Abstract
The relationships between home blood pressure (BP), masked hypertension defined by home BP, and integrated flow‐mediated vasodilation (FMD) response remain unclear. The authors enrolled 257 patients (mean age, 63.5 years; 51% men) who had at least one cardiovascular risk factor. FMD of the brachial artery was measured with a semiautomatic edge‐detection algorithm. The integrated FMD response was calculated as the area under the dilation curve during 120 seconds after deflation (FMD‐AUC 120) and the FMD magnitude as the percentage change in peak diameter (ΔFMD). Masked hypertension was defined by office BP <140/90 mm Hg and home BP ≥135 mm Hg and/or 85 mm Hg. Home systolic BP was inversely correlated with FMD‐AUC 120 and ΔFMD (FMD‐AUC 120: r=−.23, P<.001; ΔFMD: r=−.13, P=.041), and office systolic BP was inversely associated with FMD‐AUC 120 (r=−.16, P=.011), but not with ΔFMD. After adjusting for covariates, home systolic BP (β=−.27, P=.003), but not office BP, was inversely associated with FMD‐AUC 120, whereas ΔFMD was not associated with office or home systolic BP. FMD‐AUC 120 was significantly lower in patients with masked hypertension compared with those with normotension (7.7±6.7 vs 11.5±8.8 mm × s, P=.048). Home systolic BP and masked hypertension defined by home BP were associated with a decrease in FMD‐AUC 120.
In recent years, the importance of out‐of‐office blood pressure (BP) measurement has been widely known,1, 2, 3, 4 and many reports have shown that self‐measured home BP could predict cardiovascular (CV) events better than office BP.5, 6, 7 When guided by only office BP, control of BP might be insufficient to prevent CV events.8 Masked hypertension defined by normal office BP and high ambulatory BP has been associated with CV events.9, 10 On the other hand, masked hypertension defined by normal office BP and high home BP has also been shown to be associated with an increased risk of CV events,11, 12, 13 hypertensive organ damage, and atherosclerosis.14, 15 Consequently, home BP has been established as the determinant of masked hypertension.16
A decrease in flow‐mediated vasodilation (FMD) has been shown to predict CV events17 and hypertensive organ damage.18 FMD analyses have been widely performed, and a decrease of FMD has been shown to reflect endothelial dysfunction.19, 20, 21, 22 Two studies showed an association between a decrease in FMD and masked hypertension defined by normal office BP and high ambulatory BP.23, 24
To date, the integrated FMD response has been measured by time‐course analysis using continuous measurement in addition to the traditional FMD magnitude, which was calculated as the percentage change in peak diameter from the resting baseline diameter. Although there have also been some reports on the association between CV risk and the integrated FMD response,25, 26 previous reports did not investigate the association between masked hypertension and the integrated FMD response. Thus, the association between masked hypertension defined by home BP and the integrated FMD response remains unclear.
In this study, we hypothesized that home BP and masked hypertension defined by home BP would be associated with integrated FMD response in patients with a CV risk factor.
Methods
Study Participants
This study was performed as part of the Japan Morning Surge–Home Blood Pressure (J‐HOP) study.27 The protocol of the J‐HOP study has been registered at the University Hospital Medical Information Network Clinical Trials Registry Web site under the trial identifier UMIN000000894. Briefly, the J‐HOP study is a prospective observational evaluation of predictive values of home BP for CV events in Japanese outpatients with any of the following CV risk factors at the clinic or hospital: hypertension, diabetes mellitus, hyperlipidemia, smokers (including chronic obstructive pulmonary disease), chronic renal disease, atrial fibrillation, metabolic syndrome, and sleep apnea syndrome.
Hypertension was defined by office systolic BP of at least 140 mm Hg, diastolic BP of at least 90 mm Hg, or both, or as current treatment with antihypertensive medication. Hyperlipidemia was defined by a total cholesterol level >240 mg/dL or by current treatment with an oral lipid‐lowering agent. Diabetes mellitus was defined by a fasting glucose level >126 mg/dL, a random nonfasting glucose level >200 mg/dL, or current treatment with an oral hypoglycemic agent or insulin. Smoking was defined by a current smoking habit, as reported previously.27 A regular drinker was defined as someone who drank alcohol at least 5 days per week.
