Abstract
Background
Trigeminal neuralgia is a chronic disease characterized by intense facial pain that is caused by trigeminal nerve affectation. It usually affects adults from 50 years of age, and is more frequent in women. Additionally, it presents serious psychological effects that often lead to depression, which is why it is considered highly disabling. The therapeutic approach is based on the modification of nerve activity through electrical, surgical or chemical stimulation in specific regions of the nervous system.
Objective
To perform a meta-analysis of the scientific literature related to invasive and non-invasive electrical neuromodulation of trigeminal neuralgia, in order to assess their effects over pain and adverse effects.
Methods
A literature search was conducted in 4 databases, followed by a manual search of articles on invasive or non-invasive electrical neuromodulation to control the pain of trigeminal neuralgia, including the last 15 years.
Results
Regarding non-invasive methods, clinical trials did not present enough results in order to perform a meta-analysis. Regarding invasive methods, clinical trials meta-analysis showed no statistical differences between different treatment methods. In all cases, improvements in patients' pain were reported, although results regarding adverse effects were variable.
Conclusion
In the treatment of trigeminal neuralgia, the continuous radiofrequency provides better short and medium-term results, but pulsed radiofrequency shows less adverse effects after treatment, and has better results in the long-term.
Keywords: Neuromodulation, trigeminal nerve diseases, trigeminal neuralgia, transcutaneous electrical nerve stimulation, radiofrequency therapy, meta analysis
1. INTRODUCTION
Neuropathic pain is pain that arises as a direct consequence of a lesion or diseases affecting the somatosensory system [1]. Neuropathic pain is diagnosed based on common neurologic signs and symptoms. It is best treated with a combination of multiple therapeutic approaches, and treatments including conservative, complementary, medical, interventional, and surgical treatment modalities [2].
Trigeminal neuralgia is a physiopathological condition defined as sudden, severe, brief, stabbing, and recurrent pain within the distribution of one or more branches of the trigeminal nerve [3-6]. It is a severe facial pain disorder that has been studied for decades [7]. It is characterized by recurrent unilateral brief electric shock-like pains, abrupt in onset and termination, limited to the distribution of one or more divisions of the trigeminal nerve and triggered by innocuous stimuli. It may develop without apparent cause or be a result of another diagnosed disorder. Additionally, there may be concomitant continuous pain of moderate intensity within the distribution of the affected nerve division [8]. Middle-aged adults mainly suffer from it, since an important risk factor of this disease is being over 50 years of age; only 1% of those affected are under 20 years of age when trigeminal neuralgia is diagnosed. It affects more women than men, with a ratio of 2-3:1 [9, 10]. Ethnic or geographical predisposition is unknown [11-13].
Trigeminal neuralgia makes up the group of neuropathic pain conditions along with postherpetic neuralgia, painful diabetic peripheral neuropathy and glossopharyngeal neuralgia. It is is one of the most frequent conditions with an incidence of 2-13 per 100000 inhabitants per year [9, 14], and increasing up to 25 per 100,000 per year in people aged more than 70 years [6, 15, 16]. It is estimated that there are about 15000 to 40000 patients affected worldwide, though due to the misdiagnosed cases, the exact number is not known which should be much higher [17]. Trigeminal neuropathy has been seen in higher rates in those diagnosed with arterial hypertension, multiple sclerosis, Charcot-Marie-Tooth neuropathy, and glossopharyngeal neuralgia [15].
Headaches and facial pain can be difficult to diagnose and treat often because of the confluence of complex anatomic structures and sensory systems. The trigeminal cranial nerve (CN V) and its ophthalmic (CN V1), maxillary (CN V2), and mandibular (CN V3) divisions provide sensory innervation to the face via their cutaneous terminal branches. These branches relay sensory input and converge at the Gasserian ganglion (also known as trigeminal or semilunar ganglion) located in Meckel’s cave. Cutaneous branches of each such as the supraorbital, infraorbital, and mental nerves can be targeted for various therapeutic interventions [14].
The resulting facial pain, usually for a short period of time, occurs in multiple forms and can be described by the patients as “stabbing”, “electric shock”, “burning”, “pressure”, “crushing”, “explosion”, “shooting”, “shocking”, “migraine”, “drilling”, “puncture” or a combination of these [4, 16, 18-20]. Irrespective of the clinical manifestation, the quality of life of the subjects diagnosed with trigeminal neuralgia is severely affected by changing their daily activities and leading to different degrees of disability and periods of anxiety, which could go from moderate to severe. Pain can follow from an innocuous stimulus at the trigeminal nerve distribution [8]. It is mentioned that affected patients that develop certain disability degrees represent up to 45% of the total. These kinds of patients can be so affected that they need to stay away from their daily life activities up to 15 days in the last 6 months. It is important to state that the same study reported that one-third part of studied patients were having moderate to severe depression (35.7%), and half of those patients suffered from anxiety episodes directly related to trigeminal neuralgia (50%) [21]. Discomfort occurs despite the fact that in general, the pain completely disappears between episodes of crisis. Based on the aforementioned fact, trigeminal neuralgia is classified into type I and type II. The first type is characterized by pain-free periods between attacks and the second type is characterized by constant pain [19, 22-24].
It must be mentioned that anxiety attacks increase both in intensity and in frequency. It is because the episodes between crises, despite being “painless”, commonly trigger a lack of appetite or inability to speak, in addition to the fear caused by “being waiting” for the next episode of pain [23, 25].
On the other hand, an attempt has been made to establish a unified criterion for the classification of trigeminal neuralgia in such a way that establishes three criteria for its grouping: regarding the persistence of pain, which has already been mentioned, regarding the etiology of the neuralgia that will be discussed later and third, based on the type of vascular injury that precedes it [19, 26]. According to the authors, trigeminal neuralgia is classified into the usual offenders such as a superior cerebellar artery or anterior inferior cerebellar artery loop with or without a superior petrosal vein loop (group I). The unusual vascular offenders (group II, with a higher incidence of atypical pain and sensory impairment) comprise unusual arterial loops (group IIa), pure venous compressions (group IIb, which shows a higher incidence of postoperative hemorrhagic complications) and vascular diseases (group IIc) [26].
As previously mentioned, trigeminal neuralgia occurs on at least one or more of the three branches of the trigeminal nerves, in a way that in most cases, only one branch is affected, either the maxillary or mandibular nerve (60%), whereas in one-third of the cases, both branches are affected (35%). By contrast, a very small proportion of cases (4%), are related to patients with damage to the frontal branch of the trigeminal nerve [27]. Anyway, the direct contact of the vessels with specific fibres of the nerve plays a role in the genesis of the neuralgia [28]. It suggests an association between the location of the neurovascular conflict and its resulting distribution of the pain. The simplest explanation to these observations is that the nerve has some degree of the somatotopic organization of its fibres maintained along the root. As a consequence, the focal compression of the no neurovascular contact plays a role in the clinical manifestation of the neuralgia.
The suggested etiology for trigeminal neuralgia is extremely varied, including demyelination, perception irregularities at spinal cord level, axon compression, alteration in pain mechanisms at cortical and/or basal ganglia levels, particularly a decrease in the number of μ-opioid receptors, hyperpolarization of sensory neurons or neurovascular conflict, to mention a few examples [29, 30]. It is interesting to mention that those neuroplastic changes in the trigeminal neuralgia patients were confined to cortical systems associated with pain experience and modulation, especially associated with the μ-opioidergic system, arguably one of the mechanisms centrally involved in the regulation of multiple aspects of the pain experience [30, 31].
This causes trigeminal neuralgia to be classified as classical, idiopathic and secondary [32, 33]. It is important to mention that pain episodes are usually triggered by stimuli on the affected side of the face, which under normal conditions would be harmless, such as a light touch on the face, tooth brushing, chewing, cold wind on the face, and even normal activities like talking.
The idiopathic variants represent 10% of the affected patients and these, even after undergoing clinical studies such as MRI or different surgical procedures remain undiagnosed in terms of etiology [19]. The idiopathic variant is defined as no neurovascular contact (NVC) or NVC without morphological changes of the trigeminal root. In contrast, in the classical variant, the main feature is vascular conflict, especially vascular compression at the root of the trigeminal nerve, which in turn causes nerve root atrophy and/or displacement. The classical form of trigeminal neuralgia is defined as due to a neurovascular compression with morphological changes in the trigeminal root. Finally, the secondary variant is the most rare and tangential to tumors or vascular malformations, in addition to being associated with multiple sclerosis. It should be mentioned that this secondary variant is often preceded by bilateral trigeminal neuralgia, since most affected patients suffer from the classical unilateral variant. Also, two phenotypes were classified: purely paroxysmal trigeminal neuralgia (with paroxysmal pain only) and trigeminal neuralgia with concomitant continuous pain [19, 33].
