Abstract
Borderline Personality Disorder (BPD) is a chronic debilitating psychiatric disorder characterized mainly by emotional instability, chaotic interpersonal relationships, cognitive disturbance (e.g., dissociation and suicidal thoughts) and maladaptive behaviors. BPD has a high rate of comorbidity with other mental disorders and a high burden on society. In this review, we focused on two compromised brain regions in BPD - the hypothalamus and the corticolimbic system, emphasizing the involvement and potential contribution of the endocannabinoid system (ECS) to improvement in symptoms and coping. The hypothalamus-regulated endocrine axes (hypothalamic pituitary – gonadal, thyroid & adrenal) have been found to be dysregulated in BPD. There is also substantial evidence for limbic system structural and functional changes in BPD, especially in the amygdala and hippocampus, including cortical regions within the corticolimbic system. Extensive expression of CB1 and CB2 receptors of the ECS has been found in limbic regions and the hypothalamus. This opens new windows of opportunity for treatment with cannabinoids such as cannabidiol (CBD) as no other pharmacological treatment has shown long-lasting improvement in the BPD population to date. This review aims to show the potential role of the ECS in BPD patients through their most affected brain regions, the hypothalamus and the corticolimbic system. The literature reviewed does not allow for general indications of treatment with CBD in BPD. However, there is enough knowledge to indicate a treatment ratio of a high level of CBD to a low level of THC. A randomized controlled trial investigating the efficacy of cannabinoid based treatments in BPD is warranted.
Keywords: Borderline personality disorder, hypothalamus, corticolimbic system, endocannabinoid system, pharmacological treatment, cannabidiol
1. Introduction
Borderline Personality Disorder (BPD) is a chronic debilitating psychiatric disorder characterized by emotional instability, impulsive/aggressive behavior, chaotic interpersonal relationships, cognitive disturbances, including dissociation, severe functional impairment, suicidal thoughts and attempts, anhedonia and general resistance to change towards more adaptive behavior, including deficits in decision making and pursuing long term goals [1-7]. Alongside these symptoms, BPD has a high rate of comorbid mental disorders and a high burden on society [6].
There is a wide agreement in the recent scientific literature regarding the etiological pathways of BPD, showing that childhood adversity is a strong risk factor for future BPD symptoms [8-13]. Epidemiologically, the prevalence of BPD in the USA is between 0.5% and 5.9% in the general population, with a median of 1.35% [14]. A recent epidemiological study found that about 25.2% of the general population had 1-2 BPD symptoms, 3.8% had 3-4 symptoms, 1.1% had 5 and above BPD symptoms, while only 69.9% of the population had no BPD criteria [15]. Despite the prevailing assumption that BPD appears more among women than men, there is no significant difference between adult women and men in the prevalence of BPD, while the pattern of comorbidities differs [16, 17].
In an informative Cochrane review [18] based on 1742 participants in randomized controlled trials (RCTs) and other clinical trials, as well as on the APA guidelines for pharmacological treatment of BPD, the authors concluded that some beneficial effects were found for antipsychotics (flupentixol decanoate, haloperidol, thiothixene), mood stabilizers (carbamazepine, valproate semisodium, lamotrigine, topiramate) and dietary supplements (omega-3 fatty acids) and that antidepressants such as selective serotonin reuptake inhibitors (SSRIs) are not widely used, but may be useful in comorbid states, which are very prevalent. Others suggest that treatment with SSRIs has effects on corticolimbic connectivity and reduces amygdala activation, apparently related to intactness of 5-HT synaptic function 1. However, SSRIs have not been an established treatment for impulsive and aggressive behaviors among BPD patients, a major feature of BPD [19]. Saunders and Silk [20] found twenty randomized placebo-controlled pharmacological trials. However, little concordance was found among the included studies. A more recent study analyzed the pharmacological treatment of 2195 inpatients with BPD between 2001 and 2011 [21]. The authors note that contrary to the guidelines, about 90% of inpatients with BPD received psychotropic drugs [21]. According to the APA, total BPD severity is not significantly affected by any pharmacological treatment and no promising results are available for the core BPD symptoms [18]. Therefore, novel treatment approaches are urgently needed. The aims of this review are to map the most compromised brain regions in BPD and to suggest a novel treatment approach targeting the endocannabinoid system (ECS).
1.1. The Hypothalamic Perspective in BPD
The endocrine axes which are regulated by the hypothalamus [hypothalamic pituitary gonadal (HPG), thyroid (HPT), and adrenal (HPA) axes] synthesize hormones in the hypothalamus, the pituitary and in the gonads, thyroid and adrenal glands, respectively. The reproductive progress with age is modulated by the HPG via steroid hormones. Thyroid hormones regulate energy metabolism and development through the HPT axis. The HPA axis regulates the organism’s reaction to stressful conditions by the secretion of glucocorticoids (GCs) such as cortisol and corticosterone [22]. These axes were found to affect each other in a crossover manner. Proteins involved in one axis, such as hormone receptors or specific enzymes, appear on the hormone-producing tissues of the other axis. For example, androgen receptors are present in the thyroid gland [23]. Flood et al. [23] performed a promoter analysis and identified several thyroid receptor-responsive elements in genes expressed in gonadal tissue and androgen receptor-responsive elements in genes expressed in thyroid tissue. Thus, the HPG and the HPT axes are activated in concert in the manner that genes from one axis activate signaling to the other [24].
Testosterone, the major androgen hormone of the HPG axis, is found to be elevated in association with disorders characterized by impaired impulse control [25], a main feature of BPD. Another study found increased salivary testosterone levels in BPD patients compared to healthy controls [26]. In addition, increased hair testosterone levels were reported in BPD patients compared to controls [27]. Progesterone, estradiol and estrogen are regulated by the hypothalamic HPG axis. In BPD, lower levels and cyclical reductions of these hormones showed correlation with impaired cognitive functioning, including thought ruminations, negative thinking and emotional stability, which are a pivotal part of the BPD profile [28]. Another HPG endocrinological dysregulation, a higher prevalence of polycystic ovary syndrome, has also been reported in women with BPD [29]. Thus, relevant data indicate that women with BPD have higher than expected serum androgen levels and thus greater chances of having polycystic ovaries. Endocrinological dysregulation of the reproduction process in women has been found in numerous studies to be related to mood disorders, which are very common in BPD [30].
In the HPT axis, the thyroid hormones T3 and T4 are released from the thyroid gland in response to thyroid-stimulating hormone (TSH), which, in turn, is released from the anterior pituitary gland. The thyrotropin-releasing hormone stimulation test has been used to examine potential HPT endocrinological dysregulation in BPD and to test for biological differences from depressive states, including major depression [31]. However, the results do not support, in general, a blunted response in BPD patients, while the evidence is clearer (and significantly more frequent than in BPD) for Major Depression, where about 50% of the patients studied showed a blunted TSH response [32, 33].
TSH is regulated by a thyrotropin-releasing hormone produced from the hypothalamus. Stress reactions have been associated with thyroid function [34]. Increased T4 levels are part of stress arousal reactions, which are also a problematic phenomenon in BPD, showing the activity of the HPT axis in stress regulation and disorders in the stress system. Early trauma, common in BPD life history, causes changes in the reactivity to stress [35] and may cause lasting changes in the thyroid levels [36]. Specifically, altered thyroid activity levels, especially FT3/FT4, were associated with exposure to adversity in childhood in women with BPD, especially if they reported comorbidity with posttraumatic stress disorder (PTSD) [36]. Furthermore, a positive correlation between the expression of interpersonal violence and serum T3 levels in female patients with BPD in adulthood [37] has been reported, providing further support for HPT involvement in BPD.
HPA axis functioning seems to be altered in BPD as reactivity to stress is endocrinologically dysregulated in both men and women with BPD. There is a correlation between exposure to early adversity and the production of GC hormones [38]. Since the development of the hippocampus, the amygdala, and the frontal lobe continue after birth, stress events on early childhood development may lead to a high increase of GC hormones, and consequently to structural, functional and epigenetic changes on those areas [39]. The test for HPA functioning, combining dexamethasone with Corticotropin Releasing Hormone [CRH] (DEX-CRH), has been found to be sensitive to trauma history, a major etiological factor in BPD [9, 19, 40, 41].
The role of the HPA axis in BPD is complex [7, 42]. It has been proposed that BPD patients show an imbalance between activity in central noradrenergic and cholinergic symptoms associated with the HPA endocrinological dysregulation [43]. Accordingly, abnormalities in noradrenergic activity have been later noted for BPD [44]. Given the abnormalities in stress reactivity in BPD, the endocrinological dysregulation of HPA function and cortisol synthesis remains an unresolved question in the literature [27, 42]. Dettenborn et al. [27] reported no difference in cortisol levels between BPD patients and healthy controls, but they concede that higher baseline levels of cortisol were reported in BPD patients in most studies, compared to healthy controls. In addition, only BPD patients compared with cluster C personality disorders demonstrated reduced cortisol levels with a blunted cortisol and heart rate reactivity in stress tests [27, 45]. In addition, research on the HPA axis functioning in adults with BPD or adolescents with repetitive non-suicidal self-injury has shown increased cortisol awakening response indicating increased HPA axis activity [46]. Accordingly, ACTH:cortisol ratio was higher in BPD patients than in controls and cognitive evaluation of a stressor was impaired [47]. The hypothalamus modulates arousal states, including aggression, a major phenotype of BPD, as shown by research in both rodents and humans [48], thus leading to a more adaptive reaction to arousing stimuli.