BP Measurements
Office BP was measured by a nurse using a validated upper‐arm cuff oscillometric BP device (HEM‐5001; Omron, Kyoto, Japan)28 with the patient seated after 5 minutes of rest. Three office BP and pulse rate readings were taken at 15‐second intervals, and the values of office BP and pulse rate were defined by the average of 6 readings from 2 clinic visits.
Self‐measured home BP was also obtained by a validated upper‐arm cuff oscillometric device (HEM‐5001; Omron, Kyoto, Japan).28 Home BP was measured on the nondominant upper arm with the patient having remained seated after 2 minutes of rest.29 The home BP monitoring device automatically took 3 readings at 15‐second intervals on each occasion,30 and the data were stored in the monitor's memory. The rapid method (15‐second intervals between BP measurements) was shown to be as accurate as a 1‐minute interval.30 Morning BP was measured within 1 hour after waking, after urination, and before breakfast and the taking of antihypertensive medication. Evening BP was measured immediately before bedtime. Patients were instructed to avoid measuring BP just after taking a bath, drinking alcohol, or smoking. The BP data were downloaded to a computer by the physician or nurses at the time of each clinic visit. Home BP was defined by an average of morning and evening BP during the course of 2 weeks.
We defined normotension by office BP <140/90 mm Hg and home BP <135/85 mm Hg, white‐coat hypertension by office BP ≥140 mm Hg and/or 90 mm Hg and home BP<135/85 mm Hg, masked hypertension by office BP <140/90 mm Hg and home BP ≥135 mm Hg and/or 85 mm Hg, and sustained hypertension by office BP ≥140 mm Hg and/or 90 mm Hg and home BP ≥135 mm Hg and/or 85 mm Hg.
Flow‐Mediated Vasodilation
The details of FMD measurement have been published elsewhere.26 Participants were examined after 15 minutes of rest in the fasting state (ie, after 12‐hour fasting and not taking any medications), and patients were instructed to avoid exercise for at least 4 to 6 hours prior to the FMD examination.21 The brachial artery was imaged using an instrument equipped with software for monitoring the brachial artery diameter. This system was equipped with a 10‐MHz linear array transducer and a novel stereotactic probe‐holding device (UNEX EF 18G; Unex Co, Ltd, Nagoya, Japan).31 After baseline images of the brachial artery were recorded, occlusion of the forearm blood flow was initiated and maintained for a period of 5 minutes by a cuff inflated to 50 mm Hg above the participant's systolic BP. Images of the brachial artery were recorded for a 2‐minute duration after cuff deflation.21 All FMD measurements were obtained by a physician (TK) blinded to the clinical data.
FMD Time Course Analysis
As reported previously,26 the FMD magnitude (ΔFMD) was calculated as the percentage change in peak diameter from the resting baseline diameter, and the integrated FMD response (FMD‐AUC120) was calculated as the area under the dilation curve during the 120‐second dilation period after cuff deflation (Figure 1). In addition, we investigated the FMD magnitude in the percentage change at 90 seconds and 120 seconds from the resting baseline diameter (FMD at 90 seconds and 120 seconds) to examine the dilation reaction after its peak.
Figure 1.
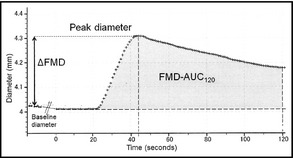
Time course analysis of flow‐mediated vasodilation (FMD). ΔFMD, FMD magnitude in percentage change in peak diameter from baseline; FMD‐AUC 120, integrated FMD response calculated as the area under the dilation curve during the 120‐second dilation period after cuff deflation.
Blood Samples
Blood samples were drawn from the antecubital vein in a fasting state at the second clinic visit. We measured the levels of total cholesterol, triglycerides, fasting glucose, and hemoglobin A1c (HbA1c). All blood samples were measured at a single laboratory (SRL Inc, Tokyo, Japan).
