Abstract
This study was performed to investigate whether intensive antihypertensive treatment with achieved blood pressure (BP) ≤140/90 mm Hg, as compared with standard treatment with achieved BP ≤150/90 mm Hg, could further improve cardiovascular outcomes in Chinese hypertensive patients older than 70 years. A total of 724 participants were randomly assigned to intensive or standard antihypertensive treatment. After a mean follow‐up of 4 years, the mean achieved BP was 135.7/76.2 mm Hg in the intensive treatment group and 149.7/82.1 mm Hg in the standard treatment group. The visit‐to‐visit variability in systolic BP and diastolic BP was lower in the intensive group than that in the standard group. Intensive antihypertensive treatment, compared with the standard treatment, decreased total and cardiovascular mortality by 41.7% and 50.3%, respectively, and reduced fatal/nonfatal stroke by 42.0% and heart failure death by 62.7%. Cox regression analysis indicated that the mean systolic BP (P=.020; 95% confidence interval, 1.006–1.069) and the standard deviation of systolic BP (P=.033; 95% confidence interval, 1.006–1.151) were risk factors for cardiovascular endpoint events. Intensive antihypertensive treatment with achieved 136/76 mm Hg was beneficial for Chinese hypertensive patients older than 70 years. Long‐term visit‐to‐visit variability in systolic BP was positively associated with the incidence of cardiovascular events.
Hypertension is one of the most common diseases associated with the elderly. It is a significant risk factor for senile congestive heart failure, stroke, coronary heart disease, renal failure, and aortic aneurysm and has become an important public‐health challenge worldwide.1 The risks associated with hypertension are greater in older than in younger patients, and antihypertensive treatment is reported to be actually more cost‐effective for the elderly.2 The results of the study for the Hypertension in the Very Elderly Trial (HYVET) suggest that antihypertensive treatment with the achieved blood pressure (BP) of 143.5/77.9 mm Hg was beneficial in hypertensive patients older than 80 years, associated with a 30% reduction in the rate of fatal or nonfatal stroke, a 39% reduction in the rate of death from stroke, a 21% reduction in the rate of death from any cause, a 23% reduction in the rate of death from cardiovascular causes, and a 64% reduction in the rate of heart failure, compared with the placebo group with the achieved BP of 158.5/84.0 mm Hg.3 Thus, BP reduction in preventing stroke and other cardiovascular events for elderly hypertensive patients has evoked great focus in the past decade. Although many guidelines for the management of hypertension proposed the goal of systolic BP (SBP) as <150 mm Hg for the elderly,4, 5 it is unclear whether further reduction is still beneficial. In addition, although it has been suggested that BP variability derived from 24‐hour ambulatory monitoring may be an independent risk factor for cardiovascular morbidity,6, 7 little is known about BP variability during long‐term follow‐up.8
Therefore, this study is designed to investigate whether the intensive antihypertensive treatment with the target BP of <140/90 mm Hg, as compared with the standard treatment with the target BP of <150/90 mm Hg, could further improve cardiovascular outcomes in Chinese hypertensive patients older than 70 years, and to assess whether the visit‐to‐visit variability in BP causes greater risk of cardiovascular events.
Methods
Study Population
Participants were eligible if they were older than 70 years and were classified as hypertensive irrespective of sex, SBP ≥150 mm Hg and/or diastolic BP (DBP) ≥90 mm Hg, measured twice in different days, or were diagnosed with hypertension and currently receiving antihypertensive treatment. Patients selected for participation all received outpatient general practice care.
Exclusion criteria included secondary hypertension, valvular heart disease, chronic kidney dysfunction (serum creatinine ≥3.0 mg/dL), previous myocardial infarction or stroke in the past 6 months, New York Heart Association (NYHA) class III or higher congestive heart failure, echocardiography determining left ventricular ejection fraction (LVEF) <40%, hepatic dysfunction, autoimmune disorders, malignant tumor, Alzheimer's disease, and other noncardiovascular diseases potentially causing death before the end of the study.
This was a prospective, randomized, open‐label, blinded‐endpoint assessment (PROBE) study, which was approved by the Medical Ethics Committee in Shanghai Songjiang Center Hospital, Shanghai 201600, China. Following the Helsinki Declaration, all enrolled participants were informed about the study in detail. Written informed consent was obtained from all eligible patients before or during the run‐in period.
