Abstract
In experimental studies, statins have been shown to lower blood pressure through increased nitric oxide bioavailability and improved arterial compliance. The clinical significance of this effect remains poorly documented. The authors performed a meta‐analysis of the effect of statins on systolic blood pressure (SBP) and diastolic blood pressure (DBP) including prospective randomized, controlled trials of statin therapy. EMBASE and MEDLINE searches for studies in which patients were randomized to treatment with a statin plus standard treatment (or placebo) vs standard treatment (or placebo) were conducted. Studies that provided data on SBP and DBP values before the initiation of the treatment and at the end of the follow‐up period were included. A total of 40 studies with 51 comparison groups examining 22,511 controls and 22,602 patients taking statins were examined. Mean SBP in the statin group decreased by 2.62 mm Hg (95% confidence interval [CI], −3.41 to −1.84; P<.001) and DBP by 0.94 mm Hg (95% CI, −1.31 to −0.57; P<.001). In studies including hypertensive patients, the decrease in blood pressures with statins was slightly greater (SBP, −3.07 mm Hg; 95% CI, −4.00 to −2.15 and DBP, 1.04; 95% CI, −1.47 to −0.61). Similarly, statins effectively reduced SBP in diabetic patients. In this large meta‐analysis of prospective controlled studies, the authors found a small but statistically significant reduction of SBP in patients taking statins. The decrease in blood pressure may contribute to the pleiotropic effect of statins in reducing cardiovascular risk.
Statins have pleiotropic effects such as improving endothelial‐dependent vasodilation, increasing bioavailability of nitric oxide, and reducing levels of endothelin‐1 (potent vasoconstrictor).1 Statins also downregulate expression of angiotensin type 1 receptors, decrease expression of NAD(P)H oxidase subunit p22phox, and reduce free radical release in the vasculature2 and have been shown to improve arterial compliance.3 These pleiotropic effects may directly lower blood pressure (BP) in addition to lowering cholesterol levels.3 Previous studies report a positive correlation between BP and cholesterol levels. Indirect evidence from several trials investigating cholesterol‐lowering regimens suggests that lowering cholesterol may simultaneously reduce BP by between 2 mm Hg and 5 mm Hg.4 However, conflicting results have been reported with respect to BP‐lowering effects of statins in humans.4 The present study was designed to systematically review prospective randomized trials and assess the antihypertensive effects of statins.
Materials and Methods
Search Strategy
We systematically searched the electronic databases MEDLINE, PubMed, EMBASE, and the Cochrane Library for Central Register of Clinical Trials using the MESH terms statins, HMG‐CoA enzyme inhibitors, hypertension, blood pressure, and the names of individual statin agents. We limited our search to studies in humans and peer‐reviewed journals in English language from 1996 to June 2012. Additionally, a manual search of all relevant references from the screened articles and reviews on statins was performed for additional clinical studies.
Study Selection
We included only prospective randomized, controlled trials published as original articles in peer‐reviewed scientific journals in English. We excluded trials where we could not extract or calculate the difference between baseline and end‐of‐treatment systolic BP (SBP) and diastolic BP (DBP) in intervention and control groups and those that did not report any of the following variables: number of patients in both the statin and control groups, length of study, and description of the main relevant features of the study population, including sex, age, hypertensive status, and description of concomitant therapy, if any.
Data Extraction and Quality
The data were independently extracted by two authors (V.A. and A.B.) using standardized protocol and reporting form (Figure 1). Disagreements were resolved by arbitration (F.M.), and consensus was reached after discussion. For our analysis we extracted characteristics of each study (type of design with duration of intervention and methods), baseline demographics, and SBP and DBP at baseline and at the end of the study. Authors of the papers were individually contacted if the data were unclear. The study quality was evaluated according to the Jadad composite score,5 which is a 5‐point quality scale, with low‐quality studies having a score of ≤2 and high‐quality studies a score of ≥3.6
Figure 1.

Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram of study selection. CI indicate 95% confidence interval.
Outcomes Assessed
Our primary outcome was the difference in SBP and DBP among the treatment and control groups compared with baseline BPs.
Data Analysis and Synthesis
An intention‐to‐treat traditional meta‐analysis was performed in line with recommendations from the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) Statement. All analyses were performed by Review Manager (RevMan) 5.1. (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011). Heterogeneity was assessed with the I 2 statistic, with I 2 <25% considered low and I 2 >75% considered high. Since we expected individual studies to differ in baseline patient characteristics, choice of statin and its dose, and the length of follow‐up, we decided a priori to use a DerSimonian‐Laird random‐effects model for relative risk (RR) estimation for all outcomes. Reported values are two‐tailed, and hypothesis testing results were considered statistically significant at P=.05. Small study effect, including publication bias, was tested using funnel plot and Egger tests. If publication bias was found, the nonparametric trim and fill method of Duvall and Tweedie was performed to add studies that appeared to be missing. We separately analyzed the various groups of statins. We separately examined whether there were any differences in the outcomes between the studies that included an up‐titration of the antihypertensive agents during the study vs the studies that kept the dosages of the antihypertensive medications stable. Meta‐regression (OpenMeta analyst) was used to assess whether age, follow‐up duration, and Jadad score were associated with the effect of statin therapy on BP.
Results
Study Selection
We identified 40 clinical studies (Table 1), with 40 control arms and 51 intervention arms, which examined the effects of statins on SBP and DBP based on our inclusion and exclusion criteria. The inter‐rater reliability was measured by the use of the Cohen's kappa test, which in our study was 0.91 (standard error, 0.035), suggesting good agreement.
Table 1.