Although GCs start adaptive coping processes, prolonged or inappropriate GC secretion as in chronic stress is dysfunctional. Inappropriate processing of stressful stimuli and environments may develop in the BPD individual, thus resulting in a risk of developing BPD [49, 50].
Gender differences have also been observed in BPD. Women with BPD had decreased cortisol levels in response to stress, whereas male BPD individuals showed an increase in cortisol levels in response to the same stress test [51]. Finally, in a large meta-analysis, it has been found that cortisol as a biomarker of the HPA axis is a crucial and helpful measure in the study of BPD, a population vulnerable to stress, while confounding factors such as comorbidities should be taken into consideration [52].
An additional genetic perspective indicates that BPD is associated with polymorphisms in the CRH receptor (CRHR) and the FK506 Binding Protein 5 (FKBP5) genes. Specifically, rs4713902 and rs9470079, two FKBP5 polymorphisms, revealed significant associations with BPD. Together, this information suggests an association between a genetically modified HPA functionality and BPD, thus supporting the role of the HPA axis in BPD development [35].
From an epigenetic perspective, the GR gene (NR3C1) may be relevant for BPD. Increased methylation of the promoter region of the NR3C1 gene has been related to both subjects with the early history of life trauma and to patients with BPD. The results of a methylation study showed a positive association between methylation of NR3C1 and the clinical severity of the BPD patients. These results show the nature-nurture perspective as they present the gene-environment interactions that serve as evidence for the association of NR3C1 to childhood trauma and to the clinical severity of BPD symptoms [10].
Although the state of the art of the research on these three hypothalamic axes in BPD is mostly correlational, there is mounting scientific evidence for this association. Basic research allowing for causal inference is warranted.
1.2. The Corticolimbic Dysfunction in Borderline Personality Disorder
There is substantial evidence for dysfunction of interconnectivity between the limbic system and the cortex in BPD [2, 53], with better corticolimbic connectivity found during instructed emotional regulation [54]. Functional neuroimaging studies have brought support for changes in temporoparietal areas (fusiform gyrus, superior temporal gyrus and inferior parietal lobule) and fronto-limbic regions (inferior frontal gyrus, amygdala, medial, dorsolateral and anterior cingulate prefrontal cortices), possibly mediating impaired affective-cognitive processing in BPD [55].
Imaging studies including MRI, fMRI and PET, showed heightened sensitivity to emotional stimuli in BPD together with the display of dysfunctions in the prefrontal cortex (PFC; a key region in decision making) and in the limbic system especially the amygdala (a key region in emotional regulation) [56-58]. This is evident while the individual is viewing or listening to emotional, fearful, or traumatic stimuli or even in anticipation of such a stimulus, in the presence of a neutral stimulus or even in the resting situation without a trigger [2, 57]. Additionally, in fMRI studies, individuals with BPD showed more hyperactivity of the limbic system to emotionally evocative stimuli such as higher amygdala reactivity to unpleasant pictures and fearful words compared to healthy controls [59]. However, no activation of the amygdala in BPD and in healthy controls has been detected in reaction to positive stimuli [60, 61]. Furthermore, Duque Alarcón et al. [12] found that the level of childhood maltreatment negatively correlated with the score in social cognitive scale, in BPD and control groups, but only the BPD patients showed hypoconnectivity among the corticolimbic areas, which play a significant role in emotion regulation and social cognition [12]. These findings highlight a neural corticolimbic network characterizing the dysfunctions of patients suffering from BPD [2, 4, 62, 63].
Theories on the biological underlying processes of aggression, which is part of the BPD profile, implicate prefrontal and limbic structures. More accurately, a brain-circuit comprising the amygdala, hypothalamus, and periaqueductal gray has been identified in a growing body of literature; heightened activation of this circuit may set off reactive aggression [64-66]. This anger-prone neural connectivity has been found in women diagnosed with BPD [67].
In addition, age would appear to play a role. In imaging clinical studies, while gray matter volume deficits in the limbic structures may become significantly apparent with advancing age in the course of BPD, parieto-occipital rather than frontal gray matter deficits are characteristics of younger aged BPD patients [68]. Moreover, some of the studies showed reduced volumes in prefrontal brain areas involved in stress regulation, especially in the anterior cingulate cortex and orbitofrontal cortex [69].
The interpersonal difficulties and instability of BPD were also apparent in functional imaging studies showing on the neural level hyperactivity of the amygdala and other limbic regions in response to social stimuli such as interpersonal scenes or facial expressions [70]. BPD patients also showed heightened sensitivity to social threat stimuli, expressed in faster eye fixation on the threat and hyperactivation of the amygdala [71]. Thus, individuals with BPD are deficient in neurocognitive control capacities regarding social situations and social stimuli.
Finally, while structural neuroimaging studies in BPD patients showed reductions in hippocampal volumes, they also reported an increase in hypothalamic volume [71]. In addition, the hypothalamic volume was associated with a history of trauma in BPD (Mauchnik & Schmahl [72]). Thus, we argued in this review that the hypothalamic-corticolimbic regions are the most relevant brain areas involved in the BPD disorder.
1.3. The Endocannabinoid System
The ECS consists of cannabinoid receptors (e.g., CB1, CB2), the endogenous ligands that are interconnected to these cannabinoid receptors [mainly the endocannabinoids anandamide (N-arachidonylethanolamine, AEA) and 2-arachidonoylglycerol (2-AG)], and enzymes by which their biosynthesis and degradation are achieved [e.g., fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL)] [73-75]. In the central nervous system, endocannabinoids are produced on demand and released from the postsynaptic neural membrane [76]. There are several brain regions that express a high density of CB receptors, including, hippocampus, amygdala and PFC regions, which show compromised functioning in BPD [77].
CB1 receptors are widely spread in the brains of mammals [78]. They are found mostly in presynaptic terminals where they decrease neurotransmitter (e.g., GABA and glutamate) secretion to the synapse. They are also present in a variety of peripheral tissues. The CB2 receptor is also a G protein-coupled receptor and is encoded by the CB2 receptor gene (CNR2) located at chromosome p36.11 [79]. CB2 receptors are found on enteric neurons and also expressed by immune and epithelial cells in the gastrointestinal (GI) tract [80-82]. They are also found in glial cells as well, and to a much lesser extent, in neurons of several brain regions such as the amygdala, hippocampus, cerebral cortex, hypothalamus and cerebellum [83, 84]. However, others reported that the comparison of CB2 with CB1 gene expression in the CNS resulted in relatively insignificant levels of CB2 receptor expression [85, 86].
During the period in which the CB1 and CB2 receptors were discovered, researchers also reported their endogenous ligands and called them endocannabinoids [87-92]. Their precursor is arachidonic acid, and in general – they are lipids. AEA, one of the two main endocannabinoids, does not only act via CB1 receptors; it has additional binding sites, including the vanilloid type 1 receptor [93]. The cannabinoid CB2 receptors are activated by AEA and 2-AG [83, 84, 91, 94].
Given that ECS receptors localization includes brain regions, which are compromised in BPD, this review aims to show that targeting the ECS system may be beneficial for BPD on top of the conventional treatment, which has been shown to be insufficient. There is a growing body of literature showing that the ECS is involved in the regulation of mood, depression and anxiety, known phenotypes of BPD [95-99]. A study [100] extensively revisited the potential role of the ECS in depression, anxiety and mood disorders, as well as our recent review on the potential treatment for mood and anxiety disorders by cannabinoids coupled with terpenes, known as the “Entourage Effect” [101]. This line of research is also supported by the existing large body of knowledge regarding the epidemiological and clinical association between cannabis use and suicidal behavior, a central phenotype of both BPD and depression [102, 103] and other psychiatric disorders [104].
Regarding impaired social interaction, which is of utmost importance for BPD daily functioning, Trezza and Vanderschuren [105] found in animals that administration of AEA transporter inhibitor increased or decreased social interaction in male rats, depending on the selectivity of the inhibitor. Specifically, the more selective inhibitor, N-arachidonoyl-(2-methyl-4-hydroxyphenyl) amine (VDM11), increased social interaction, but this effect was blocked when the CB1 antagonist SR141716A was injected 30 minutes before VDM11. A summary of research from this group and others showed a corticolimbic network modulating social play, in which the nucleus accumbens (NAcC) is a central site for opioid and dopamine modulation of social play, while endocannabinoid mediation of social play relies on basolateral amygdala [106]. Furthermore, the functional interaction between CB1 and mu opioid receptors in the NAcC in the modulation of social play was shown both in rats and in mice, including by studying receptor-knockout mice [107]. This ECS-opioid interaction may be relevant for determining the pain threshold in BPD, especially in cases of non-suicidal self-injury. In a recent pilot study, reduced levels of AEA have been found in the hair of women with BPD showing long term ECS deficiency compared to healthy controls [108].
Another report found that serum levels of AEA were higher in patients with BPD, compared to patients with PTSD and a healthy control group [109]. Overall, the relationship between plasma endocannabinoids and brain function is challenging to interpret [110, 111], but the implications of ECS in BPD are demonstrated in animal studies of BPD phenotypes and in BPD patients.