Statistical Analysis
Data are shown as the mean±standard deviation or as a percentage. The distribution of all variables was examined using the Kolmogorov‐Smirnov test of normality. To approximate normal distribution, natural log‐transformed values for triglycerides, fasting glucose, and HbA1c were used in the analysis. The associations among the continuous clinical parameters, FMD‐AUC120 and ΔFMD, were assessed using Pearson's correlation coefficient. The χ2 test was used for categoric data. Unpaired t tests were used for normally distributed data and for comparisons between two groups, and analysis of variance was used for comparisons of more than two groups. The results of multiple linear regression analysis to assess the independent associations between office BP, home systolic BP, and FMD‐AUC120 and ΔFMD were adjusted for age and sex. Variables with P<.05 in univariate analysis were included in the model. The office systolic BP and home systolic BP were included together in the same model because of the low degree of multicollinearity (the variance inflation factor was 1.65 to 1.70 when both variables were included in the same model). Bonferroni's analysis was used for comparisons of FMD‐AUC120 and ΔFMD among subgroups defined by office and home BP. The differences in FMD‐AUC120 and ΔFMD in masked hypertension and normotension were assessed by analysis of covariance with adjustment for age, sex, diabetes, hyperlipidemia, smoking status, and HbA1c, followed by the Bonferroni post hoc test. Computer software (SPSS version 16.0; SPSS Inc, Chicago, IL) was used for the analyses. A probability value <.05 was considered statistically significant.
Results
The clinical characteristics of the patients are shown in Table 1. The average age was 63.5±11.4 years and 51% of participants were men.
Table 1.
Patient Characteristics
| Parameter | Value |
|---|---|
| Patients, No. | 257 |
| Age, y | 63.5±11.4 |
| Men, % | 51 |
| Body mass index, kg/m2 | 24.4±3.7 |
| Current smoker, % | 11 |
| Regular drinker, % | 20 |
| Hyperlipidemia, % | 33 |
| Diabetes mellitus, % | 23 |
| Antihypertensive therapy, % | 76 |
| Antihypertensive medication, No. | 1.6±1.3 |
| Office systolic blood pressure, mm Hg | 138±16 |
| Office diastolic blood pressure, mm Hg | 80±12 |
| Office pulse rate, beats per min | 72±12 |
| Home systolic blood pressure, mm Hg | 135±16 |
| Home diastolic blood pressure, mm Hg | 77±12 |
| Home pulse rate, beats per min | 67±9 |
| Total cholesterol, mg/dL | 201±33 |
| Triglyceride, mg/dL | 130±84 |
| Fasting glucose, mg/dL | 110±27 |
| Hemoglobin A1c, % | 5.5±.8 |
| Brachial artery parameters | |
| FMD‐AUC120, mm × s | 9.4±7.4 |
| ΔFMD, % | 5.1±4.6 |
Values are expressed as mean±standard deviation or percentage of patients. Abbreviations: ΔFMD, flow‐mediated vasodilation (FMD) magnitude in percentage change in peak diameter from baseline; FMD‐AUC120, integrated FMD response calculated as the area under the dilation curve during the 120‐second dilation period after cuff deflation.
FMD‐AUC120 and ΔFMD values were similar between men and women, smokers and nonsmokers, drinkers and nondrinkers, and patients with and without hyperlipidemia, diabetes, antihypertensive medication use, and statin use (Table 2). We analyzed BP levels between patients who were taking antihypertensive medication and those who were not. Office systolic/diastolic BP was 138±16/83±13 mm Hg in patients taking antihypertensive medication (n=61) and 138±16/80±12 mm Hg in patients not taking antihypertensive medication (n=196). Home systolic/diastolic BP was 134±16/77±12 mm Hg in patients taking antihypertensive medication and 136±15/76±11 mm Hg in patients not taking antihypertensive medication. The differences in office and home systolic/diastolic BPs between the two groups were not significant.
Table 2.
Clinical Patient Characteristics and FMD Parameters
| Covariates | FMD AUC120, mm × s | P Value | ΔFMD, % | P Value |
|---|---|---|---|---|
| Sex | ||||
| Male (n=131) | 9.3±6.9 | .799 | 4.9±4.1 | .435 |
| Female (n=126) | 9.5±8.0 | 5.3±5.1 | ||
| Current smoker | ||||
| Yes (n=29) | 11.0±7.2 | .205 | 5.5±3.8 | .536 |
| No (n=228) | 9.2±7.5 | 4.9±4.6 | ||
| Regular drinker | ||||
| Yes (n=52) | 11.0±6.7 | .085 | 4.7±4.1 | .388 |
| No (n=205) | 9.0±7.6 | 5.2±4.8 | ||
| Hyperlipidemia | ||||
| Present (n=85) | 10.2±8.9 | .229 | 5.6±4.4 | .097 |
| Absent (n=172) | 9.0±6.6 | 4.7±4.7 | ||
| Diabetes mellitus | ||||
| Present (n=58) | 10.1±8.7 | .413 | 5.6±4.1 | .327 |
| Absent (n=199) | 9.2±7.0 | 4.9±4.7 | ||
| Antihypertensive medication | ||||
| Present (n=196) | 9.0±6.9 | .131 | 4.9±4.2 | .179 |
| Absent (n=61) | 10.7±8.8 | 5.8±5.7 | ||
| Statin use | ||||
| Present (n=64) | 10.2±8.9 | .323 | 5.5±4.1 | .414 |
| Absent (n=193) | 9.1±6.9 | 5.0±4.8 | ||
Values are expressed as mean±standard deviation or percentage of patients. Abbreviations: ΔFMD, flow‐mediated vasodilation (FMD) magnitude in percentage change in peak diameter from baseline; FMD‐AUC120, integrated FMD response calculated as the area under the dilation curve during the 120‐second dilation period after cuff deflation.