Intervention, Characteristics, and Effects on BP in Individual Studies
| Study | Up‐titration of Antihyper‐ tensives | Statin, mg/d | Follow‐ Up, mo | HTN/ Hyperlipidemia/ Diabetes | Age Treatment/ Control, SD | Treatment Group Baseline SBP/ DBP, mm Hg | Treatment Group Follow‐Up SBP/DBP, mm Hg | Control Group Baseline SBP/ DBP, mm Hg | Control Group Follow Up SBP/ DBP, mm Hg |
|---|---|---|---|---|---|---|---|---|---|
| Lavallee and colleagues7 | + | Atorvastatin (80) | 3 | +/+/− | 61/60 | 132.7 (14.6)/ 78 (8.3) | −3.9 (2.1)/ −2.8 (1.4) | 133.7 (12.8)/ 78.1 (7.7) | −0.8 (2.24)/ −1.4 (1.5) |
| Mancia and colleagues8 | − | Pravastatin (40) | 31 | +/+/− | 58.5/58.3 | 159.6 (8.9)/ 98.3 (4.1) | −19.2 (1.94)/ −12.4 (1.55) | 160 (9.1)/ 98.3 (4.4) | −18.1 (1.94)/ −12.8 (1.55 |
| Williams and colleagues10 | + | Atorvastatin (10) | 40 | +/+/− | 62.6/62.9 | 159.6 (16.7)/ 92.5 (9.7) | 133.9 (0.6)/ 79.1 (0.4) | 160.3 (17.5)/ 92.9 (9.2) | 133.8 (0.6)/ 79 (0.4) |
| Manisty and colleagues9 | + | Atorvastatin (10) | 12 | +/+/− | 64/84 | 158 (20)/93 (10) | 141 (11)/81 (8) | 164 (20)/94 (10) | 142 (12)/81 (7) |
| Orr and colleagues11 | − | Atorvastatin (80) | 3 | −/+/− | 53/55 | 129 (4)/74 (2) | 124 (4)/73 (3) | 127 (4)/75 (2) | 124 (4)/75 (2) |
| Ge and colleagues37 | − | Atorvastatin (20) | 4 | +/+/− | 64/65 | 164.3 (14.8)/ 106.4 (9.2) | 123.2 (12.4)/ 82.5 (7.8) | 162.8 (13.4)/ 105.2 (8.6) | 136.7 (11.2) 91 (7.32) |
| Kuklinska and colleagues16 | − | Atorvastatin (80) | 3 | +/+/− | 53 | 129 (11)/76 (9) | 123.3 (8.9)/ 72.1 (8.6) | 129.5 (13)/ 74 (7.6) | 128.5 (9.7)/ 74 (7.6) |
| Grimm and colleagues14 | − | Atorvastatin (20) | 1.5 | +/+/− | 56.5/55.5 | 132.3 (11.3)/ 81 (9.5) | −4 (1)/ −1.7 (0.8) | 132.9 (12.3)/ 81.9 (8.2) | −1 (1.17)/ −1.1 (0.7) |
| Koh and colleagues12 | − | Atorvastatin (20) | 2 | +/+/− | 53 (2) | 157 (1)/96 (1) | 138 (2)/85 (1) | 156 (2)/95 (1) | 139 (1)/85 (1) |
| Tonelli and colleagues19 | + | Pravastatin (40) | 24 | −/+/− | 58.5/58.7 | 128.7 (18.4)/78.5 (10.2) | 0.7 (0.4)/0.2 | 129 (17.8)/ 78.6 (10.1) | −0.2 (0.3)/ −0.4 (0.3) |
| Ichihara and colleagues39 | + | Pravastatin (10) | 12 | +/+/− | 60/60 | 142 (7)/84 (3) | 140 (5)/86 (2) | 142 (6)/89 (4) | 138 (10)/90 (3) |
| Simvastatin (5) | 12 | 60 | 145 (4)/88 (2) | 142 (4)/86 (2) | |||||
| Fluvastatin (20) | 12 | 60 | 142 (6)/84 (3) | 141 (4)/85 (2) | |||||
| Kushiro and colleagues40 | + | Pravastatin | 60 | +/+/− | 60/60 | 140.9 (16)/ 82.9 (10) | 138.6 (16)/ 80.3 (10) | 141 (15)/83 (10) | 139.1 (15)/ 81.3 (10) |
| Terzoli and colleagues18 | + | Simvastatin, Pravastatin, Atorvastatin | 2 | +/+/− | 62.7 (10.3)/ 62.7 (10.3) | 149.8 (15.9)/ 87 (9.3) | 143.6 (11.3)/ 83.7 (10.1) | 152.2 (12.4)/ 85.3 (5.8) | 150.6 (11.6)/ 87.4 (6.4) |
| 135.2 (14.6)/ 78.7 (10.7) | 132.8 (13.7)/ 77.6 (9.5) | 140.3 (11)/ 82.6 (4.6) | 138.7 (10.6)/ 83.7 (6.4) | ||||||
| Collins and colleagues49 | + | Simvastatin (40) | 56 | +/+/− | 65.5 (7.8)/ 65.1 (8.2) | −7.6 (27.5)/−4.5 (15.1) | −7.1 (28.9)/ −4.6 (14.1) | ||
| Sposito and colleagues17 | + | Pravastatin, Lovastatin | 4 | +/+/− | 52 (3)/53 (3) | 153 (9)/100 (3) | 130 (5)/81 (4) | 149 (8)/102 (2) | 137 (6)/87 (8) |
| Athyros and colleagues22 | − | Various Statins (various doses) | 36 | +/+/− | 58 (11)/58 (14) | 123 (14)/74 (8) | 122 (12)/74 (8) | 125 (16)/75 (9) | 122 (13)/73 (8) |
| Cohn and colleagues34 | + | Atorvastatin (10) | 2 | +/−/− | 54.7 (8.9)/ 55.3 (9.3) | 146.7 (11.1)/91.8 (7.2) | −7 (2)/−4.2 (1) | 147 (11)/93 (5.3) | −5 (2)/−3.4 (0.8) |
| Balletshoffer and colleagues15 | − | Cerivastatin (0.8) | 3 | +/−/+ | 59 (8)/59 (8) | 148 (22)/86 9) | 141 (22)/82 (12) | 151 (14)/85 (11) | 157 (16)/88 (11) |
| Cerivastin (0.2) | 59 (8)/59 (8) | 141 (22)/82 (12) | 149 (26)/86 (11) | ||||||
| Lewandowski and colleagues45 | − | Simvastatin (40) | 2 | +/+/− | 38.7 (10)/ 38.7 (10) | 142 (11.8)/91 (10.8) | 136 (9.5)/84 (9.8) | 136 (9.5)/ 86 (11) | 131 (12.4)/82 (7.5) |
| Danaoglu and colleagues35 | − | Simvastatin (20) | 3 | +/+/− | 52 (3)/54 (4) | 160 (11)/99 (9) | 122 (9)/76 (6) | 158 (14)/ 104 (10) | 126 (19)/82 (8) |
| Derosa and colleagues27 | − | Fluvastatin (80) | 12 | −/+/− | 50.6 (9.4)/ 52.4 (10.2) | 133 (4)/86 (5) | 127 (5)/82 (3) | 132 (5)/84 (3) | 128 (3)/82 (2) |