1.4. The Endocannabinoid System and the Hypothalamus
CB1 receptors are located in the hypothalamus and the pituitary gland, and they regulate all the hypothalamic-peripheral endocrine axes. Regarding the HPA, ECS can regulate HPA axis stress reactivity and therefore, it could play a role in both the pathophysiology and treatment of BPD [97]. In addition, the ECS system regulates panic-like defense responses, which are among BPD patients' characteristics. Accordingly, it has been found, using intra-hypothalamic microinjections, that the endocannabinoid AEA reduced the defensive behavior of rats via the CB1 receptor [112]. Altogether, endocannabinoids are important regulators of the physiological HPA reactivity to stress in pathological conditions, such as anxiety, phobias, depression, and posttraumatic stress disorders [113-115]. Endocannabinoids activate presynaptic CB1 receptors in the hypothalamus [116]. The successive activation of the CB1 receptor signaling pathway is directed to the inhibition of the excitatory neurotransmitter glutamate and its effects on the hypothalamic paraventricular and supraoptic nuclei [116]. Thus, the ECS may contribute to HPA regulation by terminating arousal states, thereby facilitating behavioral and emotional recovery in BPD.
It is well known that CB1 receptors are present at very high levels on inhibitory (GABAergic interneurons) and at to lesser extent on excitatory (glutamatergic) terminals [117], as well as on neurons expressing dopamine D1 receptors, playing a specific role in the repertoire of different emotional behaviors included in social and cognitive activities, which are affected in BPD [118-120]. Thus the unique BPD phenotype could be due to specific involvement of CB1 receptors expressed on different neuronal subpopulations.
Regarding the HPG, a recent review has described the widespread effects of the ECS on specific reproductive aspects such as the production of follicles, oocyte maturation, secretion of reproductive hormones and processing of the fertilized embryo [121]. In a perfusion experiment, the pulsatile secretion of gonadotropin-releasing hormone (GnRH) was totally antagonized by a CB1 receptor agonist (WIN 55,212-2). Thus, the release of GnRH can be inhibited by activating CB1 receptors in the hypothalamus, preventing anterior pituitary stimulation [122]. Furthermore, Scorticati et al. [123] found in male and ovariectomized female rats that the release of GnRH from the hypothalamus was decreased by the central administration of AEA. Regarding gonadotropins, in females, while follicle stimulating hormone (FSH) secretion seems to be unaffected by the administration of exogenous or endogenous cannabinoids [124], several findings suggest that cannabinoids downregulate blood luteinizing hormone (LH) levels [115]. In monkeys, chronic administration (18 d) of THC (tetrahydrocannabinol-Δ9-trans-(-), the main psychomimetic component of the cannabis plant) was shown to block estrogen and LH surges and the successive elevation in progesterone [125]. However, the same animals developed tolerance to the anti-reproductive effect of the drug after a few months of treatment [126]. A general agreement relates the LH-inhibitory action of cannabinoids to a supra-pituitary reactivity, possibly at the hypothalamic synaptic level. This may raise concerns regarding potential side effects on fertility when treating people with BPD with cannabis based medications [127].
Regarding the HPT, pioneer studies showed that marijuana is able to decrease TSH and thyroid hormones in rats [128, 129]. However, the picture is more complex. The activation of CB1 receptors blocked the secretion of TSH and a CB1 antagonist returned this important secretion. Thus, the HPT CB1 receptors are involved in BPD compromised emotional and behavioral functioning, but there is no agreement in the literature on the positive effect of CB1 receptor activation in this population [130]. Specifically, a negative effect on the optimal functioning of the HPT has been demonstrated for THC [131]. Opposite results were reported by others for THC activity on CB1 receptors within the HPT [132].
In sum, all three axes of the hypothalamic function seem to be affected by the activation of the ECS receptors involved in their signaling and regulation. Thus the ECS has the potential to contribute to the return of the optimal hypothalamic regulation, which is impaired in BPD patients, while its manipulation may cause considerable side effects.
We note that endocrinological dysregulation of these three hypothalamic axes has been documented in other psychiatric disorders such as mood disorders and anxiety [94, 133-139]. Thus the ECS may be a more general target for modulating mood, anxiety and arousal beyond age and gender considerations.
1.5. The Endocannabinoid System within the Corticolimbic Structures: A new Option of Treatment
Endocannabinoid tone in the corticolimbic system is a critical regulator of anxiety [140], another very common co-occurring disorder in BPD. Extensive expression of CB1 receptors has been found in limbic regions such as the hippocampus and the medial PFC. Many preclinical studies show that CB1 receptors in the corticolimbic system are implicated in the antidepressant and anxiolytic like phenotypes (e.g., [141, 142]). This may be directly relevant for understanding and treating the BPD population.
The ECS is affected by stress while at the same time, it modulates stress exposure [143]. Early adversity is one of the causes of the fragility of the ECS [143] and one of the developmental risk factors for the occurrence of BPD later in life. As such, the question if the ECS has a modulatory role in BPD is of importance. In addition, the double facets of the ECS, regulating stress reactivity and, at the same time, being affected by stress, may play a significant role in BPD because this disorder is characterized by functional and emotional fluctuations and vulnerability to stressful conditions. The positive impact of a functioning ECS may be apparent in remissions as well as in the reduction in ECS functionality during breakdowns. Currently, the double facets of the ECS are shown in rats exposed to restricted bedding rearing conditions. Specifically, the physiological evidence shows that early life stress attenuates the hormonal (HPA axis) response to stress, while the enhancement of anandamide levels counteracts this effect [144]. Chronic stress has complex effects on cannabinoid CB1 receptor connectivity densities in corticolimbic structures. It has been found that repeated stress produces an increase in CB1 receptor connectivity in the PFC, a reduction in CB1 receptor connectivity in the hippocampus and no effect in the amygdala in adult rats. In adult rats, a 40-day recovery from stress resulted in a return to the baseline level of CB1 receptor connectivity in the PFC and upregulation in the hippocampus, possibly pointing to a rebound effect. Adolescents showed this rebound too. In contrast, adolescents exposed to stress showed a downregulation effect in the CB1 receptor in the PFC in adulthood. These findings show the relevance of age to the timing of exposure to stress, highlighting the critical period of early life stress, a common aspect in BPD anamnesis, and showing its effect on the CB1 receptor in the corticolimbic system [145]. Recently, another contribution to BPD has been found: THC-induced reductions in adaptive decision-making processes are regulated by the reactivity of prefrontal CB1 receptors [133]. Signaling by prefrontal or ventral hippocampal CB1 receptors regulates mesolimbic dopamine pro-hedonic activity. However, both the anxiolytic effects and the anxiogenic like effects of drugs that facilitate endocannabinoid signaling are correlated with increases in AEA levels in the PFC and the hippocampus [146-148] indicating that these regions are involved in the divergent cannabinoid effects [149]. The anxiolytic-like effects [150] attributed mainly to cannabidiol (CBD), a major cannabinoid of the Cannabis sativa plant, are of major relevance for BPD emotional regulation; however, the anxiogenic-like effects, attributed mainly to THC, may raise concern. Thus a ratio of high CBD levels to low THC levels would seem to be potentially anxiolytic and optimal for CBD features.
There is currently a lack of valid animal models that translate the key features of BPD [151]. However, impairment in social behavior may be a functional outcome both in BPD and in other psychiatric disorders (such as anxiety and schizophrenia). Given that CBD has been shown to improve social withdrawal in animal models of schizophrenia [152-153] and anxiety [85], the social aspects of BPD may be positively affected by CBD.
The potential therapeutic effects of CBD for psychiatric and other medical disorders are being extensively studied because it produces desirable effects without the psychotomimetic and anxiogenic effects attributed to another derivate of the plant, THC, even in cases of co-administration of CBD and THC [154]. Neuro-imaging studies indicate that CBD is active in brain regions that express a high density of CB receptors, including, hippocampus, amygdala and PFC, which are dysfunctional in BPD patients [95, 154]. It has been recently reported that the yet unknown doses of CBD optimal for psychiatric treatment severely limit its indication, although safety has been validated. Moreover, small samples in the few published human studies on the treatment with CBD in psychiatric disorders do not yet warrant a general indication [85]. To the best of our knowledge, there is no published RCT on CBD treatment of BPD, and no established animal model for BPD. Given the potential positive effects of CBD, such an RCT on a large sample is warranted.
Conclusion
The Endocannabinoid System in Borderline Personality Disorder
The ECS system’s receptors are present in the compromised brain regions in BPD within the corticolimbic system and the hypothalamus. The conventional psychiatric treatment fails to show long-lasting improvement for BPD patients. Targeting the ECS system with novel pharmacological treatments, especially focusing on the CB1 receptor, may help the BPD population, which is in great risk and without a current tool to reduce this population’s symptoms. According to the U.S. National Library of Medicine (https://clinicaltrials.gov), there is no clinical trial investigating the usefulness of targeting the ECS system in BPD (e.g., through CB1, CB2 or cannabidiol), even though there are several clinical trials currently investigating the usefulness of cannabidiol in psychiatric disorders that have some similar symptoms to BPD-such as anxiety and emotional instability.
