Abstract
This paper provides recommendations on the treatment of orthostatic hypotension (OH) as reviewed by the American Society of Hypertension. It focuses on recent reports on the evaluation and management of OH and provides practical advice for clinicians on how to screen, diagnose, and treat patients using behavioral, nonpharmacologic, and pharmacologic strategies. The authors also provide a stepwise approach on how to apply new findings to successfully control OH and reduce the risk of syncope and falls in these patients. Treatment of OH is also discussed in special situations such as in hypertensive and hospitalized patients. It should be noted, however, that research in this area is mostly limited to studies in small numbers of patients. Unfortunately, the trials of the type needed to develop evidence‐based guidelines are not available for this condition.
Orthostatic hypotension (OH) is defined as a sustained reduction of systolic blood pressure (SBP) of at least 20 mm Hg or diastolic blood pressure (DBP) of 10 mm Hg within 3 minutes of standing or head‐up tilt to at least 60° on a tilt table.1 The diagnosis can be made easily at the bedside by measuring blood pressure (BP) and heart rate supine and after 1 and 3 minutes of standing.
The most sensitive and consistent measurements are the ones obtained early in the morning, when patients are usually more symptomatic. In patients with hypertension, a reduction of SBP of 30 mm Hg is more appropriate to define OH1 because the magnitude of the fall in BP depends on the baseline BP. However, prospective studies demonstrate that a decline in SBP of ≥20 mm Hg is a risk factor for falls, especially in elderly patients with hypertension.2, 3
Concomitant measurements of heart rate are important because the absence of adequate compensatory heart rate increase is typically of neurogenic OH, a pathologic form of OH caused by central or peripheral nervous system diseases that results in autonomic failure. On the other hand, exaggerated tachycardia (>15 beats per minute) will suggest dehydration, volume depletionn, or other transient conditions rather than neurogenic OH. In the elderly, however, cardioacceleration is less useful as a diagnostic tool because of an age‐related reduction in baroreflex sensitivity.
Typical symptoms of OH are lightheadedness or dizziness beginning within a few seconds after standing; dim, blurred, or tunnel vision; and a dull pain in the back of the neck and shoulder (coat hanger distribution). Patients may be vague about symptoms and complain only of fatigue or other nonspecific descriptors. Classically, symptoms should never occur while supine, are more prominent while standing, and should be relieved by seating or lying down. OH, detected during a patient's evaluation, may be asymptomatic, especially in patients with intact cerebral autoregulation, but the patient should still be considered at risk for falls and syncope.
OH is relatively common in elderly people. The prevalence of OH in community dwellers older than 65 years is 16.2%4 and the incidence of OH increases exponentially with age, affecting most commonly men5, 6 and institutionalized patients, such as those living in nursing homes where the prevalence of OH can be up to 50% or more. The number of prescribed medications, particularly antihypertensives, and the presence of multiple comorbidities are predictors of OH.7, 8
Multiple epidemiological studies have reported that OH is associated with incident coronary artery disease, stroke, and heart failure.9, 10 In the elderly, OH has been identified as an independent predictor of mortality11 and falls.2, 3 Elderly people with OH are more likely to be physically frail with decreased functional capacity. OH is often overlooked as a cause of frailty in geriatric patients in whom orthostatic vital signs are rarely obtained.
OH is a risk factor for syncope and falls.2, 12 OH has been documented in 24% to 31% of patients presenting to the emergency department for syncope.13, 14 This condition, therefore, represents a significant economic burden on the US healthcare system. A recent report using the National Inpatient Sample Database showed that the overall annual rate for OH‐related hospitalization was 36 per 100,000 US adults. This number increases steadily with age and it could be as high as 233 per 100,000 in patients 75 years or older. Considering that the US demographic is rapidly changing, with the elderly population representing nearly 20% of the total US population in the next 20 years, the impact of OH‐related hospitalizations will be an increasing challenge to health policy planners and the medical community.
Pathophysiology of OH
In healthy individuals, changing position from supine to upright posture results in about 700 mL of venous pooling in the lower extremities and splanchnic circulation, decreased venous return to the heart, reduced ventricular filling, and a transient decrease in cardiac output and BP. This results in a baroreflex‐mediated compensatory sympathetic activation and decreased parasympathetic activation that increases venous return, heart rate, and vascular resistance with the goal of restoring cardiac output and BP. Impairments in one or more of these compensatory mechanisms eventually results in OH (Figure 1).
