Abstract
Alzheimer’s disease (AD) is a multifarious and developing neurodegenerative disorder. The treatment of AD is still a challenge and availability of drug therapy on the basis of symptoms is not up to the mark. In the context of existence, which is getting worse for the human brain, it is necessary to take care of all critical measures. The disease is caused due to multidirectional pathology of the body, which demands the multi-target-directed ligand (MTDL) approach. This gives hope for new drugs for AD, summarized here in with the pyrimidine based natural product inspired molecule as a lead. The review is sufficient in providing a list of chemical ingredients of the plant to cure AD and screen them against various potential targets of AD. The synthesis of a highly functionalized scaffold in one step in a single pot without isolating the intermediate is a challenging task. In few examples, we have highlighted the importance of this kind of reaction, generally known as multi-component reaction. Multi-component is a widely accepted technique by the drug discovery people due to its high atom economy. It reduces multi-step process to a one-step process, therefore the compounds library can be made in minimum time and cost. This review has highlighted the importance of multicomponent reactions by giving the example of active scaffolds of pyrimidine/fused pyrimidines. This would bring importance to the fast as well as smart synthesis of bio-relevant molecules.
Keywords: Alkaloid, fused pyrimidine, multicomponent reaction, multi-target therapy, natural compound
1. INTRODUCTION
Alzheimer’s disease (AD) is a silent and dangerous problem in the world, which is worsening day by day. In most of the cases, it is affecting the cognitive zone and leading to dementia [1]. Most of the Alzheimer’s disease cases suffer from the sporadic form of the disease in which aetiology is still unknown. It involves multiple genetic, metabolic and environmental risk factors [2].
The global scenario of the Alzheimer based dementia is anticipated to be high. It is expected to double every year therefore making it the most expensive for the government to deal with in the upcoming decades [3]. A report on the growth of AD has indicated the burden of the disease. It is estimated to be more than 74 million till 2030. The number will likely double by the end of year 2050. Apart from this, the prevalence of the disease is high in East Asia and Africa with around 10 million people as compared to Western Europe with 7.5 million people. This is a quite depressive number for health personnel [4]. Based on a report on the role of genomics in AD, it was revealed that there are nine genetic factors associated with the occurrence and progression of AD [5].
The progression of the disease is one of the main factors of attraction. This disease is comparable to cancer in terms of the number of people affected throughout the world. However, currently, the funding for AD is 1/12 th of the total funding for cancer. Throughout research on AD, only 1% cost has been implemented till now [6]. Research on this disease is a need in today’s age.
1.1. Pathogenesis of AD
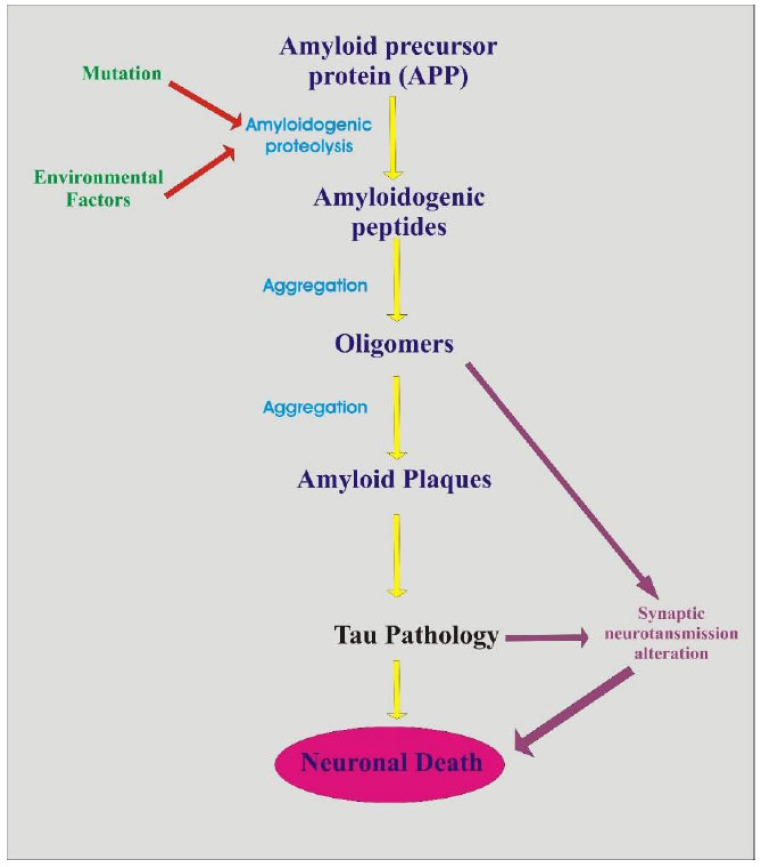
The microscopic observation reveals that the formation of amyloid and senile plague appears in the hippocampus and cerebral cortex during the progression of AD. Moreover, it gives the progressive expression as it crosses the age. AD gradually leads to deformation of Tau protein, resulting in neuronal degeneration, which eventually leads to permanent damage of neuronal cell and turns into dementia (Fig. 1) [7-10].
Fig. (1).
Pathogenesis of AD. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
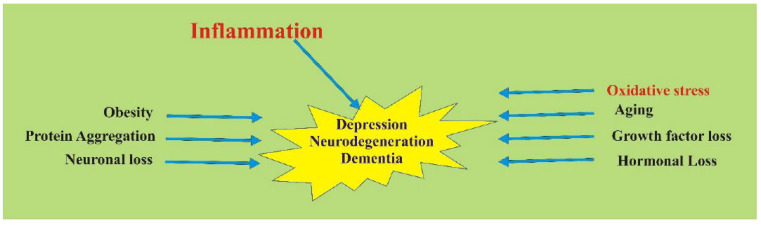
Few research papers have reported the involvement of genetic factors and one of apolipoprotein E in AD. They also described the mechanism of oxidation processes culminating in neurodegeneration [10-12]. The damage of the neuronal cell mainly involves two mechanisms; oxidative damage and inflammation (Fig. 2) [13].
Fig. (2).
Factors responsible for Neurodegeneration. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
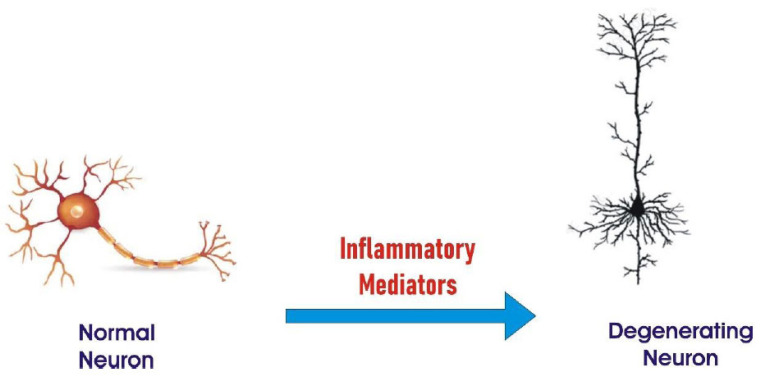
The inflammation response of the body is the result of the sequence of over production of amyloid proteins. The inflammation leads to the destruction of neuronal cells (Fig. 3) [14, 15]. There are reports that shed light on the involvement of inflammation in the degeneration of neurons or the progression of AD [16].
Fig. (3).
Inflammation mediated Neurodegeneration. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
It is known from decades that COX-2 enzymes are involved in the inflammation process. However, recently its role has been explored and a relationship has been established with the pathogenesis of a number of diseases other than pain related to inflammation [17-19] such as schizophrenia [20], depression [21], epilepsy [22], parkinsonism [23, 24], ischemic brain injury [25] and diabetic peripheral neuropathy [26].
Extensive clinical studies reported during the last decade revealed that COX-2 expression has been implicated in a number of pathophysiological conditions related with the brain. It is evident that COX-2 plays a crucial role in the pathogenesis of various degenerative diseases like Alzheimer's disease (AD), Schizophrenia, Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS) [27-29]. It has been found that COX-2 slows down the clearance of amyloid beta (Aβ) peptides, which is responsible for the progressive degeneration of brain neurones [30]. Inflammatory reactions in brain neurons often lead to neurodegeneration and further loss of cognitive abilities. Now a days the term “neuroinflammation” is used to describe any inflammation that is occurring in brain neurons.
Selective COX-2 inhibitors are being investigated for the treatment of these neurological disorders [31]. The approach of targeting COX-2 has become an alternative therapy to treat some of the afore mentioned diseases [32]. Selective targeting of COX-2 to treat some of the diseases other than inflammation mediated pain has opened a new horizon of disease management. This could provide a convincing alternative therapeutic regimen for a variety of diseases. Many new synthesis molecules or pure active constituent isolated from plant sources are widely available and reported in compound list which are not explored for pharmacological activities [33, 34].
1.2. Heterocyclic Scaffolds
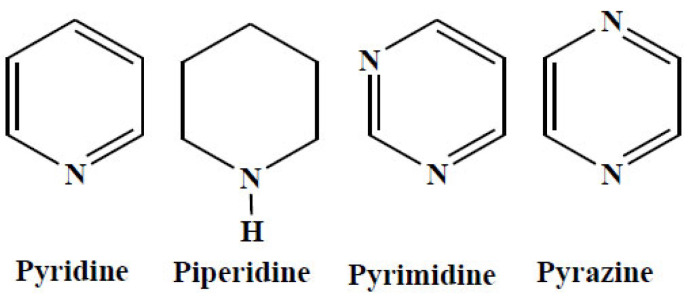
Heterocycles are the most abundant scaffold used in the pharmaceutical field. Among them N-based heterocyclics (Fig. 4) cover more than 90%, so their synthesis has been a special arena to be exploited by drug discovery scientists.
Fig. (4).
