Abstract
The aim of the study was to determine whether epicardial adipose tissue thickness (EAT), a new cardiometabolic risk factor, is associated with essential hypertension. The sample included 127 asymptomatic patients with one or more cardiovascular risk factors consecutively referred for cardiac computed tomography angiography. Data were collected retrospectively and compared between hypertensive (n=39) and normotensive (n=88) patients. The hypertensive patients had a significantly higher mean EAT thickness than the normotensive group (2.81±1.6 mm vs 2.07±1.43 mm; P=.011) and a significantly elevated mean coronary artery calcium score (316.8±512.6 vs 108.73±215; P=.0257). The odds ratio for a patient with tissue thickness ≥2.4 mm having hypertension was 1.396 (95% confidence interval, 1.033–1.922). Factors independently associated with hypertension were body mass index, low‐density lipoprotein, and age. A model score was developed using the logistic regression coefficients for calculation of individual risk. Hypertensive patients have significantly higher than normal EAT thickness. Epicardial adipose tissue thickness may serve as a risk indicator for hypertension and cardiovascular morbidity.
Hypertension is the most important modifiable risk factor for the development of coronary heart disease, stroke, congestive heart failure, and end‐stage renal disease in the Western world.1 Epicardial adipose tissue (EAT), a type of visceral adipose tissue, is considered to play an important role in the pathogenesis of coronary artery disease (CAD). EAT releases a wide range of biologically active molecules that modulate vascular smooth‐muscle contraction. Their paracrine effects might be attributable to their location being close to the adventitia and extravascular bed.2, 3, 4 Recent studies have suggested that increased EAT thickness may serve as a novel cardiometabolic risk factor and may be linked to hypertension.5, 6, 7, 8 The aim of this study was to evaluate the relationship between whether EAT thickness is increased in patients with essential hypertension. Measurements were performed using cardiac computed tomography angiography (CCTA), which, unlike echocardiography, provides simultaneous information on EAT thickness, coronary artery calcium level, and obstructive coronary artery disease (CAD).
Patient and Methods
Patients, Setting, and Design
A cross‐sectional, retrospective study design was used. The initial study group included all consecutive asymptomatic patients who underwent CCTA at MOR Institute, a diagnostic hospital‐affiliated facility of a tertiary medical center from November 2007 to January 2009 as part of a screening procedure for primary prevention of CAD. The patients were either referred by their primary physician or self‐referred. All of them had at least one risk factor for atherosclerotic CAD. Exclusion criteria comprised history of heart disease, known diabetes, body mass index (BMI) >30, chronic renal disease, thyroid disorders, alcohol or drug abuse, and secondary hypertension. Patients with allergy to contrast media, irregular heart rate, and impaired renal function (creatinine level >1.2 mg) were excluded. The studies were conducted at MOR Institute. The program included clinical evaluation, blood tests, and coronary artery calcium scoring. The data were recorded from the medical files and compared between patients with and without hypertension. The patients signed an informed consent form after receiving a full explanation of the procedure. The study was approved by the institutional ethics committee.
Screening Procedure
Prior to CCTA, a detailed medical investigation was carried out by an internist, and the clinical and laboratory data were recorded. On admission, blood samples were obtained to evaluate the levels of glucose, creatinine, cholesterol, triglycerides, and low‐density lipoprotein (LDL) and high‐density lipoprotein (HDL) using routine laboratory assays. Height and weight were measured, and BMI was calculated as weight (in kilograms) divided by height (in meters squared). Blood pressure (BP) was recorded as the average of two measurements performed 10 minutes apart using a sphygmomanometer. Arterial hypertension was defined as known but untreated BP >140/90 mm Hg or current use of antihypertensive medications. Dyslipidemia was characterized as currently receiving cholesterol‐lowering medications or known but untreated high total cholesterol level. Family history of CAD was defined as the occurrence of CAD in a first‐ or second‐degree relative before the age of 55 years.
CCTA was performed according to the method described by Bachar and colleagues.3 In short, oral β‐blockers (metoprolol 50 mg or propranolol 20 mg) were administered 3 hours before the study to patients untreated with oral β‐blockers in order to maintain a pulse heart rate <70 beats per minute and thereby improve image resolution. To facilitate adequate breath hold, patients were connected to an oxygen mask and asked to hyperventilate. Images were obtained with a 64‐slice Brilliance CT scanner (Philips, Cleveland, OH) with dedicated cardiac reconstruction software and electrocardiographic (ECG) triggering.