Figure 1.
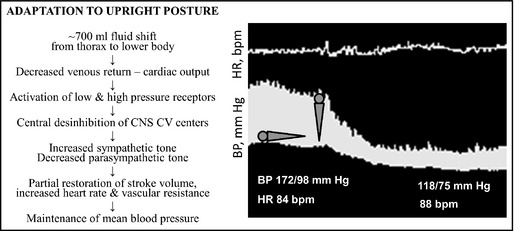
Left panel shows the normal response to upright posture. Patients with orthostatic hypotension (OH) are unable to compensate for the posture‐induced changes in venous return to the heart. The right panel shows a continuous blood pressure (BP) tracing of a patient with neurogenic OH (lower tracing) in a transition from supine to upright posture. Note the profound decrease in BP without an adequate compensatory increase in heart rate (upper tracing). Note also the presence of supine hypertension, a condition often seen in patients with OH. CNS indicates central nervous system, CV, cardiovascular; bpm, beats per minute; HR, heart rate.
Patients with autonomic failure who lack these compensatory responses experience severe OH. Autonomic failure can be caused by primary neurodegenerative autonomic disorders such as pure autonomic failure and multiple system atrophy. These conditions are rare. Most commonly, diseases associated with a peripheral damage of autonomic nerves are diabetes mellitus and Parkinson's disease. If a patient presents with a subacute onset of OH with rapid progression, a primary autoimmune process causing autoimmune autonomic failure or a paraneoplastic syndrome (small cell lung carcinoma, monoclonal gammopathies, light chain disease, or amyloid) need to be ruled out. All these conditions are part of the spectrum of autonomic neuropathies associated with OH also known as neurogenic OH.
More often OH is seen in the frail elderly with numerous pathologies and polypharmacy, without an obvious single cause. The elderly are particularly prone to develop OH because aging is associated with impairment of various compensatory mechanisms to orthostasis. Elderly individuals have decreased baroreflex sensitivity, with diminished heart rate responses15 and impaired α1‐adrenergic vasoconstriction.16 There are age‐related reductions in parasympathetic tone that result in less cardioacceleration during vagal withdrawal on standing.17 The elderly are particularly prone to dehydration, since they often have an impaired thirst response, and the aged kidney loses some of its ability to conserve salt and water during periods of fluid restriction or volume loss because of a reduction of renin, angiotensin, and aldosterone and an elevation in natriuretic peptides. The aged heart is also stiff and noncompliant, resulting in impaired diastolic filling, which reduces stroke volume, particularly during the decrease in venous return that results from orthostasis. All these age‐related physiological changes increase the risk of OH in the elderly. A period of inactivity during hospitalization or acute illness can precipitate OH. Severe symptomatic OH may develop in the face of an additional stress such as uncontrolled hypertension, which further impairs the above compensatory mechanisms, as well as certain medications such as diuretics and other situations that reduce intravascular volume acutely, eg, vomiting and diarrhea.18
Practical Considerations and Recommendations
Our approach to the evaluation and management of a patient with OH is shown in Figure 2. The initial assessment should include BP and heart rate measurement when the patient has been supine for at least 5 minutes and ideally at both 1 and 3 minutes of standing. This approach captures immediate declines in BP, which are a risk for falls during this vulnerable period, as well as cases of delayed onset of BP decline. In a busy practice this is often not possible, and single measurements in the seated (rather than supine) and standing postures are an alternative. This may reduce sensitivity but is preferable to no measurement at all.
Figure 2.
