Abstract
Background
Mulberry, including several species belonging to genus Morus, has been widely used as a traditional medicine for a long time. Extracts and active components of mulberry have many positive neurological and biological effects and can become potential candidates in the search for new drugs for neurological disorders.
Objectives
We aimed to systematically review the medical literature for evidence of mulberry effects on the central nervous system.
Methods
We conducted a systematic search in nine databases. We included all in vivo studies investigating the effect of mulberry on the central nervous system with no restrictions.
Results
We finally included 47 articles for quality synthesis. Our findings showed that mulberry and its components possessed an antioxidant effect, showed a reduction in the cerebral infarct volume after stroke. They also improved the cognitive function, learning process, and reduced memory impairment in many animal models. M. alba and its extracts ameliorated Parkinson's disease-like behaviors, limited the complications of diabetes mellitus on the central nervous system, possessed anti-convulsant, anti-depressive, and anxiolytic effects.
Conclusion
Mulberry species proved beneficial to many neurological functions in animal models. The active ingredients of each species, especially M. alba, should be deeper studied for screening potential candidates for future treatments.
Keywords: Mulberry, Morus, neurology, systematic review, memory improvement, antidepression
1. INTRODUCTION
Mulberry is the generic name of species in the genus Morus of the Moraceae family. These plants are mostly found in Asia, Europe, America, and Africa. They grow in various conditions of climate, topography, and type of soil [1]. For a long time, mulberry was widely used in Chinese as a medicinal herbal treating several disorders, and several studies determined certainly its health benefits afterward [2, 3]. Some studies recorded the presence of phenolics, flavonoids, anthocyanins, and carotenoids in deeply colored mulberry, which might be responsible for its several potential effects [4-7]. Amongst these, M. indica root, M. lhou Koidz and flavonoids from these plants could be active compounds causing antioxidant and anti-inflammatory effects, as mice consumed these interventions saw a reduction of oedema and writhing response [8, 9]. Besides, many benefits of mulberry were also reported such as the protection against obesity, diabetic, neurotoxicity and hepatotoxicity [3, 10].
With regards to the neuroprotective effects, polyphenols, anthocyanin, and other phenolic compounds might be attributed to the protection against oxidative damage in the brain resulting in the improvement of brain functions, for example, improving the learning ability via the protection against neurotoxicity and the increase in neuron cells [11]. Nevertheless, the evidence seemed to be inconclusive with the limit of evidence of possible mechanisms of action in vivo models. For instance, the aged-related memory impairment in mice was improved by M. alba fruit powder but this fruit did not entirely show their positive effect in alcoholic mice [12, 13]. Other reports revealed that M. alba leaves possessed the anxiolytic-like activity in mice assessed by elevated plus maze (EPM) and hole-board test via the histaminergic system [14]. Notably, specific compounds that were responsible for these bioactivities have not been presented. This issue led to the arguments about mulberry’s efficiency and its application for potential treatments in the future.
Neurodegenerative diseases are a burden on both human health and finance. Since human has had greater longevity than ever, the numbers of patients with Alzheimer diseases (AD) and Parkinson disease (PD) are sharply increasing. Notably, it is estimated that over 100 million AD patients globally in 2050 [15]. Similarly, the total number of PD patients in 2030 could be doubled compared with their number in 2005 [16]. Unfortunately, there is no cure for both diseases. Besides, pharmacotherapy for AD can only focus on relieving the symptoms [17]. Therefore, it is beneficial to neurodegenerative patients, especially the elderly, to use supplementary food, which can help to enhance their brain activity apart from pharmacological treatments. Interestingly, mulberry is becoming a candidate for the treatment of both AD and PD through improving their symptoms and preventing age-related neurodegeneration. For example, some motor deficits related to PD in mice were improved by consuming M. alba extract [18]. Additionally, artoindonesianin O, mulberrofuran G (MG), albanol B and kuwanon isolated from M. alba were predicted to prevent the amyloid β (Aβ)-peptide plaque via the inihibition of phospho-extracellular signal-regulated protein kinases 1 and 2 (p-ERK1/2) or via the inhibition of β-site amyloid precursor protein cleaving enzyme 1 (BACE1) in vitro models [19, 20].
Although several papers reported the advantages of various species, there is no review of their therapeutic activities on the brain. We conducted a systematic review including papers showing reliable evidence to show a thorough insight into the advantages of mulberry, confirm the effects of the mulberry extract on the brain and nervous system, and suggest the active compounds which can be investigated for further studies of mulberry applications.
2. MATERIALS AND METHODS
2.1. Protocol Registration
We followed the Recommendations of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement to conduct this systematic review, as shown in the PRISMA checklist (Table 1). The protocol could be accessed at PROSPERO (CRD42015026620).
Table 1.
PRISMA checklist.
| Section/Topic | Checklist Item | Reported on Page # |
|---|---|---|
| TITLE | ||
| Title | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | ||
| Structured summary | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1 |
| INTRODUCTION | ||
| Rationale | Describe the rationale for the review in the context of what is already known. | 1-2 |
| Objectives | Provide an explicit statement of questions being addressed concerning participants, interventions, comparisons, outcomes, and study design (PICOS). | 2 |
| METHODS | ||
| Protocol and registration |
Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 2 |
| Eligibility criteria | Specify study characteristics (e.g., PICOS, length of follow-up), and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving a rationale. | 2 |
| Information sources | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search, and date last searched. | 2 |
| Search | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 2 |
| Study selection | State the process for selecting studies (i.e., screening, eligibility, included in a systematic review, and, if applicable, included in the meta-analysis). | 2 |
| Data collection process | Describe the method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 2 |
| Data items | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 2 |
| Risk of bias in individual studies |
Describe methods used for assessing the risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 2 |
| Summary measures | State the principal summary measures (e.g., risk ratio, the difference in means). | 2 |
| Synthesis of results | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | NA |
| Risk of bias across studies | Specify any assessment of the risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | N/A |
| Additional analyses | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | NA |
| RESULTS | ||
| Study selection | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 5 |
| Study characteristics | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 5 |
| Risk of bias within studies | Present data on the risk of bias of each study and, if available, any outcome-level assessment (see item 12). | 5 |
| Results of individual studies | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 5-22 |
| RESULTS | ||
| Synthesis of results | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | NA |
| Risk of bias across studies | Present results of any assessment of the risk of bias across studies (see Item 15). | NA |
| Additional analysis | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression | NA |
| DISCUSSION | ||
| Summary of evidence |
Summarize the main findings, including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 22-23 |
| Limitations | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). |
23 |
| Conclusions | Provide a general interpretation of the results in the context of other evidence and implications for future research. |
24 |
| FUNDING | ||
| Funding | Describe sources of funding for the systematic review and other support (e.g., the supply of data); the role of funders for the systematic review. |
25 |
2.2. Selection Criteria
Our inclusion criteria were (1) studies showing neurological effects of the mulberry genus Morus, (2) only including studies of the genus Morus, (3) studies dealing with humans or animals, and (4) no restriction on language, country, gender, age or study design. Exclusion criteria were: (1) unreliably extracted data, (2) overlapped data sets, (3) in vitro studies, (4) articles without available full-text and (5) theses, book chapters, editorials, author responses, conference papers, reviews, posters, letters, and patents.
2.3. Search Strategy
We performed our search in nine electronic databases including MEDLINE (PubMed), Scopus, Google Scholar, ISI Web of Science, POPLINE, the System for Information on Grey Literature in Europe (SIGLE), Global Health Library (GHL), Virtual Health Library (VHL), and the New York Academy of Medicine Grey Literature Report (NYAM) for studies published up to September 18, 2017. Details of the search terms for each database are presented in Table 2. A manual search was also performed by screening the reference of the included studies, the similar studies proposed by PubMed, Google Scholar on the first page, and the references of reviews relevant to our topic.
Table 2.
Detailed search strategy for nine database searches.