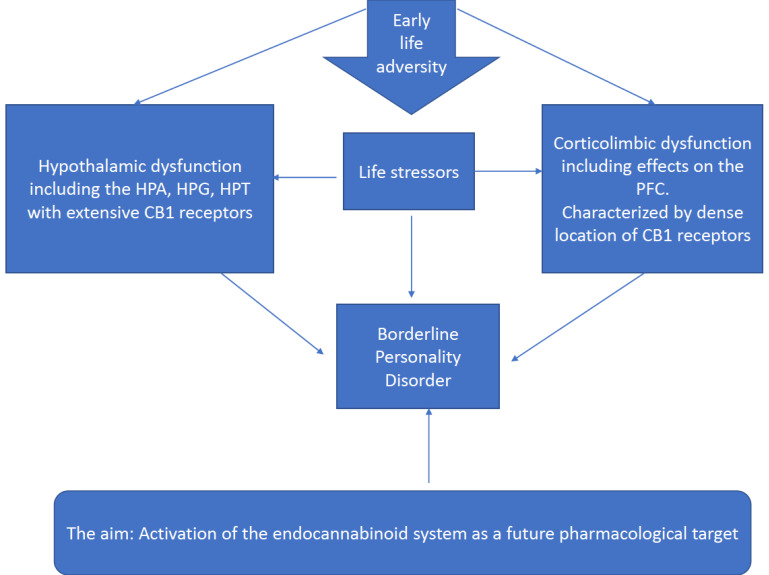
Moreover, imaging studies are needed to shed light on the distribution and activity of the CB2 receptor. This receptor holds promise to BPD, but unfortunately has been targeted less in basic studies and clinical trials. In sum, the most relevant regions for targeting the suggested new treatment with CBD in BPD patients from this review (Fig. 1), are the corticolimbic network and the organ of maintaining homeostasis, the hypothalamus and its axes which are compromised in BPD.
Fig. (1).
Adverse effects of early stress exposure are a risk factor for the development of Borderline Personality Disorder. This depends on alterations in the hypothalamus and the corticolimbic system. The Endocannabinoid system may function as a modulator through receptor crosstalk in these brain regions.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Coccaro E.F., Fanning J.R., Phan K.L., Lee R. Serotonin and impulsive aggression. CNS Spectr. 2015;20(3):295–302. doi: 10.1017/S1092852915000310. [DOI] [PubMed] [Google Scholar]
- 2.Krause-Utz A., Elzinga B.M., Oei N.Y.L., Paret C., Niedtfeld I., Spinhoven P., Bohus M., Schmahl C. Amygdala and dorsal anterior cingulate connectivity during an emotional working memory task in borderline personality disorder patients with interpersonal trauma history. Front. Hum. Neurosci. 2014;8(October):848. doi: 10.3389/fnhum.2014.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krause-Utz A., Frost R., Winter D., Elzinga B.M. Dissociation and alterations in brain function and structure: implications for borderline personality disorder. Curr. Psychiatry Rep. 2017;19(1):6. doi: 10.1007/s11920-017-0757-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leichsenring F., Leibing E., Kruse J., New A., Lancet F.L-T. 2011, U. Borderline personality disorder. Lancet. 2011;377 doi: 10.1016/S0140-6736(10)61422-5. [DOI] [PubMed] [Google Scholar]
- 5.Marissen M.A.E., Arnold N., Franken I.H.A. Anhedonia in borderline personality disorder and its relation to symptoms of impulsivity. Psychopathology. 2012;45(3):179–184. doi: 10.1159/000330893. [DOI] [PubMed] [Google Scholar]
- 6.Skodol A.E., Gunderson J.G., Pfohl B., Widiger T.A., Livesley W.J., Siever L.J. The borderline diagnosis i: psychopathology, comorbidity, and personaltity structure. Biol. Psychiatry. 2002;51(12):936–950. doi: 10.1016/S0006-3223(02)01324-0. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman D.J., Choi-Kain L.W. The hypothalamic-pituitary-adrenal axis in borderline personality disorder: a review. Harv. Rev. Psychiatry. 2009;17(3):167–183. doi: 10.1080/10673220902996734. [DOI] [PubMed] [Google Scholar]
- 8.Cirasola A., Hillman S., Fonagy P., Chiesa M. Mapping the road from childhood adversity to personality disorder: The role of unresolved states of mind. Pers. Ment. Health. 2017;11(2):77–90. doi: 10.1002/pmh.1365. [DOI] [PubMed] [Google Scholar]
- 9.Infurna M.R., Brunner R., Holz B., Parzer P., Giannone F., Reichl C., Fischer G., Resch F., Kaess M. The specific role of childhood abuse, parental bonding, and family functioning in female adolescents with borderline personality disorder. J. Pers. Disord. 2016;30(2):177–192. doi: 10.1521/pedi_2015_29_186. [DOI] [PubMed] [Google Scholar]
- 10.Martín-Blanco A., Ferrer M., Soler J., Salazar J., Vega D., Andión O., Sanchez-Mora C., Arranz M.J., Ribases M., Feliu-Soler A., Pérez V., Pascual J.C. Association between methylation of the glucocorticoid receptor gene, childhood maltreatment, and clinical severity in borderline personality disorder. J. Psychiatr. Res. 2014;57:34–40. doi: 10.1016/j.jpsychires.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Winsper C., Wolke D., Lereya T. Prospective associations between prenatal adversities and borderline personality disorder at 11-12 years. Psychol. Med. 2015;45(5):1025–1037. doi: 10.1017/S0033291714002128. [DOI] [PubMed] [Google Scholar]
- 12.Duque-Alarcón X., Alcalá-Lozano R., González-Olvera J.J., Garza-Villarreal E.A., Pellicer F. Effects of Childhood maltreatment on social cognition and brain functional connectivity in borderline personality disorder patients. Front. Psychiatry. 2019;10:156. doi: 10.3389/fpsyt.2019.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turniansky H., Ben-Dor D., Krivoy A., Weizman A., Shoval G. A history of prolonged childhood sexual abuse is associated with more severe clinical presentation of borderline personality disorder in adolescent female inpatients - A naturalistic study. Child Abuse Negl. 2019;98:104222. doi: 10.1016/j.chiabu.2019.104222. [DOI] [PubMed] [Google Scholar]
- 14.Torgersen S., Kringlen E., Cramer V. The prevalence of personality disorders in a community sample. Arch. Gen. Psychiatry. 2001;58(6):590–596. doi: 10.1001/archpsyc.58.6.590. [DOI] [PubMed] [Google Scholar]
- 15.Ten Have M., Verheul R., Kaasenbrood A., van Dorsselaer S., Tuithof M., Kleinjan M., de Graaf R. Prevalence rates of borderline personality disorder symptoms: a study based on the Netherlands Mental Health Survey and Incidence Study-2. BMC Psychiatry. 2016;16(1):249. doi: 10.1186/s12888-016-0939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant B.F., Chou S.P., Goldstein R.B., Huang B., Stinson F.S., Saha T.D., Smith S.M., Dawson D.A., Pulay A.J., Pickering R.P., Ruan W.J. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National epidemiologic survey on alcohol and related conditions. J. Clin. Psychiatry. 2008;69(4):533–545. doi: 10.4088/JCP.v69n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson D.M., Shea M.T., Yen S., Battle C.L., Zlotnick C., Sanislow C.A., Grilo C.M., Skodol A.E., Bender D.S., McGlashan T.H., Gunderson J.G., Zanarini M.C. Gender differences in borderline personality disorder: findings from the collaborative longitudinal personality disorders study. Compr. Psychiatry. 2003;44(4):284–292. doi: 10.1016/S0010-440X(03)00090-7. [DOI] [PubMed] [Google Scholar]
- 18.Stoffers J., Völlm B.A., Rücker G., Timmer A., Huband N., Lieb K. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst. Rev. 2010;(6):CD005653. doi: 10.1002/14651858.cd005653.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rinne T., de Kloet E.R., Wouters L., Goekoop J.G., DeRijk R.H., van den Brink W. Hyperresponsiveness of hypothalamic-pituitary-adrenal axis to combined dexamethasone/corticotropin-releasing hormone challenge in female borderline personality disorder subjects with a history of sustained childhood abuse. Biol. Psychiatry. 2002;52(11):1102–1112. doi: 10.1016/S0006-3223(02)01395-1. [DOI] [PubMed] [Google Scholar]
- 20.Saunders E.F.H., Silk K.R. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J. Clin. Psychopharmacol. 2009;29(5):461–467. doi: 10.1097/JCP.0b013e3181b2b9f3. [DOI] [PubMed] [Google Scholar]
- 21.Bridler R., Häberle A., Müller S.T., Cattapan K., Grohmann R., Toto S., Kasper S., Greil W. Psychopharmacological treatment of 2195 in-patients with borderline personality disorder: A comparison with other psychiatric disorders. Eur. Neuropsychopharmacol. 2015;25(6):763–772. doi: 10.1016/j.euroneuro.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Castañeda C.D.C., Langlois V.S., Fernandino J.I. Crossover of the hypothalamic pituitary-adrenal/interrenal, -thyroid, and -gonadal axes in testicular development. Front. Endocrinol. (Lausanne) 2014;5:139. doi: 10.3389/fendo.2014.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flood D.E.K., Fernandino J.I., Langlois V.S. Thyroid hormones in male reproductive development: evidence for direct crosstalk between the androgen and thyroid hormone axes. Gen. Comp. Endocrinol. 2013;192:2–14. doi: 10.1016/j.ygcen.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 24.Brüggemann M., Licht O., Fetter É., Teigeler M., Schäfers C., Eilebrecht E. http://dx.doi.org/10.1002/ 2018 doi: 10.1002/etc.3995. [DOI] [PubMed]
- 25.Cotrufo P., Monteleone P., d’Istria M., Fuschino A., Serino I., Maj M. Aggressive behavioral characteristics and endogenous hormones in women with Bulimia nervosa. Neuropsychobiology. 2000;42(2):58–61. doi: 10.1159/000026673. [DOI] [PubMed] [Google Scholar]
