Abstract
Background
People with spinal cord injuries (SCI) commonly experience pain and spasticity; limitations of current treatments have generated interest in cannabis as a possible therapy.
Objectives
We conducted this systematic review to: 1) examine usage patterns and reasons for cannabinoid use, and 2) determine the treatment efficacy and safety of cannabinoid use in people with SCI.
Methods
PubMed, Embase, Web of Science and Cumulative Index to Nursing and Allied Health Literature databases were queried for keywords related to SCI and cannabinoids.
Results
7,232 studies were screened, and 34 were included in this systematic review. Though 26 studies addressed cannabinoid usage, only 8 investigated its therapeutic potential on outcomes such as pain and spasticity. The most common method of use was smoking. Relief of pain, spasticity and recreation were the most common reasons for use. A statistically significant reduction of pain and spasticity was observed with cannabinoid use in 83% and 100% of experimental studies, respectively. However, on examination of randomized control trials (RCTs) alone, effect sizes ranged from -0.82 to 0.83 for pain and -0.95 to 0.09 for spasticity. Cannabinoid use was associated with fatigue and cognitive deficits.
Conclusion
Current evidence suggests that cannabinoids may reduce pain and spasticity in people with SCI, but its effect magnitude and clinical significance are unclear. Existing information is lacking on optimal dosage, method of use, composition and concentration of compounds. Long-term, double-blind, RCTs, assessing a wider range of outcomes should be conducted to further understand the effects of cannabinoid use in people with SCI.
Keywords: Spinal cord injury, cannabinoids, cannabis, marijuana, pain, spasticity
1. Introduction
Spinal cord injury (SCI) is a life-long condition with deleterious effects on an individual’s physical, mental and social wellbeing. Compared to the general population, people with SCI have a lower health-related quality of life due to a combination of poor physical health, stress, and secondary health conditions [1, 2]. In 2016, there were estimated one million new cases of SCI globally, with an incidence of 13 per 10, 000 individuals [3].
Following an SCI, people commonly suffer from spasticity and pain [4, 5]. In the SCI population, an estimated 65-78% of individuals report symptoms of spasticity, within the first year after injury [6, 7]. As many as 80% of people with SCI will experience neuropathic pain (NPP) [8]. Pain at the level of injury may consist of both peripheral and central NPP, while below-level pain is isolated central NPP [5]. It is also common for a person with SCI to experience difficulties performing activities of daily living, sleep disturbances, development of contractures, pressure ulcers, infections and a negative self-image [6]. These conditions are difficult to treat and interventions are often unsuccessful. The current anti-spastic and analgesic medications carry wide-ranging side-effect profiles and are costly [9, 10]. The inefficiency of the current treatment model has led people with SCI to explore alternative methods to manage spasticity and pain.
One such therapy recently garnering international attention is medicinal cannabis, currently legalized in Canada, 28 American states, the District of Columbia, Guam, and Puerto Rico [11, 12]. Public acceptance of cannabinoids for both medical and recreational purposes is increasing, with a recent survey reporting that two-thirds of medical cannabinoid users felt supported by friends and family [13].
1.1. Cannabinoids for Therapeutic Purposes
The human endocannabinoid system is comprised of cannabinoid receptors found throughout the central and peripheral nervous systems (CB1-Receptors) as well as the immune system (CB2-Receptors) [14]. Plants belonging to the genus Cannabis produce over 60 cannabinoid compounds, including the psychotropic cannabinoid Δ9-tetrahydrocannabinol (THC) and the non-psychoactive cannabidiol (CBD) [15]. THC binds to both the CB1- and CB2-Receptors with high affinity, while CBD shows little affinity for either receptor, but this may be overcome by increasing the dose [16]. These compounds mimic naturally occurring ligands at cannabinoid receptors in the human body to modulate physiological functions, and it is believed that their action on the central nervous system-located CB1-Receptors is what reduces spasticity [17]. THC and CBD can also influence other receptors, such as ion channels [18-24]. Preclinical studies have revealed that the analgesic effect of cannabinoids involves the inhibition of the release of neurotransmitters and neuropeptides from presynaptic nerve endings, modulation of postsynaptic neuron excitability, activation of descending inhibitory pain pathways and reduction of neural inflammation [25-28].
To date, the effects of cannabinoids have been studied in clinical trials to treat nausea and vomiting due to cancer chemotherapy, loss of appetite in people with HIV-induced or cancer-related weight loss, chronic pain, spasticity in people with multiple sclerosis (MS), intraocular pressure in people with glaucoma, and other conditions, such as SCI [29-34].
Despite the growing body of literature on medical cannabinoids, its use as a therapeutic alternative for SCI has not been thoroughly studied. Given the recent legalization of cannabinoids, its widespread usage, and prevalence of secondary conditions such as refractory pain and spasticity in people with SCI, it is necessary to conduct a rigorous review of the effects and therapeutic potential of cannabinoids. The purpose of this systematic review is to analyze the literature on the use of medical cannabinoids in people with SCI to answer the following: 1) characteristics of users, 2) patterns of use, 3) reasons for use, 4) therapeutic effects, and 5) associated side effects of cannabinoid use.
2. Methods
2.1. Literature Search Strategy
A systematic review of all relevant literature, published from the database inception until February 29th, 2020, was conducted using four databases (PubMed, Embase, Web of Science and Cumulative Index to Nursing and Allied Health Literature (CINAHL)) and keywords for SCI and cannabis (Table e-1) in accordance with the Cochrane Handbook for Systematic Reviews of Interventions guidelines [35]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to report our systematic review [36]. No protocol or registry entry is available for this systematic review.
Table.
e-1. Search terms.
| Search keywords for spinal cord injury | spinal cord OR spinal injur* OR SCI OR spinal cord damage OR spinal cord stroke OR spinal cord insult OR paraplegi* OR tetraplegi* OR quadriplegi* |
|---|---|
| Search keywords for cannabis | cannabis OR marijuana OR cannabinoid OR tetrahydrocannabinol OR THC OR cannabidiol OR CBD |
2.2. Study Selection
Studies were included for qualitative analysis if they met the following criteria: (1) conducted with humans; (2) included at least two adults with an SCI; and (3) examined the effects of cannabinoids (in any preparation: synthetic or natural, form, or route of administration) against any comparison product. We included all study designs except case studies, reviews (i.e. narrative reviews, book chapters), opinion papers, non-peer-reviewed work, conference abstracts or papers and studies where the full text was unavailable. Studies were also excluded if the information on patient demographics, research design, intervention, and/or results could not be extracted accurately from the article. Non-randomized studies of interventions (NRSI) were included in the systematic review as recommended by the Cochrane handbook, which consider the inclusion of NRSI when RCTs are lacking [35].
2.3. Study Appraisal
Independent reviewers (author 1 and 2) screened titles, abstracts, and full-texts; only eligible studies were included in the qualitative analysis. A third reviewer (author 3) resolved discrepancies.
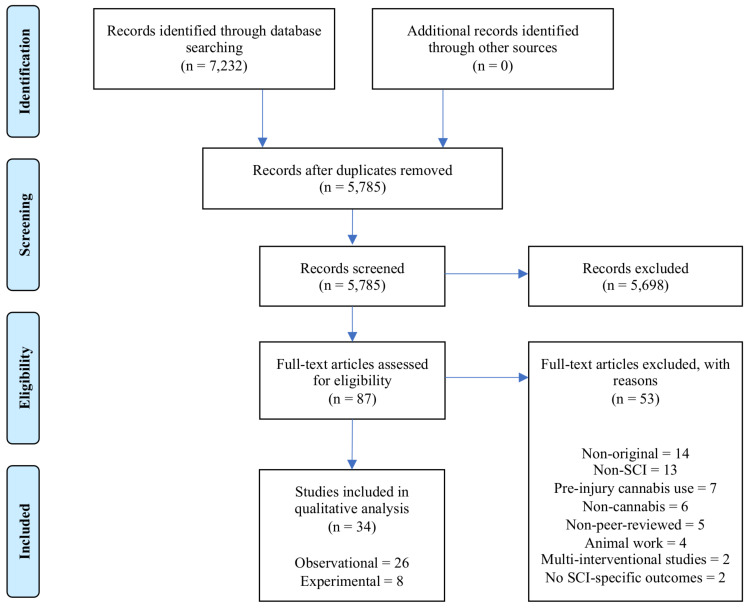
Fig. 1 illustrates the PRISMA flow diagram. A consensus was achieved (between authors 1-3) on data to be extracted from studies, which included author and year of study, study design, population characteristics (e.g., etiology, level of and time since SCI), intervention, dosage and form of cannabinoid, outcomes measured, and side effects. Data extraction from observational and experimental studies was separated and performed independently by two authors. The principal summary measure was the difference in means.
Fig. (1).
PRISMA Flow Diagram.
Reviewers (author 1 and 2) also assessed observational studies (14-item checklist), pre-post trials (12-item checklist) and randomized control trials (RCTs) (14-item checklist) for methodologic quality and bias using the National Institute of Health (NIH) assessment tool [37].
2.4. Data Analysis
A modified coding system described by Sallis et al. [38], was used to summarise the studies reporting the effect of cannabinoids on various SCI-based outcomes. If 0-33% of the studies reported a statistically significant difference between cannabinoids and placebo, the result was coded as no effect (0). If 34-59% of the studies reported a statistically significant difference, the result was categorised as inconsistent (?). If 60-100% of the studies reported a statistically significant difference, the result was rated as positive (+) or negative (-). When four or more studies supported a difference or no difference, it was coded as ++, --, 00, respectively to indicate consistent observations. The code ?? indicated a marker that has been examined in four or more studies with inconsistent findings. Coding analysis was conducted on all primary and secondary outcomes measures.
For the RCTs, the effect size was calculated based on the standardized mean difference [39]. Effect sizes were calculated for pain and spasticity because they are commonly experienced by individuals with SCI and have been studied with cannabis in other conditions such as chronic non-cancer pain and spasticity for MS [31, 40]. Effect sizes of NRSI were not calculated. These study designs substantially inflate the effect magnitude compared to control group designs, as the control group captures any non-intervention influences, for example, familiarization to the outcome measure [41].
3. Results
PubMed, Embase, Web of Knowledge, CINAHL and searches yielded 7,232 citations. In total, 34 publications were eligible and included (Fig. 1, Table e-2).
Table.
e-2. Database search results.
| Database | Date Accessed | Results Returned |
|---|---|---|
| PubMed | February 29, 2020 | 3968 |
| Embase | February 29, 2020 | 2168 |
| Web of Knowledge | February 29, 2020 | 981 |
| Cumulative Index to Nursing and Allied Health Literature | February 29, 2020 | 115 |
3.1. Description of Studies
Of the 34 studies included in this systematic review, 26 were observational and 8 were experimental. The results were grouped based on the objectives: 1) characteristics of users, 2) patterns of use, 3) reasons for use, 4) therapeutic effects of cannabinoids, and 5) side effects associated with cannabinoid use. Homogenous SCI participant populations were included in 18 out of the 22 observational studies and 4 of the 8 experimental studies. From the experimental studies, Dronabinol capsules were used in two studies, at different doses, while the other studies used variable proportions of CBD and THC as their interventions. Placebo was the most common comparator, used in six of the eight studies.
3.2. Quality of Studies
Among the observational studies, 19 were of poor quality and seven of fair quality (Table e-3). Of the seven RCTs, one study provided good evidence, four studies provided fair quality evidence and two were poor quality studies (Table e-4), while the single pre-/post-study was evaluated as poor quality (Table e-5).
Table.
e-3. Quality of the observational studies.
| Author, Year |
Research Question/
Objective Clearly Stated? |
Study Population Specified and Defined? | Participation Rate of Eligible Persons >50%? |
Subjects from Same/Similar Populations? Inclusion/
Exclusion Criteria Prespecified and Applied Uniformly? |
Sample Size Justification, Power Description, Variance and Effect Estimates Provided? | Exposure(s) Measured Prior to the Outcome(s) Measured? | Sufficient Timeframe for Association between Exposure and Outcome to be Seen? | Did the Study Examine Different Levels of the Exposure as Related to the Outcomes? | Exposure Measures Defined, Valid, Reliable and Implemented Consistently? | Exposure(s) Assessed more than Once Over Time? |
Were Outcomes Assessed Reliable and Consis-
tent? |
Were Assessors Blinded to Exposure Status of Parti-
cipants? |
Was Loss to Follow-up after Baseline <20%? | Key Potential Confounding Variables Measured and Adjusted Statistically for their Impact on Relationship between Exposure(s) and Outcome(s)? | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dunn & Davis, 1974 [63] | + | - | ? | + | - | - | - | - | + | - | - | N/A | N/A | - | POOR |
| Malec et al., 1982 [64] | + | - | + | + | - | - | - | - | + | - | + | N/A | N/A | - | POOR |
| Heinemann et al., 1991 [65] | + | - | + | + | - | N/A | + | - | - | + | + | N/A | + | + | POOR |
| Rothstein et al., 1992 [66] | + | - | ? | + | - | - | - | + | + | N/A | + | N/A | N/A | - | POOR |
| Young et al., 1995 [43] | + | - | - | + | - | - | - | + | + | - | + | N/A | N/A | + | POOR |
| Kolakowsky-Hayer et al., 2002 [67] | + | + | ? | + | - | - | - | + | + | - | + | N/A | N/A | + | FAIR |
| Warms et al., 2002 [68] | + | + | + | + | - | - | - | - | + | - | + | N/A | N/A | - | POOR |
| Grotenhermen & Schnelle, 2003 [51] | + | - | ? | + | - | - | - | + | + | - | + | N/A | N/A | - | POOR |
| Gorter, 2005 [52] | + | - | - | + | - | - | - | + | + | - | - | N/A | N/A | - | POOR |
| Cardenas & Jensens, 2006 [53] | + | + | + | + | - | - | - | + | + | - | - | N/A | N/A | - | POOR |
| Mahoney et al., 2007 [69] | + | - | + | + | N/A | - | - | - | + | - | + | N/A | N/A | N/A | POOR |
| Aggarwal et al., 2009 [46] | + | - | + | + | - | - | ? | - | + | N/A | - | N/A | N/A | N/A | POOR |
| Heutink et al., 2011 [70] | + | + | + | + | - | - | - | + | + | - | + | N/A | N/A | - | POOR |
| Hwang et al., 2012 [48] | + | - | ? | + | - | - | - | + | + | - | + | N/A | N/A | + | FAIR |
| Fekete et al., 2015 [42] | + | + | + | + | - | - | - | + | + | - | + | N/A | N/A | + | FAIR |
| Shroff, 2015 [54] | + | - | ? | + | N/A | - | - | - | + | - | + | N/A | N/A | N/A | POOR |
| Author, Year |
Research Question/ Objective Clearly Stated? |
Study Population Specified and Defined? | Participation Rate of Eligible Persons >50%? |
Subjects from Same/Similar Populations? Inclusion/ Exclusion Criteria Prespecified and Applied Uniformly? |
Sample Size Justification, Power Description, Variance and Effect Estimates Provided? | Exposure(s) Measured Prior to the Outcome(s) Measured? | Sufficient Timeframe for Association between Exposure and Outcome to be Seen? | Did the Study Examine Different Levels of the Exposure as Related to the Outcomes? | Exposure Measures Defined, Valid, Reliable and Implemented Consistently? | Exposure(s) Assessed more than Once Over Time? |
Were Outcomes Assessed Reliable and Consis- tent? |
Were Assessors Blinded to Exposure Status of Parti- cipants? |
Was Loss to Follow-up after Baseline <20%? |
Key Potential Confounding Variables Measured and Adjusted Statistically for their Impact on Relationship between Exposure(s) and Outcome(s)? |
Overall Quality |
| Drossel et al., 2016 [45] | + | - | + | + | - | - | - | + | + | - | + | N/A | N/A | - | POOR |
| Andresen et al., 2017 [44] | + | + | + | + | - | - | - | + | + | - | + | N/A | N/A | + | FAIR |
| Clark et al., 2017 [47] | + | + | + | + | - | - | - | + | + | - | + | N/A | N/A | + | FAIR |
| Patel et al., 2017 [71] | + | - | + | + | - | - | ? | - | + | N/A | + | N/A | N/A | - | FAIR |
| Bruce et al., 2018 [50] | + | - | + | + | - | - | - | + | + | - | + | N/A | N/A | N/A | POOR |
| Hawley et al., 2018 [49] | + | - | ? | + | - | - | - | + | + | - | + | N/A | N/A | - | POOR |
| Bourke et al., 2019 [72] | + | - | ? | + | N/A | - | - | - | + | - | + | N/A | N/A | N/A | POOR |
| Eldridge et al., 2019 [73] | + | - | - | + | - | - | ? | - | + | - | + | N/A | N/A | - | POOR |
| Graupensperger et al., 2019 [74] | + | - | + | + | - | - | ? | - | + | - | + | N/A | N/A | + | FAIR |
| Stillman et al., 2019 [75] | + | - | - | + | - | - | - | + | + | - | + | N/A | N/A | - | POOR |
Note: N/A: not applicable, for study designs where the question could not be applied; ?: cannot be determined; +: yes; -: no.
Table.
e-4. Quality of the randomized control trial studies.
| Author, Year |
Rando-
mization? |
Adequate Method of Rando-
mization? |
Concealed Treatment Allocation? | Participants and Providers Blinded to Group Assignment? |
Assessors Blinded to Group Assign-
ment? |
Groups Similar at Baseline? | Overall Drop-out Rate <20% at Endpoint? |
Differential Dropout Rate
<15% at Endpoint? |
High Adherence to Inter-
vention? |
Other Inter-
ventions Avoided or Similar in Groups? |
Were Outcomes Assessed Reliable and Consistent? | Was Sample Size Sufficiently Large to Detect a Difference in Main Outcome with >80% Power? | Were Outcomes Reported or Subgroups Prespecified? | Participants Analyzed to Group they were Originally Assigned? | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Karst et al., 2003 [56] | + | + | + | + | + | - | + | + | + | + | + | + | + | + | GOOD |
| Wade et al., 2003 [58] | + | + | + | + | + | N/A | + | N/A | ? | - | + | + | + | + | FAIR |
| Hagenbach et al., 2007 [55] | + | ? | + | + | ? | + | + | + | + | + | + | - | + | + | FAIR |
| Wilsey et al., 2008 [59] | + | + | + | + | + | + | + | + | - | + | + | - | + | + | FAIR |
| Rintala et al., 2010 [57] | + | + | + | + | + | + | - | ? | + | + | + | - | + | + | POOR |
| Pooyania et al., 2010 [77] | + | + | + | + | ? | - | + | + | + | - | + | - | + | + | POOR |
| Wilsey et al., 2016 [60] |
+ | + | + | + | ? | + | + | + | + | - | + | - | + | + | FAIR |
N/A: not applicable, for study designs where the question could not be applied; ?: cannot be determined; +: yes; -: no.
