Abstract
Introduction:
Some medicinal plants have shown promising therapeutic potential for the management of the diseases. We aimed to systematically review the literature wherein the therapeutic effects of saffron have been studied on eye disorders.
Methods
A systematic literature search was performed in PubMed, Scopus, Web of Science, Google scholar and other databases using eye disorders and saffron as key terms. No strict inclusion criteria were defined, and almost all clinical studies, as well as in vivo and in vitro studies were included. The reported data in each study were extracted and then qualitatively described.
Results
Finally, 78 articles were found but only 29 relevant articles were included. Nine articles were clinical trials and 20 articles were studies conducted on cellular and molecular aspects of saffron on eye disorders. According to the included studies, crocin prevented the pro-inflammatory response in retinal cells and decreased glucose levels in diabetic mice. Also, crocetin prevented retinal degeneration and saffron protected photoreceptors from light-induced damage in retinal cells. Saffron also improved visual function in age-related macular edema and decreased intraocular pressure in patients with glaucoma. In addition, it was shown that crocin can improve best corrected visual acuity and decrease central macular thickness in patients with diabetic maculopathy.
Conclusion
The results of this review indicated that saffron and its main ingredients such as crocin could be a potential candidate for the treatment of ocular disease especially eye inflammation; however, further clinical studies are needed to confirm such efficiency.
Keywords: Saffron, crocin, herbal medicine, eye disorder, eye inflammation, ocular complication
1. INTRODUCTION
Some types of eye disorders are age-related macular degeneration (AMD), Bulging eyes, Cataracts, Color Blindness, Crossed Eyes (Strabismus), Diabetic Macular Edema, Glaucoma, Keratoconus, Ocular Hypertension, Retinal Detachment, and Uveitis [1, 2].
Some common eye diseases such as AMD occur when a part of the retina that is responsible for central vision does not work properly [3, 4]. AMD happens when a part of the retina called the macula is damaged. Clinically, AMD is classified into ‘dry’ or atrophic form and ‘wet’ or neovascular form. About 1 out of 100 people between the ages of 65 and 75 have age-related macular degeneration [5, 6]. There is currently no effective treatment for dry macular degeneration. Wet AMD is typically treated with anti-vascular endothelial growth factor drug [6, 7].
Nowadays, diabetes-induced complications are a major concern for many diabetic people since they may lead to many ocular diseases, such as diabetic retinopathy, and clinically significant macular edema (CSME) [8]. It is typically divided into two types of proliferative and non-proliferative; in the case of proliferative diabetic retinopathy, the walls of the blood vessels become thin and can cause blood leakage and bleeding at the end of the retina [9, 10]. Intravitreal ranibizumab and bevacizumab are administrated for the treatment of CSME [10]. Diabetic retinopathy occurs in 1 out of 3 people with diabetes, with reported rates of DME reaching 7% in this group of patients [11, 12].
Glaucoma is a group of related eye disorders that cause damage to the optic nerve that carries information from the eye to the brain. Some treatments are available and may help in slowing disease progression, preserving existing vision, and if started early enough, recovering some lost vision. The global prevalence of glaucoma for the population aged 40-80 years is 3.54% [13, 14].
Cataract is the most frequent eye disease among older populations that should be considered at an early age by the health practitioners to provide early preventive programs [15, 16]. Cataract includes some types such as nuclear cataracts, cortical cataracts, posterior subcapsular cataracts, and congenital cataracts. Globally, cataract has remained the major cause of blindness over the years. Approximately 45 million people are blind worldwide, out of which cataract accounts for 17.6 million (39%) cases. South-East Asian region contributes to 50-80% of blindness all over the world [16-18].
Most eye diseases are due to inflammation in the eye, including diabetic retinopathy, which is an inflammation of the retina. According to studies, saffron and its active compounds (such as Crocin, Crocetin, Safranal) have antioxidant properties and can be effective in the treatment of diseases of inflammatory origin.
In this study, we aimed to systematically review all available documents on the therapeutic potential of saffron in eye diseases.
2. METHODS
2.1. Search Strategy and Selection Criteria
PubMed, Scopus, Embase, Web of Science, Medline, Ovid, Science Direct and Google scholar were searched for the defined keywords. The key terms for this systematic review were “saffron” and “eye disorder” as well as all their equivalents. The following search method ((saffron OR crocin)) AND (eye disorders OR eye diseases OR eye problems OR eye complications OR ocular complications OR ocular diseases OR ocular disorders) was used and defined to find potentially relevant documents. All included documents were double-checked, and to minimize the possibility of data loss, manual reference list screening of the included articles was also performed. However, for a comprehensive and reliable conclusion, inclusion criteria were limited to articles with English language or articles in which the data could be extractable from only the abstract. Procedures including literature search and data collection were performed in May 2019 according to the PRISMA checklist 2009 by two authors independently. In the case of disagreement between the two authors in each step, the third author performed procedures to resolve the issue.
2.2. Data Collection
All necessary data were extracted from the included articles and were categorized. The data for clinical trials include design of the study, total numbers of participated patients, age of participants, sex ratio, type of eye disorders, and all measurable parameters such as type of drug formulation, total saffron extract or the type of active ingredient, doses of medication, and the duration of therapy. The data for in vivo and in vitro studies involve type and number of animals, type of saffron extract, administered dose, duration of treatment, study variables and mechanistic pathways.
2.3. Measured Variables and Quality Assessment
The collected articles in this study were categorized into two separate sections of clinical trials and studies in vitro or on animal models. The main outcomes in the clinical trials include intraocular pressure (IOP), visual acuity (VA), best corrected visual acuity (BCVA), central macular thickness (CMT), and focal electroretinogram (fERG), in which response to the therapy was defined according to changes in each criterion. According to the type of eye disorder, the outcomes in each study differ from one another. For example, IOP is an important measurement for eye disease such as glaucoma, while measurements of BCVA, CMT and fERG are important in diabetic maculopathy and AMD.
