Abstract
The common assumption is that blood pressure (BP) will decrease on subsequent readings. The objective of this study is to examine the prevalence and direction of BP classification change with repeat measurements and compare common clinical characteristics of groups of patients who do and do not have a change in BP classification. A nationally representative subsample of 1725 adolescents aged 13 to 18 years from the National Health and Nutrition Survey were analyzed. Three BP measurements were obtained. Patients were classified based on the first and the average of 3 BP measurements as having normal BP, hypertension, and/or prehypertension. Of the 1725 adolescents, 1569 (90.9%) maintained BP classification, 107 (6.2%) had a reduction in their classification, and 49 (2.9%) had an increase in their classification. Comparing the two groups that changed BP classification to the group without change, C‐reactive protein and body mass index (BMI) z score were significantly higher in the groups that had a change in BP classification (P=.02 and <.001, respectively). After adjusting for other variables, higher BMI value was significantly associated with change in BP classification. With repeat measurements, the majority (~91%) did not have a change in classification. Obesity was a significant predictor of the 9% that had a change in classification. Repeat BP measurements in obese adolescents may lead to more accurate classification of BP status.
Several studies have documented that blood pressure (BP) in children is variable, which supports recommendations to obtain multiple readings on several visits before diagnosing hypertension.1, 2, 3, 4, 5 This practice is followed to a variable degree in different clinical settings.6, 7 Furthermore, the proportion of pediatric patients whose BP declines, remains the same, or rises with repeat measurement and the impact on BP classification is unknown. Of particular concern, incorrect classification can lead to inappropriate management of a patient's BP.
As the prevalence of pediatric obesity continues to rise, there is a parallel trend in hypertension and prehypertension in the pediatric population.8 Consequently, accurate measurement of BP becomes ever more important to ensure appropriate classification as normotensive, prehypertensive, or hypertensive. Although the direct effects on mortality from childhood hypertension are not as well established as for adults, evidence of target organ damage is well documented in the literature.
Atherosclerosis, a strong predictor of cardiovascular disease in adults, begins in childhood.9, 10, 11 Furthermore, left ventricular hypertrophy (LVH) has been found in more than one third of children and adolescents with elevated BP.2, 12, 13 Children with BP in the prehypertensive range are also at greater risk of kidney damage with decreased glomerular filtration rates (GFRs) and proteinuria.14 Pediatric patients with more severe BP elevations are at increased risk of significant target‐organ damage, morbidity, and mortality, such as neurologic damage (hypertensive encephalopathy, seizures, and stroke) and heart failure.2, 15, 16 A correct diagnosis of prehypertension and hypertension is important, because normalizing BP may reverse early signs of target organ damage, including carotid intimal‐media wall thickness (a correlate of LVH) and reduced GFR.12, 14, 17
Generally, one BP is measured in most pediatric clinical visits, particularly if the initial BP value is <95% for age, sex, and height. The impact of this practice on accurate assignment of BP classification is incompletely defined. Therefore, in this study—using BP measurements obtained during the National Health and Nutrition Survey (NHANES)—we retrospectively examined the direction of change in BP classification with repeat measurements. Furthermore, we compared demographic and biochemical characteristics of those with changing BP classification with those whose BP remained the same with repeated measurements. Finally, we examined independent determinants of change in BP classification with repeated measurements.
Methods
Study Population
Data from NHANES for the years 2005 to 2008 were analyzed. NHANES is an ongoing nationally representative cross‐sectional survey of the civilian, noninstitutionalized US population that uses a complex, stratified, multistage probability design. The survey is performed by the National Center for Health Statistics (NCHS) at the Centers for Diseases Control and Prevention (CDC) and released in 2‐year increments. Survey participants underwent standardized interviews, physical examinations, and laboratory testing in their homes and at a mobile examination center (MEC).
Health interviews are conducted in respondents' homes. Health measurements are performed in specially designed and equipped mobile centers, which travel to locations throughout the country. The study team consists of a physician, medical and health technicians, and dietary and health interviewers. Many of the study staff are bilingual (English/Spanish).
In each location, local health and government officials are notified of the upcoming survey. Households in the study area receive a letter from the NCHS director to introduce the survey. Local media may feature stories about the survey.