It does suggest that the trigeminal nerve with a clear-cut vascular compression is not a general disease of the trigeminal system but a focal one. It may also be the case that biological and genetic factors create an increased fragility of the nerve in certain subjects and that the vascular conflict is only a precipitating factor [28].
Given the difference in etiological patterns suggested for trigeminal neuralgia, the therapeutic approach is also very different. In the classical variant, the initial treatment is pharmacological, based on a single dosage of anti-epileptic drugs, mainly carbamazepine or oxcarbazepine, with an effectiveness of up to 70% in reducing pain [5, 10, 34]. However, in non-respondent patients, more invasive treatments such as nerve blocking or surgical interventions may be possible [35]. Unfortunately, although neurosurgical treatments are capable of eliminating trigeminal neuralgia entirely, in some cases, 20-30% of patients do not show improvement 1-year post-surgery [36].
Additionally, it is really important to remember that most of the affected patients are older adults so polypharmacotherapy, common for this age group, often leads to undesirable drug interactions, which can include comorbidity and changes in the pharmacokinetics and the pharmacodynamics of the drugs used. Psychosocial comorbidities are known to play a significant role in the risk of developing, and progression of, chronic neuropathic pain syndromes. It is mentioned that there is a selectively altered affective circuit in patients with trigeminal neuralgia which may be related to their experience of negative affect and the comorbidity of mood and anxiety disorders seen in this population [36-38].
On the other hand, the side effects of anti-epileptic drugs often include central nervous system alterations such as loss of balance, nausea, sickness, drowsiness, kidney dysfunction, and/or heart rhythm disturbances, to mention a few examples; these are the situations that are detrimental to the quality of life of patients suffering from trigeminal neuralgia, and which in turn, create a vicious circle when receiving pharmaceutical treatment [36]. Thus, these medications often lead to side effects that lead to a reduction in use by 27% and 18% of responding patients, respectively [39]. This is why it is very important to look for non-pharmacochemical alternatives in order to treat this condition, whether they are invasive or not.
One of the most modern and widely used invasive methods to treat trigeminal neuralgia is radiofrequency (RF) thermocoagulation, which despite being considered safe and minimally invasive, often results in neuronal destruction and collateral complications. This method is based on the generation of a radiofrequency current that passes from the electrode to the target tissue, mobilizing tissue ions to the electrical field produced, causing the heating of the tissue and the subsequent coagulation and protein denaturation, in addition to neural destruction, and finally blocking the pain-signaling cascades. A variant of this method that has been widely accepted by health professionals and patients themselves is pulsed RF. This RF system generates an alternating current of 20 milliseconds in duration and 480 milliseconds intervals. This alternating treatment is proposed to produce a modulation of the pain instead of blocking it, so it is said to be a safe and effective treatment for combatting various forms of pain. However, this invasive method can cause severe side effects such as facial discomfort, dysesthesia, anesthesia dolorosa and corneal anesthesia. A very few studies have been published to date on the effects of RF in trigeminal neuralgia [36].
On the contrary, there are also other methods to treat this pathology that are non-invasive and non-pharmacological. Non-pharmacologic therapies have become a vital part of managing chronic pain. Although these can be used as stand-alone therapies, non-pharmacologic treatments often are used to augment and complement pharmacologic treatments. Non-pharmacologic approaches can be classified as behavioral, cognitive, integrative, and physical therapies (1). Among these non-invasive methods is the use of therapeutic laser, which reduces pain through the local reduction of histamine, acetylcholine, bradykinin and prostaglandins while leading to an increase in the concentration of serotonin, acetylcholinesterase, ATP, beta endocrines, enkephalins and aerobic metabolism, lymphatic drainage and pain threshold. However, there are only a few studies on the effect of laser in the management of trigeminal neuralgia pain. Additionally, transcranial direct current stimulation (tDCS) is another non-invasive method to choose in the treatment of trigeminal neuralgia. This method modulates cortical excitability of the motor cortex based on the direction of the electric current, which could be anodal or cathodal, while maintaining the neuromodulation effect even after electrical stimulation. In this way, there is a modulation membrane potential in neurons of the stimulated cortical area, mediated by N-methyl-D-aspartate (NMDA-R) receptors [40]. As another non-invasive, non-pharmacological treatment method, we can find prolonged transcutaneous electrical nerve stimulation (TENS). TENS has been widely used to induce hypoalgesia and for pain relief for more than 40 years [41, 42]. It is effective in reducing both acute and chronic pain, like in muscle and connective tissue disorder such as arthritis, backache, cervical pain, bursitis and in neurological conditions like causalgia, carpal tunnel syndrome, peripheral neuropathy and other miscellaneous disorders, according to medical literature [43]. TENS produces electro-analgesia probably by one or several of the following mechanisms: Presynaptic inhibition in the dorsal horn of the spinal cord, endogenous pain control (via endorphins, enkephalins, and dynorphins), and direct inhibition of an abnormally excited nerve and restoration of afferent input [44]. Also, TENS is inexpensive, non-invasive and safe with no major side effects and can be self-administered by patients following simple training. Hardly any studies are reported which have specifically used or recommended the use of TENS in the treatment of trigeminal neuralgia [43].
Due to the lack of information found on the effects of the different treatments of invasive and non-invasive neuromodulation in trigeminal neuralgia and its relationship with adverse effects, it was decided to carry out research that shed light on the effect of these methods on pain, and the occurrence of side effects after its application. To this end, it was decided to perform a meta-analysis in which, the advantages and disadvantages of invasive versus non-invasive methods in the treatment of trigeminal neuralgia were analyzed.
2. MATERIALS AND METHODS
2.1. Selection Criteria
This systematic review was performed in line with PRISMA recommendations, using the acronym PICO. Population: individuals suffering from trigeminal neuralgia. Intervention: invasive or non-invasive neuromodulation. Comparison: invasive or non-invasive neuromodulation or placebo. Outcomes: Variables on the existence and measure of subjective pain. Eligible studies to be used at the revision stage were randomized clinical trials conducted up to 15 years ago. Studies on invasive and non-invasive neuromodulation were included regardless of type and dose. In addition, only studies written in English or Spanish, which received at least three points in the Jadad scale, were selected. Such criteria ensure methodological and intervention quality, and provide specific information for health professionals treating these types of patients. Exclusion criteria were animal studies, reviews, clinical cases, cohort studies, case-control studies, conference abstracts, books and dissertations
2.2. Search and Selection of Articles
Three independent authors conducted an electronic search from 14 November to 20 December 2019. The electronic article search was carried out in PubMed/Medline, Pedro, Cochrane Central Register of Controlled Trials and Virtual Health Library databases. The search of complete articles was performed using the following search terms “trigeminal neuralgia”, “Trigeminal neuralgia AND electrotherapy”, “trigeminal neuralgia AND physical therapy modalities”, “trigeminal neuralgia neuromodulation”, “(“Transcutaneous Electric Nerve Stimulation” AND “Trigeminal Neuralgia”)”, “(“trigeminal neuralgia” OR “Facial Neuralgia” OR “Trigeminal Ganglion neuralgia”) AND (“Transcutaneous Electric Nerve Stimulation” OR “Neuromodulation Therapy” OR TENS)”, “(“Transcutaneous Electric Nerve Stimulation” OR TENS OR “electric neuromodulation” OR neuromodulation OR “Neuromodulation therapy”) AND (“trigeminal neuralgia” OR “trigeminal neuropathy” OR “facial neuralgia” OR “gasser neuralgia”)”, (“trigeminal neuralgia” AND “electrical stimulation”). Studies were first analyzed by carefully reading the titles and abstracts. After excluding those articles that did not meet the inclusion criteria, the remaining full texts were read critically and some of them were selected to be included in the results section of this study. Once identified, articles were selected according to the relevance of their title and abstract, and two independent reviewers critically read the full texts in order to determine if they were included. Consulting a third reviewer solved any discrepancies. In order to find more potential articles, a search in the references of the studies included was carried out. Duplicated references were manually eliminated.
2.3. Data Extraction
Data extracted were authors, year, sample, comparison, duration, pain outcomes and adverse effects. Data concerning the pain were selected both, as subjective pain sensation measured by Visual Analogic Scale (VAS), Numeric Pain Rating Scale (NPRS) or similar scales, and as percentage pain reduction or a number of pain-free patients after the intervention. Data on adverse effects were taken as a number of patients suffering from adverse effects. Those articles that did not clearly state the different adverse effects a patient could have, it was assumed that only one adverse effect occurred for each patient. Numerical data to perform the meta- analysis were extracted by a reviewer, and checked by another one. Differences of opinion between reviewers were resolved by discussion. Values were entered into Microsoft Office Excel 2013 data management program, and analyzed with Stata 14 (StataCorp).