Table.
e-5. Quality of the pre-/post-studies.
| Author, Year | Clearly Stated Study Question? |
Clearly Described Eligibility/
Selection Criteria for Study Population? |
Were Participants Representative of the Clinical Population of Interest? | Were all Eligible Participants that Met the Prespecified Entry Criteria Enrolled? | Was the Sample Size Sufficiently Large? |
Was the Intervention Clearly Described and
Delivered Consistently Across the Study Population? |
Were the Outcomes Measures Prespecified, Clearly
Defined, Valid, Reliable, and Assessed Consistently? |
Were the People Assessing the Outcomes Blinded to the
Participants’ Exposures/Interventions? |
Was the Loss to Follow-up after Baseline 20% of Less? | Did Statistical Methods Examine Changes in Outcome Measures from before to after Intervention? Did they Provide p-values? | Were Outcome Measures of Interest Taken Multiple Times before the Intervention and Multiple Times after the Intervention? |
If the Intervention was Conducted at Group Level, did Statistical Analysis Take into Account the Use of Individual-level Data to Determine Effect at Group Level?
Assigned? |
Overall Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kogel et al., 1995 [76] | + | + | + | NR | - | + | + | - | + | +/- | + | N/A | POOR |
N/A: not applicable, for study designs where the question could not be applied; NR: not reported; +: yes; -: no.
3.3. Aim 1: Characteristics of Cannabinoid Users
Cannabinoid users were found to be younger [42-44] compared to non-users. A Danish study (n=537) by Andresen et al. [44], reported a mean age of 42.5 years for current users (over the last 2 years) as compared to 55.8 years for non-users. Cannabinoid users were also more likely to be single [44, 45] compared to non-users. Six studies demonstrated no significant difference in cannabinoid use between males and females [42, 44-48]. The results of two cross-sectional surveys by Hwang et al. [48], and Young et al. [43], suggested that low education status was associated with greater cannabinoid use, but this was not supported by other studies; Drossel et al, [45], and Hawley et al. [49]. Across studies reporting participants’ demographics, no major differences were found between users and non-users in terms of their socioeconomic status, social support or medical complications [42-43, 48].
3.4. Aim 2: Patterns of Cannabinoid Use
The results of three studies examining the frequency (monthly, weekly, daily) of cannabinoid use in people with SCI were inconsistent, as two studies (n=244, n=215) reported a larger percentage of daily users [45, 48], while one large study (n=1,619) demonstrated a higher proportion of monthly users (Table 1) [47]. Three studies reported the most common routes of administration. All studies ranked smoking as the most frequently used, followed by edibles and vapor in two studies (n=244, n=116) [45, 49], and the converse order in one smaller study (n=30) [50]. In general, inhalation (smoking, vaping) was more common than ingestible (oil, drops, food) administration [51, 52]. Other medications that were not SCI-specific were often used in combination with cannabinoids [46, 50].
Table 1.
Patterns of cannabinoid use from observational studies.
| Author, Year | Study Type | Legalization (Location) | Number of Participants (SCI/Total) |
Inclusion
Criteria |
Exclusion Criteria |
Male/Female/
Transgender |
Mean Age | Tetraplegia/ Paraplegia/ Unknown | Mean Time Since Injury | Prevalence | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dunn & Davis, 1974 [63] | Cross-sectional | Illegal (Florida, USA) | 10/10 | SCI patients using cannabis | - | 10/0/0 | NR | NR | NR | N/A- cannabis use was inclusion criteria | |||||||||||||||||
| Malec et al., 1982 [64] | Cross-sectional | Illegal (Wisconsin, USA) |
43/43 | SCI patients | - | 38/5/0 | NR | NR | NR | Within last yr: 56% | |||||||||||||||||
| Heinemann et al., 1991 [65] |
Case-series | Illegal (Illinois, USA) |
86/86 | 13-66 age, 2+ yr since tSCI, English language, no cognitive impairment | - | 59/27/0 | 39.5 (13-65) |
47/39 | 13.1 ± 10.2 | 6 mo pre-SCI: 31%; Post-SCI: 42% | |||||||||||||||||
| Rothstein et al., 1992 [66] |
Cross-sectional | MC legal (New York, USA) | 153/153 | Male veterans with SCI | - | 153/0/0 | 53 ± 1 (20-76) | NR | NR | Current (urinary cannabinoid test): 10% | |||||||||||||||||
| Young et al., 1995 [43] | Cross-sectional | Illegal (Texas, USA) | 123/123 | 17+ age, 9+ mo since tSCI, residual motor disability with assistive walking device if ambulatory | - | 82/41/0 | 36 ± 10.9 (19-76) | Complete tetra: 53, complete para: 53, incomplete: 17 | 9.7 ± 6.6 | Current (regular basis at time of study): 16% | |||||||||||||||||
| Kolakowsky-Hayner et al., 2002 [67] | Cross-sectional | Illegal (Virginia, USA) | 30/60 | SCI and brain injury patients treated in trauma centre | - | 56/4/0 | 35.0 ± 10.85 | NR | 1.4 | Past 6-12 mo: 50% among illicit drug users (n=6 SCI, n=1 TBI) | |||||||||||||||||
| Warms et al., 2002 [68] | Cross-sectional | MC legal starting Nov 1998, study V1 Feb 1997 – Jul 1998, V2 Aug 1998 – June 2000 (Washington, USA) | 471/471 | 18+ age, 6+ mo since SCI | - | 334/137/0 | 42.5 ± 13.2 (18-84) |
240/221, unknown: 9 | NR | Ever: 3% | |||||||||||||||||
| Grotenhermen & Schnellea, 2003 [51] | Cross-sectional | Dronabinol prescription and ∆9-THC special permit (Germany) and permit (Switzerland) |
4/165 | Members of Association for Cannabis as Medicine | No severe disease | 101/64/0 | Median age: 40.3 ± 12.4 (16-87) | NR | NR | Ever: 87% | |||||||||||||||||
| Gortera, 2005 [52] | Cross-sectional | MC legal (Netherlands) | ?/107 | Members of Multiple Sclerosis society | - | 48/59/0 | Median age: 58.0 | NR | NR | N/A- MC was inclusion criteria | |||||||||||||||||
| Cardenas & Jensen, 2006 [53] | Cross-sectional | MC legal (Washington, USA) | 117/117 | 18+ age, tSCI, chronic pain | Incomplete questionnaires | 85/32/0 | 48.8 ± 11.7 (21-79) |
56/61 | 17.3 ± 10.9 | Ever: 32%; Current: 20% | |||||||||||||||||
| Mahoney et al., 2007 [69] |
Interview | Illegal (Texas, USA) | 24/24 | 1+ yr since SCI, spasticity, English language | - | 17/7/0 | 45.1 (21-68) |
13/11 | 16 | NR | |||||||||||||||||
| Aggarwal et ala., 2009 [46] |
Retrospective chart review | MC legal (Washington, USA) | 5/139 | 18+ age, pain clinic patients, access to MC with valid doctor documentation | Cannabinoid receptor 1 blocker drug rimonabant | 88/51/0 | Median age: 48 (18-84) | NR | NR | N/A- MC was inclusion criteria | |||||||||||||||||
| Author, Year | Study Type | Legalization (Location) | Number of Participants (SCI/Total) |
Inclusion Criteria |
Exclusion Criteria |
Male/ Female/ Transgender |
Mean Age |
Tetraplegia/ Paraplegia/ Unknown |
Mean Time Since Injury | Prevalence | |||||||||||||||||
| Heutink et al., 2011 [70] | Cross-sectional | MC legal starting 2003, study 1990-2005 (the Netherlands) | 279/279 | 18+ age, SCI rehab patients, living in community | - | 173/106/0 | 51.3 ± 14.0 (25-81) | 103/165, unknown: 11 | 11.6 ± 10.7 | Past, discontinued: 6%; Current (at study): 3% | |||||||||||||||||
| Hwang et al., 2012 [48] | Cross-sectional | Illegal (Florida, USA) | 215/215 | SCI before age 19, current age 21-25, former hospital patient | - | 127/88/0 | 23.4 ± 0.9 | 112/101, unknown: 2 | 10.2 ± 4.9 | Current (at least mo): 11% | |||||||||||||||||
| Fekete et al., 2015 [42] | Cross-sectional | MC permit (Switzerland) | 511/511 | 16+ age, tSCI or non-tSCI; | New SCI with palliative care, neurodegenerative diseases or Guillain-Barre syndrome; congenital conditions leading to SCI | 373/138/0 | 52.9 ± 14.8 | 158/353 | 17.6 ± 13.0 | Current (last 30 d): 7% | |||||||||||||||||
| Shroff, 2015 [54] | Interview | MC legal (Canada) | 53/53 | 19-65 age, 1+ years since SCI, BC resident, member of paraplegic association | - | 42/11/0 | NR | NR | NR | NR | |||||||||||||||||
| Drossel et al., 2016 [45] | Cross-sectional | MC legal (Michigan & California, USA) | 244/244 | 18+ age, 5+ years since tSCI, English language, neurogenic bowel and/or bladder, no cognitive limitations | - | 181/63/0 | 49.7 | 134/110 | 18.6 | Ever: 23% | |||||||||||||||||
| Andresen et al., 2017 [44] | Cross-sectional | MC legal starting 2011, study 1990-2012 (Denmark) | 537/537 | Inclusion: 18+ age, acquired tSCI, rehab clinic patients | Incomplete questionnaires | 413/124/0 | 54.6 ± 14.6 (18-88) | 247/263, unknown: 27 | 18.2 ± 12.8 | Ever: 36%; Current (last 2 yrs): 9% | |||||||||||||||||
| Clark et al., 2017 [47] | Cross-sectional | MC illegal (Georgia & South Carolina, USA) | 1619/1619 | 18+ age, 1+ year since tSCI, some residual impairment | No painful condition, no prescription pain med | 1166/453/0 | 49.3 ± 14.2 | 453/1166 | 11.5 ± 9.2 | Current (mo): 16% | |||||||||||||||||
| Patel et al., 2017 [71] | Retrospective chart review | MC legal (Canada) | 19/19 | Patients of mobility clinic with documented SCI | - | 14/5/0 | 46.7 (18-89) | NR | NR | Current: 16% | |||||||||||||||||
| Bruce et al.a, 2018 [50] |
Interview | MC legal (Illinois, USA) | 6/30 | 18+ age, smoked MC in past 3 mo, qualifying health condition for MC | - | 19/11/0 | 44.6 ± 15.9 | NR | NR | N/A- MC was inclusion criteria | |||||||||||||||||
| Hawley et al., 2018 [49] |
Cross-sectional | MC and recreational legal (Colorado, USA) | 51/116 | SCI rehab patient | - | 95/21/0 | 47.1 ± 13.8 (22-74) | Tetra ABC: 38, para ABC: 31, tetra/para D: 41, unknown: 5 | 13.0 | Before injury: 67%; After injury: 53% | |||||||||||||||||
| Bourke et al., 2019 [72] |
Interview | Illegal (New Zealand) | 8/8 | 18+ age, SCI patients using cannabis for pain, residing in New Zealand, English speaking, | Comorbid conditions inhibiting communication and participation in interview | 6/2/0 | Age 20-39: n = 1, 40-59: n= 5, 60+: n=2 | Tetra: 6 Para: 2 |
NR | N/A- MC was inclusion criteria | |||||||||||||||||
| Author, Year | Study Type | Legalization (Location) | Number of Participants (SCI/Total) | Inclusion Criteria | Exclusion Criteria |
Male/ Female/ Transgender |
Mean Age |
Tetraplegia/ Paraplegia/ Unknown |
Mean Time Since Injury | Prevalence | |||||||||||||||||
| Eldridge et al., 2019 [73] |
Retrospective chart review | Illegal (Indiana, USA) |
20/20 | 18+ age, SCI patients received medical care at Eskenazi Medical Center | - | 17/3/0 | 45.05 ± 13.84 | NR | NR | Before injury: 25% | |||||||||||||||||
| Graupensperger et al., 2019 [74] | Retrospective chart review | MC legal starting 2016 and implemented Feb 2018, study Jan 1997-April 2018 (Pennsylvania, USA) | 6192/1466985 | 16+ age, patients at Penn State Hershey Medical Center | - | 3368/2824/0 | NR | NR | NR | Cannabis use disorder with SCI: 1% vs. non-SCI 0.2% | |||||||||||||||||
| Stillman et al., 2019 [75] | Cross-sectional | 39 states in USA, not disclosed; mixed legality | 353/353 | SCI patients included in mailing lists maintained by Thomas Jefferson University, University of Washington at Seattle, and Uni- versity of Alabama at Birmingham |
- | 183/107/3 | 52.74 (19-82) | NR | 17.49 | Current: 39% Past: 15% |
|||||||||||||||||
Abbreviations: d: days; freq: frequency; MC: medical cannabis; mo: monthly; N/A: not applicable; NR: not reported; qd: daily; SCI: spinal cord injury; TBI: traumatic brain injury; THC: tetrahydrocannabinol; tSCI: traumatic spinal cord injury, wk: weekly; yr: yearly. adata listed not limited to people with SCI.
3.5. Aim 3: Reasons for Cannabinoid Use
Table 2 summarizes the variety of reasons for cannabinoid use. Relief of pain, spasticity and recreation were typically the top three responses [44, 45, 49, 50, 53, 54].
Table 2.
Reasons for cannabinoid use from observational studies.
| Author, Year | Study Type | Legalization (Location) | Number of Participants (SCI/Total) |
Inclusion
Criteria |
Exclusion Criteria |
Male/
Female |
Mean Age |
Tetraplegia/
Paraplegia/ Unknown |
Mean Time Since
Injury |
Reasons for Use |
|---|---|---|---|---|---|---|---|---|---|---|
| Cardenas & Jensen, 2006 [53] | Cross-sectional | MC legal (Washington, USA) | 117/117 | 18+ age, tSCI, chronic pain | Incomplete questionnaires | 85/32 | 48.8 ± 11.7 (21-79) | 56/61 | 17.3 ± 10.9 | Chronic pain |
| Shroff, 2015 [54] | Interview | MC legal (Canada) | 53/53 | 19-65 age, 1+ years since SCI, BC resident, member of paraplegic association |
- | 42/11 | NR | NR | NR | Pain, spasm relief, relaxation, recreation |
| Drossel et al., 2016 [45] |
Cross-sectional | MC legal (Michigan & California, USA) | 244/244 | 18+ age, 5+ years since tSCI, English language, neurogenic bowel and/or bladder, no cognitive limitations |
- | 181/63 | 49.7 | 134/110 | 18.6 | Pain relief 70%, spasticity 46%, anxiety 30%, bowel 11%, recreation 9%, bladder: 6% |
| Andresen et al., 2017 [44] | Cross-sectional | MC legal starting 2011, study 1990-2012 (Denmark) | 537/537 | Inclusion: 18+ age, acquired tSCI, rehab clinic patients |
Incomplete questionnaires | 413/124 | 54.6 ± 14.6 (18-88) | 247/263, unknown: 27 | 18.2 ± 12.8 | First use: pleasure 89%; SCI medicinal: pain and/or spasticity 22%; Current use: pleasure 63%, pain 60%, party 48%, spasticity 46%, depression 31%, sleep 29%, anxiety/stress 29%, fatigue 15%, appetite 15%, weakness 13% |
| Bruce et ala., 2018 [50] |
Interview | MC legal (Illinois, USA) | 6/30 | 18+ age, smoked MC in past 3 mo, qualifying health condition for MC | - | 19/11 | 44.6 ± 15.9 | NR | NR | Medicinal cannabis use with prescription meds: alternative 60%, tapering 27%, complementary 20% |
| Hawley et al., 2018 [49] |
Cross-sectional | MC and recreational legal (Colorado, USA) | 51/116 | SCI rehab patient | - | 95/21 | 47.1 ± 13.8 (22-74) | Tetra ABC: 38, para ABC: 31, tetra/para D: 41, unknown: 5 | 13.0 | Spasticity 70%, recreation 63%, sleep 63%, pain 59%, decrease meds 52%, nausea 33%, appetite 33%, depression 33% |
Abbreviations: ABCD: American Spinal Injury Association classification A (complete injury), B (incomplete – sensory is preserved), C (incomplete – most muscle groups below the level of injury have strength <3), D (incomplete– most muscle groups below the level of injury have strength >3); BC: British Columbia; MC: medical cannabis; NR: not reported; SCI: spinal cord injury; tSCI: traumatic spinal cord injury; mo: monthly.
adata listed not limited to people with SCI.
3.6. Aim 4: Treatment Efficacy of Cannabinoids
3.6.1. Pain
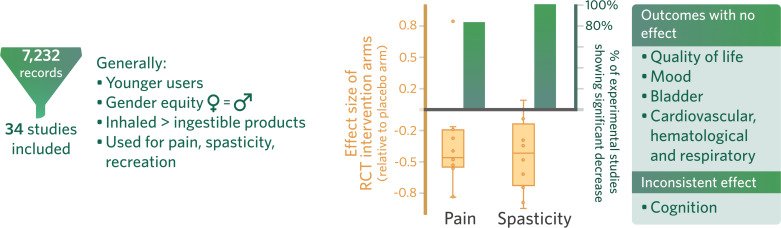
Six experimental studies, including a total of ten therapeutic intervention-arms, reported data related to a range of cannabinoids (Dronabinol; 1’,1’-dimethylheptyl-Δ8-tetrahydrocannabinol-11-oic acid (CT-3); THC cigarettes; CBD-/THC-rich sublingual spray; THC vaporized cannabinoid) for the treatment of pain in people with SCI (Table 3, Table e-6) [55-60]. Four of these studies reported pain outcomes using a visual analogue scale (VAS), a measure of pain subjectively rated on a continuum from none to an extreme amount of pain, with clinically meaningful changes in chronic pain estimated as a decrease by 2.3 points and 30-mm on the 11-point and 100-mm scales, respectively [56, 58-62]. The single study rated good-quality by Karst et al. [56], concluded that CT-3 significantly (p=0.02) reduced pain compared to placebo at three hours after oral administration. Three fair-quality RCTs investigated the analgesic effects of cannabinoids and showed that cigarettes (containing 3.5% and 6.9% THC), vaporized THC (2.9% and 6.7%) and CBD-rich or THC-rich sublingual sprays significantly (p<0.05) reduced pain compared to placebo [58-60]. Two of these studies concluded that cannabinoids significantly improved the following multidimensional pain descriptors associated with NPP: intensity, sharpness, burning, aching, sensitivity, unpleasantness, deep pain, superficial pain. Neither study found any improvement in allodynia [59, 60]. A poor-quality study found that oral Dronabinol had no significant analgesic effects compared to placebo [57]. However, an open-label pre-/post-study investigating oral Dronabinol concluded a significant decrease in pain (p=0.047) compared to baseline after one day, although this significant decrease did not persist in later follow-ups at 8 and 43 days [55]. None of the studies that investigated pain using a VAS reported clinically meaningful differences [56, 58, 60]. Overall, the effect sizes of cannabinoids on pain as studied in the RCTs (n=5) ranged from -0.82 to 0.83. A statistically significant improvement in pain was reported in 83% of all experimental studies (n=6) (Table e-6, Table e-7, Fig. 2).
Table 3.