| Fluvastatin (80) | 12 | 53.1 (10)/ 51.6 (8.3) | 132 (4)/85 (3) | 123 (4)/79 (2) | 131 (3)/85 (4) | 125 (3)/81 (2) | |||
| Fassett and colleagues36 | + | Atorvastatin (10) | 12 | +/+/− | 62.3 (16.3)/ 64.8 (15) | 147.6 (20.2)/75.8 (9.1) | −4.18 (8.35)/ −1.24 (5.22) | 148.3 (22.1)/ 79.3 (6.7) | −4.63 (8.98)/ −1.98 (3.51) |
| Glorioso and colleagues41 | − | Atorvastatin (20) | 4 | +/+/− | 53 (2)/53 (2) | 149 (6)/97 (2) | 140 (5)/90 (4) | 149 (6)/97 (2) | 147 (8)/94 (4) |
| Hjelstuen and colleagues38 | + | Fluvastatin (40) | 12 | +/+/− | 55.8 (7.9)/ 57.5 (9.2) | 141.8 (12.3)/90.5 (7.4) | −1.7 (14.25)/ 0.1 (9.1) | 140.4 (15.3)/ 88.6 (9) | −1.5 (7.68)/ −1.1 (7.68) |
| Su and colleagues46 | 0 | Pravastatin (10) | 6 | +/+/− | 61/64 | 175 (11)/105 (6) | 130 (9)/73 (6) | 174 (9)/104 (5) | 130 (8)/76 (7) |
| Teixeira and colleagues21 | + | Fluvastatin (20) | 12 | +/+/− | 51/51 | 139 (14)/87 (11) | 126 (12)/80 (7) | 138 (12)/86 (9) | 131 (11)/82 (8) |
| Sever and colleagues20 | − | Atorvastatin (10) | 36 | +/+/− | 63/63.3 | 164.3 (17.8)/95.1 (10.2) | 137.5 (17)/ 80.5 (9.5) | 164.7 (18.3)/ 95.1 (10.4) | 137.5 (16.8)/ 80.8 (9) |
| Atorvastatin (10) | 36 | +/+/− | 63.2/63 | 164.1 (17.7)/94.9 (10.4) | 140 (16.5)/ 82.5 (9.8) | 163.7 (17.7)/ 95 (10.1) | 140.5 (16.5)/ 82.6 (9.6) | ||
| Colomb and colleagues13 | − | Pravastatin (40) | 6 | −/−/− | 57.4/57.7 | 126.8/75.2 | −2.5 (21.12)/ −2.8 (17.61) | 126.5/74 | −1 (21.1)/ −0.4 (17.8) |
| Simvastatin (20) | 6 | 126.8/75.2 | −2.7 (21.05)/ −2.4 (17.2) | 0.2 (20.9)/ 0.6 (17.1) | |||||
| Lee and colleagues42 | − | Pravastatin (40) | 6 | +/−/− | 71/72 | 133 (16)/76 (10) | 130 (18)/79 (8) | 134 (15)/75 (9) | 132 (14)/79 (8) |
| Magen and colleagues43 | − | Atorvastatin (10) | 2 | +/+/− | 54.1/51.4 | 153 (4.8)/87.1 (6.7) | 136.9 (6.1)/7 8.3 (4.2) | 151.1 (7.4)/ 84.7 (5.9) | 150.9 (6.8)/ 83.2 (5.7) |
| Nakamura and colleagues25 | − | Cerivastatin (0.15) | 6 | −/−/+ | 58/55 | 122 (14)/78 (10) | 118 (16)/76 (8) | 124 (12)/76 (12) | 126 (12)/76 (10) |
| Olkinuora and colleagues44 | − | Simvastatin (10) | 3 | +/+/− | 61/62 | 156 (12)/93 (9) | 142 (10)/86 (8) | 161 (12)/95 (9) | 147 (15)/88 (9) |
| McDowell and colleagues29 | − | Simvastatin (40) | 3 | −/+/− | 18–70 | 133 (18.7)/80 (11.2) | 136 (18.7)/78 (11.2) | 130 (20.8)/76 (10.4) | 137 (17.3)/80 (7) |
| Hommel and colleagues30 | − | Simvastatin (20) | 3 | +/+/+ | 41/35 | 140 (18)/84 (11) | 135 (21)/82 (10) | 135 (19)/83 (11) | 138 (18)/86 (11) |
| Bak and colleagues26 | − | Pravastatin+diet 1 (20) | 6 | −/+/− | 55.3/54.6 | 133 (14.1)/83.1 (7.4) | 2.5 (16.1)/2 (7.14) | 134.4 (14.9)/82.9 (8) | 0.6 (18.6)/0.9 (9.1) |
| Pravastatin+diet 2 (20) | 55.6/54.6 | 137.5 (16.3)/85.4 (6.1) | −5.1 (18.21)/ −0.9 (8.9) | 137.4 (14.2)/84.1 (7.3) | −2.8 (15.7)/0.1 (7.65) | ||||
| Lee and colleagues28 | − | Pravastatin (20) | 6 | +/+/− | 52/50 | 121 (10)/70 (4) | 120 (11)/70 (6) | 117 (10)/69 (5) | 117 (8)/70 (4) |
| Hodis and colleagues48 | − | Lovastatin (80) | 48 | −/+/− | 37–67 | 124.6 (13.3)/80.5 (7.2) | 122.5 (11.9)/ 78.7 (6.7) | 122.2 (14)/79.6 (8.1) | 121.1 (12.6)/78.7 (7.6) |
| Borghi and colleagues23 | − | Simvastatin | 60 | +/+/− | 55.2 (10) | 118 (5)/78.5 (6) | 3.54 (6.14)/ −1.57 (4.7) | 119.3 (6)/79.3 (7) | 3.58 (6)/−1.98 (3.49) |
| Simvastatin | 60 | 59.9 (7) | 130.3 (1)/82 (8) | −6.5 (10.42)/ −3.28 (6.56) | 132.6 (3)/84.7 (6) | −.66 (9.78)/−1.7 (5.5) | |||
| Simvastatin | 60 | 61.2 (8) | 145.6 (6)/88.3 (8) | −11.64 (7.2)/ −5.3 (7.5) | 145.3 (4)/89.5 (7) | −2.9 (5.25/ −2.68 (6.5) | |||
| Simvastatin | 60 | 65.5 (8) | 164.7 (5)/97.2 (6) | −24.7 (7.2)/ −12.5 (7.5) | 166.8 (12)/95.6 (11) | −6.67 (11.2)/ −5/7 (9.7) | |||
| Fogari and colleagues24 | − | Atorvastatin (20) | 3 | +/+/+ | 58.7/58.7 | 160 (11)/98 (5) | 137 (8)/80 (4) | 160 (11)/98 (5) | 143 (8)/84 (4) |
| Kanaki and colleagues31 | − | Atorvastatin (10) | 6.5 | +/+/− | 59.7/58.8 | 146.7 (7.2)/92.2 (10.3) | 141.6 (7.3)/ 89.6 (9.2) | 147.5 (6.7)/91.2 (8.5) | 147.8 (6.8)/90.6 (8.9) |
Abbrevations: BP, blood pressure; DBP, diastolic blood pressure; HTN, hypertension; SBP, systolic blood pressure; SD, standard deviation.
