Abstract
Hypertension is a well‐known risk factor for the development and rupture of cerebral aneurysms. The authors conducted a study to investigate the prognostic value of admission blood pressure (BP) on prognosis in patients with subarachnoid hemorrhage (SAH). Two hundred patients with SAH were divided into two groups according to Hunt Hess score (good prognosis: 1 to 3, and poor prognosis: 4 and 5) and according to death in hospital (surveyed and died). The prognostic factors of SAH and BP changes according to Hunt Hess scores in the acute stages of the event were evaluated. Admission mean arterial BP values of the patients who died in hospital were significantly lower than in the patients who were surveyed (P=.026). The admission mean arterial BP values were found to be lower in the poor prognostic patients (Hunt Hess score of 4 and 5) (P<.001). Decreased admission BP values were found to be associated with poor prognosis and mortality.
Subarachnoid hemorrhage (SAH) is most frequently caused by rupture of a saccular aneurysm and is a life‐threatening condition associated with substantial morbidity and mortality. SAH accounts for approximately 6% to 8% of all strokes.1 The annual incidence of SAH varies between 10 and 30 cases per 100,000 people in the general population.1 Spontaneous SAH as a result of ruptured aneurysm or arteriovenous malformation occurs in approximately 90% of cases and is caused by hypertension or unknown causes.2 Although hypertension is the most important modifiable risk factor for SAH, blood pressure (BP) management and the prognostic value of BP during the acute phase of SAH are still controversial.3, 4
A low BP value at admission has been reported to be associated with poor outcomes due to vasospasms and a high BP at admission has been linked to rebleeding in SAH.5 Hypoperfusion in association with a low BP value or rebleeding in SAH could lead to neurological deterioration caused by ischemia in hypoperfused brain regions.6, 7 For this reason, it is difficult to manage BP in the acute stages of SAH. There are only a few studies on the prognostic effect of admission BP in patients with SAH, and the results of these studies are conflicting.5, 6, 7 The aim of this study was to evaluate the impact of admission BP on the short‐term prognosis of SAH patients.
Materials and Methods
Patients
The study series comprised 236 patients with SAH admitted to the Department of Emergency Medicine at our institution between January 2009 and January 2012.The diagnosis of SAH was made by the presence of a typical clinical history of spontaneous SAH and blood in the basal cisterns observed on the admission brain computed tomography (CT) scan or by xanthocromia of the cerebrospinal fluid in patients with negative CT scans. All patients underwent CT scanning and/or lumbar puncture at admission. The aneurysmal origin of the SAH was confirmed by means of cerebral angiography. Clinical grading according to the system proposed by Hunt and Hess8 was performed at the time of admission. Patients who had SAH caused by intracranial or intraspinal arteriovenous malformations (2 patients), venous hemorrhage (3 patients), head trauma (7 patients), coagulation disorders (3 patients), and bleeding in intracranial tumors (1 patient) were excluded. Twenty patients were excluded because of late admission after SAH (>6 hours of onset).
The hospital records of the remaining 200 patients were reviewed for details about age, sex, admission mean arterial blood pressure (MABP) values, initial Glasgow Coma scale (GCS) scores, smoking, alcohol usage, blood glucose levels, and comorbid conditions (eg, ischemic heart disease, previous stroke, diabetes mellitus, or a history of hypertension).
The admission BP values were obtained during transfer or immediately after the patient's arrival in the emergency department by a trained nurse using a calibrated sphygmomanometer. For almost all the patients, at least 6 BP readings during the first 6 hours after the onset of the SAH were available. Of these, the highest value was chosen as the admission BP. The MABP was calculated by adding one third of the pulse pressure (systolic/diastolic) to the diastolic pressure. The patients were considered to be hypertensive if their BP readings preceding the SAH had at least twice exceeded the classification of hypertension according to the World Health Organization/International Society of Hypertension (160/95 mm Hg) or if they were taking antihypertensive medication.9
The study complied with the Declaration of Helsinki. The research protocol was approved by the locally appointed ethics committee of our insitution. Informed consent was obtained from the patients or their relatives.
