillation, taking regular medication for any reason (include antihypertensive drugs), renal insufficiency (serum creatinine: ≥1.5 mg/dL in men and ≥1.4 mg/dL in women), major noncardiovascular diseases, and known diabetes or fasting glucose ≥126 mg/dL. The local ethics committee assessed and approved the study and written informed consent for participation in the study was obtained from all individuals.
BP Measurement and ABPM
BP was measured by using a mercury sphygmomanometer in an office setting. Systolic BP (SBP) and diastolic BP (DBP) were taken. In each patient, BP was measured on at least 3 separate days after 15 minutes of comfortably sitting and then averaged. Each patient wore an ABPM device for a single 24‐hour period. Noninvasive 24‐hour ABPM was performed with a portable compact digital recorder (Tracker NIBP2, Delmar Reynolds Ltd, Hertford, UK), and analyzed using a customized analytical software (Delmar Reynolds Medical Inc, Model 2169, Hertford, UK). The device was programmed to inflate and record BP at prespecified intervals (every 15 minutes during daytime hours and every 30 minutes during nighttime hours), which provided approximately 80 BP recordings during the 24‐hour period. The display of ABPM was inactivated so that viewing each BP reading did not distract patients. For analysis, reports generated from a session of ABPM contained BP recordings for the entire 24 hours, heart rate, mean arterial pressure, and BP load, as well as summary statistics for the overall 24‐hour, daytime, and nighttime periods. When the readings exceeded at least 80% of the total readings programmed for the testing period, the recording was considered as valid and satisfactory.
Diagnosis of Hypertension
Individuals who had an SBP ≥140 mm Hg and/or a DBP ≥90 mm Hg in the office setting, and an average 24‐hour systolic BP >130 mm Hg and/or diastolic BP >80 mm Hg, an average daytime systolic BP >135 mm Hg and/or diastolic BP >85 mm Hg or an average nighttime systolic BP >125 mm Hg and/or diastolic BP >75 mm Hg on ABPM were diagnosed as having hypertension.14 In addition, the patients who had a reduction in BP <10% from the daytime to the nighttime period were defined as nondipper, and the patients who had a reduction in BP ≥10% from the daytime to the nighttime period were considered to have DH.4
Blood Samples
After the diagnosis of hypertension based on ABPM, blood samples were drawn in the morning after a 20‐minute rest following a fasting period of 12 hours before ABPM. Glucose, creatinine, and lipid profiles for blood samples were analyzed for each patient. hs‐CRP was measured using BN2 model nephelometer. Plasma NT‐proBNP was measured by electrochemiluminescence (Roche Diagnostics, Basel, Switzerland).
hs‐cTnT was measured using a fourth‐generation assay on an Elecsys 2010/cobas e 411 instrument (Roche Diagnostics, Mannheim, Germany). The 99th percentile of the hs‐cTnT assay among healthy individuals has been described at 14 ng/L, and the lowest concentration measurable with a coefficient of variation <10% (10% CV) is 13.0 pg/L.15 Intra‐assay and inter‐assay coefficients of variation were 4.5% and 7.9%, respectively.
Echocardiography
Standard 2‐dimensional and Doppler echocardiographies were performed using a commercially available echocardiographic machine (Vivid 7R GE Medical System, Horten, Norway) with a 2.0‐ to 3.5‐MHz transducer. Measurements were made during normal breathing at end expiration. LV end‐diastolic diameter (LVDd), end‐diastolic interventricular septal thickness (IVSth), and end‐diastolic LV posterior wall thickness (PWth) were measured at end diastole according to established standards of the American Society of Echocardiography.16 LV ejection fraction (LVEF) was determined by the biplane Simpson's method.17 LV mass (LVM) was calculated using the Devereux formula18: LVM=(1.04[(LVEDD+IVSth+PWth)3−(LVEDD)3]−13.6).
The LVM index (LVMI, g/m2) was then obtained with the following formula: LVM/body surface area. Relative wall thickness (RWth) was measured at end diastole as the ratio of (2xPWth)/LVEDD.
All echocardiographic measurements were repeated by a second observer (MG) blinded to the values obtained by the first observer (MC). Interobserver variability was assessed by calculating the coefficient of variation. The coefficient of variation was <7% for all measurements. Any discrepancy was resolved by consensus. All echocardiographic measurements were repeated 1 week later by an observer (MC) blinded to the results of the previous measurements and intra‐observer variability was <5% for all measurements.