N-based heterocyclics. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
1.3. Pyrimidine
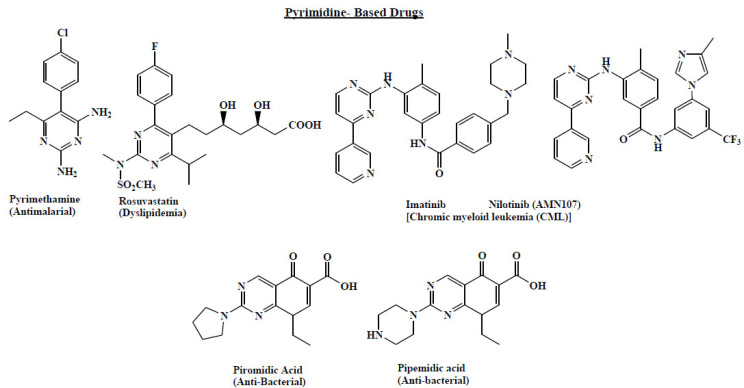
The pyrimidine ring system occupied 15% of the pharmacophores which comprised of N-heterocycles. Although it comes next to pyridine ring system in terms of importance, among the fused ring system pyrimidine ring predominates [35]. Since pyrimidine is a key component of nucleotide bases, an integral part of genetic material (DNA), therefore, the pyrimidine ring has been the centre of attraction for synthetic chemists [36]. Apart from this pyrimdine moiety is an integral part for many drugs/lead such as anti-malarial [37], HMG- CoA reductase inhibitors [38], antibacterial [39], etc. The relevance of the pyrimidine scaffold is further demonstrated by the versatile biological activities exhibited by various substituted pyrimidines. These include pyrimidinylpipe rdinyloxypyridones as GPR119 modulators [40], indolylurea-pyrimidine conjugate as PKCα inhibitors [41], functionalised pyrimidines as antimicotic agents [42] and phenylaminopyrimidines as ABL-kinase inhibitors [43]. Pyrimidine containing marketed drugs like pyrimethamine, rosuvastatin, pipemidic acid etc and recently Imitinib [44] and Nilotinib [45], a tyrosine kinases inhibitors were licensed for treatment of patients with chronic myeloid leukaemia by the U.S. Food and Drug Administration (FDA) (Fig. 5).
Fig. (5).
Pyrimidine based Drugs. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
1.4. Pyrimidines for AD
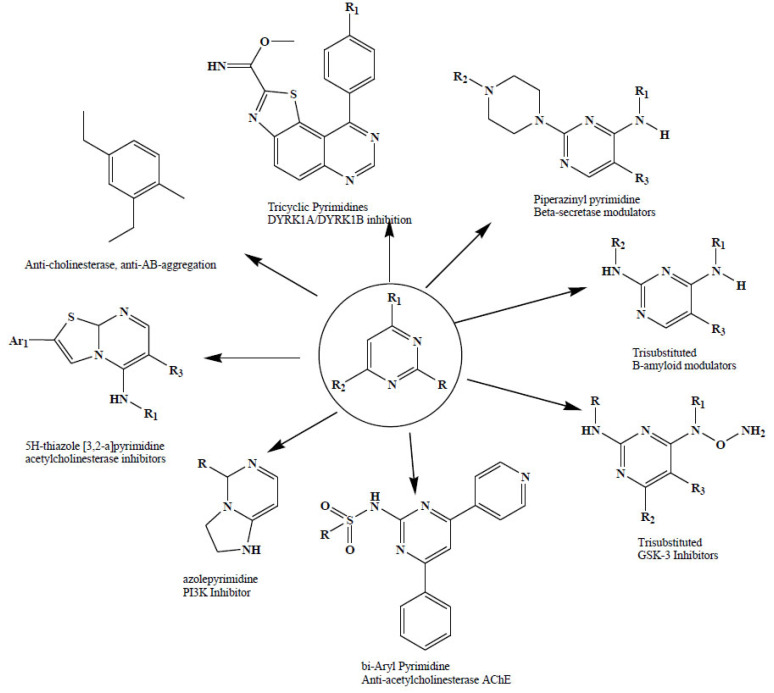
The importance of pyrimidine nucleus in the AD management has been proven and reported by several scientists. The most important among them are the trisubstituted pyrimidine moiety which was functionalized through various electron-withdrawing (Nitro, Cyano etc) and electron-donating groups (Methoxy, Methyl etc) as multi-targeted Alzheimer’s disease (AD) therapeutics [46-50].
The substituted pyrimidine derivatives were generally found with the multi target approaches like anti-cholinesterase (AChE and BuChE), anti-Aβ-aggregation (AChE- and self-induced) and anti-β-secretase (BACE-1) inhibitory activity, in an effort to identify lead, multifunctional candidates as part of our multi-targeted approach to treat AD [51].
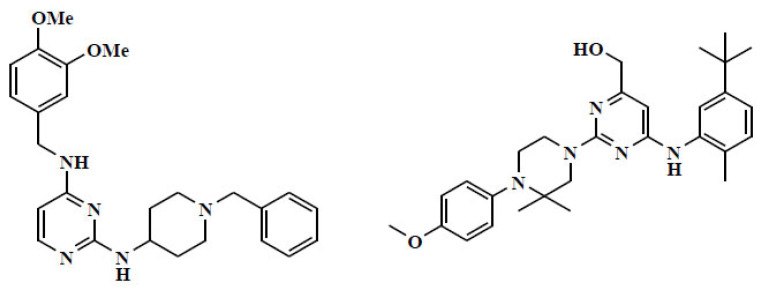
A series of piperazinyl pyrimidines as γ-secretase modulators are of potential use in the treatment of Alzheimer’s disease. This novel class of piperazinyl pyrimidines were discovered with the potent and selective inhibition of Fb42 over Ab40 production with 180-fold selectivity over inhibition of Notch cleavage. The structure-activity relationship of the derivatives was found to be significantly improved in Fb42/Ab40 selectivity via introduction of the gem-dimethyl group. Hydroxymethyl substituted derivative was the most potent which may be due to the gain of a hydrogen bond interaction (Fig. 6) [52].
Fig. (6).
Pyrimidine based Drugs. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Scientists from another lab revealed the activity of fused azole-pyrimidine for phosphotidylinositol-3-kinase (PI3K) inhibition, especially for PI3K-γ inhibition and can be used for the prophylaxis and treatment of diseases associated with PI3K, particularly with PI3K-γ activity. These derivatives were found effective in the treatment of inflammation and immuno regulatory disorders, such as asthma, atopic dermatitis, rhinitis, allergic diseases, chronic obstructive pulmonary disease (COPD), septic shock, joint diseases and autoimmune pathologies such as rheumatoid arthritis, grave’s disease, cancer, myocardial contractility disorders, heart failure, thromboembolism, ischemia, and atherosclerosis. Moreover, they showed to be useful for pulmonary hypertension, renal failure, cardiac hypertrophy, as well as neurodegenerative disorders such as PD and AD, diabetes and focal ischemia, since the diseases also relate to PI3K activity in a human or animal subject [45].
1.5. Synthetic Approach
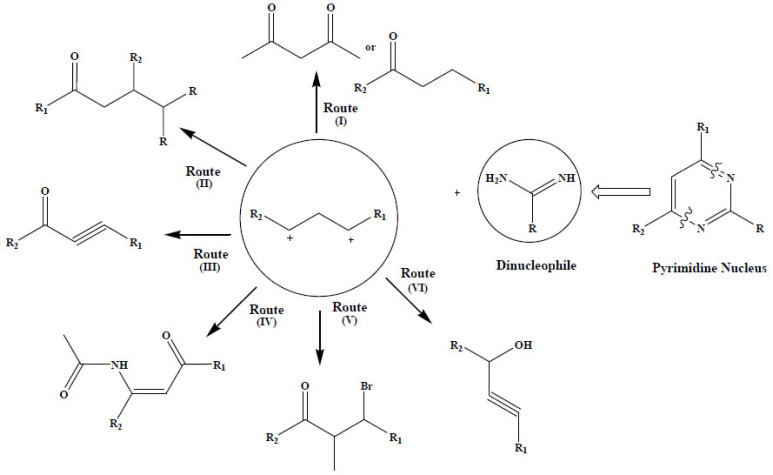
The importance of the pyrimidine nucleolus is to gain popularity to get the optimized structure for the concern disease. Moreover, many strategies were reported for the laboratory synthesis of substituted pyrimidine derivatives as depicted in (Fig. 7). Synthesis of pyrimidine in an economical way was a challenging task for organic chemists. At this point, the natural source derived molecules and their synthesis come into the picture.
Fig. (7).
Synthesis of substituted pyrimidine derivatives. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The reported procedures for the construction of the pyrimidine scaffold may be classified under two main categories; cyclo-condensation of di-electrophile with di-neucleophile (Two nucleophilic centre), and cyclopropane ring opening by di-nucleophilic system. Cyclo-condensation reaction generally involves condensation of di-neucleophile like amidine derivative with synthetic equivalent of 1,3 di- caronyl compound like alpha-beta unsaturated carbonyl [53] (Route I), enaminone [46, 54] (Route II), Ethynyl ketones [55-58] (Route III), Enammide [59] (Route IV), β- bromovinylaldehyde [60] (Route V), propargylic alcohol [61] (Route VI) (Fig. 7).
The challenge for the synthesis of naturally fused compound with the other nucleolus is still a mystery. It has been tried by various groups for targeting various diseases. The focus here is to emphasize on the natural product inspired compound for AD. It is described in a systematic manner.
1.6. Synthetic Approach for the Bioactive Pyrimidine
Pyrimidine ring based anti-cancer drug methotrexate (MTX) was used as a standard for the new series of pyrimidine derivatives for AD and anti-cancer activity [62].
The construction of highly functionalized bio-relevant heterocyclic scaffolds in convenient and cost-effective way has been a challenge for drug discovery scientists from the industry and academia. Various methods have been adopted for the synthesis of highly functionalized heterocycles. However, all the processes need longer steps to get highly functionalized heterocycles, which is costlier. This will effect the drug discovery process, because it requires a library of compounds for the HTS (high-throughput) screening to get the optimum activity scaffold out of a thousands of molecules [55-61].
