Abstract
J Clin Hypertens (Greenwich). 2012;00:00–00. ©2012 Wiley Periodicals, Inc.
Delayed blood pressure (BP) and heart rate (HR) decline at recovery post‐exercise are independent predictors of incident coronary artery disease (CAD). Delayed BP recovery and exaggerated BP response to exercise are independent predictors of future arterial hypertension (AH). This study sought to examine whether the combination of two exercise parameters provides additional prognostic value than each variable alone. A total of 830 non‐CAD patients (374 normotensive) were followed for new‐onset CAD and/or AH for 5 years after diagnostic exercise testing (ET). At the end of follow‐up, patients without overt CAD underwent a second ET. Stress imaging modalities and coronary angiography, where appropriate, ruled out CAD. New‐onset CAD was detected in 110 participants (13.3%) whereas AH was detected in 41 former normotensives (11.0%). The adjusted (for confounders) relative risk (RR) of CAD in abnormal BP and HR recovery patients was 1.95 (95% confidence interval [CI], 1.28–2.98; P=.011) compared with delayed BP and normal HR recovery patients and 1.71 (95% CI, 1.08–2.75; P=.014) compared with normal BP and delayed HR recovery patients. The adjusted RR of AH in normotensives with abnormal BP recovery and response was 2.18 (95% CI, 1.03–4.72; P=.047) compared with delayed BP recovery and normal BP response patients and 2.48 (95% CI, 1.14–4.97; P=.038) compared with normal BP recovery and exaggerated BP response individuals. In conclusion, the combination of two independent exercise predictors is an even stronger CAD/AH predictor than its components.
Electrocardiographic (ECG) exercise testing (ET) remains a valuable tool for cardiovascular risk assessment. 1 Although many clinicians typically think of ET only as a measure of ST‐segment changes that may reflect ischemia, non‐ECG parameters have emerged as stronger independent predictors of coronary artery disease (CAD). 1 , 2 , 3 , 4
Markers reflecting autonomic nervous system dysfunction can predict cardiovascular events, future arterial hypertension (AH), and mortality. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 The delayed fall in heart rate (HR) immediately after exercise (delayed HR recovery) has been associated with CAD and death. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 The insufficient decline in blood pressure (BP) during recovery has been reported as a predictor of CAD, new‐onset AH, and mortality. 12 , 13 , 14 , 15 , 16 The exaggerated BP response to exercise has been related to the risk of future AH. 16 , 17 , 18 , 19
A prospective study aiming to synthesize the clinical importance and evaluate the prognostic value of the combination of the aforementioned non‐ECG exercise parameters was conducted. Its purpose was to test the hypothesis that the combination of two established prognostic markers provides an additional predictive value than each variable alone. Specifically, the relative risk (RR) for incident CAD according to HR and BP recovery (one or both abnormal) as well as the RR for new‐onset hypertension according to BP response and recovery (one or both abnormal) were calculated and the predictive accuracy of the new “combined” predictors over the established predictors was compared head‐to‐head.
Methods
Patients and Design
The study cohort was made up of consecutive adults who were referred to our institution for ET between January 2004 and December 2005. The test was indicated due to angina‐like symptoms, a Framingham risk score consistent with at least a moderate risk of serious cardiac events, an intention to engage in vigorous exercise, or involvement in occupations in which impairment might impact public safety. 20 Patients with a history of CAD, decompensated congestive heart failure, left bundle‐branch block, pre‐excitation syndrome, electronically paced rhythm, ECG left ventricular (LV) hypertrophy, or resting ST‐segment depression >1 mm and those taking digitalis were excluded from the study. During the recruitment period, 1280 patients were examined and 974 were eligible and accepted to participate in the study. Written informed consent was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki and was approved by our hospital’s ethics committee.
Participants underwent a symptom‐limited treadmill ET (multistage Bruce protocol). Patients reaching an age‐specific target HR (220 beats per minute minus age in years) were encouraged to exercise to exhaustion. A 15‐lead ECG analysis system, including the right precordial leads 21 (GE CaseT‐2000 Series, Milwaukee, WI) was used. The ECG was recorded continuously and the BP was measured every minute at rest, during exercise, and for up to the tenth minute of recovery. An ischemic ST‐segment response was defined according to the standard ST deviation criteria. 20 Based on the exercise‐induced changes in Q‐, R‐ and S‐wave amplitudes, the QRS score was calculated. The calculating formula has been previously reported. 21
Patients with exercise‐induced angina‐like symptoms or ischemic ST‐segment response underwent stress echocardiography or thallium‐201 scintigraphy. A coronary angiography was recommended in patients with positive stress imaging results (wall motion abnormalities or perfusion defects, respectively). Patients with angiographically documented CAD at the baseline evaluation (n=77) were excluded from the study.
During a 5‐year follow‐up, participants were re‐examined during hospital visits and/or by phone‐call interviews for detecting new‐onset CAD or/and AH (in normotensives). At the end of follow‐up, a second diagnostic treadmill ET was performed in patients without documented CAD during follow‐up. Patients with a positive second ET also underwent stress imaging modalities and those with a positive result underwent an angiography. Patients lost during follow‐up (n=67) were withdrawn from the study. Finally, 830 patients were included in the analysis.
The primary endpoint during the ongoing surveillance was incident CAD defined as the first occurrence of nonfatal myocardial infarction, coronary heart disease death, or coronary revascularization procedure (coronary artery bypass grafting or percutaneous coronary intervention) at any time between the baseline ET and the final follow‐up date.
A secondary endpoint was incident AH in formerly normotensive individuals (n=374, 45.1%). These participants fulfilled criteria for new‐onset hypertension if they initiated any BP‐lowering medication during follow‐up or if the office BP was ≥140/90 mm Hg on at least 3 visits, according to European Society of Hypertension guidelines. 22
Sources of information included clinical examinations, interviews, hospital documents, ECGs, laboratory examinations, and medical and legal records. All available data were reviewed by a committee of 3 physicians to assign cardiovascular disease diagnoses. This committee had no knowledge of the patients’ exercise parameters.