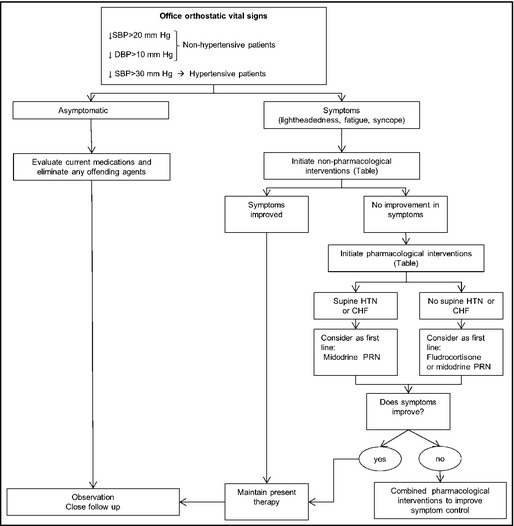
Approach to the evaluation and treatment of orthostatic hypotension. SBP indicates systolic blood pressure; DBP, diastolic blood pressure; HTN, hypertension; CHF, congestive heart failure, PRN, as needed.
Detection of OH may require multiple measurements on different days, which can be accomplished by asking the patient to maintain a BP diary with recordings of supine and standing BP at different times of the day or after particular situational stresses (eg, medications, meals, exercise, or an overnight fast). Patients typically are worse on awakening because of nighttime pressure natriuresis, so that morning orthostatic measurements are more sensitive to detect OH. Ambulatory automated BP monitoring may be useful only if the patient's posture is recorded.
Once the diagnosis of OH has been established, a careful history and physical examination should be performed. A detailed history of medication use is needed because certain medications such as α‐blockers, diuretics, vasodilators, dopamine agonists, venodilators, or tricyclic antidepressants can induce or worsen OH. Assessment of comorbidities such as volume losses (dehydration, excessive vomiting or diarrhea, and bleeding) is important to determine possible acute contributors to OH. Seemingly benign infections such as urinary tract infection can dramatically worsen OH, as will de‐conditioning (even short‐duration bed rest in a patient with impaired autonomic reflexes). Furthermore, symptoms of urinary retention, severe constipation, decreased sweating, and erectile dysfunction in men suggest neurogenic causes of OH, but these symptoms are nonspecific and common in the elderly. On the other hand, absence of erectile dysfunction in a patient with significant OH would make the diagnosis of neurogenic OH doubtful.
Some patients may complain of increased localized sweating (eg, in the face) that may be compensatory for anhidrosis elsewhere. A targeted evaluation should be performed to look for signs of amyloidosis, malignancy, or other diseases causing peripheral neuropathy. A neurological evaluation is important to assess for early signs of motor disorders that could raise suspicions of Parkinson's disease or multiple system atrophy. Lewy body dementia should be considered in the presence of dementia and parkinsonian signs (motor rigidity, increased motor tone, and a slow shuffling gait).
Autonomic function tests are helpful for the diagnosis of neurogenic OH by assessing the autonomic reflex arcs. In response to the Valsalva maneuver, autonomic impairment is characterized by an exaggerated and sustained decrease in BP without a compensatory increase in heart rate during phase 2 (strain) and lack of BP overshoot during phase 4 (release). This response is diagnostic of neurogenic OH. The continuous BP monitoring required to perform this test is now possible with noninvasive devices, but these are not widely available outside specialized centers. Nonetheless, simple orthostatic BP measurements suffice in most cases. Very few conditions other than neurogenic OH can explain a profound decrease in BP without an adequate compensatory heart rate increase.
Treatment
Treatment of OH can be challenging. We should not aim to achieve arbitrary BP goals; instead, the treatment should be directed toward improvement of symptoms and patient's functional status and in reducing the risk of falls and syncope. Treatment strategies can be divided into physiological countermeasures and pharmacologic interventions (Table 1). It should be noted that these recommendations are based mostly on studies performed in a small number of well‐defined patients with primary forms of autonomic failure, which are clinically different from the more common situation of elderly patients with multiple comorbidities who develop OH. Even in these select patients, there is limited evidence based on long‐term randomized controlled clinical trials.19
Table 1.