BP measurements were obtained on all NHANES participants aged 8 years and older. Of the 2125 adolescents between the age of 13 and 18 years, 1730 had 3 BP measurements. After resting quietly in a sitting position for 5 minutes and determining the maximum inflation level (MIL), 3 consecutive BP readings are obtained. If a BP measurement was interrupted or incomplete, a fourth attempt was made. All BP determinations (systolic and diastolic) were taken in the MEC. The participants were asked to sit all the way to the back of the chair so that their spine was straight and rest quietly for 5 minutes prior to BP measurement. The arm and back were supported with the legs uncrossed and both feet flat on the floor. The arm was bared and unrestricted by clothing with the palm of the hand turned upward and the elbow slightly flexed. The arm was positioned so that the midpoint of the upper arm was at the level of the heart. Four participants were receiving medication for hypertension and one had a missing weight value and thus eliminated from analysis. The remaining 1725 participants constituted the study cohort.
NHANES 2005–2008 was approved by the NCHS's institutional review board. All of the participants 18 years and older provided informed consent and for those participants younger than 18 years, parents/guardians provided informed consent.
Study Variables
Demographic variables included in the current study were age, sex, and self‐reported race/ethnicity (categorized as non‐Hispanic black, non‐Hispanic white, Mexican American, and other). Poverty to income ratio (PIR) is the ratio of a family's income to the poverty threshold as defined by the US Census Bureau. A PIR ≤1 was defined as below the poverty threshold by NHANES.
Physical measures were obtained in the MEC according to standardized protocol.18 Three systolic and diastolic BP measurements were obtained for each participant using a mercury sphygmomanometer. Based on the first and the average of 3 BP measurements, we classified participants as having hypertension if BP was ≥95th percentile for age, sex, and height and pre‐hypertension if BP was ≥90th percentile and <95th percentile for age, sex, and height.2 BP classification was used to determine the direction of change in BP classification as “up,” “down,” or no change based on the repeat measurement. Also, the distribution of BP classification was examined among the 3 groups (up, down, and no change).
Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). BMI z scores were calculated based on 2000 CDC growth charts.20
Laboratory procedures are described in detail in the NHANES General Information for Public Files 2005 to 2006 and 2007 to 2008 Laboratory Files.20, 21 Serum uric acid levels were measured using a colorimetric method. High‐sensitivity C‐reactive protein (hs‐CRP) was measured by latex‐enhanced nephelometry. Serum cholesterol levels were measured on the Beckman Synchron LX20 (Beckman Coulter, Brea, CA). Urinary albumin levels were measured by using a solid‐phase fluorescent immunoassay. Urinary creatinine levels were measured by using the Jaffe rate reaction with a CX3 analyzer (Beckman ASTRA, Brea, CA). The urinary albumin/creatinine ratio (ACR) was calculated as urinary albumin divided by urinary creatinine. An ACR ≥30 was used to define albuminuria. Serum creatinine was measured by means of the modified kinetic Jaffe reaction. Because differences were noted in serum creatinine values from the 1999 to 2000 survey when compared with a “gold standard,” a correction factor was applied to adjust for errors in measurement. Estimated GFR (eGFR) was measured using the Schwartz formula.22
The nutritional assessment component of the current NHANES includes a 24‐hour dietary recall interview for participants of all ages. Dietary recall interviews were conducted in person by trained dietary interviewers fluent in Spanish and English. The setting of the interview was a private room in the MEC. Each MEC dietary interview room contained a standard set of measuring guides. These tools were used to help the respondent report the volume and dimensions of the food items consumed. They were not intended to represent any one particular food, but rather were designed to help respondents estimate portion sizes. This set of measuring guides was designed specifically for use in the current NHANES setting with a target population of noninstitutionalized US civilians. Whereas the tools are helpful in portion size estimation for a wide variety of foods, culturally sensitive precautions are warranted when applying these guides to other populations.
Statistical Analysis
The proportion of adolescent study participants aged 13 to 18 years was calculated by change in BP classification (BP increase, BP decrease, or BP classification remained the same). Participant demographic characteristics and laboratory measures were calculated by BP category. Statistical tests of significance between BP categories were determined using linear and logistic regression for continuous and dichotomous variables, respectively. Multivariate logistic regression was used to examine demographic and/or laboratory determinants of change in BP classification.