Definitions
Delayed HR Recovery. HR recovery was defined as the difference between peak HR and first minute of recovery HR. Second and third minute recovery HR have also been used in previous studies. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 A certain normal limit for HR recovery has not been officially adopted because it depends on the type of recovery protocol used. 3 In ETs without a post‐exercise cool‐down period, like in the present study, a threshold of 18 beats per minute (bpm) has been proposed. 9 A simpler method dividing the population into quartiles according to the HR recovery has been adopted by others. 7 Using the latter method, a value ≤23 bpm (which corresponded to the 25th percentile for the population, defining patients of the first quartile) was considered as delayed HR recovery in this study.
Delayed BP Recovery. Diastolic BP changes are usually minimal during exercise, 23 making measurement difficult in clinical practice. Thereby, the decline in systolic BP (SBP) is typically used for delayed BP recovery definition. 12 , 13 , 14 , 15 , 16 Values >.90 15 or .95 14 for the third minute of recovery SBP to peak SBP ratio or >1.00 for the third minute of recovery SBP to the first minute of recovery SBP ratio 12 , 14 (patients in the fourth quartile) have been adopted as thresholds for abnormal BP recovery. A second minute of recovery SBP >195 mm Hg (patients in the third tertile) 13 and an age‐adjusted third minute of recovery BP exceeding the 95th percentile 16 have also been used. In the present study, a value ≥.90 for the third minute of recovery SBP to peak exercise SBP ratio (which corresponded to the 75th percentile for the population, defining patients in the fourth quartile) was considered as delayed BP recovery.
Exaggerated BP Response. The definition of exaggerated BP response is usually based on SBP measurements. 19 An age‐adjusted BP during the second stage of exercise exceeding the 95th percentile, 16 a HR‐adjusted peak exercise BP above the 75th percentile, 17 and a peak exercise BP >200/100 mm Hg 18 have been reported as thresholds for exaggerated BP response. However, individuals with increased exercise SBP are likely to have higher resting SBP, which is also a predictor of future hypertension. By calculating the acute change in SBP (ΔSBP) from rest to peak exercise, we can remove the confounding effect of resting BP on hypertension risk. 24 A ΔSBP value exceeding the 75th percentile for the population (patients in the fourth quartile) has previously defined an exaggerated BP response. 19 Using this method, a ΔSBP value ≥65 mm Hg was considered as an exaggerated BP response in the present study.
Statistical Analysis
Continuous variables are presented as mean±standard deviation and categorical variables as observed number (percentage). The median value, the 25th and 75th percentiles of HR recovery, BP recovery, and BP response were calculated. Analysis of variance was used to evaluate differences between subgroups for continuous variables once homogeneity of variance was demonstrated (Levene’s test); otherwise, a nonparametric test (Kruskal‐Wallis) was used. Differences for categorical variables were analyzed with chi‐square test. Kaplan‐Meier cumulative incidence plots of CAD and AH were constructed according to exercise BP and HR. Cox regression analysis was applied to estimate the RR of CAD and AH and the 95% confidence intervals (CIs) in patients positive for one or both exercise predictors after adjusting for established risk factors and other potential covariates (ie age, sex, family history of CAD, obesity, smoking, AH, dyslipidemia, diabetes, medication, exercise duration, exercise‐induced angina, ST‐segment response, and QRS score for CAD’s RR calculation and age, sex, family history of AH, obesity, smoking, and baseline resting BP for AH’s RR calculation). Differences were considered significant for two‐sided P<.05. Data analysis was performed with SPSS 15.0 statistical software (SPSS Inc, Chicago, IL).
Results
A total of 830 patients followed for 60±3 months were included in analysis. Baseline characteristics and exercise parameters (first ET) as well as differences between the BP and HR recovery subgroups are presented in Table I. The median value for HR recovery was 30 bpm (25–75th percentile, 23–38 bpm). An abnormal HR recovery was found in 209 patients (25.2%). The median value for BP recovery ratio was 0.82 (25–75th percentile, 0.77–0.90). A delayed BP recovery was found in 228 of the studied patients (27.5%) and in 95 of the 374 normotensives (25.4%). The median value for BP response (ΔSBP) was 55 mm Hg (25–75th percentile, 40–65 mm Hg). An abnormal BP response was found in 98 of the 374 normotensive patients (26.2%).
Table I.
Patients’ Baseline Clinical Characteristics and Exercise Parameters
| Exercise BP Recoverya and HR Recoveryb Subgroups | ||||||
|---|---|---|---|---|---|---|
| All (N=830) | Normal BP rec Normal HR rec (n=465) | Delayed BP rec Normal HR rec (n=156) | Normal BP rec Delayed HR rec (n=137) | Delayed BP rec Delayed HR rec (n=72) | P Value | |
| Clinical Characteristics | ||||||
| Age, y | 53.8±11.4 | 52.7±11.4 | 54.1±11.6 | 55.4±11.0 | 57.5±10.8 | .002 |
| Male sex, No. (%) | 550 (66.3) | 302 (64.9) | 94 (60.3) | 105 (76.6) | 49 (68.1) | .022 |
| Family history of CAD, No. (%) | 188 (22.7) | 102 (21.9) | 34 (21.8) | 34 (24.8) | 18 (25.0) | .851 |
| BMI, kg/m2 | 27.0±3.5 | 26.8±3.5 | 27.1±3.5 | 27.2±3.3 | 27.6±3.9 | .231 |
| Smoking, No. (%) | 238 (28.7) | 133 (28.6) | 36 (23.1) | 46 (33.6) | 23 (31.9) | .224 |
| Arterial hypertension, No. (%) | 456 (54.9) | 238 (51.2) | 84 (53.8) | 85 (62.0) | 49 (68.1) | .015 |