BP and FMD‐AUC120
Home systolic BP and office systolic BP were significantly inversely correlated with FMD‐AUC120 (home systolic BP: r=−.23, P<.001; office systolic BP: r=−.16, P=.011; Table 3). Home systolic BP was associated with ΔFMD, but office systolic BP was not associated with ΔFMD (Table 3). Office or home diastolic BP or pulse rate was not associated with FMD‐AUC120 or with ΔFMD (Table 3).
Table 3.
Univariate and Multivariate Regression Analysis of FMD‐AUC 120 and ΔFMD
| FMD‐AUC120 | ΔFMD | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Univariate | Multivariate | Univariate | Multivariate | ||||
| r | P Value | β | P Value | r | P Value | β | P Value | |
| Age | −.20 | .001 | −.17 | .014 | −.09 | .132 | −.06 | .435 |
| Body mass index | −.03 | .640 | – | – | −.08 | .182 | – | – |
| Office systolic blood pressure | −.16 | .011 | .01 | .921 | −.07 | .249 | −.04 | .664 |
| Office diastolic blood pressure | −.02 | .570 | – | – | .02 | .725 | – | – |
| Office pulse rate | −.02 | .805 | – | – | .02 | .793 | – | – |
| Home systolic blood pressure | −.23 | <.001 | −.27 | .003 | −.13 | .041 | −.06 | .513 |
| Home diastolic blood pressure | −.06 | .350 | – | – | −.02 | .780 | – | – |
| Home pulse rate | −.02 | .802 | – | – | −.01 | .906 | – | – |
| Total cholesterol | .07 | .282 | – | – | .02 | .721 | – | – |
| Log triglyceride | −.05 | .432 | – | – | −.02 | .703 | – | – |
| Log fasting glucose | −.08 | .184 | – | – | .00 | .984 | – | – |
| Log hemoglobin A1c | .00 | .999 | – | – | .05 | .427 | – | – |
Multiple linear regression analysis was performed to determine the variables with independent significant association with flow‐mediated vasodilation (FMD) response calculated as the area under the dilation curve during 120 seconds after deflation (FMD‐AUC120). Age, sex, office and home systolic blood pressure, and variables with P<.05 in univariate analysis were included in the model (calcium channel blocker use).
Multivariate logistic regression analysis including age, sex, antihypertensive medication, and both office and home systolic BP revealed that home systolic BP was independently associated with FMD‐AUC120 (β=−.27, P=.003), but was not independently associated with ΔFMD (Table 3).
Masked Hypertension and FMD‐AUC120
Table 4 shows patient characteristics for comparison among subgroups of office and home BP.
Table 4.