Neuroradiological Methods
The SAH was verified by CT for all the patients within an hour after admission, and a brain CT was repeated if sudden clinical deterioration occurred. All the CT scans were analyzed, and the locations of the SAHs were noted. Follow‐up CT, in addition to magnetic resonance imaging in some cases, was used to detect for secondary structural abnormalities. Angiography was performed to identify the bleeding aneurysm. The bleeding aneurysms were classified as follows: (1) middle cerebral artery territory, (2) anterior cerebral artery territory, (3) internal carotid artery territory, and (4) vertebro‐basilary circulation. The association between the admission MABP and the Hunt‐Hess scores was evaluated.
Statistical Analysis
Statistical analysis was performed using the SPSS 15.0 statistical software package (SPSS Inc, Chicago, IL). Descriptive statistics are reported as frequencies and percentages for categorical variables and as the median and range for continuous variables. Comparison of the parameters between the groups was performed using chi‐square tests for the categorical variables and using analysis of variance (ANOVA) for the continuous variables. To identify the relationship between the admission MABP and the outcome, the patients were divided into two groups according to their Hunt‐Hess scores (Hunt‐Hess score of 1 to 3 or Hunt‐Hess score of 3 or 4). Continuous values are expressed as the mean±standard deviation. A P value of <.05 was accepted as statistically significant.
There are no direct evaluations in the literature of admission BP and prognosis of patients with SAH. For this reason, the power analysis in the current study was performed according to the study by Balci and colleagues, who investigated the effect of admission BP on the prognosis of patients with intracerebral hemorrhage that occured during treatment with aspirin, warfarin, or no drugs.10 The power of the test was found to be “1” for 199 patients with one‐way ANOVA power analysis.
Results
Two hundred patients (116 women, 84 men) were reviewed during the study period. The mean age of the patients was 57.0±14.9 years (range: 22–97 years). The mean GCS value was 11.2±4.2 and the MABP value was 104.5±15.9 mm Hg. One hundred (50%) of the patients had a history of hypertension. The other risk factors for SAH were smoking in 15 patients (7.5%), alcohol usage in 10 patients (5%), heart disease in 24 patients (12%), and cerebrovascular disease in 17 patients (8.5%). The mean duration of the in‐hospital stay was 12.7±12.2 days. Aneurysms were detected in the middle cerebral artery territory in 50 patients (25%), in the anterior cerebral artery territory in 66 patients (33%), in the internal carotid artery territory in 54 patients (27%), and in the vertebro‐basilary circulation in 30 patients (15%).
When the patients were classified according to their Hunt‐Hess scores, 139 patients had a good prognosis (Hunt‐Hess score of 1–3) and 61 patients had a poor prognosis (Hunt‐Hess score of 4 or 5). There was no statistically significant difference between the patients with good and poor prognosis in terms of sex (P=.294), cigarette smoking (P=.236), history of hypertension (P=.643), or cerebrovascular disease (P=.968). However, alcohol usage was more common in patients with a poor prognosis (P=.035). The distribution of sex, cigarette smoking, alcohol usage, hypertension, and cerebrovascular disease according to poor and good prognosis are presented in Table 1. The mean blood glucose level was significantly higher in the patients with a poor prognosis (187.3±80.0 mg/dL) when compared with those with a good prognosis (151.1±67.8 mg/dL) (P=.001). The mean duration of the in‐hospital stay was shorter for the patients with a poor prognosis (11.0±15.2 days) than for those with a good prognosis (13.5±10.6 days). However, the difference between the two groups was not statistically significant (P=.197).
Table 1.
Distribution of Demographic Characteristics, Risk Factors, GCS, Hunt‐Hess Score, and MABP According to Good and Poor Prognosis
| Hunt‐Hess Score of 1–3 (n=139) | Hunt‐Hess Score of 4 and 5 (n=61) | P Value | |
|---|---|---|---|
| Age, y | 56.1±14.6 | 59.4±15.6 | .150 |
| Male/female | 55/84 | 29/32 | .294 |
| Initial GCS | 13.4±2.5 | 6.2±2.6 | <.001 |
| Hunt‐Hess score | 2.0±0.6 | 4.8±0.4 | <.001 |
| MABP, mm Hg | 107.9±15.2 | 96.8±14.9 | <.001 |
| Blood glucose, mg/dL | 151.1±67.8 | 187.3±80.1 | =.001 |
| In‐hospital stay, d | 13.5±10.6 | 11.1±15.2 | .197 |
| Smoking, No. (%) | 10 (7.2) | 5 (8.2) | .236 |
| Alcohol, No. (%) | 6 (4.3) | 4 (6.5) | .055 |
| Heart disease, No. (%) | 16 (11.5) | 8 (13.1) | .975 |
| Cerebrovascular disease, No. (%) | 11 (7.9) | 6 (9.8) | .968 |
| History of hypertension, No. (%) | 69 (49.6) | 31 (50.8) | .643 |
Abbreviations: GCS, Glasgow Coma score; MABP, mean arterial blood pressure.