Baseline Characteristics
These studies enrolled 22,511 controls and 22,602 patients taking statins, with an average follow‐up duration of 13.9 months. The mean age of patients taking statins was 57.9±5.8 years, which was not significantly different from the mean age of the control group (57.7±6.5 years). Atorvastatin was the statin used in 15 studies,7, 9, 10, 11, 12, 14, 16, 20, 24, 31, 34, 36, 37, 41, 43 pravastatin in 8 studies,8, 13, 19, 26, 28, 39, 40, 42, 46 simvastatin in 10 studies,13, 23, 29, 30, 35, 39, 44, 45, 47, 49 fluvastatin in 4 studies,21, 27, 38, 39 cerivastatin in 2 studies,15, 25 and lovastatin in 1 study.48 One study27 compared fluvastatin plus orlistat (an inhibitor of intestinal lipid digestion) with orlistat alone. Some studies included only hypertensive patients,7, 9, 10, 12, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 28, 30, 31, 35, 36, 37, 38, 39, 40, 41, 42, 44, 45 and other studies up‐titrated the antihypertensive agents.7, 9, 10, 12, 15, 17, 18, 19, 21, 36, 38, 39, 40, 49 Four studies included only type 1 or only type 2 diabetic patients.15, 25, 30, 47
Quality Assessment
The included studies were of variable quality. Twenty‐four studies were of good quality (Jadad score ≥3), with a low risk of bias, and 15 studies of low quality (Jadad score <3), with a high risk of bias. During the study selection process, we attempted to avoid duplication of data. However, the Conduit Artery Function Evaluation–Lipid‐Lowering Arm (CAFE‐LLA) study10 recruited 891 patients already randomized into the Anglo‐Scandinavian Cardiac Outcomes Trial–Lipid‐Lowering Arm (ASCOT‐LLA).20, 33 The CAFE‐LLA study examined the effects of atorvastatin on aortic pressures and aortic stiffness, an endpoint not included in the ASCOT‐LLA study. Additionally, according to the study protocol, the patients' brachial BPs and central hemodynamics were measured separately from the main ASCOT‐LLA protocol. Therefore, we decided to include the study in our analysis. A sensitivity analysis excluding the CAFE‐LLA results did not influence the effects on SBP, DBP, or heterogeneity.
Heterogeneity
For the outcomes measured, when all studies were combined, a highly significant level of statistical heterogeneity was evident, suggesting that it is more suitable to group studies by statin type. However, total heterogeneity as well as heterogeneity for the subgroups with the higher number of studies (atorvastatin and simvastatin groups) were still evident (P<.001). Additionally, we conducted a sensitivity analysis excluding small studies (with <50 patients assigned to each study group) as well as studies with extreme BP reductions or elevations during the follow‐up.23, 37, 43
Effect of Statins on SBP
The overall effect for statin therapy on SBP was a mean difference of −2.62 mm Hg (95% confidence interval [CI], −3.41 to −1.84; P<.001), with significant heterogeneity between studies (P<.001, Figure 2). The funnel plot did not show asymmetry consistent with publication bias, and Egger's test result was not significant (P=.67).
Figure 2.
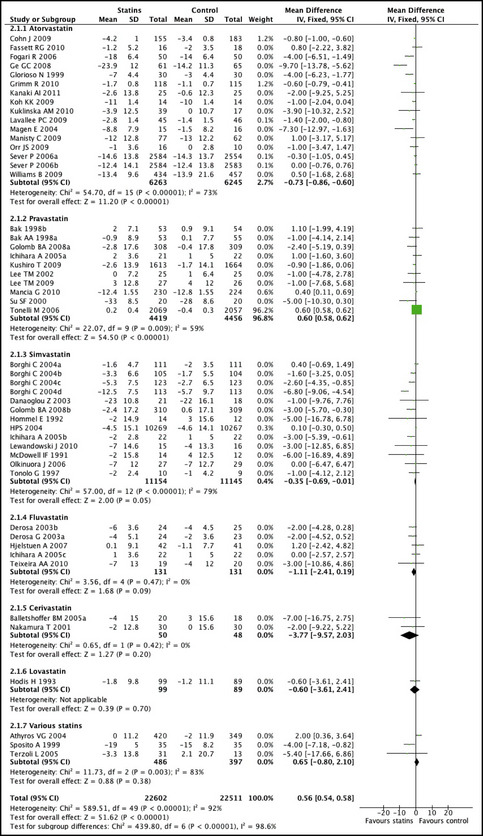
Mean differences and 95% confidence intervals (CIs) in diastolic blood pressure (DBP) achieved in patients taking a statin compared with those taking placebo or other control treatment.