| Dyslipidemia, No. (%) | 467 (56.3) | 262 (56.3) | 88 (56.4) | 84 (61.3) | 33 (45.8) | .203 |
| Diabetes mellitus, No. (%) | 56 (6.7) | 23 (4.9) | 11 (7.1) | 13 (9.5) | 9 (12.5) | .049 |
| Treatment: ACE inhibitors or ARBs, No. (%) | 265 (31.9) | 140 (30.1) | 46 (29.5) | 52 (38.0) | 27 (37.5) | .199 |
| Diuterics, No. (%) | 134 (16.1) | 57 (12.3) | 26 (16.7) | 32 (23.4) | 19 (26.4) | .001 |
| Calcium channel blockers, No. (%) | 133 (16.0) | 67 (14.4) | 25 (16.0) | 27 (19.7) | 14 (19.4) | .388 |
| β‐Blockers, No. (%) | 111 (13.4) | 61 (13.1) | 16 (10.3) | 22 (16.1) | 12 (16.7) | .401 |
| Statins, No. (%) | 199 (24.0) | 117 (25.2) | 28 (17.9) | 39 (28.5) | 15 (20.8) | .182 |
| Antiplatelet agents, No. (%) | 105 (12.7) | 52 (11.2) | 15 (9.6) | 26 (19.0) | 12 (16.7) | .042 |
| Exercise Parameters | ||||||
| Exercise duration, s | 540.5±150.1 | 563.4±145.5 | 528.0±152.6 | 510.1±147.3 | 478.3±151.1 | <.001 |
| METs | 10.7±2.7 | 11.1±2.6 | 10.6±2.7 | 10.2±2.6 | 9.7±2.6 | <.001 |
| Resting HR, bpm | 82.9±12.8 | 81.4±11.8 | 80.1±13.2 | 90.0±12.9 | 85.4±13.1 | <.001 |
| Peak exercise HR, bpm | 161.6±17.8 | 163.8±16.4 | 162.2±19.6 | 157.7±18.7 | 154.1±18.4 | <.001 |
| Achieved HR, % of predicted | 97.3±9.0 | 98.0±8.3 | 97.9±10.4 | 95.8±9.2 | 94.8±9.5 | .006 |
| Recovery HR, bpm | ||||||
| First minute | 130.4±18.4 | 128.4±17.1 | 125.7±19.0 | 139.0±18.6 | 136.8±18.5 | <.001 |
| Second minute | 109.2±16.9 | 107.7±15.8 | 104.2±17.1 | 117.2±17.5 | 114.6±16.3 | <.001 |
| Third minute | 101.0±15.1 | 99.5±13.8 | 96.6±15.1 | 109.3±16.0 | 104.7±15.0 | <.001 |
| Resting SBP, mm Hg | 118.5±15.7 | 117.7±15.0 | 116.8±15.1 | 120.8±17.5 | 122.9±17.0 | .008 |
| Peak exercise SBP, mm Hg | 172.5±23.0 | 176.2±22.4 | 161.3±22.0 | 176.7±22.7 | 164.5±20.5 | <.001 |
| Exaggerated BP response,c No. (%) | 237 (28.6) | 173 (37.2) | 22 (14.1) | 36 (26.3) | 6 (8.3) | <.001 |
| Recovery SBP, mm Hg | ||||||
| First minute | 166.2±23.9 | 165.6±23.2 | 166.8±25.0 | 164.3±25.5 | 171.8±22.0 | .154 |
| Second minute | 154.7±25.3 | 151.0±24.3 | 161.5±25.6 | 153.0±26.3 | 166.5±22.5 | <.001 |
| Third minute | 144.3±22.3 | 139.2±20.2 | 155.0±21.9 | 139.9±21.5 | 162.5±20.9 | <.001 |
| Resting RP product, bpm×mm Hg | 9844±2126 | 9592±1910 | 9354±2028 | 10,885±2315 | 10,547±2482 | <.001 |
| Peak exercise RP product, bpm×mm Hg | 27,880±4753 | 28,837±4486 | 26,224±4979 | 27,798±4452 | 25,442±4701 | <.001 |
| Recovery RP product, bpm×mm Hg | ||||||
| First minute | 21,637±4161 | 21,226±3866 | 21,052±4670 | 22,715±3979 | 23,507±4345 | <.001 |
| Second minute | 16,894±3769 | 16,278±3551 | 16,877±3971 | 17,838±3629 | 19,105±3827 | <.001 |
| Third minute | 14,581±3155 | 13,871±2876 | 15,009±3326 | 15,229±2996 | 17,010±3214 | <.001 |
| Angina‐like symptoms, No. (%) | 24 (2.9) | 11 (2.4) | 6 (3.8) | 4 (2.9) | 3 (4.2) | .385 |
| Ischemic ST‐segment response, No. (%) | 104 (12.5) | 56 (12.0) | 22 (14.1) | 17 (12.4) | 9 (12.5) | .356 |
| QRS scored | 0.7±3.4 | 1.3±3.3 | 0.2±3.7 | −0.1±3.3 | −0.8±3.6 | .001 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARBs, angiotensin receptor blockers; BMI, body mass index; BP, blood pressure; bpm, beats per minute; CAD, coronary artery disease; HR, heart rate; METs, metabolic equivalents (3.5 mL O2/kg/min); rec, recovery; RP product, rate‐pressure product (HR×SBP); SBP, systolic blood pressure. aDelayed BP recovery: third minute of recovery SBP/peak exercise SBP ≥0.90. bDelayed HR recovery: peak exercise HR – first minute of recovery HR ≤23 bpm. cExaggerated BP response: peak exercise SBP – resting SBP ≥65 mm Hg. dQRS score: (resting [R−Q−S] aVF+resting [R−Q−S] V5)−(peak exercise [R−Q−S] aVF+peak exercise [R−Q−S] V5).
During follow‐up, 72 coronary events occurred, including 10 coronary heart disease deaths, 30 nonfatal myocardial infarctions, and 32 coronary revascularization procedures. Moreover, at the end of follow‐up, 83 patients had a positive second ET: 56 with positive stress echocardiography or thallium‐201 scintigraphy underwent an angiography and 38 underwent revascularization. Overall, 110 of the 830 studied patients (13.3%) developed CAD during the surveillance. Patients positive for 1 of the 2 non‐ECG predictors (delayed BP or delayed HR recovery) in the first ET were found with a higher cumulative and 5‐year incidence of CAD compared with patients with normal BP and HR recovery (16.0% and 18.2%, respectively, vs 7.5%; P<.05 for both comparisons) (1, 2). The RR of new‐onset CAD, after adjustment for covariates, was 1.72 (95% CI, 1.12–2.78; P=.012) and 1.96 (95% CI, 1.30–3.02; P=.009), respectively (Table III, Figure 3). Even higher incidence of CAD was found in patients positive for both exercise predictive measures (34.7%; P<.05 vs all of the former subgroups) (1, 2). The adjusted (for confounders) RR of these patients for developing CAD was 3.35 (95% CI, 2.62–5.57; P<.001) compared with patients with normal BP and HR recovery, 1.95 (95% CI, 1.28–2.98; P=.011) compared with patients with delayed BP and normal HR recovery and 1.71 (95% CI, 1.08–2.75; P=.014) compared with patients with normal BP and delayed HR recovery (Table III, Figure 3). As a predictor of CAD, an abnormal value for both BP and HR recovery had a sensitivity of 23%, a specificity of 94%, a positive predictive value (PPV) of 35%, a negative predictive value (NPV) of 89%, and an accuracy of 84%. At the same time, a delayed BP recovery had a sensitivity of 46%, a specificity of 75%, a PPV of 22%, a NPV of 90%, and an accuracy of 71%, whereas a delayed HR recovery had a sensitivity of 46%, a specificity of 78%, a PPV of 24%, a NPV of 90%, and an accuracy of 74%.