Data were analyzed using Stata (SE 11.2; College Station, TX). Standard errors for all estimates were obtained with the Taylor‐linearized variance estimation. Four‐year sample weights were applied to account for the complex sampling design of NHANES. This includes unequal probabilities of selection, oversampling, and nonresponse. A P value of <.05 was considered statistically significant.
Results
A total of 1725 adolescents aged 13 to 18 years from NHANES 2005–2008, representing 18,195,503 US adolescents, were included in these analyses (Figure 1). Tables 1 and 2 describe demographic and biochemical characteristics of the study cohort. When demographic and biochemical variables were examined for differences by the sex of participants, cholesterol, uric acid, hs‐CRP, eGFR, height, and weight, but not BMI z score, were statistically different. With repeat measurements, BP classification remained the same in 1569 (90.9%), declined in 107 (6.2%), and increased in 49 (2.9%) patients. Comparing the two groups that changed BP classification to the group with no change (Tables 1 and 2), age, sex, race, urinary albumin/creatinine, eGFR, uric acid level, and total cholesterol levels were not statistically different. On the other hand, BMI z score were significantly higher in the group whose BP classification went down (group II) (P=.02), and hs‐CRP was significantly higher in the group whose BP classification changed compared with those who remained the same (P=.02).
Figure 1.
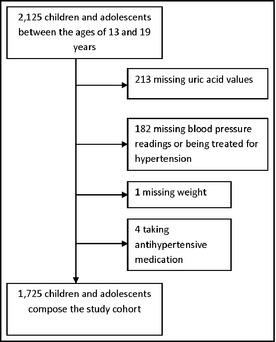
Flowchart of the study cohort.
Table 1.
| Group I BP Up 2.9% (n=49) | Group II BP Down 6.2% (n=107) | Group III BP No Change 90.9% (n=1569) | P Value | |||
|---|---|---|---|---|---|---|
| I vs III | II vs III | (I and II) vs III | ||||
| Age, y | 16.00±2.06 | 15.68±1.92 | 15.51±1.90 | .44 | .27 | .20 |
| Male | 37.32 (33) | 46.25 (64) | 50.68 (774) | .50 | .22 | .27 |
| Race | .04 | .76 | .14 | |||
| White | 66.80 (15) | 64.20 (33) | 65.28 (482) | |||
| Black | 24.60 (25) | 15.32 (32) | 12.84 (454) | |||
| Mexican American | 5.54 (6) | 8.44 (27) | 10.88 (443) | |||
| Other | 3.07 (3) | 12.03 (15) | 11.00 (190) | |||
| Poverty (yes) b | 29.32 (26) | 35.49 (66) | 38.50 (775) | .27 | .56 | .31 |
| Height, cm | 172.44±11.11 | 169.09±11.02 | 167.25±10.98 | .16 | .02 | .02 |
| Weight, kg | 78.30±27.33 | 72.07±23.28 | 65.67±20.54 | <.01 | <.001 | <.001 |
| BMI z score | 0.99±1.27 | 0.90±1.16 | 0.55±1.27 | .01 | .02 | .003 |
Abbreviations: BMI, body mass index; BP, blood pressure. aContinuous variables presented as mean±standard deviation and categorical variables presented as percentage (number). P values calculated by weighted least‐squares or weighted logistic regression analyses.
bPoverty defined as a poverty income ratio ≤1.
Table 2.
| Group I BP Up 2.9% (n=49) | Group II BP Down 6.2% (n=107) | Group III BP No Change 90.9% (n=1569) | P Value | |||
|---|---|---|---|---|---|---|
| I vs III | II vs III | (I and II) vs III | ||||
| Cholesterol, mg/dL | 171.32±29.23 | 162.84±30.03 | 160.19±35.11 | .34 | .07 | .06 |
| Uric acid, mg/dL | 5.52±1.62 | 5.34±1.42 | 5.11±1.44 | .21 | .16 | .13 |
| C‐reactive protein, mg/dL c | 0.04 (0.02–0.12) | 0.04 (0.01–0.12) | 0.03 (0.01–0.12) | .04 | .05 | .02 |
| eGFR, mL/min b | 132.31±21.96 | 136.28±35.07 | 135.58±31.67 | .82 | .31 | .88 |
| Urine albumin‐to‐creatinine ratio c | 5.3 (4.6–6.1) | 5.2 (4.5–6.3) | 5.0 (4.2–5.8) | .45 | .53 | .61 |
aContinuous variables presented as mean±standard deviation and categorical variables presented as percentage (number). P values calculated by weighted least‐squares or weighted logistic regression analyses.