In in vitro and in vivo studies that had been conducted on retinal cells or retina of animals, the diversity of measurable variables was higher. These variables included determination of oxidative stress parameters such as glutathione (GSH), superoxide dismutase (SOD), malondialdehyde (MDA), reactive oxygen species (ROS), and oxidative capacity, in addition to measurement of intracellular calcium, and apoptotic factors such as SOD and caspases (caspase 3 and 9).
For quality assessment of the included randomized controlled trials (RCT) articles, Newcastle-Ottawa and Oxford quality scoring systems (also known as Jadad score) were used. In Newcastle-Ottawa scoring system, there are three different parts including “selection”, “comparability”, and “outcome” with overall 8 questions, wherein a star is given for each item. However, a maximum of two stars can be assumed for comparability, and a fully standard paper with appropriate design can obtain a maximum of 9 stars on this scale. In Oxford quality assessment scoring systems, there are 5 questions indicating the randomization, blinding and follow-up of studies wherein a study with score ≥3 of 5 has a high quality. The questions of Newcastle-Ottawa and Jadad quality assessment scales are provided as supplementary data.
3. RESULTS
3.1. Literature Search and Study Selection
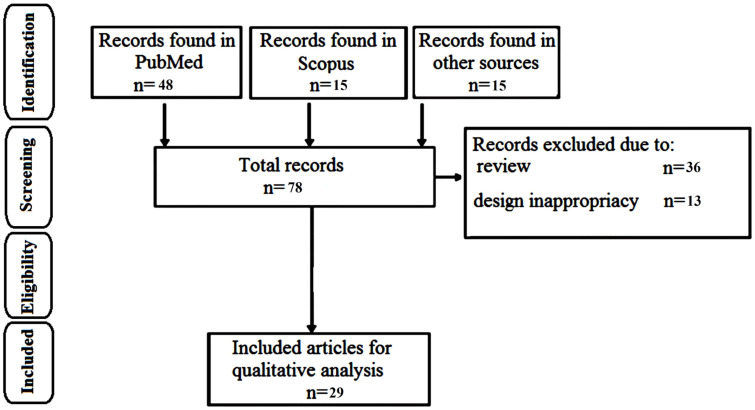
Of the 78 articles collected in the database search, 48 articles were from PubMed, 15 articles were from Scopus and 15 papers were from other sources (Google Scholar, Embase, Web of Science, Medline, Ovid, and Science Direct). After excluding review articles and other irrelevant documents, 29 documents were selected for further processing. In 9 of the 29 included literature articles, the clinical efficiency of saffron or its active constituents has been evaluated on humans, while in 20 articles, the therapeutic potential of saffron and derivatives has been evaluated on animal models or on retinal cells. Step by step process of article selection is demonstrated in Fig. 1.
Fig. (1).
The PRISMA flow diagram demonstrating the database searches, article selection process, and the number of included studies.
4. DESCRIPTION OF INCLUDED STUDIES
4.1. Animal and in vitro Studies
In this section, 20 articles from 2006 to 2019 on the therapeutic effects of saffron and its active components on animal models and/or on retinal cells were reviewed (Table 1). Of 20 papers, 8 articles studied the effect of saffron, and 9 articles evaluated the effects of crocin on eye diseases. In addition, 2 papers evaluated the effect of crocetin and one paper the effect of safranal. The average duration of treatment in animal models was 3.85 weeks (1-20 weeks) and in in vitro studies from 16 to 24 hours to 10 days. Of the 20 papers, 8 were performed both in vivo and in vitro, 8 were in vivo and 4 were in vitro. In animal studies, the lipid profile, antioxidant levels, oxidative capacity, caspases activity, glutathione, SOD, glucose level, fERG, eye vision, the internal pressure of the eye and retinal thickness were investigated. Also in in vitro studies, caspase activity, apoptosis, ROS, and locomotor activity were investigated and the main results showed that crocin prevented the oxidative stress and pro-inflammatory response and saffron was able to protect photoreceptors from light-induced damage in retinal cells.
Table 1.
General information of the in vivo and in vitro studies included in this review.