Experimental studies: effect of cannabinoids on pain.
| Author, Year | Inclusion Criteria | Exclusion Criteria | Number of Participants (SCI/Total) |
Male/
Female |
Mean Age |
Tetraplegia/
Paraplegia |
Mean Time Since Injury | Intervention | Comparison | Pain Measures | Outcome | Effect Size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randomized Control Trials (Mixed Samples) | ||||||||||||||||||||||||
| *Karst et al., 2003 [56] |
Neuropathic and somatic pain for >6mo, stable levels of pain medications for >2mo. Aged 18-65y. Consent to participate in study and follow study procedures | No N-methyl-D-aspartate receptor antagonist and cannabinoid concomitant pain-relieving medications. Severe organic or psychiatric disease, pregnancy/attempting to conceive, lactation, use of any investigational drug within 30d prior to the first dose of study drug, non-German speaking | 3/21 | 13/8 | 51y (21-65y) |
0/3 | NR | CT-3 (10.0mg–max 80.0mg) before placebo sequence f/u: 3, 8 hrs |
Placebo | VRS pain, VAS pain (100-mm scale) | ↓ Pain (3hrs: VAS p=0.02, VRS p=0.10) 8hrs: VAS p=0.21, VRS p=0.14) |
3hr VRS: ↓0.55/↓0.50 3hr VAS: ↓0.82/↓0.52 8hr VRS: ↓0.39/↓0.54 8hr VAS: ↓0.52/↓0.17 |
||||||||||||
| *Wade et al., 2003[58] |
Neurologic diagnosis and be able to identify troublesome symptoms which were stable and unresponsive to standard treatments. | History of drug or alcohol abuse, serious psychiatric illness (excluding depression associated with neurological condition), serious cardiovascular disease or active epilepsy | 4/20 | 10/10 | 48y | NR | NR | CBD-rich sublingual spray (2.5mg–max 120mg/d) f/u: 2 wks |
Placebo (Inert Plant Material) | VAS pain (daily 100-mm scale, 2wk 11-point scale) | ↓ Pain (daily VAS p<0.05) |
VAS pain/d: ↓0.45 VAS pain 2wk: ↓0.19 |
||||||||||||
| THC-rich sublingual spray (2.5mg–max 120mg/d) f/u: 2 wks |
Placebo (Inert Plant Material) | ↓ Pain (daily VAS p<0.05) |
VAS pain/d: ↓0.39 VAS pain 2wk: ↓0.82 |
|||||||||||||||||||||
| 1:1 THC:CBD sublingual spray (2.5mg–max 120mg/d) f/u: 2 wks |
Placebo (Inert Plant Material) | = Pain | VAS pain/d: ↓0.19 VAS pain 2wk: ↓0.16 |
|||||||||||||||||||||
| *Wilsey et al., 2008 [59] |
Adults with complex regional pain syndrome (CRPS type 1), SCI, peripheral neuropathy, or nerve injury. Previous cannabis exposure. Must refrain from smoking cannabis or taking oral synthetic delta-9-THC medications for 30d before study session | Candidates who met the criteria for severe major depressive disorder, or candidates with a history or diagnosis of schizophrenia or bipolar depression. Uncontrolled hypertension, cardiovascular disease, chronic pulmonary disease (asthma, chronic pulmonary obstructive disease), active substance abuse | 6/38 | 20/18 | 46y (21-71y) |
NR | 6y (10mo-24y) |
3.5% delta 9-THC cigarettes (9 puffs) f/u: 1, 2, 3, 4, 5, 6 hrs |
Placebo | VAS pain intensity (100-mm scale), VAS pain unpleasantness, Global Impression of Change, Neuropathic pain scale, VAS allodynia, Heat pain threshold | ↓ Pain (p=0.03 CI -0.0069 to -0.0003) ↓ Pain Unpleasantness (p<0.01 CI -0.33 to -0.09) ↑ Global Impression of Change of Pain (p<0.01 CI 0.064 to 0.018) ↓ Neuropathic Pain Scale (sharp, burning, aching, deep pain p<0.001; superficial p<0.04; sensitive p<0.03) |
Insufficient data | ||||||||||||
| 7% delta 9-THC cigarettes (9 puffs) f/u: 1, 2, 3, 4, 5, 6 hrs |
Placebo | ↓ Pain (p=0.04 CI -0.0068 to -0.0002) ↓ Pain Unpleasantness (p<0.01 CI -0.33 to -0.09) ↑ Global Impression of Change of Pain (p<0.01 CI 0.065 to 0.018) ↓ Neuropathic Pain Scale (sharp, burning, aching, deep pain p<0.001; superficial p<0.01; sensitive p<0.03) |
Insufficient data | |||||||||||||||||||||
| Author, Year | Inclusion Criteria | Exclusion Criteria | Number of Participants (SCI/Total) |
Male/ Female |
Mean Age |
Tetraplegia/ Paraplegia |
Mean Time Since Injury | Intervention | Comparison | Pain Measures | Outcome | Effect Size | ||||||||||||
| Randomized Control Trials (Mixed Samples) | ||||||||||||||||||||||||
| *Rintala et al., 2010 [57] |
Adults who had sustained an SCI >12 before study entry and who reported chronic (>6 mo) neuropathic pain, the intensity of which was rated as >5 at its worst on a scale of 0-10 | Previous adverse reaction to any cannabinoid or sesame oil, current or history substance abuse, serious psychological or psychiatric disorder, renal or hepatic insufficiency, history of tachycardia, pregnant or nursing | 7/7 | 5/2 | 50.1 ± 8.3y | ¾ | 21.9 ± 9.3y (4-32y) | Dronabinol (5.0mg–max 20.0mg) f/u: 2, 4 wks |
Placebo (diphenhydramine) | Brief Pain Inventory | = Pain | Brief Pain Inventory: ↑0.83 | ||||||||||||
| *Wilsey et al., 2016 [60] |
Age 18-70y, with pain intensity >4/10, who attend the UC Davis Medical Center Spinal Cord Injury Clinic | Diagnosis of bipolar depression, schizophrenia, severe depression, or affirmation to the statements “I felt life was not worth living”; “I felt like hurting myself”; “I felt like killing myself”. A history of coronary artery disease, obstructive pulmonary disease, severe liver disease, impaired renal function. Current substance use disorder. |
29/42 | 29/13 | 46.4y | NR | 11.6 ± 10.1y | 2.9% delta 9-THC vaporized cannabis (4-8 puffs) f/u: 60, 120, 180, 240, 300, 360, 420min |
Placebo | VAS 100-mm pain scale, Patient Global Impression of Change, Neuropathic Pain Scale, VAS allodynia, Heat-pain threshold | ↓ Pain Intensity (60min p<0.05, 120/240min p<0.01, 300min p<0.05, 360min p<0.05, 420min p<0.05) ↑ Pain Relief (60, 120, 240, 300, 420min p<0.0001) *given second dose at 240min ↓ all neuropathic pain except itching (p<0.0001) |
Insufficient data | ||||||||||||
| 6.7% delta 9-THC vaporized cannabis (4-8 puffs) f/u: 60, 120, 180, 240, 300, 360, 420min |
Placebo | ↓ Pain Intensity (60min p<0.05, 300min p<0.05, 360min p<0.05, 420min p<0.05) ↑ Pain Relief (60, 120, 240, 300, 360min p<0.0001) *given second dose at 240min ↓ all neuropathic pain except itching (p<0.0001) |
Insufficient data | |||||||||||||||||||||
| Pre-/Post-Studies (SCI samples) | ||||||||||||||||||||||||
| Hagenbach et al., 2007 [55] Open-label |
Terminated taking all spasmolytic medication >3 half-life periods before enrolling, free of illegal drugs. Spasticity without any spasmolytic treatment had to be >3points on the MAS in at least one muscle group | Pregnant, severe somatic and known psychiatric diseases | 22/22 | 20/2 | 40.9y (19-73y) | 11/11 | 13.3y (2-29y) |
Dronabinol capsule oral (2.5mg, 5.0mg, 10.0mg) f/u: 1, 8, 43d |
Baseline | 6-point pain scale | ↓ Pain (1d p=0.047) | |||||||||||||
Abbreviations: ↑: increase; ↓: decrease; =: no change; *: pain studied as a primary outcome; CBD: cannabidiol; CT-3: 1’,1’-dimethylheptyl-Δ8-tetrahydrocannabinol-11-oic acid in capsules; CI: confidence interval; d: day; f/u: follow-up; MAS: Modified Ashworth Scale; mo: month; NR: not reported; SCI: spinal cord injury; THC: tetrahydrocannabinol; UC: University of California; VAS: visual analog scale; VRS: verbal rating scale; wks: weeks, y: years.
Table.
e-6. Summary coding of studies examining the effect of cannabinoids on SCI-specific outcomes.
| Outcome | n/N (%) | Effect (0/-/+/?) |
|---|---|---|
| Pain | 5/6 (83%) | ++ |
| Spasticity | 5/5 (100%) | ++ |
| Quality of life and daily function | 0/3 (0%) | 00 |
| Cognition | 3/6 (50%) | ? |
| Mood and emotion | 0/3 (0%) | 00 |
| Bladder function | 0/3 (0%) | 00 |
| Cardiovascular, hematologic and respiratory | 0/3 (0%) | 00 |
Abbreviations: n: number of studies reporting a difference in the expected direction. N: number of identified studies of interest. (%): percentage of studies reporting differences in the expected direction. 0: no effect, 0–33% of studies reported significant differences. ?: inconsistent, 34–59% of studies reported significant differences. +/-: positive (+) or negative (−) effect, 60–100% of studies demonstrated significant differences. ≥4 studies: positive (++), negative (−−), no effect (00), inconsistent findings (??).
Table.
e-7. Effect sizes and relative differences of randomized control studies of the effects of cannabinoids among adults with chronic SCI.
| Outcome | Author, Year |
Outcome
Measure |
Group | Dose | Follow-up Times | Treatment | Control | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean* | n | Mean* | Effect size (d)** [CI] | |Hedges| (Δ) | ||||||||||||||||
| Pain | Karst et al., 2003 [56] |
VRS pain | CT-3 – placebo sequence AM PM |
CT-3 10.0mg-80.0mg | 3hrs 8hrs |
10 10 |
-0.36 (0.47) -0.57 (0.95) |
10 10 |
-0.11 (0.40) -0.25 (0.55) |
-0.57 (0.44) [-1.44-0.34] -0.41 (0.06) [-1.28-0.49] |
0.55 (↓) 0.39 (↓) |
||||||||||
| Placebo – CT-3 sequence AM PM |
3hrs 8hrs |
11 11 |
-0.61 (1.01) -0.62 (0.74) |
11 11 |
-0.19 (0.55) -0.29 (0.38) |
-0.52 (0.81) [-1.35-0.35] -0.56 (0.59) [-1.39-0.31] |
0.50 (↓) 0.54 (↓) |
||||||||||||||
| VAS pain, 100-mm scale | CT-3 – placebo sequence AM PM |
3hrs 8hrs |
10 10 |
-13.07 (13.76) -15.56 (23.38) |
10 10 |
-1.52 (12.98) -5.91 (14.82) |
-0.86 (13.38) [-1.74-0.09] -0.49 (19.57) [-1.36-0.42] |
0.82 (↓) 0.47 (↓) |
|||||||||||||
| Placebo – CT-3 sequence AM PM |
3hrs 8hrs |
11 11 |
-13.00 (22.14) -12.39 (14.48) |
11 11 |
-3.14 (13.11) -8.26 (29.15) |
-0.54 (18.19) [-1.37-0.33] -0.18 (23.00) [-1.01-0.66] |
0.52 (↓) 0.17 (↓) |
||||||||||||||
| Wade et al., 2003 [58] |
VAS pain, 100-mm scale (0=worst, 100=best possible) |
CBD THC CBD:THC |
2.5mg-120.0mg/d | Daily | 20 20 20 |
54.8 (22.6) 54.6 (27.4) 51.3 (27.0) |
20 | 44.5 (22.7) | 0.46 (22.6) [-0.18-0.43] 0.40 (25.1) [-0.37-2.02] 0.27(24.9) [-0.36-0.89] |
0.45 (↓) 0.39 (↓) 0.27 (↓) |
|||||||||||
| VAS pain, 11-point scale | CBD THC CBD:THC |
2wks | 20 20 20 |
3.8 (2.9) 3.5 (2.8) 3.9 (2.9) |
20 | 4.4 (3.2) | -0.20 (3.05) [-0.81-0.43] -0.90 (1.70) [-0.37-2.02] -0.16 (3.05) [-0.78-0.46] |
0.19 (↓) 0.82 (↓) 0.16 (↓) |
|||||||||||||
| Wilsey et al., 2008 [59] |
VAS pain intensity, 11-point scale | 3.5% THC 7% THC |
9 puffs | 1, 2, 3, 4, 5, 6 hrs | 36 34 |
NR NR |
33 | NR | Insufficient data | Insufficient data | |||||||||||
| Rintala et al., 2010 [57] |
Brief Pain Inventory, 11-point scale | Dronabinol | 5.0-20.0mg/d | 4wks | 7 | -0.27 (0.84) | 5 | -1.80 (2.49) | 0.90 (1.70) [-0.37-2.02] | 0.83 | |||||||||||
| Wilsey et al., 2016 [60] |
VAS pain, 100-mm scale (0=worst, 100=best possible) |
2.9% THC 6.7% THC |
4-8 puffs | 1, 2, 3, 4, 5, 6 hrs | 42 42 |
NR NR |
42 | NR | Insufficient data | Insufficient data | |||||||||||
| Spasticity | Wade et al., 2003 [58] |
NRS spasms, 100-mm scale (0=worst, 100=best possible) |
CBD THC CBD:THC |
2.5mg-120.0mg/d | Daily | 20 20 20 |
54.6 (19.1) 58.4 (22.3) 55.8 (24.4) |
20 | 47.3 (22.6) | 0.35 (20.9) [-0.28-0.97] 0.49 (22.4) [-0.14-1.11] 0.36 (23.5) [-0.27-0.98] |
0.34 (↓) 0.48 (↓) 0.35 (↓) |
||||||||||
| NRS spasticity, 100-mm scale (0=worst, 100=best possible) |
CBD THC CBD:THC |
20 20 20 |
47.8 (18.5) 57.3 (22.2) 43.8 (15.6) |
20 | 42.3 (18.1) | 0.30 (18.3) [-0.33-0.92] 0.74 (20.3) [0.09-1.37] 0.09 (16.9) [0.71-0.08] |
0.29 (↓) 0.75 (↓) 0.09 (↓) |
||||||||||||||
| Spasticity severity, 11-point scale | CBD THC CBD:THC |
2wk | 20 20 20 |
3.8 (2.0) 3.8 (2.0) 4.1 (1.8) |
20 | 5.4 (2.3) | -0.74 (2.15) [-1.37-(-0.09)] -0.74 (2.15) [-1.37-(-0.09)] -0.63 (2.07) [-1.25-(-0.57)] |
0.73 (↓) 0.73 (↓) 0.62 (↓) |
|||||||||||||
| Outcome | Author, Year | Outcome Measure | Group | Dose | Follow-up Times | Treatment | Control | ||||||||||||||
| n | Mean* | n | Mean* | Effect size (d)** [CI] | |Hedges| (Δ) | ||||||||||||||||
| - | Spasticity frequency, per day |
CBD THC CBD:THC |
20 20 20 |
4.6 (2.2) 3.4 (1.8) 3.6 (1.6) |
20 | 4.9 (2.5) | -0.36 (2.25) [-0.97-0.28] -0.97 (2.07) [-1.60-(-0.30)] -0.91 (1.98) [-1.54-(-0.24)] |
0.35 (↓) 0.95 (↓) 0.89 (↓) |
|||||||||||||
| AS | CBD THC CBD:THC |
20 20 20 |
1.7 (1.2) 1.8 (1.2) 1.7 (1.1) |
20 | 1.7 (1.0) | 0.00 (1.10) [-0.62-0.62] 0.09 (1.10) [-0.53-0.71] 0.00 (1.05) [-0.62-0.62] |
0.00 0.09 0.00 |
||||||||||||||
| Hagenbach et al., 2007 [55] |
MAS | Oral THC | (2.5mg, 5.0mg, 10.0mg |
1hr | 6 | 7.57 (7.37) | 7 | 12.00 (6.11) | -0.66 (6.71) [-1.73-0.50] | 0.61 (↓) | |||||||||||
| Pooyania et al., 2010 [77] |
AS – most involved group | Nabilone | 0.5-1.0mg/d | 4wk | 11 | 6.45 | 11 | 7.45 | Insufficient data | Insufficient data | |||||||||||
| AS – 8 muscle groups | Nabilone | 11 | 26.9 | 11 | 29.45 | Insufficient data | Insufficient data | ||||||||||||||
| VAS spasticity, 100-mm scale (0=no spasticity, 100=most spasticity) |
Nabilone | 11 | 44.09 | 11 | 53.18 | Insufficient data | Insufficient data | ||||||||||||||
| Spasm frequency scale | Nabilone | 11 | 3.45 | 11 | 3.45 | Insufficient data | Insufficient data | ||||||||||||||
| Wilsey et al., 2016 [60] |
Spasticity severity scale 11-point | 2.9% THC 6.7% THC |
4-8 puffs | 1, 2, 3, 4, 5, 6 hrs | 42 42 |
NR NR |
42 | NR | Insufficient data | Insufficient data | |||||||||||
Abbreviations: AS: Ashworth Scale, CBD: cannabidiol, MAS: modified Ashworth Scale, THC: tetrahydrocannabinol, VAS: visual analog scale, VRS: verbal rating scale, ↓: decrease. *Mean (SD), if not indicated otherwise. **Based on mean difference scores of intervention vs control group; see formula below [39]. Δ outcome change from baseline.
CG: Control Group, ES: Effect Size, M: Mean, N: number, SD: Standard Deviation, t= Time Point, TG: Treatment Group, μweighted: weighted mean, w: Weights, x: Value
Fig. (2).
Summary of the therapeutic effects of cannabinoids on patients with SCI.
Six observational studies assessed the analgesic effects of cannabinoids alone (Table 4). A small study (n=10) reported that 50% of participants experienced a decrease in headache pain and 40% in phantom pain with cannabinoid use [63]. Another trial by Andresen et al. [44], described that among participants who used cannabinoids for pain, 59% of individuals reported good (35%) or very good (24%) efficacy for pain relief, while Warms et al. [68], reported an average of 4.25 on a 5-point scale for cannabinoid pain relief. Moreover, participants in the study by Cardenas & Jensen [53] self-reported mean pain relief of 6.62 out of 10 points, with relief typically lasting several hours (for 80% of participants). Participants in two survey studies, by Cardenas & Jensen [53] and Warms et al. [68], reported that cannabinoids were the most effective analgesic out of a total of 26 and 27 pain treatments, respectively. Both studies showed that cannabinoids provided substantially more pain relief than non-steroidal anti-inflammatory drugs, baclofen, tricyclic antidepressants, and acetaminophen, among many other treatments [53, 68]. In a retrospective chart review of pain clinic patients by Aggarwal et al. [46], medical cannabinoids were the most effective treatment in 19% of patients. Five studies,
Table 4.