Protocols for the Management of Hypertension
A total of 724 hypertensive patients older than 70 years were randomly assigned to either intensive antihypertensive treatment or standard treatment by using a computer‐generated table of random numbers. Protocols for the management of hypertension are presented in Figure 1. Briefly, randomized patients were started with single‐drug treatment of an angiotensin‐converting enzyme (ACE) inhibitor (benzene enalapril 10 mg/d), a β‐blocker (bisoprolol 2.5–5 mg or metoprolol 50–100 mg/d), a calcium channel blocker (CCB) (amlodipine 5–10 mg/d), or a diuretic (indapamide 1.5–2.5 mg/d). To achieve the target BP, 1, 2, or 3 additional antihypertensive drugs could be added stepwise. If quadruple antihypertensive therapy (CCB + β‐blocker +ACE inhibitor + diuretics) failed to achieve the BP goal, increasing the dose of antihypertensive drugs was recommended. BP was measured in the follow‐up period at 4 weeks, 3 months, 6 months, and every 6 months thereafter. All efforts were made to control BP at or near the target values.
Figure 1.
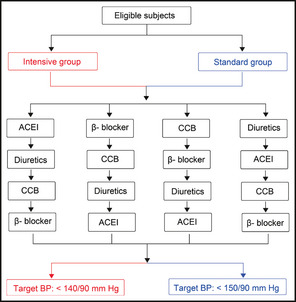
Protocols for the management of hypertension in the study. ACEI indicates angiotensin‐converting enzyme inhibitor; CCB, calcium channel blocker; BP, blood pressure.
Assessment of BP
During a run‐in period (4 weeks in untreated patients and 2–4 weeks in treated patients), patients were examined on at least two separate occasions, and BP was measured on the right upper arm at least twice per visit by the auscultatory method using a sphygmomanometer with the patients in the sitting position after 5 to 10 minutes of rest. If measured values differed by >4 mm Hg, recalibration was required. BP measurements were performed at 8 am to 11 am and averaged for each visit. BP was monitored after enrollment, which was measured in the fourth week, the third month, the sixth month, and every 6 months thereafter. By the end of the study, all patients were followed‐up an average of 10 times. Mean BP, standard deviation of SBP (SD SBP), and standard deviation of DBP (SD DBP) were calculated as the parameters implicating BP variability. Electrocardiography, echocardiography, and routine laboratory examinations, including hematological examinations, serum biochemical analyses, and urinalysis during the run‐in period were performed in all eligible patients.
Endpoint Evaluation
All investigators were required to fill out the endpoint questionnaire objectively. In order to reduce investigation bias, endpoints were evaluated by the members of the Endpoint Evaluation Committee, who were blinded to the treatment assignments and the time course of BP. The primary endpoint was the combined incidence of fatal/nonfatal stroke, acute myocardial infarction, and other cardiovascular deaths (sudden death and heart failure death). The proper diagnosis of stroke required both neurological examinations and craniocervical computed tomography, and/or magnetic resonance imaging. Acute myocardial infarction was diagnosed using the criteria as described elsewhere.9 Sudden death, defined as death from instantaneous, unanticipated circulatory collapse within 1 hour of initial symptoms, was included in the cardiovascular deaths. Secondary endpoints were deaths from any causes.
Statistical Analyses
All of the analyses were performed using SPSS statistical software version 10.0 (SPSS Inc, Cary, NC). Pearson's chi‐square test was applied to analyze count data, and measurement data were described as mean (SD) and analyzed using independent Student t test. P<.05 was considered statistically significant. An intent‐to‐treat analysis was performed to ensure that all study participants were followed until the conclusion of the study, irrespective of whether the participant was still receiving or complying with the treatment. Participants who were lost to follow‐up or died of other causes were censored and were also included in the final analyses for the actual follow‐up period. The relative risk (RR) and 95% confidence index (CI) for risks associated with the incidence of primary endpoint events were generated using Cox proportional hazards regression.
Results
Baseline Characteristics of Study Participants
A total of 745 patients were recruited, of whom 21 were excluded because of concurrent disease (n=9) or meeting the exclusion criteria (n=12). A total of 724 patients were enrolled in the study and randomly divided into two groups: intensive group (n=363) and standard group (n=361). Figure 2 presents the flow chart for the trial profile. Baseline characteristics of the studied patients are shown in Table 1. There were no differences between the two groups in age, sex, body mass index, duration of hypertension, proportion of smokers, baseline BP, serum creatinine, total cholesterol, left ventricular mass index, history of stroke, and the proportion of patients with diabetes mellitus. The mean follow‐up was 4 years.