| No. | Refs. | Type of Study | Animal Model | Number of Animal | Type of Cell | Intervene | Dosage | Duration of Treatment | Study Variables | Mechanism Pathway | Result | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [28] |
In vivo and in vitro |
Male Swiss albino mice | Mice | Retinal ganglion cells | Saffron | 200 μg/mL | 1 week | Ocular hypertension | Anti-inflammation | Saffron decreased IOP | |||||||||
| 2 | [29] | In vivo | ApoE -/- mice | 21 Mice | - | Saffron | 25 mg/kg/d | 20 weeks | Lipidemic profile, glucose, CRP, and total oxidative capacity | - | Potential protective role of saffron against retinal damage | |||||||||
| 3 | [30] | In vitro | - | - | Microglial cells | Crocin | 1 μM | 24h and 48 h | Cox-2, Reactive Oxygen Species Assay, intracellular ROS |
Activation of PI3K/Akt Signaling Pathway | Crocin prevented oxidative stress and pro-inflammatory response | |||||||||
| 4 | [31] | In vivo | Albino | 78 Rats | - | Saffron | 5 mg/kg | 2 weeks | fERG, BCL | Antagonists of selective type-1 (CB1) or type-2 (CB2) cannabinoid receptor | Neuroprotective effect of saffron | |||||||||
| 5 | [32] | In vivo | Male Wistar | 55 Rats | - | Crocin | 100 mg/kg | 8 weeks | Glucose levels, Plasma total antioxidants, glutathione levels and catalase activity | Anti α-crystallin glycation and anti-aggregation of crocin | Crocin decreased Glucose level | |||||||||
| 6 | [21] | In vitro | - | - | Primary retinal cell cultures of mice (HEK-P2X7R cells) |
Saffron | 5 mg/ml | 18h -24 h | Apoptosis, Intracellular calcium measurements | Saffron reduced the ATP-induced intracellular calcium increase in 661W cells |
Saffron protects photoreceptors from light-induced damage |
|||||||||
| 7 | [33] | In vitro | - | - | Retinal ganglion cells | Crocin | 0.1 and 1 μM | 16 h and 24 h | LDH, ROS, caspase-3 activity, nuclear factor-κB | Activation of NF-κB | Crocin protects ganglion cell against H2O2-induced damage | |||||||||
| 8 | [34] |
In vivo, In vitro |
Adult male Sprague-Dawley rats | 25 Rats | Retinal cell | Crocin | 50 mg/kg | 3 days | Caspase-3 protein levels, MDA and GSH Level, SOD | Antioxidant and antiapoptotic properties of Crocin in the retina | Crocin protects the retina from damage induced by ischemia/reperfusion | |||||||||
| 9 | [35] |
In vivo, In vitro |
Albino Rat | 54 Rats | Retinal tissues | Saffron and Light damage, Photobiomodulation | 1 mg/kg/day | 1 week | Apoptotic cell death, ONL thickness, | - | Negative interaction between PBM and saffron when given simultaneously, with a consequent reduction of the neuroprotection | |||||||||
| 10 | [19] | In vivo | Wistar rat | 26 Rats | Lens | Saffron | 60 mg/kg/day | 3 weeks | SOD, GSH, glutathione peroxidase, catalase, lipid peroxidation, malondialdehyde, and protein oxidation | Saffron prevented selenite-induced cataract formation | Saffron significantly prevented selenite-induced lipid peroxidation, protein oxidation, and increased superoxide dismutase, glutathione peroxidase, catalase, and glutathione levels | |||||||||
| No. | Refs. | Type of Study | Animal Model | Number of Animal | Type of Cell | Intervene | Dosage | Duration of Treatment | Study Variables | Mechanism Pathway | Result | |||||||||
| 11 | [26] | In vivo | Male ddY mice | - | - | Crocetin | 20mg/kg | 10 days | Electroretinogram, TUNEL | Expression of 8-OHdG, phosphorylations of MAPK, ERK, JNK and NF-κB | Crocetin prevented ischemia-induced retinal damage through its inhibition of oxidative stress | |||||||||
| 12 | [36] |
In vivo and in vitro |
P23H rat model | 3 Albino rats | Rat retinas | Safranal | 400 mg/kg/ twice a week | 1 week | Electroretinogram, photoreceptor morphology and number | a protective activity against oxidative stress | Safranal could be potentially useful to retard retinal degeneration in patients with RP. | |||||||||
| 13 | [37] | In vivo | mice | - | - | Crocin | 30 and 100 mg/kg | 2, 4 and 12 h after administration | locomotor activity | promotes non‐rapid eye movement sleep | Crocin increased the total amount of non‐REM sleep and decreased the total amount of wakefulness | |||||||||
| 14 | [27] |
In vivo and In vitro |
ddY mice and SD rats | Mice | Retinal ganglion cells | Crocetin | 10–100 mg/kg | 5 days | Caspase activity, mitochondrial membrane potential | Suppression of caspase-3 and -9 following retinal damage | Crocetin prevents retinal degeneration | |||||||||
| 15 | [20] | In vivo | Chinchilla rabbits | rabbits | - | Saffron | 0.5 mL into each eye | 20 days | Lipid peroxidation products (HP and malondialdehyde) | Antioxidative function | Saffron decreased accumulation of lipid peroxidation products | |||||||||
| 16 | [22] | In vivo | Sprague-Dawley rats | 3 rats | - | Saffron | 1 mg/kg | 1 week | fERGs, ONL, fibroblast growth factor | Cell death | Saffron may protect photoreceptors from retinal stress, maintaining both morphology and function | |||||||||
| 17 | [23] | In vitro | bovine retinas | - | Primary retinal cell cultures | Crocin | 0–160 μM | 2 weeks | Green nucleic acid stain assay, | Neuroprotective effect | Crocin protects retinal photoreceptors against light-induced cell death. | |||||||||
| 18 | [24] | In vivo | mice | - | - | Crocin | 0.25-5 mg kg | 4 weeks | visual acuity and visual contrast sensitivity |
Anti-photodamage and cytoprotective effects |
Crocin improved visual acuity and visual contrast sensitivity |
|||||||||
| 19 | [38] | In vivo | - | 32 Rats | Retinal ganglion cells | Crocin | 20 mg/kg | 4 weeks | IOP, Retinal thickness, TUNEL assay | Neuroprotective Effect | Crocin inhibited RGC apoptosis and optic nerve degeneration | |||||||||
| 20 | [25] |
In vitro and in vivo |
Sprague–Dawley Rat | Rat | Retinal ganglion cells | Crocin | 5, 25 and 50 mg/kg | 14 days | Apoptosis | PI3K/AKT signalling pathway | Crocin prevents retinal ischaemia/ reperfusion injury-induced apoptosis |
|||||||||
Abbreviations: IOP: intraocular pressure CRP: C-reactive protein, BCL: bright continuous light, LDH: lactic dehydrogenase, ROS: reactive oxygen species, MDA: malondialdehyde, GSH: Glutathione. SOD: superoxide dismutase. ONL: outer nuclear layer, 8-OHdG: 8-hydroxy-2-deoxyguanosine, MAPK: mitogen-activated protein kinases, ERK: extracellular signal-regulated protein kinases. JNK: c-Jun N-terminal kinases. NF-κB: transcription factor nuclear factor-kappa B, RP: retinitis pigmentosa. REM: rapid eye movement. HP: Hydroperoxides. RGC: retinal ganglion cell.