Reported benefits of cannabinoid use from observational studies.
| Author, Year | Study Type | Legalization (Location) | Number of Participants (SCI/Total) | Inclusion Criteria | Exclusion Criteria | Male/Female/ Transgender | Mean Age | Reported Pain Relief | Reported Spasticity Relief | Other Benefits | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dunn & Davis, 1974 [63] | Cross-sectional | Illegal (Florida, USA) |
10/10 | SCI patients using cannabis | - | 10/0/0 | NR | Relief: 50% (headache), 40% (phantom); Pain distraction (phantom): 20% |
Relief: 50% | Pleasant sensations: 50% | ||||||||
| Malec et al., 1982 [64] | Cross-sectional | Illegal (Wisconsin, USA) |
43/43 | SCI patients | - | 38/5/0 | NR | NR | Relief: 88% (Complete relief 38%, reduction to mild 46%, severe to moderate 4%) | NR | ||||||||
| Warms et al., 2002 [68] | Cross-sectional | MC legal starting Nov 1998, study V1 Feb 1997 – Jul 1998, V2 Aug 1998 – June 2000 (Washington, USA) | 471/471 | 18+ age, 6+ mo since SCI | - | 334/137/0 | 42.5 ± 13.2 (18-84) |
Pain helpfulness: 4.25 ± 0.76 (max 5); Most effective pain treatment |
NR | Pain relief greater than opioids, mexiletine, baclofen, acetaminophen, TCAs, NSAIDs, gabapentin, carbamazepine, etc | ||||||||
| Grotenhermen & Schnellea, 2003 [51] | Cross-sectional | Dronabinol prescription and ∆9-THC special permit (Germany) and permit (Switzerland) | 4/165 | Members of Association for Cannabis as Medicine | No severe disease | 101/64/0 | Median age: 40.3 ± 12.4 (16-87) | NR | NR | Large disease improvement: 75%, small improvement: 13%, no improvement: 2%, unknown: 7%, no answer: 3%; Large improvement over other drugs: 69%, small improvement: 7%, no improvement: 3%, unknown: 18%, no answer: 4% |
||||||||
| Gortera, 2005 [52] | Cross-sectional | MC legal (Netherlands) | ?/107 | Members of Multiple Sclerosis society | - | 48/59/0 | Median age: 58.0 | NR | NR | Efficacy: excellent 18%, good 47%, somewhat 18%, none 18%; Statistical significance in greater efficacy with inhalation vs. oral |
||||||||
| Cardenas & Jensen, 2006 [53] | Cross-sectional | MC legal (Washington, USA) | 117/117 | 18+ age, tSCI, chronic pain | Incomplete questionnaires | 85/32/0 | 48.8 ± 11.7 (21-79) | Relief: 6.62 ± 2.54 (max 10) Benefit duration: 9%: min, 80%: hr, 3%: days, 3%: mo, 6%: y. Most effective pain treatment |
NR | Pain relief greater than opioids, mexiletine, baclofen, acetaminophen, TCAs, NSAIDs, gabapentin, carbamazepine, etc | ||||||||
| Mahoney et al., 2007 [69] | Interview | Illegal (Texas, USA) |
24/24 | 1+ y since SCI, spasticity, English language | - | 17/7/0 | 45.1 (21-68) | NR | Prevents, modulates and stops spasms | NR | ||||||||
| Author, Year | Study Type | Legalization (Location) | Number of Participants (SCI/Total) | Inclusion Criteria | Exclusion Criteria |
Male/ Female/ Transgender |
Mean Age | Reported Pain Relief | Reported Spasticity Relief | Other Benefits | ||||||||
| Aggarwal et al.a, 2009 [46] | Retrospective chart review | MC legal (Washington, USA) | 5/139 | 18+ age, pain clinic patients, access to MC with valid doctor documentation | Cannabinoid receptor 1 blocker drug rimonabant | 88/51/0 | Median age: 48 (18-84) | Chronic pain relief; often described as the most effective pain treatment |
NR | Preferred for less side effects; adjunctive use with opioids reduced opioid dosages and 6% used to reduce opioid dependence | ||||||||
| Heutink et al., 2011 [70] |
Cross-sectional | MC legal starting 2003, study 1990-2005 (the Netherlands) | 279/279 | 18+ age, SCI rehab patients, living in community | - | 173/106/0 | 51.3 ± 14.0 (25-81) | (Alcohol and cannabis pooled) Largely effective 83%, somewhat effective 17%, not effective 0% |
NR | NR | ||||||||
| Shroff, 2015 [54] | Interview | MC legal (Canada) | 53/53 | 19-65 age, 1+ years since SCI, BC resident, member of paraplegic association | - | 42/11/0 | NR | NR | NR | Preferred for less side effects | ||||||||
| Andresen et al., 2017 [44] | Cross-sectional | MC legal starting 2011, study 1990-2012 (Denmark) | 537/537 | Inclusion: 18+ age, acquired tSCI, rehab clinic patients | Incomplete questionnaires | 413/124/0 | 54.6 ± 14.6 (18-88) | Relief: good 35%, very good 24% | Relief: good 32%, very good 27% | NR | ||||||||
| Bruce et ala., 2018 [50] |
Interview | MC legal (Illinois, USA) | 6/30 | 18+ age, smoked MC in past 3 mo, qualifying health condition for MC | - | 19/11/0 | 44.6 ± 15.9 | NR | NR | Preferred over other pain treatments for quick action, long effects, symptom relief, less side effects; adjunctive use with opioids reduced opioid dose and dependence | ||||||||
| Bourke et al., 2019 [72] |
Interview | Illegal (New Zealand) | 8/8 | 18+ age, SCI patients using cannabis for pain, residing in New Zealand, English speaking, |
Comorbid conditions inhibiting communication and participation in the interview | 6/2/0 | Age 20-39: n = 1, 40-59: n= 5, 60+: n=2 | Pain relief improving function, community participation and decreased disability | NR | Preferred for relatively lower fatigue and drowsiness as of prescribed medications Sleep improvement Quality of life improvement |
||||||||
| Stillman et al., 2019 [75] |
Cross-sectional | 39 states in USA, not disclosed; mixed legality | 353/353 | SCI patients included in mailing lists maintained by Thomas Jefferson University, University of Washington at Seattle, and University of Alabama at Birmingham | - | 183/107/3 | 52.74 (19-82) | NR | NR | Muscle relaxation: 90% Sleep promotion: 84% Well-being: 75% Anxiety relief: 70% Appetite promotion: 53% All prevalence of positive effects from cannabis were rated higher than prescription medications Cannabis use: lower prevalence of dehydration, memory loss, lethargy, drowsiness, constipation |
||||||||
Abbreviations: BC: British Columbia; d: days; hr: hours; MC: medical cannabis; min: minutes; mo: months; NR: not reported; NSAIDs: nonsteroidal anti-inflammatory drugs; SCI: spinal cord injury; TCAs: tricyclic antidepressants; ∆9-THC: delta-9-tetracannabidiol; tSCI: traumatic spinal cord injury; y: years. adata listed not limited to people with SCI.
including three interview-based studies also reported that cannabis was preferred over prescribed medications due to fewer side effects, including less dehydration, memory loss and drowsiness [46, 50, 54, 72]. Overall, cannabinoids were subjectively rated as the most effective pain relief treatment across several studies [44, 46, 50, 53, 68].
3.6.2. Spasticity
Five experimental studies, including a total of ten therapeutic intervention arms, investigated the benefits of cannabinoids on spasticity in people with SCI (Table 5, Table e-6) [55, 58, 60, 76, 77]. The Ashworth Scale (AS) (n=3) [55, 58, 77], pendulum drop test (n=2) [76, 77], spasticity numerical rating scale (NRS) (n=2) [58, 77] and the patients’ self-ratings of spasticity (severity point scales) (n=3) [55, 58, 60] were the most commonly used measures of spasticity. One study used the Modified Ashworth Scale (MAS) [55]. It is worth noting that clinically meaningful changes in spasticity measured by the MAS have been estimated to be a decrease by more than 1-point [78, 79].
Table 5.
Experimental studies: effect of cannabinoids on spasticity.
| Author, Year |
Inclusion
Criteria |
Exclusion Criteria | Number of Participants (SCI/Total) |
Male/
Female |
Mean Age |
Tetraplegia/
Paraplegia |
Mean Time Since
Injury |
Inter-
vention |
Comparison | Spasticity Measures | Outcome | Effect Size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randomized Control Trials (Mixed Samples) | ||||||||||||||||||||||||
| *Wade et al., 2003 [58] |
Neurologic diagnosis and be able to identify troublesome symptoms which were stable and unresponsive to standard treatments. | History of drug or alcohol abuse, serious psychiatric illness (excluding depression associated with neurological condition), serious cardiovascular disease or active epilepsy | 4/20 | 10/10 | 48y | NR | NR | CBD-rich sublingual spray (2.5mg–max 120mg/d) f/u: 2 wks |
Placebo (Inert Plant Material) | NRS spasticity, AS, 10-point spasticity severity scale; spasm frequency/day | ↓ Spasticity (2wk NRS p<0.05) |
NRS spasm/d: ↓ 0.34 NRS spasticity/d: ↓ 0.29 Severity 2wk: ↓ 0.73 Frequency 2wk: ↓ 0.35 |
||||||||||||
| THC-rich sublingual spray (2.5mg–max 120mg/d) f/u: 2 wks |
Placebo (Inert Plant Material) | ↓ Spasticity (daily, 2wk NRS p<0.05) | NRS spasm/d: ↓ 0.48 NRS spasticity/d: ↓ 0.75 Severity 2wk: ↓ 0.73 Frequency 2wk: ↓ 0.95 |
|||||||||||||||||||||
| 1:1 THC:CBD sublingual spray (2.5mg–max 120mg/d) f/u: 2 wks |
Placebo (Inert Plant Material) | ↓ Spasticity (daily, 2wk NRA p<0.05) | NRS spasm/d: ↓ 0.35 NRS spasticity/d: ↓ 0.09 Severity 2wk: ↓ 0.62 Frequency 2wk: ↓ 0.89 |
|||||||||||||||||||||
| *Hagenbach et al., 2007 [55] **RCT phase |
Terminated taking all spasmolytic medication >3 half-life periods before enrolling, free of illegal drugs. Spasticity without any spasmolytic treatment had to be >3points on the MAS in at least one muscle group | Pregnant, severe somatic and known psychiatric diseases | 13/13 | 11/2 | 40.9y (29-66y) |
5/8 | 14.3y (3y-29y) |
Dronabinol capsule oral (2.5mg, 5.0mg, 10.0mg) f/u: 1, 8, 43d |
Placebo (sesame oil) | MAS, 7-point spasticity severity scale | ↓ Spasticity (p=0.001 placebo of this phase vs open label of oral phase) (day one self-rating p=0.033) | MAS: ↓ 0.61 | ||||||||||||
| **Non-RCT phase | 22/22 | 20/2 | 40.9y (19-73y) | 11/11 | 13.3y (2-29y) |
Dronabinol capsule oral (2.5mg, 5.0mg, 10.0mg) f/u: 1, 8, 43d |
Baseline | ↓ Spasticity (AS at 1/8d p<0.001, 43d p<0.05) |
- | |||||||||||||||
| 8/8 | 8/0 | 48.8y (32-66y) | 5/3 | 15.5y (5-28y) |
Rectal THC (5.0mg, 10.0mg) f/u: 1, 8, 43d |
Baseline | ↓ Spasticity (AS at 1/8/43d p<0.05) | - | ||||||||||||||||
| *Pooyania et al., 2010 [77] | Aged 18-65 with a level of injury at C5 or below, and injury occurred more than 1 year previously. Stable neurologic level, with moderate spasticity (>3 AS). Spasticity medications had to be unchanged for at least 30 days before inclusion and no botulinum toxin injections >4 months |
History of heart disease, psychotic disorders, schizophrenia, or any active psychologic disorder. Previously documented sensitivity to marijuana or other cannabinoid agents, severe liver dysfunction, cognitive impairment, a major illness in another body area, fixed tendon contractures. Pregnant or nursing. History of drug dependency, smoked cannabis <30d before study onset, or unwilling to not smoke during the study | 12/12 | 12/0 | 42.4y | 6/6 | NR | Nabilone (0.5mg-1.0mg/d) f/u: 4wks |
Placebo | AS, Spasm frequency scale, VAS spasticity, Pendulum test, Global Impression of Change (subject/ clinician) |
↓ Spasticity (aAS in most spasticity group p=0.003, AS in 8 muscle groups p=0.001) | Insufficient data | ||||||||||||
| Author, Year | Inclusion Criteria | Exclusion Criteria | Number of Participants (SCI/Total) |
Male/ Female |
Mean Age |
Tetraplegia/ Paraplegia |
Mean Time Since Injury | Intervention | Comparison | Spasticity Measures | Outcome | Effect Size | ||||||||||||
| Randomized Control Trials (Mixed Samples) | ||||||||||||||||||||||||
| *Wilsey et al., 2016 [60] |
Age 18-70, with pain intensity >4/10, who attend the UC Davis Medical Center Spinal Cord Injury Clinic | Diagnosis of bipolar depression, schizophrenia, severe depression, or affirmation to the statements “I felt life was not worth living”; “I felt like hurting myself”; “I felt like killing myself”. A history of coronary artery disease, obstructive pulmonary disease, severe liver disease, impaired renal function. Current substance use disorder. | 29/42 | 29/13 | 46.4y | NR | 11.6 ± 10.1y | 2.9% delta 9-THC vaporized cannabis (4-8 puffs) f/u: 60, 120, 180, 240, 300, 360, 420min |
Placebo | 11-point spasticity severity scale (spasms, pain, muscle stiffness), Global Impression of Change | ↓ Spasticity (420min p<0.0001) ↑ Relief (p=0.0227) |
Insufficient data | ||||||||||||
| 6.7% delta 9-THC vaporized cannabis (4-8 puffs) f/u: 60, 120, 180, 240, 300, 360, 420min |
Placebo | = Spasticity | Insufficient data | |||||||||||||||||||||
| Pre-/Post-Studies (SCI samples) | ||||||||||||||||||||||||
| *Kogel et al., 1995 [76] |
SCI staff selected. Chronic problematic spasticity that has not responded to more commonly prescribed spasmolytic medications. | - | 5/5 | 5/0 | 41y (28-55y) |
5/0 | 6mo-9y | Dronabinol (15.0 mg – 60.0mg/d) f/u: 5d |
Baseline | Pendulum Drop Test | ↓ Spasticity | |||||||||||||
Note: a:clinically meaningful change in AS as defined as a decrease of 1 point. ↑: increase; ↓: decrease; =: no change; *: pain studied as a primary outcome; AS: Ashworth Scale; CBD: cannabidiol; d: day; f/u: follow-up; MAS: Modified Ashworth Scale; mo: month; NR: not reported; NRS: numerical rating scale; SCI: spinal cord injury; THC: tetrahydrocannabinol; UC: University California; wks: weeks, y: years.
Three fair-quality RCTs found cannabinoids to be effective in improving spasticity in people with SCI [55, 58, 60].
A study was automatically considered poor quality with a significant risk of bias if it included a “fatal flaw”. Examples of fatal flaws included high dropout rates, high differential dropout rates, no intention-to-treat analysis, or other unsuitable statistical analysis (e.g., completers-only analysis).
Wade et al. [58], determined that sublingual CBD, THC and 1:1 CBD:THC significantly reduced VAS scores (p<0.05) at 2 weeks. Oral Dronabinol reduced self-ratings of spasticity on day 1 (p=0.033) [55]. Wilsey et al. [60], found that 2.8% vaporized THC improved spasticity scales significantly compared to placebo (p<0.0001), while 6.7% vaporized THC did not. The single poor-quality study found that nabilone resulted in a significant reduction for those who exhibited the most spasticity, as measured by the total AS score [77]. However, the treatment group had higher spasticity at baseline. The pre-/post-studies determined that oral Dronabinol and rectal THC both improved spasticity [55, 76]. The one study that utilized the MAS demonstrated a clinically meaningful decrease in spasticity [55]. Among the RCTs (n=4), the effect size of cannabinoid use on spasticity ranged from -0.95 to 0.09; across all experimental studies, 100% of studies showed a statistically significant improvement in spasticity (n=5) (Table e-6, Table e-7, Fig. 2).
Four observational studies investigated the therapeutic effect of cannabinoids on spasticity (Table 4) [44, 63-64, 69]. Dunn and Davis [63] found that 50% of participants
3.6.3. Quality of Life and Daily Function
Mood, pain, and spasticity have been demonstrated to negatively impact activities of daily living, mobility and general health [44]. Overall, two studies [55, 58], comprised of five therapeutic intervention arms, reported the impact of cannabinoids on functional independence measures (Barthel Activities of Daily Living Index, Rivermead Mobility Index, General Health Questionnaire 28, Functional Independence Measure) and found that cannabinoids had no statistically significant effect (Table 6, Table e-6) [55, 58]. On the contrary, one interview-based study reported that the analgesic properties of cannabis use could improve the quality of life due to functional improvement [72].
Table 6.
Observational studies: reported side effects from cannabinoids.
| Author, Year | Inclusion Criteria | Exclusion Criteria | Number of Participants (SCI/Total) |
Male/
Female/ Transgender |
Mean Age |
Tetraplegia/
Paraplegia |
Mean Time Since Injury | Side Effects |
|---|---|---|---|---|---|---|---|---|
| Observational Studies | ||||||||
| Dunn & Davis, 1974 [63] | SCI patients using cannabis | - | 10/10 | 10/0 | NR | NR | NR | Urinary retention: 20% |
| Heinemann et al., 1991 [65] |
13-66 age, 2+ years since tSCI, English language, no cognitive impairment | - | 43/43 | 38/5 | NR | NR | NR | Marijuana use problems 6 months pre-SCI: 21%, post-SCI: 13% Needing help with marijuana use problems pre-and post-SCI: 1% |
| Grotenhermen & Schnellea, 2003 [51] | Members of Association for Cannabis as Medicine | No severe disease | 4/165 | 101/64 | Median age: 40.3 ± 12.4 (16-87) |
NR | NR | Side effects; none 73%, moderate 22%, no answer 4% Withdrawal; none 68%, moderate 18%, strong 3%, unknown 12% |
| Gortera, 2005 [52] | Members of Multiple Sclerosis society | - | ?/107 | 48/59 | Median age: 40.3 ± 12.4 (16-87) |
NR | NR | Dry mouth: 27%, sleepiness: 14%, euphoria: 13%, loss of concentration: 12%, feeling high: 11%; More frequent side effects in first few months of intake |
| Aggarwal et ala., 2009 [46] |
18+ age, pain clinic patients, access to MC with valid doctor documentation | Cannabinoid receptor 1 blocker drug rimonabant | 5/139 | 88/51 | Median age: 48 (18-84) | NR | NR | No side effects with MC |
| Shroff, 2015 [54] | 19-65 age, 1+ years since SCI, BC resident, member of paraplegic association | - | 53/53 | 42/11 | NR | NR | NR | Incapacitation |
| Andresen et al., 2017 [44] | Inclusion: 18+ age, acquired tSCI, rehab clinic patients | Incomplete questionnaires | 537/537 | 413/124 | 54.6 ± 14.6 (18-88) | 247/263, unknown: 27 | 18.2 ± 12.8 | Inertia: 63%, feeling subdued: 50%, absent-minded: 29%, risky behaviour: 27% |
| Clark et al., 2017 [47] | 18+ age, 1+ year since tSCI, some residual impairment | No painful condition, no prescription pain med | 1619/1619 | 1166/453 | 49.3 ± 14.2 | 453/1166 | 11.5 ± 9.2 | Frequent MC use 1.8x pain med misuse, occasional MC use 2.7x pain med misuse |
| Hawley et al., 2018 [49] | Cross-sectional | MC and recreational legal (Colorado, USA) | 51/116 | 95/21/0 | 47.1 ± 13.8 (22-74) | Tetra ABC: 38, para ABC: 31, tetra/para D: 41, unknown: 5 | 13.0 | Amotivation: 30%, social stigma: 26%, other: 22%, feeling dull: 19%, fatigue: 19%, paranoia: 19%, low blood pressure: 15%, physical instability: 11% |
| Bourke et al., 2019 [72] | 18+ age, SCI patients using cannabis for pain, residing in New Zealand, English speaking | Comorbid conditions inhibiting communication and participation in an interview | 8/8 | 6/2/0 | Age 20-39: n = 1, 40-59: n= 5, 60+: n=2 | Tetra: 6 Para: 2 |
NR | Dysphoria: detrimental effect on the mind and ability to participate within the community |
| Stillman et al., 2019 [75] | Cross-sectional | 39 states in USA, not disclosed; mixed legality | 353/353 | 183/107/3 | 52.74 (19-82) |
NR | 17.49 | Dry mouth: 55%, residual bad taste: 30%, dehydration: 29%, memory loss: 27%, lethargy: 26%, drowsiness: 22%, constipation: 17% |
Abbreviations: BC: British Columbia; MC: medical cannabis; min: minutes; NR: not reported; SCI: spinal cord injury; tSCI: traumatic spinal cord injury. adata listed not limited to people with SCI.