In order to investigate the effects of neuromodulation on trigeminal neuralgia, several analyses were performed. The effects of invasive neuromodulation were studied from different perspectives: a comparative study of pulsed versus continuous radiofrequency over time; a comparison between different voltages for pulsed radiofrequency; and the occurrence of adverse effects in continuous and pulsed radiofrequency. Due to the lack of data reported (both raw and descriptive or inferential) in studies on non-invasive neuromodulation using laser therapy, and the impossibility of making comparisons with the only study found, which met the inclusion and exclusion criteria, on anodal transcranial direct current, meta-analyses on these issues could not be performed.
In relation to the effects of radiofrequency on pain and on the occurrence of adverse effects, several meta-analyses were performed using raw data on group size, patients with and without pain, and patients with and without adverse effects extracted directly from the articles, in order to obtain the risk difference by using DerSimonian and Laird random effects method. Application of Q-homogeneity tests was not considered necessary because a random-effects model was used, since this model considers the homogeneity of the studies in the analyses performed. Relative weights were used to be clear about the relative influence of each study [45]. A value of p≤0.05 was considered significant, and a 95% confidence interval was used.
2.4. Assessment of Methodological Quality
The methodological quality of the articles found was performed using the Jadad scale [46], which is normally used to assess the methodological quality of a clinical trial. The Jadad scale considers those aspects related to biases concerning randomization, masking and description of loss to follow-up. This questionnaire is rated on the scale from zero to five; the higher the score the better the methodological quality of the clinical trial assessed. A five-point randomized clinical trial is considered rigorous. A clinical trial is of poor quality if its score is lower than three points. This study considered those articles that scored three points or higher on the Jadad scale.
3. RESULTS
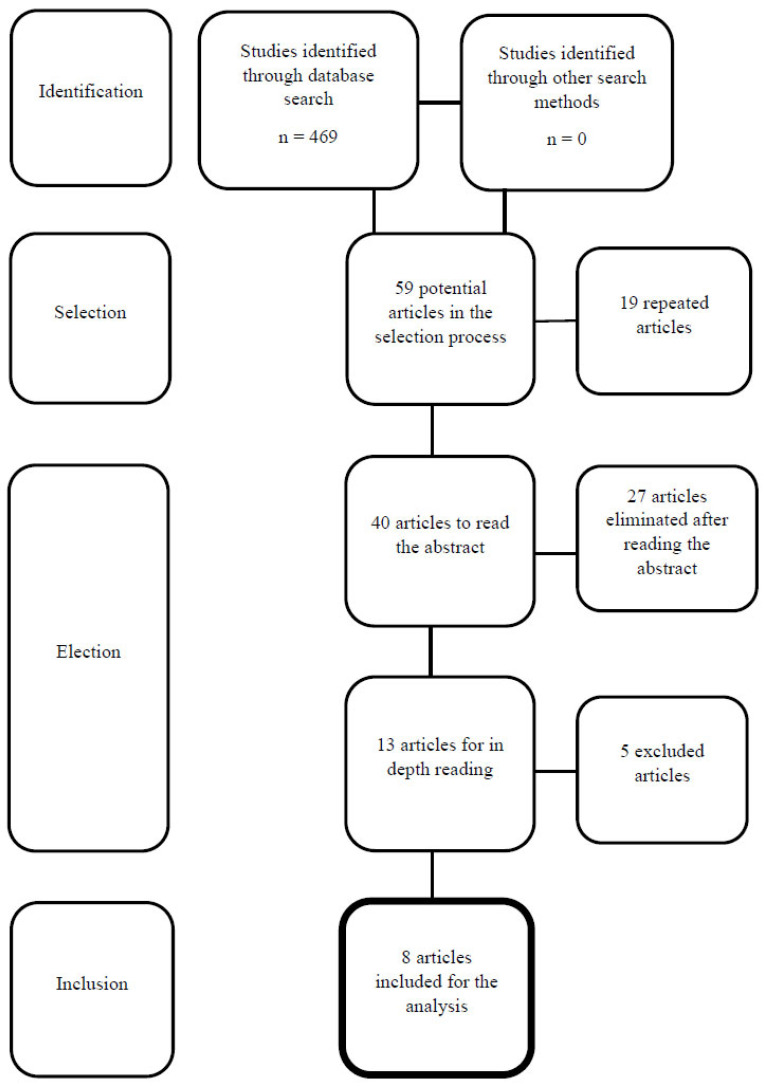
The search in the databases provided a total of 469 results. After reading the titles, 410 studies were discarded (the main reasons were the use of languages other than English or Spanish, use of other therapies or invalid types of study). Nineteen repeated articles were eliminated, and 40 studies were selected after reading the abstract. After their critical reading, 27 were discarded, as they did not meet the inclusion and exclusion criteria. Thus, 13 studies were carefully read and five of them were discarded because they did not show specific data on the subject to be reviewed. Finally, eight articles were retrieved for detailed reading and discussion. Fig. (1) shows the flowchart of the selection of articles of this review.
Fig. (1).
Study flowchart.
All of the articles included in this study obtained scores higher than three on the Jadad scale as an indicator of methodological quality. Other variables studied in the different articles, apart from the subjective pain sensation perceived by patients and the adverse effects found, were not considered, since they were not part of the objectives of this study. In general, the neuromodulation methods used were effective in reducing pain, and the research focused on the differences in the application of these methods. For invasive neuromodulation, the most usual was to investigate the effects of neuromodulation in trigeminal neuralgia using pulsed radiofrequency, either compared with conventional continuous radiofrequency or pulsed radiofrequency, but using different voltages. For non-invasive neuromodulation, the most usual was to investigate the effect of therapeutic laser in combination with medication, compared with the application of placebo laser in combination with medication. Most of the articles showed measurement results at different points in time, but in order to perform the meta-analysis, those time points with the same results were chosen. Regarding adverse effects, the most commonly reported were cephalea along the trigeminal nerve pathway, anesthesia with or without associated pain and facial dysesthesia and paresthesia. In addition, in some studies, in cases where intervention did not have the desired effect, either because of the small amount of pain reduction or due to the appearance of unpleasant adverse effects, the intervention had to be repeated but this time, the comparison intervention was applied in some cases. Adverse effects only occurred in invasive neuromodulation treatments. Studies concerning non-invasive neuromodulation did not report any adverse effects. The main characteristics and results of the selected articles are summarized in table 1. Unfortunately, no results regarding transcutaneous electrical nerve stimulation could be included in this review.
Table 1.
Summary of the analyzed articles.