In 9 included animal studies, 4 articles were performed on rats, 4 on mice and 1 on rabbit. The main variable that was evaluated in these papers included fERG, and the results indicated that saffron has neuroprotective properties and protects the eyes of the rat. The proposed mechanism in these studies for the saffron neuroprotective property was the antagonistic effect of saffron on CB1 and CB2 hybrid cannabinoid receptors. In addition, saffron significantly prevented selenite-induced lipid peroxidation, protein oxidation, and increased superoxide dismutase, glutathione peroxidase, catalase, and glutathione levels in Wistar rat. Also, Saffron decreased the accumulation of lipid peroxidation products in the rabbit [19, 20]. Saffron may protect photoreceptors from retinal stress, maintaining both morphology and function in Sprague-Dawley rats and can protect photoreceptors from light-induced damage in mice retinal cells [21, 22].
Moreover, crocin decreased glucose level and improved visual acuity and visual contrast sensitivity and protected retinal photoreceptors against light-induced cell death. Crocin also prevented apoptosis induced by retinal ischaemia/reperfusion injury [23-25].
Crocetin prevented ischemia-induced retinal damage in mice and prevented retinal degeneration [26, 27].
4.2. Clinical Trials
Of the eight RCT articles, seven have been conducted on the therapeutic effects of saffron as capsules, tablets and/or oral solutions on eye disease, and only one article evaluated the effects of crocin in the form of tablet on ocular diseases. The most recent and old documents had been published in 2019 and 2010, respectively. A total of 406 patients were studied, of which 205 were men and 201 were women, and their age range varied from 50 years to 89. Also, the average duration of treatment was 12.5 weeks. In 6 of 8 articles, the effect of saffron has been evaluated on AMD, while in one
article, therapeutic effects of saffron have been evaluated on open angle glaucoma (OAG). Also in one article, the therapeutic potential of crocin has been evaluated on diabetic maculopathy. The quality assessment of included literature was also performed and the results are demonstrated in Table 2.
Table 2.
Quality assessment of articles according to Newcastle-Ottawa and Oxford quality scoring systems (Jadad).
| No. | Authors | Newcastle-Ottawa Score | Jadad Score |
|---|---|---|---|
| 1 | Broadhead GK et al., 2019 | 8 | 5 |
| 2 | Sepahi S et al., 2018 | 7 | 5 |
| 3 | Riazi A et al., 2017 | 7 | 4 |
| 4 | Lashy A et al., 2016 | 6 | 4 |
| 5 | Jabbarpoor Bonyadi MH et al., 2014 | 7 | 4 |
| 6 | Marangoni D et al., 2013 | 6 | 4 |
| 7 | Falsini B et al., 2010 | 7 | 4 |
| 8 | Piccardi M et al., 2012 | 7 | 4 |
| 9 | Piccardi M et al., 2019 | 6 | 4 |
The maximum given score for Newcastle-Ottawa and Oxford quality scoring systems is 9 and 5, respectively.
In AMD, the effect of saffron (20 mg/day for 12 weeks) was investigated on the improvement of BCVA and fERG, and the results showed that saffron improved the retinal flicker sensitivity and BCVA in these patients compared to the control group. Also, evaluating the effect of saffron in patients with OAG showed that saffron in the form of capsule and liquid extract at a dose of 30 mg/day for 3 to 4 weeks caused a significant decrease in IOP in patients with OAG compared to the control group. In a study by Sepahi et al., the effect of crocin (5 and 15 mg/day), as the active ingredient of saffron, was investigated in diabetic maculopathy for 12 weeks. The results indicated that crocin improved BCVA and significantly reduced CMT in diabetic maculopathy patients in comparison with the control group. No specific side effects were reported for crocin and saffron in these studies.
Also, both treatment and control groups received the standard drugs (e.g. Bevacizumab, Ranibizumab, Aflibercept and Dorzolamide-timolol) for treatment of AMD, OAG and DME during the trial. Thus, the supplement therapy was reported comparatively. Further information extracted from the included RCT articles is presented in Table 3.
Table 3.
General information of the Clinical trial articles included in this review.
| No | Refs. | Design |
Sex
M/F |
Mean Age/
Age Range (Years) |
Number of Patients | Disease | Herbal Product | Formulation | Dosage | Study Variables | Duration of Treatment | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [39] | Double-blind, pilot study | 14/17 | - | 31 | Macular Dystrophy (STG/FF patients) | Saffron | Extract | 20 mg/day | visual acuity and fERG | 20 weeks | SProgression of central retinal dysfunction in ABCA4-related STG/FF |
| 2 | [40] | Double-blind | 51/49 | 73.9/51.0–89.7 | 100 | AMD | Saffron | Tablet | 20 mg/day | BCVA, mfERG | 12 weeks | Retinal flicker sensitivity and BCVA improved |
| 3 | [41] | Double-blind | 29/31 | 55.85/41-82 | 60 | DME | Crocin | Tablet | 15 and 5 mg/day | BCVA and CMT | 12 weeks | BCVA improved and CMT decreased |
| 4 | [42] | Placebo-controlled | 23/31 | 69.57 | 54 | AMD | Saffron | Capsule | 50 mg/day | BCVA and CMT | 12 weeks | Visual function, especially contrast sensitivity improved |
| 5 | [43] | Double-blind | 24/16 | 66.87 | 40 | AMD | Saffron | Capsule | 30 mg/ day | BCVA, CMT, ERG | 24 weeks | Retinal function significantly improved |
| 6 | [44] | a pilot study | 21/13 | 66.3 /Older than 50 years | 34 | POAG | Saffron | Capsule | 30 mg/day | IOP | 4 weeks | IOP was significantly decreased |
| 7 | [45] | preliminary report | 15/18 | 68.4/ 51-85 | 33 | AMD | Saffron | Tablet | 20 mg/day | fERG amplitude and sensitivity | 12 weeks | retinal flicker sensitivity improved |
| 8 | [46] | open-label study | 16/13 | 55-85 years 69.3 ± 7 |
29 | AMD | Saffron | Pill | 20 mg/day | fERG | 12- 16 weeks | The mean fERG sensitivity improved |
| 9 | [47] | Double-blind | 12/13 | 65/54-84 | 25 | AMD | Saffron | Oral | 20 mg/day | fERG, | 12 weeks | Amplitude and retinal flicker sensitivity improved |
Abbreviations: AMD: Age-related Macular degeneration, BCVA: best-corrected visual acuity, mfERG: multifocal electroretinogram, IOP: intraocular pressure. DME: Diabetic Macular Edema. CMT: central macular thickness. POAG: Primary open angle glaucoma.