Figure 2.
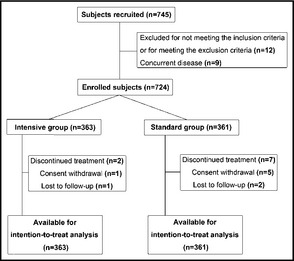
Flow chart for the trial profile.
Table 1.
Baseline Characteristics of the Study Patients
| Intensive Group (n=363) | Standard Group (n=361) | P Value | |
|---|---|---|---|
| Age, y | 76.6±4.6 | 76.5±4.5 | .826 |
| Men, No. (%) | 243 (66.9) | 237 (65.7) | .753 |
| Body mass index, kg/m2 | 23.5±3.3 | 23.2±3.4 | .352 |
| Course of hypertension, y | 13.1±7.5 | 12.9±7.1 | .822 |
| Baseline SBP, mm Hg | 158.8±16.0 | 160.3±16.9 | .201 |
| Baseline DBP, mm Hg | 83.7±9.6 | 84.8±9.5 | .107 |
| Serum creatinine, μmol/L | 86.7±9.6 | 88.3±26.9 | .410 |
| Total cholesterol, mmol/L | 4.59±1.10 | 4.45±1.11 | .101 |
| Triglyceride, mmol/L | 1.62±1.01 | 1.48±0.98 | .068 |
| HDL‐C, mmol/L | 1.41±0.47 | 1.42±0.43 | .927 |
| LDL‐C, mmol/L | 2.89±0.86 | 2.81±0.98 | .277 |
| Uric acid, μmol/L | 367.2±98.8 | 374.7±110.1 | .339 |
| Serum potassium, mmol/L | 4.04±0.50 | 3.97±0.57 | .077 |
| Left ventricular mass index, g/m2 | 128.7±34.8 | 130.3±38.4 | .192 |
| Smoking, No. (%) | 93 (25.6) | 87 (24.1) | .636 |
| Diabetes mellitus, No. (%) | 80 (22.0) | 89 (24.7) | .406 |
| History of stroke, No. (%) | 25 (6.9) | 23 (6.4) | .780 |
Abbreviations: DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure.
Drug Application
One year after enrollment, combined antihypertensive treatment was recommended for 53.7% of the patients in the intensive group and for 39.1% of the patients in the standard group (P<.01). In spite of the different intensity of antihypertensive treatment, the proportion of patients taking a constant drug was similar in the two groups: ACE inhibitor 31.5% and 29.6%, CCB 27.2% and 29.8%, β‐blockers 21.2% and 19.4%,; diuretics 21.2% and 19.4% in the intensive group and the standard group, respectively (all P values >.05).
BP Control in the Two Groups
Average SBP/DBP was lower in the intensive group (135.7±9.0/76.2±6.1 mm Hg) than that in the standard group (149.7±11.0/82.1±7.5 mm Hg) (P<.01), with an intergroup difference of 14/6 mm Hg. The mean SBP and DBP in response to the intensive and standard antihypertensive treatment during follow‐up is presented in Figure 3.
Figure 3.
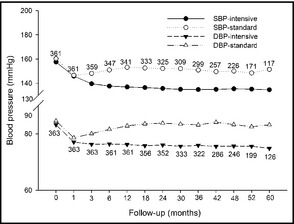
Mean systolic and diastolic blood pressure during follow‐up, in response to the intensive and standard antihypertensive treatment. SBP indicates systolic blood pressure; DBP, diastolic blood pressure.
Comparison of BP Variability in the Two Groups
BP variability was weighed by the standard deviation of SBP/DBP periodically measured during the long‐term follow‐up. Table 2 shows that both SBP and DBP variability in the intensive group was lower than that in the standard group.
Table 2.
Comparison of BP Variability in the Two Groups (Mean±SD)
| No. | SD SBP, mm Hg | SD DBP, mm Hg | |
|---|---|---|---|
| Intensive group | 363 | 8.1±3.7 | 5.1±2.1 |
| Standard group | 361 | 10.0±4.0 | 6.2±2.4 |
| P value | – | <.001 | <.001 |
Abbreviations: BP, blood pressure; SD DBP, standard deviation of diastolic blood pressure; SD, standard deviation; SD SBP, standard deviation of systolic blood pressure.