The admission MABP values were significantly lower in the patients with a poor prognosis (a Hunt‐Hess score of 4 or 5) compared with those with a good prognosis (a Hunt‐Hess score of 1–3) (P<.001). The MABP values in the patients with a Hunt‐Hess score of 5 were significantly lower compared with those in the patients with any other Hunt‐Hess score (P<.05). The MABP values for each group according to the Hunt‐Hess scores are presented in the Figure.
Figure 1.
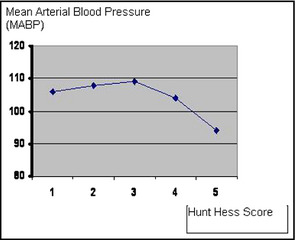
Mean arterial blood pressure values for each group according to Hunt‐Hess scores.
The in‐hospital mortality for the patients with a poor prognosis was 88.5% and for those with a good prognosis was 25.8% (54 deaths in 61 patients vs 36 deaths in 139 patients, P<.001). The mortality rates of the patients in each group according to the Hunt‐Hess scores are presented in Table 2.
Table 2.
Distribution of Demographic Characteristics, Risk Factors, GCS, Hunt‐Hess Score, and MABP According to Death During Hospital Stay
| Surveyed (n=110) | Died (n=90) | P Value | |
|---|---|---|---|
| Age, y | 54.1±14.6 | 60.7±14.5 | .002 |
| Male/female | 44/66 | 40/50 | .527 |
| Initial GCS | 13.6±2.4 | 8.2±3.9 | <.001 |
| Hunt‐Hess score | 2.1±0.9 | 3.8±1.3 | <.001 |
| MABP, mm Hg | 106.4±15.3 | 102.3±16.4 | .026 |
| Blood glucose, mg/dL | 142.6±55.2 | 185.9±85.5 | <.001 |
| In‐hospital stay, d | 13.7±10.6 | 11.6±13.8 | .220 |
| Smoking, No. (%) | 7 (6.3) | 8 (8.8) | .316 |
| Alcohol, No. (%) | 4 (3.6) | 6 (6.6) | .120 |
| Heart disease, No. (%) | 12 (10.9) | 12 (13.3) | .518 |
| Cerebrovascular disease, No. (%) | 7 (6.4) | 10 (10) | .367 |
| History of hypertension, No. (%) | 48 (43.6) | 52 (57.7) | .384 |
Abbreviations: GCS, Glasgow Coma score; MABP, mean arterial blood pressure.
Ninety of the 200 patients (45%) died during the in‐hospital period. When the patients were divided into two groups according to death in hospital, a statistically significant difference was found for age (P=.002), initial GCS (P<.001), Hunt‐Hess score (P<.001), MABP (P=.026), and blood glucose levels (P<.001). However, no significant difference was found for sex (P=.527), smoking (P=.316), alcohol usage (P=.120), heart diseases (P=.518), cerebrovascular event (P=.367), or history of hypertension (P=.384) between the patients who died or survived. The demographic characteristics and the distribution of the risk factors in the patients who died or survived are presented in Table 2.
Discussion
Nontraumatic SAHs account for 6% to 8% of all acute cerebrovascular events.1 The mortality and morbidity rates of SAH are high and the predictive factors for a poor prognosis are controversial. Rosentgart and colleagues4 reported that poor prognostic factors in SAH patients are increasing age, worsening neurological grade, a greater extent of SAH on admission CT, the presence of intracerebral hematomas or intraventricular hemorrhage, and increased systolic BP on admission, in addition to a previous diagnosis of hypertension, myocardial infarction, liver disease, or SAH. Smoking and increasing age were reported as prognostic factors in one study.11 Deruty and colleagues reported that age and the level of consciousness on admission were prognostic factors in SAH patients.12 However, in another study, Gomis and colleagues13 reported that arterial hypertension, age, and sex had no prognostic value in patients with SAH.
