Abstract
Hypertension is associated with damage to the heart, kidneys, and vascular tree. Assessment of target organ damage (TOD) allows better prediction of cardiovascular risk than conventional risk assessment. Regression of TOD during antihypertensive treatment, which depends on the blood pressure (BP) reduction and the specific ancillary properties of each drug, may indirectly indicate that BP is well controlled. It is unclear whether regression of TOD during treatment is associated with favorable outcome and should be used as a surrogate endpoint. There is evidence that regression of left ventricular hypertrophy and albuminuria are associated with a favorable outcome. However, recent studies cast doubts on this evidence. Thus, assessment of TOD is important to define cardiovascular risk, but, so far, regression of TOD cannot be regarded as a major surrogate therapeutic target. The present paper will provide a critical overview of the data available in the literature.
Hypertension is associated with increased cardiovascular (CV) morbidity and mortality. Subtle damage to certain organs can be detected in hypertensive patients early in the disease even before overt clinical events occur. Lowering blood pressure (BP) decreases the rate of CV events. The beneficial effects of lowering BP may be observed only after a long period of treatment, particularly in patients with mild to moderate risk. In order to overcome the need for long‐term studies, it has been suggested that instead of clinical events, subclinical organ damage may be a surrogate endpoint. The present paper will discuss the importance of the initial evaluation of target organ damage in hypertensive patients and will present the evidence for using changes in subclinical organ damage as surrogate endpoints.
Subclinical Organ Damage in the Initial Evaluation in Patients With Hypertension
Several arguments support the search for subclinical (or asymptomatic) organ damage in hypertensive patients to quantify total CV risk before deciding the treatment strategy. Organ damage has been indeed shown to have an independent prognostic significance, irrespectively of whether it involves the structure and/or function of the heart, brain, kidney, or vessels.1 It has also been shown that when organ damage is detected, patients usually have a high total CV risk,2 ie, a chance of having a morbid or fatal CV event within 10 years >20%. This has important implications for treatment because therapeutic strategies differ in high‐ vs moderate‐ or low‐risk hypertensive patients. Namely, at high CV risk, treatment may have to be started in the high normal BP range, rather than when BP is ≥140/90 mm Hg as in low‐ to moderate‐risk individuals. BP may have to be reduced <130/80 mm Hg rather than just <140/90 mm Hg. Combination of two drugs may be considered for treatment initiation to favor early BP control and avoid prolonged exposure to the BP‐dependent increase in risk. Finally, in high‐risk hypertensive patients, BP‐lowering drugs should be combined with antiplatelet and lipid‐lowering agents because (1) at variance from the low‐risk condition at high CV risk, reducing platelet aggregability lowers the incidence of coronary and cerebrovascular events much more than it increases the risk of major bleedings,3 and (2) in patients in whom CV risk is high, administration of a statin adds to the protection related to BP‐lowering interventions even when serum cholesterol is not elevated.4 The evidence provided so far on the benefits of additional treatment is not always univocal. This means that physicians should decide on this according to the clinical characteristics and risk profile of each single patient.1, 2
The above reasons led guidelines of the European Society of Hypertension and the European Society of Cardiology to recommend assessment of asymptomatic organ damage in the diagnostic workup of hypertensive patients, with the following subdivision of available organ damage measures: those to search for routinely, and those whose use is only desirable because of lower availability and higher cost (Figure).1 They further recommend looking for damage in different organs because the risk increases with the increase in the number of organs affected,5 and evidence exists that this happens even with different measures of damage within the same organ. In the kidney, for example, microalbuminuria and reduced estimated glomerular filtration rate are associated with a greater risk of renal and CV events than either abnormality alone.5
Figure 1.