- 26.Rausch J., Gäbel A., Nagy K., Kleindienst N., Herpertz S.C., Bertsch K. Increased testosterone levels and cortisol awakening responses in patients with borderline personality disorder: gender and trait aggressiveness matter. Psychoneuroendocrinology. 2015;55:116–127. doi: 10.1016/j.psyneuen.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Dettenborn L., Kirschbaum C., Gao W., Spitzer C., Roepke S., Otte C., Wingenfeld K. Increased hair testosterone but unaltered hair cortisol in female patients with borderline personality disorder. Psychoneuroendocrinology. 2016;71:176–179. doi: 10.1016/j.psyneuen.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Eisenlohr-Moul T.A., DeWall C.N., Girdler S.S., Segerstrom S.C. Ovarian hormones and borderline personality disorder features: Preliminary evidence for interactive effects of estradiol and progesterone. Biol. Psychol. 2015;109:37–52. doi: 10.1016/j.biopsycho.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan R.Y., Grigg J., Kulkarni J. Borderline personality disorder and polycystic ovary syndrome: A review of the literature. Aust. N. Z. J. Psychiatry. 2018;52(2):117–128. doi: 10.1177/0004867417730650. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein-Ferber S., Granot M. The association between somatization and perceived ability: roles in dysmenorrhea among Israeli Arab adolescents. Psychosom. Med. 2006;68(1):136–142. doi: 10.1097/01.psy.0000197644.95292.00. [DOI] [PubMed] [Google Scholar]
- 31.Kavoussi R.J., Coccaro E.F., Klar H., Lesser J., Siever L.J. The TRH-stimulation test in DSM-III personality disorder. Biol. Psychiatry. 1993;34(4):234–239. doi: 10.1016/0006-3223(93)90077-Q. [DOI] [PubMed] [Google Scholar]
- 32.De la Fuente J.M., Mendlewicz J. TRH stimulation and dexamethasone suppression in borderline personality disorder. Biol. Psychiatry. 1996;40(5):412–418. doi: 10.1016/0006-3223(95)00394-0. [DOI] [PubMed] [Google Scholar]
- 33.De la Fuente J.M., Bobes J., Vizuete C., Mendlewicz J. Biological nature of depressive symptoms in borderline personality disorder: endocrine comparison to recurrent brief and major depression. J. Psychiatr. Res. 2002;36(3):137–145. doi: 10.1016/S0022-3956(01)00056-5. [DOI] [PubMed] [Google Scholar]
- 34.Kioukia-Fougia N., Antoniou K., Bekris S., Liapi C., Christofidis I., Papadopoulou-Daifoti Z. The effects of stress exposure on the hypothalamic-pituitary-adrenal axis, thymus, thyroid hormones and glucose levels. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26(5):823–830. doi: 10.1016/S0278-5846(01)00297-4. [DOI] [PubMed] [Google Scholar]
- 35.Cattane N., Rossi R., Lanfredi M., Cattaneo A. Borderline personality disorder and childhood trauma: exploring the affected biological systems and mechanisms. BMC Psychiatry. 2017;17(1):221. doi: 10.1186/s12888-017-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinai C., Hirvikoski T., Nordström A.L., Nordström P., Nilsonne A., Wilczek A., Åsberg M., Jokinen J. Hypothalamic pituitary thyroid axis and exposure to interpersonal violence in childhood among women with borderline personality disorder. Eur. J. Psychotraumatol. 2014;5(Suppl.) doi: 10.3402/ejpt.v5.23911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinai C., Hirvikoski T., Nordström A.L., Nordström P., Nilsonne Å., Wilczek A., Åsberg M., Jokinen J. Thyroid hormones and adult interpersonal violence among women with borderline personality disorder. Psychiatry Res. 2015;227(2-3):253–257. doi: 10.1016/j.psychres.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 38.Raymond C., Marin M.F., Majeur D., Lupien S. Early child adversity and psychopathology in adulthood: HPA axis and cognitive dysregulations as potential mechanisms. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;85:152–160. doi: 10.1016/j.pnpbp.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 40.Ball J.S., Links P.S. Borderline personality disorder and childhood trauma: evidence for a causal relationship. Curr. Psychiatry Rep. 2009;11(1):63–68. doi: 10.1007/s11920-009-0010-4. [DOI] [PubMed] [Google Scholar]
- 41.Drews E., Fertuck E.A., Koenig J., Kaess M., Arntz A. Hypothalamic-pituitary-adrenal axis functioning in borderline personality disorder: A meta-analysis. Neurosci. Biobehav. Rev. 2019;96:316–334. doi: 10.1016/j.neubiorev.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Wingenfeld K., Wolf O.T. Effects of cortisol on cognition in major depressive disorder, posttraumatic stress disorder and borderline personality disorder - 2014 Curt Richter Award Winner. Psychoneuroendocrinology. 2015;51:282–295. doi: 10.1016/j.psyneuen.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Siever L.J., Davis K.L. A psychobiological perspective on the personality disorders. Am. J. Psychiatry. 1991;148(12):1647–1658. doi: 10.1176/ajp.148.12.1647. [DOI] [PubMed] [Google Scholar]
- 44.Paris J., Zweig-Frank H., Kin N.M., Schwartz G., Steiger H., Nair N.P.V. Neurobiological correlates of diagnosis and underlying traits in patients with borderline personality disorder compared with normal controls. Psychiatry Res. 2004;121(3):239–252. doi: 10.1016/S0165-1781(03)00237-3. [DOI] [PubMed] [Google Scholar]
- 45.Aleknaviciute J., Tulen J.H.M., Kamperman A.M., de Rijke Y.B., Kooiman C.G., Kushner S.A. Borderline and cluster C personality disorders manifest distinct physiological responses to psychosocial stress. Psychoneuroendocrinology. 2016;72:131–138. doi: 10.1016/j.psyneuen.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Kaess M., Whittle S., Simmons J.G., Jovev M., Allen N.B., Chanen A.M. The interaction of childhood maltreatment, sex, and borderline personality features in the prediction of the cortisol awakening response in adolescents. Psychopathology. 2017;50(3):188–194. doi: 10.1159/000456549. [DOI] [PubMed] [Google Scholar]
- 47.Nater U.M., Bohus M., Abbruzzese E., Ditzen B., Gaab J., Kleindienst N., Ebner-Priemer U., Mauchnik J., Ehlert U. Increased psychological and attenuated cortisol and alpha-amylase responses to acute psychosocial stress in female patients with borderline personality disorder. Psychoneuroendocrinology. 2010;35(10):1565–1572. doi: 10.1016/j.psyneuen.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Haller J. The neurobiology of abnormal manifestations of aggression--a review of hypothalamic mechanisms in cats, rodents, and humans. Brain Res. Bull. 2013;93:97–109. doi: 10.1016/j.brainresbull.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Conklin C.Z., Bradley R., Westen D. Affect regulation in borderline personality disorder. J. Nerv. Ment. Dis. 2006;194(2):69–77. doi: 10.1097/01.nmd.0000198138.41709.4f. [DOI] [PubMed] [Google Scholar]
- 50.Gratz K.L., Rosenthal M.Z., Tull M.T., Lejuez C.W., Gunderson J.G. An experimental investigation of emotion dysregulation in borderline personality disorder. J. Abnorm. Psychol. 2006;115(4):850–855. doi: 10.1037/0021-843X.115.4.850. [DOI] [PubMed] [Google Scholar]
- 51.Inoue A., Oshita H., Maruyama Y., Tanaka Y., Ishitobi Y., Kawano A., Ikeda R., Ando T., Aizawa S., Masuda K., Higuma H., Kanehisa M., Ninomiya T., Akiyoshi J. Gender determines cortisol and alpha-amylase responses to acute physical and psychosocial stress in patients with borderline personality disorder. Psychiatry Res. 2015;228(1):46–52. doi: 10.1016/j.psychres.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Thomas N., Gurvich C., Hudaib A.R., Gavrilidis E., Kulkarni J. Systematic review and meta-analysis of basal cortisol levels in Borderline Personality Disorder compared to non-psychiatric controls. Psychoneuroendocrinology. 2019;102:149–157. doi: 10.1016/j.psyneuen.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Coccaro E.F., Sripada C.S., Yanowitch R.N., Luan Phan K. Corticolimbic function in impulsive aggressive behavior. Psiquiatr. Biol. 2012;19(2):46–53. doi: 10.1016/j.psiq.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Paret C., Kluetsch R., Zaehringer J., Ruf M., Demirakca T., Bohus M., Ende G., Schmahl C. Alterations of amygdala-prefrontal connectivity with real-time fMRI neurofeedback in BPD patients. Soc. Cogn. Affect. Neurosci. 2016;11(6):952–960. doi: 10.1093/scan/nsw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krause-Utz A., Elzinga B. Current understanding of the neural mechanisms of dissociation in borderline Personality Disorder. Curr. Behav. Neurosci. Rep. 2018;5(1):113–123. doi: 10.1007/s40473-018-0146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buchheim A., Erk S., George C., Kächele H., Kircher T., Martius P., Pokorny D., Ruchsow M., Spitzer M., Walter H. Neural correlates of attachment trauma in borderline personality disorder: a functional magnetic resonance imaging study. Psychiatry Res. 2008;163(3):223–235. doi: 10.1016/j.pscychresns.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Buchheim A., Erk S., George C., Kächele H., Martius P., Pokorny D., Spitzer M., Walter H. Neural Response during the activation of the attachment system in patients with borderline personality disorder: An fMRI study. Front. Hum. Neurosci. 2016;10:389. doi: 10.3389/fnhum.2016.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Minzenberg M.J., Fan J., New A.S., Tang C.Y., Siever L.J. Frontolimbic structural changes in borderline personality disorder. J. Psychiatr. Res. 2008;42(9):727–733. doi: 10.1016/j.jpsychires.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herpertz S.C., Dietrich T.M., Wenning B., Krings T., Erberich S.G., Willmes K., Thron A., Sass H. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol. Psychiatry. 2001;50(4):292–298. doi: 10.1016/S0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- 60.Koenigsberg H.W., Denny B.T., Fan J., Liu X., Guerreri S., Mayson S.J., Rimsky L., New A.S., Goodman M., Siever L.J. The neural correlates of anomalous habituation to negative emotional pictures in borderline and avoidant personality disorder patients. Am. J. Psychiatry. 2014;171(1):82–90. doi: 10.1176/appi.ajp.2013.13070852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koenigsberg H.W., Siever L.J., Lee H., Pizzarello S., New A.S., Goodman M., Cheng H., Flory J., Prohovnik I. Neural correlates of emotion processing in borderline personality disorder. Psychiatry Res. 2009;172(3):192–199. doi: 10.1016/j.pscychresns.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buchheim A., Roth G., Schiepek G., Pogarell O., Karch S. 2013 https://psycnet.apa.org/doi/10.4414/