Effect of Statins on DBP
The effect of statin therapy on DBP in all of the studies was in the same direction as for SBP, with a mean difference of −0.94 mm Hg (95% CI, −1.31 to −0.57; P<.001), with significant heterogeneity between studies (P<.001) (Figure 3). There was no evidence of publication bias (Egger's test, P=.58).
Figure 3.
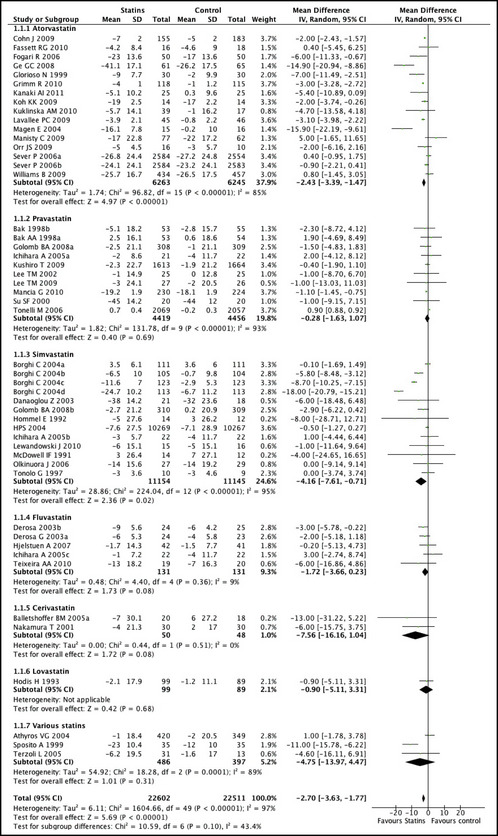
Mean differences and 95% confidence intervals (CIs) in systolic blood pressure (SBP) achieved in patients taking a statin compared with those taking placebo or other control treatment.
Subgroup Analysis
The effect of statin therapy on SBP was significant in studies that recruited patients who were hypertensive at baseline (−3.07 mm Hg; 95% CI, −4.00 to −2.15; P<.001; and DBP −1.04; 95% CI, −1.47 to −0.61; P<.001). In 4 trials that enrolled diabetic hypercholesterolemic patients, the combined effect of statins on BP was: SBP −6.50 (95% CI, −10.93 to −2.08; P=.004) and DBP −4 (95% CI, −6.26 to −1.74; P=.0005). In the studies that up‐titrated the dosages of antihypertensive medications, the effect of statins on BP was less pronounced (SBP −0.86; 95% CI, −2.41 to 0.69; P=.28; DBP −0.56; 95% CI, −1.35 to 0.24; P=.17). The effects of different types of statins on SBP and DBP are presented in Figures 2 and 3. Simvastatin and atorvastatin had a greater effect on BP (simvastatin: SBP −4.16; 95% CI, −7.61 to −0.71; DBP −2.02; 95% CI, −3.37 to −0.68), atorvastatin: −2.43; 95% CI, −3.39 to −1.47; DBP −0.96; 95% CI, −1.40 to −0.52) compared with pravastatin (SBP −0.38; −1.26 to 0.50; DBP −0.09; −0.77 to 0.58). However, the meta‐regression analysis did not show any statistically significant difference among different statin types.
In the sensitivity analysis of larger studies (study arms with >50 patients), 13 studies were included in the analysis. The effect of statins on SBP and DBP was attenuated as SBP decreased by −0.73 (95% CI, −1.53 to 0.07; P=.07) and DBP by −0.18 (95% CI, −0.58 to 0.22; P=.37). The total heterogeneity and the heterogeneity in the atorvastatin group remained significant (P<.001), suggesting that exclusion of these trials was insufficient to fully explain this residual heterogeneity. Among the conducted analyses, grouping trials by statin type provides explanation for the some of the heterogeneity seen between trials. The total heterogeneity we identified may be attributed to design, participants, interventions, and outcomes studied.
In the group of studies using simvastatin, a significant effect was seen in the study by Borghi and colleagues,23 but the remaining studies showed small effects on BP. A sensitivity analysis excluding the study by Borghi and colleagues23 showed a nonsignificant decrease in SBP by −0.60 (95% CI, −1.32 to 0.12; P=.10) and DBP by −1.47 (95% CI, −2.97 to 0.03; P=.06).
Meta‐Regression Analysis
We performed a meta‐regression analysis that demonstrated no evidence that any of the following factors were significantly related to the response to statin therapy: age (P=.75 for SBP and .687 for DBP), follow‐up duration (P=.543 and .194, respectively), and Jadad score (P=.257 and .262, respectively).
Discussion
The antihypertensive effect of statins documented by our analysis was small and reached statistical significance for SBP and DBP. The predominant reduction in SBP could not be explained on the basis of age, race, or severity of hypertension. However, we observed significant heterogeneity between trials in the efficacy of statins as antihypertensives. Much of this heterogeneity could be explained by differences in the methodological quality of the trials.
Our meta‐regression analysis did not show any statistically significant difference among different statin types. This is probably attributed to the limited number of patients included in the studies with different statin types. Besides, evidence from published randomized placebo‐controlled trials suggests that pravastatin, simvastatin, and atorvastatin, when used at their standard dosages, show no statistically significant difference in their effect on long‐term cardiovascular prevention.32
Many clinical trials have demonstrated a statin‐related reduction in morbidity and mortality in patients at risk for cardiovascular disease; however, the data on the BP‐reducing effects of statins in humans have been mixed. The main limitation of the published studies is that some of the reported results were from normotensive and some from hypertensive patients with different antihypertensive regimens. In addition, some of the studies had small numbers of patients, were unblinded, and/or allowed adjustment of antihypertensive medications throughout the trial.