| No. |
Databases
(Total 9) |
Search Terms |
Results
Total = 1187 |
|---|---|---|---|
| 1 | PubMed | (mulberry OR Morus) AND (neurotoxicity OR neurotoxic OR Neuroprotection OR neuroinflammation OR neurodegenerative OR Alzheimer OR Parkinson OR dementia OR Neuroprotective OR neurodegeneration OR Huntington OR memory OR cognitive OR cognition OR learning OR perception OR intelligence OR brain OR CNS OR (central nervous system) | 118 |
| 2 | Scopus | (TITLE-ABS-KEY (mulberry OR Morus) AND TITLE-ABS-KEY (neurotoxicity OR neurotoxic OR Neuroprotection OR neuroinflammation OR neurodegenerative OR Alzheimer OR Parkinson OR dementia OR Neuroprotective OR neurodegeneration OR Huntington OR memory OR cognitive OR cognition OR learning OR perception OR intelligence OR brain OR CNS OR (central nervous system) | 203 |
| 3 | ISI (WOS) | (mulberry OR Morus) AND (neurotoxicity OR neurotoxic OR Neuroprotection OR neuroinflammation OR neurodegenerative OR Alzheimer OR Parkinson OR dementia OR Neuroprotective OR neurodegeneration OR Huntington OR memory OR cognitive OR cognition OR learning OR perception OR intelligence OR brain OR CNS OR (central nervous system) | 610 |
| 4 | WHO GHL | (mulberry OR Morus) AND (neurotoxicity OR neurotoxic OR Neuroprotection OR neuroinflammation OR neurodegenerative OR Alzheimer OR Parkinson OR dementia OR Neuroprotective OR neurodegeneration OR Huntington OR memory OR cognitive OR cognition OR learning OR perception OR intelligence OR brain OR CNS OR (central nervous system) | 97 |
| 5 | VHL | (mulberry OR Morus) AND (neurotoxicity OR neurotoxic OR Neuroprotection OR neuroinflammation OR neurodegenerative OR Alzheimer OR Parkinson OR dementia OR Neuroprotective OR neurodegeneration OR Huntington OR memory OR cognitive OR cognition OR learning OR perception OR intelligence OR brain OR CNS OR (central nervous system) | 93 |
| 6 | POPLINE | (mulberry OR Morus) AND (neurotoxicity OR neurotoxic OR Neuroprotection OR neuroinflammation OR neurodegenerative OR Alzheimer OR Parkinson OR dementia OR Neuroprotective OR neurodegeneration OR Huntington OR memory OR cognitive OR cognition OR learning OR perception OR intelligence OR brain OR CNS OR (central nervous system) | 0 |
| 7 | SIGLE | (mulberry OR Morus) AND (neurotoxicity OR neurotoxic OR Neuroprotection OR neuroinflammation OR neurodegenerative OR Alzheimer OR Parkinson OR dementia OR Neuroprotective OR neurodegeneration OR Huntington OR memory OR cognitive OR cognition OR learning OR perception OR intelligence OR brain OR CNS OR (central nervous system) | 1 |
| 8 | Google Scholar | (1) with all of the words: mulberry with at least one of the words: neurotoxicity OR neurotoxic OR Neuroprotection OR neuroinflammation OR neurodegenerative OR Alzheimer OR Parkinson OR dementia OR Neuroprotective OR neurodegeneration OR Huntington OR memory OR cognitive OR cognition OR learning OR perception OR intelligence OR brain OR CNS OR (central nervous system) where words occur: title of the article |
44 |
| (2) with all of the words: Morus with at least one of the words: neurotoxicity OR neurotoxic OR Neuroprotection OR neuroinflammation OR neurodegenerative OR Alzheimer OR Parkinson OR dementia OR Neuroprotective OR neurodegeneration OR Huntington OR memory OR cognitive OR cognition OR learning OR perception OR intelligence OR brain OR CNS OR (central nervous system) where words occur title of the article |
21 | ||
| 9 | NYAM | (1) Mulberry (2) Morus |
0 |
The search results were then imported into Endnote X7 (Thomson Reuters, USA) software to remove duplications. The references were screened based on the title and abstract with specified criteria by three independent reviewers. The full-texts of the remained papers were downloaded and separately screened for eligibility. We translated the articles in foreign languages into English. Discussions between reviewers and, if necessary, the consultation from the supervisor (NTH) resolved all discrepancies during the screening phases
2.4. Data Extraction
Randomly included studies were used to develop a pilot extraction sheet. Three independent reviewers extracted all data, and the supervisor (NTH) resolved any disagreement related to the data. We extracted articles’ essential characteristics (first author, year of publication, study design) along with essential characteristics of patient/animal characteristics (race, gender, age). Also, information including species, plants, compound, solvent for extraction, the dosage of each experiment were concomitantly retrieved. Additionally, tests of measuring the neurological effect of the mulberry species, as well as their outcomes and times of evaluation, were also reported.
2.5. Quality Assessment
Three independent reviewers assessed the quality of each paper based on SYRCLE tools, which were developed to assess methodological quality in animal experiments [21].
We consecutively evaluated ten domains, including sequence generation, baseline characteristics, allocation concealments, random housing, performance bias blinding, random outcome assessment, detection bias blinding, incomplete outcome data, selective outcome reporting and other sources of bias. We categorized the judgment of each reviewer on each domain as “low risk,” “high risk,” or “unclear risk” of bias. Any disagreement was resolved by discussions between reviewers and by consultation from a supervisor (NTH) to reach a consensus.
3. RESULTS
3.1. Search Results
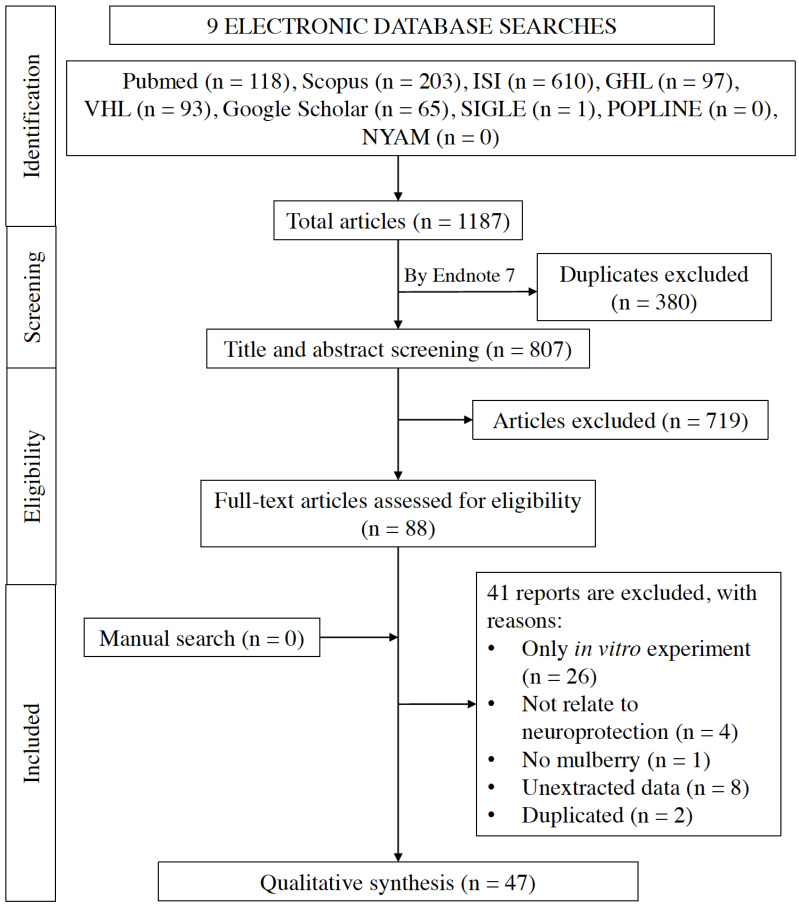
Our search retrieved 1187 studies. We performed title and abstract screening removed duplicates, screened full texts for inclusion according to our inclusion and exclusion criteria. After that, we performed a manual search in the reference of included studies, and we included 47 studies in the qualitative synthesis. We excluded the rest of the studies with reasons in the PRISMA flow diagram (Fig. 1).
Fig. (1).
PRISMA flow diagram of the included studies. ISI: Web of Science, SIGLE: The System for Information on Grey Literature in Europe, GHL: Global Health Library, VHL: Virtual Health Library, NYAM; The New York Academy of Medicine Grey Literature Report. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.2. Baseline Characteristics Of Included Studies
A summary of the included studies is presented in Table 3. All included studies are in vivo, no clinical trial on humans was found. There was a variety of mulberry species used, amongst which M. alba was the most popular one (33 studies). Other species such as M. nigra, M. atropurpurea, M. laevigata, and M. rubra were reported randomly. Methanol and ethanol extractions were the most regularly used solvents for extraction. The used doses of mulberry used and its active ingredients for neuroprotective effects varied considerably among the included studies from 0.2 mg/kg/day up to 10 g/kg twice a day. The administration was mostly via oral, except ten study treated animals via intraperitoneal injection (i.p). The treatments of mulberry fruits often saw positive effects including cognitive function improvement, anti-oxidant, anxiolytic, anti-depressant, and anti-ischemic activities. Multiple tests consisting of infarct volume measurement, cell viability, Morris water maze, Hole-Board Test, Horizontal Wire Test, Open Field Test, Forced swim test, etc. were performed to support the statements and findings of each study.
Table 3.
Baseline characteristics of included studies.