Statistical Analysis
All analyses were conducted using SPSS 17.0 (SPSS for Windows 17.0; Chicago, IL). Continuous variables were expressed as mean±standard deviation and categorical variables were expressed as percentages. Comparisons of categorical and continuous variables between the two groups were performed using the chi‐square test and independent samples t test, respectively. Multivariate, stepwise, backward conditional logistic regression analysis was used to determine the independent predictors of NDH. All significant parameters in the univariate analysis were selected in the multivariate model. A receiver operator characteristic (ROC) curve analysis was performed to identify the optimal cutoff point of hs‐cTnT to predict NDH in patients with hypertensive patients. The area under the curve (AUC) value was calculated as a measure of the accuracy of the test. The Pearson correlation analysis was used to establish the association between hs‐cTnT with clinical and laboratory parameters. All significant parameters in the bivariate analysis were selected in the multivariate model. To avoid overfitting and collinearity in assessing the multivariate model, independent variables were tested for intercorrelation. Collinearity between variables was excluded before modelling. Finally, hs‐CRP, office SBP, NT‐proBNP, and nighttime SBP were selected in the multivariate model. A multivariate stepwise linear regression analysis was performed to identify the independent associations of hs‐cTnT in hypertensive patients. A P value of <.05 was considered significant.
Results
From the initial 374 hypertensive patients, 57 patients were excluded because they had normal ABPM (51 patients) and extreme dipper HTN (6 patients). Our data were prospectively collected from patients with 198 dipper and 119 nondipper newly diagnosed hypertensive patients. None of the patients received antihypertensive drugs before participation in the present study and during ABPM.
Comparison of Baseline, Laboratory, and Echocardiographic Findings
Comparison of baseline, laboratory, and echocardiographic findings were shown in Table 1. Age, hs‐CRP, NT‐proBNP, and hs‐cTnT values were higher in the NDH group compared with the DH group (P<.05, for all). LVEF was lower and LVMI was higher in the NDH group compared with the DH group (P<.05, for all).
Table 1.
Comparison of Baseline, Clinical, Laboratory, and Echocardiographic Characteristics Between Groups
| Variables | Dipper HTN Group (n=198) | Nondipper HTN Group (n=119) | P Value |
|---|---|---|---|
| Age, y | 51.7±5.1 | 53.4±7.6 | .019 |
| Male sexa | 94 (47.5%) | 48 (40.3%) | .131 |
| BMI, kg/m2 | 29.1±3.7 | 28.5±3.5 | .189 |
| BSA, m2 | 1.83±0.16 | 1.80±0.15 | .090 |
| Heart rate, beats per min | 76.6±11.8 | 77.5±11.9 | .562 |
| Current smokinga | 74 (37.4%) | 35 (29.4%) | .092 |
| Laboratory findings | |||
| Fasting glucose, mg/dL | 89.6±8.1 | 88.4±9.8 | .531 |
| Total cholesterol, mg/dL | 192.4±29.4 | 189.5±33.4 | .412 |
| Triglycerides, mg/dL | 176.7±47.1 | 166.4±42.3 | .049 |
| HDL cholesterol, mg/dL | 40.1±6.2 | 41.4±6.2 | .090 |
| LDL cholesterol, mg/dL | 115.4±24.9 | 113.8±27.3 | .584 |
| Creatinin, mg/dL | 0.77.0±0.12 | 0.79.8±0.14 | .077 |
| hs‐CRP, mg/dL | 0.70±0.26 | 0.86±0.34 | <.001 |
| NT‐proBNP, pg/mL | 89.9±33.1 | 127.2±53.0 | <.001 |
| hs‐cTnT, ng/L | 5.7±3.0 | 9.0±2.7 | <.001 |
| Hemoglobin, g/dL | 13.9±2.7 | 13.8±3.1 | .672 |
| Echocardiography | |||
| Left atrial diameter, mm | 34.1±3.5 | 33.8±3.9 | .512 |
| LVID, mm | 45.4±4.3 | 46.0±3.8 | .262 |
| IVSt, mm | 10.9±1.7 | 11.5±2.7 | .015 |
| PWt, mm | 10.2±1.5 | 10.4±1.9 | .297 |
| Ejection fraction, % | 67±4.9 | 65.4±4.0 | .002 |
| RWt, mm | 0.45±0.1 | 0.46±0.1 | .665 |
| LVMI, g/m2 | 109.9±27.2 | 119.7±31.6 | .004 |
Abbreviations: BMI, body mass index; BSA, body surface area; HDL, high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; hs‐cTnT, high‐sensitivity cardiac troponin T; HTN, hypertension; IVSt, interventricular septal thickness; LDL, low‐density lipoprotein; LVID, left ventricular internal diameter; LVMI, left ventricular mass index; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PWt, posterior wall thickness, RWt, relative wall thickness. aChi‐square. Bold values indicate significance. Values are expressed as mean±standard deviation or number (percentage).