Since it is probable that statins exert some hypotensive effects, the evaluation of their impact on BP in association with standard hypotensive drugs is relevant. Sposito and colleagues17 suggested a beneficial effect of combined 16‐week therapy with statins (pravastatin or lovastatin) both on SBP and DBP in comparison to monotherapy with enalapril or lisinopril. Authors also observed a correlation between the magnitude of diastolic (but not systolic) BP reduction and the reduction in serum cholesterol. Moreover, a decrease in heart left ventricular mass was demonstrated in patients treated with statins. Results by Borghi and colleagues23 suggested that the addition of statin treatment (pravastatin or simvastatin) to conventional antihypertensive therapy might improve BP control in hypertensive patients with hypercholesterolemia.
In one of the first large randomized trials on the topic, the University of California San Diego (UCSD) Statin Study, evaluated the impact of statins on BP in 1016 patients with increased levels of serum cholesterol.13 A significant decrease in BP with statin therapy was observed, compared with the placebo group. However, the observed effect for SBP in the pravastatin group and for DBP in the simvastatin group disappeared during the 2‐month follow‐up period.
A previous meta‐analysis4 included 20 controlled clinical trials, which had enrolled 828 normotensive and hypertensive patients. The antihypertensive effect was more pronounced in studies in which the initial SBP was >130 mm Hg (mean difference for SBP −4.0; 95% CI, −5.8 to −2.2). In addition, there was a tendency toward lower DBP values in statin‐treated patients compared with the control groups (mean difference −0.9 mm Hg; 95% CI, −2.0 to −0.2) and greater reduction of DBP in patients with baseline DBP >80 mm Hg (mean difference, −1.2 mm Hg; 95% CI, −2.6 to −0.1). The authors concluded that the impact of statins on BP was dependent on the initial BP, with higher initial values associated with a more pronounced influence of statins. Although we showed comparable BP reductions with the previous meta‐analysis, methodological differences between the two studies exist: (1) we used 12 studies that were included in the meta‐analysis by Strazzullo and colleagues, (2) we did not exclude studies in which concomitant antihypertensive treatment remained unchanged throughout the study, (3) we included large randomized studies published after 2007,13, 40 and (4) we excluded studies with follow‐up <8 weeks as well as prospective crossover studies with or without an insufficient wash‐out period.
The beneficial effects of statins on the vasculature are present early after statin administration and appear to be independent of their cholesterol‐lowering actions.50 Statins up‐regulate the expression and activity of endothelial nitric oxide synthase via activation of phosphatidylinositol 3‐kinase,51 inhibition of geranylgeranylation of the small G‐protein Rho,52 and of vascular Rac1‐mediated activation of NADPH‐oxidase.50 Statins have also been shown to inhibit several angiotensin II–activated intracellular signaling systems, delay hypertension‐induced vascular alterations,53 reduce large artery stiffness, and improve systemic arterial compliance.3 These mechanisms may, in part, explain the BP effects of statins suggested by our analysis.
Limitations
Despite the substantial data on the effectiveness of statin therapy in the primary and secondary prevention of cardiovascular events in patients with hypercholesterolemia, it is difficult to assess the antihypertensive effects of statins and the impact on cardiovascular risk. This lack of clarity is caused by several factors: (1) BP effect is not one of the primary endpoints of clinical trials, (2) statistical power to assess antihypertensive effects is insufficient, and (3) study groups were composed of both hypertensive and normotensive patients. Additionally, the effects of antihypertensive therapies may have varied between trials because of variations in how and when the BP was measured. Also, the results are subject to limitations inherent to any meta‐analysis based on pooling of data from different trials with different inclusion criteria, different designs, variable follow‐up duration with differing attrition rates, and different patient populations. As in other meta‐analyses, given the lack of data in each trial, we did not adjust our analyses for compliance to assigned therapy.
Outlook
Further studies evaluating the magnitude of the antihypertensive effects of statins should: (1) use 24‐hour ambulatory BP measurements to accurately determine the extent and duration of antihypertensive effects, (2) have BP changes as one of the primary endopoints (3) keep the dosages of other antihypertensives steady during the study, (4) assess the effects of different statin dosages, (5) examine the effects of statins on hypertensive subgroups (eg, dippers vs nondippers and diabetics vs nondiabetics), (6) determine the onset and duration of antihypertensive actions of statins, and (7) evaluate possible synergistic effects of statin and other antihypertensive agents.
Conclusions
The available data support only a modest BP‐lowering effect of statins that is most prominent in patients with poorly controlled hypertension. Overall, the small antihypertensive effect may add to the reduction in cardiovascular risk conferred by statin therapy and may be clinically significant in patients with intermediate to high cardiovascular risk.
Acknowledgments and Disclosures
No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
J Clin Hypertens (Greenwich). 2013;15:310–320. ©2013 Wiley Periodicals, Inc.23614844
References
- 1. Haug C, Schmid‐Kotsas A, Zorn U, et al. Endothelin‐1 synthesis and endothelin B receptor expression in human coronary artery smooth muscle cells and monocytederived macrophages is up‐regulated by low density lipoproteins. J Mol Cell Cardiol. 2001;33:1701–1712. [DOI] [PubMed] [Google Scholar]