| Author/Year |
Plant/
Compounds |
Solvent for Extraction | Dose | Positive Control | Study Design | Effect | Tests | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nade/2010 [22] | M. alba leaves | Methanol | 100 - 300 mg/kg | N/A | Haloperidol-induced oxidative stress in mice | Antidopaminergic effect Antioxidant effect |
Behavioral testing Biochemical analysis |
||||||||||||||||
| Bauomy/2014 [42] | M. alba leaves | 70% ethanol | 200; 400; 800 mg/kg | N/A | Mice infected with S. mansoni | Antioxidant effect Neuroprotection against damage from S. mansoni |
Biochemical analysis | ||||||||||||||||
| Rebai/2017 [44] | M. alba leaves | 70% acetone | 100 μg/mL/kg | N/A | Glyphosate-induced toxicity in brain mice | Antioxidant effect | Biochemical analysis | ||||||||||||||||
| Choi/2000 [47] | M. alba leaves | NA | 100 and 300mg/kg | N/A | Healthy rats | Anti-oxidant effect | Biochemical analysis | ||||||||||||||||
| Choi/2000 [46] | M. alba leaves | NA | 100 - 300 mg/kg | N/A | Healthy rats | Antioxidant effect | Oxygen radical formation | ||||||||||||||||
| Kang/2006 [49] | M. alba leaves | 85% Methanol | 1, 10, 50 mg/ml | N/A | MCAO mice | Protection against ischemia | Infarct volume measurement Cells viability |
||||||||||||||||
| Tamtaj/2016 [51] | M. alba leaves | Alcoholic | 100, 200, 400 mg/kg | N/A | Healthy rats | Improve cognitive function | Morris water maze test | ||||||||||||||||
| Nade/2015 [30] | M. alba leaves | methanol | 25, 50, 100 mg/kg | Ondansetron | Scopolamine-induced cognitive deficits mice | Improve cognitive function | Elevated plus maze Morris water maze task |
||||||||||||||||
| Sattayasai/2008 [55] | M. alba leaves | NA | 100, 200, 500 or 1000 mg/kg | Desipramine, diazepam | Healthy mice | Antidepressant- without an anxiolytic-like effect | The chronic forced swimming test The elevated plus-maze The climbing test The coordination test The rota-rod test Sieve test. |
||||||||||||||||
| Yadav/2008 [28] | M. alba leaves | Methanol | 50, 100, 200 mg/kg | Diazepam | Healthy mice | Anxiolytic effect | Hole-Board Test Elevated plus maze test Open Field Test |
||||||||||||||||
| Lee/2013 [14] | M. alba leaves | 85% Methanol | 200 or 400 mg/kg | Diazepam | Healthy mice | Anxiolytic effect | Elevated plus maze test Hole-Board Test Horizontal Wire Test Open Field Test, |
||||||||||||||||
| Yadav/2008 [23] | M. alba leaves | Methanol | 50, 100, 200 mg/kg | N/A | Catalepsy model | Anti-dopaminergic effect | Footshock-induced aggression Sleeping time |
||||||||||||||||
| Kim/2003 [52] | M. alba leaves | NA | 10 mg/kg and 100 mg/kg | N/A | Healthy mice | Recovery from the central nervous system complications of diabetes mellitus. | New cell formation | ||||||||||||||||
| Nade/2010 [41] | M. alba root | Methanol | 25, 50 and 100mg/kg | Diazepam | Mice suffered chronic restraint stress | Anti-stress Antioxidant effect |
Passive shock avoidance test Elevated plus maze Open field test Biochemical analysis |
||||||||||||||||
| Nade/2009 [31] | M. alba root | Methanol | 25, 50, 100 mg/kg | Diazepam | Healthy mice | Adaptogenic activity Anti-stress activity Antioxidant |
Elevated plus maze Biochemical analysis |
||||||||||||||||
| Lee/2013 [56] | M. alba root | NA | 50, 100, and 200 mg/kg | RU486 (mifepristone) | Healthy mice | Antidepressant-like effects | Forced swim test Tail suspension test |
||||||||||||||||
| Lim/2014 [26] | M. alba root bark | NA | 30 and 100 mg/kg | RU486 (mifepristone) | Healthy mice | Antidepressant-like effects | Forced swim test | ||||||||||||||||
| Ye/2017 [57] | M. alba root bark | NA | 10 g/kg | N/A | Diabetes mice | Antidepressant-like effects | Forced swim test Open-field test Locomotor activity assessment |
||||||||||||||||
| Wattanathorn/2012 [12] | M. alba fruit | NA | 2, 10, 50 mg/kg | Donepezil | Cholinotoxin-induced cognitive decline in mice | Improve cognitive function Neuroprotection |
Morris water maze | ||||||||||||||||
| Kaewkaen/2012 [25] | M. alba fruit | Ethyl alcohol | 2, 10, 50 mg/kg | Vitamin C, Donepezil | MCAO mice | Improve cognitive function Neuroprotection |
Morris water maze | ||||||||||||||||
| Kaewkaen/2012 [50] | M. alba fruits | NA | 2, 10, 50 mg/kg | Vitamin C | MCAO mice | Improve cognitive function Neuroprotection |
Morris water maze Hot plate test |
||||||||||||||||
| Wattanathorn/2012 [13] | M. alba fruit | NA | 2, 10, 50 mg/kg | Vitamin C, Donepezil | Alcoholic mice | Improve cognitive function Neuroprotection |
Morris water maze | ||||||||||||||||
| Kaewkaen/2012 [32] | M. alba fruit | Ethyl alcohol | 2, 10, 50 mg/kg | Donepezil | MCAO mice | Improve cognitive function Neuroprotection |
Morris water maze | ||||||||||||||||
| Kim/2013 [52] | M. alba fruit | Ethanol | 20, 100, 500 mg/kg | Donepezil | Healthy mice | Improve cognitive function | Object recognition test Step-through passive avoidance test |
||||||||||||||||
| Kim/2015 [53] | M. alba fruit | Ethanol | 0.1, 1, 10, 100 microgram/ml | N/A | Alzheimer disease-like models | Improve cognitive function | Novel object recognition test Y maze test |
||||||||||||||||
| Gu/2017 [18] | M. alba fruit | 70% ethanol | 250 mg/kg | N/A | Parkinson disease model | Protection against PD-like symptoms | Olfactory test Pole test Open filed test |
||||||||||||||||
| Kim/2010 [61] | M. alba fruit | 70% ethanol | 500 mg/kg | N/A | Parkinson disease model | Protection against PD-like symptoms | Behavioral test | ||||||||||||||||
| Hwang/2004 [63] | M. alba fruit | Not reported | Not reported | N/A | Healthy mice | MAO activity modulation | Biochemical analysis | ||||||||||||||||
| Khan/2015 [59] | M. alba stem bark | Methanol | 100, 200, 250, 500 mg/kg | Diazepam | Healthy mice | Sedative effect | Open field test Hole cross test |
||||||||||||||||
| Kim/2015 [54] | M. alba leaves and fruit mixture | Ethanol | 0.2, 0.5, 1 g/kg/day | N/A | Obese mice | Improve cognitive function | Novel object recognition test | ||||||||||||||||
| Turgut/2015 [33] | M. nigra leaves | Methanol | 50, 100 mg/kg | N/A | ᴅ-galactose-induced aging mice | Improve cognitive function | Morris water maze | ||||||||||||||||
| Dalmagro/2017 [24] | M. nigra leaves | Hot water | 3–100 mg/kg | Fluoxetine | Healthy mice | Antidepressant-like effects | Forced swim test Tail suspension test Biochemical analysis |
||||||||||||||||
| Syringic acid | 0.1 – 10 mg/kg | ||||||||||||||||||||||
| Shih/2010 [43] | M. atropurpurea fruit | Methanol | NA | N/A | Aging mice | Improve cognitive function Antioxidant effect |
Avoidance response tests. Oxidant status assays |
||||||||||||||||
| Srikanta/2016 [34] | M. rubra fruit | NA | 20 mg/kg | Resveratrol | Streptozotocin-induced diabetic rats | Antioxidant effect | Physicochemical analysis Antioxidant Status |
||||||||||||||||
| Tubaş/2017 [60] | M. rubra fruit | Not reported | 5, 10 mg/kg | N/A | Penicillin-induced epileptiform mice | Anti-epileptic activity | Electrocorticogram records | ||||||||||||||||
| Barman/1980 [9] | M. indica leaves | Methanol | 200 mg/kg | N/A | Healthy mice | Sedative effect | Spontaneous activity Anti-convulsant effect |
||||||||||||||||
| Samuel/2016 [48] | Mulberry variety AR-14 leaves | NA | 100 mg/kg p.o. | Resveratrol | MCAO mice | Protection against focal cerebral ischemia | Neurobehavioral test Histological studies |
||||||||||||||||
| Samuel/2016 [40] | Nine varieties of M. alba and M. indica | Water | 100 mg/kg | N/A | Rotenone- induced oxidative stress | Antioxidant effect | Biochemical analysis | ||||||||||||||||
| El-baz/2016 [45] |
M. alba fruit M. rubra fruit |
Ethanol | 300 mg /Kg | Donepezil | Alzheimer induced rats | Neuroprotection against Alzheimer disease | 8-OHdG/2-dG ratio DHCR24 and FKBP1B genes ROS level Apoptotic related enzymes |
||||||||||||||||
| Aditya Rao/2012 [29] |
M. alba leaves M. laevigata leaves |
Petroleum, ether, chloroform, methanol | 200 and 400 mg/kg | N/A | Healthy mice | Sedative effect | Locomotor activity | ||||||||||||||||
| Hong/2017 [39] | Mulberrofuran G the root bark of M. bombycis |
NA | 0.2, 1, and 5 mg/kg | N/A | MCAO mice | Protection against ischemia | Infarct volume measurement | ||||||||||||||||
| Kang/2006 [37] | Cyanidin-3-O-beta-ᴅ-glucopyranoside from M. alba | 1% HCl–MeOH | 10, 20, 30 µg/ml | N/A | MCAO mice | Protection against ischemia | Infarct volume measurement Cells viability |
||||||||||||||||
| Andrabi/2004 [38] | Oxyresveratrol from mulberry wood | NA | 2, 10, 20 and 30 mg/kg | N/A | MCAO mice | Protection against ischemia | Infarct volume measurement Histological analysis |
||||||||||||||||
| Lim/2016 [35] | Sanggenon G isolated from the root bark of M. alba | Ethyl acetate | 5, 10 and 20 mg/kg |
Yohimbine | Healthy mice | Antidepressant-like effects | Forced swim test Open-field test |
||||||||||||||||
| Lim/2015 [36] | Sanggenon G isolated from the root bark of M. alba | Ethyl acetate | 30 mg/kg | Imipramine | Healthy mice | Antidepressant-like effects | Forced swim test | ||||||||||||||||
| Gupta/2014 [58] | Morusin from M. alba stem bark | NA | 5, 10 mg/kg | Diazepam | Healthy mice | Sedative effect Anticonvulsant activity |
Convulsion model Locomotor activity |
||||||||||||||||
| Ma/2014 [27] | Mulberry flavonoid from M. alba leaves | NA | 0.3 g/kg | Methycobal | Alloxan-induced diabetic rats | Recovery of peripheral nerve injury in diabetic rats |
Histopathological examination | ||||||||||||||||
MCAO: Middle Cerebral Artery Occlusion, N/A: Not applied.
3.3. Quality Assessments
We evaluated almost all included studies (44/47) as a high risk of bias via SYRCLE tools evaluation. The three categories of selection bias, performance bias, and detection bias were frequently determined as high risk. In particular, all studies did not report a method to randomly divide animals into groups and pick them for assessing outcomes. Only six studies performed the blinding of caregivers and researchers [22-27]. Two studies stating the observators were blind in assessing the outcomes were considered low bias for blinding of detection bias [22, 26].
Additionally, 43 among 47 studies reporting the results of all experiments are rated with the low bias of selective outcome reporting. Table 4 represents the details of each item evaluation.
Table 4.
