Abstract
Restless legs syndrome (RLS)/Willis-Ekbom disease is a neurologic disorder characterized by a strong desire to move when at rest (usually in the evening) and paraesthesia in their lower legs. The most widely used therapies for first-line treatment of RLS are dopaminergic drugs; however, their long-term use can lead to augmentation. α2δ Ligands, opioids, iron, glutamatergic drugs, adenosine, and sleep aids have been investigated as alternatives. The pathogenesis of RLS is not well understood. Despite the efficacy of dopaminergic drugs in the treatment of this disorder, unlike in Parkinson’s disease dopaminergic cell loss in the substantia nigra has not been observed in RLS. The etiology of RLS is likely complex, involving multiple neural pathways. RLS-related genes identified in genome-wide association studies can provide insight into the mechanistic basis and pathophysiology of RLS. Here we review the current treatments and knowledge of the mechanisms underlying RLS.
Keywords: Restless legs syndrome, dopaminergic drugs, nondopaminergic drugs, mechanisms, genetic factors, augmentation
1. INTRODUCTION
Restless legs syndrome (RLS)/Willis-Ekbom disease (WED) is a neurologic disorder characterized by the desire to move when at rest (usually in the evening) and paraesthesia in the lower legs [1]. RLS is underdiagnosed, and it is only recently that it has become widely recognized and studied [2-4]. In 1982, levodopa became the first drug used to treat RLS [5]; while effective, it has augmentation as a side effect at all doses [5-7]. Dopamine agonists such as pramipexole, ropinirole, and rotigotine were approved for RLS treatment between 2004 and 2009 [8, 9], and are now used in the first-line setting [10]. However, augmentation still occurs to varying degrees with dopaminergic drugs. To address this problem, neurologists have sought to optimize drug usage or seek alternatives such as α2δ ligands [11] and opioids [12]. The former—which include gabapentin enacarbil, gabapentin, and pregabalin—have been used for the treatment of RLS since 2000, although they are less frequently prescribed than dopaminergic drugs. Pregabalin is a relatively new drug and has been the focus of just a few clinical studies since 2010 [13-15]. Nonetheless, owing to their efficacy and the fact that they do not cause augmentation, α2δ ligands hold promise for the treatment of RLS. Opioids were approved as second-line therapy to treat refractory RLS in 2013 [16].
Most research on the pathologic mechanisms of RLS has focused on the dopaminergic system, iron deficiency, or genomic imprinting [17-20]. Despite their demonstrated efficacy, the mechanism of action of dopaminergic drugs in the treatment of RLS is not well understood. One positron emission tomography (PET) study reported a decreased number of dopamine transporters in RLS [19] and another PET study showed that mesolimbic D2/3 receptor binding may be reduced, which is supported by the observation that the D2/3 receptor agonist pramipexole is effective as a treatment [21]. However, dopaminergic neuron loss in the substantia nigra (SN) or related A11 hypothalamic region, which is a feature of neurodegenerative diseases such as Parkinson’s disease (PD) that are managed with dopaminergic drugs, has not been observed in autopsy studies of patients with RLS [22, 23]. Moreover, unlike in PD [24], iron levels in the SN were found to be reduced in RLS [23, 25-27]. Iron deficiency activates the hypoxia pathway in the central nervous system (CNS) [28]. Iron is a cofactor for tyrosine hydroxylase (TH) and is important for D2 receptor function [29]. Thus, alterations in the dopaminergic system caused by iron deficiency may contribute to RLS. A meta-analysis of 3 genome-wide association studies (GWASs) that included a total of 15,126 patients with RLS identified 19 risk gene loci for RLS [30]. Rare variants of the Meis homeobox 1 (MEIS1) gene have been identified in RLS; MEIS1 was shown to influence the dopaminergic system at both the spinal and supraspinal levels in mice, suggesting a possible mechanism underlying RLS pathology [31]. Recently, the role of BTB domain-containing 9 (BTBD9) gene in the pathogenesis of RLS has been increasingly noticed. It is known that mutations in BTBD9 are closely associated with high risk of RLS [32], while knockout of BTBD9 in mice may cause RLS-like phenotypes, and dysfunction of the dopaminergic system, which contribute to develop RLS [33]. However, the mechanisms of BTBD9 is not fully understood. New therapeutic options are also emerging for RLS including glutamatergic or adenosine therapy [34]. This review discusses the drugs that are currently used for RLS treatment and what is known of their mechanisms of action.
2. Dopaminergic drugs
2.1. Dopaminergic Drugs
Levodopa has demonstrated efficacy in the treatment of RLS [5-7]. However, augmentation often occurs with prolonged daily use, especially at a dose of 200 mg or higher [6, 7, 35]. Because of the short half-life of levodopa, symptom rebound often occurs in the early morning in patients [36]. The intermittent use of low-dose levodopa has been proposed for the treatment of intermittent RLS. Dopamine agonists such as pramipexole, ropinirole, and rotigotine were approved by the U.S. Food and Drug Administration and European Medicines Agency between 2004 and 2009 as first-line treatments for RLS [8, 9]. Patients with moderate to severe RLS can be treated with pramipexole, which is well tolerated at daily doses of 0.25–0.75 mg [8]; the most common adverse events (AEs) are augmentation, dizziness, somnolence, and gastrointestinal symptoms, with long-term use and higher doses known to cause augmentation [13]. Ropinirole is also well tolerated, with effective daily doses of 0.78–4 mg [8]; the most common AEs are augmentation, nausea, dizziness, headache, and daytime somnolence. In long-standing RLS (mean duration of symptoms=26.1 years), the incidence of augmentation was ≤4% after 26 weeks on ropinirole [37]. Rotigotine is well tolerated, and transdermal administration at doses of 2–3 mg was shown to be effective for managing RLS symptoms. Skin reactions, augmentation, and nausea are the most common AEs, although these are mostly of low grade [38].
PD is associated with a loss of dopaminergic neurons; exogenous dopaminergic drugs such as levodopa and dopamine agonists alleviate PD symptoms by increasing brain dopamine levels [39]. Given the efficacy of these drugs for RLS treatment, it has been suggested that loss of dopaminergic neurons in the SN or related A11 hypothalamic region may also be a feature of RLS. However, there has been no such evidence from autopsy studies [22, 23]. This implies that dopaminergic drugs alleviate RLS not through simple dopamine supplementation but by altering neural circuits, although the precise mechanism remains unclear. RLS/WED comprises both motor and sensory symptoms; the relevant neural circuits along with sensorimotor integration are controlled by descending monoaminergic neuron clusters with spinal cord projections in the dorsal raphe (5-hydroxytryptamine), locus coeruleus (norepinephrine), and the A11 area of the posterior hypothalamus (dopamine) [40]. Injury to the A11 area leads to hyperactivity in mice, which serves as an animal model of RLS [41]. Hypersensitivity of corticostriatal neuron terminals was demonstrated in a rodent RLS model, which was counteracted by local perfusion of pramipexole or ropinirole [42]. Long-term levodopa administration reduced hyperalgesia in patients with RLS, suggesting that the underlying pathophysiology is related to the regulation of supraspinous pain involving the basal ganglia and/or dopaminergic pathway [43]. The characteristics of dopaminergic drugs used to treat RLS are summarized in Table 1.
Table 1.