5. DISCUSSION AND CONCLUSION
Herbal medicines are commonly used in the management of most diseases; however, the treatment of eye disorders with medicinal plants and herbal products has been less considered. Due to the importance of eye diseases and considering the effect of treatment method on visual acuity, optimal medicine regimen and treatment are recommended. Herbal medicines have usually fewer side effects, compared to chemical agents, and a low-risk therapeutic approach is accompanied with more acceptances by the patients. Thus, the benefits of herbal medicines led to more investigations on their pharmacological properties [48-50].
In this review, the results of in vitro studies showed that crocin protects retinal cells through neuroprotective effect and inhibition of oxidative stress. Mechanistic studies on retino-protective effects of crocin have shown that it can inhibit oxidative stress, inflammation, and cell apoptosis by activating the PI3K / AKT signaling pathway [25, 30]. In addition, the results of animal studies showed that crocin improves visual activity, protects retinal photoreceptors and inhibits oxidative stress in the rat's eye through neuroprotective, antiphotodamage, and cytoprotective effects [20, 23, 24, 36, 38].
Studies have also shown that crocin protects the retinal ganglion cells against H2O2-induced ischemia through mitochondrial pathway and activation of nuclear factor κB (NF-κB) and inhibition of ROS and LDH [29, 33, 35]. Also, crocin with its neuroprotective effect protects retinal photoreceptors from light-induced cell death [35]. A similar study on the effect of saffron on eye cellular photoreceptors showed that saffron at a dose of 5 mg/kg inhibited apoptosis and reduced ATP-induced intracellular calcium [21]. On the other hand, crocetin protected cells from retinal degeneration in the eye of the mouse model by inhibiting caspase activity [27]. Moreover, Safranal, another component of saffron, was shown to inhibit oxidative stress in the eyes of rat by protecting cells against retinal degeneration [36].
Animal studies on the effects of crocin and saffron showed that saffron improved fERG in the eyes of rat through mechanisms such as CB1 and CB2 receptor antagonism and its neuroprotective effect [29]. Also, saffron at a dose of 25 mg/kg and crocin at a dose of 100 mg/kg improved lipid profiles, reduced blood glucose and oxidative capacity, inhibited alpha-crystallization and glycation, and improved visual acuity in mice and rat by inhibiting oxidative stress. Saffron also protects the retina photoreceptors of rat against oxidative stress by alleviating the morphology and function of eye photoreceptors [31, 32]. It could prevent cataract (at the dose of 60 mg/kg) through protection of the eye cells against selenite-induced cataract (selenite depletion), lipid peroxidation, SOD, and malondialdehyde, in Wistar rats [19].
Moreover saffron (25 mg/kg/day) has a protective role against oxidative stress-induced retinal damage in the eyes of mice [29]. In addition, findings showed that crocin at the doses of 30 and 100 mg/kg/day has a critical role in locomotor activity in the eye of mice in sleep mode [37].
Investigation of the effect of different crocin analogues (crocin 1, crocin 2, crocin 3 and crocin 4 and crocetin) on ocular blood flow and retinal function showed that all crocin analogues except crocin 3 increased retinal blood flow after 120 min of drug instillation. Also all crocin analogues had a remarkable effect on choroidal blood flow. The results of this study showed that crocin analogues can be used in the treatment of ischemic retinopathy and AMD [51].
The results of the clinical section of this systematic study showed that saffron and its active compounds, such as crocin and crocetin, in their oral form, have been effective in reducing inflammation in eye diseases, such as diabetic retinopathy, OAG, and age-related macular edema. Of course, the effect of crocin, is dose-dependent, since crocin at a dose of 15 mg compared to 5 mg per day significantly reduced the thickness of macular degeneration in patients with diabetic retinopathy [41]. Also, the data showed that saffron (20-30 mg/day) has neuroprotective effects and reduces inflammation in ocular diseases such as AMD and OAG [40, 43-47]. The variables measured in these studies include BCVA, CMT, fERG and IOP. The BCVA was improved by saffron (20 mg/day) and crocin (15 mg/day) tablets. Crocin (15 mg/day) significantly reduced the CMT after 90 days’ clinical trial. Also, the capsule and aqueous extract of saffron (30 mg/day) led to a significant decrease in the IOP compared to the placebo group [41, 44].
The pathogenesis of diabetic retinopathy (DR) has not been completely elucidated; accumulating evidence suggests that the inflammatory reactions may play a major role. Microglia are the primary innate resident immune cells in the retina that are involved in the inflammatory changes causing DR. According to more studies, crocin significantly reduced gene expression of the pro-inflammatory markers IL6, CCL2, and iNOS in LPS-challenged BV-2 microglial cells and potently blocked NO production in these microglia cells [30, 52, 53].
High blood glucose, lack of control of fat and blood pressure, alcohol consumption and even gestational diabetes are the most important risk factors that cause diabetes-induced inflammatory eye diseases [54]. Therefore, it is expected that treatment modalities to improve the risk factors of eye diseases may help to prevent the occurrence of many ocular complications.