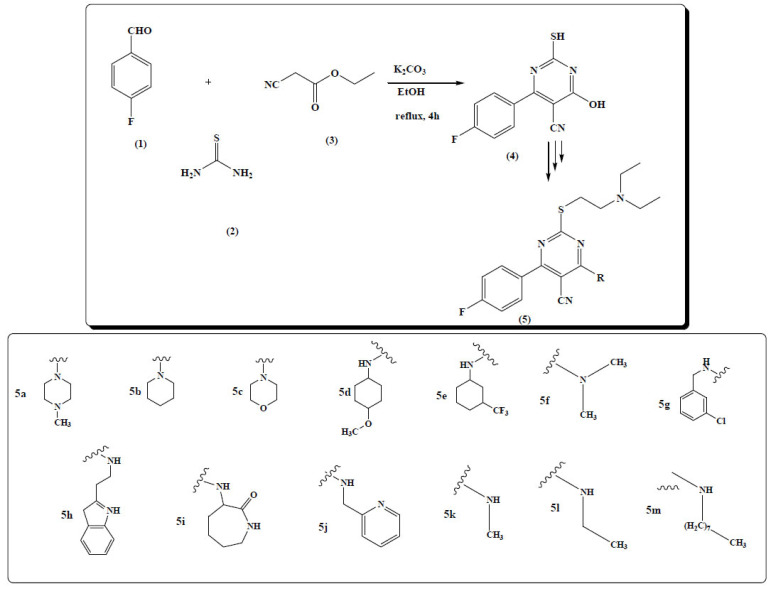
Senthil kumar et al. synthesized a pyrimidine ring. The substrate for the reaction was 4-flourobenzaldehyde (1), thiourea (2) and ethylcyanoacetate (3) in methanol with K2CO3 base. The pyrimidine scaffold (4) was further functionalised through three steps to get the final pyrimidine derivatives (5) (Fig. 8) [62].
Fig. (8).
Pyrimidine derivatives (5a-m). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
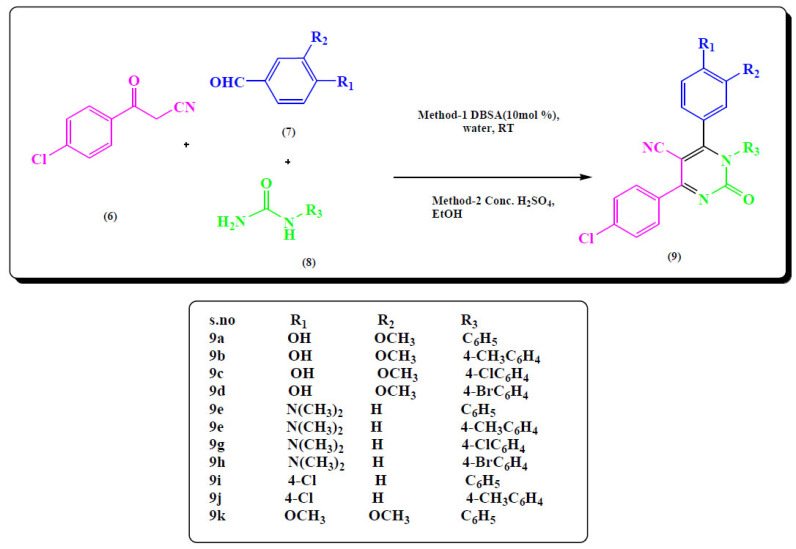
More et al. synthesized 5-carbonitrile derivatives by multi component reaction. They developed two methods for the synthesis. First was the conc. H2SO4 in ethanol and other was dodecylbenzenesulfonic acid (DBSA) in water. The substrate was p-chlorobenzoylacetonitrile (6), aldehydes (7), and urea derivatives (8) for the multicomponent reactions to get the different derivatives of pyrimidine-5-carbonitrile (9). The method with DBSA in water was more efficient than the first one. Bioactive of pyrimidine derivatives was assessed through in-vitro studies (Fig. 9) [63].
Fig. (9).
Pyrimidine-5-carbonitrile derivatives (9a-k). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
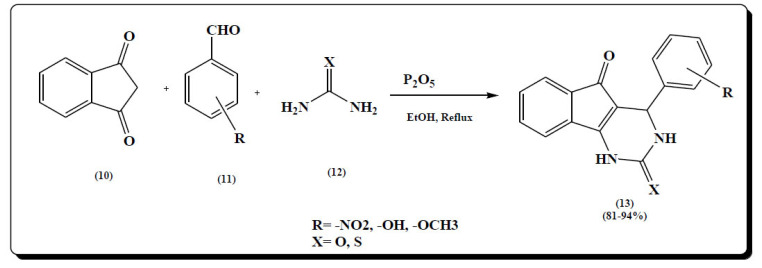
Warekar et al. synthesized 3,4-dihydropyrimidines-2 (1H)-one 1,3-indanedione (10), aldehydes (11) and urea/thiourea (12) derivatives by Biginelli type reactions, where P2O5 was used as acid catalyst and dehydrating agent was taken in a pot with ethanol. Di-hydro pyrimidine derivatives (13) were synthesized with good to excellent yield. It was anticipated that the reaction proceeded through Knoevenagel condensation reaction of 1,3-indanedione (10) and aldehydes (11) followed by Michael reaction (Fig. 10) [64].
Fig. (10).
Di-hydro pyrimidine derivatives (13). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
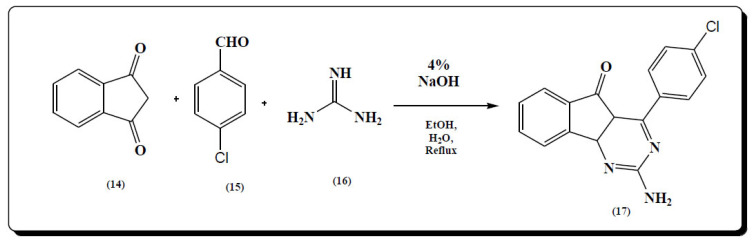
Patravale and group utilized multicomponent reaction for the synthesis of substituted indenopyrimidine. The approach for multicomponent synthesis was greener. One pot synthesis using 1,3-indandione (14), aromatic aldehydes (15) and guanidine hydrochloride (16) in the presence of catalytic amount of NaOH in ethanol:water gave different 2-amino-4-phenyl-5H-indeno[1,2-d] pyrimidine-5-one derivatives (17) (Fig. 11) [65].
Fig. (11).
Synthesis of 2-amino-4-phenyl-5H-indeno[1,2-d] pyrimidine-5-one derivatives (17). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
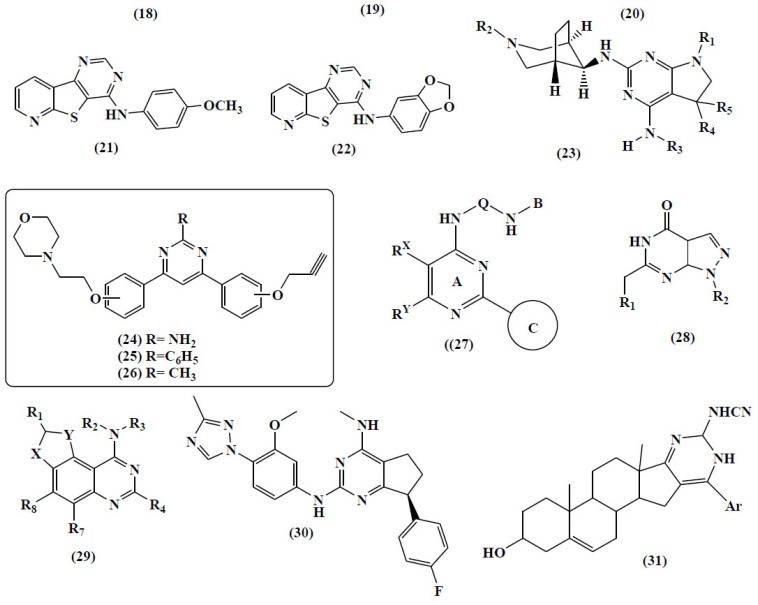
Loidreau and group synthesized two series of novel N-aryl-7- methoxybenzo[b]furo[3,2-d]pyrimidin-4-amines and their N-aryl-7- methoxybenzo[b]thieno[3,2-d]pyrimidin-4-amine analogues. Their inhibition potential on five different kinases-CDK5/p25 (cyclin-dependent kinase), CK1δ/ε (casein kinase 1), GSK3α/β (glycogen synthase kinase 3), DYRK1A (dual-specificity tyrosine phosphorylation regulated kinase) and CLK1 (cdc2-like kinase 1) were studied. The most effective compounds on these two kinase families are the benzo-thieno[3,2-d] pyrimidines (18, 19) which showed interesting sub-micromolar inhibition and selectivity towards CLK1 and DYRK1A over the other tested kinases [66].
The same group also tried with the N-aryl benzo[b]thieno[3,2-d]pyrimidin-4-amines and synthesized their pyrido and pyrazino analogues (20-22). The inhibitory potency of the final products was tested against same five Ser/Thr kinases, and some of the lead was taken up for further studies [66].
A patent based on the synthesis of naturally inspired azabicyclic fused Pyrimidine derivatives (23) for the targeting MAP-activated protein kinase 2 (MK2), which reduced the rate of neurodegeneration was published [67].
The 4,6-Diphenylpyrimidine derivatives (the most active compounds (24-26) were also found effective for the inhibition of the Monoamine Oxidisase and Acetylcholinesterase for the treatment of AD. The work was conducted by Kumar B and co-workers [68].
Recently, a research group found the magical activity of pyrimidine-based compound (27) for the inhibition of CSK-3, which is a potential target for AD [69].
One of the pioneer research groups found the effectiveness of 6-cyclylmethyl- and 6-alkylmethyl-substituted pyrazolo[3,4-d] pyrimidines (28) for the improvement of learning and memory. These are the major symptoms of AD. Moreover, one of the research based on the tricyclic pyrimidines revealed their anti-AD action through DYRK1A/DYRK1B inhibition [70].
Another patent was published on tricyclic pyrimidines (29) as inhibitors of DYRK1A/DYRK1B for the treatment of AD by Gerard Rosse [71].
Recently in 2019, a research group developed a molecule “BMS-932481” (30) for the treatment of Alzheimer’s which gave hope to AD patients. This was through the reduction of Aβ1–42 and Aβ1–40 in the plasma, brain, and cerebrospinal fluid. It is in clinical trial and gave a lot of hope to the world. [72].
The anti-Alzheimer activity of some synthesized heterocyclic pyrimidine and thiopyrimidine derivatives fused with steroidal structure was reported and conveniently screened for their anti-Alzheimer activities using of Flurbiprofen as the reference drug compound (31) was demonstrated to exhibit remarkable activity and amyloid (A) lowering results [73, 74] (Fig. 12 for compound 18-31).