Dopaminergic drugs for the treatment of RLS.
| - | Longest Study Period (N) | Effective Daily Dose | Adverse Effects | Clinical Benefits | Mechanism of Action |
|---|---|---|---|---|---|
| Levodopa | 30 weeks [7] (N=361) |
<200 mg [6, 7] |
Augmentation, symptom rebound in the early morning [36] | Improves nighttime RLS symptoms; reduces pain; well tolerated by controlling augmentation [7] | Unknown; may alter motor neuron excitability by reducing inhibition [43] |
| Dopamine agonists | Unknown; may counteract corticostriatal hypersensitivity or alter D2/3 receptor activation [42] | ||||
| Pramipexole | 52 weeks (N=719) [13] |
0.25, 0.50, 0.75 mg [8] | Augmentation, dizziness, somnolence, headache, nausea, abdominal discomfort [13] | Improves nighttime RLS symptoms and subjective nighttime sleep; pain reduction; well tolerated by controlling augmentation [13] | - |
| Ropinirole | 26 weeks (N=404) [37] |
0.78-4 mg [8] | Augmentation, nausea, dizziness, headache, daytime somnolence [37] | Improves nighttime RLS symptoms and subjective nighttime sleep; reduces pain; well tolerated by controlling augmentation [37] | - |
| Rotigotine | 24 weeks (N=505) [38] |
2-3 mg [8] | Skin reactions, nausea, augmentation [38] | Improve nighttime RLS symptoms and subjective nighttime sleep; reduces pain; well tolerated by controlling augmentation and skin reactions [38] | - |
3. NONDopaminergic drugs
3.1. α2δ Ligands
α2δ Ligands drugs have been used for the treatment of RLS since the 2000s. Representative drugs are gabapentin enacarbil, gabapentin, and pregabalin. Gabapentin enacarbil is effective at dose of 1200 mg and well tolerated, and is a pro-drug of gabapentin, which has received regulatory approval in the U.S. and Japan [44]. On the other hand, only one study has shown that a dose of 600 mg was effective [45], while 2 studies found it to be ineffective in relieving RLS symptoms [46, 47]. Somnolence and dizziness are the most common side effects of gabapentin enacarbil [44]. Gabapentin at a dose of 800 mg was found to alleviate RLS, and a dose of 200 mg has been used in patients undergoing hemodialysis. The most common AEs associated with gabapentin are dizziness, somnolence, and peripheral edema [6, 48-50]. Pregabalin is a relatively new drug for the treatment of RLS that has been investigated in 3 clinical studies. At doses of 150–450 mg/day, pregabalin produced good outcomes in moderate to severe idiopathic RLS, with lower rates of augmentation compared to pramipexole [13]. The most common AEs are dizziness, somnolence, fatigue, and headache [13-15].
The mechanism of action of α2δ ligands is thought to be related to the inhibition of glutamatergic neurotransmission. α2δ ligands bind with high affinity to l-type voltage-regulated calcium channels that regulate Ca2+ influx at nerve endings in response to depolarization, leading to a reduction in excitatory neurotransmitter (mainly glutamate) release [51, 52].
3.2. Opioid Drugs
In Europe, oxycodone–naloxone is approved as second-line therapy for severe RLS. A 12-week double-blind and 40-week open-label trial of 306 patients with severe RLS showed that 5.0 mg oxycodone and 2.5 mg naloxone twice daily—which was up titrated to a maximum of 40 and 20 mg twice daily, respectively—was effective in patients with severe RLS who did not respond to dopaminergic drugs [16]. In a small open-label study evaluating the use of methadone in 27 RLS patients who failed on dopaminergic drugs, 17 patients remained on methadone for 23±12 months at a dose of 15.5±7.7 mg (range, 5–40 mg), with a 75% reduction in symptoms and no occurrence of augmentation [53]. In other studies, oxycodone (mean dose of 15.9 mg/day) improved RLS symptoms [54] and demonstrated long-term efficacy in the treatment of RLS [55]. In a postmortem study, there was no difference in the number of opioid receptors in the SN between patients with RLS and controls [56]. However, opioid and dopaminergic systems are known to interact: functional imaging of dopamine receptor occupancy in humans showed that stimulation of opioid receptors especially of the mu type promotes dopamine release [57].
3.3. Iron Therapy
Intravenous ferric carboxymaltose (1000 mg) is a first-line treatment in adult patients with RLS with iron deficiency or serum ferritin <100 µg/l [58, 59]. Brain iron levels may be low even if the serum iron is normal [60]; therefore, iron supplementation can be beneficial for alleviating RLS symptoms, although there is insufficient evidence for the efficacy of oral or intravenous iron in RLS treatment [61].
Iron levels in the SN were found to be reduced in autopsy studies of RLS [22], which was substantiated by imaging data and cerebrospinal fluid examination [25-27, 60]. Iron is a cofactor for TH and is important for D2 receptor function [29]; thus, iron deficiency can lead to changes in the dopaminergic system and RLS. Iron-deficient rats exhibited alterations in dopaminergic function including a decrease in D2 receptors and dopamine transporter function and elevated levels of extracellular dopamine [62]. Iron deficiency also activates the CNS hypoxia pathway. Autopsy of the SN of RLS patients revealed nitric oxide-mediated hypoxia-inducible factor 1α (HIF-1α) activation in the cerebral microvasculature along with increased vascular endothelial growth factor (VEGF) expression. HIF-1α regulates the transcription of various genes involved in iron metabolism [28]. Interestingly, in a mouse model of PD induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine showing excessive levels of iron, HIF-1α, TH, and VEGF were upregulated in the SN and striatum in the presence of an iron chelator [24]. Consistent with these findings, reducing iron content activated the CNS hypoxia pathway. In animal models, iron deficiency in the brain resulted in downregulation of adenosine A1 receptors in the striatum and cortex [41].
3.4. Glutamatergic and Adenosine Therapy
Perampanel has significant therapeutic effects on both sensory and motor symptoms in RLS patients [63]. One study evaluated the efficacy of perampanel at doses of 3.8 mg/day in 20 RLS patients over a period of 8 weeks, and a small open-label controlled clinical trial that enrolled 13 untreated idiopathic RLS patients showed that treatment with dipyridamole (starting at 100 mg, with uptitration to 400 mg if necessary) had significant clinical benefits in RLS [64]. Perampanel acts as a noncompetitive selective antagonist of postsynaptic ionic α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) glutamate receptor (AMPA-R), reducing excessive glutamate stimulation [63]. The nonselective equilibrative nucleoside transporter 1 (ENT1)/ENT2 inhibitor dipyridamole may exert pharmacologic effects through hypoadenosinergic mechanisms. Ultimately, hypoadenosinergic may lead to enhanced cortical dopaminergic and glutamatergic functions of RLS/WED [64].
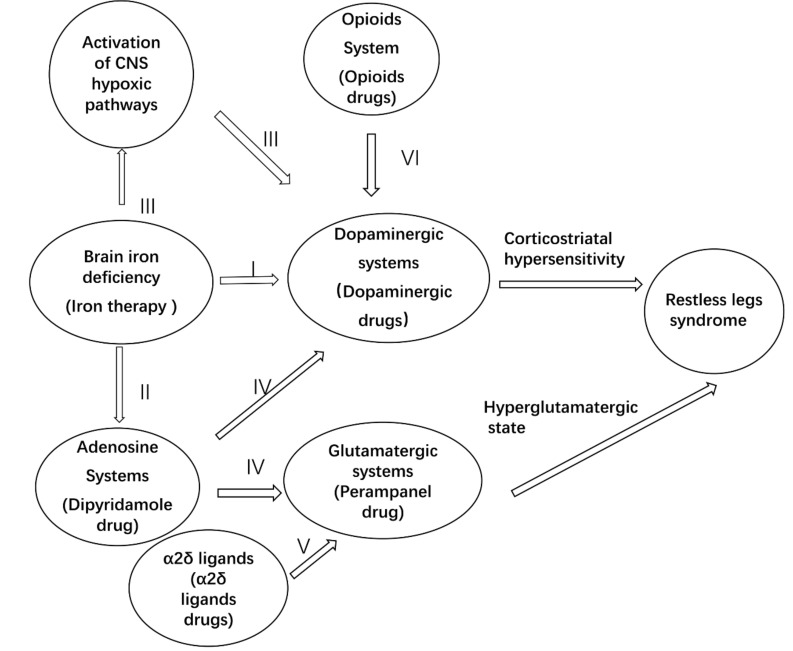
The interactions between dopaminergic, adenosine, opioid, and glutamatergic systems and iron in RLS are illustrated in Fig. 1.
Fig. (1).