Quality assessment of included studies by using SYRCLE tool.
|
Author/
Year |
Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Other | Overall Assessment | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sequence generation |
Baseline characteristics | Allocation concealment | Random housing | Blinding | Random outcome assessment | Blinding | Incomplete outcome data | Selective outcome reporting | Other sources of bias |
||
| Nade/2010 [22] | + | + | + | + | - | + | - | - | - | - | High risk |
| Samuel/2016 [40] | + | + | + | + | + | + | + | - | - | ? | High risk |
| Nade/2010 [41] | + | + | + | + | + | + | + | - | - | - | High risk |
| Turgut/2015 [33] | + | - | + | + | + | + | + | + | ? | ? | High risk |
| Bauomy/2014 [42] | + | + | + | + | + | + | + | - | - | ? | High risk |
| Shih/2010 [43] | + | - | + | + | ? | + | + | ? | - | - | High risk |
| Rebai/2017 [44] | + | - | + | - | + | + | + | - | - | ? | High risk |
| El-baz/2016 [45] | + | + | + | + | + | + | + | - | - | ? | High risk |
| Wattanathorn/2012 [12] | + | + | + | + | ? | + | + | ? | ? | ? | High risk |
| Kaewkaen/2012 [25] | + | + | + | + | - | + | + | - | - | - | High risk |
| Choi/2000 [47] | + | + | + | + | + | + | + | - | - | - | High risk |
| Choi/2000 [46] | + | + | + | + | + | + | + | - | - | - | High risk |
| Srikanta/2016 [34] | + | - | + | + | + | + | + | + | - | ? | High risk |
| Dalmagro/2017 [24] | + | - | + | + | - | + | + | + | + | - | High risk |
| Hong/2017 [39] | + | - | + | + | + | + | + | - | - | ? | High risk |
| Samuel/2016 [48] | + | - | + | + | + | + | + | - | - | ? | High risk |
| Kang/2006 [49] | + | + | ? | + | ? | + | + | + | - | ? | High risk |
| Kang/2006 [37] | + | + | + | + | + | + | + | + | - | ? | High risk |
| Andrabi/2004 [38] | + | - | ? | + | ? | + | + | + | - | ? | High risk |
| Kaewkaen/2012 [50] | + | + | + | + | + | + | + | ? | - | ? | High risk |
| Wattanathorn/2012 [13] | + | + | + | + | + | + | + | - | - | - | High risk |
| Kaewkaen/2012 [32] | + | - | + | + | ? | + | + | ? | - | - | High risk |
| Tamtaj/2016 [51] | + | - | + | ? | + | - | + | + | - | - | High risk |
| Nade/2015 [30] | + | + | + | + | + | + | + | - | - | - | High risk |
| Nade/2009 [31] | + | + | + | + | + | + | + | - | - | - | High risk |
| Kim/2013 [52] | + | + | + | + | ? | + | + | ? | - | - | High risk |
| Kim/2015 [53] | + | + | + | + | ? | + | + | - | - | - | High risk |
| Kim/2015 [54] | + | + | + | + | ? | + | + | - | - | - | High risk |
| Sattayasai/2008 [55] | + | + | + | + | + | + | + | ? | - | ? | High risk |
| Lim/2016 [35] | + | + | + | + | + | + | + | + | ? | - | High risk |
| Lee/2013 [56] | + | + | + | + | ? | + | + | ? | - | - | High risk |
| Lim/2014 [26] | + | + | - | ? | - | + | - | - | - | - | Low risk |
| Lim/2015 [36] | + | - | + | + | + | + | + | - | - | + | High risk |
| Ye/2017 [57] | + | - | + | + | + | + | + | ? | - | - | High risk |
| Yadav/2008 [28] | + | + | + | + | + | + | + | - | - | - | High risk |
| Lee/2013 [14] | + | + | + | + | + | + | + | + | - | - | High risk |
| Gupta/2014 [58] | + | + | + | + | + | + | + | - | - | ? | High risk |
| Aditya Rao/2012 [29] | + | - | + | + | + | + | + | - | - | - | High risk |
| Khan/2015 [59] | + | + | + | + | + | + | + | - | - | ? | High risk |
| Barman/1980 [9] | + | + | + | + | + | + | + | ? | - | ? | High risk |
| Tubaş/2017 [60] | + | - | + | ? | + | + | + | + | - | - | High risk |
| Yadav/2008 [23] | + | + | + | + | - | + | + | - | - | - | High risk |
| Gu/2017 [18] | + | - | + | + | + | + | + | ? | - | - | High risk |
| Kim/2010 [61] | + | + | + | + | + | + | + | ? | - | - | High risk |
| Kim/2003 [52] | + | + | + | + | + | + | + | ? | - | ? | High risk |
| Ma/2014 [27] | + | - | ? | + | - | + | + | - | - | - | Low risk |
| Hwang/2004 [63] | + | + | + | + | + | + | + | ? | - | ? | High risk |
+: high risk. -: low risk. ?: unclear.
chromatography column, and finally the residual resin was extracted with chloroform (yield = 0.34% of the wood weight). Finally, MG (a prenylated flavonoid) was isolated from the methanol extract of dried root bark of M. bombycis [39]. The purified process involved in varied solvents including n-hexane, chloroform, and ethyl acetate, then fractionalized by methanol via a chromatography column. Detail of constituents of the extracts in this review is presented in Table 5.
3.4. Phytochemical Screening Of Studied Extracts
The methanol extract of M. alba leaves contains a wide range of phytochemical groups, including phenolic, flavonoid, tannin, sterol, alkaloid, saponin, anthocyanin, anthraquinone, carbohydrate, protein, and amino acid [22, 23, 28, 29]. From this extract, it could be found the presence of tannins, alkaloids, glycosides, and flavonoids in the ethyl acetate soluble fraction (EASF) [30]. Extracting its leaves with nonpolar solvents such as petroleum ether or chloroform, we could observe the presence of steroids and glycosides [29]. However, the petroleum ether leaves extract had saponins, flavonoids and tannins while chloroform leaves extract showed terpenoids, alkaloids and carbohydrates. The EASF of M. alba methanol root extract had fewer phytochemical groups compared to the leaves extract, as only phenolics, flavonoids and alkaloids were reported [31]. For M. alba fruit powder, phenolics and anthocyanidins were found [12]. Extracting M. alba fruit with ethanol, we could obtain a high amount of anthocyanins, a smaller quantity of flavonoids, and phenolics [32].
M. nigra leaves extract mainly contains phenolics [24, 33]. However, the major compound of hot water extract is syringic acid (80.57%), while the methanol extract only has a small concentration of this compound. Instead, the methanol leaves extract of M. nigra has a significant quantity of vanillic acid and chlorogenic acid [33]. Other phenolic acid are gallic, protocatechuic, p-hydroxybenzoic, caffeic, p-coumaric, ferulic, o-coumaric, rosmarinic, and trans-cinnamic acids.
The constituents of M. laevigata leaves were also varied [29]. The methanol extract consists of steroids, terpenoids, saponins, alkaloids, flavonoids, tannins, carbohydrates, proteins and amino acids. In the chloroform extract, there are steroids, terpenoids, alkaloids and carbohydrates while the petroleum ether extract has saponins, flavonoids, tannins, and carbohydrates.
In the wine made from M. rubra fruit, they determined the presence of flavonoids and phenolics, in which several polyphenols were determined namely gallic acid, gallocatechin, catechin, caffeic acid, ferulic acid, p-coumaric acid, cinnamic acid, trans-resveratrol, trans-piceid, cis-resveratrol, cis-piceid [34].
Some purified compounds that showed positive effects in this review could be extracted from mulberry as followed. Sanggenon G was extracted from root or root bark of M. alba by methanol, then fractionalized the parent extract by ethyl acetate [35, 36]. Its concentration was indicated of 0.446 ± 0.007 mg/g. An anthocyanin, cyanidin-3-O-β-ᴅ-glucopyranoside (C3G), could be extracted from M. alba fruit by the isolation from 1% HCl-Methanol extract [37]. Oxyresveratrol (a hydroxystilbene) was obtained from M. alba wood after several steps [38]. First, the M. alba wood was extracted with 96% ethanol, then purified by a mixture of chloroform-methanol via a silica vacuum liquid
3.5. The Anti-Oxidant Effect In The Brain
Different extracts from all parts of types of mulberry (M. alba, M. nigra, M. rubra and M. atropurpurea) showed their antioxidant effect on a wide range of animal models (Table 6).
Table 6.
Antioxidant effect of Morus on brain.