Incidence of the Primary Endpoint Event
During follow‐up, there were 107 cases of cardiovascular events, with 40 cases (11.0%) in the intensive group, which was obviously less than those in the standard group (67 cases, 18.6%) (P=.004) (Table 3). Figure 4 indicates that Kaplan‐Meier estimates of cumulative rates of cardiovascular events (A) and stroke (B) during follow‐up were lower in the intensive group than in the standard group. Intensive antihypertensive treatment, compared with the standard treatment, decreased total and cardiovascular mortality by 41.7% (P=.001) and 50.3% (P=.002), respectively, and reduced the incidence of the primary composite outcome by 40.6% (P=.004), fatal/nonfatal stroke by 42.0% (P=.036), heart failure death by 62.7% (P=.029), and cardiovascular death by 50.3% (P=.002). However, the two groups showed no difference in the incidence of acute myocardial infarction (P=.991).
Table 3.
Number of Events and Deaths from the Primary Endpoint
| Intensive group (n=363) | Standard group (n=361) | P Value | |||
|---|---|---|---|---|---|
| No. (%) | Per 1000 py | No. (%) | Per 1000 py | ||
| Stroke (total) | 21 (5.8) | 13.3 | 36 (10.0) | 25.1 | .036 |
| Hemorrhagic stroke | 4 (1.1) | 2.5 | 8 (2.2) | 5.6 | .240 |
| Ischemic stroke | 17 (4.7) | 10.8 | 28 (7.8) | 19.5 | .087 |
| All cardiovascular events | 40 (11.0) | 25.3 | 67 (18.6) | 46.8 | .004 |
| Acute myocardial infarction | 9 (2.5) | 5.7 | 9 (2.5) | 6.2 | .991 |
| Heart failure death | 6 (1.7) | 3.8 | 16 (4.4) | 11.2 | .029 |
| Cardiovascular death | 25 (6.9) | 15.8 | 50 (13.9) | 34.9 | .002 |
Abbreviation: py, patient‐years. The primary endpoint was the combined incidence of fatal/nonfatal stroke, acute myocardial infarction, and other cardiovascular deaths (sudden death and heart failure death).
Figure 4.
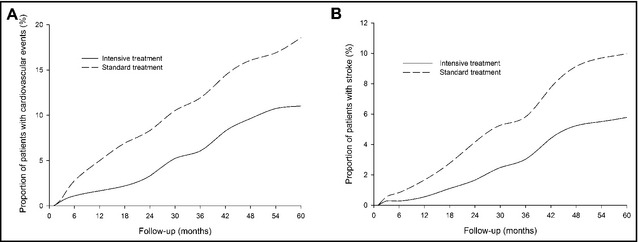
Kaplan‐Meier estimates of cumulative rates of cardiovascular events (A) and stroke (B).
Risks for the Incidence of Primary Endpoint Events
Cox regression analysis indicated that mean SBP (P=.020, 95% confidence interval [CI], 1.006–1.069) and standard deviation of SBP (P=.033, 95% CI, 1.006–1.151) were risk factors for the incidence of primary endpoint events (Table 4).
Table 4.
Cox Regression Analysis of Risks for the Incidence of Primary Endpoint Events
| B | SE | Wald | P | Exp (B) | 95% CI for Exp (B) | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age, y | 0.011 | 0.027 | 0.167 | 0.683 | 1.011 | 0.959 | 1.066 |
| Course of hypertension | −0.006 | 0.013 | 0.253 | 0.615 | 0.994 | 0.970 | 1.018 |
| Serum creatinine | 0.001 | 0.005 | 0.060 | 0.807 | 1.001 | 0.991 | 1.011 |
| Total cholesterol | 0.068 | 0.100 | 0.465 | 0.495 | 1.071 | 0.880 | 1.303 |
| Uric acid | 0.000 | 0.001 | 0.054 | 0.816 | 1.000 | 0.997 | 1.151 |
| Left ventricular mass index | 0.004 | 0.003 | 2.202 | 0.128 | 1.004 | 0.999 | 1.010 |
| Diabetes mellitus | −0.107 | 0.078 | 1.884 | 0.170 | 0.899 | 0.970 | 1.018 |
| Average SBP | 0.036 | 0.016 | 5.381 | 0.020 | 1.037 | 1.006 | 1.069 |
| Average DBP | −0.004 | 0.030 | 0.014 | 0.905 | 0.996 | 0.940 | 1.056 |
| SD SBP | 0.073 | 0.034 | 4.539 | 0.033 | 1.076 | 1.006 | 1.151 |
| SD DBP | 0.055 | 0.053 | 1.057 | 0.304 | 1.056 | 0.970 | 1.018 |
Abbreviations: CI, confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation; SD DBP, standard deviation of diastolic blood pressure; SD SBP, standard deviation of systolic blood pressure; SE, standard error.