Potential mechanisms of restless legs syndromes (RLS). Although the precise mechanism remains unclear, the etiology of RLS is likely complex, involving multiple neural pathways. I. In iron-deficient rats of RLS, exhibited alterations in dopaminergic function including a decrease in D2 receptors and dopamine transporter function and elevated levels of extracellular dopamine. II. Iron-deficient resulted in downregulation of adenosine A1 receptors in the striatum and cortex. III. Iron-deficient activates the CNS hypoxia pathway, then upregulation of tyrosine hydroxylase (TH) in the CNS. IV. Hypoadenosinergic may enhance cortical dopaminergic and glutamatergic functions, thus lead to RLS. V. The mechanism of action of α2δ ligands is thought to be related to the inhibition of excitatory neurotransmitter (mainly glutamate) release. VI. Stimulation of opioid receptors especially of the mu type promotes dopamine release. Finally, corticostriatal hypersensitivity and hyperglutamatergic state may result in RLS.
3.5. OTHER Therapies
Vitamins C (200 mg) and E (400 mg) were used to treat uremic patients with RLS a randomized, controlled study RLS [65]. In another report of 21 RLS patients with vitamin D deficiency, vitamin D supplementation improved RLS symptoms [66]; and in an open-label randomized study comparing the efficacy of clonazepam (0.5 mg) and nortriptyline (25 mg) in women with RLS, both drugs resulted in clinical improvement, with the latter showing greater benefits [67]. Benzodiazepines, as a group of gamma-aminobutyric acid (GABA)-ergic sedative-hypnotic agents, are also used for treating RLS to improve the sleep quality, alleviate periodic leg movements in sleep [68] and reduce RLS-related anxiety [69]. Kurlan and Rabin found that benzodiazepines are benefit for augmentation during the RLS treatment [70]. However, benzodiazepines were not recommended as the first-line treatment for RLS as per the American Academy of Sleep Medicine Practice Guideline [71]. Carlos et al. conducted a systematic review concerning the efficacy and safety of benzodiazepines for RLS, they could not obtain compelling evidence regarding the efficacy and safety of benzodiazepines for RLS, however, they believed that treatment of benzodiazepines is a useful adjuvant therapy for treating RLS [72]. Nondopaminergic drugs used in the treatment of RLS are summarized in Table 2.
Table 2.
Nondopaminergic drugs for the treatment of RLS.
| - | Longest Study Period (N) |
Effective
Daily Dose |
Adverse Effects | Clinical Benefits | Mechanism of Action |
|---|---|---|---|---|---|
| α2δ Ligands | Well tolerated | Regulates Ca2+ influx and reduces excitatory neurotransmitter levels [51, 52] | |||
| Gabapentin enacarbil | 24 weeks (N=327) [44] |
600 or 1200 mg [8] | Somnolence, dizziness [44-47] |
Improves nighttime RLS symptoms and subjective nighttime sleep; reduces pain [44-47] | - |
| Gabapentin | 6 weeks (N=24) [50] |
800 mg; uremic RLS: 200 mg [8] | Somnolence, dizziness, peripheral edema [6, 48-50] | Improves nighttime RLS symptoms and subjective nighttime sleep; reduces pain [6, 48-50] | - |
| Pregabalin | 52 weeks (N=719) [13] |
150–450 mg [8] | Somnolence, dizziness, fatigue, headache [13-15] | Improves nighttime RLS symptoms and subjective nighttime sleep; reduces pain [13-15] | - |
| Opioids | Well tolerated but side effects should be monitored | Stimulates dopamine release [57] | |||
| Oxycodone–naloxone | 12 weeks plus 1 year open trial (N=306) [16] |
Mean dose, 21.9 mg [8] | Addictive tendency, possible respiratory problems [16] | Improves nighttime RLS symptoms and subjective nighttime sleep; reduces pain [16] | - |
| Methadone | 92 weeks N=27 [53] |
Mean dose, 15.6 mg [8] | Addictive tendency, possible respiratory problems [53] | Improves nighttime RLS symptoms and subjective nighttime sleep; reduces pain [53] | - |
| Oxycodone | 2 weeks (N=11)[54] |
Likely efficacious Mean dose:15.9 mg [8] |
Addictive tendency, possible respiratory problems [54] | Improves nighttime RLS symptoms and subjective nighttime sleep; reduces pain [54] | - |
| Iron supplementation | |||||
| Intravenous ferric carboxymaltose |
12 weeks (N=110) [58] |
Likely efficacious [8] | Nausea, headache; requires more time to stabilize RLS; lack of pain and subjective nighttime sleep assessments [58] | Improves nighttime RLS symptoms; well tolerated [58] | Regulates dopaminergic system; controls central nervous system hypoxia pathways; regulates adenosine A1 receptors [28, 29, 41, 62] |
| Glutamatergic therapy | |||||
| Perampanel | 8 weeks (N=20) [63] |
Likely efficacious Mean dose: 3.8 mg [63] |
Somnolence, dizziness, headache, irritability; lack of pain assessment [63] | Improves nighttime RLS symptoms and subjective nighttime sleep; well tolerated [63] | Inhibits glutamate release [63] |
| Adenosine therapy | Regulates hypoadenosinergic state [64] | ||||
| Dipyridamole | 8 weeks (N=13) [64] |
Likely efficacious Mean dose: 281.8 mg [64] |
Abdominal cramps, diarrhea, dizziness, flushing; lack of pain assessment [64] | Improves nighttime RLS symptoms and subjective nighttime sleep; well tolerated [64] | - |
4. GENETIC FACTORS
A meta-analysis of 3 GWAS studies involving 15,126 patients with RLS identified 19 risk genes for RLS [30]. These genes are related to dopaminergic neurotransmission, brain iron metabolism, or neurodevelopmental processes [30, 73]. The functions of the candidate genes MEIS1, BTB domain-containing 9 (BTBD9), and protein tyrosine phosphatase receptor type D and their involvement in dopaminergic neurotransmission and iron metabolism have been investigated in animal models [74-77]. MEIS1 gene mutation has been observed in RLS patients and may be associated with impaired neurodevelopment [31]. In RLS model mice, MEIS1 was shown to act on the dopaminergic system at both the spinal and supraspinal levels [31]. D2 receptor activity was reduced in BTBD9 mutant mice, which may contribute to RLS [33]; BTBD9 deficiency results in changes in the cerebral cortex—specifically, in cortical projections to D1 medium spinous neurons (MSNs) [32], and increased striatum MSN activity may contribute to RLS onset through modulation of striatum cholinergic interneurons [78].
5. augmentation
The phenomenon of augmentation was first described in 1996 following the treatment of RLS with carbidopa/levodopa [79]. It is regarded as one of the most severe complications of RLS, which is characterized by worsening symptoms, such as an increase in the symptom severity or earlier onset time [80]. Even after dopaminergic agonists replaced levodopa as first-line therapy for RLS, augmentation remains a significant clinical challenge. Augmentation is thought to be related to the dopaminergic system. The D3 receptor has a high affinity for dopamine; in the spinal cord, low dopamine concentrations activate the D2/3 receptor to mediate dorsal spinal cord inhibition. However, high concentrations stimulate the D1 receptor, which induces locomotor activity. Dopaminergic drugs used to treat RLS activate D1-like receptors, which can lead to augmentation [81]. Augmentation occurs when patients who have been treated with a stable drug dose for at least 6 months require more medication to achieve a response [82]. Clinically, it is important to distinguish augmentation from “loss of efficacy”, which may be caused by “progression of RLS” or “tolerance” [83]. Although augmentation and loss of efficacy” exhibit a worsening of symptoms, and sometimes it might be difficult to distinguish in an individual patient. The progression of RLS is a relatively chronic process over time, and the increase in severity of symptoms is progressive; whereas the increase of symptom severity is reported “more dramatic” in augmentation [83]. With respect to “tolerance”, the symptoms cannot be severer than the situation when the treatment was initiated. Reversely, augmentation commonly causes more severe symptoms in comparison with the situation when the treatment was initiated. Moreover, Williams and García-Borreguero pointed out that augmentation should be differentiated with neuroleptic-induced akathisia and early morning rebound [83]. Prevention and treatment are important for avoiding augmentation, as discussed below.