Effective Interventions to Treat Orthostatic Hypotension
| Nonpharmacologic interventions |
| Eliminate any offending agents (α‐blockers, diuretics) |
| Increase fluid and salt intake |
| Avoid getting up quickly or standing motionless |
| Use of abdominal binder or compressive waist‐high stockings |
| Raise head of the bed by 6 to 9 inches during nighttime |
| Avoid prolonged standing and exposure to hot environment (hot showers) |
| Leg crossing while standing (cocktail party posture) |
| 16 oz of tap water (drink as a bolus) |
| Exercise program (swimming, recumbent bicycle, rowing) |
| Pharmacologic interventions |
| Single agents |
| Increase intravascular volume |
| Fludrocortisone 0.1–0.3 mg/d |
| Adrenergic agonists and sympathomimetics (prescribe as a PRN indication rather than at fixed intervals) |
| Midodrine 2.5–10 mg |
| Pyridostigmine 60 mg |
| Pseudoephedrine 30 mg |
| Atomoxetine 18 mg |
| Splanchnic vasoconstrictor |
| Octreotide 12.5–25 µg subcutaneous |
| Investigational therapy |
| Droxidopa (L‐DOPS) |
| Combination therapy |
| Combined use of fludrocortisone (0.1–0.3 mg each morning) and midodrine (5–10 mg) |
| Combined use of midodrine (5–10 mg) or pseudoephedrine (30 mg) and water bolus (16 ounces) |
Physiological Countermeasures
Physiological countermeasures are the first line of treatment for OH. They should be instituted in every patient, and may be the only treatment needed in many patients. Patients with more severe forms of OH may find these measures insufficient to improve their symptoms and may feel discouraged to use them. Nonetheless, even if medications are needed, they should be added to, rather than replace, physiological countermeasures.
The first step involves the removal of any potential medication that could precipitate or contribute to OH. Among common offenders are α‐blockers commonly used to treat symptoms of prostate hyperplasia, diuretics, and tricyclic antidepressants. In patients with hypertension and OH, certain medications should be avoided, but antihypertensive treatment should not be abandoned (refer to Treatment in Special Situations).
Patients should be educated about the use of simple physical countermeasures aimed at reducing venous pooling in the lower extremities and thereby improving cardiac output on standing. Such maneuvers include moving from the supine to standing position in gradual stages, avoiding standing motionless, crossing one leg in front of the other while standing (the cocktail posture), squatting, and tensing the leg muscles. These maneuvers have been shown to improve orthostatic tolerance and can be applied to relieve symptoms instantaneously while standing.20 Custom‐fitted compression stockings that apply graded pressure to the lower body can be used to decrease venous pooling on standing. It is best to use thigh‐ or waist‐high stockings that produce at least 15‐ to 20‐mm Hg pressure. Unfortunately, they are difficult to put on, limiting compliance. An abdominal binder, worn as tight as possible, may be easier to use and equally effective because most of the pooling occurs in the splanchnic circulation.21
Patients should be encouraged to increase salt consumption up to 6 to 10 g of sodium chloride daily and 1.5 to 2 L of water a day. If needed, patients can use sodium chloride tablets (1 g with each meal). Physical activity is recommended to avoid de‐conditioning and improve functional status. Exercise should be encouraged, as tolerated. Water exercises are particularly helpful because of the improvement in venous return produced by the pressure of the water. Otherwise, reclining exercises (recumbent biking or rowing) are preferable to upright ones (treadmill).
Another strategy to achieve a rapid increase in BP consists of the rapid ingestion of tap water (16 ounces in 3 to 4 minutes). This can be used as a rescue measure when patients are symptomatic due to excessive hypotension on standing. The BP effect is observed in the first 5 to 10 minutes and peaks around 30 minutes after ingestion. This is thought to be a sympathetic reflex induced by the hypotonicity of the water rather than a volume effect.22
Pharmacologic Interventions
If pharmacologic agents are needed for the treatment of OH, the presence of hypertension should be considered when selecting the appropriate agent. In the nonhypertensive patient, either fludrocortisone or midodrine can be tried. Fludrocortisone is a synthetic mineralocorticoid analog that expands intravascular volume by increasing renal sodium reabsorption. Treatment is usually initiated with 0.1 mg/d, together with a high‐sodium diet, and then increased up to 0.3 mg/d. Higher doses (>0.3 mg/d) may cause corticosteroid‐like effects and should be avoided. Heart failure is a relative contraindication to the use of fludrocortisone.
The effect of fludrocortisone on plasma volume is only transient. Its long‐term benefit may be related to potentiation of the pressor effect of norepinephrine and angiotensin II.23, 24 Common side effects include hypokalemia, supine hypertension, heart failure, and headaches. Patients should be monitored for volume overload and hypokalemia. Potassium supplements may be required to maintain a normal serum potassium level.