The patients underwent scanning twice in a supine position. The first scan was performed without contrast medium to calculate the calcium score. Noncontrast scans were performed during a single breath hold at a slice thickness of 3 mm and at intervals of 1.5 mm. The calcium score was determined by the method of Agatston and colleagues9 using dedicated software (Extended Brilliance Workspace V4.2, Philips Medical System, Best, the Netherlands).
Thereafter, non‐ionic contrast agent (Ultravist 370; Schering AG, Berlin‐Wedding, Germany), 85–100 mL, was injected into the antecubital vein at a rate of 4 to 5 mL/s, followed by a saline flush using a dual‐head automatic injector (Stellant, Medrad, PA). Scan volume was determined from the tracheal bifurcation to the diaphragmatic surface of the heart. Scan parameters were as follows: 140 kV, 400 mA, 0.4‐second rotation speed, and 64×0.625‐mm detector array, with 1‐mm single‐slice collimation with 50% overlap. Pitch, which was dependent on the heart rate, averaged 0.3. Images were reconstructed in different phases of the cardiac cycle using a retrospective ECG‐gated algorithm with 1‐mm–thick sections and 0.4‐mm intervals.
All images were transferred to a workstation with dedicated cardiac reconstruction software (Extended Brilliance Workspace V4.2, Philips Medical System, Eindhoven, Netherlands) and reviewed by a blinded manner by two experienced CT radiologists, by consensus. The initial retrospective ECG‐gated reconstruction was generated with the reconstruction window, starting at the end‐diastolic phase (ie, 75% of the R‐peak interval). In the case that data were insufficient because of motion artifacts, additional reconstruction data were obtained in increments and decrements of 10%. All segments with a diameter of ≥2 mm belonging to the left main artery, left anterior descending, left circumflex, and right coronary arteries were included according to the modified classification of the American College of Cardiology/American Heart Association. The images were evaluated by axial scans, curved multiplanar reformations through the lumen of the coronary vessels, and 3‐dimensional volume‐rendered visualization. Significant CAD was defined as ≥1 stenosis of ≥50% in diameter in a major epicardial vessel.
Epicardial fat was characterized as presence of adipose tissue between the surface of the heart and the visceral pericardium surrounding the three main coronary arteries. Epicardial fat thickness was measured on the right ventricular anterior free wall, at the base of the ventricles on a short‐axis view, according to the method described by Bachar and colleagues.3 Specifically, measurements were made at the 25%, 50%, and 75% level of the wall, from the visceral pericardium to the epicardium of the myocardium and perpendicular to the surface of the heart. The mean of the 3 measurements at each point was used for analysis.
Statistical Analysis
Statistical analyses were performed using the SAS, version 9.3 (SAS Institute, Cary, NC). Significance was set at 5%. All statistical tests were two‐sided. Nominal P values are presented. Where confidence limits were appropriate, two‐sided 95% confidence intervals were constructed. Data were recorded as mean±standard deviation. For comparison of means (continuous variables), the two‐sample t test or Wilcoxon rank‐sum test was used. For comparison of proportions (categorical variables), chi‐square test or Fisher exact test were used as appropriate. Descriptive statistics of the different parameters were plotted against hypertension status and compared using t test or chi‐square test. Pearson correlation coefficients between the different parameters were calculated. Logistic regression modeling was performed to identify risk factors for hypertension. Model performance was assessed by receiver operating characteristic (ROC) curve analysis.
Results
Sixty‐three patients from the original group were found to be obese (BMI >30) or diabetic on evaluation and were therefore excluded from the study, leaving a total sample of 127 patients. A total of 110 were men (86.6%) and 17 were women with a mean age of 56±9 years. Thirty‐nine of the patients were hypertensive and 88 normotensive. The baseline demographic, clinical, and biochemical characteristics are presented in Table 1. There were no significant differences between the groups in smoking habits or mean serum glucose level, diastolic BP, or lipid parameters. Additionally, no differences were found between the hypertensive and normotensive patients concerning significant CAD (35.9% and 24%) or dyslipidemia (69.2% and 52.2%), as well as family history of CAD (43.6% and 47%, respectively). The hypertensive patients were characterized by significantly older age (62.2±7.89 vs 53.2±8.11 years; P<.0001), higher BMI (27.58±1.99 vs 26.7±2.21; P=.043) (Figure 1), lower LDL level (115.9±29.5 vs 130.9±40.6 mg/dL; P=.0225), and higher mean calcium score (316.8±512.6 vs 108.73±215 mg/dL; P=.0257). Mean EAT thickness was statistically significantly higher in the hypertensive group (2.81±1.6 vs 2.07±1.43 mm; P=.011) (Figure 2). On Pearson correlation analysis, EAT thickness was significantly associated with age (r=0.418, P<.0001), systolic BP (r=0.228, P=.011), and calcium score (r=0.459, P<.0001). There was also a significant correlation between age and systolic BP (r=0.406, P<.0001), diastolic BP (r=0.191, P=.034), HDL level (r=0.230, P=.01), and calcium score (r=0.32, P=.0003), as well as between calcium score and systolic BP (r=0.185, P=.04).