3.6.4. Cannabinoids and Opioids
Four observational studies compared the efficacy and safety profile of cannabinoids with opioids [46, 50, 53, 68]. Cannabinoids were noted to provide greater pain relief than all other pain medications, including opioids, such as codeine, methadone, oxycodone, Percodan, Percocet, and Vicodin. In particular, participants of semi-structured interviews reported quicker onset, longer duration of action, greater symptom relief and fewer side effects for cannabinoids compared to opioids, when prescribed for chronic conditions (e.g. rheumatoid arthritis, SCI, fibromyalgia) [50]. These findings were corroborated by two previously described cross-sectional studies that reported analgesic superiority of cannabinoids among people with SCI, including greater pain relief than opioids; however, no statistical analyses were conducted [53, 68].
Many participants reported fewer side effects of cannabinoids compared with opioid use (i.e. constipation, nausea, incapacitation and allergies) [46, 50, 54]. Opioids were also least likely to be continued as pain medication [53]. Finally, patients perceived cannabinoids as a means of harm reduction with respect to the addictive potential of opioids. Patients described using cannabinoids either alternatively or in conjunction with opioids reduced their opioid dose and dependence [46, 50].
3.7. Aim 5: Side Effects Associated with Cannabinoid Use
The specific side effects of cannabinoids varied between the experimental studies, but were not uncommon (Table 7). Dry mouth, fatigue and increased hunger were the most commonly noted and were associated with both CBD and THC therapy [55-58]. Most of these side effects were rated as mild (dry mouth, drowsiness, itchiness, weakness, dizziness, confusion, incoordination, rash). However, a substantial number of side effects were reported as moderate (scale of mild, moderate and severe), such as constipation, fatigue and abdominal discomfort [57].
Table 7.
Experimental studies: reported side effects from cannabinoids.
| Author, Year | Inclusion Criteria | Exclusion Criteria | Intervention | Comparison | Side Effects | ||
|---|---|---|---|---|---|---|---|
| Randomized Control Trials (Mixed Samples) | |||||||
| Karst et al., 2003 [56] | Neuropathic and somatic pain for >6mo, stable levels of pain medications for >2mo. Aged 18-65y. Consent to participate in study and follow study procedures | No N-methyl-D-aspartate receptor antagonist and cannabinoid concomitant pain-relieving medications. Severe organic or psychiatric disease, pregnancy/attempting to conceive, lactation, use of any investigational drug within 30d prior to first dose of study drug, non-German speaking | CT-3 (10.0mg–max 80.0mg) f/u: 3, 8 hrs |
Placebo | ↑ Fatiguef; ↑ Dry mouthf ; ↑ Limited power of concentrationf; ↑ Painf; = Objective concentration; = Vitals (RR, HR, BP, wt, temp, ECG, hematologic and blood chemistry) | ||
| Wade et al., 2003 [58] | Neurologic diagnosis and be able to identify troublesome symptoms which were stable and unresponsive to standard treatments | History of drug or alcohol abuse, serious psychiatric illness (excluding depression associated with neurological condition), serious cardiovascular disease or active epilepsy | CBD-rich sublingual spray (2.5mg–max 120mg/d) f/u: 2 wks |
Placebo (Inert Plant Material) | = Objective concentration; = Bladder function; = Daily functioning | ||
| THC-rich sublingual spray (2.5mg–max 120mg/d) f/u: 2 wks |
Placebo (Inert Plant Material) | ↓ Objective Concentration (SOMC) j; ↑ Appetite (daily VAS) j; = Bladder function; = Daily functioning | |||||
| 1:1 THC:CBD sublingual spray (2.5mg–max 120mg/d) f/u: 2 wks |
Placebo (Inert Plant Material) | = Objective concentration; = Bladder function; = Daily functioning; ↑ Sleep (daily VAS) j |
|||||
| Hagenbach et al., 2007 [55] *RCT phase |
Terminated taking all spasmolytic medication >3 half-life periods before enrolling, free of illegal drugs. Spasticity without any spasmolytic treatment had to be >3points on the MAS in at least one muscle group | Pregnant, severe somatic and known psychiatric diseases | Dronabinol capsule oral (2.5mg, 5.0mg, 10.0mg) f/u: 1, 8, 43d |
Placebo (sesame oil) | ↑ Reaction Time; = Vitals (HR, BP, ECG, hematologic and blood chemistry); = Mood; = Functional independence | ||
| *Non-RCT phase | Dronabinol capsule oral (2.5mg, 5.0mg, 10.0mg) f/u: 1, 8, 43d |
Baseline | ↓ Systolic BP; ↑ Vital capacity (43d) g; = Mood; = Functional independence; = objective concentration; = Bladder function; ↑ Fatigue (36%); ↑ Dry mouth (32%); ↑ Anxiety (32%); ↑ Disturbance of attention (27%); ↑ Pain (23%); ↑ Dizziness (23%) | ||||
| Rectal THC (5.0mg, 10.0mg) f/u: 1, 8, 43d |
Baseline | ↑MCC (43d) k; = Vitals (HR, BP, ECG, hematologic and blood chemistry); = Mood; = Functional independence | |||||
| Wilsey et al., 2008 [59] | Adults with complex regional pain syndrome (CRPS type 1), SCI, peripheral neuropathy, or nerve injury. Previous cannabis exposure. Must refrain from smoking cannabis or taking oral synthetic delta-9-THC medications for 30d before study session | Candidates who met the criteria for severe major depressive disorder, or candidates with a history or diagnosis of schizophrenia or bipolar depression. Uncontrolled hypertension, cardiovascular disease, chronic pulmonary disease (asthma, chronic pulmonary obstructive disease), active substance abuse | 3.5% delta 9-THC cigarettes (9 puffs) f/u: 1, 2, 3, 4, 5, 6 hrs |
Placebo | ↑ “Feeling high”j; ↑ “Feeling stoned”j; ↑ “Impaired”d; ↑ Sedationd; ↑ Hungerb; ↓ Attention; ↓ Learning/memory; ↓ Psychomotor speed; ↑ “Good drug effect”b; ↑ Calmnessi | ||
| 7% delta 9-THC cigarettes (9 puffs) f/u: 1, 2, 3, 4, 5, 6 hrs |
Placebo | ↑ “Feeling high”b; ↑ “Feeling stoned”c; ↑ “Bad drug effect”d; ↑ “Impaired”d; ↑ Sedatione; ↑ Hungere; ↓ Learning/memory; ↑ “Good drug effect”e; ↑ HR (immediately); = Mood; = Spasticity ; = Neurocognition (overall) | |||||
| Pooyania et al., 2010 [77] |
Aged 18-65 with a level of injury at C5 or below, and injury occurred more than 1 year previously. Stable neurologic level, with moderate spasticity (>3 AS). Spasticity medications had to be unchanged for at least 30 days before inclusion and no botulinum toxin injections >4mo | History of heart disease, psychotic disorders, schizophrenia, or any active psychologic disorder. Previous documented sensitivity to marijuana or other cannabinoid agents, severe liver dysfunction, cognitive impairment, a major illness in another body area, fixed tendon contractures. Pregnant or nursing. History of drug dependency, smoked cannabis <30d before study onset, or unwilling to not smoke during the study | Nabilone (0.5mg-1.0mg/d) f/u: 4wks |
Placebo | ↑ Drowsiness (27.2%); ↑ Dry mouth (18.1%); ↑ Asthenia (18.1%); ↑ Vertigo (18.1%) | ||
| Author, Year | Inclusion Criteria | Exclusion Criteria | Intervention | Comparison | Side Effects | ||
| Randomized Control Trials (Mixed Samples) | |||||||
| Rintala et al., 2010 [57] | Adults who had sustained an SCI >12 before study entry and who reported chronic (>6mo) neuropathic pain, the intensity of which was rated as >5 at its worst on a scale of 0-10 | Previous adverse reaction to any cannabinoid or sesame oil, current or history substance abuse, serious psychological or psychiatric disorder, renal or hepatic insufficiency, history of tachycardia, pregnant or nursing | Dronabinol (5.0mg–max 20.0mg) f/u: 2, 4 wks |
Placebo (diphenhydramine) | ↑ Constipation; ↑ Fatigue; ↑ Dry mouth; ↑ Abdominal discomfort | ||
| Wilsey et al., 2016 [60] | Age 18-70, with pain intensity >4/10, who attend the UC Davis Medical Center Spinal Cord Injury Clinic | Diagnosis of bipolar depression, schizophrenia, severe depression, or affirmation to the statements “I felt life was not worth living”; “I felt like hurting myself”; “I felt like killing myself”. A history of coronary artery disease, obstructive pulmonary disease, severe liver disease, impaired renal function. Current substance use disorder. | 2.9% delta 9-THC vaporized cannabis (4-8 puffs) f/u: 60, 120, 180, 240, 300, 360, 420min |
Placebo | ↑ “Good Drug Effect”a; ↑ “Bad Drug Effect”a; ↑ Higha; ↑ Drunka; ↑ Stoneda; ↑ Sedateda; ↑ Nauseaa; ↑ Changes Perceiving Time/Spacea; ↑ HR (immediately); ↑ calmness; = Neurocognition (overall) | ||
| 6.7% delta 9-THC vaporized cannabis (4-8 puffs) f/u: 60, 120, 180, 240, 300, 360, 420min |
Placebo | ↑ Confuseda; ↑ Desires Morea; ↑ Hungrya; ↑ Difficulty Paying Attention/ Remembering Thingsa; ↑ “Good Drug* Effect”a; ↑ “Bad Drug Effect”a; ↑ High*a; ↑ Drunk*a; ↑ Impaired*a; ↑ Stoned*a; ↑ Sedated*a; ↑ Nauseaa; ↑ Changes Perceiving Space*/Timea | |||||
| Pre-/Post-Studies (SCI samples) | |||||||
| Kogel et al., 1995 [76] | SCI staff selected. Chronic problematic spasticity that has not responded to more commonly prescribed spasmolytic medications. | NR | Dronabinol (15.0 mg - 60.0mg/d) f/u: 5d |
Baseline | ↓ Subjective Concentration; ↓ vigor; = objective concentration; ↑ >1 dysphoric mood scale | ||
Abbreviations: ↑: increase; ↓: decrease; =: no change; adata listed not limited to people with SCI; AS: Ashworth Scale; BP: blood pressure; CBD: cannabidiol; CT-3: 1’,1’-dimethylheptyl-Δ8-tetrahydrocannabinol-11-oic acid in capsules; d: day; ECG: electrocardiogram; f/u: follow-up; HR: heart rate; MAS: Modified Ashworth Scale; MC: Medical Cannabis; MCC: maximal cystometric capacity; mo: month; N/A: not applicable; NR: not reported; RR: respiratory rate; SOMC: short orientation-memory-cognition test; temp: temperature; THC: tetrahydrocannabinol; UC: University California; VAS: visual analog scale; wt: weight; y: year. *denotes that higher dose was significant vs lower dose; adenotes p<0.0001; bdenotes p<0.001; cdenotes p=0.001; ddenotes p=0.003; edenotes p<0.01; fdenotes p=0.02; gdenotes p=0.028; hdenotes p=0.03; idenotes p<0.03; jdenotes p<0.05; kdenotes p=0.075.
3.7.1. Cognition
Six experimental studies, comprised of ten therapeutic intervention arms, investigated the effect of cannabinoids on cognition in people with SCI, as secondary outcomes (Table 6, Table e-6) [55-56, 58-60, 76]. Wade et al. [58], assessed cognition using the short orientation-memory-concentration test (SOMC), a measure of concentration. They found that
THC-rich sublingual spray alone caused decreased concentration (p<0.05), while CBD-rich and 1:1 CBD:THC had no effect on SOMC scores. Karst et al. [56], measured changes in processing speed, visual attention and task switching with the Trail Making Test (TMT), and found CT-3 to have no effect on time to completion. Hagenbach et al. [55], used the Continuous Performance Test, Divided Attention Test, and the Deux Barrages tests to measure the effect of 2.5 – 10.0 mg Dronabinol. In the placebo-controlled double-blind, parallel trial, Dronabinol increased reaction times, while the open-label phase of the study showed no change in scores after Dronabinol administration. Kogel et al. [76], measured the effects of 15.0 – 60.0 mg Dronabinol and found no change in concentration measured with the Weschler Memory Scale. Vaporized THC (2.9%, 6.7%) had no effect on neurocognition based on the Grooved Pegboard Test (GPT), Weschler Adult Intelligence Scale Digit Symbol Test (WAIS-III), TMT, Paced Auditory Serial Addition Test [60]. Wilsey et al. [59], studied cognition changes using the WAIS-III, GPT and the Hopkins Verbal Learning Test-Revised. 3.5% THC cigarettes resulted in decreased attention, learning and memory, and decreased psychomotor speed compared to placebo. The 7% THC cigarettes also decreased learning and memory [59]. The two studies by Wilsey et al., reported subjective effects such as “slowed down mentally ” or “difficulty paying attention or remembering things ” [59, 60]. Significantly more participants reported “feeling high”, “feeling stoned ”, “feeling impaired ”, or having difficulty concentrating when on the active treatment compared to the placebo [56, 59, 60, 76].
Among the three SCI-specific observational studies that investigated cognition, cannabinoids were associated with negative cognitive states. These included reports of participants experiencing inertia or executive dysfunction (63%), feeling subdued or dull (50% from a sample of 537 and 19% from a sample of 51), absent-minded (29%), memory loss (27%), lethargy (26%), and drowsiness or fatigue (22% from a sample of 353 and 19% from a sample of 51) [44, 49, 75].
3.7.2. Mood and Emotion
Three experimental studies, including five therapeutic intervention arms, investigated the effects of cannabinoids on mood as a secondary outcome measure in people with SCI who did not have a history of psychological or psychiatric disorders [55, 59, 76] (Table 6, Table e-6). Hagenbach et al. [55], found that Dronabinol (2.5 – 10.0 mg) had no significant effect on mood based on the Hamilton Rating Scale for Depression. Kogel et al. [76], reported a decrease in vigor and an increase in at least one dysphoric mood (anger, tension) with Dronabinol based on the Profile of Mood States questionnaire [55, 59, 76]. Wilsey et al. [59], found that neither 3.5% nor 7%-THC cigarettes affected VAS scores for any parameter (sad vs. happy; anxious vs. relaxed; jittery vs. calm; bad vs. good; paranoid vs. self-assured; fearful vs. unafraid). One observational study described that 13% of participants were “feeling depressed ” after cannabinoid use [44].
3.7.3. Bladder function
Wade et al. [58], assessed the effects of sublingual THC-rich, CBD-rich and 1:1 CBD:THC spray on bladder function using subjective severity scales for incontinence and bladder urgency, as well as incontinence frequency per day and nocturia frequency per night, and reported no effect (Table 6, Table e-6). Rectal THC increased the maximum cystometric capacity (MCC) in five of six participants, but there was no significant change in any of the other bladder function parameters (first desire to void, intra-vesical pressure, bladder compliance, postvoid residual urine volume, volume at first detrusor contraction). Administration of oral THC yielded mixed effects on MCC, with no significant change in other bladder function parameters [55]. Survey data from Dunn and Davis [63] demonstrated a 20% increase in urinary retention (from a sample of ten males) (Table 6, Table e-6).
3.7.4. Cardiovascular, Hematologic and Respiratory Effects
Overall, three studies reported that cannabinoids did not impact electrocardiogram (ECG) findings, while not specifically noting if cannabinoids affected ECG parameters such as rhythm, speed or axis that might indicate tachycardia, bradycardia or an arrhythmia (Table 6, Table e-6) [55, 56, 60]. Karst et al. [56], reported that CT-3 caused no significant changes in the measured respiratory rate (RR), heart rate (HR), or blood and hematologic chemistry (chloride, sodium, potassium, creatinine, total bilirubin, alkaline phosphatase (ALP),
γ-glutamyl transferase (γ-GT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and whole blood cell count). No significant change from baseline was found in blood pressure (BP) at 3 and 8 hours after administration of CT-3 [56].
Hagenbach et al. [55], reported that Dronabinol and rectal-THC had no effect on HR, blood tests (hemogram, C-reactive protein, AST, ALT, γ-GT, ALP, creatinine, uric acid, sodium, potassium) or pulmonary function tests. At the 6-week follow-up, Dronabinol was associated with a reduction in BP from baseline, while BP increased with the placebo; this difference was marginally significant [55]. Wilsey et al. [60], implemented single-day dosing titration strategies for vaporized 2.9%- and 6.7%-THC, and reported that neither potency had an effect on RR, but caused significant increases in HR (p<0.0001) at 1 hour and 4 hours compared to placebo. Vaporized cannabinoids decreased both systolic and diastolic BP compared to placebo, but was not statistically significant [60]. Their study population consisted of current
users (50%), ex-users (40%), and cannabinoid-naïve (10%), and found that ongoing cannabinoid use had no effect on HR, RR or BP [60]. One observational study described hypotension (15%) [49] as a notable side effect.
3.7.5. Other Side Effects
Karst et al. [56], reported that CT-3 led to no significant changes in weight or temperature. Other side effects reported across observational studies included dry mouth (55%), residual bad tastes (30%), amotivation (30%), dehydration (29%), risky behaviour (27%), paranoia (19%), related financial issues (19%), constipation (17%), and physical instability (11%) [44, 49, 75] (Table 6). Less common effects also included health-related problems (6%), work-related problems (4%), nausea (4%), weight gain (4%) and hallucinations (2%) [44, 49].