Comparison of ABPM
Mean nighttime SBP, mean nighttime DBP, and mean 24‐hour SBP values of NDH patients were higher than DH patients (P<.05, for all) (Table 2).
Table 2.
Comparison of Ambulatory Blood Pressure Measurements Between Groups
| Variables | Dipper HTN Group (n=198) | Nondipper HTN Group (n=119) | P Value |
|---|---|---|---|
| Office SBP, mm Hg | 152.1±17.7 | 153.8±15.5 | .406 |
| Office DBP, mm Hg | 96.6±13.1 | 98.2±13.3 | .295 |
| Daytime SBP, mm Hg | 146.8±12.8 | 145.6±12.7 | .417 |
| Nighttime SBP, mm Hg | 132.5±11.0 | 143.6±13.8 | <.001 |
| Daytime DBP, mm Hg | 89.7±10.4 | 88.1±9.5 | .178 |
| Nighttime DBP, mm Hg | 79.5±11.7 | 85.2±10.4 | <.001 |
| 24‐h SBP, mm Hg | 139.6±11.3 | 144.6±12.8 | <.001 |
| 24‐h DBP, mm Hg | 84.6±10.4 | 86.6±9.5 | .081 |
Abbreviations: DBP, diastolic blood pressure; HTN, hypertension, SBP, systolic blood pressure. Bold values indicate significance.
Independent Predictors of NDH
hs‐cTnT (odds ratio [OR], 1.409; 95% confidence interval [CI], 1.276–1.556; P<.001) and NT‐proBNP (OR, 1.012; 95% CI, 1.005–1.020; P=.001) were independent predictors of NDH in logistic regression analysis (Table 3).
Table 3.
Independent Predictors of Nondipper Hypertension in Logistic Regression Analysis
| Variables | Odds Ratio | 95% CI (Lower–Upper) | P Value |
|---|---|---|---|
| Age, y | 1.026 | 0.982–1.072 | .253 |
| Triglycerides, mg/dL | 0.995 | 0.989–1.002 | .154 |
| hs‐cTnT, ng/L | 1.409 | 1.276–1.556 | <.001 |
| hs‐CRP, mg/dL | 1.060 | 0.938–1.198 | .353 |
| NT‐proBNP, pg/mL | 1.012 | 1.005–1.020 | .001 |
| Ejection fraction, % | 0.941 | 0.883–1.003 | .060 |
| LVMI, g/m2 | 0.988 | 0.976–1.000 | .055 |
Abbreviations: CI, confidence interval; hs‐CRP, high‐sensitivity C‐reactive protein; hs‐cTnT, high‐sensitivity cardiac troponin T; LVMI, left ventricular mass index; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide. Bold values indicate significance.
The cutoff value of hs‐cTnT obtained by the ROC curve analysis was 7.55 ng/L for predicting NDH (sensitivity: 79%, specificity: 70%). The AUC was 0.814 (95% CI, 0.769–0.860; P<.001). The ROC curve of hs‐cTnT level for predicting the presence of NDH is shown in Figure 1.
Figure 1.
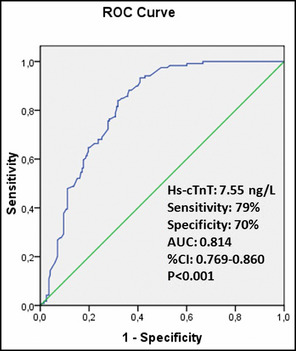
The receiver operator characteristic (ROC) curve of high‐sensitivity cardiac troponin T (hs‐cTnT) level for predicting the presence of nondipper hypertension. AUC indicates area under the curve; CI, confidence interval.