bEstimated glomerular filtration rate (eGFR) estimated using Schwartz formula.
cPresented as median (interquartile range).
Using multivariate logistic regression models, determinants of change in BP classification change vs no change (as the outcome variable) were examined. There were no significant associations found between change in BP classification and age, sex, race, urinary albumin/creatinine, eGFR, uric acid, and total cholesterol levels. BMI z score and hs‐CRP were the only factors significantly associated with any change in BP classification (either up or down) after adjusting for age and sex (odds ratio [OR] for BMI z score: 1.45; 95% confidence interval [CI], 1.17–1.84; P<.01; OR for hs‐CRP, 0.39; 95% CI, 0.16–0.97; P=.04). Finally, BMI z score was the only factor that remained significant for a decrease in BP after adjusting for age and sex (OR, 1.59; 95% CI, 1.05–2.38; P=.03).
With repeat measurements, BP classification remained the same in 1569 (90.9%), declined in 107 (down in 6.2%), and rose in 49 (up in 2.9%) patients. We examined the distribution of BP classification in each group (Figure 2). Among adolescents for whom BP classification went down (n=107), 85.1% (n=91) were classified the as prehypertensive and 15% (n=16) as hypertensive on the first BP reading. Among those for whom BP went up (n=49), 81.6% (n=40) were classified as normotensive and 18.4% (n=9) as prehypertensive on the first BP reading. Finally, for those in whom BP did not change classification (same) (n=1569), 86.6% (n=1359) were classified as normotensive, 11.2% (n=175) as prehypertensive, and 2.2% (n=35) as hypertensive on the first BP reading.
Figure 2.
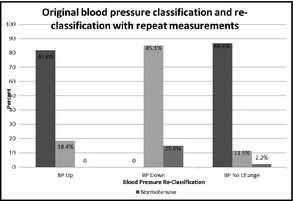
First blood pressure classification and reclassification with repeat measurements.
It is important to note that there was no difference in sodium intake between those whose BP went down vs no change (P=.424) and those whose BP went up and those whose stayed the same (P=.168) (data no shown). Also, there was no significant difference in water intake between those whose BP went down vs no change (P=.072) and those whose BP went up and those whose stayed the same (P=.926) (data not shown). Moreover, there was no difference in BP change by sex (P=.35). Of the sample that was eligible and responded to the questionnaire about reproductive health, 61.5% were taking birth control pills (n=61). Of whom, 9.9% had a reduction in BP, 89.7% had no BP change, and 9.4% had higher BP with repeat measurements.
Discussion
Hypertension guidelines recommend using the average of ≥2 BP measurements for clinical purposes, yet most patients have only 1 BP measurement in the usual routine office encounter.23 While health care providers mostly expect a reduction in BP with repeat measurements, in this analysis of NHANES data, we shed light on a small percentage (2.9%) of adolescents who have a higher BP classification with repeat testing and may be missed with single BP measurement. In addition, our study shows that BMI z score and CRP are independent correlates of change in BP classification with repeat measurements. Furthermore, a larger percentage of adolescents who changed their classification “up” were prehypertensive on their first BP measurement. Our study also shows that the majority of pediatric patients (~91%) do not change BP classification with repeat BP measurements. BP classification went down for 6.2% of adolescents.