Comparison of Subgroups Defined by Office and Home Blood Pressure
| Covariate | Normotension (n=90) | White‐Coat Hypertension (n=41) | Masked Hypertension (n=41) | Sustained Hypertension (n=85) | P Value |
|---|---|---|---|---|---|
| Age, y | 63.7±10.2 | 63.9±12.6 | 65.3±9.6 | 62.4±12.9 | .606 |
| Men, % | 47 | 59 | 44 | 55 | .386 |
| Body mass index, kg/m2 | 23.9±3.2 | 24.3±3.4 | 24.8±3.6 | 24.9±3.4 | .238 |
| Current smoker, % | 6.7 | 9.8 | 15 | 15 | .282 |
| Regular drinker, % | 17 | 27 | 24 | 19 | .505 |
| Antihypertensive medication, % | 73 | 71 | 80 | 76 | .793 |
| Hyperlipidemia, % | 46 | 17a | 32 | 27 | .006 |
| Diabetes mellitus, % | 26 | 12 | 34 | 19 | .078 |
| Office systolic blood pressure, mm Hg | 124±10 | 147±6b | 130±7b,c | 152±12b,d | <.001 |
| Office diastolic blood pressure, mm Hg | 74±8 | 83±8b | 75±9e | 88±13b,d | <.001 |
| Office pulse rate, beats per min | 72±10 | 74±13 | 69±13 | 74±13 | .211 |
| Home systolic blood pressure, mm Hg | 122±9 | 128±6f | 144±9b,c | 148±14b,c | <.001 |
| Home diastolic blood pressure, mm Hg | 71±8 | 72±7 | 79±11b,g | 84±13b,c | <.001 |
| Home pulse rate, beats per min | 66±9 | 67±9 | 67±10 | 69±10 | .467 |
| Total cholesterol, mg/dL | 196±33 | 202±31 | 198±33 | 208±32 | .100 |
| Triglycerides, mg/dL | 130±86 | 117±75 | 131±69 | 137±91 | .667 |
| Fasting glucose, mg/dL | 108±26 | 107±14 | 116±31 | 109±31 | .318 |
| Hemoglobin A1c, % | 5.5±.8 | 5.3±.6 | 5.9±1.0e,f | 5.5±.8 | .007 |
a P<.01. b P<.001 vs normotension. c P<.001 vs white‐coat hypertension. d P<.001 vs masked hypertension. e P<.01 vs. white‐coat hypertension. f P<.05 vs. normotension. g P<.05 vs. white‐coat hypertension by Bonferroni's analysis. [Correction added after online publication 25‐Jun‐2013: The P value information has been updated.]
Adjusting for age, sex, diabetes, hyperlipidemia, smoking status, and HbA1c, FMD‐AUC120 was significantly lower in patients with masked hypertension than in those with normotension (7.7±6.7 vs 11.5±8.8 mm × s, P=.048; Figure 2, Panel A). Meanwhile, ΔFMD in patients with masked hypertension did not significantly differ from that in patients with normotension (4.5%±4.2% vs 5.7%±4.8%, P=1.00; Figure 2, Panel B).
Figure 2.
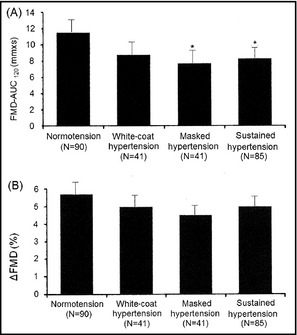
Integrated flow‐mediated vasodilation (FMD) response calculated as the area under the dilation curve during the 120‐second dilation period after cuff deflation (FMD‐AUC 120) and FMD magnitude in percentage change in peak diameter from baseline (ΔFMD) in patients with normotension, white‐coat hypertension, masked hypertension, and sustained hypertension. *P<.05 vs normotension by Bonferroni's analysis.
We showed two typical cases (Figure 3, Panels A and B). Panel A shows high FMD‐AUC120 and ΔFMD in a patient with normotension, and panel B shows reduced FMD‐AUC120 and ΔFMD in a patient with masked hypertension.
Figure 3.
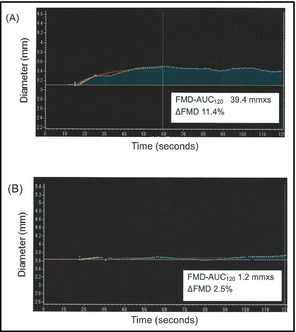
Two cases of real‐time semiautomatic analysis of integrated flow‐mediated vasodilation (FMD) response calculated as the area under the dilation curve during the 120‐second dilation period after cuff deflation (FMD‐AUC 120). (A) A 52‐year‐old woman. Office blood pressure 122/72 mm Hg, home blood pressure 101/59 mm Hg, hyperlipidemia (+), diabetes mellitus (−), nonsmoker. (B) A 66‐year‐old woman. Office blood pressure 122/68 mm Hg, home blood pressure 167/81 mm Hg, hyperlipidemia (+), diabetes mellitus (+), nonsmoker. ΔFMD indicates FMD magnitude in percentage change in peak diameter from baseline.