- 63.Krause-Utz A., Winter D., Niedtfeld I., Schmahl C. The latest neuroimaging findings in borderline personality disorder. Curr. Psychiatry Rep. 2014;16(3):438. doi: 10.1007/s11920-014-0438-z. [DOI] [PubMed] [Google Scholar]
- 64.McLott J., Jurecic J., Hemphill L., Dunn K.S. Development of an amygdalocentric neurocircuitry-reactive aggression theoretical model of emergence delirium in posttraumatic stress disorder: an integrative literature review. AANA J. 2013;81(5):379–384. https://search.proquest.com/docview/1503497698?accountid=14483 [PubMed] [Google Scholar]
- 65.Raine A. The neuromoral theory of antisocial, violent, and psychopathic behavior. Psychiatry Res. 2019;277:64–69. doi: 10.1016/j.psychres.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 66.Walker S.E., Papilloud A., Huzard D., Sandi C. The link between aberrant hypothalamic-pituitary-adrenal axis activity during development and the emergence of aggression-Animal studies. Neurosci. Biobehav. Rev. 2018;91:138–152. doi: 10.1016/j.neubiorev.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 67.Bertsch K., Roelofs K., Roch P.J., Ma B., Hensel S., Herpertz S.C., Volman I. Neural correlates of emotional action control in anger-prone women with borderline personality disorder. J. Psychiatry Neurosci. 2018;43(3):161–170. doi: 10.1503/jpn.170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kimmel C.L., Alhassoon O.M., Wollman S.C., Stern M.J., Perez-Figueroa A., Hall M.G., Rompogren J., Radua J. Age-related parieto-occipital and other gray matter changes in borderline personality disorder: A meta-analysis of cortical and subcortical structures. Psychiatry Res. Neuroimaging. 2016;251:15–25. doi: 10.1016/j.pscychresns.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Rodrigues E., Wenzel A., Ribeiro M.P., Quarantini L.C., Miranda-Scippa A., de Sena E.P., de Oliveira I.R. Hippocampal volume in borderline personality disorder with and without comorbid posttraumatic stress disorder: a meta-analysis. Eur. Psychiatry. 2011;26(7):452–456. doi: 10.1016/j.eurpsy.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 70.Donegan N.H., Sanislow C.A., Blumberg H.P., Fulbright R.K., Lacadie C., Skudlarski P., Gore J.C., Olson I.R., McGlashan T.H., Wexler B.E. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol. Psychiatry. 2003;54(11):1284–1293. doi: 10.1016/S0006-3223(03)00636-X. [DOI] [PubMed] [Google Scholar]
- 71.Kuhlmann A., Bertsch K., Schmidinger I., Thomann P.A., Herpertz S.C. Morphometric differences in central stress-regulating structures between women with and without borderline personality disorder. J. Psychiatry Neurosci. 2013;38(2):129–137. doi: 10.1503/jpn.120039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mauchnik J., Schmahl C. The latest neuroimaging findings in borderline personality disorder. Curr. Psychiatry Rep. 2010;12(1):46–55. doi: 10.1007/s11920-009-0089-7. [DOI] [PubMed] [Google Scholar]
- 73.Chye Y., Christensen E., Solowij N., Yücel M. The endocannabinoid system and cannabidiol’s promise for the treatment of substance use disorder. Front. Psychiatry. 2019;10:63. doi: 10.3389/fpsyt.2019.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Svízenská I., Dubový P., Sulcová A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures--a short review. Pharmacol. Biochem. Behav. 2008;90(4):501–511. doi: 10.1016/j.pbb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 75.Piomelli D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003;4(11):873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 76.Wilson R. I., Nicoll R. A. 2002.
- 77.Glass M., Dragunow M., Faull R.L. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77(2):299–318. doi: 10.1016/S0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 78.Herkenham M. Cannabinoid receptor localization in brain: relationship to motor and reward systems. Ann. N. Y. Acad. Sci. 1992;654:19–32. doi: 10.1111/j.1749-6632.1992.tb25953.x. [DOI] [PubMed] [Google Scholar]
- 79.Colino L., Herranz-Herrer J., Gil-Benito E., Ponte-Lopez T., Del Sol-Calderon P., Rodrigo-Yanguas M., Gil-Ligero M., Sánchez-López A.J., de Leon J., Blasco-Fontecilla H. Cannabinoid receptors, mental pain and suicidal behavior: a systematic review. Curr. Psychiatry Rep. 2018;20(3):19. doi: 10.1007/s11920-018-0880-4. [DOI] [PubMed] [Google Scholar]
- 80.Trautmann S.M., Sharkey K.A. The endocannabinoid system and its role in regulating the intrinsic neural circuitry of the gastrointestinal tract. Int. Rev. Neurobiol. 2015;125:85–126. doi: 10.1016/bs.irn.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 81.Wright K., Rooney N., Feeney M., Tate J., Robertson D., Welham M., Ward S. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129(2):437–453. doi: 10.1016/j.gastro.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 82.Wright K.L., Duncan M., Sharkey K.A. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br. J. Pharmacol. 2008;153(2):263–270. doi: 10.1038/sj.bjp.0707486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Sickle M. D., Duncan M., Kingsley P. J., Mouihate A., Urbani P., Mackie K., Stella N., Makriyannis A., Piomelli D., Davison J. S., Marnett L. J., Di Marzo V., Pittman Q. J., Patel K. D., Sharkey K. A. 2005. [DOI] [PubMed]
- 84.Gong J.P., Onaivi E.S., Ishiguro H., Liu Q.R., Tagliaferro P.A., Brusco A., Uhl G.R. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071(1):10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 85.Dos Santos R.G., de Lima Osório F., Martin-Santos R., Zuardi A.W., Hallak J.E.C., Crippa J.A.S. Modulation of the endocannabinoid and oxytocinergic systems as a potential treatment approach for social anxiety disorder. CNS Drugs. 2019;33(10):1031–1038. doi: 10.1007/s40263-019-00669-5. [DOI] [PubMed] [Google Scholar]
- 86.Marco E.M., Echeverry-Alzate V., López-Moreno J.A., Giné E., Peñasco S., Viveros M.P. Consequences of early life stress on the expression of endocannabinoid-related genes in the rat brain. Behav. Pharmacol. 2014;25(5-6):547–556. doi: 10.1097/FBP.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 87.Brusco A., Tagliaferro P.A., Saez T., Onaivi E.S. Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Ann. N. Y. Acad. Sci. 2008;1139:450–457. doi: 10.1196/annals.1432.037. [DOI] [PubMed] [Google Scholar]
- 88.Devane W. A., Hanuš L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. 1992. [DOI] [PubMed]
- 89.Li Y., Kim J. Deletion of CB2 cannabinoid receptors reduces synaptic transmission and long-term potentiation in the mouse hippocampus. Hippocampus. 2016;26(3):275–281. doi: 10.1002/hipo.22558. [DOI] [PubMed] [Google Scholar]
- 90.Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N.E., Schatz A.R., Gopher A., Almog S., Martin B.R., Compton D.R., Pertwee R.G., Griffin G., Bayewitch M., Barg J., Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50(1):83–90. doi: 10.1016/0006-2952(95)00109-D. [DOI] [PubMed] [Google Scholar]
- 91.Scarante F.F., Vila-Verde C., Detoni V.L., Ferreira-Junior N.C., Guimarães F.S., Campos A.C. Cannabinoid modulation of the stressed hippocampus. Front. Mol. Neurosci. 2017;10:411. doi: 10.3389/fnmol.2017.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stempel A.V., Stumpf A., Zhang H.Y., Özdoğan T., Pannasch U., Theis A.K., Otte D.M., Wojtalla A., Rácz I., Ponomarenko A., Xi Z.X., Zimmer A., Schmitz D. Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron. 2016;90(4):795–809. doi: 10.1016/j.neuron.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aguiar D.C., Moreira F.A., Terzian A.L., Fogaça M.V., Lisboa S.F., Wotjak C.T., Guimaraes F.S. Modulation of defensive behavior by transient receptor potential vanilloid type-1 (TRPV1) channels. Neurosci. Biobehav. Rev. 2014;46(Pt 3):418–428. doi: 10.1016/j.neubiorev.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 94.Micale V., Drago F. Endocannabinoid system, stress and HPA axis. Eur. J. Pharmacol. 2018;834:230–239. doi: 10.1016/j.ejphar.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 95.Crippa J.A.S., Derenusson G.N., Ferrari T.B., Wichert-Ana L., Duran F.L.S., Martin-Santos R., Simões M.V., Bhattacharyya S., Fusar-Poli P., Atakan Z., Santos Filho A., Freitas-Ferrari M.C., McGuire P.K., Zuardi A.W., Busatto G.F., Hallak J.E.C. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J. Psychopharmacol. (Oxford) 2011;25(1):121–130. doi: 10.1177/0269881110379283. [DOI] [PubMed] [Google Scholar]