Figure 1.
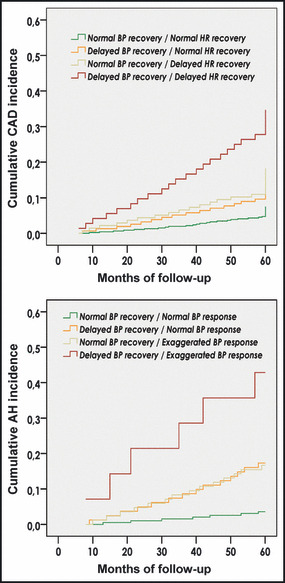
Kaplan‐Meier curves. Cumulative incidence of coronary artery disease (CAD) and arterial hypertension (AH) according to exercise blood pressure (BP) and heart rate (HR) (P<.05 between subgroups).
Figure 2.
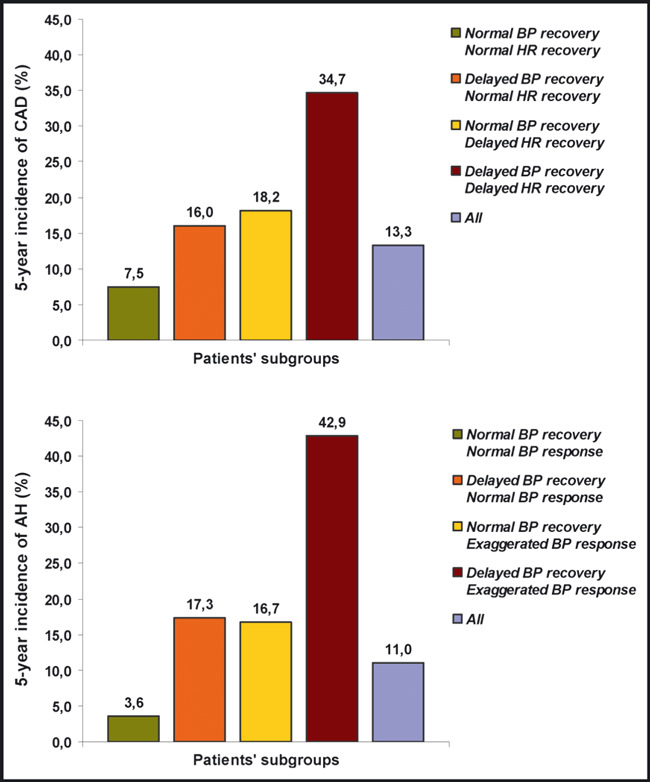
Incidence diagrams. Five‐year incidence of coronary artery disease (CAD) and arterial hypertension (AH) according to exercise blood pressure (BP) and heart rate (HR) (P<.05 between subgroups).
Figure 3.
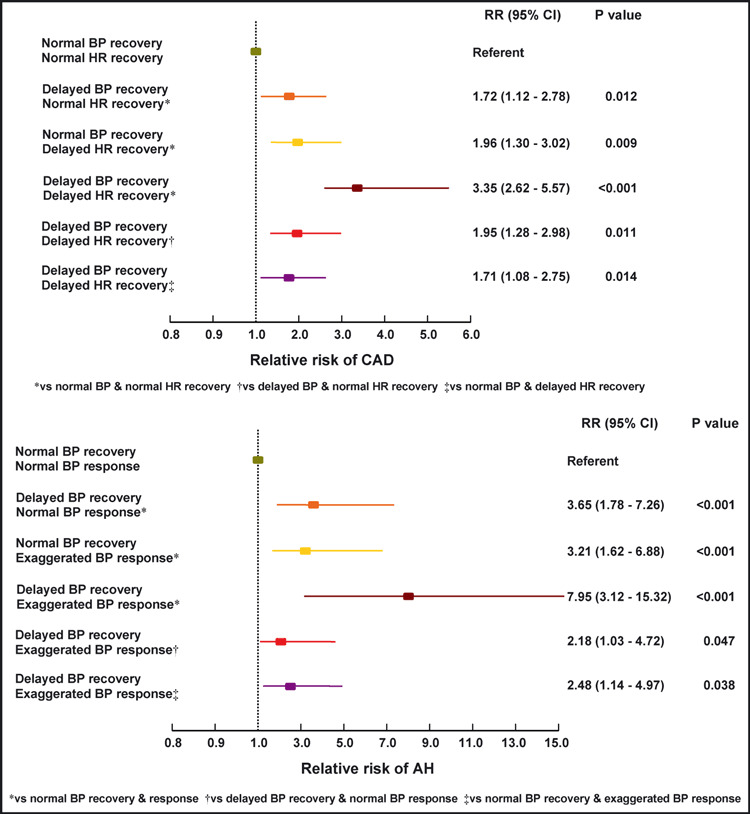
Relative risk (RR) diagrams. RR of coronary artery disease (CAD) (adjusted for age, sex, family history of CAD, obesity, smoking, arterial hypertension [AH], dyslipidemia, diabetes, medication, exercise duration, exercise‐induced angina, ST‐segment response, and QRS score) and RR of AH (adjusted for age, sex, family history of AH, obesity, smoking, and baseline resting blood pressure [BP]) according to exercise BP and heart rate (HR).