5.1. Prevention
The choice of initial treatment for RLS is critical. α2δ Ligands improved symptoms without resulting in augmentation compared to pramipexole in a 1-year study [11]. Therefore, α2δ ligands may be selected as an initial treatment option, although the common side effects of dizziness, somnolence, fatigue, weight gain, and constipation should be taken into account [8]. If dopaminergic drugs are used as the starting treatment, certain points must be considered. Firstly, the maximum recommended dose should not be exceeded (pramipexole, 0.5–0.75 mg; ropinirole, 4 mg; rotigotine, 3 mg) [84]. Secondly, longer-acting dopamine agonists may be preferable: in a 12-week double-blind, placebo-controlled study, transdermal rotigotine continuously released over 24 h was shown to improve daytime symptoms without augmentation [85]. Thirdly, if the frequency of RLS is <1–2 per week, intermittent medication is recommended and levodopa can be considered; daily use of dopaminergic drugs should only be started when symptoms significantly affect the quality of life. However, larger clinical trials are needed to compare the efficacy and safety of the various treatment options for RLS.
5.2. Treatment
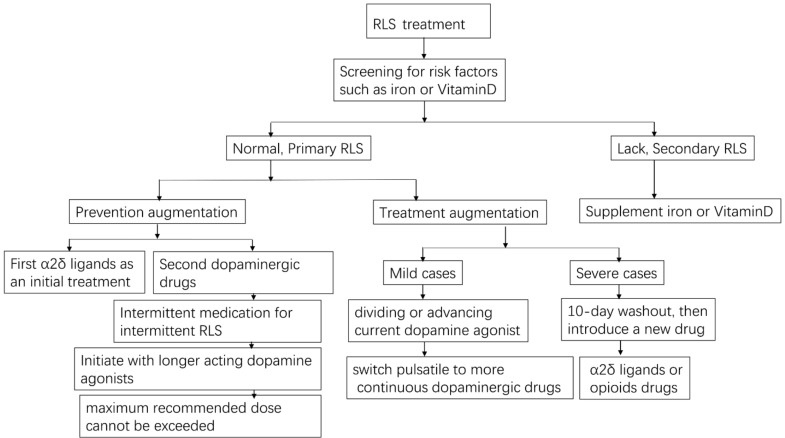
In mild cases of RLS, dopamine agonist therapy can be continued after augmentation by dividing or advancing the dose before symptom onset while ensuring that the maximum recommended dose is not exceeded. Moreover, a switch from pulsatile to continuous delivery (e.g., using transdermal rotigotine patches) may be beneficial. There are no RCT studies verifying the effect of pramipexole ER on RLS by far. However, some available literatures indicated a satisfactory efficacy of pramipexole ER [82, 86, 87]. Maestri et al. observed the efficacy of long-acting, extended-release (ER) formula of pramipexole in 24 consecutive RLS outpatients suffered from augmentation. They found that pramipexole ER has persisted efficacy against augmentation in patients with RLS [86]. The efficacy and safety of pramipexole ER for RLS require further verification by the future RCTs. Adding α2δ ligand may or may not be useful [87]. In severe cases, a 10-day washout of dopaminergic drugs followed by a different drug such as α2δ ligand or opioid is recommended [82]. However, the potential emergence of transient, extremely severe RLS symptoms during the washout period and risks associated with reintroduction of low-dose dopamine agonists should be taken into account. Low doses of opioid (e.g., prolonged-release oxycodone–naloxone [16] or methadone [53]) are effective for the treatment of severe RLS; however, possible side effects include addiction and respiratory depression. The therapeutic options for RLS treatment are illustrated in Fig. 2.
Fig. (2).
RLS treatment and the prevention and treatment of augmentation.
6. non-pharmacologic and alterative treatments for RLS
In addition to the pharmacologic treatments we introduced above, many non-pharmacologic treatments are also introduced for RLS. Giannaki et al. enrolled 24 RLS patients who underwent hemodialysis to investigate the efficacy of progressive exercise for RLS. They found that a 6-month intradialytic progressive exercise can improve the functional capacity, sleep quality, depression score, and RLS severity. No adverse events were reported [88]. Another RCT enrolled 41 patients with RLS also verified that exercise contributes to ameliorate the RLS symptoms [89]. Lettieri and Eliasson conducted a RCT to verify the efficacy and safety of pneumatic compression devices (PCDs). They found that PCDs significantly improved the RLS-related indices, which may be an effective adjunctive therapy for RLS [90]. Lin et al. reported that repetitive transcranial magnetic stimulation (rTMS) in high frequency remarkably ameliorates RLS-related motor symptoms, sleep disturbance and anxiety [91]. In addition, other alternative therapies, such as acupuncture [92], infrared device [93, 94], vibration pads [95], cold air chamber [96] and yoga [97] were also employed to treat RLS. However, these studies could not provide convincing evidence regarding the efficacy and safety of alternative treatments because of low study quality [98]. Our previous study pointed out that the flaws of experimental design might confuse the placebo/nocebo effects with identifying efficacy/adverse events during the study verifying the efficacy/safety of a certain complementary therapy [99]. Furthermore, well-designed studies are therefore desired for verifying these alternative treatments.
7. discussion
In patients who meet the diagnostic criteria for RLS, it is important first to determine whether the syndrome is primary or secondary. In the latter case, risk factors such as iron deficiency [58, 59] and vitamin D deficiency [66] must be actively treated. For primary RLS, the initial treatment choice is important. While α2δ ligands are a first-line option [8], gabapentin enacarbil is approved for RLS treatment only in the U.S. and Japan. Dopaminergic drugs are also the first-line treatment for primary RLS even though augmentation can occur with long-term use. Hence, dosing is critical and the maximum recommended doses should not be exceeded. Longer-acting dopamine agonists may have benefits. Finally, once augmentation occurs, the dose of dopaminergic drugs should be reduced while introducing low-dose α2δ ligand, or else patients should be switched to a long-term dopaminergic drug regimen. If transdermal rotigotine is used, the shorter-acting dopamine agonist should be discontinued. Opioids are recommended when α2δ ligand is ineffective in severe cases. Augmentation should be monitored for any drug that is administered over the long term.
Clarifying the pathogenesis of RLS can help to identify alternative therapeutics to dopaminergic drugs. For instance, iron deficiency directly affects the dopaminergic system [62], but also caused downregulation of adenosine receptors in the striatum and cortex in an animal model, thereby decreasing adenosine energy activation, which could result in enhanced cortical dopaminergic and glutamatergic neurotransmission in RLS [84]. Iron deficiency leads to activation of CNS hypoxia pathways and upregulation of TH in the CNS, which may be implicated in RLS [100]. Additionally, the opioid and dopaminergic systems interact with each other [57] and with the adenosine and glutamatergic systems. Investigations of genomic imprinting (e.g., of the RLS-related MEIS1 and BTBD9 genes) have provided insight into the mechanisms of RLS; impairment of neurodevelopmental processes and dysregulation of the dopaminergic system are presumed to be involved based on experiments using MEIS1 and BTBD9 mutants [31-33, 78].
CONCLUSION
Although RLS was first described in the 1940s, a detailed understanding of the disease emerged in recent years. RLS pathogenesis is considered to be a complex process involving multiple signaling pathways. Application of molecular technologies will provide clarification of the etiology of RLS and a basis for the development of individualized treatment approaches. Augmentation needs to be given special consideration in the clinical management of RLS. While dopaminergic drugs will continue to be useful, the therapeutic potential of iron, glutamatergic, and adenosine therapies and sleep medicines for the treatment of RLS warrants further investigation.