| Invasive neuromodulation |
Study | Jadad scale | Sample | Comparison | Duration | Results on pain | Adverse effects |
|---|---|---|---|---|---|---|---|
| Erdine et al. (2007) [47] | 4 | 2 groups of 20 patients |
Pulsed vs. conventional Radiofrequency | 1 intervention - Pulsed Radiofrequency group: 2 minutes at 42º Conventional Radiofrequency group: 60 seconds at 70º |
- Pulsed radiofrequency group: Pain decreased only in 2 of the 20 patients and recurred 3 months later. - Conventional radiofrequency group: patient’s pain decreased in a statistically significant way (p<0.001) from an average of 9 (7-10) to an average of 1 (0-5). |
Pulsed radiofrequency group: 3 cases of cephalea Conventional radiofrequency group: - 5 cases of cephalea - 1 case de anesthesia dolorosa - 20 cases of hypoesthesia and paresthesia |
|
| Li et al. (2012) [48] | 4 | 3 groups of 20 patients |
Continuous pulsed Radiofrequency vs. long-duration continuous radiofrequency vs. short-duration continuous radiofrequency | 1 Ten minute intervention at 42º | There were no statistically significant differences between groups, but there were intra-group differences (p<0.001). In all three groups, the percentage of pain-free patients was over 70% in 12 months. |
100% of the cases showed: - facial dysesthesia - paresthesia - ipsilateral dry eye |
|
| Fang et al. (2015) [49] | 4 | 2 groups of 30 patients |
Standard voltage pulsed radiofrequency vs. high voltage pulsed radiofrequency | 1 intervention - High voltage group: 4 minutes at 42º, highest voltage tolerated by each patient - Standard voltage group: 4 minutes at 42º at 2Hz |
The immediate efficiency of treatment in the high voltage group was higher, but not statistically significant. However, differences were observed at one month, 3 months, 6 months (p=0.037) and one year (p=0.000): - Standard voltage pulsed radiofrequency group: 6 months after treatment, 41% of patients showed a favorable progress, although at the end of the year only 19% efficacy was achieved. - High voltage pulsed radiofrequency group: after one year, a significant decrease of pain was achieved in 69% of the patients, without any recurrence. |
No adverse effects were found in any case | |
| Luo et al. (2017) [50] | 5 | 2 groups of 30 patients |
Standard voltage pulsed radiofrequency vs. high voltage pulsed radiofrequency | 1 intervention - High voltage group: 2 minutes, 2 times, at 42º, highest voltage tolerated by each patient - Standard voltage group: 2 minutes, 2 times at 42º at 2Hz |
There were statistically significant differences between both groups at one month (p=0.028), 3 months (p=0.028), 6 months (p=0.015) and one year (p=0.007) after the application of the technique: - High voltage group: 7 patients with more than 50% pain decrease after one month, 2 patients after 3 months, 3 patients after 3 months and 7 patients after one year. The treatment response rate was 90%. - Standard voltage group: 2 patients with more than 50% pain decrease after one month, 2 patients after 3 months, 4 patients after 3 months y 5 patients after one year. The treatment response rate was 60-67%. |
- High voltage group: 8 cases of anesthesia in the infraorbital nerve area. - Standard voltage group: 4 cases of anesthesia in the infraorbital nerve area. |
|
| Elawami et al. (2017) [51] |
3 | 3 groups of 20, 11 and 12 patients respectively |
Continuous and pulsed radiofrequency vs. continuous radiofrequency at 75º vs. pulsed radiofrequency at 42º | 1 intervention - Continuous and pulsed radiofrequency group: 270 seconds at 60º - 75º continuous radiofrequency group: 270 seconds - 42º pulsed radiofrequency group: 10 minutes |
There were no statistically significant differences between groups, but there were intra-group differences: - Continuous and pulsed radiofrequency group: pain decreased from 9.15±1.13 to 0, 2 years after the intervention (p=0.000). - 75º continuous radiofrequency group: pain decreased from 9±0.89 to 1.18±0.18, 2 years after the intervention (p=0.0001). - 42º pulsed radiofrequency group: pain decreased from 8.67±2.53 to 1.83±0.36, 2 years after the intervention (p=0.0001). |
- Continuous and pulsed radiofrequency group: 5 cases of adverse effects. - 75º continuous radiofrequency group: 4 cases of adverse effects. - 42º pulsed radiofrequency group: 3 cases of adverse effects. Bleeding, “fits”, hematomas, neuralgia, masseter weakness, dysesthesia, vomiting and the need to repeat the surgery were reported. |
|
|
Non-invasive
neuromodulación |
Amanat et al. (2013) [52] | 3 | 2 groups of 12 and 14 patients respectively |
Laser in combination with carbamazepine vs. placebo laser in combination with carbamazepine | 10 sessions 3 times a week. - Carbamazepine: According to doctor´s prescription to each patient - Laser: 5 minutes at 12 mW and 12.73 J/cm2 |
The difference between the two groups was not significant. Both groups showed a significant decrease in pain over time (p<0.0001). In the group where laser was used in combination with carbamazepine: pain decreased from 7.7±1.9 to 3.5±2.8 after 2-4 months. In the group where placebo laser was used in combination with carbamazepine: pain decreased from 7.5±2.3 to 3.8±3.7 after 2-4 months. |
No adverse effects were found in any case |
| Ebrahimi et al. (2018) [40] | 3 | 2 groups of 15 patients |
Laser in combination with carbamazepine vs. placebo laser in combination with carbamazepine | 9 Sessions 3 times a week. - Carbamazepine: 100 mg at the beginning and 100 mg after 2 days, to control pain. - Laser: 25 seconds at 200 mW and 5 J/cm2 |
The severity of pain was less at the end of the treatment in the group where laser was used in combination with carbamazepine, than in the placebo group (p=0.003). Pain in both groups decreased over time (from the beginning to the end of the treatment) and this decrease was statistically significant (in the experimental group from 6/8 to 1/ 2 and in the control group from 6/6 to 2/7) (p=0.003). Positive results only lasted 4 months (p=0.003). |
No adverse effects were found in any case | |
| Hagenacker et al. (2014) [53] | 3 | 2 groups of 10 patients |
Anodal transcraneal direct current vs. placebo | - 20 minutes a day for 14 days. | The difference in pain reduction between both groups was 29% (p=0.008) - Transcraneal current group: 18%±29 pre-post pain decrease - Placebo group: 11%±30.8 pre- post pain decrease |
No adverse effects were found in any case |
Of the articles selected, three did not show enough data to be included in a statistical analysis. Finally, five studies were included in the meta-analysis, in which the effects of continuous and pulsed radiofrequency, and standard and high voltage pulsed radiofrequency, were compared: Erdine et al. (2007) [47], Li et al. (2012) [48], Fang et al. (2015) [49], Luo et al. (2017) [50] and Elawamy et al. (2017) [51]. Analyses were performed to compare the risk of suffering from pain immediately after treatment (there seems to be a 51,92% greater risk of suffering from pain by using pulsed radiofrequency, but the difference is not statistically significant p=0.4117); the risk of suffering from pain one to three months after treatment (there seems to be a 28.36% greater risk of suffering from pain by using pulsed radiofrequency, although the difference is not statistically significant p=0.3393); and, the risk of suffering from pain six to 12 months after treatment (in this case, there seems to be a 9.34% lower risk of suffering from pain by using pulsed reradiofrequency, although the difference is not statistically significant p=0.2846) (Table 2). Regarding the different voltage in pulsed radiofrequency, there seems to be a 26,67% and 30% greater risk of suffering from pain at three and six months after treatment respectively by using standard voltage pulsed radiofrequency, which provided a statistically significant difference (p=0.0009 and p=0.0002) (Table 3). In relation to the risk of suffering from adverse effects, pulsed radiofrequency showed a 31.66% lower risk of occurrence, although the difference was not significant (p=0.2997) (Table 4). No comparisons could be made between studies concerning non-invasive neuromodulation since the necessary data to perform a meta-analysis was not shown.
Table 2.
Risk of suffering from pain after treatment. Pulse radiofrequency vs. continuous radiofrequency.
| Measures were taken immediately after treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Risk difference | 95% Confidence interval | Sample weights Random-effects | Significance | ||||
| Lower limit | Upper limit | |||||||
| Erdine et al. (2007) | 0.85 | 0.69 | 1.01 | 50% | 0.4117 | |||
| Elawamy et al. (2017) | -0.08 | -0.24 | 0.07 | 50% | ||||
| Global | 0.5192 | 0.3202 | 0.7181 | 4.59 | ||||
| Chi-square test for heterogeneity =1 | 0.32 | |||||||
| - | ||||||||
| Measures were taken 1 to 3 months after treatment | ||||||||
| Study | Risk difference | 95% Confidence interval | Sample weights Random-effects | Significance | ||||
| Lower limit | Upper limit | |||||||
| Erdine et al. (2007) | 1 | 1 | 1 | 36.6% | 0.3393 | |||
| Li et al. (2012) | -0.03 | -0.19 | 0.14 | 34.4% | ||||
| Elawamy et al. (2017) | -0.57 | -0.90 | -0.23 | 29.0% | ||||
| Global | 0.2836 | 0.1193 | 0.4479 | 24.57 | ||||
| Chi-square test for heterogeneity = 10.39 | Variation % I2 due to heterogeneity= 80.75 | 0.0055 | ||||||
| - | ||||||||
| Measures were taken 6 to 12 months after treatment | ||||||||
| Study | Risk difference | 95% Confidence interval | Sample weights Random-effects | Significance | ||||
| Lower limit | Upper limit | |||||||
| Li et al. (2012) | -0.04 | -0.14 | 0.07 | 52.6% | 0.2846 | |||
| Elawamy et al. (2017) | -0.66 | -0.96 | -0.36 | 47.4% | ||||
| Global | -0.0934 | -0.2137 | 0.027 | 10.38 | ||||
| Chi-square test for heterogeneity = 1 | 0.3173 | |||||||
Table 3.
Risk of suffering from pain after treatment. High voltage pulsed radiofrequency vs. standard voltage pulsed radiofrequency.
| Measures were taken 3 months after treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Risk difference | 95% Confidence interval | Sample weights Random-effects | Significance | ||||
| Lower limit | Upper limit | |||||||
| Fang et al. (2015) | 0.3 | 0.08 | 0.52 | 49.4% | 0.0009 | |||
| Luo et al. (2017) | 0.23 | 0.01 | 0.45 | 50.6% | ||||
| Global | 0.2667 | 0.094 | 0.439 | 156.54 | ||||
| Chi-square test for heterogeneity = 0.17 | 0.6767 | |||||||
| - | ||||||||
| Measures were taken 6 months after treatment | ||||||||
| Study | Risk difference | 95% Confidence interval | Sample weights Random-effects | Significance | ||||
| Lower limit | Upper limit | |||||||
| Fang et al. (2015) | 0.3 | 0.08 | 0.52 | 51.4% | 0.0002 | |||
| Luo et al. (2017) | 0.3 | 0.07 | 0.53 | 48.6% | ||||
| Global | 0.3 | 0.1294 | 0.4706 | 150.53 | ||||
| Chi-square test for heterogeneity = 2.38e-31 | 1 | |||||||
Table 4.