Table 1.
Baseline Clinical, Biochemical, and Demographic Characteristics of Patients Referred for CCTA With and Without Hypertension
| Characteristics | Total Sample (N=127) | Normotensive (n=88) | Hypertensive (n=39) | P Value |
|---|---|---|---|---|
| Age, y | 55.98±9.03 | 53.2±8.11 | 62.2±7.89 | .0001 |
| Smoking, pack‐years | 14.79±22.52 | 15.76±21.2 | 12.6±25.3 | .479 |
| BMI, kg/m2 | 26.98±2.17 | 26.7±2.21 | 27.58±1.94 | .043 |
| Systolic BP, mm Hg | 125.54±13.71 | 122.3±11.6 | 133.03±15.4 | .0004 |
| Diastolic BP, mm Hg | 78.25±7.38 | 77.3±6.3 | 80.4±9.16 | .068 |
| Blood glucose, mg/dL | 99.87±6.16 | 100±17.89 | 99.47±11.45 | .83 |
| Cholesterol, mg/dL | 201.05±40.48 | 205.7±42.7 | 190.6±33.1 | .054 |
| LDL, mg/dL | 126.27±7.81 | 130.9±40.6 | 119.9±29.5 | .022 |
| HDL, mg/dL | 45.24±10.11 | 44.85±10.0 | 46.13±10.3 | .5169 |
| Triglycerides, mg/dL | 151.36±86.68 | 151.9±85.9 | 150.24±89.6 | .923 |
| EAT thickness, mm | 2.07±1.43 | 2.81±1.6 | .0111 | |
| Calcium score | 168.92±340.91 | 108.73±215 | 316.8±512.6 | .025 |
Values are expressed as mean±standard deviation. Abbreviations: BMI, body mass index; BP, blood pressure; CCTA, cardiac computed tomography, angiography; EAT, epicardial adipose tissue; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Figure 1.
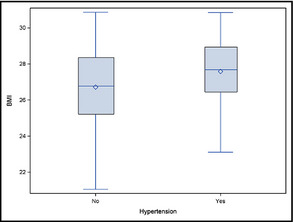
Boxplot of body mass index (BMI) comparing hypertensive and normotensive groups.
Figure 2.
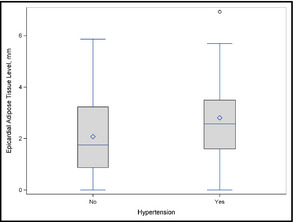
Boxplot of epicardial adipose tissue thickness in hypertensive and normotensive groups.
Using a logistic regression analysis, a predictive model was constructed with EAT as a predictor along with other parameters that remained significant when entered together with EAT, maximizing the power of the model (area under the ROC curve) (Tables 2 and 3). On ROC curve analysis, EAT thickness ≥2.4 mm predicted the presence of hypertension with a sensitivity of 86.5%, specificity of 59.5%, positive predictive value of 48.5%, and negative predictive value of 90.9% (area under the curve, 0.77). The odds ratio (OR) for a patient with an EAT thickness of ≥2.4 mm having hypertension was 1.396. The parameters independently associated with hypertension were BMI (OR, 1.297), LDL (OR, 0.985), and age (<50 years>) (OR, 3.901). The model estimates of the parameter coefficients (Table 2) were transformed into the components of a risk score (or probability index), P=e Y/(1+e Y), where Y is a linear combination of the model coefficients:
Table 2.
Multivariate Logistic Regression Analysis to Identify the Independent Determinants of Hypertension
| Coefficient | SE | P Value | |
|---|---|---|---|
| EAT | 0.3333 | 0.1633 | .04 |
| BMI | 0.2599 | 0.1112 | .02 |
| LDL | −0.0152 | 0.0066 | .02 |
| Age >50< | 1.3612 | 0.6083 | .02 |
Abbreviations: BMI, body mass index; EAT, epicardial adipose tissue; LDL, low‐density lipoprotein; SE, standard error.