Comparison of the Causes of Death
During a mean of 4 years of follow‐up, we identified 138 cases of incident death, including 51 cases (14.0%) in the intensive group and 87 cases (24.1%) in the standard group. Intensive antihypertensive treatment with the target BP <140/90 mm Hg reduced total deaths and cardiovascular death by 41.7%(P=.001) and 50.3% (P=.002), respectively, when compared with the standard treatment with a target BP <150/90 mm Hg (Table 5). There were no significant differences in uremia, tumor, pulmonary infection, and other causes of death between the two groups. Figure 5 indicates that Kaplan‐Meier estimates of cumulative rates of all‐cause (A) and cardiovascular (B) death during follow‐up were lower in the intensive group than in the standard group.
Table 5.
Causes of Death
| Intensive Group (n=363) | Standard Group (n=361) | P Value | |
|---|---|---|---|
| Cardiovascular death | 25 (6.9) | 50 (13.9) | .002 |
| Stroke | 7 (1.9) | 21 (5.8) | .007 |
| Acute myocardial infarction | 8 (2.2) | 7 (1.9) | .803 |
| Sudden death | 4 (1.1) | 6 (1.7) | .743 |
| Heart failure death | 6 (1.7) | 16 (4.4) | .029 |
| Uremia | 1 (0.3) | 4 (1.1) | .366 |
| Tumor | 12 (3.3) | 14 (3.9) | .679 |
| Pulmonary infection | 6 (1.7) | 7 (1.9) | .772 |
| Other causes of death | 7 (1.9) | 12 (3.3) | .240 |
| Total deaths | 51 (14.0) | 87 (24.1) | .001 |
Values are expressed as number (percentage).
Figure 5.
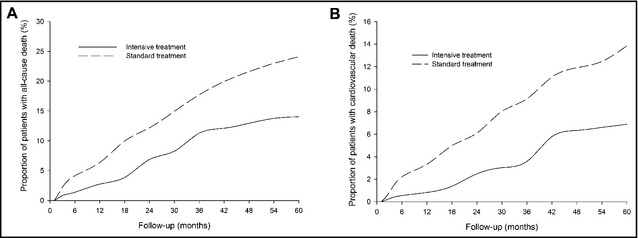
Kaplan‐Meier estimates of cumulative rates of all‐cause (A) and cardiovascular (B) death.
Incidence of Other Events
Five patients (1.4%) in the intensive group and 6 (1.7%) patients in the standard group received percutaneous coronary intervention (P=.754). There were 3 (0.8%)and 5 (1.3%) cases of femoral fracture (P=.716) and 2(0.6%) and 3 (0.8%)cases of vascular dementia (P=.995)in the intensive group and the standard group, respectively.
Discussion
The major findings of this study are that intensive antihypertensive treatment with a target BP <140/90 mm Hg and the final achieved BP of 135.7/76.2 mm Hg substantially reduced fatal/nonfatal stroke and heart failure death in Chinese hypertensive patients older than 70 years, compared with standard treatment with a target BP <150/90 mm Hg and the final achieved BP of 149.7/82.1 mm Hg. The reduction in the rate of deaths from any cause is unexpected. Here, we demonstrate that it is safe and valuable for elderly hypertensive patients in China to receive the intensive treatment to achieve a BP of 135.7/76.2 mm Hg. Furthermore, this study also indicates that long‐term visit‐to‐visit variability in SBP is positively associated with the incidence of cardiovascular events in elderly hypertensive patients, implicating the potential to be applied in risk stratification for elderly hypertension.