Clinical characteristics and exercise parameters (first ET) of patients with normal baseline resting BP (n=374) as well as differences between the BP response and recovery subgroups are presented in Table II. During follow‐up, 41 patients (11.0%) developed AH. Patients positive for 1 of the 2 non‐ECG prognostic markers (delayed BP recovery or exaggerated BP response) in the first ET were found with a higher cumulative and 5‐year incidence of AH compared with patients with normal BP recovery and response (17.3% and 16.7%, respectively, vs 3.6%; P<.001 for both comparisons) (1, 2). The RR of new‐onset AH, after adjustment for covariates, was 3.65 (95% CI, 1.78–7.26; P<.001) and 3.21 (95% CI, 1.62–6.88; P<.001), respectively (Table III, Figure 3). An even higher incidence of AH was found in patients with a cluster of both exercise predictors (42.9%; P<.05 vs every other subgroup) (1, 2). The adjusted (for confounders) RR of new‐onset AH in these individuals was 7.95 (95% CI, 3.12–15.32; P<.001) compared with patients with normal BP recovery and response, 2.18 (95% CI, 1.03–4.72; P=.047) compared with patients with delayed BP recovery and normal BP response and 2.48 (95% CI, 1.14–4.97; P=.038) compared with patients with normal BP recovery and exaggerated BP response (Table III, Figure 3). As a predictor of AH, an abnormal value for both BP response and recovery had a sensitivity of 15%, a specificity of 98%, a PPV of 43%, an NPV of 90%, and an accuracy of 89%. At the same time, a delayed BP recovery had a sensitivity of 49%, a specificity of 78%, a PPV of 21%, a NPV of 93%, and an accuracy of 74% whereas an exaggerated BP response had a sensitivity of 49%, a specificity of 77%, a PPV of 20%, a NPV of 92%, and an accuracy of 74%.
Table II.
Baseline Clinical Characteristics and Exercise Parameters of Normotensive Patients
| Exercise BP Recoverya and BP Responseb Subgroups | ||||||
|---|---|---|---|---|---|---|
| All (N=374) | Normal BP rec Normal BP resp (n=195) | Delayed BP rec Normal BP reps (n=81) | Normal BP rec Exaggerated BP resp (n=84) | Delayed BP rec Exaggerated BP resp (n=14) | P Value | |
| Clinical Characteristics | ||||||
| Age, y | 49.6±11.0 | 48.6±10.8 | 49.5±12.1 | 50.8±10.1 | 55.5±9.2 | .079 |
| Male sex, No. (%) | 254 (67.9) | 124 (63.6) | 52 (64.2) | 69 (82.1) | 9 (64.3) | .018 |
| Family history of AH, No. (%) | 65 (17.4) | 33 (16.9) | 14 (17.3) | 15 (17.9) | 3 (21.4) | .632 |
| BMI, kg/m2 | 26.1±3.3 | 25.9±3.4 | 26.2±3.2 | 26.5±3.0 | 25.8±3.0 | .469 |
| Smoking, No. (%) | 120 (32.1) | 59 (30.2) | 26 (32.1) | 31 (36.9) | 4 (28.6) | .735 |
| Dyslipidemia, No. (%) | 193 (51.6) | 99 (50.8) | 38 (46.9) | 48 (57.1) | 8 (57.1) | .578 |
| Diabetes mellitus, No. (%) | 7 (1.9) | 4 (2.1) | 1 (1.2) | 2 (2.4) | 0 (0.0) | .897 |
| Treatment: Statins, No. (%) | 80 (21.4) | 42 (21.5) | 8 (9.9) | 27 (32.1) | 3 (21.4) | .013 |
| Antiplatelet agents, No. (%) | 32 (8.6) | 16 (8.2) | 4 (4.9) | 9 (10.7) | 3 (21.4) | .240 |
| Exercise Parameters | ||||||
| Exercise duration, s | 584.3±145.9 | 589.5±130.8 | 545.0±142.8 | 614.5±165.7 | 557.3±194.0 | .016 |
| METs | 11.5±2.6 | 11.5±2.4 | 10.9±2.6 | 12.0±3.1 | 11.3±3.3 | .047 |
| Resting HR, bpm | 83.4±12.3 | 84.9±12.2 | 82.6±14.0 | 81.7±11.1 | 78.4±8.4 | .061 |
| Peak exercise HR, bpm | 166.2±16.4 | 168.0±15.9 | 162.5±19.3 | 165.0±13.6 | 169.0±17.3 | .057 |
| Achieved HR, % of predicted | 97.6±8.3 | 98.1±8.0 | 95.4±10.1 | 97.6±6.7 | 102.7±8.1 | .008 |
| Recovery HR, bpm | ||||||
| First minute | 133.6±18.1 | 135.5±17.3 | 131.5±22.4 | 131.0±15.5 | 134.1±14.4 | .183 |
| Second minute | 111.3±17.0 | 113.3±16.5 | 108.5±20.1 | 109.4±14.8 | 110.3±13.8 | .110 |
| Third minute | 102.8±15.4 | 104.8±15.6 | 99.3±17.8 | 101.4±12.1 | 103.1±11.4 | .044 |
| Resting SBP, mm Hg | 111.7±13.0 | 112.3±13.2 | 111.8±12.4 | 110.9±13.9 | 107.9±10.5 | .596 |
| Peak exercise SBP, mm Hg | 164.9±21.3 | 160.3±16.5 | 151.6±19.6 | 185.6±17.1 | 180.4±14.7 | <.001 |
| ΔSBP,c mm Hg | 53.2±17.3 | 48.1±10.9 | 39.8±14.2 | 74.7±9.5 | 72.5±8.5 | <.001 |
| Recovery SBP, mm Hg | ||||||
| First minute | 157.3±22.4 | 149.6±17.9 | 156.9±23.7 | 171.2±21.1 | 183.9±20.4 | <.001 |
| Second minute | 146.2±23.8 | 137.7±19.3 | 150.9±23.5 | 156.0±25.0 | 177.9±21.4 | <.001 |
| Third minute | 136.8±20.7 | 128.2±17.0 | 146.3±19.6 | 142.3±19.0 | 170.4±14.7 | <.001 |
| Resting RP product, bpm×mm Hg | 9326±1817 | 9534±1795 | 9246±2009 | 9066±1702 | 8445±1177 | .052 |
| Peak exercise RP product, bpm×mm Hg | 27,418±4458 | 26,963±3915 | 24,719±4653 | 30,573±3372 | 30,439±3629 | <.001 |
| Recovery RP product, bpm×mm Hg | ||||||
| First minute | 21,004±3666 | 20,258±3451 | 20,757±4993 | 22,355±3318 | 24,670±3818 | <.001 |
| Second minute | 16,289±3683 | 15,656±3385 | 16,462±4259 | 17,024±3325 | 19,684±3736 | <.001 |
| Third minute | 14,072±3014 | 13,458±2813 | 14,601±3540 | 14,403±2432 | 17,567±2513 | <.001 |
| Angina‐like symptoms, No. (%) | 7 (1.9) | 3 (1.5) | 2 (2.5) | 2 (2.4) | 0 (0.0) | .872 |
| Ischemic ST‐segment response, No. (%) | 28 (7.5) | 14 (7.2) | 6 (7.4) | 7 (8.3) | 1 (7.1) | .907 |
| QRS scored | 1.7±3.0 | 2.0±2.9 | 1.2±3.2 | 1.7±3.1 | 1.0±3.0 | .469 |
Abbreviations: AH, arterial hypertension; BP, blood pressure; bpm, beats per minute; BMI, body mass index; HR, heart rate; METs, metabolic equivalents (3.5 mL O2/kg/min); rec, recovery; resp, response; RP product, rate‐pressure product (HR×SBP); SBP, systolic blood pressure. aDelayed BP recovery: third minute of recovery SBP / peak exercise SBP ≥0.90. bExaggerated BP response: peak exercise SBP – resting SBP ≥65 mm Hg. cΔSBP: peak exercise SBP – resting SBP. dQRS score: (resting [R−Q−S] aVF+resting [R−Q−S] V5)−(peak exercise [R−Q−S] aVF+peak exercise [R−Q−S] V5).