Table 3.
Multiple Logistic Analysis of Hypertension Risk Factors in Patients Referred for CCTA
| Risk Factors | Odds Ratio (95% CI) | P Value |
|---|---|---|
| EAT mean | 1.396 (1.013–1.922) | .04 |
| BMI | 1.297 (1.043–1.613) | .019 |
| LDL | 0.985 (0.972–0.998) | .02 |
| Age >50< | 3.901 (1.184–12.85 | .02 |
Abbreviations: BMI, body mass index; CCTA, cardiac computed tomography angiography; CI, confidence interval; EAT, epicardial adipose tissue; LDL, low‐density lipoprotein.
Y=−7.8269+0.3333*EAT+0.2599*BMI – 0.0152*LDL+1.3612* (1–if age >50 or 0 if age <50). The risk score scale ranged from 0 to 1, where the higher the score, the more likely the patient to have hypertension.
For example, we entered the parameters for patient number 55 of our study into the model, namely, age younger than 50 years, BMI=27.46, LDL=158 mg/dL, EAT thickness=2.533 mm, as follows:
Y=−7.8269+0.3333*2.533+0.2599*27.46–0.0152*158+1.3612*0=−2.25, for a risk score of 0.095. The result indicated that this patient is at low risk for hypertension.
Patients with EAT thickness <2.4 mm had a mean calcium score of 58.3±116.8 compared with 326.1±471.4 in patients with EAT thickness ≥2.4 mm (P<.0001). Patients with EAT thickness <2.4 mm had a 13.7% rate of significant CAD compared with 47.2% for patients with EAT thickness ≥2.4 mm (P<.0001). Among the patients with hypertension, 87% had a BMI >25, whereas 13% had a BMI <25 (P<.001).
Discussion
The present study on the predictive value of EAT thickness for the development of hypertension yielded several significant findings: (1) The hypertensive patients showed higher EAT thickness on CCTA than the normotensive patients; (2) the hypertensive patients had a higher calcium score than the normotensive patients, indicating the presence of more calcified plaques in the coronary vessels; and (3) the hypertensive patients had higher BMI value and were older than the normotensive individuals. Altogether, EAT thickness ≥2.4 mm was associated with a high calcium score, increased systolic BP, and older age. It is notable that EAT thickness ≥2.4 mm predicted the presence of hypertension with 86.5% sensitivity, 59.5% specificity, 48.5%, positive predictive value, and 90.9% negative predictive value. On multivariate analysis, EAT thickness was independently associated with hypertension, along with age, LDL, and BMI. Using these parameters, we developed a model and created a risk score (Y) to determine the probability of a patient having hypertension [P=e Y/(1+e Y), calculated as follows: Y=−7.8269+0.3333*EAT+0.2599*BMI – 0.0152*LDL+1.3612* (1 if age >50, 0 if age <50).
Similar to our results, several studies have demonstrated a relationship between increased EAT thickness and the development of hypertension.4, 5, 6, 7 Gastaldelli and colleagues4 reported the existence of a link between EAT and hypertension, atherosclerosis, and coronary heart disease. In a study of 13 nondiabetic men with newly detected, untreated essential hypertension, Sironi and colleagues5 showed that fat was selectively accumulated in the visceral abdominal, intrathoracic, and epicardial regions. Others have noted an association with epicardial ectopic fat along with visceral fat in patients with early hypertension.6 Interestingly, Ertas and colleagues7 demonstrated that in hypertensive individuals, echocardiographic EAT in hypertensive individuals was independently associated with impaired diurnal BP profiles. Sengul and colleagues8 showed that patients with increased epicardial fat thickness have a high circadian BP and an increased prevalence of a nondipper pattern, with significant differences in EAT between normotensive, dipper, and nondipper hypertensive groups. Given that several clinical imaging studies have shown a strong direct correlation between EAT and abdominal visceral adiposity and BMI,10 we excluded patients classified as obese (BMI >30). Nevertheless, the BMI in the overweight range was statistically higher in the patients with hypertension. Accordingly, several studies have demonstrated that increased BMI is correlated with BP levels and that individuals with abdominal obesity are at risk for developing hypertension.11, 12 In the Framingham Offspring Study, Garrison and colleagues13 reported that nearly 70% of new cases of essential hypertension were related to excess of body fat.