Risk of suffering from adverse effects after treatment. Pulse radiofrequency vs. continuous radiofrequency.
| Measures were taken after treatment | |||||
|---|---|---|---|---|---|
| Study | Risk difference | 95% Confidence interval | Sample weights Random-effects | Significance | |
| Lower limit | Upper limit | ||||
| Erdine et al. (2007) | -0.85 | -1.01 | -0.69 | 25.4% | 0.2997 |
| Li et al. (2012) | 0 | 0 | 0 | 26.2% | |
| Fang et al. (2015) | 0 | 0 | 0 | 26.2% | |
| Elawamy et al. (2017) | -0.11 | -0.49 | 0.26 | 22.2% | |
| Global | -0.3166 | -0.4543 | -0.1788 | 18.49 | |
| Chi-square test for heterogeneity = 0.17 | 0.4991 | ||||
4. DISCUSSION
This investigation was conducted aiming at bringing some clarity in the treatment of trigeminal neuralgia through the different neuromodulation techniques, both in terms of the effects on pain and the adverse effects of the treatment.
Eight articles were selected to perform the meta-analysis, although only five of them were finally included. All of the articles received a high methodological quality, scoring three or more points on the Jadad scale [46].
The five articles included in the analysis were clinical trials assessing the effectiveness of radiofrequency and its different types, as a treatment for trigeminal neuralgia. Regarding the remaining articles, two studies assessed the effectiveness of laser therapy in combination with pharmacology; and the other one used anodal transcranial direct current as treatment for trigeminal neuralgia. These studies did not have enough data to be included in the meta-analysis, therefore it has not been possible to analyze whether or not there are differences between invasive and non-invasive neuromodulation treatments (Table 1).
Articles studying laser therapy in combination with pharmacology reported their efficiency and lack of adverse effects, which could make it an ideal therapy for this type of condition. However, the poor quality of the methodology when reporting the results makes it impossible to extract the data in order to establish a comparative statistical analysis. In both cases, a decrease in pain was significant, but it is noteworthy that the effects did not last more than four months; however, duration was reported for only this type of therapy (Table 2). In the case of anodal transcranial direct current, the nature of the therapy in combination with the fact that it was the only article found that studied this treatment and passed the Jadad scale, makes it inappropriate to include its numerical data in a comparative meta-analysis with a completely different technique. Making a comparison between two such different techniques would not lead to interesting results, or allow any useful inferences to be made for clinicians.
Radiofrequency ablation is currently one of the most widely used invasive neuromodulation techniques to treat trigeminal neuralgia, especially when the medical treatments have failed. Radiofrequency produces a rise in tissue temperature through a high-frequency current that circulates between two electrodes (one placed on the external surface and the other one on the tissue to be treated). This current generates an electric field that causes ionic vibrations, which produce thermal heat that consequently leads to the thermocoagulation of the tissue [50, 54, 55]. The procedure is easy and quick, lasting for about 10 minutes. However, computerized tomography equipment is necessary to guide the intervention as well as all the surgical material in order to correctly perform the technique [47-53, 55-59]. It is also a very recurrent technique for painful syndromes symptoms, such as headache. Despite the fact that it is not a cause of trigeminal nerve affectation, it has shown optimal results when compared with other therapies [58, 60, 61].
Several types of radiofrequency have been categorized in this study: pulsed radiofrequency, continuous radiofrequency, high voltage pulsed radiofrequency and standard voltage pulsed radiofrequency.
Regarding the results of the studies analyzed, in the study by Li et al. (2012) [48], it was found that both pulsed and continuous radiofrequency, of long and short duration, are effective in decreasing pain in trigeminal neuralgia. Furthermore, although 100% of participants suffered from side effects, 70% of them did not register pain relapse at twelve months. On the other hand, Erdine et al. (2007) [47] observed a statistically significant improvement in the conventional radiofrequency group, which obtained a decrease in pain average from nine to one, according to the VAS scale. Side effects were more frequent in conventional radiofrequency, but the treatment was more effective.
When comparing studies, pulsed radiofrequency was found to have a 51.92% greater risk of suffering from pain immediately after treatment than continuous radiofrequency. However, this data cannot be conclusive because a 51.92% is not indicative of a difference beyond possible chance, it is like flipping a coin (Table 2), and so a clear recommendation towards any technique cannot be established.
When assessing the risk of suffering from pain between the first and third month after treatment, Li et al. (2012) [48], Erdine et al. (2007) [47] and Elawamy et al. (2017) [51] observed 80.75% heterogeneity and therefore, the similarity between them cannot be accepted because the methodology used in the studies was highly diverse (Table 2). Thus, the results of these tests have to be taken with caution, without jumping to any hasty conclusions. At medium-term, it seems that it is more likely to suffer from pain after treatment using pulsed radiofrequency than continuous.
In contrast with the assessment at short and medium-term, when analyzing the data obtained on the risk of suffering from pain between six and twelve months after treatment, pulsed radiofrequency was observed to have a 9.34% lower risk (Table 2). In addition, when analyzing the risk of suffering from side effects between pulsed and continuous radiofrequency, pulsed radiofrequency showed a 31.66% risk of side effects occurrence after its treatment (Table 4).
In the light of the data reported in the analyses, and considering that they must be taken with caution since no statistically significant results were obtained for these variables, there seems that pulsed radiofrequency is worse at reducing pain at immediate and medium-term. However, pulsed radiofrequency shows better results than continuous radiofrequency in reducing pain in the long-term, and also has fewer adverse effects (Tables 2 and 4).
Fang et al. (2015) [49] and Luo et al. (2017) [50] tried to improve the approach to radiofrequency treatment by further standardizing the action protocol, more specifically the voltage in the application of the technique. In both protocols, standard and high voltage treatments were applied in groups. Temperature in all cases was 42 Celsius degrees; while standard voltage of 2Hz was applied, and the maximum tolerated by each patient was applied at high voltage. Not knowing the exact voltage applied on each patient and not establishing a standard protocol make the replication of the technique complex and increase the bias in the results.
In the first study, the immediate efficiency at medium and long-term was higher in the high voltage group, reaching a significant decrease in pain in 69% of the patients. In the second study, the same differences were found with the high voltage group having the best response rate after treatment (90%). In this latter study, patients whose pain reduced to zero according to the NRS (numerical rating scale), or those who had a reduction of pain of at least 50% were included. Of the 90% response rate, most reduced their pain to zero (20,25,24,20; at one month, three months, six months and one year respectively) however, a minority was also observed who only reduced it by 50% (7,2,3,7; at one month, three months, six months and one year respectively) [50].
After analyzing these studies, it seems that standard voltage pulsed radiofrequency treatment shows a greater risk of suffering from pain at three and six months after completing the treatment by 26.27% and 30% respectively, showing significant differences as well. Only this data showed enough statistic quality to draw a strong conclusion.
Other studies also evaluated patient satisfaction with regard to the temperature used during the application of radiofrequency; the lowest temperature was the most satisfactory with less side effects (75 degrees vs. 68 degrees) [57].
Hagenacker et al. (2014) [53] carried out an experimental study to assess the effectiveness of pain treatment in trigeminal neuralgia through anodal transcranial direct current. This study could not be included in the meta-analysis due to the lack of comparable data with the rest of the selected articles. This therapy stimulated a primary motor cortex daily for 20 minutes over 14 days using intensity 1 mA. In this case, pain intensity was reduced significantly after two weeks of treatment without any reported side effects (Table 1). It should also be added that this method has been used for decades with the aim of improving psychiatric conditions such as depression, pain that does not improve with the use of conventional therapies and post-stroke disorders. Furthermore, good results have been obtained in terms of reducing pain in fibromyalgia, painful phantom limb syndromes and multiple sclerosis [62-65]. Continuous transcranial direct current is a promising, non-invasive treatment that guarantees minimum adverse effects in patients who have already failed medical treatment; however, the same idea regarding lack of research and the need for more homogeneous protocols for the different studies is highlighted in all articles [65].
The two remaining articles, Ebrahimi et al. (2018) [40] and Amanat et al. (2013) [52], included in the analysis, assessed the treatment of trigeminal neuralgia with laser therapy. Both treatment protocols are different regarding the number of sessions, duration, dose and power used (9/weeks- 10/weeks, 25s - 5min, 5J/cm2- 12.73J/cm2, 200mW- 12mW; respectively). This makes it impossible to compare results in both studies, and we cannot assess which line of investigation is better to follow.