ACKNOWLEDGEMENTS
Declared none
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This study was supported by grants from the National Natural Science Foundation of China (81671103).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Allen R.P., Picchietti D., Hening W.A., Trenkwalder C., Walters A.S., Montplaisi J. Restless Legs Syndrome Diagnosis and Epidemiology workshop at the National Institutes of Health; International Restless Legs Syndrome Study Group. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. doi: 10.1016/S1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 2.Earley C.J., Heckler D., Allen R.P. Repeated IV doses of iron provides effective supplemental treatment of restless legs syndrome. Sleep Med. 2005;6(4):301–305. doi: 10.1016/j.sleep.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Earley C.J., Silber M.H. Restless legs syndrome: understanding its consequences and the need for better treatment. Sleep Med. 2010;11(9):807–815. doi: 10.1016/j.sleep.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Winkelman J.W., Redline S., Baldwin C.M., Resnick H.E., Newman A.B., Gottlieb D.J. Polysomnographic and health-related quality of life correlates of restless legs syndrome in the Sleep Heart Health Study. Sleep. 2009;32(6):772–778. doi: 10.1093/sleep/32.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akpinar S. Treatment of restless legs syndrome with levodopa plus benserazide. Arch. Neurol. 1982;39(11):739. doi: 10.1001/archneur.1982.00510230065027. [DOI] [PubMed] [Google Scholar]
- 6.Micozkadioglu H., Ozdemir F.N., Kut A., Sezer S., Saatci U., Haberal M. Gabapentin versus levodopa for the treatment of restless legs syndrome in hemodialysis patients: an open-label study. Ren. Fail. 2004;26(4):393–397. doi: 10.1081/JDI-120039823. [DOI] [PubMed] [Google Scholar]
- 7.Trenkwalder C., Benes H., Grote L., Happe S., Högl B., Mathis J., Saletu-Zyhlarz G.M., Kohnen R., Group C.S., CALDIR Study Group Cabergoline compared to levodopa in the treatment of patients with severe restless legs syndrome: results from a multi-center, randomized, active controlled trial. Mov. Disord. 2007;22(5):696–703. doi: 10.1002/mds.21401. [DOI] [PubMed] [Google Scholar]
- 8.Winkelmann J., Allen R.P., Högl B., Inoue Y., Oertel W., Salminen A.V., Winkelman J.W., Trenkwalder C., Sampaio C. Treatment of restless legs syndrome: Evidence-based review and implications for clinical practice (Revised 2017)§. Mov. Disord. 2018;33(7):1077–1091. doi: 10.1002/mds.27260. [DOI] [PubMed] [Google Scholar]
- 9.Winkelman J.W., Armstrong M.J., Allen R.P., Chaudhuri K.R., Ondo W., Trenkwalder C., Zee P.C., Gronseth G.S., Gloss D., Zesiewicz T. Practice guideline summary: Treatment of restless legs syndrome in adults: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2016;87(24):2585–2593. doi: 10.1212/WNL.0000000000003388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trenkwalder C., Winkelmann J., Inoue Y., Paulus W. Restless legs syndrome-current therapies and management of augmentation. Nat. Rev. Neurol. 2015;11(8):434–445. doi: 10.1038/nrneurol.2015.122. [DOI] [PubMed] [Google Scholar]
- 11.Wanner V., Garcia M.C., Romero S., Cano-Pumarega I., García-Borreguero D. Non-dopaminergic vs. dopaminergic treatment options in restless legs syndrome. Adv. Pharmacol. 2019;84:187–205. doi: 10.1016/bs.apha.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Silber M.H., Becker P.M., Buchfuhrer M.J., Earley C.J., Ondo W.G., Walters A.S., Winkelman J.W. Scientific and medical advisory board, restless legs syndrome foundation. the appropriate use of opioids in the treatment of refractory restless legs syndrome. Mayo Clin. Proc. 2018;93(1):59–67. doi: 10.1016/j.mayocp.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Allen R.P., Chen C., Garcia-Borreguero D., Polo O., DuBrava S., Miceli J., Knapp L., Winkelman J.W. Comparison of pregabalin with pramipexole for restless legs syndrome. N. Engl. J. Med. 2014;370(7):621–631. doi: 10.1056/NEJMoa1303646. [DOI] [PubMed] [Google Scholar]
- 14.Allen R., Chen C., Soaita A., Wohlberg C., Knapp L., Peterson B.T., García-Borreguero D., Miceli J. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512–519. doi: 10.1016/j.sleep.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Borreguero D., Larrosa O., Williams A.M., Albares J., Pascual M., Palacios J.C., Fernandez C. Treatment of restless legs syndrome with pregabalin: a double-blind, placebo-controlled study. Neurology. 2010;74(23):1897–1904. doi: 10.1212/WNL.0b013e3181e1ce73. [DOI] [PubMed] [Google Scholar]
- 16.Trenkwalder C., Beneš H., Grote L., García-Borreguero D., Högl B., Hopp M., Bosse B., Oksche A., Reimer K., Winkelmann J., Allen R.P., Kohnen R., Group R.S., Reloxyn Study Group Prolonged release oxycodone-naloxone for treatment of severe restless legs syndrome after failure of previous treatment: a double-blind, randomised, placebo-controlled trial with an open-label extension. Lancet Neurol. 2013;12(12):1141–1150. doi: 10.1016/S1474-4422(13)70239-4. [DOI] [PubMed] [Google Scholar]
- 17.Winkelmann J., Schormair B., Lichtner P., Ripke S., Xiong L., Jalilzadeh S., Fulda S., Pütz B., Eckstein G., Hauk S., Trenkwalder C., Zimprich A., Stiasny-Kolster K., Oertel W., Bachmann C.G., Paulus W., Peglau I., Eisensehr I., Montplaisir J., Turecki G., Rouleau G., Gieger C., Illig T., Wichmann H.E., Holsboer F., Müller-Myhsok B., Meitinger T. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat. Genet. 2007;39(8):1000–1006. doi: 10.1038/ng2099. [DOI] [PubMed] [Google Scholar]
- 18.Daubian-Nosé P., Frank M.K., Esteves A.M. Sleep disorders: A review of the interface between restless legs syndrome and iron metabolism. Sleep Sci. 2014;7(4):234–237. doi: 10.1016/j.slsci.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Earley C.J., Kuwabara H., Wong D.F., Gamaldo C., Salas R., Brasic J., Ravert H.T., Dannals R.F., Allen R.P. The dopamine transporter is decreased in the striatum of subjects with restless legs syndrome. Sleep (Basel) 2011;34(3):341–347. doi: 10.1093/sleep/34.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H., Zhu W., Pan T., Xie W., Zhang A., Ondo W.G., Le W. Spinal cord dopamine receptor expression and function in mice with 6-OHDA lesion of the A11 nucleus and dietary iron deprivation. J. Neurosci. Res. 2007;85(5):1065–1076. doi: 10.1002/jnr.21207. [DOI] [PubMed] [Google Scholar]
- 21.Oboshi Y., Ouchi Y., Yagi S., Kono S., Nakai N., Yoshikawa E., Futatsubashi M., Terada T., Kim K., Harada K. In vivo mesolimbic D2/3 receptor binding predicts posttherapeutic clinical responses in restless legs syndrome: a positron emission tomography study. J. Cereb. Blood Flow Metab. 2012;32(4):654–662. doi: 10.1038/jcbfm.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connor J.R., Boyer P.J., Menzies S.L., Dellinger B., Allen R.P., Ondo W.G., Earley C.J. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61(3):304–309. doi: 10.1212/01.WNL.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- 23.Earley C.J., Allen R.P., Connor J.R., Ferrucci L., Troncoso J. The dopaminergic neurons of the A11 system in RLS autopsy brains appear normal. Sleep Med. 2009;10(10):1155–1157. doi: 10.1016/j.sleep.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mochizuki H., Choong C.J., Baba K. Parkinson’s disease and iron. J. Neural Transm. (Vienna) 2020;127(2):181–187. doi: 10.1007/s00702-020-02149-3. [DOI] [PubMed] [Google Scholar]