| Refs. | Species | Part Used | Solvent |
Dose*
(Administration) |
Positive Control | Animal Model | Model | Test Duration | Main Results | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nade et al. [22] | M. alba | Leaves | Methanol | 100-300 mg/kg/day (p.o) | N/A | Male Wistar strain rats, 170–220g | Haloperidol-induced oxidative stress | 21 days | ↑ CAT and SOD levels ↓ LPO and NO levels |
||||
| Choi et al. [47] | M. alba | Leaves | Methanol | 100-300 mg/kg/day (p.o) | N/A | Male Sprague Dawley, 160±10g | Healthy rats | 6 weeks | ↓ BORs levels by 25.1%, IORs levels by 16.5%, LPO levels by 18.1% and OP levels by 14.2%. | ||||
| Choi et al. [46] | M. alba | Leaves | Methanol | 100-300 mg/kg/day (p.o) | N/A | Male Sprague Dawley, 260±20g | Healthy rats | 6 weeks | ↓ Hydroxyl radical by 21.1%, superoxide radical by 12%, LPO by 12.26%, and OP levels by 13.77%. ↑ Mn-SOD activity by 18.6%, Cu/Zn-SOD activity by 17.7%, and GPx activity by 23.9%. |
||||
| Bauomy et al. [42] | M. alba | Leaves | Methanol | 200, 400, 800 mg/kg/day (p.o) | N/A | 9-11 weeks male Swiss albino mice | Mice infected with Schistosoma mansoni | 10 days | ↑GSH and CAT levels in normal and infected mice in a dose-dependent manner. ↑ TAC in infected mice. |
||||
| Rebai et al. [44] | M. alba | leaves | Cold acetone | 100 μg/kg/day (i.p) | N/A | Female Wistar rats, 180–240g | Glyphosate-induced toxicity in brain mice | 15 days | ↓ LDH activity, PC and MDA levels ↑ SOD activity |
||||
| Nade et al.. [41] | M. alba | root | methanol | 25, 50 and 100mg/kg/day (p.o) | Diazepam | Male Wistar rats, 150–180g | Chronic restraint stress | 10 days | ↑ CAT, GSH, SOD level ↓ LPO level |
||||
| Kaewkaen et al. [32] | M. alba | fruits | Ethanol | 2, 10 and 50 mg/kg/day (p.o) | Donepezil | 8 weeks male Wistar rats, 300–350g | Vascular dementia | 28 days | ↓MDA level and ↑ SOD and GSH-Px activity. ↑ CAT insignificantly |
||||
| Wattanathorn et al. [12] | M. alba | fruits | N/A | 2, 10 and 50 mg/kg/day (p.o) | Donepezil | Male Wistar rats, 180-200g | Cholinotoxin-induced cognitive decline in mice | 2 weeks | ↓MDA level | ||||
| Turgut et al. [33] | M. nigra | leaves | methanol | 50,100 mg/kg/day (p.o) | N/A | 8 weeks male BALB/c mice | ᴅ-galactose-induced aging mice | 5 days | ↓ MDA levels, and ↑SOD, GPx and CAT activities | ||||
| Dalmagro et al. [24] | M. nigra | leaves | Water | 3, 10, 30, 100 mg/kg/day (p.o) | Fluoxetine | Male Swiss mice, 30-40g | Healthy mice | Acute: 1 day Chronic: 7 days |
Acute and chronic treatment did not change the levels of TBARS, NPHS levels. ↓ PC level only at 30 mg/kg. ↓ NO level in the brain at 30 and 100 mg/kg with subchronic treatment. |
||||
| Syringic acid from M. nigra | N/A | N/A | 0.1, 1, 10, 100 mg/kg/day (p.o) | ↑ TBARS in the brain ↓ PC and NO levels in the brain |
|||||||||
| El-baz et al. [45] |
M. alba M. rubra |
Fruit | Ethanol | 300 mg/kg/day (p.o) | Donepezil | Male Albino rats, 180-200 g | Alzheimer induced rats | 6 weeks | ↑109.54 – 118.09% of LPO levels and 55.17 – 54.6% of GSH levels compared with AD-induced mice | ||||
| Srikanta et al. [34] | Wine made from M. rubra |
fruit | N/A | 20 mg/kg/day (p.o) | Resveratrol | 8 weeks male Wistar rats, 200g | Streptozotocin-induced diabetic rats | 6 weeks | No insignificant change of antioxidant capacity in the brain of diabetic rats | ||||
| Shih et al. [43] | M. atropurpurea | fruit | Methanol | 100; 500 mg/kg/day (p.o) | N/A | 6 months male SAMR1 and SAMP8 mice | Senescence-accelerated mice | 12 weeks | ↑ GST and CAT levels at 100 mg/kg, and further GPx level at 500 mg/kg No significant improvement of GRd was observed |
||||
| Samuel et al. [40] | Nine varieties of M. alba and M. indica |
leaves | Water | 100 mg/kg/day (p.o) | N/A | Male Sprague Dawley rats, 200±10g | Rotenone- induced oxidative stress | 1 hours (pretreatment) | ↓ MDA levels by 50.49% and 41.36% when treating with S-146 and BR-2 extract, respectively ↓ SOD level by 54.01% and 40.18% when treating with S-146 and AR-14 extract |
||||
BOR: basal oxygen radical, CAT: catalase, GPx: glutathione peroxidase, GRd: glutathione reductase, GSH: glutathione, GST: glutathione S-transferase, IOR: Induced oxygen radical, i.p.: intraperitoneal injection, LDH: lactate dehydrogenase, LPO: lipid peroxide, MDA: malonyldialdehyde, N/A: not applied, NPHS: non-protein sulfhydryls, NO: Nitrite, PC: Protein carbonyl, p.o: per oral, TAC: total antioxidant capacity, TBARS: thiobarbituric acid reactive substance. *weight of extract per body weight of the animal.
Our study showed that mulberry reversed the disorder of redox system in brain caused by rotenone [40], chronic stress [41], haloperidol [22], ᴅ-galactose [33], Schistosoma mansoni infection [42], aging [43], glyphosate [44], Alzheimer [45], and cholinotoxins [12]. These triggers led to the decrease in levels of antioxidant enzymes in the body including catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione S-transferase (GST), glutathione reductase (GRd), and the contents of reduced glutathione (GSH). Also, they increased lactate dehydrogenase (LDH) activity, nitrite (NO), and malonyldialdehyde (MDA) levels, which are formed by the oxidation. There was an exception that SOD, CAT, and peroxidase activities increased in order to respond to stress [41], and the neurotoxicity caused by glyphosate [44]. However, all those changes were almost normalized by the acute or subchronic consumption of mulberry except the case of GRd activity reported by Shih et al. [43]. This study indicated that methanol extract of M. alba fruit insignificantly increased that enzyme even at a high dose (500 mg/kg/day after 12 weeks of treatment). Besides, 10 days of treatment at all doses of M. alba leaves extract improved total antioxidant capacity (TAC) in mice after 46 days infected with S. mansoni [42].
In the model of vascular dementia, mice were pretreated with the ethanol extract of M. alba fruit at 10 and 50 mg/kg 7 days before and 21 after occlusion of the right middle cerebral artery (MCAO). The results showed the enhanced activities of SOD, CAT, and GPx, although this elevation of CAT activity was not remarkable [32]. Interestingly, the antioxidant effect of mulberry was also observed in normal mice. Choi et al. [46, 47] demonstrated that treating 100 and 300 mg/kg/day after 6 weeks of methanol extract of M. alba leaves could reduce hydroxyl radical, superoxide radical, lipid peroxide, basal and induced oxygen levels in both mitochondrial and microsome in the brain. Moreover, the results from this group of authors clarified that M. alba leaves extract rose the activities of both Mn-SOD in brain mitochondrial and Cu/Zn-SOD in brain cytosol [46].
There were only two studies that did not show the positive antioxidant effect of mulberry in the brain. Srikanta et al. [34] observed that 6 weeks of the treatment of wine made from M. rubra fruit did not improve the total antioxidant capacity after the oxidation caused by streptozotocin in diabetic mice. Similarly, Dalmagro et al. [24] also reported that almost all dose of the aqueous extract of M. nigra did not dramatically influence the contents of oxidant markers in the brain, like protein carbonyl (PC), non-protein thiol groups (NPSH), thiobarbituric acid reactive substances (TBARS), and NO level compared to normal mice. The subacute treatment of its primary compound - syringic acid (SA) acted as a pro-oxidant compound illustrated by the downgrade of NO level in the brain.
3.6. Protection Against Ischemia
M. alba leaves and the riched gamma-aminobutyric acid (GABA) leaves, M. alba fruit extract (MLE) are positive candidates to screen for the prevention of ischemic injury.
Regarding the neuroprotective effect of mulberry against ischemia, Samuel et al. [48] compared the strength between MLE-AR-14, a freeze-dried solid leaf extracted from the mulberry variety AR-14, and resveratrol. Using doses of 50 and 100 mg/kg of MLE- AR-14 orally one hour before middle cerebral artery occlusion/reperfusion (MCAO/R) induced a similar reduction of infarct size in mice compared to 50 and 100 mg of resveratrol (34%, 65% vs. 55%, 76%, respectively). This study also pointed out a notable active effect of those two interventions, even on a post-ischemic injury. Namely, after 6 hours of ischemic injury, treatment with MLE-AR-14 (50 mg and 100 mg) provided a neuroprotective effect of about 28% and 54%, respectively; whereas the percentage of ischemic brain reduction was a bit higher, by 53% and 68% for doses of 50 mg and 100 mg by resveratrol, respectively. The effect against neural cell death induced by cerebral ischemia was hypothesized to be involved in the free radical scavenging activity. The authors observed the attenuation of MDA level (an oxidative stress marker) and the upregulation of glutathione levels (an endogenous antioxidant) in the blood in the presence of MLE-AR-14 or resveratrol, although resveratrol showed significantly more effective.
However, Kang et al. reported that oral treatment of methanol M. alba leaves extract (200 mg/kg) 30 minutes after the MCAO did not reduce the infarct volume of the mice brain. The neuroprotective effect against MCAO- induced mice was only enhanced when conducting the accumulation of GABA in M. alba leaves (GAML). GAML shortened the cerebral injury size by 31% using a dose of 200 mg/kg orally compared with the control group [49].
This result was quite similar to the effect of positive control (5 mg/kg intravenously injected edaravone). The mulberry fruit extracted with 1% HCl-MeOH also reduced the ischemic brain volume by 26% [37].
Additionally, purified compounds extracted from M. alba fruit (C3G), M. alba fruit (oxyresveratrol), and M. bombycis root bark (MG) showed neuroprotective effects against cerebral ischemia [37]. Treatment of C3G 30 minutes after MCAO (10 mg/kg per orally), successfully reduced the ischemic brain volume by 18%. Similarly, intraperitoneally injecting 10 and 20 mg/kg of oxyresveratrol (twice: before and after MCAO) decreased the injured brain volume at days 3 after stroke by 54% and 63%, respectively [38]. The intraperitoneal administration of MG (0.2, 1, and 5 mg/kg) 30 minutes before MCAO/R showed a similar effective impact on the reduction of injured brain zone in mice compared to carnosine (25, 50, and 75 mg/kg, i.p.) [39]. The injured brain zone was 39.0 ± 6.4%, 26.0 ± 7.4%, and 19.0 ± 4.3% by the dose of 0.2, 1, and 5 mg/kg, respectively, in MG group; and the cerebral infarct size was 50.6 ± 6.2%, 40.0 ± 6.5%, and 19.6 ± 4.2% at dose 25, 50, and 75 mg/kg, respectively, in carnosine group.