Table III.
Relative risk of New‐Onset CAD and AH According to Exercise BPa and HRb
| Unadjusted RR | 95% CI | P Value | Adjusted RRc | 95% CI | P Value | |
|---|---|---|---|---|---|---|
| New‐onset CAD | ||||||
| Delayed BP and normal HR recoveryd | 2.13 | 1.32–3.44 | .002 | 1.72 | 1.12–2.78 | .012 |
| Normal BP and delayed HR recoveryd | 2.42 | 1.51–3.91 | .001 | 1.96 | 1.30–3.02 | .009 |
| Delayed BP and delayed HR recoveryd | 4.61 | 2.94–7.25 | <.001 | 3.35 | 2.62–5.57 | <.001 |
| Delayed BP and delayed HR recoverye | 2.17 | 1.34–3.50 | .002 | 1.95 | 1.28–2.98 | .011 |
| Delayed BP and delayed HR recoveryf | 1.90 | 1.18–3.06 | .008 | 1.71 | 1.08–2.75 | .014 |
| New‐onset AH | ||||||
| Delayed BP recovery and normal BP responseg | 4.81 | 2.02–11.49 | <.001 | 3.65 | 1.78–7.26 | <.001 |
| Normal BP recovery and exaggerated BP responseg | 4.64 | 1.95–11.11 | <.001 | 3.21 | 1.62–6.88 | <.001 |
| Delayed BP recovery and exaggerated BP responseg | 11.94 | 4.63–30.30 | <.001 | 7.95 | 3.12–15.32 | <.001 |
| Delayed BP recovery and exaggerated BP responseh | 2.48 | 1.15–5.35 | .038 | 2.18 | 1.03–4.72 | .047 |
| Delayed BP recovery and exaggerated BP responsei | 2.57 | 1.19–5.56 | .025 | 2.48 | 1.14–4.97 | .038 |
Abbreviations: AH, arterial hypertension; BP, blood pressure; CAD, coronary artery disease; CI, confidence interval; HR, heart rate; RR, relative risk; SBP, systolic blood pressure. aDelayed BP recovery: third minute of recovery SBP / peak exercise SBP ≥0.90. Exaggerated BP response: peak exercise SBP – resting SBP ≥65 mm Hg. bDelayed HR recovery: peak exercise HR – first minute of recovery HR ≤23 bpm. cConfounders for new‐onset CAD relative risk were age, sex, family history of CAD, obesity, smoking, arterial hypertension, dyslipidemia, diabetes, medication, exercise duration, exercise‐induced angina, ST‐segment response, and QRS score, whereas confounders for new‐onset AH relative risk were age, sex, family history of AH, obesity, smoking, and baseline resting BP. dCompared with normal BP and normal HR recovery. eCompared with delayed BP and normal HR recovery. fCompared with normal BP and delayed HR recovery. gCompared with normal BP recovery and normal BP response. hCompared with delayed BP recovery and normal BP response. iCompared with normal BP recovery and exaggerated BP response.
Discussion
This prospective study, which aimed to evaluate the prognostic value of non‐ECG exercise markers, indicates that patients with abnormally delayed BP and HR recovery after exercise are at a 3.35‐fold greater 5‐year risk for new‐onset CAD compared with individuals with normal BP and HR recovery after adjustment for traditional risk factors. Meanwhile, patients with delayed BP and normal HR recovery are at a 1.72‐fold greater risk whereas patients with delayed HR and normal BP recovery are at a 1.96‐fold greater risk compared with patients with normal BP and HR recovery. Thus, the combined criterion seems to be a much stronger predictor than its components: the additional value on the estimated risk of CAD (RR increase) is calculated at 95% compared with delayed BP and normal HR recovery and at 71% compared with delayed HR and normal BP recovery. It is noteworthy that the prevalence of established CAD predictors such as exercise‐induced ST‐segment deviation and exercise‐induced angina, which are incorporated in the Duke prognostic treadmill score, 20 is similar between the studied subgroups. Significant differences are noted only in the exercise duration, another standard predictor, 20 , 21 and the QRS score 21 (Table I), pointing to the emerging utility of the recently reported M‐score, which is a combination of exercise duration and QRS score. 21 Moreover, patients with exaggerated BP response to exercise and delayed BP decrease in the recovery are at a 7.95‐fold greater 5‐year risk for new‐onset AH compared with individuals with normal BP response and recovery after adjustment for covariates. At the same time, patients with delayed BP recovery and normal BP response are at a 3.65‐fold greater risk whereas patients with exaggerated BP response and normal BP recovery are at a 3.21‐fold greater risk compared with patients with normal BP response and recovery. Thereby, the combined criterion seems to be a much better predictor than its components: the additional value on the estimated risk of AH (RR increase) is 118% compared with delayed BP recovery and normal BP response and 148% compared with exaggerated BP response and normal BP recovery.