Fig. (12).
Bioactive Pyrimidines. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The present scenario related to the pyrimidine ring based natural product or inspired molecule created an open platform for research of the multi targeting plant extract, which is not discovered so far. The future related to the plant and Alzehimer’s treatment showed a positive direction for the drug discovery scientists to work on for the wellbeing of sufferer. The present review is sufficient in providing an attractive area for research to get the data of all the chemical ingredients of the plant and further screen them against various potential targets of AD.
Library of compounds cannot be made by a multi-step process. To overcome this challenge a multi-component reaction is needed. It is given below with examples for the synthesis of highly functionalized scaffold in one step in one pot without isolating the intermediate. Thus, the multi-component reaction came into existence and quickly gained momentum in the field of drug discovery. The multi-component is widely used by drug discovery scientists due to its high atom economy. It also reduces multi-step process to one step process, so the compounds library can be made in minimum time and cost.
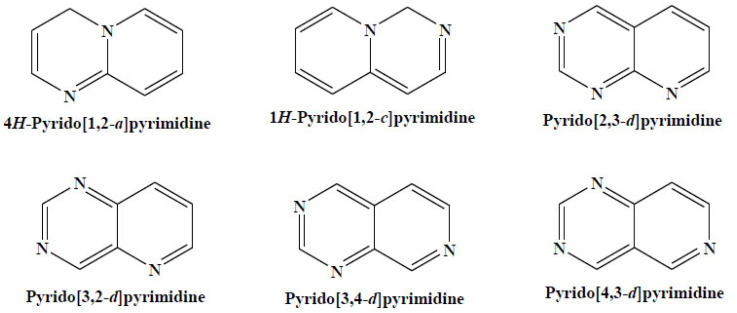
The pyridopyrimidines are class of 6–6 bicyclic systems containing two or three nitrogen atoms in both six-membered rings. The compounds are also named as diaza- or triaza-naphthalenes (Fig. 13).
Fig. (13).
The pyridopyrimidines. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Multicomponent reactions were used in the synthesis of pyridopyrimidines and in the last years, the interest in the synthesis of pyrido[1,2-a]pyrimidines from the condensation of 2-aminopyridines with different nucleophiles is growing due to their highly efficient and adequate products. Ettar and co-workers reported the diverse methodologies that have been reported on the chemistry of pyrido[1,2-a]pyrimidines [75].
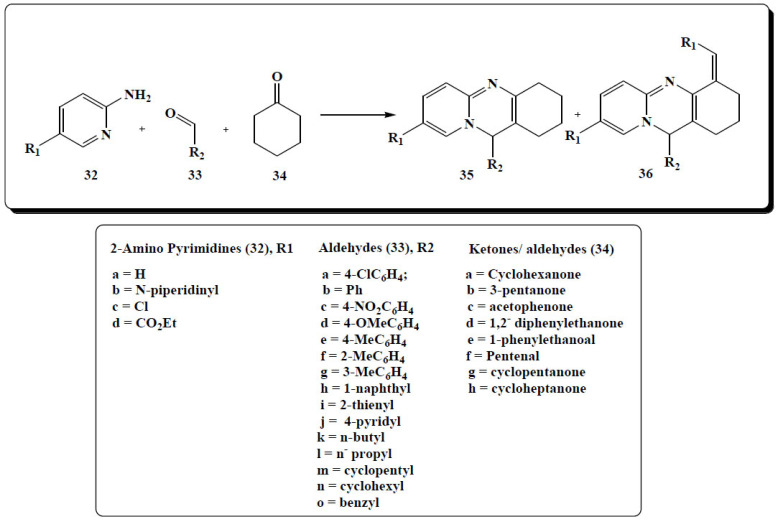
Three-component condensation reaction of equimolar amounts of 2-aminopyridines (32), aldehyde or ketone derivatives (33) and cyclohexanone (34) gave a mixture of pyrido[1,2-a]pyrimidines (35, 36) as reported by Yang and co- workers in 2013, (Fig. 14) [76].
Fig. (14).
Synthesis procedure for the mixture of pyrido[1,2-a]pyrimidines. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
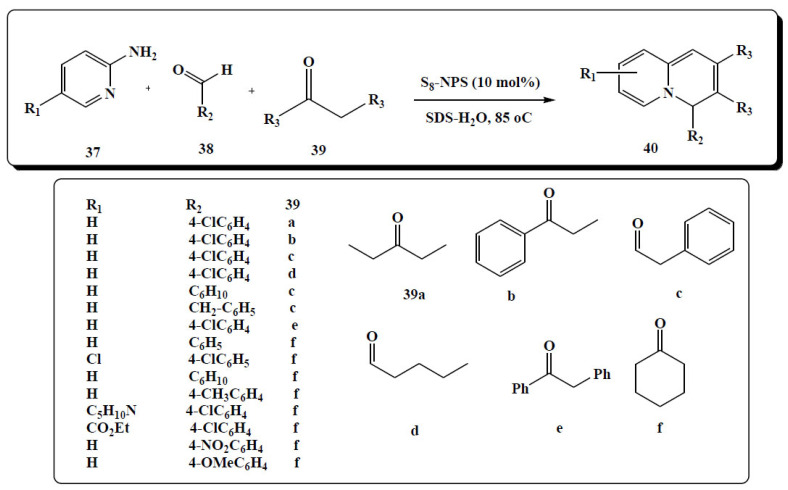
Multicomponent reactions of 2-aminopyridines (37), aldehyde (38), and aldehyde/ketones (39) in aqueous solution of sodium dodecyl sulfate (SDS) gave substituted 4H-pyrido[1,2-a]pyrimidines (40) (Fig. 15) [77].
Fig. (15).
Synthesis of substituted 4H-pyrido[1,2-a] pyrimidines. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
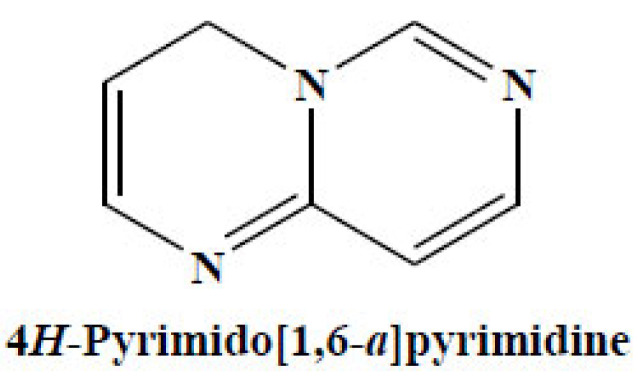
4H-Pyrimido[1,6-a]pyrimidines are a type of 6–6 bicyclic systems which are rarely reported despite the fact that they provide an exceptional ring structure and multiple substitution designs, polarities, and H-bonding proficiencies (Fig. 16).
Fig. (16).
4H-Pyrimido[1,6-a]pyrimidine. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
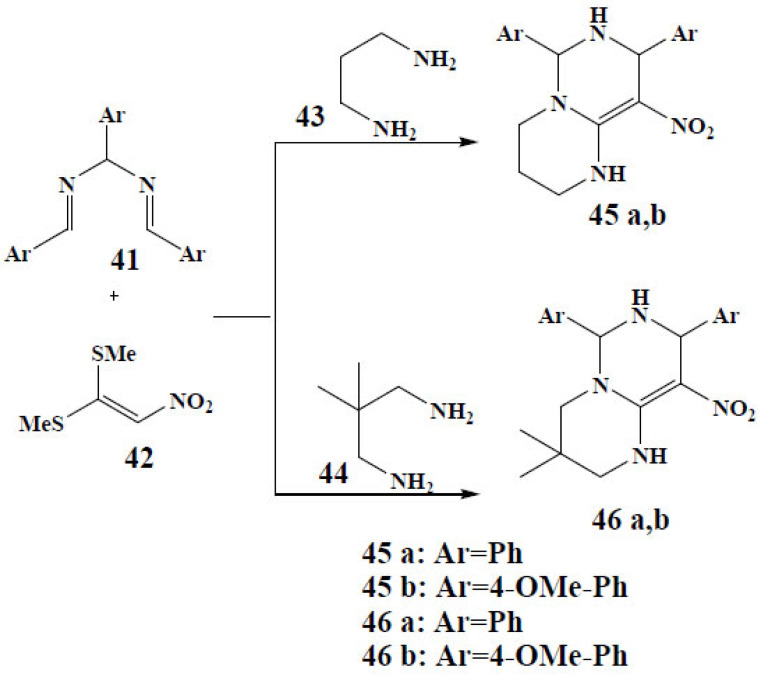
Alizadeh et al. reported a very proficient route for the synthesis of diarylhexahydro-2H-pyrimidopyrimidines through a multicomponent procedure. (2-nitroethene- 1,1-diyl)bis(methylsulfane) (42) with propane-1,3-diamine (43) or 2,2-dimethylpropane-1,3-diamine (44) followed by reaction with N,N’-(arylmethylene)bis(1-arylmethanimine) (41) yielded the desired diarylhexahydro-2H-pyrimidopyrimidines (45-a,b, 46-a,b). This is an alternative technique for application in drug discovery (Fig. 17) [78].
Fig. (17).
Multicomponent synthetic route for diarylhexahydro-2H- pyrimidopyrimidines. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
A detailed review was published by Elattar et al. for the synthesis of substituted pyrimidopyrimidine, pyrimido[1,6-a]pyrimidin-diones and pyrimidoquinazolines by various routes. Common among them are from acyclic reactants, multicomponent synthesis, from 4-aminopyrimidines, ring annulation, from 6-aminopyrimidine-2,4-diol, pyrimidine -2,4(1H,3H)-dione, from 4,6-dichloropyrimidine and various other methods are available [79].