Regarding their probable mechanism, these compounds protected the brain cells after MCAO via varied pathways. C3G and MLE prevented the polymorphonuclear leukocytes from infiltrating into cerebral focal ischemic tissue after stroke, which might be helpful for cell survival [37]. Meanwhile, oxyresveratrol prevented the cell death via the inactivation of apoptotic markers including cytosolic cytochrome c release and caspase-3 [38], and MG potentially inhibited the reactive oxygen species (ROS) generation via the decrease in nicotinamide adenine dinucleotide phosphate oxidase (NOX) enzyme activation and NOX4 protein expression [39].
3.7. Effect On Cognitive Functions
Fourteen articles reported the therapeutic activities of mulberry on cognitive impairment using various models, including the Morris water maze test, object recognition test, passive or active avoidance test, and elevated plus-maze model. Various forms of mulberry such as ethanol extracts of M. alba leaves and fruit, methanol extract of M. alba leaves, M. nigra leaves and M. atropurpurea fruit, M. alba fruit powders and a mixture of M. alba leaves and fruit (2:1) showed their effect on memory improvement at a wide range of dosages (Table 7).
Table 7.
The activities of mulberry on learning and memory.
| Refs. | Species | Part Used | Solvent | Dose* (Administration) | Positive Control | Animal Model | Model of Study (Duration) | Main Results | Conclusion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wattanathorn et al. [12] | M. alba | Fruit | N/A | 2,10, 50 mg/kg/day (p.o) x 2 weeks | Donepezil | Male Wistar rats, 180-200g | MMT (4 days) | ↓ Escape latency time at all doses ↑ Retention time at 2, 50 mg/kg |
Enhancing memory in ageing mice. | ||||||||
| Wattanathorn et al. [13] | M. alba | Fruits | N/A | 2,10, 50 mg/kg/day (p.o) x 2 weeks | Vitamin C Donepezil | 8 weeks male Wistar rats, 180-220g | MMT (14 days) | ↓ Escape latency at all doses in single-dose administration and on days 7, 14 No significant change of retention time |
Enhance spatial memory in alcoholic mice. | ||||||||
| Kaewkaen et al. [50] | M. alba | Fruit | N/A | 2, 10, 50 mg/kg/day (p.o) x 2 weeks | Vitamin C | 8 weeks Male Wistar rats, 300-350g | MMT (14 days) | ↓ Escape latency time at 2, 10 mg/kg in a healthy condition in a single dose and after 7 days. No changes in retention time. ↑Retention time at 2, 10 mg/kg in stroke condition 14 days after stroke. No changes in escape latency. |
Protect against memory impairment in MCAO mice and improve neuron density in the hippocampus. | ||||||||
| Kaewkaen et al. [25] | M. alba | Fruits | Ethanol | 2,10 and 50 mg/kg/day (p.o) x 28 days | Vitamin C Donepezil | 8 weeks male Wistar rats, 300-350g | MMT (21 days) | ↓ Escape latency time at 50 mg/ kg in single-dose administration in healthy/stroke condition 7 days after stroke ↑ Retention time at 2, 10 mg/kg on a single dose in healthy condition ↑ Retention time at 2, 10, 50 mg/kg stroke condition after days 7 and 14. No change observed in 21 days. |
Enhance cognitive functions in the MCAO rats. | ||||||||
| Kaewkaen et al. [32] | M. alba | Fruit | Ethanol | 2, 10, 50 mg/kg/day (p.o) x 28 days | Donepezil | 8 weeks male Wistar rats, 300-350g | MMT (21 days) | ↓ Escape latency time at 5 and 10 mg/kg after 21 days. No change in retention time |
Enhance memory of MCAO mice | ||||||||
| Kim et al. [52] | M. alba | Fruit | Ethanol | 20, 100 and 500 mg/kg/day (p.o) x 7 days | N/A | 6 weeks male ICR mice, 25–28 g | PAT | ↑ Retention time at 100 and 500 mg/kg | Enhance memory via up-regulating nerve growth factor. | ||||||||
| ORT | ↑ Recognition time at 100 and 500 mg/kg | ||||||||||||||||
| Kim et al. [53] | M. alba | Fruit | 70% Ethanol | 20, 100, and 500 mg/kg/day (p.o) x 14 days | N/A | 6 weeks male ICR mice, 25–28 g | NORT | ↑ Novel object recognition index in a dose-dependent manner | Protect cognitive function and survival neurons in Alzheimer disease-like models. | ||||||||
| Y-maze test (14 days) | ↑ Spontaneous alteration | ||||||||||||||||
| Tamtaj et al. [51] | M. alba | Leaves | Ethanol | 100, 200, 400 mg/kg/day (p.o) x 1 month | N/A | Male Wistar rats, 250 g | MMT (4 days) | ↓ Time to find the hidden platform at all doses in the learning stage ↓ Time to find the hidden platform at 400 mg/kg in rehearsal stage |
Improve the learning process at all dose Improve spatial memory at 400 mg/kg |
||||||||
| Kim et al. [54] | M. alba | Leaves and fruits | 70% Ethanol | 1 g/kg/day (p.o) x 12 weeks | N/A | 4 weeks male C57BL/6 mice, 23-25 g | NORT | ↑ Memory index by 78.63% | Recover memory function in obese mice. | ||||||||
| Nade et al. [31] | M. alba | Root | Methanol/ Ethyl acetate |
25, 50 and 100 mg/kg/day (p.o) x 21 days | Diazepam | Male Wistar rats, 150-180g | EPM (21 days) | ↓ Transfer latency on days 7, 10, 21 at all doses | Recover cognitive function in mice suffering chronic footshock stress |
||||||||
| Nade et al. [41] | M. alba | Root | Methanol/ Ethyl acetate |
25, 50 and 100 mg/kg/day (p.o) x 10 days | Diazepam | Male Wistar rats, 150-180g | EPM (5 and 10 days) | ↓ Transfer latency on days 5, 10 at all doses | Recover cognitive function in mice suffering chronic restraint stress | ||||||||
| Nade et al. [30] | M. alba | Leaves | Methanol/ Ethyl acetate |
25, 50 and 100 mg/kg/day (p.o) x 9 days | Ondansetron | Male Swiss albino mice, 22 - 25 g and male Wistar rats, 120-150 g | ORT | ↑ Discrimination index | Improve learning and memory in scopolamine-induced cognitive deficits mice | ||||||||
| EPM (4 days) | ↓ Transfer latency | ||||||||||||||||
| MMT (4 days) | ↑Swimming time in the target quadrant | ||||||||||||||||
| Shih et al. [43] | M. atropurpurea | Fruit | Methanol | 100, 500 mg/kg/day (p.o) x 12 weeks | N/A | 6 months male SAMR1 and SAMP8 mice | PAT (7 days) | ↑ Latency time on days 3, 7 at 500 mg/kg | Improve memory in aging mice | ||||||||
| AAT (7 days) | ↑ Latency time on days 2, 3, 4 at all doses | ||||||||||||||||
| Turgut et al. [33] | M. nigra | Leaves | Methanol | 50, 100 mg/kg/day (p.o) x 8 weeks | N/A | 8 weeks male BALB/c mice | MMT (4 days) | ↓ Time for escape latency ↑ Time spent to find the target quadrant ↑ Time swimming in the target quadrant ↑ Times crossed the platform location |
Improve cognitive deficits in aging mice induce by ᴅ-galactose. | ||||||||
AAT: Active avoidance test, EPM: Elevated plus maze, MCAO: Middle Cerebral Artery Occlusion, MMT: Moris Maze Test, N/A: Not applied, NORT: Novel object recognition test, OTR: Object recognition test, PAT: Passive avoidance test, p.o: per oral. *weight of extract per body weight of the animal.
M. alba fruit powder and M. alba ethanol fruit extract at 2, 10 mg/kg enhanced the learning and memory process in models of MCAO, alcohol intoxication-induced memory impairment, or age-related cognitive impairment induced by cholinotoxin [12, 13, 25, 32, 50]. Almost all results showed that mice using mulberry spent less time to reach the hidden platform, and spent more time in the target quadrant (retention time) in Moris Maze Test. Only alcoholic rats consuming mulberry powder, and MCAO mice consuming mulberry ethanol extract had no change of retention time. The healthy mice also enhanced their retention memory when consuming these forms of mulberry [25, 50].
Several leaves’ extracts from M. alba and M. nigra proved their efficacy on improving retention memory via Moris Maze Test. This was reflected by the reduction of time to find the target quadrant and of time for escape latency as well as by the increase in retention time and times that mice came across the platform location [30, 33, 51].
These improvements were in a dose-dependent manner and differed according to the mulberry form. For instance, in stroke condition, mulberry fruit powder at the high dose of 50 mg/kg exhibited no positive effects on retention time,
whereas it was still active in another model [12, 50]. Also, all doses of M. alba fruit extract increased retention time 14 days after stroke, but 50 mg/kg of M. alba fruit powder failed to show that effect [25, 50].
Apart from Moris Maze test, Nade et al. [30, 31, 41] emphasized the memory enhancement of ethyl acetate soluble fraction (EASF) of M. alba methanol extract by the increase in discrimination index via object recognition test, and by the reduction of transfer latency in EPM test on days 7, 14, 21. The extract might be effective than diazepam against chronic footshock stress, as this drug only showed improvement on the 1st day [31].