Elderly patients with hypertension often coexist with a variety of diseases, such as coronary heart disease, heart failure, cerebrovascular disease, renal insufficiency, and diabetes. BP reduction is one of the most powerful and effective pharmacologic interventions to reduce the incidence of major cardiovascular events and mortality. There is evidence that lowering SBP and DBP by 20 and 10 mm Hg, respectively, may reduce stroke by 40% to 50% and the risk of coronary heart disease by 15% to 30%.10 A meta‐analysis of 8 placebo‐controlled trials in 15,693 elderly patients followed for 4 years indicated that active antihypertensive treatment reduced coronary events by 23%, strokes by 30%, all cardiovascular complications by 26%, cardiovascular deaths by 18%, and total mortality by 13%.11 Distinctly different than middle‐aged hypertension, elderly hypertension has the characteristics of high SBP, increased pulse pressure, large BP fluctuation, high incidence of orthostatic hypotension, postprandial hypotension caused by an increase in the atherosclerotic arterial stiffness, and dysfunction of central neural regulation of BP.12 Therefore, lots of physicians have concerns about the undesirable consequences accompanied by BP reduction, especially the intensive antihypertensive treatment in hypertensive patients older than 70 years. For example, lowering SBP would also lower DBP to a level that may jeopardize coronary blood flow and increase coronary heart events. In the active treatment group of the Systolic Hypertension of the Elderly Program (SHEP) trial, a decrease of 5 mm Hg in DBP increased the risk for stroke by 14%, for coronary heart disease by 8%, and for cardiovascular disease by 11%.13 Meanwhile, this study showed that greater reductions in BP in the treated group (SBP, 143 mm Hg vs 155 mm Hg) and reduced primary endpoints including stroke by 36%, heart failure by 49%, and coronary events by 27%, indicating that BP lowering was effective and beneficial for elderly hypertensive patients.13 Such findings were verified by the following trials preformed in Western populations: the Systolic Hypertension in Europe (Syst‐Eur) study14 and the Hypertension in the Very Elderly Trial (HYVET).3 Similar results were also obtained in the studies performed in Chinese populations, such as the Elderly Systolic Hypertension in China (Syst‐China) trial,15 the Shanghai Trial of Hypertension in the Elderly (STONE),16 and the Felodipine Event Reduction (FEVER) study.17 However, how far SBP should be reduced in elderly hypertensive patients remains controversial.
Whether Chinese elderly hypertensive patients can benefit from intensive antihypertensive treatment with a target BP of <140 mm Hg needs to be established. Our data show that in elderly patients older than 70 years (average age, 76.6 years) with hypertension, lowering BP <140/90 mm Hg (136/76 mm Hg about), compared with <150/90 mm Hg, significantly reduced the incidence of stroke by 41.9% and heart failure deaths by 62.7%, and did not alter the incidence of acute myocardial infarction. Total mortality was significantly reduced by 41.3% without increasing adverse events. The results of our study suggest that it is safe and valuable for elderly patients to achieve a BP of 135.7/76.2 mm Hg if they can tolerate medications. However, whether a lower target BP has further benefits is uncertain. In contrast to our study, the recent Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS) compared moderately intense antihypertensive treatment with less intense antihypertensive treatment and found no difference in incidence of cardiovascular events between patients with achieved SBP <140 mm Hg or >140 mm Hg,18 and the investigators suggested that a reduction of mean SBP to 146 mm Hg might be adequate in most elderly hypertensive patients. Comparisons of our study findings with currently available studies on antihypertensive treatment for elderly hypertension (JATOS, HYVET, and FEVER) are presented in Table 6. The underlying explanations for the different conclusions arrived from JATOS and our study included the following. The mean age of enrolled patients and the proportion of diabetes, previous coronary heart disease, and stroke were higher in our study than in JATOS (Table 6), which are risk factors for cardiovascular events and could get more benefits from antihypertensive treatment. Our study included 18% of patients with atrial fibrillation. However, JATOS claimed to remove patients with atrial fibrillation. The coexistence of hypertension and atrial fibrillation significantly increased the annual risk of stroke to an individual.19 Therefore, the incidence of stroke is higher in our study than that in JATOS. Similarly to our study, HYVET and FEVER did not exclude patients with atrial fibrillation either. The pre‐trial of HYVET showed, compared with placebo, that antihypertensive treatment could prevent 19 cases of stroke per 1000 patients, but increased the total mortality, which predicted that elderly hypertensive patients would not get benefits from antihypertensive therapy.20 However, the later formal study with increased sample size and follow‐up came to the opposite conclusion.3 The follow‐up of JATOS was only 2 years. If the trial was extended to 4 years, would the result be the same? Intergroup (observed‐control) difference in SBP and DBP for JATOS was less than HYVET and our study, but greater than FEVER. Four studies had similarly achieved SBP (135–143 mm Hg) and incidence of stroke (11.2–13.7 per 1000 patient‐years) in the observed group, but only JATOS indicated no difference in the incidence of stroke between the observed and control groups, with the lowest incidence of stroke in the control group. JATOS also had a lower incidence of myocardial infarction (1.3 per 1000 patient‐years) and heart failure (1.8 per 1000 patient‐years) than HYVET, FEVER, and our study. The recent evidence is scanty for the BP target recommendation on elderly hypertension. Therefore, further large‐scale prospective multicenter randomized controlled trials are expected to verify the most beneficial target BP for elderly hypertensive patients in China.