- 96.Hen-Shoval D., Amar S., Shbiro L., Smoum R., Haj C.G., Mechoulam R., Zalsman G., Weller A., Shoval G. Acute oral cannabidiolic acid methyl ester reduces depression-like behavior in two genetic animal models of depression. Behav. Brain Res. 2018;351:1–3. doi: 10.1016/j.bbr.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 97.Hill M.N., Ho W.S.V., Sinopoli K.J., Viau V., Hillard C.J., Gorzalka B.B. Involvement of the endocannabinoid system in the ability of long-term tricyclic antidepressant treatment to suppress stress-induced activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2006;31(12):2591–2599. doi: 10.1038/sj.npp.1301092. [DOI] [PubMed] [Google Scholar]
- 98.Shbiro L., Hen-Shoval D., Hazut N., Rapps K., Dar S., Zalsman G., Mechoulam R., Weller A., Shoval G. Effects of cannabidiol in males and females in two different rat models of depression. Physiol. Behav. 2019;201:59–63. doi: 10.1016/j.physbeh.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 99.Shoval G., Shbiro L., Hershkovitz L., Hazut N., Zalsman G., Mechoulam R., Weller A. Prohedonic effect of cannabidiol in a rat model of depression. Neuropsychobiology. 2016;73(2):123–129. doi: 10.1159/000443890. [DOI] [PubMed] [Google Scholar]
- 100.Micale V., Tabiova K., Kucerova J., Drago F. 2015. Role of the endocannabinoid system in depression: from preclinical to clinical evidence. [Google Scholar]
- 101.Ferber S.G., Namdar D., Hen-Shoval D., Eger G., Koltai H., Shoval G., Shbiro L., Weller A. The “Entourage Effect”: Terpenes coupled with cannabinoids for the treatment of mood disorders and anxiety disorders. Curr. Neuropharmacol. 2020;18(2):87–96. doi: 10.2174/1570159X17666190903103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Borges G., Bagge C.L., Orozco R. A literature review and meta-analyses of cannabis use and suicidality. J. Affect. Disord. 2016;195:63–74. doi: 10.1016/j.jad.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 103.Shalit N., Shoval G., Shlosberg D., Feingold D., Lev-Ran S. The association between cannabis use and suicidality among men and women: A population-based longitudinal study. J. Affect. Disord. 2016;205:216–224. doi: 10.1016/j.jad.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 104.Shoval G., Zalsman G., Apter A., Diller R., Sher L., Weizman A. A 10-year retrospective study of inpatient adolescents with schizophrenia/schizoaffective disorder and substance use. Compr. Psychiatry. 2007;48(1):1–7. doi: 10.1016/j.comppsych.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 105.Trezza V., Vanderschuren L.J.M.J. Divergent effects of anandamide transporter inhibitors with different target selectivity on social play behavior in adolescent rats. J. Pharmacol. Exp. Ther. 2009;328(1):343–350. doi: 10.1124/jpet.108.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vanderschuren L.J.M.J., Achterberg E.J.M., Trezza V. The neurobiology of social play and its rewarding value in rats. Neurosci. Biobehav. Rev. 2016;70:86–105. doi: 10.1016/j.neubiorev.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Manduca A., Lassalle O., Sepers M., Campolongo P., Cuomo V., Marsicano G., Kieffer B., Vanderschuren L.J.M.J., Trezza V., Manzoni O.J.J. Interacting cannabinoid and opioid receptors in the nucleus accumbens core control adolescent social play. Front. Behav. Neurosci. 2016;10(NOV):211. doi: 10.3389/fnbeh.2016.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wingenfeld K., Dettenborn L., Kirschbaum C., Gao W., Otte C., Roepke S. Reduced levels of the endocannabinoid arachidonylethanolamide (AEA) in hair in patients with borderline personality disorder - a pilot study. Stress. 2018;21(4):366–369. doi: 10.1080/10253890.2018.1451837. [DOI] [PubMed] [Google Scholar]
- 109.Schaefer C., Enning F., Mueller J.K., Bumb J.M., Rohleder C., Odorfer T.M., Klosterkötter J., Hellmich M., Koethe D., Schmahl C., Bohus M., Leweke F.M. Fatty acid ethanolamide levels are altered in borderline personality and complex posttraumatic stress disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2014;264(5):459–463. doi: 10.1007/s00406-013-0470-8. [DOI] [PubMed] [Google Scholar]
- 110.Hillard C.J. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology. 2018;43(1):155–172. doi: 10.1038/npp.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kolla N.J., Mishra A. The endocannabinoid system, aggression, and the violence of synthetic cannabinoid use, borderline personality disorder, antisocial personality disorder, and other psychiatric disorders. Front. Behav. Neurosci. 2018;12:41. doi: 10.3389/fnbeh.2018.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dos Anjos-Garcia T., Ullah F., Falconi-Sobrinho L.L., Coimbra N.C. 2017. [DOI] [PubMed]
- 113.Crowe M.S., Nass S.R., Gabella K.M., Kinsey S.G. The endocannabinoid system modulates stress, emotionality, and inflammation. Brain Behav. Immun. 2014;42:1–5. doi: 10.1016/j.bbi.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 114.Marco E.M., Rapino C., Caprioli A., Borsini F., Laviola G., Maccarrone M., Lodola A. Potential therapeutic value of a novel faah inhibitor for the treatment of anxiety. PLoS One. 2015;10(9):e0137034. doi: 10.1371/journal.pone.0137034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pagotto U., Marsicano G., Cota D., Lutz B., Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr. Rev. 2006;27(1):73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- 116.Di S., Malcher-Lopes R., Halmos K.C., Tasker J.G. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J. Neurosci. 2003;23(12):4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marsicano G., Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 1999;11(12):4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- 118.Terzian A.L.B., Micale V., Wotjak C.T. Cannabinoid receptor type 1 receptors on GABAergic vs. glutamatergic neurons differentially gate sex-dependent social interest in mice. Eur. J. Neurosci. 2014;40(1):2293–2298. doi: 10.1111/ejn.12561. [DOI] [PubMed] [Google Scholar]
- 119.Terzian A.L., Drago F., Wotjak C.T., Micale V. The dopamine and cannabinoid interaction in the modulation of emotions and cognition: assessing the role of cannabinoid cb1 receptor in neurons expressing dopamine D1 receptors. Front. Behav. Neurosci. 2011;5:49. doi: 10.3389/fnbeh.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Micale V., Stepan J., Jurik A., Pamplona F.A., Marsch R., Drago F., Eder M., Wotjak C.T. Extinction of avoidance behavior by safety learning depends on endocannabinoid signaling in the hippocampus. J. Psychiatr. Res. 2017;90:46–59. doi: 10.1016/j.jpsychires.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 121.Walker O.S., Holloway A.C., Raha S. The role of the endocannabinoid system in female reproductive tissues. J. Ovarian Res. 2019;12(1):3. doi: 10.1186/s13048-018-0478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gammon C.M., Freeman G.M., Jr, Xie W., Petersen S.L., Wetsel W.C. Regulation of gonadotropin-releasing hormone secretion by cannabinoids. Endocrinology. 2005;146(10):4491–4499. doi: 10.1210/en.2004-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scorticati C., Fernández-Solari J., De Laurentiis A., Mohn C., Prestifilippo J.P., Lasaga M., Seilicovich A., Billi S., Franchi A., McCann S.M., Rettori V. The inhibitory effect of anandamide on luteinizing hormone-releasing hormone secretion is reversed by estrogen. Proc. Natl. Acad. Sci. USA. 2004;101(32):11891–11896. doi: 10.1073/pnas.0404366101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tyrey L. delta-9-Tetrahydrocannabinol suppression of episodic luteinizing hormone secretion in the ovariectomized rat. Endocrinology. 1978;102(6):1808–1814. doi: 10.1210/endo-102-6-1808. [DOI] [PubMed] [Google Scholar]
- 125.Asch R.H., Smith C.G., Siler-Khodr T.M., Pauerstein C.J. Effects of delta 9-tetrahydrocannabinol during the follicular phase of the rhesus monkey (Macaca mulatta). J. Clin. Endocrinol. Metab. 1981;52(1):50–55. doi: 10.1210/jcem-52-1-50. [DOI] [PubMed] [Google Scholar]
- 126.Smith C. G., Almirez R. G., Berenberg J., Asch R. H. 1983.