Hypertension is known to be a major risk factor for the development and rupture of cerebral aneurysms.11, 14 Kleinpert and colleagues11 reported that patients with hypertension had a nearly 7‐fold higher risk of an aneurysmal SAH. Despite the well‐known relationship between SAH and hypertension, BP changes during the acute phase of the SAH are not clear, with only a few studies investigating the prognostic effects of BP in SAH patients.4, 11, 13, 15, 16 Rosentgart and colleagues4 reported that elevated systolic BP on admission was an independent prognostic factor. However, Gomis and colleagues13 found no association between BP and the prognosis of SAH patients.
The present study aimed to look separately at the effect of admission MABP on the prognosis of patients with SAH by dividing them into two groups according to their Hunt‐Hess scores (patients with good or a poor prognosis) and their prognosis (survival or death in hospital). We used MABP instead of systolic BP and we found a slight increase in the MABP values of the patients with grade 1 to 3 Hunt‐Hess scores. In the patients with a grade 4 Hunt‐Hess score, the MABP decreased slightly. MABP also decreased in patients with a grade 5 Hunt‐Hess score, and most of the deaths occured in this group. In light of these findings, we conclude that decreased BP is associated with a poor prognosis and mortality in SAH patients. The study revealed no significant difference between the patients with a good (Hunt‐Hess score of 1–3) or a poor prognosis (a Hunt‐Hess score of 4 or 5) and age, history of hypertension, cerebrovascular diseases, or ischemic heart diseases.
Brain energy metabolic insufficiency and elevations in the lactate‐pyruvate ratio have been shown to be linked to a poor outcome in SAH patients.17, 18 Helbok and colleagues16 reported that acute reductions in serum glucose might be associated with a brain energy metabolic crisis and lead to poor prognosis in SAH patients. In the current study, the patients with a poor prognosis had increased blood glucose levels compared with those with a good prognosis. The increased blood glucose levels may be a reaction to the metabolic energy demands of the brain.
In previous studies the use of prophylactic or therapeutic hypervolemia or prophylactic‐induced hypertension was found in association with a lower risk of poor prognosis.4, 6 Raabe and colleagues6 compared the relative importance of hypertension with hypervolemia in relation to increasing cerebral oxygenation in patients with cerebral vasospasms after SAH. They reported that moderate hypertension (cerebral perfusion pressure 80–120 mm Hg) in a normovolemic hemodiluted patient is an effective method for improving cerebral oxygenation and is associated with a lower complication rate compared with hypervolemia.
Tabuchi and colleagues5 studied the relationship between hypotension and cerebral vasospasm in SAH patients. They measured systemic arterial BP every 2 hours in each of 125 patients for a period of more than 2 weeks. They found a decrease in systemic BP (>40 mm Hg) in 41.6% of the patients and 5.5% of the decreases in BP occured just before the onset of symptomatic vasospasm. Tabuchi and colleagues5 concluded that decreased BP might result from delayed cerebral vasospasms. However, as presented in our study, Tabuchi and colleagues5 reported that symptomatic vasospasm occured in some of the SAH patients following decreased BP.
Conclusions
Hypervolemia and hypertension therapy are routinely used for prophylaxis and treatment of symptomatic cerebral vasospasms at many institutions.19, 20 The present study demonstrated that SAH patients with a poor prognosis had decreased admission MABP values. Although most prognostic factors for outcomes after SAH are present on admission and are not modifiable, it is possible to begin therapeutic hypervolemia in emergency departments at the early stages of events to prevent a poor prognosis.
Disclosures
The present study has no contributions or funding to disclose.
J Clin Hypertens (Greenwich). 2013;15:737–741. ©2013 Wiley Periodicals, Inc.