In both studies, laser therapy is supplemented with the pharmacological treatment of Carbamazepine. Ebrahimi et al. (2018) [40] recommend a dose of 100mg at the beginning of the treatment and another 100mg after two days if necessary, while Amanat et al. (2013) [52] indicate that the treatment with carbamazepine will depend on the patient and his needs. This fact causes a great bias when defining whether the treatment using laser therapy was effective or perhaps, just the pharmacology was enough to relieve the symptoms. It has to be emphasized that none of these studies registered adverse effects after treatment and that both, the experimental and the control groups improved over time. Ebrahimi et al. (2018) [40] showed less severity of pain in the experimental group than in the control, but these results only lasted for four months.
In both treatments, laser therapy was applied using different parameters. In diode laser, power can range between 0.1 and 5W. It is known that for more serious chronic processes, a higher power must be used rather than for acute processes; however, in this case, power ranged in very low values (0.2W and 0.12W) [66, 67].
On the other hand, carbamazepine blocks voltage-gated Na+ channels and reduces synaptic transmission. It is very effective in diabetic neuropathy but is most commonly prescribed to treat trigeminal neuralgia. It is considered as the first line of drug for the pain management of trigeminal neuralgia (95% CI: 1.2-2.2), having an effect on the frequency and intensity of pain. However, its complex pharmacology may interact with other drugs and result in a long list of moderate adverse effects such as nausea, drowsiness, loss of balance, dizziness and loss of appetite; or in more severe ones such as leukopenia, thrombocytopenia, hepatitis, skin toxicity and hyponatremia [40, 52, 54].
This study on laser therapy in combination with carbamazepine included participants suffering from trigeminal neuralgia, who were already receiving pharmacological therapy without success, but during the treatment, they were administered the same medication that is generally used in the treatment of this condition. Oxcarbazepine, structurally similar to carbamazepine, seems to have the same effects on trigeminal neuralgia, although having better patient satisfaction and fewer side effects. Perhaps the decrease in side effects and the increase in patient treatment adherence may report better results in future research [52, 68].
There are other neuromodulation therapies such as the use of TENS (transcutaneous electrical nerve stimulation), ultrasound, magneto-therapy, galvanotherapy, deep brain stimulation or direct motor cortex stimulation that entail the implantation of electrodes over the primary motor cortex, but insufficient scientific evidence was found on these therapies during the literature search period, or the methodological quality was not enough to include them in the analysis [58-60, 69].
Nowadays, thanks to the great progress in the field of technology, numerous instruments are emerging with the aim of improving the quality of life of people suffering from pain. For instance, such is the case of NEAS® X SIGNAL that emits low voltage electrical stimuli and is capable of stimulating the nervous system through its galvanic conductors. There is no scientific data or research on this technique at present, but it is important to note that it acts upon the autonomic nervous system improving the quality of sleep, increasing cell recovery and decreasing pain.
Electroacupuncture uses the TENS system in combination with traditional acupuncture, both in specific pressure points indicated by traditional Chinese medicine, and in myofascial trigger points described by Travell & Simons, thus treating painful syndromes [70-73]. Also, there is no evidence of this technique as a treatment for trigeminal neuralgia in one of the languages stipulated in the inclusion criteria of this paper, therefore it could not be discussed.
Numerous articles were found, which finally did not have the required methodological quality and had to be rejected for analysis (the score in the Jadad scale was lower than three). The remaining eight articles were analyzed, only obtaining claims from five of them. The other three did not have enough data to be included in the meta-analysis. This emphasizes the poor methodological quality of the different clinical trials recently published on invasive and non-invasive neuromodulation in trigeminal neuralgia. Systematic reviews of interventional treatments for patients with medically refractory trigeminal neuralgia have been published a few years ago [74-78]. Considered together, these evidence-based reviews conclude that surgical procedures directed at the peripheral trigeminal nerve are either ineffective or that there is insufficient evidence to demonstrate their effectiveness [79]. Sadly, we have no option but to agree, as the poor methodological quality shown in these articles is not enough to affirm results.
Much more research with higher methodological quality standards is needed, aimed at improving the approach to patients suffering from trigeminal neuralgia, in order to improve the impact of neuropathic pain on their lives.
CONCLUSION
There seems that continuous radiofrequency provides better short and medium-term results in the treatment of trigeminal neuralgia, but pulsed radiofrequency shows less adverse effects after treatment, and has better results in the long-term. Similarly, no data can be concluded since there were no significant differences in all results, and samples were very heterogeneous.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Xu L., Zhang Y., Huang Y. doi: 10.1007/978-94-017-7537-3_9. Translational Research in Pain and Itch. Advances in Experimental Medicine and Biology; Ma, C.; Huang, Y., Eds.; Springer: Dordrecht, 2016, Vol. 904, pp. 117-129. [DOI] [Google Scholar]
- 2.Jones R.C., III, Lawson E., Backonja M. Managing neuropathic pain. Med. Clin. North Am. 2016;100(1):151–167. doi: 10.1016/j.mcna.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Zurak N., Mahovic D. Idiopathic Trigeminal Neuralgia (ITN): Facts and Fiction. Psychiatr. Danub. 2019;31(Suppl. 5):724–731. [PubMed] [Google Scholar]
- 4.Maarbjerg S., Di Stefano G., Bendtsen L., Cruccu G. Trigeminal neuralgia - diagnosis and treatment. Cephalalgia. 2017;37(7):648–657. doi: 10.1177/0333102416687280. [DOI] [PubMed] [Google Scholar]
- 5.Gambeta E., Chichorro J.G., Zamponi G.W. Trigeminal neuralgia: An overview from pathophysiology to pharmacological treatments. Mol. Pain. 2020;16:1744806920901890. doi: 10.1177/1744806920901890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feller L., Khammissa R.A.G., Fourie J., Bouckaert M., Lemmer J. Postherpetic neuralgia and trigeminal neuralgia. Pain Res. Treat. 2017;2017:1681765. doi: 10.1155/2017/1681765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber K. Neuromodulation and devices in trigeminal neuralgia. Headache. 2017;57(10):1648–1653. doi: 10.1111/head.13166. [DOI] [PubMed] [Google Scholar]
- 8.Arnold M. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2008;38(1):1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 9.Türp J.C., Gobetti J.P. Trigeminal neuralgia versus atypical facial pain. A review of the literature and case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996;81(4):424–432. doi: 10.1016/S1079-2104(96)80018-7. [DOI] [PubMed] [Google Scholar]
- 10.Katusic S., Beard C.M., Bergstralh E., Kurland L.T. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945-1984. Ann. Neurol. 1990;27(1):89–95. doi: 10.1002/ana.410270114. [DOI] [PubMed] [Google Scholar]
- 11.Di Stefano G., Maarbjerg S., Truini A. Trigeminal neuralgia secondary to multiple sclerosis: from the clinical picture to the treatment options. J. Headache Pain. 2019;20(1):20. doi: 10.1186/s10194-019-0969-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Quliti K.W. Update on neuropathic pain treatment for trigeminal neuralgia. The pharmacological and surgical options. Neurosciences (Riyadh) 2015;20(2):107–114. doi: 10.17712/nsj.2015.2.20140501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oomens M.A.E.M., Forouzanfar T. Pharmaceutical management of trigeminal neuralgia in the elderly. Drugs Aging. 2015;32(9):717–726. doi: 10.1007/s40266-015-0293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antony A.B., Mazzola A.J., Dhaliwal G.S., Hunter C.W. Neurostimulation for the treatment of chronic head and facial pain: A literature review. Pain Physician. 2019;22(5):447–477. [PubMed] [Google Scholar]