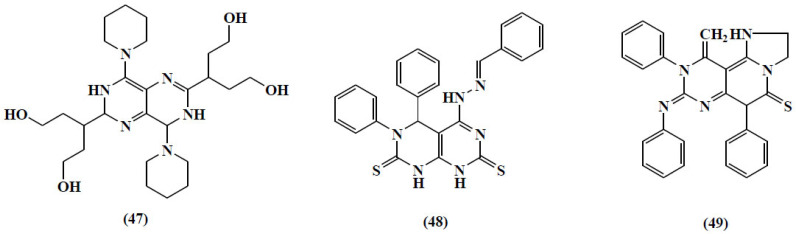
Another review was published by Monier and co-workers for the various synthetic routes for pyrimidopyrimidines or tetra-azanaphthalenes. These were the two fused pyrimidine rings with four possible structural isomers. The main emphasis was on the chemistry and biological significance of pyrimido[4,5-d] pyrimidine and pyrimido[5,4-d]pyrimidine analogs as types of bicyclic [6 + 6] systems. They focused on synthetic methods, the reactivities of the substituents linked to the ring carbon and nitrogen atoms and biological applications. The potent bioactive components of the class are compound (47-49) (Fig. 18) [80].
Fig. (18).
Structure of the potent bioactive components of Pyrimidopyrimidines. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
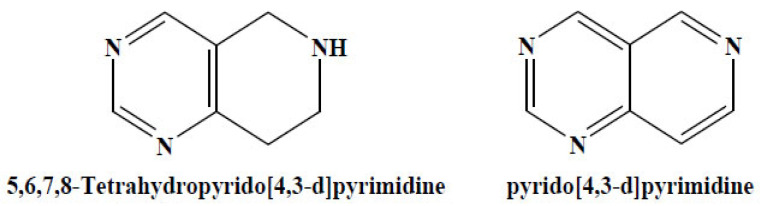
A rewiew was published earlier in 2016 by Ettar and his co-workers. It summarized the developments in the chemistry of bicyclic 6-6 systems with more emphasis was on the structural features, reactions, and synthetic methodologies of pyrido[4,3-d]pyrimidines. 5,6,7,8-Tetrahydropyrido[4,3-d] pyrimidine (Fig. 19). Related compounds have been used as starting materials for the multi-step synthesis of tetrahydropteroic acid derivatives [81].
Fig. (19).
Pyridopyrimidine derivatives. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
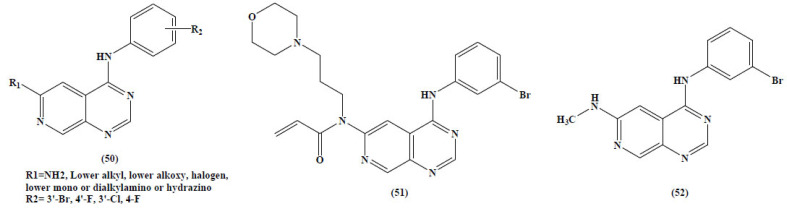
Pyrido[3,4-d]pyrimidines is another class of derivatives which is one of the most important heterocyclic compounds with remarkable synthetic, biological and medical applications. Recently, an overview was published by Monier et al. They elaborated the chemistry of heterocyclic compounds which incorporated the pyrido[3,4-d]pyrimidine scaffold (50-52) (Fig. 20) [82].
Fig. (20).
Biologically active pyrido[3,4-d]pyrimidines. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
This review has highlighted the importance of multi-component reactions by using the example of active scaffolds of pyrimidine/fused pyrimidines. This would highlight the importance of the fast as well as smart synthesis of the bio-relevant molecules. The multi-component reactions are widely accepted in academia, the pharmaceutical sciences, and pharmaceutical industries. It is probable in the future that multi- component reactions will be a powerful tool in synthesis for drug discovery scientists.
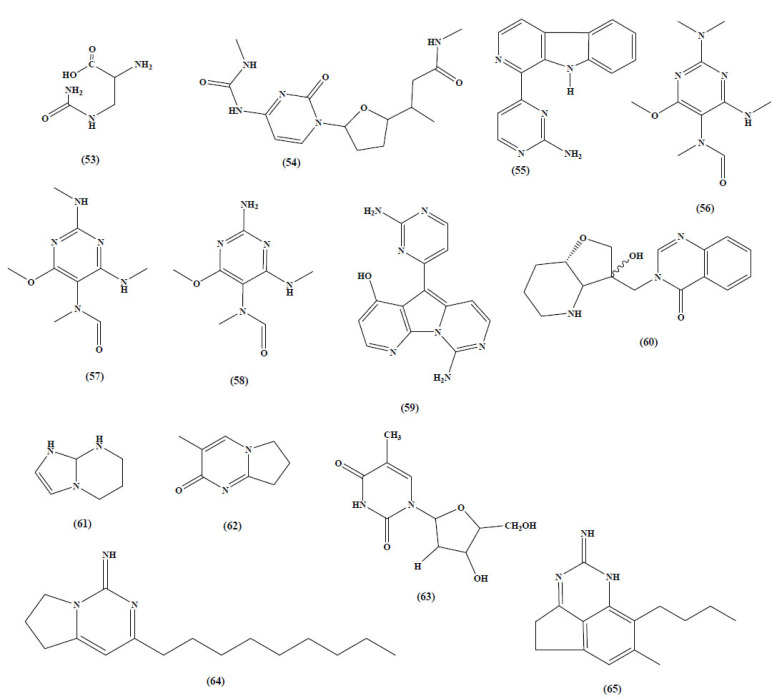
The aim of this review is to bridge the gap between the naturally inspired pyrimidine ring analogues and their role in the treatment of the Alzheimer’s disease (AD) (53-65). We need to understand how Alzheimer’s disease occurs and what factors induce Alzheimer’s with comorbidities. Various natural plants are available in the list of medicinal plants, but few of them are explored for CNS activities. Alkaloids are one of the famous categories of active constitutes useful for the treatment of Alzheimer’s disease. Few plants unexplored for brain treatment are mentioned in Table (1).
Table 1.
Unexplored plants for the treatment of alzheimer disease (Fig. 21).
| S. No | Plant Sources | Chemical Name | Str. No. | Chemical Constituent |
Pharmacological Activity | Refs. |
|---|---|---|---|---|---|---|
| 1. |
Albizzia Julibrissin (Non-Proteinogenic Amino Acid) |
2-amino-3-ureidopropanoic acid | 53 | Albizziin | Anxiety, Depression, Insomnia, Cancer, | [83-85] |
| 2. | Tadehagi Triquetrum | (5-(4-[(methylcarbamoyl) amino]-2-oxopyrimidin-1(2H)-yl) Tetrahydrofuran-2-Yl) Methyl Methylcarbamate |
54 | New pyrimdine alkaloid | Anti oxidant | [86] |
| 3. | Annona foetida | Pyrimidine-β-carboline alkaloid | 55 | N-hydroxy annomontine, Annomontine, | Anti acetyl cholinesterase |
[87,88] |
| 4. | Heterostemma brownii | 6-methoxy-4-(N-methylamino)-2-(N,N-dimethylamino)-5- (N-methylformamido)pyrimidine, 6-methoxy-2,4-bis(N- methylamino)-5-(N-methylformamido)pyrimidine, and 2-amino-6-methoxy-4-(N-methylamino)-5-(N-methylformamido)pyrimidine |
56, 57, 58 | Heteromine A and Heteromine B | Treatment of Cancer, Anti convulsants |
[89,90] |
| 5. | Kirkpatrickia varialosa | 9-Amino-5-(2-aminopyrimidin-4-yl)pyrido | 59 | Variolin analogues | Antiviral activity, neurodegenerative disorders | [91,92] |
| 6. | Dichroa febrifuga | 3-{3-[(2R,3S)-3-Hydroxypiperidin-2-yl]-2-oxopropyl} quinazolin-4(3H)-one |
60 | Febrifugine | Antimalaria, neuroprotective |
[93,94] |
| 7. | Alchornea javanensis | Hexahydroimidazo [1,2-a]pyrimidine | 61 | Alchorneine, alchornidine | Antifungal | [95] |
| 8. | Glycyrrhiza uralennsis | 3-methy-6,7,8-trihydro-pyrrolo [1,2-a]pyrimidine-2-one | 62 | uridine | Cytotoxic activity against human cancer cell | [96] |
| 9. | Acanthostrongylophora Ingens | Pyrimidine-β-carboline alkaloid | 55 | Acanthomine A, Ingenines A and B | Anti leishmanial | [97,98] |
| 10. | Eudistoma vannamei | 1-(4-Hydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl- 1H-pyrimidine-2,4-dione |
63 | staurosporine | Cytotoxic | [99] |
| 11. | Monanchora arbuscula | 3-Nonyl-6,7-dihydro-5H-pyrrolo [1,2-c]pyrimidin-1- ylideneamine, 8-Butyl-7-methyl-4,5-dihydro-1H-cyclopenta[de] quinazolin-2-ylideneamine |
64, 65 | monalidine A, arbusculidine A |
anti-parasitic activity |
[100,101] |
CONCLUSION
This review highlighted the pyrimidine alkaloids isolated from natural products for the development of multifunctional potential anti-AD agents because of their basic structure pyrimidine ring and fused counter parts. SAR for trisubstituted Pyrimidine (Fig. 22) has also been given. Alzheimer-based dementia is anticipated to increase globally and double every year. Treatment of this disease will be the most expensive to deal with for the government in the upcoming twenty years. This review is comprised of many studies which may show the importance of curing AD with a multi targeting approach. Naturally occurring pyrimidine is so far not in the market or research. Many natural plants are having pyrimidines alkaloids which are unexplored and hidden for the treatment of Alzheimer’s disease. This review gives a platform to researchers for further study so a novel compound which adds advantage to drug therapy for treatment of neurodegenerative diseases can be developed. Further, preclinical and clinical experimental data requires establishing an evidence to develop a formulation.
Fig. (22).
SAR for trisubstituted Pyrimidine. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (21).
Naturally inspired Pyrimidine alkaloids. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
ACKNOWLEDGEMENTS
We would like to acknowledge all the authors for ideas, execution and implementation. We would also like to appreciate management for giving the opportunity to write this manuscript.