In patients with OH and supine hypertension or heart failure, short‐acting pressor agents such as the α1‐adrenergic agonist midodrine, are preferable. Treatment should begin with a single 2.5‐mg dose, since occasional patients are hypersensitive even to low doses of pressor agents. Then it can be increased up to 10 mg. These agents will increase BP for 2 to 3 hours at a time and are best given on an as‐needed basis, to be taken 30 to 45 minutes before upright activities. The goal of treatment is to improve orthostatic symptoms, and pressor agents should not be used if patients are going to remain seated or supine. Evening doses should be avoided because of increased risk of causing supine hypertension.
An alternative pharmacologic strategy to direct vasoconstrictors is to harness any residual sympathetic activity the patients may have and raise BP by increasing plasma norepinephrine. Among these strategies, pharmacologic inhibition of the norepinephrine transporter (NET) with atomoxetine has shown promising results. NET inhibition will increase synaptic norepinephrine that is tonically released, which should result in an increase in BP. Indeed, a proof‐of‐concept study found that atomoxetine was an effective pressor agent in patients with autonomic failure and acutely improved orthostatic tolerance even in pediatric doses (18 mg).25 Alternatively, one can use ephedra alkaloids such as pseudoephedrine, a sympathomimetic amine. A comparative assessment of the effect of various pressor agents, including pseudoephedrine, on BP in autonomic failure patients was previously published.26
Another potential therapeutic agent is pyridostigmine, a cholinesterase inhibitor that facilitates cholinergic neurotransmission at the level of autonomic ganglia and, therefore, may increase BP preferentially on standing, when residual sympathetic tone is increased. Indeed, 60 mg of pyridostigmine seems to increase orthostatic BP and improve symptoms without a significant increase in supine BP.27 Pyridostigmine appears to be less effective in patients with severe forms of neurogenic OH.28 Also, its use may be limited by side effects, including nausea, vomiting, loose stools, urinary urgency, frequency, and abdominal cramping.
Octreotide is also very effective, even when other agents fail, in part because of its ability to constrict the splanchnic circulation, where most of the orthostatic blood pooling occurs.21 Its use is limited by the need for parenteral administration and by gastrointestinal side effects such as abdominal pain and nausea. Patients with type 2 diabetes mellitus are at higher risk for these side effects and rarely tolerate this drug.
Droxidopa (l‐dihydroxyphenylserine) is an investigational therapy for the treatment of OH. Droxidopa has a structure similar to norepinephrine but has a carboxyl group. It can be administered orally and is converted to norepinephrine through the enzyme dopa‐decarboxylase that is ubiquitous in tissues. The optimal dose varies between 200 mg and 2000 mg and patients require careful titration. This medication has not been approved by the Food and Drug Administration, and further studies need to be done before it is brought to market.
In patients with OH refractory to treatment, combination therapy should be considered (Table 1). Fludrocortisone and midodrine in low doses can be used in combination if these agents fail to improve symptoms as standalone therapy. Midodrine and other sympathomimetic agents such as ephedra alkaloids, ie, 30 mg of ephedrine, can be combined with water bolus (480 mL) to produce an additive effect on BP, which, if used cautiously, can also be an alternative therapy for patients with refractory OH.29
Treatment in Special Situations
OH in the Hypertensive Patient
Patients with a history of hypertension may also experience OH. Antihypertensive medications should not be stopped in patients with OH. Even though there is a conventional belief that lowering and controlling BP with antihypertensive medications may exacerbate OH and increase falling, existing data do not support this theory. A recent study showed that the risk of falls is nearly 2.5 times higher in elderly with uncontrolled hypertension and OH.3 In addition, withholding antihypertensive treatment often worsens OH (through pressure diuresis). Instead, antihypertensive agents should be used judiciously. Angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers may improve BP regulation and cerebral blood flow in elderly patients and prevent OH.3, 30 These agents should be started in low doses and slowly increased at intervals of 1 to 2 weeks, while carefully monitoring orthostatic BP responses.