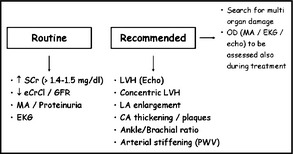
Routine and recommended examinations for evaluation of target organ damage (OD) according to European Society of Hypertension/European Society of Cardiology 2007 guidelines. SCr indicates serum creatinine; CrCl, creatinine clearance; GFR, glomerular filtration rate; MA, microalbuminuria; EKG, electrocardiography; LVH, left ventricular hypertrophy; LA, left atrial; CA, carotid artery; PWV, pulse wave velocity.
Assessment of Organ Damage During Antihypertensive Treatment
With very few exceptions (see below), whether regression of organ damage is associated with a reduction of morbid and fatal CV events has never been investigated in randomized clinical trials. This may be difficult to do, however, because in a given individual, regression of organ damage is unpredictable and thus randomization to groups with expected greater or smaller improvements of organ damage is hardly achievable, unless different treatment strategies (eg, greater vs smaller BP reductions and/or use of different drugs) are planned and systematically employed. This would prevent, however, CV protective effects to be properly attributed to the modification of organ damage per se rather than to the concomitant treatment differences.
The association between treatment‐induced changes in organ damage and risk of CV events has thus necessarily been studied by post hoc analysis of trials, ie, by retrospectively calculating whether the incidence rates and risk of CV events are decreased in patients with vs those without (or with smaller) improvement or delayed progression of organ damage. Some results have shown that this may be the case, but the evidence is inconclusive.
Cardiac Subclinical Target Organ Damage
Left ventricular hypertrophy (LVH) is highly prevalent among hypertensive patients. The association of LVH and increased CV morbidity and mortality has been previously widely documented.6 Electrocardiographic (ECG) findings of LVH were found to be prognostic indicators for CV complications among hypertensive patients.7 LVH can be assessed either by the cheap and accessible, albeit less sensitive, ECG, or more accurately by echocardiography and even more so by cardiac magnetic resonance (CMR) imaging. Compared with ECG, echocardiography has the advantage of providing additional information, such as quantitative estimation of left ventricular mass (LVM), as well as abnormal left ventricular diastolic function and irregular geometric patterns. The prognostic significance of these echocardiographic characteristics has been previously demonstrated.8 A recent meta‐analysis showed that estimated LVM and LVH by either echocardiography or CMR imaging are reliable CV risk predictors.9
In the Losartan Intervention for Endpoint Reduction in Hypertension Study (LIFE) a predefined secondary endpoint was the assessment of CV death, myocardial infarction, or stroke in relation to severity of electrocardiographic LVH obtained at enrollment and annually thereafter. In‐treatment regression of ECG voltage parameters was associated with reduction of the composite end point, CV mortality, myocardial infarction, and stroke.10 In an additional subanalysis of the LIFE study, it was shown that for every 1 standard deviation of regression of the ECG parameters, there was a 19% lower adjusted risk of sudden cardiac death.11 It has also been demonstrated among the same population that lack of specific electrocardiographic LVH regression during treatment was associated with an elevated incidence rate of coronary and peripheral revascularization. Furthermore, a recent subanalysis of the LIFE study demonstrated an association of ECG‐LVH regression with lower risk of new‐onset heart failure and mortality.12
Most of the data on the CV benefit of LVM reduction derived from the LIFE study which ECG were used to diagnose and assess the regression of LVH, but ECG is not sensitive enough to estimate LVM. Only few studies evaluated the effect of LVH regression by echocardiography on CV events. In a small study, Verdecchia and colleagues evaluated 880 untreated hypertensive patients who underwent echocardiography and 24‐hour ambulatory BP monitoring at baseline and after a median of 3.5 years. The risk for a future cerebrovascular event was 2.8 times higher in those who exhibited lack of regression or new development of LVH than in those who exhibited LVH regression or persistently normal LVM.13 It is noteworthy that in this small study, serial ECG changes failed to define groups at different risk. In a substudy of the LIFE trial, CV death, myocardial infarction, and stroke were related to treatment‐induced echocardiographic left ventricular geometric pattern modifications.14 In a meta‐analysis of 4 studies including 1064 hypertensive patients, echocardiographic LVM was reported before and during antihypertensive treatment with subsequent assessment of CV events. The results showed that compared with patients displaying lack of regression or newly