As often occurs with selected variables, the sensitivity of exercise HR and BP measures for adverse outcome is not very high in this study; however, similar values have been previously reported by others. 6 , 8 , 14 The notably further decreased sensitivity of the novel combined criteria is the expected inevitable cost for the parallel significant increase in specificity, PPV, and accuracy.
The Interplay Between the Non‐ECG Exercise Predictors
The mechanisms implicated between the non‐ECG exercise indices and the cardiovascular risk are not quite clear. Vagal reactivation is the principal determinant of the decrease in HR during recovery. 25 Slow HR recovery has been associated with several risk factors for atherosclerosis, 26 worsening degrees of CAD, 6 , 10 and mortality. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 HR recovery could identify patients with ischemia who might benefit from revascularization 27 whereas formal exercise training of cardiac patients improves HR recovery. 28
Patients with severe CAD develop LV dysfunction during exercise, which is rapidly ameliorated immediately post‐exercise, leading to cardiac output improvement and thus increase or delayed decrease in BP. 29 , 30 Peripheral vasoconstriction may also occur during exercise, as a compensatory response to ischemic‐induced LV dysfunction, and persist during recovery. Furthermore, a blunted decline in peripheral vascular resistance could explain the elevated recovery BP in individuals predisposed to hypertension. Autonomic dysregulation and subtle pathophysiological features have been described in the early stages of hypertension. 31
In patients with exaggerated BP response during exercise, peripheral resistance doesn’t adequately fall to compensate for the cardiac output rise. 32 This can be explained by a sympathetic hyper‐reactivity and an increased vascular response to adrenergic stimulation or by an arteriolar wall thickening that alters its responsiveness to vasoconstrictor stimuli. 33 Normotensive patients with exaggerated BP response present structural and functional heart changes that are observed in the early course of hypertension. 34
Based on the mechanisms previously discussed, the obvious link between the non‐ECG markers evaluated in this study is probably an impaired regulation of the autonomic nervous system function. The immediate post‐exercise period is associated with sympathetic tone withdrawal and a rebound vagal tone increase. 35 Autonomic control and vasoreactivity abnormalities during exercise could extend into the early recovery. Consequently, an exaggerated BP response to exercise could be followed by an elevated recovery BP and a delayed HR restoration.
Clinical Implications
The current premise underlining the use of ET in clinical practice is that by identifying patients likely to have CAD, and thus being at increased risk for premature events, aggressive preventive interventions can be initiated appropriately. 3 According to the results of the present study, the incorporation of BP response, BP recovery, and HR recovery in ET interpretation could improve the test ability to define high‐risk patients. The more parameters that are analyzed, the better the risk stratification. These indices are easily calculated from data already contained in a standard ET. Since treadmill test is a popular, inexpensive, widely available procedure, and given the health costs of AH and CAD and the importance of early detection of these disorders, treadmill ET may become an even more cost‐effective strategy. Although ET is not recommended for mass screening, if exercise HR and BP information is available, these data should be taken into account in patients’ clinical evaluation. However, the clinical interest would be greater if prognosis could be directly modified on the basis of these findings. Chen and colleagues report that exercise HR data may effectively guide appropriate patients’ selection for revascularization. 27 Franz and colleagues 36 showed that endurance training of hypertensive patients not only induced a significant fall in resting BP but also a marked reduction of exercise BP and HR, suggesting a beneficial effect on risk factors modification.
Limitations
There are potential limitations in this study. First, the methods and thresholds used for abnormal HR recovery, BP recovery, and BP response definition have not been adopted by all researchers; however, since definitions were not uniform in previous studies, the simplicity of defining values of the first quartile as abnormal HR recovery and of the fourth quartile as exaggerated BP response and abnormal BP recovery was preferred in the present study. Second, BP measurements obtained by arm‐cuff sphygmomanometer at peak exercise may be subject to a degree of error due to the patient's movement and the ambient noise; however, this method reflects real‐life practice. Third, coronary angiography—the gold standard for CAD diagnosis—was performed in only 41 patients (4.9%) at the baseline evaluation and in 116 patients (14.0%) during or at the end of follow‐up; the absence of CAD in the remaining patients was based on noninvasive methods. Nevertheless, it would probably be considered malpractice to perform an angiography in patients with normal ET, stress echocardiography, or thallium imaging testing.
Conclusions
Exercise ECG continues to have substantial diagnostic and prognostic value, especially when parameters beyond simple ST‐segment depression are considered. 3 Its additional predictive value is primarily based on HR and BP measures. This recent accumulated knowledge has transformed the standard ET report. 4 Instead of describing a test as “normal” or “abnormal,” typically centered on ST‐segment changes, a state‐of‐the‐art exercise report includes the major prognostic findings along with their implications. According to this study, the combination of established independent HR and BP predictors provides new stronger CAD and AH predictors with higher prognostic accuracy.