Patients with neurogenic OH may have severe hypertension in the supine position. This can be treated during the day simply by avoiding the supine posture, and by raising the head of the bed by 6 to 9 inches during the night, or even advising the patient to sleep in a reclining chair. Short‐acting antihypertensive drugs given at bedtime may be needed in these cases.31, 32
OH in the Hospitalized Patient
OH is a relative common condition among hospitalized elderly patients.6 Hospitalized patients with OH are often confined to the bed by medical personnel because of their acute illness or risk of falls. In this situation, pressor agents should not be prescribed because they can worsen supine hypertension and provoke pressure diuresis. Patients should be encouraged to use a recliner or remain in the seated position during the day, and pressor agents should be used only as needed in preparation for upright activities (eg, 30 minutes prior to inpatient rehabilitation sessions).
Who, When, and Where to Refer a Patient With OH for Autonomic Evaluation
Referral to a specialized autonomic dysfunction center may be indicated in patients with disabling OH without an appropriate compensatory heart rate increase that are unresponsive to the treatment options outlined above. Situations that should alert clinicians for an early referral are acute or subacute presentations of OH with severe presyncopal symptoms and/or significant gastrointestinal impairment (gastroparesis, ileus), which could raise suspicious for autoimmune or paraneoplastic syndromes.
A list of clinicians and centers specializing in the evaluation and management of patients with autonomic disorders can be found at the American Autonomic Society Web site (http://www.americanautonomicsociety.org/).
Final Recommendations
The diagnosis of OH can be made easily at the office by measuring heart rate and BP supine (or seated) and after 1 and 3 minutes of standing. Patients often have greater OH early in the morning.
Treatment of OH should be focused on alleviating symptoms and reducing risk of falls and syncope rather than achieving an arbitrary BP goal.
Management of OH should start with behavioral and physiological countermeasures such as removal of offending agents (α‐blockers, antidepressants, diuretics), the use of waist‐high compression stockings or abdominal binders, water drinking, high‐salt diet, and elevation of the head of the bed.
Fludrocortisone should be considered as the first line of pharmacotherapy in the nonhypertensive patient. In the hypertensive patient or in patients with history of heart failure midodrine is the drug of choice because of its short half‐life and because it does not produce excessive fluid retention.
In the refractory patient, combination therapy either with two pharmacologic agents or with water drinking should be considered.
J Clin Hypertens (Greenwich). 2013;15:147–153. ©2013 Wiley Periodicals, Inc.23458585
References
- 1. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. [DOI] [PubMed] [Google Scholar]
- 2. Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med. 2000;108:106–111. [DOI] [PubMed] [Google Scholar]
- 3. Gangavati A, Hajjar I, Quach L, et al. Hypertension, orthostatic hypotension, and the risk of falls in a community‐dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992;19(6 Pt 1):508–519. [DOI] [PubMed] [Google Scholar]
- 5. Masaki KH, Schatz IJ, Burchfiel CM, et al. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98:2290–2295. [DOI] [PubMed] [Google Scholar]
- 6. Shibao C, Grijalva CG, Raj SR, et al. Orthostatic hypotension‐related hospitalizations in the United States. Am J Med. 2007;120:975–980. [DOI] [PubMed] [Google Scholar]
- 7. Kamaruzzaman S, Watt H, Carson C, Ebrahim S. The association between orthostatic hypotension and medication use in the British Women's Heart and Health Study. Age Ageing. 2010;39:51–56. [DOI] [PubMed] [Google Scholar]
- 8. Poon IO, Braun U. High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther. 2005;30:173–178. [DOI] [PubMed] [Google Scholar]
- 9. Luukinen H, Koski K, Laippala P, Airaksinen KE. Orthostatic hypotension and the risk of myocardial infarction in the home‐dwelling elderly. J Intern Med. 2004;255:486–493. [DOI] [PubMed] [Google Scholar]