- 25.Moon H.J., Chang Y., Lee Y.S., Song H.J., Chang H.W., Ku J., Cho Y.W. T2 relaxometry using 3.0-tesla magnetic resonance imaging of the brain in early- and late-onset restless legs syndrome. J. Clin. Neurol. 2014;10(3):197–202. doi: 10.3988/jcn.2014.10.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon H.J., Chang Y., Lee Y.S., Song H., Chang H.W., Ku J., Allen R.P., Earley C.J., Cho Y.W. A comparison of MRI tissue relaxometry and ROI methods used to determine regional brain iron concentrations in restless legs syndrome. Med. Devices (Auckl.) 2015;8:341–350. doi: 10.2147/MDER.S83629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizzo G., Manners D., Testa C., Tonon C., Vetrugno R., Marconi S., Plazzi G., Pizza F., Provini F., Malucelli E., Gramegna L.L., Lodi R. Low brain iron content in idiopathic restless legs syndrome patients detected by phase imaging. Mov. Disord. 2013;28(13):1886–1890. doi: 10.1002/mds.25576. [DOI] [PubMed] [Google Scholar]
- 28.Patton S.M., Ponnuru P., Snyder A.M., Podskalny G.D., Connor J.R. Hypoxia-inducible factor pathway activation in restless legs syndrome patients. Eur. J. Neurol. 2011;18(11):1329–1335. doi: 10.1111/j.1468-1331.2011.03397.x. [DOI] [PubMed] [Google Scholar]
- 29.Allen R.P., Earley C.J. Restless legs syndrome: a review of clinical and pathophysiologic features. J. Clin. Neurophysiol. 2001;18(2):128–147. doi: 10.1097/00004691-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Schormair B., Zhao C., Bell S., Tilch E., Salminen A.V., Pütz B., Dauvilliers Y., Stefani A., Högl B., Poewe W., Kemlink D., Sonka K., Bachmann C.G., Paulus W., Trenkwalder C., Oertel W.H., Hornyak M., Teder-Laving M., Metspalu A., Hadjigeorgiou G.M., Polo O., Fietze I., Ross O.A., Wszolek Z., Butterworth A.S., Soranzo N., Ouwehand W.H., Roberts D.J., Danesh J., Allen R.P., Earley C.J., Ondo W.G., Xiong L., Montplaisir J., Gan-Or Z., Perola M., Vodicka P., Dina C., Franke A., Tittmann L., Stewart A.F.R., Shah S.H., Gieger C., Peters A., Rouleau G.A., Berger K., Oexle K., Di Angelantonio E., Hinds D.A., Müller-Myhsok B., Winkelmann J. 23andMe Research Team; DESIR study group. Identification of novel risk loci for restless legs syndrome in genome-wide association studies in individuals of European ancestry: a meta-analysis. Lancet Neurol. 2017;16(11):898–907. doi: 10.1016/S1474-4422(17)30327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salminen A.V., Lam D.D., Winkelmann J. Role of MEIS1 in restless legs syndrome: From GWAS to functional studies in mice. Adv. Pharmacol. 2019;84:175–184. doi: 10.1016/bs.apha.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Lyu S., Xing H., DeAndrade M.P., Perez P.D., Zhang K., Liu Y., Yokoi F., Febo M., Li Y. The role of BTBD9 in the cerebral cortex and the pathogenesis of restless legs syndrome. Exp. Neurol. 2020;323:113111. doi: 10.1016/j.expneurol.2019.113111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyu S., Doroodchi A., Xing H., Sheng Y., DeAndrade M.P., Yang Y., Johnson T.L., Clemens S., Yokoi F., Miller M.A., Xiao R., Li Y. BTBD9 and dopaminergic dysfunction in the pathogenesis of restless legs syndrome. Brain Struct. Funct. 2020;225(6):1743–1760. doi: 10.1007/s00429-020-02090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferré S., Earley C., Gulyani S., Garcia-Borreguero D. In search of alternatives to dopaminergic ligands for the treatment of restless legs syndrome: iron, glutamate, and adenosine. Sleep Med. 2017;31:86–92. doi: 10.1016/j.sleep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Collado-Seidel V., Kazenwadel J., Wetter T.C., Kohnen R., Winkelmann J., Selzer R., Oertel W.H., Trenkwalder C. A controlled study of additional sr-L-dopa in L-dopa-responsive restless legs syndrome with late-night symptoms. Neurology. 1999;52(2):285–290. doi: 10.1212/WNL.52.2.285. [DOI] [PubMed] [Google Scholar]
- 36.Guilleminault C., Cetel M., Philip P. Dopaminergic treatment of restless legs and rebound phenomenon. Neurology. 1993;43(2):445. doi: 10.1212/WNL.43.2.445. [DOI] [PubMed] [Google Scholar]
- 37.Giorgi L., Asgharian A., Hunter B. Ropinirole in patients with restless legs syndrome and baseline IRLS total scores ≥ 24: efficacy and tolerability in a 26-week, double-blind, parallel-group, placebo-controlled study followed by a 40-week open-label extension. Clin. Ther. 2013;35(9):1321–1336. doi: 10.1016/j.clinthera.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Hening W.A., Allen R.P., Ondo W.G., Walters A.S., Winkelman J.W., Becker P., Bogan R., Fry J.M., Kudrow D.B., Lesh K.W., Fichtner A., Schollmayer E., Group S.P.S. SP792 Study Group. Rotigotine improves restless legs syndrome: a 6-month randomized, double-blind, placebo-controlled trial in the United States. Mov. Disord. 2010;25(11):1675–1683. doi: 10.1002/mds.23157. [DOI] [PubMed] [Google Scholar]
- 39.Kalia L.V., Lang A.E. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 40.Koblinger K., Füzesi T., Ejdrygiewicz J., Krajacic A., Bains J.S., Whelan P.J. Characterization of A11 neurons projecting to the spinal cord of mice. PLoS One. 2014;9(10):e109636. doi: 10.1371/journal.pone.0109636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ondo W.G., He Y., Rajasekaran S., Le W.D. Clinical correlates of 6-hydroxydopamine injections into A11 dopaminergic neurons in rats: a possible model for restless legs syndrome. Mov. Disord. 2000;15(1):154–158. doi: 10.1002/1531-8257(200001)15:1<154:AID-MDS1025>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 42.Yepes G., Guitart X., Rea W., Newman A.H., Allen R.P., Earley C.J., Quiroz C., Ferré S. Targeting hypersensitive corticostriatal terminals in restless legs syndrome. Ann. Neurol. 2017;82(6):951–960. doi: 10.1002/ana.25104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stiasny-Kolster K., Magerl W., Oertel W.H., Möller J.C., Treede R.D. Static mechanical hyperalgesia without dynamic tactile allodynia in patients with restless legs syndrome. Brain. 2004;127(Pt 4):773–782. doi: 10.1093/brain/awh079. [DOI] [PubMed] [Google Scholar]
- 44.Bogan R.K., Bornemann M.A., Kushida C.A., Trân P.V., Barrett R.W., Group X.P.S. XP060 Study Group. Long-term maintenance treatment of restless legs syndrome with gabapentin enacarbil: a randomized controlled study. Mayo Clin. Proc. 2010;85(6):512–521. doi: 10.4065/mcp.2009.0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee D.O., Ziman R.B., Perkins A.T., Poceta J.S., Walters A.S., Barrett R.W., Group X.P.S. XP053 Study Group. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J. Clin. Sleep Med. 2011;7(3):282–292. doi: 10.5664/JCSM.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inoue Y., Hirata K., Uchimura N., Kuroda K., Hattori N., Takeuchi M. Gabapentin enacarbil in Japanese patients with restless legs syndrome: a 12-week, randomized, double-blind, placebo-controlled, parallel-group study. Curr. Med. Res. Opin. 2013;29(1):13–21. doi: 10.1185/03007995.2012.746217. [DOI] [PubMed] [Google Scholar]
- 47.Walters A.S., Ondo W.G., Kushida C.A., Becker P.M., Ellenbogen A.L., Canafax D.M., Barrett R.W., Group X.P.S. XP045 Study Group. Gabapentin enacarbil in restless legs syndrome: a phase 2b, 2-week, randomized, double-blind, placebo-controlled trial. Clin. Neuropharmacol. 2009;32(6):311–320. doi: 10.1097/WNF.0b013e3181b3ab16. [DOI] [PubMed] [Google Scholar]
- 48.Happe S., Sauter C., Klösch G., Saletu B., Zeitlhofer J. Gabapentin versus ropinirole in the treatment of idiopathic restless legs syndrome. Neuropsychobiology. 2003;48(2):82–86. doi: 10.1159/000072882. [DOI] [PubMed] [Google Scholar]