Dilation Reaction After Its Peak and the Time in Seconds Needed to Achieve Peak Diameter After Cuff Release
Office and home systolic BP were significantly associated with FMD at 90 seconds (office systolic BP: r=−.14, P=.025; home systolic BP: r=−.20, P=.001), and home systolic BP, but not office systolic BP, was associated with FMD at 120 seconds (home systolic BP: r=−.18, P=.004; office systolic BP: r=−.11, P=.08).
Discussion
The major findings of the present study were (1) home systolic BP was associated with FMD‐AUC120 (a new measure of FMD) independently of office systolic BP, and (2) masked hypertension defined by normal office BP and high home BP was significantly associated with FMD‐AUC120 in patients with CV risk.
To the best of our knowledge, there are no data on the association between home systolic BP, masked hypertension defined by home BP, and a decrease in FMD. In this study, home systolic BP, but not office BP, was associated with a decrease in FMD‐AUC120. It has been shown that left ventricular mass and carotid wall thickening are associated with a decrease in FMD.32, 33 Left ventricular hypertrophy and carotid wall thickening have been associated with home BP in earlier studies,14, 34, 35 and the significant association between home BP and FMD‐AUC120 observed in this study is consistent with the findings in these previous reports. Endothelial dysfunction evaluated by FMD is a manifestation of the early stage of atherosclerosis,36 and might precede left ventricular hypertrophy and carotid wall thickening. There have been few papers on the use of antihypertensive therapy to reduce home BP and hypertensive organ damage.37 Further studies will be needed to investigate the usefulness of FMD‐AUC120 as a marker of hypertensive organ damage, and whether FMD‐AUC120 can be increased by a reduction in home BP.
Home systolic BP was significantly associated with FMD‐AUC120 after adjustment for covariates. Both home systolic BP and masked hypertension were significantly associated with FMD‐AUC120, but not with ΔFMD. This might be because FMD‐AUC120 was an integral value that included the dilation reaction after its peak. The time in seconds needed to achieve peak diameter was 71±25 seconds, and home systolic BP was associated with FMD at 90 and 120 seconds. The dilation reaction after its peak might be associated with CV risk factors,25 and further studies will be needed to clarify the association between CV risk factors and the dilation reaction after its peak.
In this study, masked hypertension defined by normal office BP and high home BP was significantly associated with a decrease in FMD‐AUC120. Masked hypertension defined by normal office BP and high home BP has been shown to be associated with an increased risk of CV events,11, 12, 13 hypertensive organ damage, and atherosclerosis.14, 15 The association between decreased FMD and masked hypertension defined by normal office BP and high home BP is also consistent with the findings of these previous reports. Although those studies showed an association between decreased FMD and masked hypertension defined by normal office BP and high ambulatory BP,23, 24 there is no evidence of an association between a decrease in FMD and masked hypertension defined by normal office BP and high home BP.
As reported previously,26 FMD‐AUC120 was associated with CV risk in patients at risk for experiencing CV events. In addition, it was established that ΔFMD is useful for predicting the prognosis in patients at low CV risk, but it might not be as useful in patients at high CV risk38 or in patients 50 years or older.30 In this study, we enrolled relatively older and higher‐risk patients. Instead of the traditional FMD magnitude, FMD‐AUC120 might be a useful marker of hypertensive organ damage in patients with a CV risk factor. We have already shown a positive association between the CV risk score and FMD‐AUC120 26. Further studies will be needed to clarify the association between FMD‐AUC120, CV risk factors, and subsequent CV events.
Limitations
This study has some limitations. First, the associations between FMD‐AUC120 and risk factors (hyperlipidemia and diabetes mellitus) other than hypertension were not significant in this study. We recruited patients with CV risk; in particular, most of them were hypertensive. The reason why BP strongly affected FMD‐AUC120 might be the high prevalence of hypertension, and the contribution of CV risk factors other than hypertension (hyperlipidemia and diabetes mellitus) might become weak. Population‐based studies including patients of different ethnic groups will be needed. Secondly, this study was cross‐sectional,26 and the causal relationship was unclear. Finally, we did not measure nitroglycerine‐mediated dilation and therefore could not compare FMD and nitric oxide–independent vasodilation.