developed LVH, those with LVH regression exhibited a 59% reduced risk of CV disease.15 Furthermore, Pierdomenico and associates showed in a meta‐analysis that included 5 studies and 3149 patients with 333 CV events that regression of echocardiographic LVH in hypertension is associated with a reduction of CV events.16 However, in a recent large meta‐analysis that included 14 studies and 12,809 participants with 2259 events, Costanzo and his coworkers failed to show a significant continuous relationship between LVH changes and clinical events.17 This recent meta‐analysis has some flaws; nevertheless, it casts doubt on the importance of LVH regression as a predictor of CV events. Moreover, in the Telmisartan Randomized Assessment Study in ACE‐Intolerant Subjects With Cardiovascular Disease (TRANSCEND), prevalence of LVH was reduced after 2 and 5 years by telmisartan compared with placebo, and new‐onset LVH occurred less frequently with telmisartan.18 Yet, the beneficial effect of telmisartan on regression of LVH was not translated to benefit in CV events since the primary outcome was the same in the telmisartan and the placebo groups.19 These results may have various explanations but they suggest that there is not enough evidence to justify regression of LVH as a surrogate therapeutic target.
Renal Subclinical Organ Damage
Data accumulated during the past decade has provided evidence for the pivotal role of urinary albumin excretion (UAE) as a prognostic indicator for CV risk among diabetic as well as nondiabetic and hypertensive patients. In a recently published meta‐analysis, patients with albuminuria exhibit a significantly increased risk for coronary artery disease.20
While the association between UAE and the risk for CV events is well established, its role as an independent therapeutic target requires further clarification. During the past decade, several studies have directly addressed this issue. In the Prevention of Renal and Vascular End‐Stage Disease Intervention Trial (PREVEND‐IT), fosinopril treatment significantly reduced UAE and was associated with a 40%, albeit nonsignificant, reduction of the primary endpoint.21 Similarly, in the Irbesartan in Patients With Type‐2 Diabetes and Microalbuminuria (IRMA‐2) study, patients treated with irbesartan demonstrated a dose‐dependent significant decrease of UAE compared with placebo but only a trend towards less frequent nonfatal CV events.22 A post hoc analysis of the Reduction in Endpoint in Noninsulin‐Dependent Diabetes Mellitus With the Angiotensin II Antagonist Losartan (RENAAL) study has shown that for a 50% reduction of UAE, there was an 18% reduction in CV risk.23 In the LIFE study, the reduction in UAE was estimated to explain one fifth of the beneficial effects of losartan.24 Moreover, further analysis of the LIFE trial demonstrated that in the first year of antihypertensive treatment, a stepwise increase in UAE was translated into a significant increase in CV risk, independent of the BP‐lowering effects.25 The prognostic importance of baseline and serial changes in UAE, particularly among patients with resistant hypertension, was also demonstrated in a prospective cohort consisting of 531 patients with resistant hypertension. Each 10‐fold increase in baseline UAE was associated with a significant 50% increase in CV morbidity and mortality. Patients with UAE regression had a nonsignificant 27% lower CV risk, compared with those with persistent UAE. Patients who developed UAE had a nonsignificant 65% increased risk for a CV event compared with persistent normoalbuminuric participants.26
The association of UAE regression with reduction of CV morbidity and mortality, as well as its role as an independent therapeutic target has been challenged by recent large long‐term studies in which UAE regression was not associated with reduction in CV events.27, 28, 29 In the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET), although the rate of UAE increased less among patients treated with the combined regimen, this effect was not accompanied by a reduction of CV events but was rather associated with an increase in renal events and death.29 Similar results were observed in the Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints (ALTITUDE), which was terminated prematurely despite a decrease in UAE, because of increased CV events in the aliskiren arm.28 These studies included high‐risk patients, and in these patients, regression of microalbuminuria may not have a strong influence on subsequent events. The results cannot be explained by the powerful double renin‐angiotensin system blockade, because in the recent Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) trial, olmesartan delayed onset of microalbuminuria but was associated with a higher rate of fatal CV events among patients with preexisting coronary heart disease.27 Thus, UAE regression is not always associated with improved CV events and therefore cannot be a major surrogate therapeutic target.