- 10. Rose KM, Tyroler HA, Nardo CJ, et al. Orthostatic hypotension and the incidence of coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Hypertens. 2000;13(6 Pt 1):571–578. [DOI] [PubMed] [Google Scholar]
- 11. Luukinen H, Koski K, Laippala P, Kivela SL. Prognosis of diastolic and systolic orthostatic hypotension in older persons. Arch Intern Med. 1999;159:273–280. [DOI] [PubMed] [Google Scholar]
- 12. Jonsson PV, Lipsitz LA, Kelley M, Koestner J. Hypotensive responses to common daily activities in institutionalized elderly. A potential risk for recurrent falls. Arch Intern Med. 1990;150:1518–1524. [PubMed] [Google Scholar]
- 13. Sarasin FP, Louis‐Simonet M, Carballo D, et al. Prevalence of orthostatic hypotension among patients presenting with syncope in the ED. Am J Emerg Med. 2002;20:497–501. [DOI] [PubMed] [Google Scholar]
- 14. Atkins D, Hanusa B, Sefcik T, Kapoor W. Syncope and orthostatic hypotension. Am J Med. 1991;91:179–185. [DOI] [PubMed] [Google Scholar]
- 15. Gribbin B, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circ Res. 1971;29:424–431. [DOI] [PubMed] [Google Scholar]
- 16. Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension. 1998;32:298–304. [DOI] [PubMed] [Google Scholar]
- 17. Maddens M, Lipsitz LA, Wei JY, et al. Impaired heart rate responses to cough and deep breathing in elderly patients with unexplained syncope. Am J Cardiol. 1987;60:1368–1372. [DOI] [PubMed] [Google Scholar]
- 18. Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med. 2007;120:841–847. [DOI] [PubMed] [Google Scholar]
- 19. Logan IC, Witham MD. Efficacy of treatments for orthostatic hypotension: a systematic review. Age Ageing. 2012;41:587–594. [DOI] [PubMed] [Google Scholar]
- 20. Krediet CT, van Lieshout JJ, Bogert LW, et al. Leg crossing improves orthostatic tolerance in healthy subjects: a placebo‐controlled crossover study. Am J Physiol Heart Circ Physiol. 2006;291:H1768–H1772. [DOI] [PubMed] [Google Scholar]
- 21. Diedrich A, Biaggioni I. Segmental orthostatic fluid shifts. Clin Auton Res. 2004;14:146–147. [DOI] [PubMed] [Google Scholar]
- 22. Jordan J, Shannon JR, Grogan E, et al. A potent pressor response elicited by drinking water. Lancet. 1999;353:723. [DOI] [PubMed] [Google Scholar]
- 23. Hickler RB, Thompson GR, Fox LM, Hamlin JT III. Successful treatment of orthostatic hypotension with 9‐alpha‐fluorohydrocortisone. N Engl J Med. 1959;261:788–791. [DOI] [PubMed] [Google Scholar]
- 24. van Lieshout JJ, Ten Harkel AD, Wieling W. Fludrocortisone and sleeping in the head‐up position limit the postural decrease in cardiac output in autonomic failure. Clin Auton Res. 2000;10:35–42. [DOI] [PubMed] [Google Scholar]
- 25. Shibao C, Raj SR, Gamboa A, et al. Norepinephrine transporter blockade with atomoxetine induces hypertension in patients with impaired autonomic function. Hypertension. 2007;50:47–53. [DOI] [PubMed] [Google Scholar]
- 26. Jordan J, Shannon JR, Biaggioni I, et al. Contrasting actions of pressor agents in severe autonomic failure. Am J Med. 1998;105:116–124. [DOI] [PubMed] [Google Scholar]
- 27. Singer W, Opfer‐Gehrking TL, McPhee BR, et al. Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. J Neurol Neurosurg Psychiatry. 2003;74:1294–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shibao C, Okamoto LE, Gamboa A, et al. Comparative efficacy of yohimbine against pyridostigmine for the treatment of orthostatic hypotension in autonomic failure. Hypertension. 2010;56:847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jordan J, Shannon JR, Diedrich A, et al. Water potentiates the pressor effect of ephedra alkaloids. Circulation. 2004;109:1823–1825. [DOI] [PubMed] [Google Scholar]
- 30. Lipsitz LA, Gagnon M, Vyas M, et al. Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension. 2005;45:216–221. [DOI] [PubMed] [Google Scholar]
- 31. Jordan J, Shannon JR, Pohar B, et al. Contrasting effects of vasodilators on blood pressure and sodium balance in the hypertension of autonomic failure. J Am Soc Nephrol. 1999;10:35–42. [DOI] [PubMed] [Google Scholar]
- 32. Arnold AC, Biaggioni I. Management approaches to hypertension in autonomic failure. Curr Opin Nephrol Hypertens. 2012;21:481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]