We sought to examine the evidence regarding the use of saffron and its constituents in age-related macular degeneration (AMD), diabetic retinopathy and glaucoma. We conclude that saffron and crocin play an important role in the inhibition of oxidative stress in more eye diseases. However, these results have shown that saffron and its constituents have therapeutic effects on eye disorder with neuroprotective and antioxidant activity. The efficacy of saffron is based on experiences, clinical outcomes, including animal testing, and clinical trials. It looks like that the therapeutic use of herbal molecules on ocular treatment could become realistic in the near future.
Acknowledgements
The support by Mashhad University of Medical Sciences is gratefully acknowledged.
list of Abbreviations
- AMD
Age-related Macular Degeneration
- BCVA
Best Corrected Visual Acuity
- CMT
Central Macular Thickness
- CSME
Clinically Significant Macular Edema
- fERG
focal Electroretinogram
- GSH
Glutathione
- IOP
Intraocular Pressure
- MDA
Malondialdehyde
- OAG
Open Angle Glaucoma
- ROS
Reactive Oxygen Species
- SOD
Superoxide Dismutase
- VA
Visual Acuity
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Qi L., Cai J., Mao D., Wang M., Ge X., Wu W., Jin X., Li C., Hua Y., Li M. Use of contrast-enhanced computed tomographic imaging to diagnose and evaluate Behçet’s disease with vascular complications. Exp. Ther. Med. 2019;18(6):4265–4272. doi: 10.3892/etm.2019.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozmen M.C. Is gabapentin effective in dry eye disease and neuropathic ocular pain? Acta Neurol. Belg. 2019;120(5):1215–1216. doi: 10.1007/s13760-019-01251-y. [DOI] [PubMed] [Google Scholar]
- 3.Sunness J.S. The natural history of geographic atrophy, the advanced atrophic form of age-related macular degeneration. Mol. Vis. 1999;5:25. [PubMed] [Google Scholar]
- 4.Subramanian M.L., Ness S., Abedi G., Ahmed E., Daly M., Feinberg E., Bhatia S., Patel P., Nguyen M., Houranieh A. Bevacizumab vs ranibizumab for age-related macular degeneration: early results of a prospective double-masked, randomized clinical trial. Am. J. Ophthalmol. 2009;148(6):875–882. doi: 10.1016/j.ajo.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Olalla M., Laura G-Q., Andrea L-R., Anxo F-F., Ana L-P., Maximino J.A., María J.L., Ángel C. Anti-VEGF Treatment and response in age-related macular degeneration: disease’s susceptibility, pharmacogenetics and pharmacokinetics. Curr. Med. Chem. 2019;26:1–20. doi: 10.2174/0929867326666190711105325. [DOI] [PubMed] [Google Scholar]
- 6.Papapostolou I., Lommatzsch A.P., Farecki M.L., Ziegler M., Gutfleisch M., Pauleikhoff D. Are there different phenotypes in geographic atrophy of amd? - pilot study on differentiation using multimodal imaging. Klin. Monbl. Augenheilkd; 2019. [Epub a head of Print] [DOI] [PubMed] [Google Scholar]
- 7.Ba J., Peng R.S., Xu D., Li Y.H., Shi H., Wang Q., Yu J. Intravitreal anti-VEGF injections for treating wet age-related macular degeneration: a systematic review and meta-analysis. Drug Des. Devel. Ther. 2015;9:5397–5405. doi: 10.2147/DDDT.S86269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas R.L., Halim S., Gurudas S., Sivaprasad S., Owens D.R. IDF Diabetes Atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res. Clin. Pract. 2019;157:107840. doi: 10.1016/j.diabres.2019.107840. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Q., Guo C., You A., Wang D., Wang W., Zhang X. One-year outcomes of novel VEGF decoy receptor therapy with intravitreal conbercept in diabetic retinopathy-induced macular edema. Mol. Vis. 2019;25:636–644. [PMC free article] [PubMed] [Google Scholar]
- 10.Singh R.P., Elman M.J., Singh S.K., Fung A.E., Stoilov I. Advances in the treatment of diabetic retinopathy. J. Diabetes Complications. 2019;33(12):107417. doi: 10.1016/j.jdiacomp.2019.107417. [DOI] [PubMed] [Google Scholar]
- 11.Sadat M.N.G., Razeghinejad R., Janghorbani M., Mohamadian A., Hassan J.M., Bazdar S., Salehi A., Molavi V.H. Prevalence, incidence and ecological determinants of diabetic retinopathy in iran: systematic review and meta-analysis. J. Ophthalmic Vis. Res. 2019;14(3):321–335. doi: 10.18502/jovr.v14i3.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liew G., Wong V.W., Saw M., Tsang T.E., Nolan T., Ong S., Ho I.V. Profile of a population-based diabetic macular oedema study: the Liverpool Eye and Diabetes Study (Sydney). BMJ Open. 2019;9(1):e021884–e021884. doi: 10.1136/bmjopen-2018-021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tham Y.C., Li X., Wong T.Y., Quigley H.A., Aung T., Cheng C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Ichhpujani P., Thakur S., Spaeth G.L. Contrast sensitivity and glaucoma. J. Glaucoma. 2019;29(1):71–75. doi: 10.1097/IJG.0000000000001379. [DOI] [PubMed] [Google Scholar]
- 15.Nodehi- Moghadam A.; Goudarzian, M.; Azadi, F.; Nasiri, A.; Hosseini, SM.; Geranmayeh, S.; Larne, Y.; Habibi, M.; Yaghmaei, P. Prevalence of eye disorders in elderly population of Tehran, Iran. Elder. Health. J. 2015;1(2):46–51. [Google Scholar]
- 16.Matsuura K., Miyazaki D., Sasaki S.I., Inoue Y., Sasaki Y., Shimizu Y. Effectiveness of intraoperative iodine in cataract surgery: cleanliness of the surgical field without preoperative topical antibiotics. Jpn. J. Ophthalmol. 2019;64(1):37–44. doi: 10.1007/s10384-019-00703-5. [DOI] [PubMed] [Google Scholar]
- 17.World Health, O. State of the world’s sight: VISION 2020: the Right to Sight: 1999-2005. Geneva: World Health Organization; 2005. [Google Scholar]