4. Discussion
The aims of this systematic review were to analyze cannabinoid usage patterns, reasons for use, and treatment efficacy and safety, in people with SCI. The reviewed evidence shows cannabinoid users tended to be single, male, and younger compared to non-users, and the preferred route of administration was smoking. The observational studies reported that cannabinoids were preferred over traditional analgesics due to an earlier onset and longer duration of action, greater therapeutic efficacy, and a relatively limited side effect profile. Interestingly, many studies involved adjunct use of cannabinoids with concurrent substances, including opioids. Preliminary results from both observational and experimental studies suggest that cannabinoids may effectively manage pain and spasticity in people with SCI. The most significant side effects were “fatigue”, “feeling high” or “feeling stoned ”, and “difficulty concentrating”. However, experimental studies did not show changes in objectively measured parameters; such as BP, RR, temperature, and blood biomarkers. Acute responses to cannabinoids, including euphoria, feelings of detachment and relaxation could be hypothesized as the basis for subjective decreases in pain [80]. This was addressed by Wilsey et al. [60], who controlled for psychoactive side-effects and determined that the main effect of THC remained significant. Instead, THC-mediated analgesia may be related to reduced subjective unpleasantness associated with amygdala activity [81] in acute pain, and decreased functional connectivity between sensorimotor and affective cortical regions in acute pain [81] and chronic NPP [82].
The effect of cannabinoids on BP is important to reveal because people with SCI at or above the sixth thoracic level commonly experience orthostatic hypotension (OH) and low resting BP [83]. The studies that investigated the effect of cannabinoids on BP in people with SCI included samples slightly biased towards lower levels of injury (tetraplegia, n=11, paraplegia, n=14), which may suggest more stable baseline BP, and may explain the relatively low incidence of hypotensive events.
SCI is also associated with metabolic dysfunctions, and as much as 50% of the SCI population have abnormal fatty infiltrates of the liver [84]. Preliminary studies have suggested that cannabinoids and synthetic analogues were possibly hepatotoxic; however, cannabinoid use has been associated with lower levels of non-alcoholic liver disease [85, 86]. Despite no significant changes in liver enzymes across these studies, the mechanisms of cannabinoids’ effect on the liver are not well understood and warrant further investigation, particularly in the SCI population.
Concrete conclusions regarding the efficacy of cannabinoids could not be made due to the poor quality of the studies. The observational studies included in this systematic review shared common pitfalls. Samples were often small or lacked justification for their sample size, and sometimes were not SCI-specific. Moreover, these studies were cross-sectional, did not measure different levels of cannabis exposure, did not account for potential confounders and often lacked basic study information, including injury demographics. For the experimental studies, major methodological issues include small and heterogeneous samples, varying cannabinoid formulations and doses, varying concurrent medication use among participants, diverse and missing outcome measures, inconsistent comparison treatments, and missing chronic follow-up. For these reasons, a meta-analysis of these quantitative data and additional Grading of Recommendations, Assessment, Development and Evaluations was deemed ill-suited. Given the low level of evidence from existing literature, the NIH assessment tool was apt to provide an indication of the quality of studies and biases for a range of study designs. Moreover, despite similar study outcomes, the tools used to measure these outcomes and timepoints of measurement were inconsistent between studies, making it difficult to compare mean differences. Cannabinoids showed a statistically significant reduction of 83% in pain and 100% in spasticity among the experimental studies. However, clinically meaningful changes in VAS pain scores were not reported by any studies, whereas, the one study that measured spasticity through MAS showed a clinically meaningful decrease [55]. Further, few studies reported effect sizes or sufficient data to calculate effect sizes, thus precluding a comprehensive meta-analysis (Table e-7). Of the effect sizes calculated from RCTs, the overall effects of cannabinoids on decreasing pain and spasticity were inconsistent [39]. While the magnitude of the effect sizes varied considerably, all experimental studies showed cannabinoids significantly decreased pain, except for one poor-quality study, where they were unable to show any statistically significant change in pain. This paradoxical relationship between statistical and clinical significance, as well as effect size magnitudes, further confounds physicians’ recommendations for the use of cannabinoids in persons with SCI.
Other work on this topic has come to similar, inconclusive interpretations of the therapeutic effects of cannabinoids in the SCI population. Empirical data is currently not robust enough, coupled with the difficulty of conducting rigorous randomized research in individuals with symptom complexes that are challenging to measure precisely [87]. A systematic review by Hagen et al. [88], which focused on both NPP and spasticity-related pain after SCI, included three studies which investigated the effects of cannabinoids. Hagen et al. [88], determined those studies to be too limited to allow certain conclusions regarding the efficacy of cannabinoids. Similarly, The National Academy of Sciences concluded there was insufficient evidence to support or refute the efficacy of cannabinoids as a treatment for spasticity in patients with paralysis due to SCI [87]. While sufficient conclusions cannot be made due to the low-quality evidence and small sample sizes that prevented a comprehensive meta-analysis, this systematic review presents an up-to-date search of the evidence, with a meaningful quantitative analysis given the limited data and a summary of the ongoing clinical trials. Furthermore, this systematic review, after synthesis of the available evidence, highlights the requirement for rigorous RCTs in the future.
The sample sizes of the experimental studies ranged from 5 to 42 participants, and among those, the number of SCI participants ranged from 3 to 29. These studies with heterogenous populations did not specify symptomatic relief in the SCI participants, therefore, there was a lack of SCI-specific conclusions. Furthermore, demographic data was often unreported, including the level of injury, time since injury, the ratio of males to females, and age. As a result, we were unable to distinguish the efficacy of cannabinoids for specific injury characteristics or participant demographics.
None of the observational studies examined the relationship between dosages and efficacy; only two of 22 studies reported mean dose. Among the experimental studies, there was high variability in the formulations and doses of cannabinoid interventions tested. This dose variability was present between and within studies. Most studies used self-titrated, variable dosing strategies due to the variability in individual responses to cannabinoids, balancing symptomatic efficacy and safety. For those studies that compared interventions of different potencies of THC [59, 60], there was no significant difference in analgesic effects between the two THC potencies (3.5% vs. 7%; 2.9% vs. 6.7%), but there were significant differences in the prevalence of side effects, suggesting that individuals may use lower therapeutic THC doses, while avoiding common side effects. In experimental studies that use purified forms of synthetic THC alone, it is possible that the results may not corroborate the subjective reports of observational studies. Recreational cannabis formulations, which are often smoked, contain hundreds of cannabinoids and in different ratios. It has been proposed that THC and CBD have synergistic effects that modulate treatment efficacy [89]. Therefore, further research is required to delineate specific cannabinoids and ratios of these to optimize therapeutic effects.
A significant number of trials involved the adjunct use of cannabinoids with other substances (n=6), allowing participants to continue their current anti-spastic or analgesic pharmacologic regimens. It is therefore, unclear, whether the therapeutic effects or side effects are due to cannabinoids themselves, or potential interactions with other medications. A study conducted on physically healthy individuals showed that concurrent use of cannabinoids with opioids can decrease the necessary opioid dose for comparable analgesic effects, without the increased potential of cannabinoid abuse [90]. Therefore, this suggests that the combination of cannabinoids and opioids may result in the safest and most effective analgesic effect. Moreover, cannabinoid use as an alternative or adjunctive treatment may have the potential as a harm reduction method due to the considerable side effects, toxicity, and addiction potential associated with opioids and other pain medications [46, 50]. However, clinicians may be reluctant to combine these medications until more information on their interactions is provided in humans with SCI. In future cannabinoid studies where concomitant medications are allowed, concomitant medications should be recorded for additional analysis. In the United States, there appears to be a correlation between states that have legalized cannabis and a decline in opioid-related overdose deaths, although the data is difficult to interpret without a thorough understanding of the interactions of these drugs [91].
A possible limitation of the systematic review itself was the narrow search terms for cannabinoids. While many synonyms for cannabis were included in the search, given the vast synthetic forms of cannabinoid receptor ligands, possible papers with interventions with molecular-based names may have been missed. Furthermore, part of the inclusion criteria required included studies to be peer-reviewed, excluding grey literature that could have been valuable since the medicinal properties of cannabis are still highly debated. This inclusion criteria also eliminated clinical trial results that had not yet been published in peer-reviewed journals. However, a review of the clinical trials on clinicaltrials.gov, International Standard Randomised Controlled Trial Number, and Australian New Zealand Clinical Trials Registries involving participants with SCI and cannabinoid interventions have been summarized (Table e-8). Furthermore, our systematic review protocol was not registered, which may have introduced unintentional bias [92].
Table.
e-8. Clinical trials conducted on adults with SCI with cannabinoids interventions searched February 29th, 2020.
| Clinical Trial Name |
Registry, Identifier
(Status) |
Phase | Conditions | Interventions | Inclusion Criteria | Exclusion Criteria | Primary Outcomes Measured | Secondary Outcomes Measured |
|---|---|---|---|---|---|---|---|---|
| Cannabinoids and an Anti-inflammatory Diet for the Treatment of Neuropathic Pain after Spinal Cord Injury | Clinicaltrials.gov, NCT04057456 (Not yet recruiting) |
2 | Spinal Cord Injuries Neuropathic Pain |
Placebo diet Anti-inflammatory diet THC/CBD Capsules High CBD Capsules Placebo capsules |
Informed consent; SCI >12mo duration; neuropathic pain >3/10 in severity on NRS with average >3/10 pain over the past 7d on screening; ongoing constant pain for >3mo or relapsing/remitting pain for >6mo; dosing of other pain medications stable for >1mo; cannabinoids stopped >7d prior to screening | History of psychotic disorder/convulsive disorder/substance abuse, current SI, intolerance to cannabinoids, traumatic SCI superimposed on prior congenital stenosis; pregnancy; unwilling to stop PRN pain medications; other medical conditions that confound the assessment of neuropathic pain | Average Pain intensity; Sensory Changes; Pain relief | Patient global impression of change; Work productivity and activity; Mood; Depression; Sleep; Spasticity; Pro-inflammatory Biomarkers (IL-2, IL-6, IL-1β, tumor necrosis factor alpha (TNF-α), interferon-gamma (IFN-γ) and prostaglandin E2 (PGE2)); Anti-inflammatory Biomarkers (IL-4, IL-10 and IL-1a) |
| Effect of Cannabinoids on Spasticity and Neuropathic Pain in Spinal Cord Injured Persons | Clinicaltrials.gov, NCT01222468 (Completed) |
2 | Muscle Spasticity as a Result of Spinal Cord Injury | Nabilone 0.5mg Placebo |
SCI; 12mo post-injury; C2-T12, ASIA A-D, stable level of injury; moderate to severe spasticity or moderate to severe neuropathic pain; no cognitive impairment; medications unchanged for >30d or inadequate pain control at a stabilized dose of gabapentin/pregabalin for >30d; no botulinum toxin injections <6mo | Significant CVS; major illness in another body area; history of psychological disorders or predisposition to psychosis; sensitivity to cannabinoids; severe liver disfunction; history of drug dependency; fixed tendon contractures; used cannabis <30d; unwilling to refrain from smoking cannabis during the study; pregnant or nursing mother | Ashworth Scale; VAS | Spasticity; Sleep; Subject's Global Impression of Change; Clinician's Global Impression of Change; Pain |
| A Study of Cannabis Based Medicine Extracts and Placebo in Patients with Pain Due to Spinal Cord Injury | Clinicaltrials.gov, NCT01606202 (Completed) |
3 | Pain | GW-1000-02 (THC 27mg/ml: CBD 25mg/ml) in 100uL Placebo |
Informed consent; >18yrs; diagnosis of non-acute SCI with central neuropathic pain not wholly relieved by current therapy; central neuropathic pain with mean severity NRS >4 during last 7d of baseline period; stable neurology >6mo; stable medication regimen >4wk; use of contraception during study; no use of cannabinoids >7d, willing to abstain from any use during the study; clinically acceptable laboratory results at visit 2; willingness to comply with all study requirements | History of significant psychiatric disorder other than depression associated with their underlying condition; history of alcohol/substance abuse; severe CVS disorder, (other than atrial fibrillation), poorly controlled htn or severe HF; history of AD, epilepsy; pregnant or nursing mother; significant renal/hepatic impairment; procedures requiring GA during the study; terminal illness; inappropriate for placebo medication; significant disease or disorder in the opinion of the investigator; regular levodopa therapy <7d; known or suspected hypersensitivity/adverse reaction to cannabinoids, intention to travel internationally during the study; intention to donate blood during the study; participation in another research <12wks to study entry; previous randomisation into this study; <18yrs | Mean Central Neuropathic Pain; | Spasticity; Concentration; Quality of Life; Patient Global Impression of Change; Pain; Caregiver Strain; Sleep; Incidence of Adverse Events; Use of Escape Medication |
Abbreviations: AD: autonomic dysreflexia; AS: Ashworth Scale; COPD: chronic obstructive pulmonary disease; CVS: cardiovascular disease; d: day; GA: general anesthetic; HF: heart failure; htn: hypertension; IL: interleukin; mo: month; NRS: numerical rating scale, PRN: as needed; SCI: spinal cord injury; SI: suicidal ideation; SZA: schizophrenia; TB: tuberculosis; TBI: traumatic brain injury; wk: week; yrs: year.
4.1. Future Directions and Considerations
Overall, the search yielded very few RCTs that evaluated the efficacy of cannabinoids for pain and spasticity in people with SCI. Several inconsistencies may be attributed to variability in doses and formulations, routes of administration and the outcome measures tested. Nevertheless, the results are promising and implicate the need for chronic use, longitudinal studies in the future. While this systematic review provides preliminary evidence that the short-term use of cannabinoids has beneficial effects in people with SCI, further research is warranted. Additional trials that adhere to Consolidated Standards of Reporting Trials should be followed, use double-blinding, have standardized outcome measures and dosage conditions, and have more homogenous SCI-specific populations with larger sample sizes are warranted. At this time, there is not enough good quality evidence to help clinicians decide when or how to use cannabinoids for their patients with SCI.
The studies included in this systematic review often did not report bladder, bowel, and sexual functioning or BP as outcome measures, an important limitation and an area of development for future studies. These are common secondary complications following SCI [93] as a result of either partial or total loss of supraspinal control [94]. Bladder irritation, bowel distention, sexual arousal/ejaculation and pain after SCI can trigger life-threatening episodes of hypertension (≥ 20 mmHg) known as autonomic dysreflexia (AD) [95] in people with SCI at or above the sixth thoracic level [96]. Cannabinoid use can lead to reduced visceral sensation and abdominal pain [97], but it is unclear if it can inhibit visceral stimuli that trigger AD during bowel management in people with SCI. If cannabinoid use has the potential to modulate afferent inputs, it could reduce the incidence and/or severity of AD experienced due to bladder/bowel distension and/or sexual intercourse. Cannabinoid receptors have been identified in the gut [97] and bladder, likely affecting micturition [98], but there is a paucity of data for their effects with SCI. The benefits of cannabinoids have been shown in other neurological conditions like MS, with reduced urinary incontinence [99], urgency, frequency and nocturia [100]. Increased complete spontaneous bowel movement and relief in constipation severity and evacuation strain, resulted from hemp seed pill use, compared to placebo [101]. Men and women also self- report improved sexual pleasure and satisfaction with cannabinoid use [102], but erectile dysfunction has been observed with men [103]. Furthermore, people with SCI consistently ranked recovery in sexual and bowel/bladder function and reducing cardiovascular complications more importantly than regaining the ability to walk [104]. Thus, the lack of data and the importance of these outcomes to quality of life substantiate the need to include bladder, bowel, sexual function and BP measures in future trials examining the potential of cannabinoids to treat these secondary health issues in people with SCI.
BP should also be routinely monitored among those with cervical and high thoracic SCI due to a decreased capacity of the arterial baroreflex to efficaciously trigger vasoconstriction and maintain BP [105]. OH, a decrease in systolic BP of at least 20 mmHg or diastolic BP of at least 10 mmHg upon postural changes from supine to upright [106], commonly occurs following high-level SCI [105]. OH may lead to dizziness, light-headedness, blurred vision, fatigue, nausea, dyspnea or cognitive deficits [107, 108], and can lead to incapacitation upon the use of cannabinoids [109]. There have been reports that adults with SCI experience lowered BP with cannabinoids [49], which could have profound implications through the exacerbation of existing hypotensive conditions.
Among all the experimental studies, only one (n=20) examined effects of CBD as the main component [58]. Wade et al. [58], demonstrated efficacy of THC- and CBD-containing products for pain and spasticity relief, but only CBD-specific products lacked effects of intoxication and decreased concentration. This corroborates findings of studies that have demonstrated the safety of CBD among humans [110]. Moreover, when used in conjunction with THC, CBD may inhibit THC metabolism and decrease THC-related side effects [111]. Therefore, more research needs to be conducted to examine the potential role of CBD as a safer therapeutic agent.
Moreover, all of these studies evaluated symptomatic relief provided by cannabinoids in comparison to placebo. However, greater clinical relevance would be obtained with comparisons to analgesics and anti-spastic medications commonly prescribed to people with SCI.
Another major recommendation for future studies is to investigate chronic cannabinoid use, an area of study that is absent in current research trials. It has been shown in able-bodied individuals that psychotic disorders and cognitive decline may be associated with heavy cannabinoid use [112], which is relevant in people with SCI who often experience significant deficits across cognitive domains such as reasoning, memory, attention, concentration and problem solving [113]. There is also evidence that able-bodied individuals can develop tolerance with long-term cannabinoid use, resulting in decreased side effects and therapeutic effects; therefore, longitudinal studies are warranted [114]. The effects of chronic cannabinoids use across delivery mechanisms on pulmonary function is another pertinent avenue of research in people with SCI, as higher levels of injury are associated with decreased forced vital capacity and forced expired volume [115]. In particular, chronic cannabinoid smoking is associated with dose-related impairments of large airway function resulting in airflow obstruction and hyperinflation [116, 117] and vaporized cannabis, as used in the study by Wilsey et al. [60], has been associated with respiratory failure [118].
Conclusion
The results of these studies suggest that people with SCI use cannabinoids both recreationally and for its therapeutic effects, primarily for pain and spasticity. The existing evidence also suggests that cannabinoids may help reduce pain and spasticity in people with SCI, at least in the short-term, but the clinical significance and magnitude of its effects appear unclear. Side effects were variable among participants, and were rated as mild to moderate. However, sufficient conclusions cannot be made due to the low quality of evidence and small sample sizes that prevented a meta-analysis. Future studies should be designed as SCI-specific, double-blind RCTs that incorporate larger sample sizes, long-term follow-up, and a wider range of outcomes important to SCI, including BP, bladder, bowel, and sexual function. Moreover, the implementation of standardized outcome measures and cannabinoid formulations, alongside comparisons with traditional therapy, should be utilized to further our understanding of the beneficial and detrimental effects of cannabinoids in people with SCI.