Epicardial fat functions as a metabolically active endocrine organ in direct contact with the myocardium including buffering coronary arteries against torsion induced by the arterial pulse wave and cardiac contraction, thermal regulation via the heat‐generating brown fat components, and facilitating coronary artery remodeling and lipid storage for the energy needs of the myocardium. However, when the thickness of the adipose tissue is increased, it may become hypoxic and start to secrete a large number of inflammatory cytokines and vasoactive peptides, including free fatty acids, interleukin 6, tumor necrosis factor‐α, angiotensin II, and plasminogen activator inhibitor‐I, all of which may independently increase BP.14, 15, 16 Paolisso and colleagues17 explained the rise in BP associated with a high level of EAT thickness by an increase in plasma catecholamine concentration induced by cardiac fatty acids. The increased BP may also be caused by endothelial dysfunction and heightened sympathetic activity caused by the paracrine effect of EAT.18 Others related the change in BP to EAT‐derived hypoadinopectinemia, which leads to a decrease in arterial elastic properties and consequent impairment of endothelial vasodilation.19 It is conceivable that this observation could explain our findings. Interestingly, Can and colleagues20 reported that EAT thickness was increased in women with preeclampsia in comparison with controls, and the more severe the preeclampsia, the higher the increase in fat thickness. A large study by Natale and colleagues21 showed that the carotid artery stiffness characteristic of patients with hypertension was independently related to EAT. Accordingly, in our study, the mean calcium score was statistically higher in the patients with hypertension and was directly correlated with EAT thickness. Nakanishi and colleagues22 reported that increased epicardial fat volume measured by CT is associated with greater progression of coronary artery calcification. We have demonstrated that patients with EAT thickness ≥2.4 mm have a significantly higher rate of CAD than patients with EAT thickness <2.4 mm. Along the same lines, EAT may play a pathogenetic role in CAD, given the close anatomic relationship of epicardial fat and the coronary arteries and the positive correlation between the amount of epicardial fat and the presence of coronary atherosclerosis. The ability of these cardiometabolic tissues to secrete free fatty acids, hormones, and cytokines could contribute to the progression of CAD by maintaining chronic inflammation and modulating coronary artery atherothrombosis.3, 10, 23 The pro‐inflammatory milieu of EAT‐induced changes in adipokine expression may also be involved in the progression of coronary atherosclerosis and CAD.24, 25, 26, 27 Although in our study most of the patients were men, in the studies by Ertas and colleagues7 and Sengual and colleagues,8 there was an association between epicardial fat thickness and hypertension in women similar to men. Therefore, we think that our results are also applicable for women.
Study Limitations
The present study has several limitations. The single‐center, retrospective design and inclusion of self‐referred patients may have harbored an inherent bias. Second, measurements of volumetric EAT are considered more reproducible than measurements of EAT thickness. However, EAT thickness is easier to measure, is less time‐consuming, and allows the evaluation of calcium score and the extent of coronary stenosis simultaneously. We are aware of the fact that our study was observational and was carried out on a relatively limited number of patients.
Conclusions
The present work shows that hypertensive patients have higher than normal EAT thickness. EAT thickness ≥2.4 mm predicts the presence of hypertension and is associated with an increased calcium score and presence of CAD. We present a model based on the parameters of EAT, LDL, BMI, and age for calculation of the probability that a patient with increased EAT has hypertension.
J Clin Hypertens (Greenwich). 2013;15:893–898. DOI: 10.1111/jch.12201. ©2013 Wiley Periodicals, Inc.