- 18.Amaniti A., Dalakakis I., Gkinas D., Sapalidis K., Grosomanidis V., Papazisis G. Corrigendum to “Bradycardia Leading to Asystole following dexmedetomidine infusion during cataract surgery: dexmedetomidine-induced asystole for cataract surgery”. Case Rep. Anesthesiol. 2019;2019:7254218. doi: 10.1155/2019/7254218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makri O.E., Ferlemi A.V., Lamari F.N., Georgakopoulos C.D. Saffron administration prevents selenite-induced cataractogenesis. Mol. Vis. 2013;19:1188–1197. [PMC free article] [PubMed] [Google Scholar]
- 20.Shukurova P., Babaev R. A study into the effectiveness of the application of saffron extract in ocular pathologies in experiment. Georgian Med. News. 2010;(182):38–42. [PubMed] [Google Scholar]
- 21.Corso L., Cavallero A., Baroni D., Garbati P., Prestipino G., Bisti S., Nobile M., Picco C. Saffron reduces ATP-induced retinal cytotoxicity by targeting P2X7 receptors. Purinergic Signal. 2016;12(1):161–174. doi: 10.1007/s11302-015-9490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maccarone R., Di Marco S., Bisti S. Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. Invest. Ophthalmol. Vis. Sci. 2008;49(3):1254–1261. doi: 10.1167/iovs.07-0438. [DOI] [PubMed] [Google Scholar]
- 23.Laabich A., Vissvesvaran G.P., Lieu K.L., Murata K., McGinn T.E., Manmoto C.C., Sinclair J.R., Karliga I., Leung D.W., Fawzi A., Kubota R. Protective effect of crocin against blue light- and white light-mediated photoreceptor cell death in bovine and primate retinal primary cell culture. Invest. Ophthalmol. Vis. Sci. 2006;47(7):3156–3163. doi: 10.1167/iovs.05-1621. [DOI] [PubMed] [Google Scholar]
- 24.Liou J-C., Yang S-L., Wang P-H., Wu J-L., Huang Y-P., Chen B-Y., Lee M-C. Protective effect of crocin against the declining of high spatial frequency-based visual performance in mice. J. Funct. Foods. 2018;49:314–323. doi: 10.1016/j.jff.2018.08.031. [DOI] [Google Scholar]
- 25.Qi Y., Chen L., Zhang L., Liu W.B., Chen X.Y., Yang X.G. Crocin prevents retinal ischaemia/reperfusion injury-induced apoptosis in retinal ganglion cells through the PI3K/AKT signalling pathway. Exp. Eye Res. 2013;107:44–51. doi: 10.1016/j.exer.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Ishizuka F., Shimazawa M., Umigai N., Ogishima H., Nakamura S., Tsuruma K., Hara H. Crocetin, a carotenoid derivative, inhibits retinal ischemic damage in mice. Eur. J. Pharmacol. 2013;703(1-3):1–10. doi: 10.1016/j.ejphar.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi M., Tsuruma K., Imai S., Nakanishi T., Umigai N., Shimazawa M., Hara H. Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. Eur. J. Pharmacol. 2011;650(1):110–119. doi: 10.1016/j.ejphar.2010.09.081. [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Albarral J.A., Ramírez A.I., de Hoz R., López-Villarín N., Salobrar-García E., López-Cuenca I., Licastro E., Inarejos-García A.M., Almodóvar P., Pinazo-Durán M.D., Ramírez J.M., Salazar J.J. Neuroprotective and anti-inflammatory effects of a hydrophilic saffron extract in a model of glaucoma. Int. J. Mol. Sci. 2019;20(17):4110. doi: 10.3390/ijms20174110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doumouchtsis E.K., Tzani A., Doulamis I.P., Konstantopoulos P., Laskarina-Maria K., Agrogiannis G., Agapitos E., Moschos M.M., Kostakis A., Perrea D.N. Effect of saffron on metabolic profile and retina in apolipoprotein e-knockout mice fed a high-fat diet. J. Diet. Suppl. 2018;15(4):471–481. doi: 10.1080/19390211.2017.1356417. [DOI] [PubMed] [Google Scholar]
- 30.Yang X., Huo F., Liu B., Liu J., Chen T., Li J., Zhu Z., Lv B. Crocin inhibits oxidative stress and pro-inflammatory response of microglial cells associated with diabetic retinopathy through the activation of pi3k/akt signaling pathway. J. Mol. Neurosci. 2017;61(4):581–589. doi: 10.1007/s12031-017-0899-8. [DOI] [PubMed] [Google Scholar]
- 31.Maccarone R., Rapino C., Zerti D., di Tommaso M., Battista N., Di Marco S., Bisti S., Maccarrone M. Modulation of Type-1 and Type-2 cannabinoid receptors by saffron in a rat model of retinal neurodegeneration. PLoS One. 2016;11(11):e0166827. doi: 10.1371/journal.pone.0166827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahmani F., Bathaie S.Z., Aldavood S.J., Ghahghaei A. Inhibitory effect of crocin(s) on lens α-crystallin glycation and aggregation, results in the decrease of the risk of diabetic cataract. Molecules. 2016;21(2):143–143. doi: 10.3390/molecules21020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lv B., Chen T., Xu Z., Huo F., Wei Y., Yang X. Crocin protects retinal ganglion cells against H2O2-induced damage through the mitochondrial pathway and activation of NF-κB. Int. J. Mol. Med. 2016;37(1):225–232. doi: 10.3892/ijmm.2015.2418. [DOI] [PubMed] [Google Scholar]
- 34.Chen L., Qi Y., Yang X. Neuroprotective effects of crocin against oxidative stress induced by ischemia/reperfusion injury in rat retina. Ophthalmic Res. 2015;54(3):157–168. doi: 10.1159/000439026. [DOI] [PubMed] [Google Scholar]
- 35.Di Marco F., Di Paolo M., Romeo S., Colecchi L., Fiorani L., Spana S., Stone J., Bisti S. Combining neuroprotectants in a model of retinal degeneration: no additive benefit. PLoS One. 2014;9(6):e100389. doi: 10.1371/journal.pone.0100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernández-Sánchez L., Lax P., Esquiva G., Martín-Nieto J., Pinilla I., Cuenca N. Safranal, a saffron constituent, attenuates retinal degeneration in P23H rats. PLoS One. 2012;7(8):e43074. doi: 10.1371/journal.pone.0043074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masaki M., Aritake K., Tanaka H., Shoyama Y., Huang Z.L., Urade Y. Crocin promotes non-rapid eye movement sleep in mice. Mol. Nutr. Food Res. 2012;56(2):304–308. doi: 10.1002/mnfr.201100181. [DOI] [PubMed] [Google Scholar]
- 38.Lyu B., Xu Z., Chen T., Shi J., Yang X. 2016.