- 2. Wassmann S, Laufs U, Baumer AT, et al. HMG‐CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–1457. [DOI] [PubMed] [Google Scholar]
- 3. Ferrier KE, Muhlmann MH, Baguet JP, et al. Intensive cholesterol reduction lowers blood pressure and large artery stiffness in isolated systolic hypertension. J Am Coll Cardiol. 2002;39:1020–1025. [DOI] [PubMed] [Google Scholar]
- 4. Strazzullo P, Kerry SM, Barbato A, et al. Do statins reduce blood pressure? A meta‐analysis of randomized, controlled trials. Hypertension. 2007;49:792–798. [DOI] [PubMed] [Google Scholar]
- 5. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 6. Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Ann Intern Med. 2001;135:982–989. [DOI] [PubMed] [Google Scholar]
- 7. Lavallée PC, Labreuche J, Gongora‐Rivera F, et al. Placebo‐controlled trial of high‐dose atorvastatin in patients with severe cerebral small vessel disease. Stroke. 2009;40:1721–1728. [DOI] [PubMed] [Google Scholar]
- 8. Mancia G, Parati G, Revera M, et al. Statins, antihypertensive treatment, and blood pressure control in clinic and over 24 hours: evidence from PHYLLIS randomised double blind trial. BMJ. 2010;340:c1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manisty C, Mayet J, Tapp RJ, et al. Atorvastatin treatment is associated with less augmentation of the carotid pressure waveform in hypertension: a substudy of the Anglo‐Scandinavian Cardiac Outcome Trial (ASCOT). Hypertension. 2009;54:1009–1013. [DOI] [PubMed] [Google Scholar]
- 10. Williams B, Lacy PS, Cruickshank JK, et al. Impact of statin therapy on central aortic pressures and hemodynamics: principal results of the Conduit Artery Function Evaluation‐Lipid‐Lowering Arm (CAFE‐LLA) Study. Circulation. 2009;119:53–61. [DOI] [PubMed] [Google Scholar]
- 11. Orr JS, Dengo AL, Rivero JM, Davy KP. Arterial destiffening with atorvastatin in overweight and obese middle‐aged and older adults. Hypertension. 2009;54:763–768. [DOI] [PubMed] [Google Scholar]
- 12. Koh KK, Quon MJ, Han SH, et al. Additive beneficial effects of atorvastatin combined with amlodipine in patients with mild‐to‐moderate hypertension. Int J Cardiol. 2011;146:319–325. [DOI] [PubMed] [Google Scholar]
- 13. Golomb BA, Dimsdale JE, White HL, et al. Reduction in blood pressure with statins: results from the UCSD Statin Study, a randomized trial. Arch Intern Med. 2008;168:721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grimm R, Malik M, Yunis C, et al.; TOGETHER Investigators . Simultaneous treatment to attain blood pressure and lipid goals and reduced CV risk burden using amlodipine/atorvastatin single‐pill therapy in treated hypertensive participants in a randomized controlled trial. Vasc Health Risk Manag. 2010;6:261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balletshofer BM, Goebbel S, Rittig K, et al. Intense cholesterol lowering therapy with a HMG‐CoA reductase inhibitor does not improve nitric oxide dependent endothelial function in type‐2‐diabetes–a multicenter, randomised, double‐blind, three‐arm placebo‐controlled clinical trial. Exp Clin Endocrinol Diabetes. 2005;113:324–330. [DOI] [PubMed] [Google Scholar]
- 16. Kuklinska AM, Mroczko B, Musial WJ, et al. Influence of atorvastatin on blood pressure control in treated hypertensive, normolipemic patients—An open, pilot study. Blood Press. 2010;19:260–266. [DOI] [PubMed] [Google Scholar]
- 17. Spósito AC, Mansur AP, Coelho OR, et al. Additional reduction in blood pressure after cholesterol‐lowering treatment by statins (lovastatin or pravastatin) in hypercholesterolemic patients using angiotensin‐converting enzyme inhibitors (enalapril or lisinopril). Am J Cardiol. 1999;83:1497–1499. [DOI] [PubMed] [Google Scholar]
- 18. Terzoli L, Mircoli L, Raco R, Ferrari AU. Lowering of elevated ambulatory blood pressure by HMG‐CoA reductase inhibitors. J Cardiovasc Pharmacol. 2005;46:310–315. [DOI] [PubMed] [Google Scholar]
- 19. Tonelli M, Sacks F, Pfeffer M, et al. Effect of pravastatin on blood pressure in people with cardiovascular disease. J Hum Hypertens. 2006;20:560–565. [DOI] [PubMed] [Google Scholar]
- 20. Sever PS, Poulter NR, Dahlof B, Wedel H; ASCOT Investigators . Antihypertensive therapy and the benefits of atorvastatin in the Anglo‐Scandinavian Cardiac Outcomes Trial: lipid‐lowering arm extension. J Hypertens. 2009;27:947–954. [DOI] [PubMed] [Google Scholar]
- 21. Teixeira AA, Buffani A, Tavares A, et al. Effects of fluvastatin on insulin resistance and cardiac morphology in hypertensive patients. J Hum Hypertens. 2011;25:492–499. [DOI] [PubMed] [Google Scholar]
- 22. Athyros VG, Mikhailidis DP, Papageorgiou AA, et al. Effect of statins and ACE inhibitors alone and in combination on clinical outcome in patients with coronary heart disease. J Hum Hypertens. 2004;18:781–788. [DOI] [PubMed] [Google Scholar]
- 23. Borghi C, Dormi A, Veronesi M, et al; Brisighella Heart Study Working Party . Association between different lipid‐lowering treatment strategies and blood pressure control in the Brisighella Heart Study. Am Heart J. 2004;148:285–292. [DOI] [PubMed] [Google Scholar]
- 24. Fogari R, Derosa G, Lazzari P, et al. Effect of amlodipine‐atorvastatin combination on fibrinolysis in hypertensive hypercholesterolemic patients with insulin resistance. Am J Hypertens. 2004;17:823–827. [DOI] [PubMed] [Google Scholar]
- 25. Nakamura T, Ushiyama C, Hirokawa K, et al. Effect of cerivastatin on urinary albumin excretion and plasma endothelin‐1 concentrations in type 2 diabetes patients with microalbuminuria and dyslipidemia. Am J Nephrol. 2001;21:449–454. [DOI] [PubMed] [Google Scholar]
- 26. Bak AA, Huizer J, Leijten PA, et al. Diet and pravastatin in moderate hypercholesterolaemia: a randomized trial in 215 middle‐aged men free from cardiovascular disease. J Intern Med. 1998;244:371–378. [DOI] [PubMed] [Google Scholar]