Acknowledgements
Dr. Krassioukov’s laboratory is supported by funds from the Canadian Institute for Health Research, Heart and Stroke Foundation, Canadian Foundation for Innovation and the Craig H. Neilsen Foundation. Emmanuel Tse was a recipient of the 2019 Florence E. Heighway Summer Research Award provided by the UBC Faculty of Medicine. Dr. Nightingale is supported by a 2018-2020 Michael Smith Foundation for Health Research Trainee Award, in conjunction with the International Collaboration on Repair Discoveries (ICORD). Dr. Walter was supported by a 2017-2019 Michael Smith Foundation for Health Research Trainee Award, in partnership with the Rick Hansen Foundation. We would like to acknowledge other members of the Spinal Cord Injury Research Evidence (SCIRE) Team for their support with literature searching and study appraisals. Special thanks to Adam Mesa of ICORD for his work on the graphical abstract.
list of Abbreviations
- AD
Autonomic Dysreflexia
- ALP
Alkaline Phosphatase
- ALT
Alanine Aminotransferase
- AS
Ashworth Scale
- AST
Aspartate Aminotransferase
- BP
Blood Pressure
- CBD
Cannabidiol
- CINAHL
Cumulative Index to Nursing and Allied Health Literature
- CT-3
1’,1’-dimethylheptyl-Δ8-tetrahydrocannabinol-11-oic acid
- ECG
Electrocardiogram
- GPT
Grooved Pegboard Test
- HR
Heart Rate
- MAS
Modified Ashworth Scale
- MCC
Maximum Cystometric Capacity
- MS
Multiple Sclerosis
- NIH
National Institute of Health
- NPP
Neuropathic Pain
- NRS
Numerical Rating Scale
- NRSI
Non-Randomized Studies of Interventions
- OH
Orthostatic Hypotension
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCTs
Randomized Control Trials
- RR
Respiratory Rate
- SCI
Spinal Cord Injury
- SOMC
Short Orientation-Memory-Concentration Test
- THC
Δ9-tetrahydrocannabinol
- TMT
Trail-Making Test
- VAS
Visual Analogue Scale
- WAIS-III
Weschler Adult Intelligence Scale Digit Symbol Test
- γ-GT
γ-glutamyl transferase
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
Kylie Nabata – Reports no disclosures. Emmanuel Tse – Reports no disclosures. Amanda Lee – Reports no disclosures. Janice Eng – Reports no disclosures. Matthew Querée – Reports no disclosures. Matthias Walter – Reports no disclosures. Tom Nightingale and Andrei Krassioukov have previously received funding and/or are waiting for decisions on pertinent pending grants from cannabis industry sponsors (Canopy Growth Corporation Ltd and Tetra Bio-Pharma Inc).
References
- 1.Boakye M., Leigh B.C., Skelly A.C. Quality of life in persons with spinal cord injury: comparisons with other populations. J. Neurosurg. Spine. 2012;17(1) Suppl.:29–37. doi: 10.3171/2012.6.AOSPINE1252. [DOI] [PubMed] [Google Scholar]
- 2.Guilcher S.J., Craven B.C., Lemieux-Charles L., Casciaro T., McColl M.A., Jaglal S.B. Secondary health conditions and spinal cord injury: an uphill battle in the journey of care. Disabil. Rehabil. 2013;35(11):894–906. doi: 10.3109/09638288.2012.721048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James S.L., Theadom A., Ellenbogen R.G., Bannick M.S., Montjoy-Venning W., Lucchesi L.R., Abbasi N., Abdulkader R., Abraha H.N., Adsuar J.C., Afarideh M., Agrawal S., Ahmadi A., Ahmed M.B., Aichour A.N., Aichour I., Aichour M.T.E., Akinyemi R.O., Akseer N., Alahdab F., Alebel A., Alghnam S.A., Ali B.A., Alsharif U., Altirkawi K., Andrei C.L., Anjomshoa M., Ansari H., Ansha M.G., Antonio C.A.T., Appiah S.C.Y., Ariani F., Asefa N.G., Asgedom S.W., Atique S., Awasthi A., Ayala Q.B.P., Ayuk T.B., Azzopardi P.S., Badali H., Badawi A., Balalla S., Banstola A., Barker-Collo S.L., Bärnighausen T.W., Bedi N., Behzadifar M., Bekele B.B., Belachew A.B., Belay Y.A., Bennett D.A., Bensenor I.M., Berhane A., Beuran M., Bhalla A., Bhaumik S., Bhutta Z.A., Biadgo B., Biffino M., Bijani A., Bililign N., Birungi C., Boufous S., Brazinova A., Brown A.W., Car M., Cárdenas R., Carrero J.J., Carvalho F., Castañeda-Orjuela C.A., Catalá-López F., Chaiah Y., Champs A.P., Chang J-C., Choi J-Y.J., Christopher D.J., Cooper C., Crowe C.S., Dandona L., Dandona R., Daryani A., Davitoiu D.V., Degefa M.G., Demoz G.T., Deribe K., Djalalinia S., Do H.P., Doku D.T., Drake T.M., Dubey M., Dubljanin E., El-Khatib Z., Ofori-Asenso R., Eskandarieh S., Esteghamati A., Esteghamati S., Faro A., Farzadfar F., Farzaei M.H., Fereshtehnejad S-M., Fernandes E., Feyissa G.T., Filip I., Fischer F., Fukumoto T., Ganji M., Gankpe F.G., Gebre A.K., Gebrehiwot T.T., Gezae K.E., Gopalkrishna G., Goulart A.C., Haagsma J.A., Haj-Mirzaian A., Haj-Mirzaian A., Hamadeh R.R., Hamidi S., Haro J.M., Hassankhani H., Hassen H.Y., Havmoeller R., Hawley C., Hay S.I., Hegazy M.I., Hendrie D., Henok A., Hibstu D.T., Hoffman H.J., Hole M.K., Homaie Rad E., Hosseini S.M., Hostiuc S., Hu G., Hussen M.A., Ilesanmi O.S., Irvani S.S.N., Jakovljevic M., Jayaraman S., Jha R.P., Jonas J.B., Jones K.M., Jorjoran S. Z.; Jozwiak, J.J.; Jürisson, M.; Kabir, A.; Kahsay, A.; Kahssay, M.; Kalani, R.; Karch, A.; Kasaeian, A.; Kassa, G.M.; Kassa, T.D.; Kassa, Z.Y.; Kengne, A.P.; Khader, Y.S.; Khafaie, M.A.; Khalid, N.; Khalil, I.; Khan, E.A.; Khan, M.S.; Khang, Y-H.; Khazaie, H.; Khoja, A.T.; Khubchandani, J.; Kiadaliri, A.A.; Kim, D.; Kim, Y-E.; Kisa, A.; Koyanagi, A.; Krohn, K.J.; Defo, K. B.; Kucuk Bicer, B.; Kumar, G. A.; Kumar, M.; Lalloo, R.; Lami, F. H.; Lansingh, V. C.; Laryea, D. O.; Latifi, A.; Leshargie, C. T.; Levi, M.; Li, S.; Liben, M. L.; Lotufo, P. A.; Lunevicius, R.; Mahotra, N. B.; Majdan, M.; Majeed, A.; Malekzadeh, R.; Manda, A.-L.; Mansournia, M. A.; Massenburg, B. B.; Mate, K. K. V.; Mehndiratta, M. M.; Mehta, V.; Meles, H.; Melese, A.; Memiah, P. T. N.; Mendoza, W.; Mengistu, G.; Meretoja, A.; Meretoja, T. J.; Mestrovic, T.; Miazgowski, T.; Miller, T. R.; Mini, G. K.; Mirica, A.; Mirrakhimov, E. M.; Moazen, B.; Mohammadi, M.; Molokhia, M.; Monasta, L.; Mondello, S.; Moosazadeh, M.; Moradi, G.; Moradi, M.; Moradi-Lakeh, M.; Moradinazar, M.; Morrison, S. D.; Moschos, M. M.; Mousavi, S. M.; Murthy, S.; Musa, K. I.; Mustafa, G.; Naghavi, M.; Naik, G.; Najafi, F.; Nangia, V.; Nascimento, B. R.; Negoi, I.; Nguyen, T. H.; Nichols, E.; Ningrum, D. N. A.; Nirayo, Y. L.; Nyasulu, P. S.; Ogbo, F. A.; Oh, I.-H.; Okoro, A.; Olagunju, A. T.; Olagunju, T. O.; Olivares, P. R.; Otstavnov, S. S.; Owolabi, M. O.; P A, M.; Pakhale, S.; Pandey, A. R.; Pesudovs, K.; Pinilla-Monsalve, G. D.; Polinder, S.; Poustchi, H.; Prakash, S.; Qorbani, M.; Radfar, A.; Rafay, A.; Rafiei, A.; Rahimi-Movaghar, A.; Rahimi-Movaghar, V.; Rahman, M.; Rahman, M. A.; Rai, R. K.; Rajati, F.; Ram, U.; Rawaf, D. L.; Rawaf, S.; Reiner, R. C.; Reis, C.; Renzaho, A. M. N.; Resnikoff, S.; Rezaei, S.; Rezaeian, S.; Roever, L.; Ronfani, L.; Roshandel, G.; Roy, N.; Ruhago, G. M.; Saddik, B.; Safari, H.; Safiri, S.; Sahraian, M. A.; Salamati, P.; Saldanha, R. d. F.; Samy, A. M.; Sanabria, J.; Santos, J. V.; Santric Milicevic, M. M. M.; Sartorius, B.; Satpathy, M.; Savuon, K.; Schneider, I. J. C.; Schwebel, D. C.; Sepanlou, S. G.; Shabaninejad, H.; Shaikh, M. A. A.; Shams-Beyranvand, M.; Sharif, M.; Sharif-Alhoseini, M.; Shariful, I. S. M.; She, J.; Sheikh, A.; Shen, J.; Sheth, K. N.; Shibuya, K.; Shiferaw, M. S.; Shigematsu, M.; Shiri, R.; Shiue, I.; Shoman, H.; Siabani, S.; Siddiqi, T. J.; Silva, J. P.; Silveira, D. G. A.; Sinha, D. N.; Smith, M.; Soares, F. A. M.; Sobhani, S.; Soofi, M.; Soriano, J. B.; Soyiri, I. N.; Stein, D. J.; Stokes, M. A.; Sufiyan, M. a. B.; Sunguya, B. F.; Sunshine, J. E.; Sykes, B. L.; Szoeke, C. E. I.; Tabarés-Seisdedos, R.; Te Ao, B. J.; Tehrani-Banihashemi, A.; Tekle, M. G.; Temsah, M.-H.; Temsah, O.; Topor-Madry, R.; Tortajada-Girbés, M.; Tran, B. X.; Tran, K. B.; Tudor Car, L.; Ukwaja, K. N.; Ullah, I.; Usman, M. S.; Uthman, O. A.; Valdez, P. R.; Vasankari, T. J.; Venketasubramanian, N.; Violante, F. S.; Wagnew, F. W. S.; Waheed, Y.; Wang, Y.-P.; Weldegwergs, K. G.; Werdecker, A.; Wijeratne, T.; Winkler, A. S.; Wyper, G. M. A.; Yano, Y.; Yaseri, M.; Yasin, Y. J.; Ye, P.; Yimer, E. M.; Yip, P.; Yisma, E.; Yonemoto, N.; Yoon, S.-J.; Yost, M. G.; Younis, M. Z.; Yousefifard, M.; Yu, C.; Zaidi, Z.; Zaman, S. B.; Zamani, M.; Zenebe, Z. M.; Zodpey, S.; Feigin, V. L.; Vos, T.; Murray, C. J. L. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown A., Weaver L.C. The dark side of neuroplasticity. Exp. Neurol. 2012;235(1):133–141. doi: 10.1016/j.expneurol.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryce T.N., Biering-Sørensen F., Finnerup N.B., Cardenas D.D., Defrin R., Lundeberg T., Norrbrink C., Richards J.S., Siddall P., Stripling T., Treede R.D., Waxman S.G., Widerström-Noga E., Yezierski R.P., Dijkers M. International spinal cord injury pain classification: part I. Background and description. March 6-7, 2009. Spinal Cord. 2012;50(6):413–417. doi: 10.1038/sc.2011.156. [DOI] [PubMed] [Google Scholar]
- 6.Sköld C., Levi R., Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch. Phys. Med. Rehabil. 1999;80(12):1548–1557. doi: 10.1016/S0003-9993(99)90329-5. [DOI] [PubMed] [Google Scholar]
- 7.Maynard F.M., Karunas R.S., Waring W.P., III Epidemiology of spasticity following traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 1990;71(8):566–569. [PubMed] [Google Scholar]
- 8.Siddall P.J., McClelland J.M., Rutkowski S.B., Cousins M.J. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103(3):249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 9.Holtz K.A., Szefer E., Noonan V.K., Kwon B.K., Mills P.B. Treatment patterns of in-patient spasticity medication use after traumatic spinal cord injury: a prospective cohort study. Spinal Cord. 2018;56(12):1176–1183. doi: 10.1038/s41393-018-0165-0. [DOI] [PubMed] [Google Scholar]
- 10.Becker W.J., Harris C.J., Long M.L., Ablett D.P., Klein G.M., DeForge D.A. Long-term intrathecal baclofen therapy in patients with intractable spasticity. Can. J. Neurol. Sci. 1995;22(3):208–217. doi: 10.1017/S031716710003986X. [DOI] [PubMed] [Google Scholar]
- 11.Fischer B., Ala-Leppilampi K., Single E., Robins A. Cannabis law reform in Canada: Is the” Saga of promise, hesitation and retreat” coming to an end? 1. Can. J. Criminol. Crim. Justice. 2003;45(3):265–298. doi: 10.3138/cjccj.45.3.265. [DOI] [Google Scholar]
- 12. https://medicalmarijuana.procon.org/
- 13.Leos-Toro C., Shiplo S., Hammond D. Perceived support for medical cannabis use among approved medical cannabis users in Canada. Drug Alcohol Rev. 2018;37(5):627–636. doi: 10.1111/dar.12823. [DOI] [PubMed] [Google Scholar]
- 14.Pertwee R.G. Endocannabinoids and their pharmacological actions. Handb. Exp. Pharmacol. 2015;231:1–37. doi: 10.1007/978-3-319-20825-1_1. [DOI] [PubMed] [Google Scholar]
- 15.Small E., Cronquist A. A practical and natural taxonomy for Cannabis. Taxon. 1976;•••:405–435. doi: 10.2307/1220524. [DOI] [Google Scholar]
- 16.Mackie K. Cannabinoid receptors: where they are and what they do. J. Neuroendocrinol. 2008;20(Suppl. 1):10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 17.Pryce G., Baker D. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br. J. Pharmacol. 2007;150(4):519–525. doi: 10.1038/sj.bjp.0707003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrot R.J., Hubbard J.R. Cannabinoids: Medical implications. Ann. Med. 2016;48(3):128–141. doi: 10.3109/07853890.2016.1145794. [DOI] [PubMed] [Google Scholar]
- 19.Russo E.B., Burnett A., Hall B., Parker K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005;30(8):1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- 20.Staton P.C., Hatcher J.P., Walker D.J., Morrison A.D., Shapland E.M., Hughes J.P., Chong E., Mander P.K., Green P.J., Billinton A., Fulleylove M., Lancaster H.C., Smith J.C., Bailey L.T., Wise A., Brown A.J., Richardson J.C., Chessell I.P. The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain. 2008;139(1):225–236. doi: 10.1016/j.pain.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Ahrens J., Demir R., Leuwer M., de la Roche J., Krampfl K., Foadi N., Karst M., Haeseler G. The nonpsychotropic cannabinoid cannabidiol modulates and directly activates alpha-1 and alpha-1-Beta glycine receptor function. Pharmacology. 2009;83(4):217–222. doi: 10.1159/000201556. [DOI] [PubMed] [Google Scholar]
- 22.Burstein S. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg. Med. Chem. 2015;23(7):1377–1385. doi: 10.1016/j.bmc.2015.01.059. [DOI] [PubMed] [Google Scholar]
- 23.De Petrocellis L., Ligresti A., Moriello A.S., Allarà M., Bisogno T., Petrosino S., Stott C.G., Di Marzo V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011;163(7):1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales P., Hurst D.P., Reggio P.H. Molecular targets of the phytocannabinoids: a complex picture. Prog. Chem. Org. Nat. Prod. 2017;103:103–131. doi: 10.1007/978-3-319-45541-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pernía-Andrade A.J., Kato A., Witschi R., Nyilas R., Katona I., Freund T.F., Watanabe M., Filitz J., Koppert W., Schüttler J., Ji G., Neugebauer V., Marsicano G., Lutz B., Vanegas H., Zeilhofer H.U. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science. 2009;325(5941):760–764. doi: 10.1126/science.1171870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross R.A. Anandamide and vanilloid TRPV1 receptors. Br. J. Pharmacol. 2003;140(5):790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill K.P., Palastro M.D., Johnson B., Ditre J.W. Cannabis and pain: A clinical review. Cannabis Cannabinoid Res. 2017;2(1):96–104. doi: 10.1089/can.2017.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andre C.M., Hausman J.F., Guerriero G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016;7:19. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell F.A., Tramèr M.R., Carroll D., Reynolds D.J.M., Moore R.A., McQuay H.J. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ. 2001;323(7303):13–16. doi: 10.1136/bmj.323.7303.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gates P.J., Albertella L., Copeland J. The effects of cannabinoid administration on sleep: a systematic review of human studies. Sleep Med. Rev. 2014;18(6):477–487. doi: 10.1016/j.smrv.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Lynch M.E., Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br. J. Clin. Pharmacol. 2011;72(5):735–744. doi: 10.1111/j.1365-2125.2011.03970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machado R.F.C., Stéfano S.C., De Cássia H.R., Rosa Oliveira L.M., Da Silveira D.X. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: systematic review and meta-analysis. Eur. J. Cancer Care (Engl.) 2008;17(5):431–443. doi: 10.1111/j.1365-2354.2008.00917.x. [DOI] [PubMed] [Google Scholar]
- 33.Martín-Sánchez E., Furukawa T.A., Taylor J., Martin J.L.R. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med. 2009;10(8):1353–1368. doi: 10.1111/j.1526-4637.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- 34.Tramèr M.R., Carroll D., Campbell F.A., Reynolds D.J.M., Moore R.A., McQuay H.J. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323(7303):16–21. doi: 10.1136/bmj.323.7303.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins J., Wells G. Cochrane handbook for systematic reviews of interventions. 2011.
- 36.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lung N. H., Institute B. 2018.