LIST OF ABBREVIATIONS
- AD
Alzheimer’s Disease
- DNA
Deoxyribonucleic Acid
- CSK-3
C-Terminal Src Kinase
- CDK5
Cyclin-dependent Kinase
- CK1
Ccasein Kinase 1
- GSK-3
Glycogen Synthase Kinase 3
- DYRK1A
Dual-specificity tyrosine phosphorylation Regulated Kinase
- CLK1
Cdc2-like Kinase 1
- MK2
MAP-activated protein Kinase
- DBSA
Dodecylbenzenesulfonic Acid
- COX
Cyclooxygenase
- PI3K
Phosphotidylinositol-3-ki
AUTHOR’S CONTRIBUTION
All coauthors contributed at different stages. Saloni Kakkar, Shivani Singh and Deepak Kumar have worked on the conception and design of the study. Prerna Sharma searched for plant literature. Meenakshi Dhanawat drew the structures and Inderjeet Verma compiled the table. Sumeet Gupta and Anroop Nair drafted the article, revised it critically and submitted the final manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Alzheimers Disease fact and figure https://www.alz.org/media/Documents/facts-and-figures-2018-r.pdf. 2018 [Google Scholar]
- 2.Poddar J., Singh S., Kumar P., Bali S., Gupta S., Chakrabarti S. Inhibition of complex I-III activity of brain mitochondria after intracerebroventricular administration of streptozotocin in rats is possibly related to loss of body weight. Heliyon. 2020;6(7):e04490. doi: 10.1016/j.heliyon.2020.e04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reitz C., Brayne C., Mayeux R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011;7(3):137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzhiemer report https://n.neurology.org/content/proteomics-and-alzheimer-disease-ad
- 5. https://www.alz.co.uk/research/world-report-2018
- 6. https://www.alz.org/alzheimers-dementia/facts-figure
- 7.Todd A., Moore A., Ashton M., Van S. Current research and development of treatments for Alzheimer’s disease. Pharm. J. 2019;19(14):20. [Google Scholar]
- 8.Kunz L., Schröder T.N., Lee H., Montag C., Lachmann B., Sariyska R., Reuter M., Stirnberg R., Stöcker T., Messing-Floeter P.C., Fell J., Doeller C.F., Axmacher N. Reduced grid-cell-like representations in adults at genetic risk for Alzheimer’s disease. Science. 2015;350(6259):430–433. doi: 10.1126/science.aac8128. [DOI] [PubMed] [Google Scholar]
- 9.Goedert M., Spillantini M.G., Crowther R.A. A brief history of Tau. Clin. Chem. 2015;61(11):1417–1418. doi: 10.1373/clinchem.2015.245142. [DOI] [PubMed] [Google Scholar]
- 10.Cabezas-Opazo F.A., Vergara-Pulgar K., Pérez M.J., Jara C., Osorio-Fuentealba C., Quintanilla R.A. Mitochondrial dysfunction contributes to the pathogenesis of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2015;2015:509654. doi: 10.1155/2015/509654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prado D., Cardoso I.L. Apolipoproteina E e Doenca de Alzheimer. Rev. Neurosci. 2013;21(1):118–125. doi: 10.34024/rnc.2013.v21.8211. [DOI] [Google Scholar]
- 12.Stancu I.C., Vasconcelos B., Terwel D., Dewachter I. Models of β-amyloid induced Tau-pathology: the long and “folded” road to understand the mechanism. Mol. Neurodegener. 2014;9:51. doi: 10.1186/1750-1326-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galasko D., Montine T.J. Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomarkers Med. 2010;4(1):27–36. doi: 10.2217/bmm.09.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuppo E.E., Arias H.R. The role of inflammation in Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2005;37(2):289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Rogers J., Webster S., Lue L.F., Brachova L., Civin W.H., Emmerling M., Shivers B., Walker D., McGeer P. Inflammation and Alzheimer’s disease pathogenesis. Neurobiol. Aging. 1996;17(5):681–686. doi: 10.1016/0197-4580(96)00115-7. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y.J., Han S.B., Nam S.Y., Oh K.W., Hong J.T. Inflammation and Alzheimer’s disease. Arch. Pharm. Res. 2010;33(10):1539–1556. doi: 10.1007/s12272-010-1006-7. [DOI] [PubMed] [Google Scholar]
- 17. https://clinicaltrials.gov
- 18.Bazan N., Botting J., Vane S. J new targets in inflammation inhibitors of COX-2 or adhesion molecules. Springer Science+Business Media. (1st edition. ), 1996. [Google Scholar]
- 19.Keller W.R., Kum L.M., Wehring H.J., Koola M.M., Buchanan R.W., Kelly D.L. A review of anti-inflammatory agents for symptoms of schizophrenia. J. Psychopharmacol. 2013;27(4):337–342. doi: 10.1177/0269881112467089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller N., Riedel M., Scheppach C., Brandstätter B., Sokullu S., Krampe K., Ulmschneider M., Engel R.R., Möller H.J., Schwarz M.J. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am. J. Psychiatry. 2002;159(6):1029–1034. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- 21.Katyal J., Kumar H., Gupta Y.K. Anticonvulsant activity of the cyclooxygenase-2 (COX-2) inhibitor etoricoxib in pentylenetetrazole-kindled rats is associated with memory impairment. Epilepsy Behav. 2015;44:98–103. doi: 10.1016/j.yebeh.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 22.Niranjan R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: focus on astrocytes. Mol. Neurobiol. 2014;49(1):28–38. doi: 10.1007/s12035-013-8483-x. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell J.A., Warner T.D. COX isoforms in the cardiovascular system: understanding the activities of non-steroidal anti-inflammatory drugs. Nat. Rev. Drug Discov. 2006;5(1):75–86. doi: 10.1038/nrd1929. [DOI] [PubMed] [Google Scholar]
- 24.Van Dam P.S., Cotter M.A., Bravenboer B., Cameron N.E. Pathogenesis of diabetic neuropathy: focus on neurovascular mechanisms. Eur. J. Pharmacol. 2013;719(1-3):180–186. doi: 10.1016/j.ejphar.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Dhanawat M., Mehta D.K., Gupta S., Das R. Understanding the pathogenesis involved in parkinson’s disease and potential therapeutic treatment strategies. Cent. Nerv. Syst. Agents Med. Chem. 2020;20(2):88–102. doi: 10.2174/1871524920666200705222842. [DOI] [PubMed] [Google Scholar]
- 26.Fondel E., Eilis J.O., Fitzgerald K.C., Falcone G.J., Mccullough M.L., Thun M.J. park, Y.; Colonel, L. N.; ascheri, a. Non-steroidal antiinflammatory drugs and amyotrophic lateral sclerosis: Results from five prospective cohort studies. Amyo. Lat. Sclerosis. 2012;13:573–579. doi: 10.3109/17482968.2012.703209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palumbo S., Bosetti F. Alterations of brain eicosanoid synthetic pathway in multiple sclerosis and in animal models of demyelination: role of cyclooxygenase-2. Prostaglandins Leukot. Essent. Fatty Acids. 2013;89(5):273–278. doi: 10.1016/j.plefa.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Morales I., Guzmán-Martínez L., Cerda-Troncoso C., Farías G.A., Maccioni R.B. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 2014;8:112. doi: 10.3389/fncel.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang J., Yang M.S., Quan Y., Gueorguieva P., Ganesh T., Dingledine R. Therapeutic window for cyclooxygenase-2 related anti-inflammatory therapy after status epilepticus. Neurobiol. Dis. 2015;76:126–136. doi: 10.1016/j.nbd.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I.R., McGeer P.L., O’Banion M.K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F.L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21(3):383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FitzGerald G.A. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat. Rev. Drug Discov. 2003;2(11):879–890. doi: 10.1038/nrd1225. [DOI] [PubMed] [Google Scholar]
- 32.Brown D.J., The Pyrimidines: Supplements-II, Wiley-interscience; New York The Chemistry of Heterocyclic Compounds. 1970;16:261–268. [Google Scholar]
- 33.Chen X., Chong C.R., Shi L., Yoshimoto T., Sullivan D.J., Jr, Liu J.O. Inhibitors of Plasmodium falciparum methionine aminopeptidase 1b possess antimalarial activity. Proc. Natl. Acad. Sci. USA. 2006;103(39):14548–14553. doi: 10.1073/pnas.0604101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta S., Nair A., Jhawat V., Mustaq N., Sharma A., Dhanawat M., Khan S.A. Unwinding complexities of diabetic alzheimer by potent novel molecules. Am. J. Alzheimers Dis. Other Demen. 2020;35:1533317520937542. doi: 10.1177/1533317520937542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma A., Gupta S., Chauhan S., Nair A., Sharma P. Astilbin: a promising unexplored compound with multidimensional medicinal and health benefits. Pharmacol. Res. 2020;158:104894. doi: 10.1016/j.phrs.2020.104894. [DOI] [PubMed] [Google Scholar]
- 36.Hanefeld M. Clinical rationale for rosuvastatin, a potent new HMG-CoA reductase inhibitor. Int. J. Clin. Pract. 2001;55(6):399–405. [PubMed] [Google Scholar]