- 49.Thorp M.L., Morris C.D., Bagby S.P. A crossover study of gabapentin in treatment of restless legs syndrome among hemodialysis patients. Am. J. Kidney Dis. 2001;38(1):104–108. doi: 10.1053/ajkd.2001.25202. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Borreguero D., Larrosa O., de la Llave Y., Verger K., Masramon X., Hernandez G. Treatment of restless legs syndrome with gabapentin: a double-blind, cross-over study. Neurology. 2002;59(10):1573–1579. doi: 10.1212/WNL.59.10.1573. [DOI] [PubMed] [Google Scholar]
- 51.Taylor C.P., Angelotti T., Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res. 2007;73(2):137–150. doi: 10.1016/j.eplepsyres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Hendrich J., Van Minh A.T., Heblich F., Nieto-Rostro M., Watschinger K., Striessnig J., Wratten J., Davies A., Dolphin A.C. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc. Natl. Acad. Sci. USA. 2008;105(9):3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ondo W.G. Methadone for refractory restless legs syndrome. Mov. Disord. 2005;20(3):345–348. doi: 10.1002/mds.20359. [DOI] [PubMed] [Google Scholar]
- 54.Walters A.S., Wagner M.L., Hening W.A., Grasing K., Mills R., Chokroverty S., Kavey N. Successful treatment of the idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993;16(4):327–332. doi: 10.1093/sleep/16.4.327. [DOI] [PubMed] [Google Scholar]
- 55.Walters A.S., Winkelmann J., Trenkwalder C., Fry J.M., Kataria V., Wagner M., Sharma R., Hening W., Li L. Long-term follow-up on restless legs syndrome patients treated with opioids. Mov. Disord. 2001;16(6):1105–1109. doi: 10.1002/mds.1214. [DOI] [PubMed] [Google Scholar]
- 56.Walters A.S., Ondo W.G., Zhu W., Le W. Does the endogenous opiate system play a role in the Restless Legs Syndrome? A pilot post-mortem study. J. Neurol. Sci. 2009;279(1-2):62–65. doi: 10.1016/j.jns.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 57.Hagelberg N., Kajander J.K., Någren K., Hinkka S., Hietala J., Scheinin H. Mu-receptor agonism with alfentanil increases striatal dopamine D2 receptor binding in man. Synapse. 2002;45(1):25–30. doi: 10.1002/syn.10078. [DOI] [PubMed] [Google Scholar]
- 58.Trenkwalder C., Winkelmann J., Oertel W., Virgin G., Roubert B., Mezzacasa A., FCM-RLS Study Investigators Ferric carboxymaltose in patients with restless legs syndrome and nonanemic iron deficiency: A randomized trial. Mov. Disord. 2017;32(10):1478–1482. doi: 10.1002/mds.27040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho Y.W., Allen R.P., Earley C.J. Clinical efficacy of ferric carboxymaltose treatment in patients with restless legs syndrome. Sleep Med. 2016;25:16–23. doi: 10.1016/j.sleep.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 60.Mizuno S., Mihara T., Miyaoka T., Inagaki T., Horiguchi J. CSF iron, ferritin and transferrin levels in restless legs syndrome. J. Sleep Res. 2005;14(1):43–47. doi: 10.1111/j.1365-2869.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 61.Allen R.P., Picchietti D.L., Auerbach M., Cho Y.W., Connor J.R., Earley C.J., Garcia-Borreguero D., Kotagal S., Manconi M., Ondo W., Ulfberg J., Winkelman J.W. International Restless Legs Syndrome Study Group (IRLSSG). Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis-Ekbom disease in adults and children: an IRLSSG task force report. Sleep Med. 2018;41:27–44. doi: 10.1016/j.sleep.2017.11.1126. [DOI] [PubMed] [Google Scholar]
- 62.Nelson C., Erikson K., Piñero D.J., Beard J.L. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J. Nutr. 1997;127(12):2282–2288. doi: 10.1093/jn/127.12.2282. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Borreguero D., Cano I., Granizo J.J. Treatment of restless legs syndrome with the selective AMPA receptor antagonist perampanel. Sleep Med. 2017;34:105–108. doi: 10.1016/j.sleep.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Borreguero D., Guitart X., Garcia Malo C., Cano-Pumarega I., Granizo J.J., Ferré S. Treatment of restless legs syndrome/Willis-Ekbom disease with the non-selective ENT1/ENT2 inhibitor dipyridamole: testing the adenosine hypothesis. Sleep Med. 2018;45:94–97. doi: 10.1016/j.sleep.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 65.Sagheb M.M., Dormanesh B., Fallahzadeh M.K., Akbari H., Sohrabi Nazari S., Heydari S.T., Behzadi S. Efficacy of vitamins C, E, and their combination for treatment of restless legs syndrome in hemodialysis patients: a randomized, double-blind, placebo-controlled trial. Sleep Med. 2012;13(5):542–545. doi: 10.1016/j.sleep.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 66.Tutuncu M., Tutuncu M. The effect of vitamin D on restless legs syndrome: prospective self-controlled case study. Sleep Breath. 2020;24(3):1101–1106. doi: 10.1007/s11325-019-01984-3. [DOI] [PubMed] [Google Scholar]
- 67.Roshi; Tandon, V.R.; Mahajan, A.; Sharma, S.; Khajuria, V. Comparative efficacy and safety of clonazepam versus nortriptyline in restless leg syndrome among forty plus women: a prospective, open-label randomized study. J Midlife Health. 2019;10(4):197–203. doi: 10.4103/jmh.JMH_26_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saletu M., Anderer P., Saletu-Zyhlarz G., Prause W., Semler B., Zoghlami A., Gruber G., Hauer C., Saletu B. Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo-controlled sleep laboratory studies with clonazepam. Eur. Neuropsychopharmacol. 2001;11(2):153–161. doi: 10.1016/S0924-977X(01)00080-3. [DOI] [PubMed] [Google Scholar]
- 69.Brand S., Beck J., Hatzinger M., Holsboer-Trachsler E. Patients suffering from restless legs syndrome have low internal locus of control and poor psychological functioning compared to healthy controls. Neuropsychobiology. 2013;68(1):51–58. doi: 10.1159/000350957. [DOI] [PubMed] [Google Scholar]
- 70.Kurlan R., Rabin M. Augmentation in restless legs syndrome Poor response to sudden withdrawal of dopaminergic therapy. J Parkinsonism Restless Legs Synd. 2013;3:49–52. doi: 10.2147/JPRLS.S47648. [DOI] [Google Scholar]
- 71.Aurora R.N., Kristo D.A., Bista S.R., Rowley J.A., Zak R.S., Casey K.R., Lamm C.I., Tracy S.L., Rosenberg R.S. American Academy of Sleep Medicine. The treatment of restless legs syndrome and periodic limb movement disorder in adults--an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep (Basel) 2012;35(8):1039–1062. doi: 10.5665/sleep.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carlos K., Prado G.F., Teixeira C.D., Conti C., de Oliveira M.M., Prado L.B., Carvalho L.B. Benzodiazepines for restless legs syndrome. Cochrane Database Syst. Rev. 2017;3:CD006939. doi: 10.1002/14651858.CD006939.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tilch E., Schormair B., Zhao C., Salminen A.V., Antic Nikolic A., Holzknecht E., Högl B., Poewe W., Bachmann C.G., Paulus W., Trenkwalder C., Oertel W.H., Hornyak M., Fietze I., Berger K., Lichtner P., Gieger C., Peters A., Müller-Myhsok B., Hoischen A., Winkelmann J., Oexle K. Identification of Restless Legs Syndrome Genes by Mutational Load Analysis. Ann. Neurol. 2020;87(2):184–193. doi: 10.1002/ana.25658. [DOI] [PubMed] [Google Scholar]