Disclosure: The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
References
- 1. Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guidelines for assessment of cardiovascular risk in asymptomatic adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–e636. [DOI] [PubMed] [Google Scholar]
- 2. Palatini P. Exercise testing in asymptomatic subjects: from diagnostic test to prognostic tool? Eur Heart J. 2008;29:1803–1806. [DOI] [PubMed] [Google Scholar]
- 3. Kligfield P, Lauer M. Exercise electrocardiogram testing: beyond the ST segment. Circulation. 2006;114:2070–2082. [DOI] [PubMed] [Google Scholar]
- 4. Lauer M, Froelicher ES, Williams M, Kligfield P. Exercise testing in asymptomatic adults: a statement for professionals from the American Heart Association Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention. Circulation. 2005;112:771–776. [DOI] [PubMed] [Google Scholar]
- 5. Kokkinos P, Myers J, Doumas M, et al. Heart rate recovery, exercise capacity, and mortality risk in male veterans. Eur J Prev Cardiol. 2012;19:177–184. [DOI] [PubMed] [Google Scholar]
- 6. Ghaffari S, Kazemi B, Aliakbarzadeh P. Abnormal heart rate recovery after exercise predicts coronary artery disease severity. Cardiol J. 2011;18:47–54. [PubMed] [Google Scholar]
- 7. Adabag AS, Grandits GA, Prineas RJ, et al. MRFIT Research Group . Relation of heart rate parameters during exercise test to sudden death and all‐cause mortality in asymptomatic men. Am J Cardiol. 2008;101:1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol. 2003;42:831–838. [DOI] [PubMed] [Google Scholar]
- 9. Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation. 2001;104:1911–1916. [PubMed] [Google Scholar]
- 10. Shetler K, Marcus R, Froelicher VF, et al. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001;38:1980–1987. [DOI] [PubMed] [Google Scholar]
- 11. Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart‐rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. [DOI] [PubMed] [Google Scholar]
- 12. Huang CL, Su TC, Chen WJ, et al. Usefulness of paradoxical systolic blood pressure increase after exercise as a predictor of cardiovascular mortality. Am J Cardiol. 2008;102:518–523. [DOI] [PubMed] [Google Scholar]
- 13. Laukkanen JA, Kurl S, Salonen R, et al. Systolic blood pressure during recovery from exercise and the risk of acute myocardial infarction in middle‐aged men. Hypertension. 2004;44:820–825. [DOI] [PubMed] [Google Scholar]
- 14. McHam SA, Marwick TH, Pashkow FJ, Lauer MS. Delayed systolic blood pressure recovery after graded exercise: an independent correlate of angiographic coronary disease. J Am Coll Cardiol. 1999;34:754–759. [DOI] [PubMed] [Google Scholar]
- 15. Tsuda M, Hatano K, Hayashi H, et al. Diagnostic value of postexercise systolic blood pressure response for detecting coronary artery disease in patients with or without hypertension. Am Heart J. 1993;125:718–725. [DOI] [PubMed] [Google Scholar]
- 16. Singh JP, Larson MG, Manolio TA, et al. Blood pressure response during treadmill testing as a risk factor for new‐onset hypertension: the Framingham Heart Study. Circulation. 1999;99:1831–1836. [DOI] [PubMed] [Google Scholar]
- 17. Miyai N, Arita M, Miyashita K, et al. Blood pressure response to heart rate during exercise test and risk of future hypertension. Hypertension. 2002;39:761–766. [DOI] [PubMed] [Google Scholar]
- 18. Sharabi Y, Ben‐Cnaan R, Hanin A, Martonovitch G, Grossman E. The significance of hypertensive response to exercise as a predictor of hypertension and cardiovascular disease. J Hum Hypertens. 2001;15:353–356. [DOI] [PubMed] [Google Scholar]
- 19. Miyai N, Arita M, Morioka I, et al. Exercise BP response in subjects with high‐normal BP: exaggerated blood pressure response to exercise and risk of future hypertension in subjects with high‐normal blood pressure. J Am Coll Cardiol. 2000;36:1626–1631. [DOI] [PubMed] [Google Scholar]
- 20. Gibbons RJ, Balady GJ, Beasley JW, et al. ACC/AHA guidelines for exercise testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol. 1997;30:260–311. [DOI] [PubMed] [Google Scholar]
- 21. Michaelides AP, Tousoulis D, Raftopoulos LG, et al. The impact of novel exercise criteria and indices for the diagnostic and prognostic ability of exercise testing. Int J Cardiol. 2010;143:119–123. [DOI] [PubMed] [Google Scholar]
- 22. Mancia G, DeBacker G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: the task force for the management of Arterial Hypertension of the European Society of Hypertension and of the European Society of Cardiology. J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 23. Palatini P. Blood pressure behaviour during physical activity. Sports Med. 1988;5:353–374. [DOI] [PubMed] [Google Scholar]
- 24. Bassertt DR, Duey WJ, Walker AJ, et al. Exaggerated blood pressure response to exercise: importance of resting blood pressure. Clin Physiol. 1998;18:457–462. [DOI] [PubMed] [Google Scholar]
- 25. Imai K, Sato H, Hori M, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–1535. [DOI] [PubMed] [Google Scholar]
- 26. Kizilbash MA, Carnethon MR, Chan C, et al. The temporal relationship between heart rate recovery immediately after exercise and the metabolic syndrome: the CARDIA Study. Eur Heart J. 2006;27:1592–1596. [DOI] [PubMed] [Google Scholar]
- 27. Chen MS, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery and impact of myocardial revascularization on long‐term mortality. Circulation. 2004;110:2851–2857. [DOI] [PubMed] [Google Scholar]
- 28. Hao SC, Chai A, Kligfield P. Heart rate recovery response to symptom‐limited treadmill exercise after cardiac rehabilitation in patients with coronary artery disease with and without recent events. Am J Cardiol. 2002;90:763–765. [DOI] [PubMed] [Google Scholar]
- 29. Miyahara T, Yokota M, Iwase M, et al. Mechanisms of abnormal postexercise systolic blood pressure response and its diagnostic value in patients with coronary artery disease. Am Heart J. 1990;120:40–49. [DOI] [PubMed] [Google Scholar]
- 30. Rozanski A, Elkayam U, Berman DS, et al. Improvement of resting myocardial asynergy with cessation of upright bicycle exercise. Circulation. 1983;67:529–535. [DOI] [PubMed] [Google Scholar]
- 31. Julius S. Abnormalities of autonomic nervous control in human hypertension. Cardiovasc Drugs Ther. 1994;8:11–20. [DOI] [PubMed] [Google Scholar]
- 32. Wilson MF, Sung BH, Pincomb GA, Lovallo WR. Exaggerated pressure response to exercise in men at risk for systemic hypertension. Am J Cardiol. 1990;66:731–736. [DOI] [PubMed] [Google Scholar]
- 33. Kavey RW, Kveselis DA, Gaum WE. Exaggerated blood pressure response to exercise in children with increased low‐density lipoprotein cholesterol. Am Heart J. 1997;133:162–168. [DOI] [PubMed] [Google Scholar]
- 34. Gottdiener JS, Brown J, Zoltick J, Fletcher RD. Left ventricular hypertrophy in men with normal blood pressure: relation to exaggerated blood pressure response to exercise. Ann Int Med. 1990;112:161–166. [DOI] [PubMed] [Google Scholar]
- 35. Arai Y, Saul JP, Albrecht P, et al. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol. 1989;256:H132–H141. [DOI] [PubMed] [Google Scholar]
- 36. Franz I‐W. Ergometry in Hypertensive Patients: Implications for Diagnosis and Treatment. Berlin, New York: Springer‐Verlag; 1986:113. [Google Scholar]