- 127.Bergamaschi M.M., Queiroz R.H., Zuardi A.W., Crippa J.A. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr. Drug Saf. 2011;6(4):237–249. doi: 10.2174/157488611798280924. [DOI] [PubMed] [Google Scholar]
- 128.Hillard C.J., Farber N.E., Hagen T.C., Bloom A.S. The effects of delta 9-tetrahydrocannabinol on serum thyrotropin levels in the rat. Pharmacol. Biochem. Behav. 1984;20(4):547–550. doi: 10.1016/0091-3057(84)90303-4. [DOI] [PubMed] [Google Scholar]
- 129.Lomax P. The effect of marihuana on pituitary-thyroid activity in the rat. Agents Actions. 1970;1(5):252–257. doi: 10.1007/BF01968699. [DOI] [PubMed] [Google Scholar]
- 130.Liberato Costa Da Veiga M.A., Fonseca Bloise F. Henrique Costa-E-Sousa, R. Lopes Souza, L.; Aparecida, N.; Almeida, S.; Oliveira, K. J.; Cabanelas, Pazos-Moura, C. Acute effects of endocannabinoid anandamide and CB1 receptor antagonist, AM251 in the regulation of thyrotropin secretion. J. Endocrinol. 2008;199:235–242. doi: 10.1677/JOE-08-0380. [DOI] [PubMed] [Google Scholar]
- 131.Brown T.T., Dobs A.S. Endocrine effects of marijuana. J. Clin. Pharmacol. 2002;42(S1):90S–96S. doi: 10.1002/j.1552-4604.2002.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 132.Porcella A., Marchese G., Casu M.A., Rocchitta A., Lai M.L., Gessa G.L., Pani L., Sc N. Evidence for functional CB1 cannabinoid receptor expressed in the rat thyroid. Eur. J. Endocrinol. 2002;147(2):255–261. doi: 10.1530/eje.0.1470255. [DOI] [PubMed] [Google Scholar]
- 133.Brummelte S., Galea L.A. Postpartum depression: Etiology, treatment and consequences for maternal care. Horm. Behav. 2016;77:153–166. doi: 10.1016/j.yhbeh.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 134.Ferrer A., Labad J., Salvat-Pujol N., Monreal J.A., Urretavizcaya M., Crespo J.M. Hypothalamic-pituitary-adrenal axis-related genes and cognition in major mood disorders and schizophrenia: a systematic review. Prog. Neuro-Psychopharmacol. Biol. Psych. 2020;101:109929. doi: 10.1016/j.pnpbp.2020.109929. [DOI] [PubMed] [Google Scholar]
- 135.Fischer S., Ehlert U. Hypothalamic–Pituitary–Thyroid (HPT) axis functioning in anxiety disorders. a systematic review. Depress. Anxiety. 2018;35(1):98–110. doi: 10.1002/da.22692. [DOI] [PubMed] [Google Scholar]
- 136.Fischer S., Ehlert U., Castro R.A. Hormones of the hypothalamic-pituitary-gonadal (hpg) axis in male depressive disorders–a systematic review and meta-analysis. Front. Neuroendocrinol. 2019;•••:100792. doi: 10.1016/j.yfrne.2019.100792. [DOI] [PubMed] [Google Scholar]
- 137.Min W., Liu C., Yang Y., Sun X., Zhang B., Xu L., Sun X. Alterations in hypothalamic–pituitary–adrenal/thyroid (hpa/hpt) axes correlated with the clinical manifestations of depression. Prog. Neuro-Psychopharmacol. Biol. Psych. 2012;39(1):206–211. doi: 10.1016/j.pnpbp.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 138.Seidman S.N. Estosterone deficiency and mood in aging Men: Pathogenic and therapeutic interactions. W. J. Biol. Psych. 2003;4(1):14–20. doi: 10.3109/15622970309167905. [DOI] [PubMed] [Google Scholar]
- 139.Xie X., Liu P., Chen T., Wang Y., Liu X. Influence of the hypothalamus–pituitary–gonadal axis reactivation and corresponding surging sex hormones on the amplitude of low-frequency oscillations in early pubertal girls: a resting state FMRI study. J. Affect. Disord. 2019;256:288–294. doi: 10.1016/j.jad.2019.05.062. [DOI] [PubMed] [Google Scholar]
- 140.Silveira M.M., Arnold J.C., Laviolette S.R., Hillard C.J., Celorrio M., Aymerich M.S., Adams W.K. 2017. [DOI] [PMC free article] [PubMed]
- 141.Bahi A., Al Mansouri S., Al Memari E., Al Ameri M., Nurulain S.M., Ojha S. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol. Behav. 2014;135:119–124. doi: 10.1016/j.physbeh.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 142.Lee T.T.Y., Hill M.N., Lee F.S. Developmental regulation of fear learning and anxiety behavior by endocannabinoids. Genes Brain Behav. 2016;15(1):108–124. doi: 10.1111/gbb.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Goldstein F.S., Trezza V., Weller A. Early life stress and development of the endocannabinoid system: A bidirectional process in programming future coping. 2019. [DOI] [PubMed]
- 144.McLaughlin R.J., Verlezza S., Gray J.M., Hill M.N., Walker C.D. Inhibition of anandamide hydrolysis dampens the neuroendocrine response to stress in neonatal rats subjected to suboptimal rearing conditions. Stress. 2016;19(1):114–124. doi: 10.3109/10253890.2015.1117448. [DOI] [PubMed] [Google Scholar]
- 145.Lee T.T.Y., Hill M.N. Age of stress exposure modulates the immediate and sustained effects of repeated stress on corticolimbic cannabinoid CB1 receptor binding in male rats. Neuroscience. 2013;249:106–114. doi: 10.1016/j.neuroscience.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 146.Draycott B., Loureiro M., Ahmad T., Tan H., Zunder J., Laviolette S.R. Cannabinoid transmission in the prefrontal cortex bi-phasically controls emotional memory formation via functional interactions with the ventral tegmental area. J. Neurosci. 2014;34(39):13096–13109. doi: 10.1523/JNEUROSCI.1297-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tan H., Lauzon N.M., Bishop S.F., Chi N., Bechard M., Laviolette S.R. Cannabinoid transmission in the basolateral amygdala modulates fear memory formation via functional inputs to the prelimbic cortex. J. Neurosci. 2011;31(14):5300–5312. doi: 10.1523/JNEUROSCI.4718-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rubino T., Realini N., Castiglioni C. Guidali, C.; Vigano´1, D.; Marras, E.; Petrosino, S.; Perletti, G.; Maccarrone, M.; Marzo, V. Di; Parolaro, D. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb. Cortex. 2008;18:1292–1301. doi: 10.1093/cercor/bhm161. [DOI] [PubMed] [Google Scholar]
- 149.Lisboa S.F., Borges A.A., Nejo P., Fassini A., Guimarães F.S., Resstel L.B. Cannabinoid CB1 receptors in the dorsal hippocampus and prelimbic medial prefrontal cortex modulate anxiety-like behavior in rats: additional evidence. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2015;59:76–83. doi: 10.1016/j.pnpbp.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 150.Rey A.A., Purrio M., Viveros M.P., Lutz B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37(12):2624–2634. doi: 10.1038/npp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Corniquel M.B., Koenigsberg H.W., Likhtik E. Toward an animal model of borderline personality disorder. Psychopharmacology (Berl.) 2019;236(8):2485–2500. doi: 10.1007/s00213-019-05289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kucerova J., Tabiova K., Drago F., Micale V. Therapeutic potential of cannabinoids in schizophrenia. Recent Patents CNS Drug Discov. 2014;9(1):13–25. doi: 10.2174/1574889809666140307115532. [DOI] [PubMed] [Google Scholar]
- 153.Stark T., Ruda-Kucerova J., Iannotti F.A., D’Addario C., Di Marco R., Pekarik V., Drazanova E., Piscitelli F., Bari M., Babinska Z., Giurdanella G., Di Bartolomeo M., Salomone S., Sulcova A., Maccarrone M., Wotjak C.T., Starcuk Z., Jr, Drago F., Mechoulam R., Di Marzo V., Micale V. Peripubertal cannabidiol treatment rescues behavioral and neurochemical abnormalities in the MAM model of schizophrenia. Neuropharmacology. 2019;146:212–221. doi: 10.1016/j.neuropharm.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 154.Bhattacharyya S., Morrison P.D., Fusar-Poli P., Martin-Santos R., Borgwardt S., Winton-Brown T., Nosarti C., O’ Carroll C.M., Seal M., Allen P., Mehta M.A., Stone J.M., Tunstall N., Giampietro V., Kapur S., Murray R.M., Zuardi A.W., Crippa J.A., Atakan Z., McGuire P.K. Opposite effects of δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35(3):764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]