- 37.Shimizu M., Takase Y., Nakamura S., Katae H., Minami A., Nakata K., Inoue S., Ishiyama M., and Kubo Y. Pipemidic acid, a new antibacterial agent active against pseudomonas aeruginosa: in vitro properties. Antmicrob. Agents Chemother. 1975;8(2):132–138. doi: 10.1128/aac.8.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joensuu H., Dimitrijevic S. Tyrosine kinase inhibitor imatinib (STI571) as an anticancer agent for solid tumours. Ann. Med. 2001;33(7):451–455. doi: 10.3109/07853890109002093. [DOI] [PubMed] [Google Scholar]
- 39.Blass B. Pyrimidinylpiperdinyloxypyridone analogues as GPR119 modulators. ACS Med. Chem. Lett. 2014;5(5):458–459. doi: 10.1021/ml500089c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal N., Raghuwanshi S.K., Upadhyay D.N., Shukla P.K., Ram V.J. Suitably functionalised pyrimidines as potential antimycotic agents. Bioorg. Med. Chem. Lett. 2000;10(8):703–706. doi: 10.1016/S0960-894X(00)00091-3. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann J., Buchdunger E., Mett H., Meyer T., Lydon N.B. Potent and selective inhibitors of the Abl-kinase: phenylamino-pyrimidine (PAP) derivatives. Bioorg. Med. Chem. Lett. 1997;7(2):187–192. doi: 10.1016/S0960-894X(96)00601-4. [DOI] [Google Scholar]
- 42.Manley P.W., Cowan-Jacob S.W., Buchdunger E., Fabbro D., Fendrich G., Furet P., Meyer T., Zimmermann J. Imatinib: a selective tyrosine kinase inhibitor. Eur. J. Cancer. 2002;38(5) Suppl. 5:S19–S27. doi: 10.1016/S0959-8049(02)80599-8. [DOI] [PubMed] [Google Scholar]
- 43.le Coutre P., Ottmann O.G., Giles F., Kim D.W., Cortes J., Gattermann N., Apperley J.F., Larson R.A., Abruzzese E., O’Brien S.G., Kuliczkowski K., Hochhaus A., Mahon F.X., Saglio G., Gobbi M., Kwong Y.L., Baccarani M., Hughes T., Martinelli G., Radich J.P., Zheng M., Shou Y., Kantarjian H. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is active in patients with imatinib-resistant or -intolerant accelerated-phase chronic myelogenous leukemia. Blood. 2008;111(4):1834–1839. doi: 10.1182/blood-2007-04-083196. [DOI] [PubMed] [Google Scholar]
- 44.Loidreau Y., Marchand P., Dubouilh-Benard C., Nourrisson M.R., Duflos M., Loaëc N., Meijer L., Besson T. Synthesis and biological evaluation of N-aryl-7-methoxybenzo[b]furo[3,2-d]pyrimidin-4-amines and their N-arylbenzo[b]thieno[3,2-d]pyrimidin-4-amine analogues as dual inhibitors of CLK1 and DYRK1A kinases. Eur. J. Med. Chem. 2013;59:283–295. doi: 10.1016/j.ejmech.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 45.Bornemann K. Trisubstituted pyrimidines. U.S. Patent No. 7,166,599, 2007. [Google Scholar]
- 46.Bornemann K., Briem H., Dorner-Clossek C., Fechteler K., Fuchs K., Himmelsbach F., Klinder K., Kostka M. Boehringer Ingelheim Pharma GmbH and Co KG, assignee. Trisubstituted pyrimidines. United States patent application US 10/272,160, 2003. [Google Scholar]
- 47.Zhi H., Chen L.M., Zhang L.L., Liu S.J., Wan D.C., Lin H.Q., Hu C. Design, synthesis, and biological evaluation of 5H-thiazolo [3, 2-a] pyrimidine derivatives as a new type of acetylcholinesterase inhibitors. ARKIVOC. 2008;1(13):266–277. doi: 10.3998/ark.5550190.0009.d29. [DOI] [Google Scholar]
- 48.Arvidsson P.I., Burrows J.N., Yngve U., Tjerneld E. AstraZeneca AB, assignee. Imidazole substituted pyrimidines. United States patent US 8,178,529, 2012. [Google Scholar]
- 49.Rehman T.U., Khan I.U., Ashraf M., Tarazi H., Riaz S., Yar M. An efficient synthesis of bi-aryl pyrimidine heterocycles: potential new drug candidates to treat alzheimer’s disease. Arch. Pharm. (Weinheim) 2017;350(3-4):1600304. doi: 10.1002/ardp.201600304. [DOI] [PubMed] [Google Scholar]
- 50.Mohamed T., Yeung J.C., Vasefi M.S., Beazely M.A., Rao P.P. Development and evaluation of multifunctional agents for potential treatment of Alzheimer’s disease: application to a pyrimidine-2,4-diamine template. Bioorg. Med. Chem. Lett. 2012;22(14):4707–4712. doi: 10.1016/j.bmcl.2012.05.077. [DOI] [PubMed] [Google Scholar]
- 51.Rivkin A., Ahearn S.P., Chichetti S.M., Kim Y.R., Li C., Rosenau A., Kattar S.D., Jung J., Shah S., Hughes B.L., Crispino J.L., Middleton R.E., Szewczak A.A., Munoz B., Shearman M.S. Piperazinyl pyrimidine derivatives as potent γ-secretase modulators. Bioorg. Med. Chem. Lett. 2010;20(3):1269–1271. doi: 10.1016/j.bmcl.2009.11.101. [DOI] [PubMed] [Google Scholar]
- 52.Bornemann K., Briem H., Dorner-Ciossek C., Fechteler K., Fuchs K., Himmelsbach F., Klinder K., Kostka M. Boehringer Ingelheim Pharma GmbH and Co KG, assignee. Trisubstituted pyrimidines. United States patent US 7,166,599, 2007. [Google Scholar]
- 53.Goswami S., Jana S., Dey S., Adak A.K. Microwave-expedited one-pot, two-component, solvent-free synthesis of functionalized pyrimidines. Aus. J. Chem. 2006;60:120–123. [Google Scholar]
- 54.Karpov A.S., Müller T.J. New entry to a three-component pyrimidine synthesis by TMS-Ynones via Sonogashira coupling. Org. Lett. 2003;5(19):3451–3454. doi: 10.1021/ol035212q. [DOI] [PubMed] [Google Scholar]
- 55.Radi M., Schenone S., Botta M. Recent highlights in the synthesis of highly functionalized pyrimidines. Org. Biomol. Chem. 2009;7(14):2841–2847. doi: 10.1039/b906445a. [DOI] [PubMed] [Google Scholar]
- 56.Bagley M.C., Hughes D.D., Taylor P.H. Highly efficient synthesis of pyrimidines under microwave-assisted conditions. Org. Lett. 2003;5(19):3451–3454. [Google Scholar]
- 57.Bagley M.C., Hughes D.D., Lubinu M.C., Merritt E.A., Taylor P.H., Tomkinson N.C. Microwave‐assisted synthesis of pyrimidine libraries. QSAR Comb. Sci. 2004;23(10):859–867. doi: 10.1002/qsar.200420044. [DOI] [Google Scholar]
- 58.Sagar R., Kim M.J., Park S.B. An improved synthesis of pyrimidine-and pyrazole-based acyclo-C-nucleosides as carbohybrids. Tetrahedron Lett. 2008;49(34):5080–5083. doi: 10.1016/j.tetlet.2008.06.032. [DOI] [Google Scholar]
- 59.Polshettiwar V., Varma R.S. Microwave-assisted organic synthesis and transformations using benign reaction media. Acc. Chem. Res. 2008;41(5):629–639. doi: 10.1021/ar700238s. [DOI] [PubMed] [Google Scholar]
- 60.Bagley M.C., Hughes D.D., Lloyd R., Powers V.E. A new and highly expedient synthesis of pyrido [2, 3-d] pyrimidines. Tetrahedron Letters. 2001 Sep 10;42(37):6585-8. (b) Bagley MC, Lin Z, Pope SJ. Barium manganate in microwave-assisted oxidation reactions: synthesis of solvatochromic 2, 4, 6-triarylpyrimidines. Tetrahedron Lett. 2009;50(49):6818–6822. doi: 10.1016/j.tetlet.2009.09.116. [DOI] [Google Scholar]
- 61.Harbaugh R.E. Novel CNS-directed drug delivery systems in Alzheimer’s disease and other neurological disorders. Neurobiol. Aging. 1989;10(5):623–629. doi: 10.1016/0197-4580(89)90155-3. [DOI] [PubMed] [Google Scholar]
- 62.Senthilkumar N., Ravichandran Y.D., Kumar K.M., Ramaiah S. Synthesis of a new series of pyrimidine derivatives: exploration of anti-proliferative activity on EAT cells and molecular docking. Res. Chem. Intermed. 2015;42:1295–1313. doi: 10.1007/s11164-015-2086-2. [DOI] [Google Scholar]
- 63.Bharatkumar M., Sapkal D., More H. One-pot three-component synthesis of pyrimidine-5-carbonitrile derivatives in water using p-dodecylbenzenesulfonic acid as catalyst and evaluation of in vitro anti-inflammatory and anthelmintic activities. Der Pharma Chem. 2015;7:167–173. [Google Scholar]
- 64.Warekar P.P., Kolekar G.B., Deshmukh M.B., Anbhule P.V. An Efficient and Modified Biginelli-Type Synthesis of 3,4-Dihydro-1H-indeno[1,2-d]pyrimidine-2,5-dione Using Phosphorous Pentoxide. Synth. Commun. 2014;44:3594–3601. doi: 10.1080/00397911.2014.947652. [DOI] [Google Scholar]
- 65.Patravale A.A., Gore A.H., Patil D.R., Kolekar G.B., Deshmukh M.B., Anbhule P. Trouble-free multicomponent method for combinatorial synthesis of 2-Amino-4-phenyl-5-H-indeno[1,2-d]pyrimidine-5-one and their screening against cancer cell lines. Ind. Eng. Chem. Res. 2014;53:16568–16578. doi: 10.1021/ie5013618. [DOI] [Google Scholar]
- 66.Loidreau Y., Marchand P., Dubouilh-Benard C., Nourrisson M.R., Duflos M., Lozach O., Loaëc N., Meijer L., Besson T. Synthesis and biological evaluation of N-arylbenzo[b]thieno[3,2-d]pyrimidin-4-amines and their pyrido and pyrazino analogues as Ser/Thr kinase inhibitors. Eur. J. Med. Chem. 2012;58:171–183. doi: 10.1016/j.ejmech.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Blass B.E. Azabicyclic fused pyrimidine derivatives useful for the treatment of alzheimer’s disease. ACS Med. Chem. Lett. 2018;9(3):165–166. doi: 10.1021/acsmedchemlett.8b00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar B., Dwivedi A.R., Sarkar B., Gupta S.K., Krishnamurthy S., Mantha A.K., Parkash J., Kumar V. 4,6-diphenylpyrimidine derivatives as dual inhibitors of monoamine oxidase and acetylcholinesterase for the treatment of alzheimer’s disease. ACS Chem. Neurosci. 2019;10(1):252–265. doi: 10.1021/acschemneuro.8b00220. [DOI] [PubMed] [Google Scholar]