In a similar designed model, Kim et al. [52-54] showed that ethanol extract of M. alba fruit and mixture extract of M. alba leaves and fruit (2:1) improved the time spent to discover the novel object in healthy mice, in obese mice, and Aβ25–35-injected mice. Particularly, M. alba fruit extract at 100 and 500 mg/kg could increase time spent on a novel object by 66.88 ± 72.57%, and 69.14 ± 72.84%, respectively, compared with Aβ25–35-injected mice [53]. There was a significant improvement in memory of obese mice by 78.63% as well [54].
Finally, the latency time mice spent to avoid the electrical foot shock in both passive and active avoidance test was raised by treating with EASF of M. alba methanol root extract, M. alba ethanol fruit extract and M. atropurpurea methanol fruit extract showing that the learning process of memory-impaired mice, was improved [41, 43, 52].
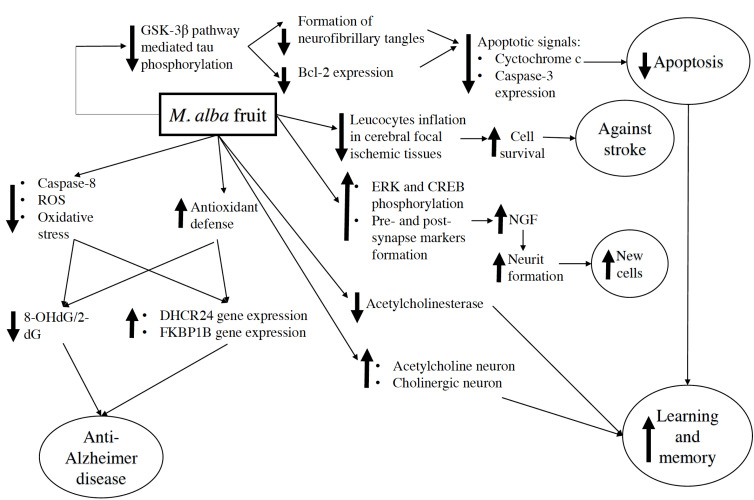
The positive effect on the cognitive functions might be chronic with the duration of mulberry exposure ranging from 9 days – 12 weeks. In addition, memory and learning improvement resulted from mulberry activities were associated with neuroprotection. This was shown in the increase of antioxidant capacity in the body [13, 32, 33, 41], the increase in density and differentiation of survival hippocampal cells [12, 13, 50, 52, 54], the inhibition of acetylcholinesterase (AchE) [13, 25], the increase in the cholinergic neuron and the acetylcholine formation [32], and the reduction of apoptotic markers in the hippocampus [32, 53].
There was a suggestion that the molecule mechanism of the anti-apoptotic activity of mulberry in vivo relating to the reduction of glycogen synthase kinase-3β (GSK-3β) pathway-mediated tau phosphorylation. This metabolite resulted in the formation of the neurofibrillary tangles [53] as well as the increase in B-cell lymphoma 2 (Bcl-2) expression in the hippocampus which led to the amplification of signals of apoptotic cascade such as cytochrome c release and the activation of caspase-3 [32, 53]. Therefore, preventing this reaction caused the reduction of apoptosis and the protection of brain cells [53]. Nerve growth factor (NGF) content in the hippocampus was also enhanced in a dose-dependent manner after the treatment of mulberry resulting in the induction of neurite and synapse formation, via the promoting extracellular-signal-regulated kinase (ERK) and cyclic AMP response element-binding protein (CREB) phosphorylation as well as pre- and post-synapse markers formation [52]. As a result, new cells generation caused memory improvement effect.
3.8. Antidepressant, Anxiolytic And Sedative Effects
Previous studies have reported the antidepressant-like effects of mulberry extracts such as sanggenon G from root bark, methanolic extract from leaves, ethyl acetate fraction, and n-butanol fraction from methanol extract of M. alba root, and alcohol extract of M. alba root (Table 8).
Table 8.
Anti-depression, anxiolytic, anti-stress effects of mulberry
| Refs. | Species | Part Used | Solvent |
Dose*
(Administration) |
Positive Control |
Animal
Model |
Model of Study | Main Results | Conclusion | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ye et al. [57] | M. alba | Root bark | N/A | 10 g/kg twice daily (p.o) x 4 weeks | N/A | 2 months male Sprague-Dawley rats | OFT | ↑ Number of rearing ↑ Number of line crossing insignificantly |
Reserve depressant behaviors in diabetes mice | |||||||||||||||
| LAT | ↑ Locomotor activity insignificantly | |||||||||||||||||||||||
| FST | ↓Immobility time | |||||||||||||||||||||||
| Lee et al. [56] | M. alba | Root bark | Ethanol | 50, 100, 200 mg/kg/day (p.o) (p.o) x 5 days | RU486 (mifepristone) | Male Wistar rats, 180–220g | FST | ↓Immobility time at 100 and 200 mg/kg ↑Climbing time at 200 mg/kg. No significant change of swimming time |
Antidepressant-like effects | |||||||||||||||
| TST | ↓Immobility time at 100 mg/kg | |||||||||||||||||||||||
| Lim et al. [26] | M. alba | Root bark | Methanol/ EtOAc Methanol/ n-butanol |
30, 100 mg/kg/day (p.o) x 7 days | RU486 (mifepristone) | 8 weeks Male Wistar rats, 180–210g | FST | ↓Immobility time, ↑climbing time, ↑swimming time at 100 mg/kg of EtOAc fraction No change observed with an n-butanol fraction |
Antidepressant-like effects | |||||||||||||||
| Nade et al. [41] | M. alba | Root | Methanol/ EtOAc |
25, 50, 100 mg/kg/day (p.o) x 10 days | Diazepam | Male Wistar rats, 150-180g | OFT | ↑ Number of squares crossed at all doses on day 10 ↓ Latency at all doses ↑ Number of rearings at 50 and 100 mg/kg |
Anxiolytic effect | |||||||||||||||
| Nade et al. [31] | M. alba | Root | Methanol | 25, 50, 100 mg/kg/day (p.o) x 28 days | Diazepam | Male Wistar rats, 150-180g | DST (21 days) | ↓ Immobility time at day 1, 14, 21 | Antidepressant-like effects | |||||||||||||||
| Khan et al. [59] | M. alba | Stem bark | Methanol | 250, 500 mg/kg/day (p.o) | Diazepam | 4 weeks male and female Swiss albino mice, 40-45 g |
OFT | ↓ Number of movement at all dose after 120 minutes of administration | Sedative effect | |||||||||||||||
| HCT | ↓ Locomotor activity at high dose | |||||||||||||||||||||||
| Lee et al. [14] | M. alba | Leaves | Methanol | 50, 100, 200, 400 mg/kg (p.o) | Diazepam | 5 weeks male ICR mice, 23–25 g | LAT | No alternation in locomotor activities or rearing frequencies after 1 hour of administration | Anxiolytic effect | |||||||||||||||
| EPM | ↑ Time spent in the open arms after 1 hour of administration ↑ Entries into open arms after 1 hour of administration |
|||||||||||||||||||||||
| HBT | ↑ Head-dips at doses of 200 and 400 mg/kg after 1 hour of administration |
|||||||||||||||||||||||
| Aditya Rao et al. [29] |
M. alba M. laevigata |
Leaves | Petroleum ether, chloroform, methanol |
200 and 400 mg/kg/day (p.o) | N/A | Male and female albino mice, 25-30 g |
LAT (5 mins) | ↓ Locomotor activity after 1 hour of administration | Sedative effect | |||||||||||||||
| Sattayasai et al. [55] | M. alba | Leaves | Boiling water | 100, 200, 500, 1000 mg/kg (i.p.) | Desipramine, diazepam | Male IRC mice | FST | ↓ Immobility time at 100 and 200 mg/kg after 30 minutes of administration | Antidepressant-like effect at low dose (100, 200 mg/kg) Sedative effect at high dose (500, 1000 mg/kg) |
|||||||||||||||
| CT | ↓ Climbing activity at 500 and 100 mg/kg after 30 minutes of administration | |||||||||||||||||||||||
| OFT | ↓ Time spent in open arms and the number of entry at 500 and 100 mg/kg after 30 minutes of administration | |||||||||||||||||||||||
| RRT | ↓ Time spent on the rod after 30 minutes of administration | |||||||||||||||||||||||
| Yadav et al. [28] | M. alba | Leaves | Methanol | 50, 100, 200 mg/kg/day (i.p.) | Diazepam | Male Swiss albino mice, 18-22 g |
OFT | ↑Square traversed at all doses after 30 minutes of administration ↑Rearing and self-rearing at 100 and 200 mg/kg after 30 minutes of administration |
Anxiolytic effect | |||||||||||||||
| HBT | ↑ The number of a head poking at 100 and 200 mg/kg after 30 minutes of administration ↑Duration of a head poking at all doses after 30 minutes of administration |
|||||||||||||||||||||||
| EPM | ↑Time spent in open arms at 100 and 200 mg/kg after 30 minutes of administration ↓ Time spent in closed arms and ↑ The entries to open arms at 200 mg/kg after 30 minutes of administration |
|||||||||||||||||||||||
| LDP | ↑Time spent in lightboxes and ↓the time spent in dark boxes at 100 and 200 mg/kg after 30 minutes of administration No change of crossings and transfer latency. |
|||||||||||||||||||||||
| Dalmagro et al. [24] | M. nigra, | Leaves | Water | 3–100 mg/kg/day (p.o) x 1 day (for acute test, and x 7 days for subchronic test | Fluoxetine | Male Swiss mice, 30–40 g | FST | ↓ Immobility time at all doses in acute test | The antidepressant-like property might occur due to syringic acid | |||||||||||||||
| TST | ↓ Immobility time at 3, 10, 30 mg/kg in acute test, and at 3, 10, 30, 100 mg/kg in subchronic test | |||||||||||||||||||||||
| OFT | No significant changes in the number of crossings, rearing, and fecal boluses in both tests | |||||||||||||||||||||||
| Syringic acid | N/A | N/A | 0.1 – 100 mg/kg/day (p.o) | TST | ↓ Immobility time at 1, 10 mg/kg in both acute and subchronic tests | |||||||||||||||||||
| OFT | No significant changes in the number of crossings, rearing, and fecal boluses in both tests | |||||||||||||||||||||||
| Barman et al. [9] | M. indica | Root | Methanol | 200 mg/kg/day (i.p.) | N/A | Male adult albino rats (150-165 g) |
SAT | ↓ Spontaneous activity by 72.78% after 30 minutes of administration | Sedative effect | |||||||||||||||
|
Gupta et al. [58] |
Morusin | N/A | N/A | 5, 10 mg/kg (i.p.) | Diazepam | Wistar albino rats (150–200 g) | LAT | ↓ Locomotor activity by 48.82% and 70.20% at 5 and 10 mg/kg, respectively after 30 minutes of administration | Sedative effect | |||||||||||||||
| Lim et al. [36] | Sanggenon G | N/A | N/A | 3, 10, 30 mg/kg/day (i.p.) | Imipramine | 8 weeks male Sprague Dawley rats, 180–210g | FST | ↓ immobility time at 30 mg/kg after 60 minutes of administration ↑ swimming time at all dose after 60 minutes of administration No change of climbing time |
Antidepressant-like effects mediated serotonergic system. | |||||||||||||||
| Lim et al. [35] | Sanggenon G | N/A | N/A | 5, 10, 20 mg/kg/day (i.p.) | Yohimbine | 8 weeks male Sprague–Dawley rats, 180–210g | FST (6 mins) | ↓ Immobility time at 20 mg/kg after 60 minutes of administration | Antidepressant-like effect | |||||||||||||||
CT: Climbing test, DST: Despair swim test, EPM: Elevated plus maze, EtOAc: Ethyl acetate, FST: forced swimming test, HCT: Hole cross test, HBT: Hold board test, HWT: Horizontal Wire Test, i.p.: intraperitoneal injection, LAT: locomotor activity test, LDP: Light/dark paradigm, N/A: Not applied, OFT: Open field test, p.o: per oral, RRT: Rota-rod test, SAT: Spontaneous activity test, TST: Tail suspension test. * weight of extract per body weight of the animal.