Vascular Subclinical Organ Damage
It has been shown that carotid intima‐media thickness (CIMT) values are associated with the incidence of CV events, both coronary and cerebrovascular, among hypertensive as well as normotensive patients.30 In the European Lacidipine Study on Atherosclerosis (ELSA), the incidence of all CV events were significantly related to baseline CIMT values at both vascular sites and that lacidipine treatment had a significant effect on regression of CIMT compared with atenolol treatment.31 Measurements of CIMT seem to be an attractive biomarker and a potential independent therapeutic target for high‐risk CV patients. Nevertheless, data regarding the interpretation of CIMT changes as a surrogate clinical endpoint for CV events have not been promising thus far. While the results of the ELSA study indicated a predictive value for baseline CIMT values, further analyses in which baseline and on‐treatment data were used failed to prove a significant predictive role of CIMT for any type of CV outcome.31 A recent meta‐analysis of 41 randomized trials enrolling 18,307 participants evaluated the prediction capacity of CIMT regression for CV events. There was no significant relationship between CIMT regression and coronary heart disease, cerebrovascular events, and all‐cause mortality.32 Thus, despite the clear association between CIMT values and CV events, changes in CIMT are weakly associated with changes in CV events and therefore are not an ideal surrogate marker in the treatment of hypertension.
Coronary artery calcification (CAC) is a well‐accepted surrogate marker for coronary atherosclerosis burden.33 Electron beam computed tomography and multi‐detector computed tomography (CT) are the primary imagery modalities for CAC evaluation, and currently a CT study for CAC measurements requires minimal scanning time. There is compelling evidence from clinical and epidemiologic studies that CAC scores predict future CV events.34, 35 Moreover, Shemesh and his colleagues recently were able to stratify hypertensive diabetic patients into a high or low CV risk category according to the presence or absence of CAC.36 Furthermore, CAC scores have been previously shown to have an additive prognostic value when combined with other risk stratification methods, such as the Framingham risk score, particularly among patients with intermediate CV risk.37
Whether CAC measurements can be applied as an independent surrogate target endpoint for CV events among hypertensive patients is not entirely clear. In the coronary calcification side arm of the International Nifedipine Study: Intervention as Goal for Hypertension Therapy (INSIGHT), Motro and colleagues showed a significant slower progression of CAC with nifedipine compared with a diuretic.38 However, these findings were not translated to improved CV outcomes with nifedipine.39
Large artery stiffness is regarded as an independent predictor of CV morbidity and mortality in patients with essential hypertension, diabetes, and end‐stage renal disease.40 Because of technical obstacles, the evaluation of aortic stiffness is performed through surrogate indices, such as pulse pressure, carotid‐femoral pulse wave velocity, and ambulatory arterial stiffness. Pulse wave velocity is associated with an increased risk of CV events,41 pulse pressure is a strong predictor of coronary events,42 and correlation between ambulatory arterial stiffness and subclinical target organ damage, such as LVH, CIMT, UAE, and glomerular filtration rate has also been observed.43
Several other emerging surrogate end points for target organ damage among hypertensive patients have also been proposed. For example, increased levels of asymmetric dimethylarginine and high‐sensitivity C‐reactive protein, which are known surrogates of endothelial dysfunction and inflammation, have been shown to be associated with microalbuminuria.44 However, the lack of information on the effect of improvement in these markers on CV events prevents their use as surrogate endpoints.