Table 6.
Comparisons of Our Study With JATOS, HYVET, and FEVER
| Our Study | JATOS | HYVET | FEVER | |
|---|---|---|---|---|
| Population | Chinese | Japanese | European | Chinese |
| Observed‐Control | Intensive‐ Standard | Intensive‐ Standard | Treatment‐No treatment | Treatment‐No treatment |
| Age, y | 76.6 | 73.6 | 83.6 | 61.5 |
| Baseline SBP, mm Hg | 159 | 171.6 | 173 | 158.7 |
| Baseline DBP, mm Hg | 84 | 89.1 | 90.8 | 92.4 |
| Stroke,% | 6.9 | 4.2 | 6.7 | 14.2 |
| Diabetes mellitus,% | 23 | 11.8 | 6.8 | 11.3 |
| Coronary heart disease,% | 7.5 | 3 | 3.1 | 13.3 |
| Atrial fibrillation,% | 18 | Excluded | Unclear | Unclear |
| Smoking,% | 25 | 14 | 6.4 | 29 |
| Achieved SBP in observed group, mm Hg | 135.7 | 135.9 | 143.5 | 137.3 |
| Achieved SBP in control group, mm Hg | 149.7 | 145.6 | 158.5 | 142.5 |
| Achieved DBP in observed group, mm Hg | 76.2 | 74.8 | 77.9 | 82.5 |
| Achieved DBP in control group, mm Hg | 82.1 | 78.1 | 84 | 85 |
| Intergroup difference in SBP | 14 | 9.7 | 15 | 5.2 |
| Intergroup difference in DBP | 6 | 3.3 | 6.1 | 2.5 |
| Incidence of stroke in observed group, per 1000 patient‐years | 13.3 | 13.7 | 12.5 | 11.2 |
| Incidence of stroke in control group, per 1000 patient‐years | 25.1 | 12.9 | 17.7 | 15.9 |
Abbreviations: DBP, diastolic blood pressure; FEVER, Felodipine Event Reduction trial; HYVET, the Hypertension in the Very Elderly Trial; JATOS, Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients; SBP, systolic blood pressure.
Another interesting finding in our study was the BP variability during long‐term follow‐up. Currently, the methods applied to assess BP variability are limited. Some studies indicate that BP variability assessed by 24‐hour ambulatory BP monitoring is an independent risk factor for cardiovascular disease.21, 22 But other studies indicate that visit‐to‐visit of BP variability is the strongest cardiovascular outcome predictor.7, 23, 24 The results of this study is consistent with the comprehensive analysis by Rothwell, which showed that the average SBP (P=.020, 95% CI, 1.006–1.069) and followed‐up SBP variability (P=.033, 95% CI, 1.006–1.151) were risk factors for endpoint events.25 Although the recently reported data on BP variability have been shown to give prognostic information, how to identify the optimal strategy for taking BP variability into account in routine practice requires more research. Current evidence indicates that different antihypertensive agents had different effects on BP variability, such as the most effective agent in reducing BP variability was a CCB, having maximum extent to prevent stroke, and such effect did not depend on the average SBP.26, 27 In our study, the proportion of the application of different classes of antihypertensive drugs was similar between the two groups. That BP variability differed between the intensive treatment and the standard treatment might be attributed to a variable daily compliance with antihypertensive treatment.
Conclusions
This study demonstrates that intensive BP control with a target of 136/76 mm Hg can not only reduce BP fluctuation and variability but also reduce the incidence of cardiovascular and cerebrovascular events in Chinese hypertensive patients older than 70 years.
J Clin Hypertens (Greenwich). 2013;15:420–427. ©2013 Wiley Periodicals, Inc.23730991
[Correction made after online publication 04‐Apr‐2013: The address for correspondence has been updated.]