- 27. Derosa G, Mugellini A, Ciccarelli L, Fogari R. Randomized, doubleblind, placebo‐controlled comparison of the action of orlistat, fluvastatin or both, on anthropometric measurements, blood pressure and lipid profile in obese patients with hypercholesterolemia prescribed a standardized diet. Clin Ther. 2003;25:1107–1122. [DOI] [PubMed] [Google Scholar]
- 28. Lee TM, Chou TF, Tsai CH. Association of pravastatin and left ventricular mass in hypercholesterolemic patients: role of 8‐iso‐prostaglandin F2_ formation. J Cardiovasc Pharmacol. 2002;40:868–874. [DOI] [PubMed] [Google Scholar]
- 29. McDowell IF, Smye M, Trinick T, et al. Simvastatin in severe hypercholesterolaemia: a placebo controlled trial. Br J Clin Pharmacol. 1991;31:340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hommel E, Andersen P, Gall MA, et al. Plasma lipoproteins and renal function during simvastatin treatment in diabetic nephropathy. Diabetologia. 1992;35:447–451. [DOI] [PubMed] [Google Scholar]
- 31. Kanaki AI, Sarafidis PA, Georgianos PI, et al. Low‐dose atorvastatin reduces ambulatory blood pressure in patients with mild hypertension and hypercholesterolaemia: a double‐blind, randomized, placebo‐controlled study. J Hum Hypertens. 2011;26:577–584 doi: 10.1038/jhh.2011.80. [DOI] [PubMed] [Google Scholar]
- 32. Zhou Z, Rahme E, Pilote L. Are statins created equal? Evidence from randomized trials of pravastatin, simvastatin, and atorvastatin for cardiovascular disease prevention. Am Heart J. 2006;151:273–281. [DOI] [PubMed] [Google Scholar]
- 33. Sever PS, Dahlof B, Poulter NR, et al., on behalf of the ASCOT investigators . Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Lipid Lowering Arm (ASCOT‐LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149–1158. [DOI] [PubMed] [Google Scholar]
- 34. Cohn JN, Wilson DJ, Neutel J, et al. Coadministered amlodipine and atorvastatin produces early improvements in arterial wall compliance in hypertensive patients with dyslipidemia. Am J Hypertens. 2009;22:137–144. [DOI] [PubMed] [Google Scholar]
- 35. Danaoğlu Z, Kültürsay H, Kayikçioğlu M, et al. Effect of statin therapy added to ACE‐inhibitors on blood pressure control and endothelial functions in normolipidemic hypertensive patients. Anadolu Kardiyol Derg. 2003;3:331–337. [PubMed] [Google Scholar]
- 36. Fassett RG, Robertson IK, Ball MJ, et al. Effects of atorvastatin on arterial stiffness in chronic kidney disease: a randomised controlled trial. J Atheroscler Thromb. 2010;17:235–241. [DOI] [PubMed] [Google Scholar]
- 37. Ge CJ, Lu SZ, Chen YD, et al. Synergistic effect of amlodipine and atorvastatin on blood pressure, left ventricular remodeling, and C‐reactive protein in hypertensive patients with primary hypercholesterolemia. Heart Vessels. 2008;23:91–95. [DOI] [PubMed] [Google Scholar]
- 38. Hjelstuen A, Anderssen SA, Holme I, et al. Effect of lifestyle and/or statin treatment on soluble markers of atherosclerosis in hypertensives. Scand Cardiovasc J. 2007;41:313–320. [DOI] [PubMed] [Google Scholar]
- 39. Ichihara A, Hayashi M, Koura Y, et al. Long‐term effects of statins on arterial pressure and stiffness of hypertensives. J Hum Hypertens. 2005;19:103–109. [DOI] [PubMed] [Google Scholar]
- 40. Kushiro T, Mizuno K, Nakaya N, et al. Pravastatin for cardiovascular event primary prevention in patients with mild‐to‐moderate hypertension in the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) Study. Hypertension. 2009;53:135–141. [DOI] [PubMed] [Google Scholar]
- 41. Glorioso N, Troffa C, Filigheddu F, et al. Effect of the HMG‐CoA reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia. Hypertension. 1999;34:1281–1286. [DOI] [PubMed] [Google Scholar]
- 42. Lee TM, Chen CC, Shen HN, Chang NC. Effects of pravastatin on functional capacity in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Clin Sci (Lond). 2009;116:497–505. [DOI] [PubMed] [Google Scholar]
- 43. Magen E, Viskoper R, Mishal J, et al. Resistant arterial hypertension and hyperlipidemia: atorvastatin, not vitamin C, for blood pressure control. Isr Med Assoc J. 2004;6:742–746. [PubMed] [Google Scholar]
- 44. Olkinuora JT, Viikari J, Vanhanen H, et al. Effects of celiprolol and simvastatin on the calculated risk of coronary heart disease (the Celisimva study). Scand Cardiovasc J. 2006;40:160–166. [DOI] [PubMed] [Google Scholar]
- 45. Lewandowski J, Siński M, Bidiuk J, et al. Simvastatin reduces sympathetic activity in men with hypertension and hypercholesterolemia. Hypertens Res. 2010;33:1038–1043. [DOI] [PubMed] [Google Scholar]
- 46. Su SF, Hsiao CL, Chu CW, et al. Effects of pravastatin on left ventricular mass in patients with hyperlipidemia and essential hypertension. Am J Cardiol. 2000;86:514–518. [DOI] [PubMed] [Google Scholar]
- 47. Tonolo G, Ciccarese M, Brizzi P, et al. Reduction of albumin excretion rate in normotensive microalbuminuric type 2 diabetic patients during long‐term simvastatin treatment. Diabetes Care. 1997;20:1891–1895. [DOI] [PubMed] [Google Scholar]
- 48. Hodis HN, Blankenhorn DH, Azen SP, et al; MARS Research Group . Coronary angiographic changes with lovastatin therapy. The Monitored Atherosclerosis Regression Study (MARS). Ann Intern Med. 1993;119:969–976. [DOI] [PubMed] [Google Scholar]
- 49. Collins R, Armitage J, Parish S, et al. Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high‐risk conditions. Lancet. 2004;363:757–767. [DOI] [PubMed] [Google Scholar]
- 50. Antoniades C, Bakogiannis C, Leeson P, et al. Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin‐mediated endothelial nitric oxide synthase coupling. Circulation. 2011;124:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sun W, Lee TS, Zhu M, et al. Statins activate AMP‐activated protein kinase in vitro and in vivo. Circulation. 2006;114:2655–2662. [DOI] [PubMed] [Google Scholar]
- 52. Laufs U, Liao JK. Post‐transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. [DOI] [PubMed] [Google Scholar]
- 53. Rupérez M, Rodrigues‐Díez R, Blanco‐Colio LM, et al. HMG‐CoA reductase inhibitors decrease angiotensin II‐induced vascular fibrosis: role of RhoA/ROCK and MAPK pathways. Hypertension. 2007;50:377–383. [DOI] [PubMed] [Google Scholar]