The Critical Position
There is strong opposition to the possibility that in hypertension, treatment‐induced changes in organ damage predict outcomes based on the argument that (1) “post hoc” results are scientifically weak because comparisons involve nonrandomized groups that may differ for variables that cannot be entirely taken care of by statistical adjustments, and (2) LVH and proteinuria have not been shown to be associated with CV and renal events in some major trials.45 However, given the variety of the clinical substudies and the complexity and number of the factors involved, 100% consistency of the results is hardly achievable in clinical research. Furthermore, in one of the trials in which discrepancy has been observed,27, 28, 29, 46, 47 ie, an antihypertensive effect was accompanied by an increased incidence of CV events. However, the number of CV events was so small as to prevent any safe conclusion.27 Finally, in another negative trial on patients at high CV risk, the antiproteinuric effect was not accompanied by a greater CV and renal protection when treatment consisted of an angiotensin‐converting enzyme inhibitor plus an angiotensin receptor antagonist.29, 47 This was not the case in the general trial population48 in which patients with an antihypertensive effect exhibited a reduced risk of renal events and of CV morbidity or mortality compared with patients in whom proteinuria increased during treatment. This suggests that in the group under dual blockade of the renin‐angiotensin system, the prognostic value of reducing urinary protein excretion might have been masked by the now well‐known adverse consequences of a drastic blockade of the renin angiotensin system. The possibility exists, however, that treatment‐induced changes in urinary protein excretion may have prognostic value in some but not in all clinical and therapeutic conditions.
Conclusions
Undisputable evidence exists that diagnostic assessment of organ damage allows better prediction of CV risk, compared with conventional risk assessment,49 with a more precise identification of high‐ risk individuals in whom a more intense treatment is needed. Evidence is less compelling on the prognostic value of the modification of organ damage induced by treatment. Yet, for some markers of cardiac and renal damage, data are highly supportive that this is the case. If conclusive demonstration is achieved, this will carry several important advantages for both CV research and clinical practice.
Author Contribution and Author Duality
GS reviewed the literature and wrote the first part of the manuscript. GG reviewed the literature and wrote the second part of the manuscript. EG reviewed the literature, wrote the first part of the manuscript, and reviewed the whole manuscript. GM reviewed the literature, wrote the second part of the manuscript, and reviewed the whole manuscript. GS, GG, EG, and GM declare no conflicts of interest regarding this manuscript.
J Clin Hypertens (Greenwich). 2013;15:742–747. ©2013 Wiley Periodicals, Inc.
References
- 1. Mancia G, Laurent S, Agabiti‐Rosei E, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–2158. [DOI] [PubMed] [Google Scholar]
- 2. Mancia G, De Backer G, Dominiczak A, et al. 2007 ESH‐ESC Practice Guidelines for the Management of Arterial Hypertension: ESH‐ESC Task Force on the Management of Arterial Hypertension. J Hypertens. 2007;25:1751–1762. [DOI] [PubMed] [Google Scholar]
- 3. Rothwell PM, Price JF, Fowkes FG, et al. Short‐term effects of daily aspirin on cancer incidence, mortality, and non‐vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. [DOI] [PubMed] [Google Scholar]
- 4. Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations, in the Anglo‐Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT‐LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. [DOI] [PubMed] [Google Scholar]
- 5. Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. [DOI] [PubMed] [Google Scholar]
- 6. Levy D, Salomon M, D'Agostino RB, et al. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation. 1994;90:1786–1793. [DOI] [PubMed] [Google Scholar]