In a cross‐sectional study that focused on adolescent high school students, the prevalence rates of prehypertension and hypertension in a cohort of 6790 adolescents (age range, 11–17 years) were determined by using the recommended repeated measurements of BP. The study was conducted in schools in Houston, Texas, and repeat measurements (obtained at separate sessions) were obtained for at‐risk adolescents. BP distribution at initial screen showed 81.1% of patients with normal BP, 9.5% with prehypertension, and 9.4% with hypertension. Prevalence after 3 screenings was 81.1% normal, 15.7% prehypertension, and 3.2% hypertension. Only classification as overweight (defined in the study as BMI ≥95%) was associated with hypertension.5
Study Limitations and Strengths
It is generally believed that with repeat BP measurements, especially after a brief period of rest, BP readings tend to go down. The practice of obtaining multiple BP readings is widely accepted to help reduce the likelihood of the white‐coat effect and classifying anxious and active children with hypertension. Very little is known about a small group of children whose BP readings go up with repeat BP measurements. In this study, we examined the 2.9% of our study population for whom BP went up with repeat measurements and found that those adolescents had higher BMI and CRP levels. While most children calm down after a brief period of rest, it is possible that some get more anxious from the process of obtaining multiple measurements. Another possible explanation comes from the statistical phenomenon called “regression to the mean,” where with repeat measurements, readings tend to converge toward the mean (low readings go up with repeat measurements). Although regression to the mean is a possible explanation, a noteworthy observation was that a higher percentage of adolescents in that group were in the prehypertension range and were overweight and had significantly different CRP levels. This raises the possibility that this group represents a high‐risk group with a more labile BP compared with their lean counterparts. Indeed, obesity is an inflammatory state, characterized by higher CRP levels, cytokine release, and hormonal dysregulation, leading to activation of the sympathetic nervous system. Decreased adiponectin levels, for example, are associated with insulin resistance, atherosclerosis, and hypertension in obese adolescents.24, 25 In our cohort, although the group that changed BP classification had a statistically different CRP levels, the differences were not clinically significant as all 3 groups had levels within normal limits for age.
Despite the intrinsic variability of BP measurements, many studies tracking BP from childhood to adulthood have shown the best predictors of future BP to be initial BP, initial BMI, and changes in BMI.26, 27 In an analysis of data from the National Childhood Blood Pressure Database examining the longitudinal BP outcomes for adolescents classified after a single measurement of BP, high BMI at initial presentation and increasing BMI during the follow‐up period predicted sustained BP elevations.4 Although our study utilized BP measurements obtained at one occasion, which is not ideal to make the diagnosis of hypertension, the 3 measurements obtained after 5 minutes of rest were sufficient to show the direction of BP classification change with repeat measurements in the same setting.
To our knowledge, this is the first study to shed light on this small percentage of children with higher BPs on repeat measurements. Also, it stresses the importance of paying close attention to BP measurements in overweight children.
Whether these findings represent an increased cardiovascular risk to the “up” group or just a statistical phenomenon, the current recommendations are to obtain multiple measurements. In the setting of a busy clinical practice, the primary care provider with limited resources may choose to focus on a subset of patients for repeat BP measurements. Our findings suggest that overweight children are more likely to change their BP classification.
Conclusions
With repeat measurements, the majority of pediatric patients (~91%) do not change classification. BP classification went down for 6.2% of adolescents. A smaller percentage of adolescents have a higher BP classification with repeat testing and may be missed with single BP measurement. Higher BMI was significantly associated with change in BP classification. Further studies comparing cardiovascular risk and target organ damage in groups that change BP classification are needed.
Disclosure
The authors have no financial relationships relevant to this article to disclose. All authors have no conflicts of interest relevant to this article to disclose.
J Clin Hypertens (Greenwich). 2013;15:717–722. ©2013 Wiley Periodicals, Inc.