The cutoff value of NT‐proBNP obtained by the ROC curve analysis was 96.3 pg/mL for predicting NDH (sensitivity: 67.2%, specificity: 66.2%). The AUC was 0.711 (95% CI, 0.749–0.774; P<.001). The ROC curve of NT‐proBNP level for predicting the presence of NDH is shown in Figure 2.
Figure 2.
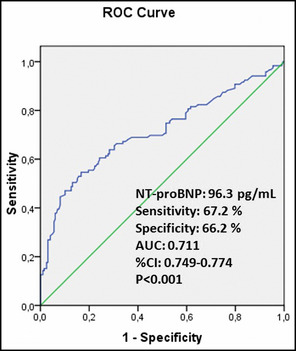
The receiver operator characteristic (ROC) curve of N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) level for predicting the presence of nondipper hypertension. AUC indicates area under the curve; CI, confidence interval.
Bivariate and Multivariate Analysis of hs‐cTnT
hs‐cTnT was associated with hs‐CRP (r=0.158, P=.005), office SBP (r=0.113, P=.045), NT‐proBNP (r=0.280, P<.001), nighttime SBP (r=0.190, P=.001), RWt (r=0.235, P<.001), and LVMI (r=0.379, P<.001) in bivariate analysis (Table 4).
Table 4.
Bivariate and Multivariate Relationships of hs‐cTnT in all Hypertensive Patients
| Variables | Pearson Correlation Coefficient | P Value | Standardized ß Regression Coefficients | P Value |
|---|---|---|---|---|
| hs‐CRP, mg/dL | 0.158 | .005 | 0.089 | .101 |
| Office SBP, mm Hg | 0.113 | .045 | 0.065 | .224 |
| NT‐proBNP, pg/mL | 0.280 | <.001 | 0.259 | <.001 |
| Nighttime SBP, mg/dL | 0.190 | .001 | 0.141 | .04 |
| RWt, mm | 0.235 | <.001 | – | – |
| LVMI, g/m2 | 0.379 | <.001 | – | – |
Abbreviations: hs‐CRP, high‐sensitivity C‐reactive protein; hs‐cTnT, high‐sensitivity cardiac troponin T; LVMI, left ventricular mass index; NT‐proBNP, N terminal pro‐brain natriuretic peptide; RWt, relative wall thickness; SBP, systolic blood pressure.
Multiple linear regression analysis showed that hs‐cTnT was independently associated with NT‐proBNP (β=0.259, P<.001) and nighttime SBP (β=0.141, P=.04).
Discussion
This is the first study to investigate the relationship between hs‐cTnT and NDH in newly diagnosed hypertensive patients. In this study, we found that patients with NDH were older; had higher levels of hs‐CRP, NT‐proBNP, and hs‐cTnT; and had lower values of LVEF compared with patients with DH. The patients with NDH also had higher values of LVMI. The main finding of this study is that (1) hs‐cTnT and NT‐proBNP were independent predictors for NDH, and (2) hs‐cTnT was independently associated with NT‐proBNP and nighttime SBP.
Newly identified biomarkers allow for the noninvasive identification of subclinical myocardial injury.19 A novel hs‐cTnT assay allows for the identification of previously undetected early stages of myocardial injury. It has been shown that hs‐cTnT levels within the normal range increase in hypertensive patients.11, 13 The exact mechanisms underlying increased hs‐cTnT levels in hypertensive patients without ischemic heart disease are unclear. Increased BP may impair coronary microvascular function due to the remodeling of vascular structures and the increased extravascular compressive force with elevated systolic and diastolic wall stress.20 LV hypertrophy increases LV end‐diastolic pressure and wall stresses, which results in the decrease of subendocardial coronary blood flow and reduced tolerance to myocardial ischemia.20 Increased myocardial ischemia may cause myocardial scarring and LV dysfunction, leading to activation of hs‐cTnT release.20, 21 In addition, the conditions that might cause cardiomyocyte apoptosis in overloaded hearts include mechanical and oxidative stress, neurohumoral activation, hypoxia, and cytokines, which might consequently lead to elevated circulating hs‐cTnT levels in hypertension.22
Previously, hs‐cTnT levels were not investigated in DH and NDH patients. In the present study, hs‐cTnT levels of NDH patients were higher than in DH patients. Also, hs‐cTnT and NT‐proBNP were an independent predictor for NDH. This result may be plausible because the patients with NDH had poorer prognosis than patients with DH.