- 74.Salminen A.V., Garrett L., Schormair B., Rozman J., Giesert F., Niedermeier K.M., Becker L., Rathkolb B., Rácz I., Klingenspor M., Klopstock T., Wolf E., Zimmer A., Gailus-Durner V., Torres M., Fuchs H., Hrabě de Angelis M., Wurst W., Hölter S.M., Winkelmann J., German Mouse Clinic Consortium Meis1: effects on motor phenotypes and the sensorimotor system in mice. Dis. Model. Mech. 2017;10(8):981–991. doi: 10.1242/dmm.030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meneely S., Dinkins M.L., Kassai M., Lyu S., Liu Y., Lin C.T., Brewer K., Li Y., Clemens S. Differential Dopamine D1 and D3 receptor modulation and expression in the spinal cord of two mouse models of restless legs syndrome. Front. Behav. Neurosci. 2018;12:199. doi: 10.3389/fnbeh.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drgonova J., Walther D., Wang K.J., Hartstein G.L., Lochte B., Troncoso J., Uetani N., Iwakura Y., Uhl G.R. Mouse model for protein tyrosine phosphatase D (PTPRD) associations with restless leg syndrome or Willis-Ekbom disease and addiction: reduced expression alters locomotion, sleep behaviors and cocaine-conditioned place preference. Mol. Med. 2015;21(1):717–725. doi: 10.2119/molmed.2015.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Allen R.P., Donelson N.C., Jones B.C., Li Y., Manconi M., Rye D.B., Sanyal S., Winkelmann J. Animal models of RLS phenotypes. Sleep Med. 2017;31:23–28. doi: 10.1016/j.sleep.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lyu S., Xing H., DeAndrade M.P., Liu Y., Perez P.D., Yokoi F., Febo M., Walters A.S., Li Y. The Role of BTBD9 in striatum and restless legs syndrome. 2019. [DOI] [PMC free article] [PubMed]
- 79.Allen R.P., Earley C.J. Augmentation of the restless legs syndrome with carbidopa/levodopa. Sleep. 1996;19(3):205–213. doi: 10.1093/sleep/19.3.205. [DOI] [PubMed] [Google Scholar]
- 80.Leu-Semenescu S., Petiau C., Charley Monaca C., Dauvilliers Y. French consensus: Augmentation syndrome in restless legs syndrome. Rev. Neurol. (Paris) 2018;174(7-8):532–539. doi: 10.1016/j.neurol.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 81.Trenkwalder C., Allen R., Högl B., Clemens S., Patton S., Schormair B., Winkelmann J. Comorbidities, treatment, and pathophysiology in restless legs syndrome. Lancet Neurol. 2018;17(11):994–1005. doi: 10.1016/S1474-4422(18)30311-9. [DOI] [PubMed] [Google Scholar]
- 82.Garcia-Borreguero D., Silber M.H., Winkelman J.W., Högl B., Bainbridge J., Buchfuhrer M., Hadjigeorgiou G., Inoue Y., Manconi M., Oertel W., Ondo W., Winkelmann J., Allen R.P. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med. 2016;21:1–11. doi: 10.1016/j.sleep.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 83.Williams A.M., Garcia-Borreguero D. Management of restless legs syndrome augmentation. Curr. Treat. Options Neurol. 2009;11(5):327–332. doi: 10.1007/s11940-009-0036-2. [DOI] [PubMed] [Google Scholar]
- 84.Ferré S., García-Borreguero D., Allen R.P., Earley C.J. New Insights into the neurobiology of restless legs syndrome. Neuroscientist. 2019;25(2):113–125. doi: 10.1177/1073858418791763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garcia-Borreguero D., Allen R., Hudson J., Dohin E., Grieger F., Moran K., Schollmayer E., Smit R., Winkelman J. Effects of rotigotine on daytime symptoms in patients with primary restless legs syndrome: a randomized, placebo-controlled study. Curr. Med. Res. Opin. 2016;32(1):77–85. doi: 10.1185/03007995.2015.1103216. [DOI] [PubMed] [Google Scholar]
- 86.Maestri M., Fulda S., Ferini-Strambi L., Zucconi M., Marelli S., Staedler C., Bassetti C.L., Manconi M. Polysomnographic record and successful management of augmentation in restless legs syndrome/Willis-Ekbom disease. Sleep Med. 2014;15(5):570–575. doi: 10.1016/j.sleep.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 87.Garcia-Borreguero D., Cano-Pumarega I., Marulanda R. Management of treatment failure in restless legs syndrome (Willis-Ekbom disease). Sleep Med. Rev. 2018;41:50–60. doi: 10.1016/j.smrv.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Giannaki C.D., Hadjigeorgiou G.M., Karatzaferi C., Maridaki M.D., Koutedakis Y., Founta P., Tsianas N., Stefanidis I., Sakkas G.K. A single-blind randomized controlled trial to evaluate the effect of 6 months of progressive aerobic exercise training in patients with uraemic restless legs syndrome. Nephrol. Dial. Transplant. 2013;28(11):2834–2840. doi: 10.1093/ndt/gft288. [DOI] [PubMed] [Google Scholar]
- 89.Aukerman M.M., Aukerman D., Bayard M., Tudiver F., Thorp L., Bailey B. Exercise and restless legs syndrome: a randomized controlled trial. J. Am. Board Fam. Med. 2006;19(5):487–493. doi: 10.3122/jabfm.19.5.487. [DOI] [PubMed] [Google Scholar]
- 90.Lettieri C.J., Eliasson A.H. Pneumatic compression devices are an effective therapy for restless legs syndrome: a prospective, randomized, double-blinded, sham-controlled trial. Chest. 2009;135(1):74–80. doi: 10.1378/chest.08-1665. [DOI] [PubMed] [Google Scholar]
- 91.Lin Y.C., Feng Y., Zhan S.Q., Li N., Ding Y., Hou Y., Wang L., Lin H., Sun Y., Huang Z.Y., Xue Q., Wang Y.P. Repetitive transcranial magnetic stimulation for the treatment of restless legs syndrome. Chin. Med. J. (Engl.) 2015;128(13):1728–1731. doi: 10.4103/0366-6999.159344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pan W., Wang M., Li M., Wang Q., Kwak S., Jiang W., Yamamoto Y. Actigraph evaluation of acupuncture for treating restless legs syndrome. Evid. Based Complement. Alternat. Med. 2015;2015:343201. doi: 10.1155/2015/343201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mitchell U.H., Myrer J.W., Johnson A.W., Hilton S.C. Restless legs syndrome and near-infrared light: An alternative treatment option. Physiother. Theory Pract. 2011;27(5):345–351. doi: 10.3109/09593985.2010.511440. [DOI] [PubMed] [Google Scholar]
- 94.Mitchell U.H., Johnson A.W., Myrer B. Comparison of two infrared devices in their effectiveness in reducing symptoms associated with RLS. Physiother. Theory Pract. 2011;27(5):352–359. doi: 10.3109/09593985.2010.502210. [DOI] [PubMed] [Google Scholar]
- 95.Burbank F., Buchfuhrer M.J., Kopjar B. Sleep improvement for restless legs syndrome patients. Part I: pooled analysis of two prospective, double-blind, sham-controlled, multi-center, randomized clinical studies of the effects of vibrating pads on RLS symptoms. Res. Rev. Parkinsonism. 2013;3:1–10. [Google Scholar]
- 96.Happe S., Evers S., Thiedemann C., Bunten S., Siegert R. Whole body and local cryotherapy in restless legs syndrome: A randomized, single-blind, controlled parallel group pilot study. J. Neurol. Sci. 2016;370:7–12. doi: 10.1016/j.jns.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 97.Innes K.E., Selfe T.K. The effects of a gentle yoga program on sleep, mood, and blood pressure in older women with restless legs syndrome (RLS): A Preliminary Randomized Controlled Trial. Evid. Based Complement. Alternat. Med. 2012;2012:294058. doi: 10.1155/2012/294058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harrison E.G., Keating J.L., Morgan P.E. Non-pharmacological interventions for restless legs syndrome: a systematic review of randomised controlled trials. Disabil. Rehabil. 2019;41(17):2006–2014. doi: 10.1080/09638288.2018.1453875. [DOI] [PubMed] [Google Scholar]
- 99.Chen Q., Wang Q., Ding S., Li S., Zhang Y., Chen S., Lin X., Li C., Asakawa T. Problems lowering the study quality in traditional medicine, introspection from an example of meta-analysis of acupuncture. BMC Complement. Med. Ther. 2020;20(1):41. doi: 10.1186/s12906-019-2806-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brewer K.L., Baran C.A., Whitfield B.R., Jensen A.M., Clemens S. Dopamine D3 receptor dysfunction prevents anti-nociceptive effects of morphine in the spinal cord. Front. Neural Circuits. 2014;8:62. doi: 10.3389/fncir.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]