References
- 1. Qureshi AI, Suri MF, Kirmani JF, et al. Is prehypertension a risk factor for cardiovascular diseases? Stroke. 2005;36:1859–1863. [DOI] [PubMed] [Google Scholar]
- 2. Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci (Lond). 2012;122:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bachar GN, Dicker D, Kornowski R, Atar E. Epicardial adipose tissue as a predictor of coronary artery disease in asymptomatic subjects. Am J Cardiol. 2012;110:534–538. [DOI] [PubMed] [Google Scholar]
- 4. Gastaldelli A, Basta G. Ectopic fat and cardiovascular disease: what is the link? Nutr Metab Cardiovasc Dis. 2010;20:481–490. [DOI] [PubMed] [Google Scholar]
- 5. Sironi AM, Gastaldelli A, Mari A, et al. Visceral fat in hypertension: influence on insulin resistance and beta‐cell function. Hypertension. 2004;44:127–133. [DOI] [PubMed] [Google Scholar]
- 6. Sironi AM, Pingitore A, Ghione S, et al. Early hypertension is associated with reduced regional cardiac function, insulin resistance, epicardial, and visceral fat. Hypertension. 2008;51:282–288. [DOI] [PubMed] [Google Scholar]
- 7. Ertas F, Kaya H, Acet H, et al. Increased echocardiographic epicardial fat thickness is related to impaired diurnal blood pressure profiles. Blood Press. 2012;21:202–208. [DOI] [PubMed] [Google Scholar]
- 8. Sengul C, Cevik C, Ozveren O, et al. Epicardial fat thickness is associated with non‐dipper blood pressure pattern in patients with essential hypertension. Clin Exp Hypertens. 2012;34:165–170. [DOI] [PubMed] [Google Scholar]
- 9. Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 10. Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev. 2007;8:253–261. [DOI] [PubMed] [Google Scholar]
- 11. Chuang SY, Chou P, Hsu PF, et al. Presence and progression of abdominal obesity are predictors of future high blood pressure and hypertension. Am J Hypertens. 2006;19:788–795. [DOI] [PubMed] [Google Scholar]
- 12. Rhéaume C, Arsenault BJ, Bélanger S, et al. Low cardiorespiratory fitness levels and elevated blood pressure: what is the contribution of visceral adiposity? Hypertension. 2009;54:91–97. [DOI] [PubMed] [Google Scholar]
- 13. Garrison RJ, Kannel WB, Stokes J3, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham offspring study. Prev Med. 1987;16:235–251. [DOI] [PubMed] [Google Scholar]
- 14. Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro‐ and anti‐inflammatory organ. Horm Metab Res. 2008;40:442–445. [DOI] [PubMed] [Google Scholar]
- 15. Iacobellis G, Gao YJ, Sharma AM. Do cardiac and perivascular adipose tissue play a role in atherosclerosis? Curr Diab Rep. 2008;8:20–24. [DOI] [PubMed] [Google Scholar]
- 16. Sacks HS, Fain JN. Human epicardial fat: what is new and what is missing? Clin Exp Pharmacol Physiol. 2011;38:879–887. [DOI] [PubMed] [Google Scholar]
- 17. Paolisso G, Manzella D, Rizzo MR, et al. Elevated plasma fatty acid concentrations stimulate the cardiac autonomic nervous system in healthy subjects. Am J Clin Nutr. 2000;72:723–730. [DOI] [PubMed] [Google Scholar]
- 18. Aydin H, Toprak A, Deyneli O, et al. Epicardial fat tissue thickness correlates with endothelial dysfunction and other cardiovascular risk factors in patients with metabolic syndrome. Metab Syndr Relat Disord. 2010;8:229–234. [DOI] [PubMed] [Google Scholar]
- 19. Teijeira‐Fernandez E, Eiras S, Grigorian‐Shamagian L, et al. Epicardial adipose tissue expression of adiponectin is lower in patients with hypertension. J Hum Hypertens. 2008;22:856–863. [DOI] [PubMed] [Google Scholar]
- 20. Can MM, Can E, Ozveren O, et al. Epicardial fat tissue thickness in preeclamptic and normal pregnancies. ISRN Obstet Gynecol. 2012;2012:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Natale F, Tedesco MA, Mocerino R, et al. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur J Echocardiogr. 2009;10:549–555. [DOI] [PubMed] [Google Scholar]
- 22. Nakanishi R, Rajani R, Cheng VY, et al. Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: a serial study using non‐contrast cardiac CT. Atherosclerosis. 2011;218:363–368. [DOI] [PubMed] [Google Scholar]
- 23. Shmilovich H, Otaki Y, Hayes SW, et al. Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: a serial study using non‐contrast cardiac CT. Atherosclerosis. 2011;218:363–368. [DOI] [PubMed] [Google Scholar]
- 24. Ueno K, Anzai T, Jinzaki M, et al. Increased epicardial fat volume quantified by 64‐multidetector computed tomography is associated with coronary atherosclerosis and totally occlusive lesions. Circ J. 2009;73:1927–1933. [DOI] [PubMed] [Google Scholar]
- 25. de Vos AM, Prokop M, Roos CJ, et al. Peri‐coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post‐menopausal women. Eur Heart J. 2008;29:777–783. [DOI] [PubMed] [Google Scholar]
- 26. Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. [DOI] [PubMed] [Google Scholar]
- 27. Iacobellis G, Pistilli D, Gucciardo M, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–255. [DOI] [PubMed] [Google Scholar]