In the present study, NDH patients had greater LV hypertrophy. Previous studies showed that NDH patients have higher LVM than DH patients.6, 23 Also, previous studies reported that hs‐cTnT was independently associated with LV hypertrophy in hypertensive patients.12 On the other hand, the present study showed that hs‐cTnT level was associated with nighttime SBP. NDH patients have greater extravascular compressive force than DH patients because of greater nighttime BP. Elevated systolic and diastolic wall stress throughout the day as a result of increased extravascular compressive force may be responsible for elevated hs‐cTnT release in NDH patients.
The present study showed that NDH patients had higher NT‐proBNP levels, and NT‐proBNP was an independent predictor for NDH. A similar result was observed in a previous study by Dogan and colleagues23 who reported that NDH patients have higher NT‐proBNP levels compared with DH patients. BNP is secreted by ventricular myocytes in response to volume expansion and pressure overload, and plasma concentrations are elevated in patients with ventricular hypertrophy.24, 25 In the present study, higher NT‐proBNP values in NDH patients may be related to greater LV hypertrophy compared with DH patients.
Rubin and colleagues26 showed that chronic hyperglycemia assessed by hemoglobin A1c is independently associated with subclinical myocardial injury measured by elevated hs‐cTnT in diabetic patients without clinically evident coronary artery disease or heart failure. They suggested that hyperglycemia contributes to myocardial injury beyond its effects on development of clinical atherosclerotic coronary disease. Yiu and colleagues27 also showed that subclinical myocardial injury assessed by high‐sensitivity troponin I is positively correlated with SBP in diabetic patients, independent of clinically overt atherosclerotic disease. However, they did not investigate the relationship between DH and NDH status and subclinical myocardial injury in diabetic patients. In our study, diabetic patients were excluded from the study. We supposed that diabetic patients with NDH might have higher subclinical myocardial injury; however, further studies are needed to confirm this suggestion.
Study Limitations
Coronary artery disease may affect hs‐cTnT levels in this patient group. Coronary angiography was not performed in our patients although patients with coronary artery disease were excluded according to clinical characteristics and patient history, electrocardiography, and treadmill exercise test.
Conclusions
hs‐cTnT and NT‐proBNP are independent predictors for NDH patients. In addition, hs‐cTnT was independently associated with NT‐proBNP and nighttime SBP in hypertension. Therefore, hs‐cTnT might be a prognostic marker in newly diagnosed hypertension.
Declaration of Interest
The authors report no conflicts of interest.
J Clin Hypertens (Greenwich). 2013;15:731–736. ©2013 Wiley Periodicals, Inc.