- 7. Havranek EP, Emsermann CD, Froshaug DN, et al. Thresholds in the relationship between mortality and left ventricular hypertrophy defined by electrocardiography. J Electrocardiol. 2008;41:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zanchetti A, Cuspidi C, Comarella L, et al. Left ventricular diastolic dysfunction in elderly hypertensives: results of the APROS‐diadys study. J Hypertens. 2007;25:2158–2167. [DOI] [PubMed] [Google Scholar]
- 9. Armstrong AC, Gidding S, Gjesdal O, et al. LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice. JACC Cardiovasc Imaging. 2012;5:837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okin PM, Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. [DOI] [PubMed] [Google Scholar]
- 11. Wachtell K, Okin PM, Olsen MH, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE Study. Circulation. 2007;116:700–705. [DOI] [PubMed] [Google Scholar]
- 12. Larstorp AC, Okin PM, Devereux RB, et al. Regression of ECG‐LVH is associated with lower risk of new‐onset heart failure and mortality in patients with isolated systolic hypertension: the LIFE study. Am J Hypertens. 2012;25:1101–1109. [DOI] [PubMed] [Google Scholar]
- 13. Verdecchia P, Angeli F, Gattobigio R, et al. Regression of left ventricular hypertrophy and prevention of stroke in hypertensive subjects. Am J Hypertens. 2006;19:493–499. [DOI] [PubMed] [Google Scholar]
- 14. Gerdts E, Cramariuc D, de Simone G, et al. Impact of left ventricular geometry on prognosis in hypertensive patients with left ventricular hypertrophy (the LIFE study). Eur J Echocardiogr. 2008;9:809–815. [DOI] [PubMed] [Google Scholar]
- 15. Verdecchia P, Angeli F, Borgioni C, et al. Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: a meta‐analysis. Am J Hypertens. 2003;16:895–899. [DOI] [PubMed] [Google Scholar]
- 16. Pierdomenico SD, Cuccurullo F. Risk reduction after regression of echocardiographic left ventricular hypertrophy in hypertension: a meta‐analysis. Am J Hypertens. 2010;23:876–881. [DOI] [PubMed] [Google Scholar]
- 17. Costanzo P, Savarese G, Rosano G, et al. Left ventricular hypertrophy reduction and clinical events. A meta‐regression analysis of 14 studies in 12809 hypertensive patients. Int J Cardiol. 2012;Jul 12 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18. Verdecchia P, Sleight P, Mancia G, et al. Effects of telmisartan, ramipril, and their combination on left ventricular hypertrophy in individuals at high vascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial and the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease. Circulation. 2009;120:1380–1389. [DOI] [PubMed] [Google Scholar]
- 19. Yusuf S, Teo K, Anderson C, et al. Effects of the angiotensin‐receptor blocker telmisartan on cardiovascular events in high‐risk patients intolerant to angiotensin‐converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174–1183. [DOI] [PubMed] [Google Scholar]
- 20. Perkovic V, Verdon C, Ninomiya T, et al. The relationship between proteinuria and coronary risk: a systematic review and meta‐analysis. PLoS Med. 2008;5:e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Asselbergs FW, Diercks GF, Hillege HL, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809–2816. [DOI] [PubMed] [Google Scholar]
- 22. Parving HH, Lehnert H, Brochner‐Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. [DOI] [PubMed] [Google Scholar]
- 23. de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110:921–927. [DOI] [PubMed] [Google Scholar]
- 24. Ibsen H, Wachtell K, Olsen MH, et al. Does albuminuria predict cardiovascular outcome on treatment with losartan versus atenolol in hypertension with left ventricular hypertrophy? A LIFE substudy. J Hypertens. 2004;22:1805–1811. [DOI] [PubMed] [Google Scholar]
- 25. Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension. 2005;45:198–202. [DOI] [PubMed] [Google Scholar]
- 26. Salles GF, Cardoso CR, Fiszman R, Muxfeldt ES. Prognostic importance of baseline and serial changes in microalbuminuria in patients with resistant hypertension. Atherosclerosis. 2011;216:199–204. [DOI] [PubMed] [Google Scholar]
- 27. Haller H, Ito S, Izzo JL Jr, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917. [DOI] [PubMed] [Google Scholar]