- 15.Manzoni G.C., Torelli P. Epidemiology of typical and atypical craniofacial neuralgias. Neurol. Sci. 2005;26(Suppl. 2):s65–s67. doi: 10.1007/s10072-005-0410-0. [DOI] [PubMed] [Google Scholar]
- 16.Khan M., Nishi S.E., Hassan S.N., Islam M.A., Gan S.H. Trigeminal neuralgia, glossopharyngeal neuralgia, and myofascial pain dysfunction syndrome: An update. Pain Res. Manag. 2017;2017:7438326. doi: 10.1155/2017/7438326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tait R.C., Ferguson M., Herndon C.M. Chronic orofacial pain: Burning mouth syndrome and other neuropathic disorders. J Pain Manag Med. 2017;3(1):120. doi: 10.35248/2684-1320.17.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montano N., Conforti G., Di Bonaventura R., Meglio M., Fernandez E., Papacci F. Advances in diagnosis and treatment of trigeminal neuralgia. Ther. Clin. Risk Manag. 2015;11:289–299. doi: 10.2147/TCRM.S37592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruccu G., Finnerup N.B., Jensen T.S., Scholz J., Sindou M., Svensson P., Treede R.D., Zakrzewska J.M., Nurmikko T. Trigeminal neuralgia: New classification and diagnostic grading for practice and research. Neurology. 2016;87(2):220–228. doi: 10.1212/WNL.0000000000002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore D., Chong M.S., Shetty A., Zakrzewska J.M. A systematic review of rescue analgesic strategies in acute exacerbations of primary trigeminal neuralgia. Br. J. Anaesth. 2019;123(2):e385–e396. doi: 10.1016/j.bja.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zakrzewska J.M., Wu J., Mon-Williams M., Phillips N., Pavitt S.H. Evaluating the impact of trigeminal neuralgia. Pain. 2017;158(6):1166–1174. doi: 10.1097/j.pain.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 22.Debta P., Sarode G., Sarode S., Gadbail A., Debta F.M., Swain S.K., Mishra E., Sahu M.C. Natural history of trigeminal neuralgia-A hospital-based retrospective study. Oral Dis. 2019;26(3):647–655. doi: 10.1111/odi.13263. [DOI] [PubMed] [Google Scholar]
- 23.Obermann M. Recent advances in understanding/managing trigeminal neuralgia. F1000 Res. 2019;17(8) doi: 10.12688/f1000research.16092.1. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheshire W.P. Trigeminal neuralgia feigns the terrorist. Cephalalgia. 2003;23(3):230. doi: 10.1046/j.1468-2982.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- 25.Tölle T., Dukes E., Sadosky A. Patient burden of trigeminal neuralgia: Results from a cross-sectional survey of health state impairment and treatment patterns in six European countries. Pain Pract. 2006;6(3):153–160. doi: 10.1111/j.1533-2500.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- 26.Kumar K., Das K.K., Singh S., Khatri D., Deora H., Singh J., Bhaisora K., Srivastava A.K., Jaiswal A.K., Behari S. Vascular offenders in trigeminal neuralgia: A unified classification and assessment of the outcome of microvascular decompression. World Neurosurg. 2019;127:e366–e375. doi: 10.1016/j.wneu.2019.03.128. [DOI] [PubMed] [Google Scholar]
- 27.Henssen D., Dijk J., Knepflé R., Sieffers M., Winter A., Vissers K. Alterations in grey matter density and functional connectivity in trigeminal neuropathic pain and trigeminal neuralgia: A systematic review and meta-analysis. Neuroimage Clin. 2019;24:102039. doi: 10.1016/j.nicl.2019.102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sindou M., Brinzeu A. Topography of the pain in classical trigeminal neuralgia: Insights into somatotopic organization. Brain. 2020;143(2):531–540. doi: 10.1093/brain/awz407. [DOI] [PubMed] [Google Scholar]
- 29.DosSantos M.F., Love T.M., Martikainen I.K., Nascimento T.D., Fregni F., Cummiford C., Deboer M.D., Zubieta J.K., Dasilva A.F. Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Front. Psychiatry. 2012;3:93. doi: 10.3389/fpsyt.2012.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DosSantos M.F., Martikainen I.K., Nascimento T.D., Love T.M., Deboer M.D., Maslowski E.C., Monteiro A.A., Vincent M.B., Zubieta J.K., DaSilva A.F. Reduced basal ganglia μ-opioid receptor availability in trigeminal neuropathic pain: A pilot study. Mol. Pain. 2012;8:74. doi: 10.1186/1744-8069-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zubieta J.K., Smith Y.R., Bueller J.A., Xu Y., Kilbourn M.R., Jewett D.M., Meyer C.R., Koeppe R.A., Stohler C.S. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293(5528):311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 32.Kawasaki K., Sugawara S., Watanabe K., Hong C., Tu T.T.H., Watanabe T., Sakamoto J., Yoshino N., Suga T., Mikuzuki L., Takenoshita M., Takada S., Kurabayashi T., Toyofuku A. Differences in the clinical characteristics of persistent idiopathic facial pain (atypical odontalgia) patients with or without neurovascular compression of the trigeminal nerve. Pain Med. 2020;21(4):814–821. doi: 10.1093/pm/pnz300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bendtsen L., Zakrzewska J.M., Abbott J., Braschinsky M., Di Stefano G., Donnet A., Eide P.K., Leal P.R.L., Maarbjerg S., May A., Nurmikko T., Obermann M., Jensen T.S., Cruccu G. European academy of neurology guideline on trigeminal neuralgia. Eur. J. Neurol. 2019;26(6):831–849. doi: 10.1111/ene.13950. [DOI] [PubMed] [Google Scholar]
- 34.Jones M.R., Urits I., Ehrhardt K.P., Cefalu J.N., Kendrick J.B., Park D.J., Cornett E.M., Kaye A.D., Viswanath O. A comprehensive review of trigeminal neuralgia. Curr. Pain Headache Rep. 2019;23(10):74. doi: 10.1007/s11916-019-0810-0. [DOI] [PubMed] [Google Scholar]
- 35.Bick S.K.B., Eskandar E.N. Surgical treatment of trigeminal neuralgia. Neurosurg. Clin. N. Am. 2017;28(3):429–438. doi: 10.1016/j.nec.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Hayes D.J., Chen D.Q., Zhong J., Lin A., Behan B., Walker M., Hodaie M. Affective circuitry alterations in patients with trigeminal neuralgia. Front. Neuroanat. 2017;11:73. doi: 10.3389/fnana.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turk D.C., Audette J., Levy R.M., Mackey S.C., Stanos S. Assessment and treatment of psychosocial comorbidities in patients with neuropathic pain. Mayo Clin. Proc. 2010;85(3) Suppl.:S42–S50. doi: 10.4065/mcp.2009.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simons L.E., Elman I., Borsook D. Psychological processing in chronic pain: A neural systems approach. Neurosci. Biobehav. Rev. 2014;39:61–78. doi: 10.1016/j.neubiorev.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Stefano G., La Cesa S., Truini A., Cruccu G. Natural history and outcome of 200 outpatients with classical trigeminal neuralgia treated with carbamazepine or oxcarbazepine in a tertiary centre for neuropathic pain. J. Headache Pain. 2014;15:34. doi: 10.1186/1129-2377-15-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebrahimi H., Najafi S., Khayamzadeh M., Zahedi A., Mahdavi A. Therapeutic and analgesic efficacy of laser in conjunction with pharmaceutical therapy for trigeminal neuralgia. J. Lasers Med. Sci. 2018;9(1):63–68. doi: 10.15171/jlms.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long D.M., Hagfors N. Electrical stimulation in the nervous system: The current status of electrical stimulation of the nervous system for relief of pain. Pain. 1975;1(2):109–123. doi: 10.1016/0304-3959(75)90096-2. [DOI] [PubMed] [Google Scholar]
- 42.Bates J.A., Nathan P.W. Transcutaneous electrical nerve stimulation for chronic pain. Anaesthesia. 1980;35(8):817–822. doi: 10.1111/j.1365-2044.1980.tb03926.x. [DOI] [PubMed] [Google Scholar]
- 43.Yameen F., Shahbaz N.N., Hasan Y., Fauz R., Abdullah M. Efficacy of transcutaneous electrical nerve stimulation and its different modes in patients with trigeminal neuralgia. J. Pak. Med. Assoc. 2011;61(5):437–439. [PubMed] [Google Scholar]
- 44. Emedicine Medscape Overview: Transcutaneous Electrical Nerve Stimulation, https://emedicine.medscape.com/article/325107-overview2020.