References
- 1. O'Brien ET, Murphy J, Tyndall A, et al. Twenty‐four‐hour ambulatory blood pressure in men and women aged 17 to 80 years: the Allied Irish Bank Study. J Hypertens. 1991;9:355–360. [DOI] [PubMed] [Google Scholar]
- 2. Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension: systolic hypertension in Europe trial investigators. JAMA. 1999;282:539–546. [DOI] [PubMed] [Google Scholar]
- 3. Krause T, Lovibond K, Caulfield M, et al; Guideline Development Group . Management of hypertension: summary of NICE guidance. BMJ. 2011;343:d4891. [DOI] [PubMed] [Google Scholar]
- 4. Verdecchia P, Schillaci G, Porcellati C. Dippers versus non‐dippers. J Hypertens. 1991;9:42–48. [PubMed] [Google Scholar]
- 5. Higashi Y, Nakagawa K, Kimura M, et al. Circadian variation of blood pressure and endothelial function in patients with essential hypertension: a comparison of dippers and non‐dippers. J Am Coll Cardiol. 2002;40:239–243. [DOI] [PubMed] [Google Scholar]
- 6. Syrseloudis D, Tsioufis C, Aragiannis D, et al. The dominant role of the systolic component of nondipping status on target‐organ damage in never‐treated hypertensives. Am J Hypertens. 2011;24:292–298. [DOI] [PubMed] [Google Scholar]
- 7. Cicek Y, Durakoglugil ME, Kocaman SA, et al. Non‐dipping pattern in untreated hypertensive patients is related to increased pulse wave velocity independent of raised nocturnal blood pressure. Blood Press. 2013;22:34–38. [DOI] [PubMed] [Google Scholar]
- 8. Erden M, Kocaman SA, Poyraz F, et al. Incremental effects of serum uric acid levels, autonomic dysfunction, and low‐grade inflammation on nocturnal blood pressure in untreated hypertensive patients and normotensive individuals. Turk Kardiyol Dern Ars. 2011;39:531–539. [DOI] [PubMed] [Google Scholar]
- 9. Gür M, Elbasan Z, Şahin DY, et al. DNA damage and oxidative status in newly diagnosed, untreated, dipper and non‐dipper hypertensive patients. Hypertens Res. 2013;36:166–171. [DOI] [PubMed] [Google Scholar]
- 10. Ohkubo T, Imai Y, Tsuji I, et al. Relation between nocturnal decline in blood pressure and mortality, the Ohasama Study. Am J Hypertens. 1997;10:1201–1207. [DOI] [PubMed] [Google Scholar]
- 11. Sato Y, Yamamoto E, Sawa T, et al. High‐sensitivity cardiac troponin T in essential hypertension. J Cardiol. 2011;58:226–231. [DOI] [PubMed] [Google Scholar]
- 12. de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hellemons ME, Lambers Heerspink HJ, Gansevoort RT, et al. High‐sensitivity troponin T predicts worsening of albuminuria in hypertension; results of a nested case–control study with confirmation in diabetes. J Hypertens. 2013;31:805–812. [DOI] [PubMed] [Google Scholar]
- 14. Stergiou GS, Salgami EV; World Health Organization‐International Society of Hypertension (WHO‐ISH) , USA Joint National Committee on Prevention, Detection, Evalutiaon, Treatment of High Blood Pressure (JNC‐7) , European Society of Hypertension‐European Society of Cardiology (ESH‐ESC) . New European, American and International guidelines for hypertension management: agreement and disagreement. Expert Rev Cardiovasc Ther. 2004;2:359–368. [DOI] [PubMed] [Google Scholar]
- 15. Hoshide S, Fukutomi M, Eguchi K, et al. clinical and change in high‐sensitive cardiac troponin T on hypertensive treatment. Clin Exp Hypertens. 2013;35:40–44. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Bierig M, Devereux RB, et al; Chamber Quantification Writing Group . Recommendations for Chamber Quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 17. Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 18. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man, anatomic validation of the method. Circulation. 1977;55:613–618. [DOI] [PubMed] [Google Scholar]
- 19. Melander O, Newton‐Cheh C, Almgren P, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 21. Hickman PE, Potter JM, Aroney C, et al. Cardiac troponin may be released by ischemia alone, without necrosis. Clin Chim Acta. 2010;411:318–323. [DOI] [PubMed] [Google Scholar]
- 22. Fortu˜no MA, Ravassa S, Fortu˜no A, et al. Cardiomyocyte apoptotic cell death in arterial hypertension: mechanisms and potential management. Hypertension. 2001;38:1406. [DOI] [PubMed] [Google Scholar]
- 23. Dogan SM, Aydin M, Gursurer M, et al. N‐terminal probrain natriuretic peptide predicts altered circadian variation in essential hypertension. Coron Artery Dis. 2007;18:347–352. [DOI] [PubMed] [Google Scholar]
- 24. Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. [DOI] [PubMed] [Google Scholar]
- 25. Cowie MR, Mendez GF. BNP and congestive heart failure. Prog Cardiovasc Dis. 2002;44:293–321. [DOI] [PubMed] [Google Scholar]
- 26. Rubin J, Matsushita K, Ballantyne CM, et al. Chronic hyperglycemia and subclinical myocardial injury. J Am Coll Cardiol. 2012;59:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yiu KH, Zhao CT, Chen Y, et al. Association of subclinical myocardial injury with arterial stiffness in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2013;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]