References
- 1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 2. Black HR. New concepts in hypertension: focus on the elderly. Am Heart J. 1998;135:S2–S7. [DOI] [PubMed] [Google Scholar]
- 3. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 4. Liu LS; Writing Group of 2010 Chinese Guidelines for the Management of Hypertension . 2010 Chinese Guidelines for the Management of Hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39:579–615. [PubMed] [Google Scholar]
- 5. Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 6. Burr ML, Dolan E, O'Brien EW, et al. The value of ambulatory blood pressure in older adults: the Dublin outcome study. Age Ageing. 2008;37:201–206. [DOI] [PubMed] [Google Scholar]
- 7. Hansen TW, Thijs L, Li Y, et al. Prognostic value of reading‐to‐reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–1057. [DOI] [PubMed] [Google Scholar]
- 8. Dolan E, Stanton AV, Thom S, et al. Ambulatory blood pressure monitoring predicts cardiovascular events in treated hypertensive patients–an Anglo‐Scandinavian cardiac outcomes trial substudy. J Hypertens. 2009;27:876–885. [DOI] [PubMed] [Google Scholar]
- 9. Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST‐segment elevation: the Task Force on the Management of ST‐Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–2945. [DOI] [PubMed] [Google Scholar]
- 10. Daskalopoulou SS, Khan NA, Quinn RR, et al. The 2012 Canadian hypertension education program recommendations for the management of hypertension: blood pressure measurement, diagnosis, assessment of risk, and therapy. Can J Cardiol. 2012;28:270–287. [DOI] [PubMed] [Google Scholar]
- 11. Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta‐analysis of outcome trials. Lancet. 2000;355:865–872. [DOI] [PubMed] [Google Scholar]
- 12. Ogihara T, Hiwada K, Morimoto S, et al. Guidelines for treatment of hypertension in the elderly–2002 revised version. Hypertens Res. 2003;26:1–36. [DOI] [PubMed] [Google Scholar]
- 13. Somes GW, Pahor M, Shorr RI, et al. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med. 1999;159:2004–2009. [DOI] [PubMed] [Google Scholar]
- 14. Fagard RH, Staessen JA. Treatment of isolated systolic hypertension in the elderly: the Syst‐Eur trial. Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Clin Exp Hypertens. 1999;21:491–497. [DOI] [PubMed] [Google Scholar]
- 15. Liu L, Wang JG, Gong L, et al. Comparison of active treatment and placebo for older patients with isolated systolic hypertension. J Hypertens. 1998;16:1823–1829. [DOI] [PubMed] [Google Scholar]
- 16. Gong L, Zhang W, Zhu Y, et al. Shanghai trial of nifedipine in the elderly (STONE). J Hypertens. 1996;14:1237–1245. [DOI] [PubMed] [Google Scholar]
- 17. Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) Study: a randomized long‐term placebo‐controlled trial in Chinese hypertensive patients. J Hypertens. 2005;23:2157–2172. [DOI] [PubMed] [Google Scholar]
- 18. JATOS Study Group . Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115–2127. [DOI] [PubMed] [Google Scholar]
- 19. Manolis AJ, Kallistratos MS, Poulimenos LE. Recent clinical trials in atrial fibrillation in hypertensive patients. Curr Hypertens Rep. 2012;14:350–359. [DOI] [PubMed] [Google Scholar]
- 20. Bulpitt CJ, Beckett NS, Cooke J, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens. 2003;21:2409–2417. [DOI] [PubMed] [Google Scholar]
- 21. Mancia G, Facchetti R, Bombelli M, et al. Long‐term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension. 2006;47:846–853. [DOI] [PubMed] [Google Scholar]
- 22. Björklund K, Lind L, Zethelius B, et al. Prognostic significance of 24‐h ambulatory blood pressure characteristics for cardiovascular morbidity in a population of elderly men. J Hypertens. 2004;22:1691–1697. [DOI] [PubMed] [Google Scholar]
- 23. Muntner P, Shimbo D, Tonelli M, et al. The relationship between visit‐to‐visit variability in systolic blood pressure and all‐cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57:160–166. [DOI] [PubMed] [Google Scholar]
- 24. Rossignol P, Cridlig J, Lehert P, et al. Visit‐to‐visit blood pressure variability is a strong predictor of cardiovascular events in hemodialysis: insights from FOSIDIAL. Hypertension. 2012;60:339–346. [DOI] [PubMed] [Google Scholar]
- 25. Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. [DOI] [PubMed] [Google Scholar]
- 26. Rothwell PM, Howard SC, Dolan E, et al. Effects of beta blockers and calcium‐channel blockers on within‐individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480. [DOI] [PubMed] [Google Scholar]
- 27. Webb AJ, Fischer U, Mehta Z, et al. Effects of antihypertensive‐drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta‐analysis. Lancet. 2010;375:906–915. [DOI] [PubMed] [Google Scholar]