- 69.Choquette D., Davies R., Wannamaker M. Vertex Pharmaceuticals Inc. Pyrimidine-based compounds useful as GSK-3 inhibitors. United States patent application US 10/314,905, 2003. [Google Scholar]
- 70.Hendrix M., Bärfacker L., Erb C., Hafner F.T., Heckroth H., Karthaus D., Tersteegen A., van der Staay F.J., Van Kampen M. 6-cyclylmethyl-and 6-alkylmethyl pyrazolo [3, 4-D] pyrimidines, methods for their preparation and methods for their use to treat impairments of perception, concentration learning and/or memory. United States patent US 8,044,060, 2011. [Google Scholar]
- 71.Rosse G. Tricyclic pyrimidines as inhibitors of DYRK1A/DYRK1B as potential treatment for Down’s syndrome or Alzheimer’s disease. ACS Med. Chem. Lett. 2013;4(6):502–503. doi: 10.1021/ml400137s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boy K.M., Guernon J.M., Zuev D.S., Xu L., Zhang Y., Shi J., Marcin L.R., Higgins M.A., Wu Y.J., Krishnananthan S., Li J., Trehan A., Smith D., Toyn J.H., Meredith J.E., Burton C.R., Kimura S.R., Zvyaga T., Zhuo X., Lentz K.A., Grace J.E., Denton R., Morrison J.S., Mathur A., Albright C.F., Ahlijanian M.K., Olson R.E., Thompson L.A., Macor J.E. Identification and preclinical evaluation of the bicyclic pyrimidine γ-secretase modulator BMS-932481. ACS Med. Chem. Lett. 2019;10(3):312–317. doi: 10.1021/acsmedchemlett.8b00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abdalla M.M., Al-Omar M.A., Al-Salahi R.A., Amr A.G., Sabrye N.M. A new investigation for some steroidal derivatives as anti-Alzheimer agents. Int. J. Biol. Macromol. 2012;51(1-2):56–63. doi: 10.1016/j.ijbiomac.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 74.Monier M., El-Mekabaty A., Abdel-Latif D., Doğru Mert B., Elattar K.M. Heterocyclic steroids: Efficient routes for annulation of pentacyclic steroidal pyrimidines. Steroids. 2020;154:108548. doi: 10.1016/j.steroids.2019.108548. [DOI] [PubMed] [Google Scholar]
- 75.Elattar K.M., Rabie R., Hammouda M.M. Recent progress in the chemistry of bicyclic 6–6 systems: chemistry of pyrido[1,2-a]pyrimidines. Monatsh. Chem. 2017;148:601–627. doi: 10.1007/s00706-016-1852-1. [DOI] [Google Scholar]
- 76.Yang K., Xiang J., Bao G., Dang Q., Bai X. Synthesis of highly substituted 4H-pyrido[1,2-a]pyrimidines via a one-pot three- component condensation reaction. ACS Comb. Sci. 2013;15(9):519–524. doi: 10.1021/co400086u. [DOI] [PubMed] [Google Scholar]
- 77.Sagir H., Rai P., Neha S., Singh P.K., Tiwaria S., Siddiqui I.R. S-Nanoparticle/SDS: an efficient and recyclable catalytic system for synthesis of substituted 4H-pyrido[1,2-a]pyrimidines in aqueous admicellar medium. RSC Adv. 2016;6:73924–73932. doi: 10.1039/C6RA07085J. [DOI] [Google Scholar]
- 78.Alizadeh A., Mokhtari J., Ahmadi M. Synthesis of the novel pyrimido[1,6-a]pyrimidine and imidazo[1,2-c]pyrimidine derivatives based on heterocyclic ketene aminals. Tetrahedron. 2012;68(1):319–322. doi: 10.1016/j.tet.2011.10.035. [DOI] [Google Scholar]
- 79.Elattar K.M., Mert B.D., Monier M., El-Mekabaty A. Advances in the chemical and biological diversity of heterocyclic systems incorporating pyrimido[1,6-a]pyrimidine and pyrimido[1,6-c]pyrimidine scaffolds. RSC Advances. 2020;10:15461–15492. doi: 10.1039/D0RA00411A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Monier M., Abdel-Latif D., El-Mekabaty A., Elattar K.M. Bicyclic 6 + 6 systems: the chemistry of pyrimido[4,5-d]pyrimidines and pyrimido[5,4-d]pyrimidines. RSC Advances. 2019;9:30835–30867. doi: 10.1039/C9RA05687D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elattar K.M., Mert B.D. Recent developments in the chemistry of bicyclic 6-6 systems: chemistry of pyrido[4,3-d]pyrimidines. RSC Advances. 2016;6:71827–71851. doi: 10.1039/C6RA12364C. [DOI] [Google Scholar]
- 82.Monier M., Abdel-Latif D., El-Mekabaty A., Elattar K.M. Bicyclic 6 + 6 systems: advances in the chemistry of heterocyclic compounds incorporated pyrimido[1,2-a]pyrimidine skeleton. Mini Rev. Org. Chem. 2020;17(6):717–739. doi: 10.2174/1389557519666190925161145. [DOI] [Google Scholar]
- 83.Lau C.S., Carrier D.J., Beitle R.R., Bransby D.I., Howard L.R., Lay J.O., Jr, Liyanage R., Clausen E.C. Identification and quantification of glycoside flavonoids in the energy crop Albizia julibrissin. Bioresour. Technol. 2007;98(2):429–435. doi: 10.1016/j.biortech.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 84.Kang T.H., Jeong S.J., Kim N.Y., Higuchi R., Kim Y.C. Sedative activity of two flavonol glycosides isolated from the flowers of Albizzia julibrissin Durazz. J. Ethnopharmacol. 2000;71(1-2):321–323. doi: 10.1016/S0378-8741(99)00202-0. [DOI] [PubMed] [Google Scholar]
- 85.Kang J., Huo C.H., Li Z., Li Z.P. New ceramides from the flower of Albizia julibrissin. Chin. Chem. Lett. 2007;18:181–184. doi: 10.1016/j.cclet.2006.12.042. [DOI] [Google Scholar]
- 86.Jupudi S.; Jubie, S.; Deepika, N.P; Dhanabal, S.P A new pyrimidines alkaloid from the roots of Tadehagi triquetrum (L.) H. Ohashi. . Nat. Prod. Research. 2019:16-18. doi: 10.1080/14786419.2019.1634716. [DOI] [PubMed] [Google Scholar]
- 87.Costa E.V., Pinheiro M.L.B., Xavier C.M., Silva J.R.A., Amaral A.C.F., Souza A.D.L., Barison A., Campos F.R., Ferreira A.G., Machado G.M.C., Leon L.L.P. A pyrimidine-β-carboline and other alkaloids from Annona foetida with antileishmanial activity. J. Nat. Prod. 2006;69(2):292–294. doi: 10.1021/np050422s. [DOI] [PubMed] [Google Scholar]
- 88.Brandio E., Monteiro A.P., Novaes P.S. Alves, D.Y. Alkaloids from Annona. JSM Biochem. Mol. Biol. 2017;4:1031. [Google Scholar]
- 89.Lin Y.L., Huang R.L., Chang C.M., Kuo Y.H. Two new puriniums and three new pyrimidines from heterostemma brownie. J. Nat. Prod. 1997;60:982–985. doi: 10.1021/np970159y. [DOI] [PubMed] [Google Scholar]
- 90.Pandhurnekar C.P., Meshram E.M., Chopde H.N., Batra R.J. Synthesis, characterization and biological activity of 4-(2-hydroxy-5(aryl-diazenyl)phenyl)-6-(aryl)pyrimidines-2-ols derivatives. organic chemistry international. 2013 [Google Scholar]
- 91.Imperatore C., Aiello A., D’Aniello F., Senese M., Menna M. Alkaloids from marine invertebrates as important leads for anticancer drugs discovery and development. Molecules. 2014;19(12):20391–20423. doi: 10.3390/molecules191220391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Plisson F., Piggott A.M. Predicting blood-brain barrier permeability of marine-derived kinase inhibitors using ensemble classifies reveals potential hits for neurodegenerative disorders. Mar. Drugs. 2019;17(2):81. doi: 10.3390/md17020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koepfli J.B., Mead J.F., Brockman J.A. Jr. Alkaloids of Dichroa febrifuga; isolation and degradative studies. J. Am. Chem. Soc. 1949;71(3):1048–1054. doi: 10.1021/ja01171a080. [DOI] [PubMed] [Google Scholar]
- 94.Ningthoujam S.S., Talukdar A.D., Nath D., Basar N., Singh K., Choudhary M.D. Febrifugine and its analogs: studies for their anti-malarial and other therapeutics properties. Studies in natural products chemistry. 2015;44:93–112. [Google Scholar]
- 95.Lamberton J.A., Johns S.R., Hart N.K., Willing R.J. Hexahydroimidazol-pyrimidines, a new class of alkaloids from Alchornea Javanensis. J. Chem. Soc. D. 1969;24:1484–1485. [Google Scholar]
- 96.Chung M.S., Han Y.N. A pyrrolo-pyrimidine alkaloid from glycyrrhiza uralensis. Arch. Pharm. Res. 1990;13:103–104. doi: 10.1023/A:1015804129806. [DOI] [Google Scholar]
- 97.Ibrahim S.R.M., Ebel R., Ebel R., Proksch P. Acanthomine A, a new Pyrimidine-β-Carboline Alkaloid from the Sponge Acanthostrongylophora Ingens. Nat. Prod. Commun. 2008;3(2):175–178. doi: 10.1177/1934578X0800300213. [DOI] [Google Scholar]
- 98.Ibrahim S.R.M., Mohamed G.A., Zayed M.F., Sayed H.M. Ingenines A and B, two new alkaloids from the Indonesian sponge Acanthostrongylophora ingens. Arzneimittel-Forschung/drug research. 2014;65:07. doi: 10.1055/s-0034-1384577. [DOI] [PubMed] [Google Scholar]
- 99.Taekara R., Basso T.O., Jimenez P.C., Costa-Lotufo L.V., Lopes N.P., Lopes J.L.C. Pyrimdine alkaloids from eudistoma vannamei. Rev. Bras. Farmacogn. 2015;25(6):698–700. doi: 10.1016/j.bjp.2015.08.001. [DOI] [Google Scholar]
- 100.Santos M.F., Harper P.M., Williams D.E., Mesquita J.T., Pinto E.G., da Costa-Silva T.A., Hajdu E., Ferreira A.G., Santos R.A., Murphy P.J., Andersen R.J., Tempone A.G., Berlinck R.G. Anti-parasitic guanidine and pyrimidine alkaloids from the marine sponge monanchora arbuscula. J. Nat. Prod. 2015;78(5):1101–1112. doi: 10.1021/acs.jnatprod.5b00070. [DOI] [PubMed] [Google Scholar]
- 101.Tadeusz A. Alkaloids - Secrets of Life- Alkaloid Chemistry. Biological Significance, Applications and Ecological Role; 2007, 334. [Google Scholar]