- 45. Delgado, M. Revisión sistemática de estudios. Metaanálisis, 7th ed; Signo: Barcelona, 2017. [Google Scholar]
- 46.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J.M., Gavaghan D.J., McQuay H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 47.Erdine S., Ozyalcin N.S., Cimen A., Celik M., Talu G.K., Disci R. Comparison of pulsed radiofrequency with conventional radiofrequency in the treatment of idiopathic trigeminal neuralgia. Eur. J. Pain. 2007;11(3):309–313. doi: 10.1016/j.ejpain.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Li X., Ni J., Yang L., Wu B., He M., Zhang X., Ma L., Sun H. A prospective study of Gasserian ganglion pulsed radiofrequency combined with continuous radiofrequency for the treatment of trigeminal neuralgia. J. Clin. Neurosci. 2012;19(6):824–828. doi: 10.1016/j.jocn.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 49.Fang L., Tao W., Jingjing L., Nan J. Comparison of high-voltage-with standard-voltage pulsed radiofrequency of Gasserian ganglion in the treatment of idiopathic trigeminal neuralgia. Pain Pract. 2015;15(7):595–603. doi: 10.1111/papr.12227. [DOI] [PubMed] [Google Scholar]
- 50.Luo F., Wang T., Shen Y., Meng L., Lu J., Ji N. High voltage pulsed radiofrequency for the treatment of refractory neuralgia of the infraorbital nerve: A prospective double-blinded Randomized Controlled Study. Pain Physician. 2017;20(4):271–279. [PubMed] [Google Scholar]
- 51.Elawamy A., Abdalla E.E.M., Shehata G.A. Effects of pulsed versus conventional versus combined radiofrequency for the treatment of trigeminal neuralgia: A prospective study. Pain Physician. 2017;20(6):E873–E881. [PubMed] [Google Scholar]
- 52.Amanat D., Ebrahimi H., Lavaee F., Alipour A. The adjunct therapeutic effect of lasers with medication in the management of orofacial pain: Double blind randomized controlled trial. Photomed. Laser Surg. 2013;31(10):474–479. doi: 10.1089/pho.2013.3555. [DOI] [PubMed] [Google Scholar]
- 53.Hagenacker T., Bude V., Naegel S., Holle D., Katsarava Z., Diener H.C., Obermann M. Patient-conducted anodal transcranial direct current stimulation of the motor cortex alleviates pain in trigeminal neuralgia. J. Headache Pain. 2014;15(1):78. doi: 10.1186/1129-2377-15-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peter B., Janey C., Tony P. Manual Oxford de gestión y tratamiento del dolor, 1st ed; Grupo Aula Médica: Madrid, 2013. [Google Scholar]
- 55.Huang B., Xie K., Chen Y., Wu J., Yao M. Bipolar radiofrequency ablation of mandibular branch for refractory V3 trigeminal neuralgia. J. Pain Res. 2019;12(12):1465–1474. doi: 10.2147/JPR.S197967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jia Y., Pan Y., Ren H., Ji N., Luo F. Effectiveness and safety of high-voltage pulsed radiofrequency to treat patients with primary trigeminal neuralgia: A multicenter, randomized, double-blind, controlled study protocol. Pain Physician. 2018;21(5):469–481. [PubMed] [Google Scholar]
- 57.Yao P., Hong T., Wang Z.B., Ma J.M., Zhu Y.Q., Li H.X., Ding Y.Y., Jiang C.L., Pan S.N. Treatment of bilateral idiopathic trigeminal neuralgia by radiofrequency thermocoagulation at different temperatures. Medicine (Baltimore) 2016;95(29):e4274. doi: 10.1097/MD.0000000000004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehrkens J.H., Steude U. Chronic electrostimulation of the trigeminal ganglion in trigeminal neuropathy: Current state and future prospects. Acta Neurochir. Suppl. (Wien) 2007;97(Pt 2):91–97. doi: 10.1007/978-3-211-33081-4_11. [DOI] [PubMed] [Google Scholar]
- 59.Yadav Y.R., Nishtha Y., Sonjjay P., Vijay P., Shailendra R., Yatin K. Trigeminal Neuralgia. Asian J. Neurosurg. 2017;12(4):585–597. doi: 10.4103/ajns.AJNS_67_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abd-Elsayed A., Kreuger L., Wheeler S., Robillard J., Seeger S., Dulli D. Radiofrequency ablation of pericranial nerves for treating headache conditions: A promising option for patients. Ochsner J. 2018;18(1):59–62. [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y., Yang L., Ni J., Dou Z. Microvascular decompression and radiofrequency for the treatment of trigeminal neuralgia: A meta-analysis. J. Pain Res. 2019;12:1937–1945. doi: 10.2147/JPR.S203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang N., Wei J., Li G., Wei B., Zhu F.F., Hu Y. Effect of dry- electrode-based transcranial direct current stimulation on chronic low back pain and low back muscle activities: A double-blind sham-controlled study. Restor. Neurol. Neurosci. 2020;38(1):41–54. doi: 10.3233/RNN-190922. [DOI] [PubMed] [Google Scholar]
- 63.David M.C.M.M., Moraes A.A., Costa M.L.D., Franco C.I.F. Transcranial direct current stimulation in the modulation of neuropathic pain: A systematic review. Neurol. Res. 2018;40(7):555–563. doi: 10.1080/01616412.2018.1453190. [DOI] [PubMed] [Google Scholar]
- 64.Lloyd D.M., Wittkopf P.G., Arendsen L.J., Jones A.K.P. Is transcranial direct current stimulation (tDCS) effective for the treatment of pain in fibromyalgia? A systematic review and meta-analysis. J. Pain. 2020;21(11-12):1085–1100. doi: 10.1016/j.jpain.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Obermann M., Bude V., Holle D., Naegel S., Hagenacker T., Diener H.C., Katsarava Z. EHMTI -0072. Anodal transcranial direct current stimulation alleviates pain in trigeminal neuralgia. J. Headache Pain. 2014;15(Suppl. 1):E21. doi: 10.1186/1129-2377-15-S1-E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. 66. Rodriguez-Martín, J.M. Electroterapia en Fisioterapia, 3rd ed; Panamericana: Barcelona, 2014. [Google Scholar]
- 67.Falaki F., Nejat A.H., Dalirsani Z. The effect of low-level laser therapy on trigeminal neuralgia: A review of literature. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014;8(1):1–5. doi: 10.5681/joddd.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Attal N., Cruccu G., Baron R., Haanpää M., Hansson P., Jensen T.S., Nurmikko T. European federation of neurological societies. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur. J. Neurol. 2010;17(9):1113–e88. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 69.Barbarisi M., Pace M.C., Passavanti M.B., Maisto M., Mazzariello L., Pota V., Aurilio C. Pregabalin and transcutaneous electrical nerve stimulation for postherpetic neuralgia treatment. Clin. J. Pain. 2010;26(7):567–572. doi: 10.1097/AJP.0b013e3181dda1ac. [DOI] [PubMed] [Google Scholar]
- 70.Quintner J.L., Bove G.M., Cohen M.L. A critical evaluation of the trigger point phenomenon. Rheumatology (Oxford) 2015;54(3):392–399. doi: 10.1093/rheumatology/keu471. [DOI] [PubMed] [Google Scholar]
- 71.Gerwin R.D. Myofascial trigger point pain syndromes. Semin. Neurol. 2016;36(5):469–473. doi: 10.1055/s-0036-1586262. [DOI] [PubMed] [Google Scholar]
- 72.Seo S.Y., Lee K.B., Shin J.S., Lee J., Kim M.R., Ha I.H., Ko Y., Lee Y.J. Effectiveness of acupuncture and electroacupuncture for chronic neck pain: A systematic review and meta-analysis. Am. J. Chin. Med. 2017;45(8):1573–1595. doi: 10.1142/S0192415X17500859. [DOI] [PubMed] [Google Scholar]
- 73.Abd-Elsayed, A.; Nguyen, S.; Fiala, K. Radiofrequency ablation for treating headache: A follow up study. Curr. Pain Headache Rep. 2020;24(4):15. doi: 10.1007/s11916-020-0843-4. [DOI] [PubMed] [Google Scholar]
- 74.Dworkin R.H., O’Connor A.B., Kent J., Mackey S.C., Raja S.N., Stacey B.R., Levy R.M., Backonja M., Baron R., Harke H., Loeser J.D., Treede R.D., Turk D.C., Wells C.D. International association for the study of pain neuropathic pain special interest group. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain. 2013;154(11):2249–2261. doi: 10.1016/j.pain.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cetas J.S., Saedi T., Burchiel K.J. Destructive procedures for the treatment of nonmalignant pain: A structured literature review. J. Neurosurg. 2008;109(3):389–404. doi: 10.3171/JNS/2008/109/9/0389. [DOI] [PubMed] [Google Scholar]
- 76.Cruccu G., Gronseth G., Alksne J., Argoff C., Brainin M., Burchiel K., Nurmikko T., Zakrzewska J.M. American academy of neurology society. European federation of neurological society. AAN-EFNS guidelines on trigeminal neuralgia management. Eur. J. Neurol. 2008;15(10):1013–1028. doi: 10.1111/j.1468-1331.2008.02185.x. [DOI] [PubMed] [Google Scholar]
- 77.Lopez B.C., Hamlyn P.J., Zakrzewska J.M. Systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia. Neurosurgery. 2004;54(4):973–982. doi: 10.1227/01.NEU.0000114867.98896.F0. [DOI] [PubMed] [Google Scholar]
- 78.Zakrzewska J.M., Akram H. Neurosurgical interventions for the treatment of classical trigeminal neuralgia. Cochrane Database Syst. Rev. 2011;9(9):CD007312. doi: 10.1002/14651858.CD007312.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gronseth G., Cruccu G., Alksne J., Argoff C., Brainin M., Burchiel K., Nurmikko T., Zakrzewska J.M. Practice parameter: The diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008;71(15):1183–1190. doi: 10.1212/01.wnl.0000326598.83183.04. [DOI] [PubMed] [Google Scholar]