Conclusions
Home systolic BP was associated with a decrease in FMD‐AUC120. In particular, FMD‐AUC120 was significantly lower in patients with masked hypertension defined by home BP than in those with normotension. FMD‐AUC120 well reflected home systolic BP rather than office systolic BP.
Disclosures
There are no conflicts of interest to disclose.
Acknowledgments
We thank the other investigators, the staff, and the participants of the J‐HOP study for their valuable contributions.
J Clin Hypertens (Greenwich). 2013;15:630–636. ©2013 Wiley Periodicals, Inc.24034655
References
- 1. Pickering TG, White WB, Giles TD, et al. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens. 2010;4:56–61. [DOI] [PubMed] [Google Scholar]
- 2. Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. [DOI] [PubMed] [Google Scholar]
- 3. Kario K, Matsuo T, Kobayashi H, et al. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27:130–135. [DOI] [PubMed] [Google Scholar]
- 4. Kario K, Pickering TG, Matsuo T, et al. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. [DOI] [PubMed] [Google Scholar]
- 5. Ohkubo T, Imai Y, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population‐based observation in Ohasama, Japan. J Hypertens. 1998;16:971–975. [DOI] [PubMed] [Google Scholar]
- 6. Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow‐up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. [DOI] [PubMed] [Google Scholar]
- 7. Niiranen TJ, Hänninen MR, Johansson J, et al. Home‐measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn‐Home study. Hypertension. 2010;55:1346–1351. [DOI] [PubMed] [Google Scholar]
- 8. Stergiou GS, Bliziotis IA. Home blood pressure monitoring in the diagnosis and treatment of hypertension: a systematic review. Am J Hypertens. 2011;24:123–134. [DOI] [PubMed] [Google Scholar]
- 9. Björklund K, Lind L, Zethelius B, et al. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation. 2003;1:107. [DOI] [PubMed] [Google Scholar]
- 10. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white‐coat” hypertension detected by 24‐h ambulatory blood pressure monitoring 10‐year follow‐up from the Ohasama study. J Am Coll Cardiol. 2005;46:508–515. [DOI] [PubMed] [Google Scholar]
- 11. Bobrie G, Chatellier G, Genes N, et al. Cardiovascular prognosis of “masked hypertension” detected by blood pressure self‐measurement in elderly treated hypertensive patients. JAMA. 2004;291:1342–1349. [DOI] [PubMed] [Google Scholar]
- 12. Mancia G, Facchetti R, Bombelli M, et al. Long‐term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension. 2006;47:846–853. [DOI] [PubMed] [Google Scholar]
- 13. Pickering TG, Eguchi K, Kario K. Masked hypertension: a review. Hypertens Res. 2007;30:479–488. [DOI] [PubMed] [Google Scholar]
- 14. Matsui Y, Eguchi K, Ishikawa J, et al. Subclinical arterial damage in untreated masked hypertensive subjects detected by home blood pressure measurement. Am J Hypertens. 2007;20:385–391. [DOI] [PubMed] [Google Scholar]
- 15. Hara A, Ohkubo T, Kikuya M, et al. Detection of carotid atherosclerosis in individuals with masked hypertension and white‐coat hypertension by self‐measured blood pressure at home: the Ohasama study. J Hypertens. 2007;25:321–327. [DOI] [PubMed] [Google Scholar]
- 16. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:10–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inoue T, Matsuoka H, Higashi Y, et al. Flow‐mediated vasodilation as a diagnostic modality for vascular failure. Hypertens Res. 2008;31:2105–2113. [DOI] [PubMed] [Google Scholar]
- 18. Korkmaz H, Onalan O. Evaluation of endothelial dysfunction: flow‐mediated dilation. Endothelium. 2008;15:157–163. [DOI] [PubMed] [Google Scholar]
- 19. Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 20. Moens AL, Goovaerts I, Claeys MJ, et al. Flow‐mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest. 2005;127:2254–2263. [DOI] [PubMed] [Google Scholar]
- 21. Muiesan ML, Salvetti M, Paini A, et al. Prognostic role of flow‐mediated dilatation of the brachial artery in hypertensive patients. J Hypertens. 2008;26:1612–1618. [DOI] [PubMed] [Google Scholar]