- 28. Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–2213. [DOI] [PubMed] [Google Scholar]
- 29. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. [DOI] [PubMed] [Google Scholar]
- 30. Polak JF, Pencina MJ, Pencina KM, et al. Carotid‐wall intima‐media thickness and cardiovascular events. N Engl J Med. 2011;365:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zanchetti A, Hennig M, Hollweck R, et al. Baseline values but not treatment‐induced changes in carotid intima‐media thickness predict incident cardiovascular events in treated hypertensive patients: findings in the European Lacidipine Study on Atherosclerosis (ELSA). Circulation. 2009;120:1084–1090. [DOI] [PubMed] [Google Scholar]
- 32. Costanzo P, Perrone‐Filardi P, Vassallo E, et al. Does carotid intima‐media thickness regression predict reduction of cardiovascular events? A meta‐analysis of 41 randomized trials. J Am Coll Cardiol. 2010;56:2006–2020. [DOI] [PubMed] [Google Scholar]
- 33. Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force. Circulation. 2007;115:402–426. [DOI] [PubMed] [Google Scholar]
- 34. Raggi P, Gongora MC, Gopal A, et al. Coronary artery calcium to predict all‐cause mortality in elderly men and women. J Am Coll Cardiol 2008;52:17–23. [DOI] [PubMed] [Google Scholar]
- 35. Shemesh J, Motro M, Morag‐Koren N, et al. Coronary artery calcification predicts long‐term mortality in hypertensive adults. Am J Hypertens. 2011;24:681–686. [DOI] [PubMed] [Google Scholar]
- 36. Shemesh J, Motro M, Morag‐Koren N, et al. Relation of coronary artery calcium to cardiovascular risk in patients with combined diabetes mellitus and systemic hypertension. Am J Cardiol. 2012;109:844–850. [DOI] [PubMed] [Google Scholar]
- 37. Greenland P, LaBree L, Azen SP, et al. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. [DOI] [PubMed] [Google Scholar]
- 38. Motro M, Shemesh J. Calcium channel blocker nifedipine slows down progression of coronary calcification in hypertensive patients compared with diuretics. Hypertension 2001;37:1410–1413. [DOI] [PubMed] [Google Scholar]
- 39. Brown MJ, Palmer CR, Castaigne A, et al. Morbidity and mortality in patients randomised to double‐blind treatment with a long‐acting calcium‐channel blocker or diuretic in the international nifedipine gits study: intervention as a goal in hypertension treatment (INSIGHT). Lancet. 2000;356:366–372. [DOI] [PubMed] [Google Scholar]
- 40. Triantafyllidi H, Tzortzis S, Lekakis J, et al. Association of target organ damage with three arterial stiffness indexes according to blood pressure dipping status in untreated hypertensive patients. Am J Hypertens. 2010;23:1265–1272. [DOI] [PubMed] [Google Scholar]
- 41. Sehestedt T, Jeppesen J, Hansen TW, et al. Which markers of subclinical organ damage to measure in individuals with high normal blood pressure? J Hypertens. 2009;27(6):1165–1171. [DOI] [PubMed] [Google Scholar]
- 42. Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation. 1999;100:354–360. [DOI] [PubMed] [Google Scholar]
- 43. Garcia‐Garcia A, Gomez‐Marcos MA, Recio‐Rodriguez JI, et al. Relationship between ambulatory arterial stiffness index and subclinical target organ damage in hypertensive patients. Hypertens Res. 2011;34:180–186. [DOI] [PubMed] [Google Scholar]
- 44. Tsioufis C, Dimitriadis K, Andrikou E, et al. ADMA, C‐reactive protein, and albuminuria in untreated essential hypertension: a cross‐sectional study. Am J Kidney Dis. 2010;55:1050–1059. [DOI] [PubMed] [Google Scholar]
- 45. Lea J, Greene T, Hebert L, et al. The relationship between magnitude of proteinuria reduction and risk of end‐stage renal disease: results of the African American study of kidney disease and hypertension. Arch Intern Med. 2005;165:947–953. [DOI] [PubMed] [Google Scholar]
- 46. Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double‐blind, controlled trial. Lancet. 2008;372:547–553. [DOI] [PubMed] [Google Scholar]
- 48. Schmieder RE, Mann JF, Schumacher H, et al. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol. 2011;22:1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten‐year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]