- 38.Sallis J.F., Prochaska J.J., Taylor W.C. A review of correlates of physical activity of children and adolescents. Med. Sci. Sports Exerc. 2000;32(5):963–975. doi: 10.1097/00005768-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J. Statistical power analysis for the behavioral sciences. Academic press; 2013. [Google Scholar]
- 40.Corey-Bloom J., Wolfson T., Gamst A., Jin S., Marcotte T.D., Bentley H., Gouaux B. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ. 2012;184(10):1143–1150. doi: 10.1503/cmaj.110837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipsey M.W., Wilson D.B. The efficacy of psychological, educational, and behavioral treatment. Confirmation from meta-analysis. Am. Psychol. 1993;48(12):1181–1209. doi: 10.1037/0003-066X.48.12.1181. [DOI] [PubMed] [Google Scholar]
- 42.Fekete C., Weyers S., Siegrist J., Michel G., Gemperli A. Poor nutrition and substance use in a Swiss cohort of adults with spinal cord injury. J. Public Health-Heidelberg. 2015;23(1):25–35. doi: 10.1007/s10389-015-0653-z. [DOI] [Google Scholar]
- 43.Young M.E., Rintala D.H., Rossi C.D., Hart K.A., Fuhrer M.J. Alcohol and marijuana use in a community-based sample of persons with spinal cord injury. Arch. Phys. Med. Rehabil. 1995;76(6):525–532. doi: 10.1016/S0003-9993(95)80506-0. [DOI] [PubMed] [Google Scholar]
- 44.Andresen S.R., Biering-Sørensen F., Hagen E.M., Nielsen J.F., Bach F.W., Finnerup N.B. Cannabis use in persons with traumatic spinal cord injury in Denmark. J. Rehabil. Med. 2017;49(2):152–160. doi: 10.2340/16501977-2105. [DOI] [PubMed] [Google Scholar]
- 45.Drossel C., Forchheimer M., Meade M.A. Characteristics of individuals with spinal cord injury who use cannabis for therapeutic purposes. Top. Spinal Cord Inj. Rehabil. 2016;22(1):3–12. doi: 10.1310/sci2201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aggarwal S.K., Carter G.T., Sullivan M.D., ZumBrunnen C., Morrill R., Mayer J.D. Characteristics of patients with chronic pain accessing treatment with medical cannabis in Washington State. J. Opioid. Manag. 2009;5(5):257–286. doi: 10.5055/jom.2009.0028. [DOI] [PubMed] [Google Scholar]
- 47.Clark J.M., Cao Y., Krause J.S. Risk of pain medication misuse after spinal cord injury: the role of substance use, personality, and depression. J. Pain. 2017;18(2):166–177. doi: 10.1016/j.jpain.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 48.Hwang M., Chlan K.M., Vogel L.C., Zebracki K. Substance use in young adults with pediatric-onset spinal cord injury. Spinal Cord. 2012;50(7):497–501. doi: 10.1038/sc.2012.8. [DOI] [PubMed] [Google Scholar]
- 49.Hawley L.A., Ketchum J.M., Morey C., Collins K., Charlifue S. Cannabis use in individuals with spinal cord injury or moderate to severe traumatic brain injury in Colorado. Arch. Phys. Med. Rehabil. 2018;99(8):1584–1590. doi: 10.1016/j.apmr.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Bruce D., Brady J.P., Foster E., Shattell M. Preferences for medical marijuana over prescription medications among persons living with chronic conditions: Alternative, complementary, and tapering uses. J. Altern. Complement. Med. 2018;24(2):146–153. doi: 10.1089/acm.2017.0184. [DOI] [PubMed] [Google Scholar]
- 51.Grotenhermen F., Schnelle M. Survey on the medical use of cannabis and THC in Germany. J. Cannabis Ther. 2003;3(2):17–40. doi: 10.1300/J175v03n02_03. [DOI] [Google Scholar]
- 52.Gorter R.W., Butorac M., Cobian E.P., van der Sluis W. Medical use of cannabis in the Netherlands. Neurology. 2005;64(5):917–919. doi: 10.1212/01.WNL.0000152845.09088.28. [DOI] [PubMed] [Google Scholar]
- 53.Cardenas D.D., Jensen M.P. Treatments for chronic pain in persons with spinal cord injury: A survey study. J. Spinal Cord Med. 2006;29(2):109–117. doi: 10.1080/10790268.2006.11753864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shroff F.M. Experiences with holistic health practices among adults with spinal cord injury. Rehabilitation Process and Outcome. 2015;4:27–34. doi: 10.4137/RPO.S12363. [DOI] [Google Scholar]
- 55.Hagenbach U., Luz S., Ghafoor N., Berger J.M., Grotenhermen F., Brenneisen R., Mäder M. The treatment of spasticity with Delta9-tetrahydrocannabinol in persons with spinal cord injury. Spinal Cord. 2007;45(8):551–562. doi: 10.1038/sj.sc.3101982. [DOI] [PubMed] [Google Scholar]
- 56.Karst M., Salim K., Burstein S., Conrad I., Hoy L., Schneider U. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. JAMA. 2003;290(13):1757–1762. doi: 10.1001/jama.290.13.1757. [DOI] [PubMed] [Google Scholar]
- 57.Rintala D.H., Fiess R.N., Tan G., Holmes S.A., Bruel B.M. Effect of dronabinol on central neuropathic pain after spinal cord injury: a pilot study. Am. J. Phys. Med. Rehabil. 2010;89(10):840–848. doi: 10.1097/PHM.0b013e3181f1c4ec. [DOI] [PubMed] [Google Scholar]
- 58.Wade D.T., Robson P., House H., Makela P., Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin. Rehabil. 2003;17(1):21–29. doi: 10.1191/0269215503cr581oa. [DOI] [PubMed] [Google Scholar]
- 59.Wilsey B., Marcotte T., Tsodikov A., Millman J., Bentley H., Gouaux B., Fishman S. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. Pain. 2008;89(10):840–848. doi: 10.1016/j.jpain.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilsey B., Marcotte T.D., Deutsch R., Zhao H., Prasad H., Phan A. An exploratory human laboratory experiment evaluating vaporized cannabis in the treatment of neuropathic pain from spinal cord injury and disease. Pain. 2016;17(9):982–1000. doi: 10.1016/j.jpain.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olsen M.F., Bjerre E., Hansen M.D., Tendal B., Hilden J., Hróbjartsson A. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: systematic review of empirical studies. J. Clin. Epidemiol. 2018;101:87–106.e2. doi: 10.1016/j.jclinepi.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Lee J.S., Hobden E., Stiell I.G., Wells G.A. Clinically important change in the visual analog scale after adequate pain control. Acad. Emerg. 2003;10(10):1128–1130. doi: 10.1111/j.1553-2712.2003.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 63.Dunn M., Davis R. The perceived effects of marijuana on spinal cord injured males. Paraplegia. 1974;12(3):175. doi: 10.1038/sc.1974.28. [DOI] [PubMed] [Google Scholar]
- 64.Malec J., Harvey R.F., Cayner J.J. Cannabis effect on spasticity in spinal cord injury. Arch. Phys. Med. Rehabil. 1982;63(3):116–118. [PubMed] [Google Scholar]
- 65.Heinemann A.W., Doll M.D., Armstrong K.J., Schnoll S., Yarkony G.M. Substance use and receipt of treatment by persons with long-term spinal cord injuries. Arch. Phys. Med. Rehabil. 1991;72(7):482–487. [PubMed] [Google Scholar]
- 66.Rothstein J.L., Levy E., Fecher R., Gordon S.K., Bauman W.A. Drug use and abuse in an urban veteran spinal cord injured population. J. Am. Paraplegia Soc. 1992;15(4):217–220. doi: 10.1080/01952307.1992.11761521. [DOI] [PubMed] [Google Scholar]
- 67.Kolakowsky-Hayner S.A., Gourley E.V., III, Kreutzer J.S., Marwitz J.H., Meade M.A., Cifu D.X. Post-injury substance abuse among persons with brain injury and persons with spinal cord injury. Brain Inj. 2002;16(7):583–592. doi: 10.1080/02699050110119475. [DOI] [PubMed] [Google Scholar]
- 68.Warms C.A., Turner J.A., Marshall H.M., Cardenas D.D. Treatments for chronic pain associated with spinal cord injuries: many are tried, few are helpful. Clin. J. Pain. 2002;18(3):154–163. doi: 10.1097/00002508-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Mahoney J.S., Engebretson J.C., Cook K.F., Hart K.A., Robinson-Whelen S., Sherwood A.M. Spasticity experience domains in persons with spinal cord injury. Arch. Phys. Med. Rehabil. 2007;88(3):287–294. doi: 10.1016/j.apmr.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 70.Heutink M., Post M.W., Wollaars M.M., van Asbeck F.W. Chronic spinal cord injury pain: pharmacological and non-pharmacological treatments and treatment effectiveness. Disabil. Rehabil. 2011;33(5):433–440. doi: 10.3109/09638288.2010.498557. [DOI] [PubMed] [Google Scholar]
- 71.Patel T., Milligan J., Lee J. Medication-related problems in individuals with spinal cord injury in a primary care-based clinic. J. Spinal Cord Med. 2017;40(1):54–61. doi: 10.1179/2045772315Y.0000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bourke J.A., Catherwood V.J., Nunnerley J.L., Martin R.A., Levack W.M.M., Thompson B.L., Acland R.H. Using cannabis for pain management after spinal cord injury: a qualitative study. Spinal Cord Ser. Cases. 2019;5:82. doi: 10.1038/s41394-019-0227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eldridge L.A., Piatt J.A., Agley J., Gerke S. Relationship between substance use and the onset of Spinal Cord Injuries: A medical chart review. Top. Spinal Cord Inj. Rehabil. 2019;25(4):316–321. doi: 10.1310/sci2504-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Graupensperger S., Corey J.J., Turrisi R.J., Evans M.B. Individuals with spinal cord injury have greater odds of substance use disorders than non-sci comparisons. Drug Alcohol Depend. 2019;205:107608. doi: 10.1016/j.drugalcdep.2019.107608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stillman M., Capron M., Mallow M., Ransom T., Gustafson K., Bell A., Graves D. Utilization of medicinal cannabis for pain by individuals with spinal cord injury. Spinal Cord Ser. Cases. 2019;5:66. doi: 10.1038/s41394-019-0208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kogel R.W., Johnson P.B., Chintam R., Robinson C.J., Nemchausky B.A. Treatment of spasticity in spinal cord injury with dronabinol, a tetrahydrocannabinol derivative. Am. J. Ther. 1995;2(10):799–805. doi: 10.1097/00045391-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 77.Pooyania S., Ethans K., Szturm T., Casey A., Perry D. A randomized, double-blinded, crossover pilot study assessing the effect of nabilone on spasticity in persons with spinal cord injury. Arch. Phys. Med. Rehabil. 2010;91(5):703–707. doi: 10.1016/j.apmr.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 78.Shaw L., Rodgers H., Price C., van Wijck F., Shackley P., Steen N., Barnes M., Ford G., Graham L. BoTULS investigators. 2010. [DOI] [PubMed]
- 79.Meseguer-Henarejos A.B., Sánchez-Meca J., López-Pina J.A., Carles-Hernández R. Inter- and intra-rater reliability of the modified ashworth scale: a systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2018;54(4):576–590. doi: 10.23736/S1973-9087.17.04796-7. [DOI] [PubMed] [Google Scholar]
- 80.Degenhardt L., Hall W., Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98(11):1493–1504. doi: 10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 81.Lee M.C., Ploner M., Wiech K., Bingel U., Wanigasekera V., Brooks J., Menon D.K., Tracey I. Amygdala activity contributes to the dissociative effect of cannabis on pain perception. Pain. 2013;154(1):124–134. doi: 10.1016/j.pain.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weizman L., Dayan L., Brill S., Nahman-Averbuch H., Hendler T., Jacob G., Sharon H. Cannabis analgesia in chronic neuropathic pain is associated with altered brain connectivity. Neurology. 2018;91(14):e1285–e1294. doi: 10.1212/WNL.0000000000006293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krassioukov A., Eng J.J., Warburton D.E., Teasell R. Spinal cord injury rehabilitation evidence research team. A systematic review of the management of orthostatic hypotension after spinal cord injury. Arch. Phys. Med. Rehabil. 2009;90(5):876–885. doi: 10.1016/j.apmr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barbonetti A., Caterina Vassallo M.R., Cotugno M., Felzani G., Francavilla S., Francavilla F. Low testosterone and non-alcoholic fatty liver disease: Evidence for their independent association in men with chronic spinal cord injury. J. Spinal Cord Med. 2016;39(4):443–449. doi: 10.1179/2045772314Y.0000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borini P., Guimarães R.C., Borini S.B. Possible hepatotoxicity of chronic marijuana usage. Sao Paulo Med. J. 2004;122(3):110–116. doi: 10.1590/S1516-31802004000300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adejumo A.C., Alliu S., Ajayi T.O., Adejumo K.L., Adegbala O.M., Onyeakusi N.E., Akinjero A.M., Durojaiye M., Bukong T.N. Cannabis use is associated with reduced prevalence of non-alcoholic fatty liver disease: A cross-sectional study. PLoS One. 2017;12(4):e0176416. doi: 10.1371/journal.pone.0176416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.National Academies of Sciences . E.; Medicine, The health effects of cannabis and cannabinoids: The current state of evidence and recommendations for research. National Academies Press; 2017. [PubMed] [Google Scholar]
- 88.Hagen E.M., Rekand T. Management of Neuropathic Pain Associated with Spinal Cord Injury. Pain Ther. 2015;4(1):51–65. doi: 10.1007/s40122-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Russo E.B. The case for the entourage effect and conventional breeding of clinical cannabis: No “Strain,” No Gain. Front. Plant Sci. 2019;9:1969. doi: 10.3389/fpls.2018.01969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cooper Z.D., Bedi G., Ramesh D., Balter R., Comer S.D., Haney M. Impact of co-administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology. 2018;43(10):2046–2055. doi: 10.1038/s41386-018-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bachhuber M.A., Saloner B., Cunningham C.O., Barry C.L. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999-2010. JAMA Intern. Med. 2014;174(10):1668–1673. doi: 10.1001/jamainternmed.2014.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stewart L., Moher D., Shekelle P. Why prospective registration of systematic reviews makes sense. Syst. Rev. 2012;1:7–7. doi: 10.1186/2046-4053-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schöps T.F., Schneider M.P., Steffen F., Ineichen B.V., Mehnert U., Kessler T.M. Neurogenic lower urinary tract dysfunction (NLUTD) in patients with spinal cord injury: long-term urodynamic findings. BJU Int. 2015;115(Suppl. 6):33–38. doi: 10.1111/bju.13085. [DOI] [PubMed] [Google Scholar]
- 94.Fowler C.J., Griffiths D., de Groat W.C. The neural control of micturition. Nat. Rev. Neurosci. 2008;9(6):453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wan D., Krassioukov A.V. Life-threatening outcomes associated with autonomic dysreflexia: a clinical review. J. Spinal Cord Med. 2014;37(1):2–10. doi: 10.1179/2045772313Y.0000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krassioukov A., Warburton D.E., Teasell R., Eng J.J. Spinal cord injury rehabilitation evidence research team. A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch. Phys. Med. Rehabil. 2009;90(4):682–695. doi: 10.1016/j.apmr.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salaga M., Abalo R., Fichna J. Cannabis and cannabinoids and the effects on gastrointestinal function: an overview. In: Preedy V.R., editor. Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment. Academic Print; 2017. pp. 471–480. [Google Scholar]
- 98.Hedlund P. Cannabinoids and the endocannabinoid system in lower urinary tract function and dysfunction. Neurourol. Urodyn. 2014;33(1):46–53. doi: 10.1002/nau.22442. [DOI] [PubMed] [Google Scholar]
- 99.Freeman R.M., Adekanmi O., Waterfield M.R., Waterfield A.E., Wright D., Zajicek J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicentre, randomised placebo-controlled trial (CAMS-LUTS). Int. Urogynecol. J. Pelvic Floor Dysfunct. 2006;17(6):636–641. doi: 10.1007/s00192-006-0086-x. [DOI] [PubMed] [Google Scholar]
- 100.Brady C.M., DasGupta R., Dalton C., Wiseman O.J., Berkley K.J., Fowler C.J. An open-label pilot study of cannabis-based extracts for bladder dysfunction in advanced multiple sclerosis. Mult. Scler. 2004;10(4):425–433. doi: 10.1191/1352458504ms1063oa. [DOI] [PubMed] [Google Scholar]
- 101.Cheng C.W., Bian Z.X., Zhu L.X., Wu J.C., Sung J.J. Efficacy of a Chinese herbal proprietary medicine (Hemp Seed Pill) for functional constipation. Am. J. Gastroenterol. 2011;106(1):120–129. doi: 10.1038/ajg.2010.305. [DOI] [PubMed] [Google Scholar]
- 102.Halikas J., Weller R., Morse C. Effects of regular marijuana use on sexual performance. J. Psychoactive Drugs. 1982;14(1-2):59–70. doi: 10.1080/02791072.1982.10471911. [DOI] [PubMed] [Google Scholar]
- 103.Cohen S. Cannabis and sex: multifaceted paradoxes. J. Psychoactive Drugs. 1982;14(1-2):55–58. doi: 10.1080/02791072.1982.10471910. [DOI] [PubMed] [Google Scholar]
- 104.Anderson K.D. Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 105.Claydon V.E., Steeves J.D., Krassioukov A. Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord. 2006;44(6):341–351. doi: 10.1038/sj.sc.3101855. [DOI] [PubMed] [Google Scholar]
- 106.The Consensus Committee of the American Autonomic Society and the American Academy of Neurology Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46(5):1470. doi: 10.1212/WNL.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 107.Freeman R., Abuzinadah A.R., Gibbons C., Jones P., Miglis M.G., Sinn D.I. Orthostatic hypotension: JACC State-of-the-Art review. J. Am. Coll. Cardiol. 2018;72(11):1294–1309. doi: 10.1016/j.jacc.2018.05.079. [DOI] [PubMed] [Google Scholar]
- 108.Mathias C.J. Orthostatic hypotension and paroxysmal hypertension in humans with high spinal cord injury. Prog. Brain Res. 2006;152:231–243. doi: 10.1016/S0079-6123(05)52015-6. [DOI] [PubMed] [Google Scholar]
- 109.Jones R.T. Cardiovascular system effects of marijuana. J. Clin. Pharmacol. 2002;42(S1):58S–63S. doi: 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- 110.Bergamaschi M.M., Queiroz R.H., Zuardi A.W., Crippa J.A. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr. Drug Saf. 2011;6(4):237–249. doi: 10.2174/157488611798280924. [DOI] [PubMed] [Google Scholar]
- 111.Huestis M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007;4(8):1770–1804. doi: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bolla K.I., Brown K., Eldreth D., Tate K., Cadet J.L. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–1343. doi: 10.1212/01.WNL.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 113.Sachdeva R., Gao F., Chan C.C.H., Krassioukov A.V. Cognitive function after spinal cord injury: A systematic review. Neurology. 2018;91(13):611–621. doi: 10.1212/WNL.0000000000006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jones R.T., Benowitz N., Bachman J. Clinical studies of cannabis tolerance and dependence. Ann. N. Y. Acad. Sci. 1976;282(1):221–239. doi: 10.1111/j.1749-6632.1976.tb49901.x. [DOI] [PubMed] [Google Scholar]
- 115.Linn W.S., Adkins R.H., Gong H., Jr, Waters R.L. Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Arch. Phys. Med. Rehabil. 2000;81(6):757–763. doi: 10.1016/S0003-9993(00)90107-2. [DOI] [PubMed] [Google Scholar]
- 116.Jett J., Stone E., Warren G., Cummings K.M. Cannabis use, lung cancer, and related issues. J. Thorac. Oncol. 2018;13(4):480–487. doi: 10.1016/j.jtho.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 117.Aldington S., Williams M., Nowitz M., Weatherall M., Pritchard A., McNaughton A., Robinson G., Beasley R. Effects of cannabis on pulmonary structure, function and symptoms. Thorax. 2007;62(12):1058–1063. doi: 10.1136/thx.2006.077081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dicpinigaitis P.V., Trachuk P., Fakier F., Teka M., Suhrland M.J. Vaping-Associated acute respiratory failure due to acute lipoid pneumonia. Lung. 2019;198(1):31–33. doi: 10.1007/s00408-019-00277-6. [DOI] [PubMed] [Google Scholar]