References
- 1. Feigin VL, Rinkel GJ, Lawes CM, et al. Risk factors for subarachnoid hemorrhage: an update systemic review of epidemiological studies. J Stroke. 2005;36:2773–2780. [DOI] [PubMed] [Google Scholar]
- 2. VanGijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. [DOI] [PubMed] [Google Scholar]
- 3. Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354:387–396. [DOI] [PubMed] [Google Scholar]
- 4. Rosengart AJ, Schultheiss KE, Tolentino J, et al. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2315–2321. [DOI] [PubMed] [Google Scholar]
- 5. Tabuchi S, Hirano N, Tanabe M, et al. Relationship of hypotension and cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neurol Res. 2006;28:196–199. [DOI] [PubMed] [Google Scholar]
- 6. Raabe A, Beck J, Keller M, et al. Relative importance of hypertension compared with hypervolemia for increasing cerebral oxygenation in patients with cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg. 2005;103:974–981. [DOI] [PubMed] [Google Scholar]
- 7. Schubert GA, Seiz M, Hegewald AA, et al. Hypoperfusion in the acute phase of subarachnoid hemorrhage. Acta Neurochir Suppl. 2011;110:35–38. [DOI] [PubMed] [Google Scholar]
- 8. Hunt WE, Hess RH. Surgical risk as related to time of intervention in the repair of intracranial aneurysm. J Neurosurg. 1968;28:14–19. [DOI] [PubMed] [Google Scholar]
- 9. Whitworth JA. World Health Organisation (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. [DOI] [PubMed] [Google Scholar]
- 10. Balci K, Utku U, Asil T, et al. The Effect of Admission Blood Pressure on the Prognosis of Patients with Intracerebral Hemorrhage That Occurred during Treatment with Aspirin, Warfarin, or No Drugs . Clin Exp Hypertens. 2012;34:118–124. [DOI] [PubMed] [Google Scholar]
- 11. Kleinpeter G, Lehr S. Is hypertension a major risk factor in aneurysmal subarachnoid hemorrhage? Wien Klin Wochenschr. 2002;15:114. [PubMed] [Google Scholar]
- 12. Deruty R, Pelissou‐Guyotat I, Mottolese C, et al. Level of consciousness and age as prognostic factors in aneurysmal SAH. Acta Neurochir (Wien). 1995;132:1–8. [DOI] [PubMed] [Google Scholar]
- 13. Gomis P, Rousseaux P, Jolly D, et al. Initial prognostic factors of aneurysmal subarachnoid hemorrhage. Neurochirurgie. 1994;40:18–30. [PubMed] [Google Scholar]
- 14. Güresir E, Beck J, Vatter H, et al. Subarachnoid hemorrhage and intracerebral hematoma: incidence, prognostic factors, and outcome. Neurosurgery. 2008;63:1088–1093. [DOI] [PubMed] [Google Scholar]
- 15. Lagares A, Gomez PA, Lobato RD, et al. Prognostic factors on hospital admission after spontaneous subarachnoid haemorrhage. Acta Neurochir (Wien). 2001;143:665–672. [DOI] [PubMed] [Google Scholar]
- 16. Ostabal MMI, Sanz C, SSuarez MA, et al. The study of prognostic factors of spontaneous subarachnoid hemorrhage. Rev Neurol. 1997;25:58–60. [PubMed] [Google Scholar]
- 17. Helbok R, Schmidt JM, Kurtz P, et al. Systemic glucose and brain energy metabolism after subarachnoid hemorrhage. Neurocrit Care. 2010;12:317–323. [DOI] [PubMed] [Google Scholar]
- 18. Sato M, Nakano M, Asari J, et al. Admission blood glucose levels and early change of neurological grade in poor‐grade patients with aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien). 2006;148:623–626. [DOI] [PubMed] [Google Scholar]
- 19. Tamargo RJ, Walter KA, Oshiro EM. Aneurysmal subarachnoid hemorrhage: prognostic features and outcomes. New Horiz. 1997;5:364–375. [PubMed] [Google Scholar]
- 20. Ildan F, Tuna M, Erman T, et al. Prognosis and prognostic factors for unexplained subarachnoid hemorrhage: review of 84 cases. Neurosurgery. 2002;50:1015–1024. [PubMed] [Google Scholar]