- 39.Piccardi M., Fadda A., Martelli F., Marangoni D., Magli A., Minnella A.M., Bertelli M., Di Marco S., Bisti S., Falsini B. Antioxidant saffron and central retinal function in abca4-related stargardt macular dystrophy. Nutrients. 2019;11(10):2461. doi: 10.3390/nu11102461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broadhead G.K., Grigg J.R., McCluskey P., Hong T., Schlub T.E., Chang A.A. Saffron therapy for the treatment of mild/moderate age-related macular degeneration: a randomised clinical trial. Graefes Arch. Clin. Exp. Ophthalmol. 2019;257(1):31–40. doi: 10.1007/s00417-018-4163-x. [DOI] [PubMed] [Google Scholar]
- 41.Sepahi S., Mohajeri S.A., Hosseini S.M., Khodaverdi E., Shoeibi N., Namdari M., Tabassi S.A.S. Effects of crocin on diabetic maculopathy: a placebo-controlled randomized clinical trial. Am. J. Ophthalmol. 2018;190:89–98. doi: 10.1016/j.ajo.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Riazi A., Panahi Y., Alishiri A.A., Hosseini M.A., Zarchi A.A.K., Sahebkar A. The impact of saffron (Crocus sativus) supplementation on visual function in patients with dry age-related macular degeneration. Ital. J. Med. 2017;11(2):196–201. [Google Scholar]
- 43.Lashay A., Sadough G., Ashrafi E., Lashay M., Movassat M., Akhondzadeh S. Short-term outcomes of saffron supplementation in patients with age-related macular degeneration: a double-blind, placebo-controlled, randomized trial. Med. Hypothesis Discov. Innov. Ophthalmol. 2016;5(1):32–38. [PMC free article] [PubMed] [Google Scholar]
- 44.Jabbarpoor Bonyadi M.H., Yazdani S., Saadat S. The ocular hypotensive effect of saffron extract in primary open angle glaucoma: a pilot study. BMC Complement. Altern. Med. 2014;14:399. doi: 10.1186/1472-6882-14-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marangoni D., Falsini B., Piccardi M., Ambrosio L., Minnella A.M., Savastano M.C., Bisti S., Maccarone R., Fadda A., Mello E., Concolino P., Capoluongo E. Functional effect of Saffron supplementation and risk genotypes in early age-related macular degeneration: a preliminary report. J. Transl. Med. 2013;11:228. doi: 10.1186/1479-5876-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piccardi M., Marangoni D., Minnella A.M., Savastano M.C., Valentini P., Ambrosio L., Capoluongo E., Maccarone R., Bisti S., Falsini B. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: sustained benefits to central retinal function. Evid. Based Complement. Alternat. Med. 2012;2012:429124. doi: 10.1155/2012/429124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falsini B., Piccardi M., Minnella A., Savastano C., Capoluongo E., Fadda A., Balestrazzi E., Maccarone R., Bisti S. Influence of saffron supplementation on retinal flicker sensitivity in early age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2010;51(12):6118–6124. doi: 10.1167/iovs.09-4995. [DOI] [PubMed] [Google Scholar]
- 48.Aziz S., Aeron A., Kahil T. Health benefits and possible risks of herbal medicine. Microbes in Food and Health. Springer; 2016. pp. 97–116. [Google Scholar]
- 49.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karimi A., Majlesi M., Rafieian-Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J. Nephropharmacol. 2015;4(1):27–30. [PMC free article] [PubMed] [Google Scholar]
- 51.Xuan B., Zhou Y.H., Li N., Min Z.D., Chiou G.C. Effects of crocin analogs on ocular blood flow and retinal function. J. Ocul. Pharmacol. Ther. 1999;15(2):143–152. doi: 10.1089/jop.1999.15.143. [DOI] [PubMed] [Google Scholar]
- 52.Yorgun M.A., Rashid K., Aslanidis A., Bresgen C., Dannhausen K., Langmann T. Crocin, a plant-derived carotenoid, modulates microglial reactivity. Biochem. Biophys. Rep. 2017;12:245–250. doi: 10.1016/j.bbrep.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang J.W., Chung H., Chan Kim H. Correlation of optical coherence tomographic hyperreflective foci with visual outcomes in different patterns of diabetic macular edema. Retina. 2016;36(9):1630–1639. doi: 10.1097/IAE.0000000000000995. [DOI] [PubMed] [Google Scholar]
- 54.Kim C. Gestational diabetes: risks, management, and treatment options. Int. J. Womens Health. 2010;2:339–351. doi: 10.2147/IJWH.S13333. [DOI] [PMC free article] [PubMed] [Google Scholar]