References
- 1. Levine RS, Hennekens CH, Klein B, et al. A longitudinal evaluation of blood pressure in children. Am J Public Health. 1979;69:1175–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114(Supplement 2): 555–576 [PubMed] [Google Scholar]
- 3. Belsha CW, Wells TG, McNiece KL, et al. Influence of diurnal blood pressure variations on target organ abnormalities in adolescents with mild essential hypertension. Am J Hypertens 1998;1:410–417. [DOI] [PubMed] [Google Scholar]
- 4. Faulkner B, Gidding SS, Portman R, Rosner B. Blood pressure variability and classification of prehypertension and hypertension in adolescence. Pediatrics 2008;122:238–242. [DOI] [PubMed] [Google Scholar]
- 5. McNiece KL, Poffenbarger TS, Turner JL, et al. Prevalence of hypertension and pre‐hypertension among adolescents. J Pediatr 2007;150:640–644. [DOI] [PubMed] [Google Scholar]
- 6. Brady TM, Solomon BS, Neu AM, et al. Patient‐, provider‐, and clinic‐level predictors of unrecognized elevated blood pressure in children. Pediatrics 2010;125:e1286–e1293. [DOI] [PubMed] [Google Scholar]
- 7. Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA 2007;298:874–879. [DOI] [PubMed] [Google Scholar]
- 8. Din‐Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963–2002. Circulation 2007;116:1488–1496. [DOI] [PubMed] [Google Scholar]
- 9. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011;128(Suppl 5): s213–s256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGill HC Jr, McMahan CA, Zieske AW, et al. for the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group . Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth. Arterioscler Thromb Vasc Biol 2000;20: 1998–2004 [DOI] [PubMed] [Google Scholar]
- 11. Berenson GS, Wattigney WA, Tracy RE, et al. Atherosclerosis of the aorta and coronary arteries and cardiovascular risk factors in persons aged 6–30 years and studied at necropsy (The Bogalusa Heart Study). Am J Cardiol 1992;70:851–858. [DOI] [PubMed] [Google Scholar]
- 12. Sorof JM, Alexandrov AV, Cardwell G, Portman RJ. Carotid artery intimal‐medial thickness and left ventricular hypertrophy in children with elevated blood pressure. Pediatrics 2003;111:61–66. [DOI] [PubMed] [Google Scholar]
- 13. Daniels SR, Loggie JM, Khoury P, Kimball TR. Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation 1998;97:1907–1911. [DOI] [PubMed] [Google Scholar]
- 14. Lubrano R, Travasso E, Raggi C, et al. Blood pressure load, proteinuria and renal function in pre‐hypertensive children. Pediatr Nephrol 2009;24:823–831. [DOI] [PubMed] [Google Scholar]
- 15. Still JL, Cottom D. Severe hypertension in childhood. Arch Dis Child 1967;42:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gill DG, Medes dC, Cameron JS, et al. Analysis of 100 children with severe and persistent hypertension. Arch Dis Child 1976;51:951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meyer AA, Kundt G, Lenschow U, et al. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six‐month exercise program. J Am Coll Cardiol 2006;48:1865–1870. [DOI] [PubMed] [Google Scholar]
- 18. National Health and Nutrition Examination Survey: Physician Examination Procedures Manual. Atlanta: Centers for Disease Control and Prevention; c2003. CDC/National Center for Health Statistics. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/PE.pdf.
- 19. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002;246:1–190. [PubMed] [Google Scholar]
- 20. National Health and Nutrition Examination Survey: General Information for the Public Files of the 2005–2006 Laboratory Data. Atlanta: Centers for Disease Control and Prevention; c2007. CDC/National Center for Health Statistics. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/lab_d_generaldoc.pdf.
- 21. National Health and Nutrition Examination Survey: General Information for the Public Files of the 2007–2008 Laboratory Data. Atlanta: Centers for Disease Control and Prevention; c2010. CDC/National Center for Health Statistics. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2007–2008/labdoc_e.htm.
- 22. Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009;3:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 24. Ouchi N, Kihara S, Funahashi T, et al. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol 2003;14:561–566. [DOI] [PubMed] [Google Scholar]
- 25. Shatat IF, Freeman KD, Vuguin PM, et al. Relationship between adiponectin and ambulatory blood pressure in obese adolescents. Pediatr Res 2009;65:691–695. [DOI] [PubMed] [Google Scholar]
- 26. Cook NR, Gillman MW, Rosner BA, et al. Prediction of young adult blood pressure from childhood blood pressure, height and weight. J Clin Epidemiol 1997;50:571–579. [DOI] [PubMed] [Google Scholar]
- 27. Lauer RM, Clarke WR, Mahoney LT, Witt J. Childhood predictors for high adult blood pressure The Muscatine Study. Pediatr Clin North Am 1993;40:23–40. [DOI] [PubMed] [Google Scholar]