- 22. Xu JZ, Zhang Y, Wu SN, et al. Impaired endothelial function in hypertensive patients with target organ damage. J Hum Hypertens. 2009;23:751–757. [DOI] [PubMed] [Google Scholar]
- 23. Veerabhadrappa P, Diaz KM, Feairheller DL, et al. Endothelial‐dependent flow‐mediated dilation in African Americans with masked‐hypertension. Am J Hypertens. 2011;24:1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takeno K, Mita T, Nakayama S, et al. Masked hypertension, endothelial dysfunction, and arterial stiffness in type 2 diabetes mellitus: a pilot study. Am J Hypertens. 2012;25:165–170. [DOI] [PubMed] [Google Scholar]
- 25. Donald AE, Halcox JP, Charakida M, et al. Methodological approaches to optimize reproducibility and power in clinical studies of flow‐mediated dilation. J Am Coll Cardiol. 2008;51:1959–1964. [DOI] [PubMed] [Google Scholar]
- 26. Kabutoya T, Hoshide S, Ogata Y, et al. The time course of flow‐mediated vasodilation and endothelial dysfunction in patients with a cardiovascular risk factor. J Am Soc Hypertens. 2012;6:109–116. [DOI] [PubMed] [Google Scholar]
- 27. Ishikawa J, Haimoto H, Hoshide S, et al. An increased visceral‐subcutaneous adipose tissue ratio is associated with difficult‐to‐treat hypertension in men. J Hypertens. 2010;28:1340–1346. [DOI] [PubMed] [Google Scholar]
- 28. Anwar YA, Giacco S, McCabe EJ, et al. Evaluation of the efficacy of the Omron HEM‐737 IntelliSense device for use on adults according to the recommendations of the Association for the Advancement of Medical Instrumentation. Blood Press Monit. 1998;3:261–265. [PubMed] [Google Scholar]
- 29. Imai Y, Otsuka K, Kawano Y, et al. Japanese Society of Hypertension (JSH) guidelines for self‐monitoring of blood pressure at home. Hypertens Res. 2003;26:771–782. [DOI] [PubMed] [Google Scholar]
- 30. Yarows SA, Patel K, Brook R. Rapid oscillometric blood pressure measurement compared to conventional oscillometric measurement. Blood Press Monit. 2001;6:145–147. [DOI] [PubMed] [Google Scholar]
- 31. Tomiyama H, Matsumoto C, Yamada J, et al. The relationships of cardiovascular disease risk factors to flow‐mediated dilatation in Japanese subjects free of cardiovascular disease. Hypertens Res. 2008;31:2019–2025. [DOI] [PubMed] [Google Scholar]
- 32. Sung J, Ouyang P, Bacher AC, et al. Peripheral endothelium‐dependent flow‐mediated vasodilatation is associated with left ventricular mass in older persons with hypertension. Am Heart J. 2002;144:39–44. [DOI] [PubMed] [Google Scholar]
- 33. Suzuki M, Takamisawa I, Suzuki K, et al. Close association of endothelial dysfunction with insulin resistance and carotid wall thickening in hypertension. Am J Hypertens. 2004;17:228–232. [DOI] [PubMed] [Google Scholar]
- 34. Matsui Y, Eguchi K, Shibasaki S, et al. Morning hypertension assessed by home monitoring is a strong predictor of concentric left ventricular hypertrophy in patients with untreated hypertension. J Clin Hypertens (Greenwich). 2010;12:776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Niiranen T, Jula A, Kantola I, et al. Home‐measured blood pressure is more strongly associated with atherosclerosis than clinic blood pressure: the Finn‐HOME Study. J Hypertens. 2007;25:1225–1231. [DOI] [PubMed] [Google Scholar]
- 36. Ter AvestE, Stalenhoef AF, de Graaf J. What is the role of non‐invasive measurements of atherosclerosis in individual cardiovascular risk prediction? Clin Sci (Lond). 2007;112:507–516. [PubMed] [Google Scholar]
- 37. Kario K, Matsui Y, Shibasaki S, et al. An alpha‐adrenergic blocker titrated by self‐measured blood pressure recordings lowered blood pressure and microalbuminuria in patients with morning hypertension: the Japan Morning Surge‐1 Study. J Hypertens. 2008;26:1257–1265. [DOI] [PubMed] [Google Scholar]
- 38. Witte DR, Westerink J, de Koning EJ, et al. Is the association between flow‐mediated dilation and cardiovascular risk limited to low‐risk populations? J Am Coll Cardiol. 2005;45:1987–1993. [DOI] [PubMed] [Google Scholar]