In general, all parts of mulberry (M. alba leaves green tea, M. alba root bark, M. nigra leaves, sanggenon G and syringic acid extracted from mulberry) could decrease the immobility time that mice spent in forced swim test (FST) [24, 26, 31, 35, 36, 55-57], and in tail suspension test (TST) [24, 56]. These indicated that mulberry possessed an antidepressant-like effect. The antidepressant-like effect of M. alba green tea extract at 200 mg/kg was even comparable with 10 mg/kg of desipramine [55]. The antidepressant-like effect of mulberry could be acute (measured 30 – 60 minutes after administration) or subchronic (measured after 7 days of administration) or chronic (measured after 28 days). The doses of extracts ranged from 3 mg per day to 10 g twice a day orally, or 3 – 100 mg/day by intraperitoneal injection. Sanggenon G showed effective antidepression at higher doses (20 and 30 mg/kg, i.p.), whereas syringic acid was better at average doses (1 and 10 mg/kg, p.o.) The modulation of the limbic hypothalamic–pituitary–adrenocortical (HPA) axis, which reported by few studies, clarified this effect [26, 31, 36, 56]. Accordingly, ethyl acetate fraction of M. alba methanol root bark extract, sanggenon G extract from the root bark, and ethanol extract of Cortex Mori Radicis (CMR) prevented the promotion of corticosterone response and c-fos immunoreactivity in the dentate gyrus or hippocampus under FST-induced depressive condition. These could be associated with the increase in glucocorticoid receptors (GR) expression in the hippocampus through the promotion of phosphorylation at S232 and S246 of GR [56]. Moreover, the anti-depressive effect of sanggenon G might be mediated by an interaction with the serotonergic system as well, as Lim et al. indicated pretreating with a selective 5-hydroxytryptamine1A (5-HT1A) receptor antagonist could reserve this positive effect [36].
Apart from that, several other effects related to anti-depressive like effects were observed. Lim et al. indicated that the practical impact of sanggenon G at 5 - 10 mg/kg was also promoted by the presence of the α2-antagonist (yohimbine) [35]. Otherwise, M. alba root bark extraction could reserve the depressive-like behaviors in diabetic mice assessed via FST [57].
The anxiolytic effect of mulberry had controversial results via different experiments. Additionally, mulberry could show the acute anxiolytic effect after 30 – 60 minutes of administration, regardless of oral administration or intraperitoneally injection. In open field test (OFT), two extracts of M. alba (M. alba leaves methanol extract and M. alba root methanol extract) decreased the latency time to enter the main area and increased the number of squares crossed as well as the number of rearings in both standards and stressed mice indicating that mulberry extracts had an anxiolytic effect [14, 28, 41]. However, only Lee et al. showed no change in the frequencies of rearing observed in mice treated with M. alba methanol leaves extract [14]. Mulberry extracts also showed their anxiolytic effect via the EPM test, light/dark exploration test, and hold board test. In the EPM test, more time was spent in the open arms along with the short transfer latency to the closed arms [14, 28, 41]. For instance, methanol extract of M. alba leaves increased up to 49.9 ± 3.1% of time spent on the open arms and 63.3 ± 1.3% of the number of entries into open arms compared with the control group [14]. Additionally, mulberry enhanced the exploratory head-dipping behaviors and time spent in lightbox in hold board test, and light/dark exploration test, respectively [14, 28]. However, M. alba root bark only tended to improve the results in diabetic mice insignificantly, and the aqueous extract of M. alba leaves showed no anxiolytic effect at 100 - 200 mg/kg [55, 57]. Lee et al. suggested that the anxiolytic activity might relate to the histaminergic system in the central nervous system, as a histamine H3 receptor antagonist abolished this effect [14].
The decreased movement of mice in the locomotor activity test showed that M. alba leaves and stem bark methanol extract, M. alba steam bark morusin, several extracts of M. laevigata possessed sedative effect [28, 29, 58, 59]. The methanol extract of M. indica root bark at 1000 mg/kg also showed the same effect, as it decreased the spontaneous motility up to 72.78% [9]. As same as the anxiolytic effect, the sedative effect of mulberry was acute. These outcomes above were observed after 30 – 60 minutes of administration.
On the other hand, the anxiolytic and sedative effects of mulberry were in a dose-dependent manner. No significant anxiolytic effect was shown by M. alba leaves methanol extract at a low dose (below 100 mg/kg) [14, 28, 52], and aqueous extract of M. alba leaves only had a sedative effect at high dose (over 500 mg/kg) [55]. The petroleum ether, chloroform and methanol fractions obtained from aqueous extract of both M. alba and M. laevigata leaves decreased over 50% of locomotor activity. Nevertheless, the petroleum ether fractions exhibited stronger effect compared to methanol fractions, then followed by chloroform fraction in both cases, indicating that solvents for extraction affected the effectiveness of mulberry [29].
3.9. Other Effects
Two studies also evaluated the anticonvulsant effect of mulberry. Tubas et al. showed that intraperitoneal treatment of M. ruba fruit extract at 10 mg/kg significantly decreased the spike frequencies of convulsions in penicillin-induced epileptiform mice from the 80th minute observed during 120 minutes tested although no change of the amplitude was observed [60]. This result was reflected in the study of Gupta et al. [58] who showed that morusin – a compound extract from M. alba dramatically increased the onset of convulsive time caused by isoniazid, from 306.16 ± 22.16 (s) to 491.42 ± 29.07 (s) (at 5 mg/kg) and 659.10 ± 31.28 (s) (at 10 mg/kg). There was a significant reduction in the duration of convulsions and the percentage of mortality as well. In maximal electroshock-induced convulsion rats, morusin intraperitoneal injected administration at 5 mg/kg led to a decrease in the duration of tonic hind limb extension (seconds) whereas 10 mg/kg of this compound even abolished this reaction. Regarding the mechanism of action, the anticonvulsant effect results from the reduction of MDA levels in erythrocytes and plasma, and the preservation of GABA in the brain [58, 60]. These anticonvulsant effects were acute observed after 30 minutes of treatments. However, a report of Barman et al. showed a different result, as M. indica root failed to stop pentylene tetrazole-induced convulsion in mice [9].
Besides, mulberry also showed its effect on sleep medicines, and this effect was dose-dependent. The sleeping time caused by two barbiturates (pentobarbitone and phenobarbitone) was extended by pretreating (i.p.) with M. indica root and M. alba leaves at 200 mg/kg 30 minutes prior the barbiturates treatments [9, 23]. This extension, however, was not significant when pretreating with M. alba leaves at the dose of 100 mg/kg. On the other hand, the use of 100 and 200 mg/kg of M. alba leaves also reduced the onset time of sleeping while there was no change with 50 mg/kg of this extract.
Regarding PD, Gu et al. [18] studied the effect of 70% ethanol extract of mulberry (M. alba) fruit against PD-like symptoms caused by 1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine/probenecid (MPTP/p), which is a neurotoxic agent. They found that the extract at the dose of 250 mg/kg/day inhibited the motor deficits in mice after 38 days of treatment, that proved by longer staying of mice on the rod in the rotarod test, less locomotor activity time when mice descended to the floor in the pole test and thus, improved bradykinesia and increased locomotor activity in the open field test. Also, non-motor deficits were improved, as pellet retrieval time was shortened in the olfactory test in the treated group. This result was concordant with the report of Kim et al. [61] who also showed the improvement of bradykinesia in behavioral tests in mice intraperitoneally administrated 500 mg/kg/day of 70% ethanol extract of M. alba fruit during 15 days period. This protection against bradykinesia was hypothesized to be associated with the protection of dopamine neurons in the substantia nigra pars compacta and striatum through the inhibition of Bax protein (an apoptotic protein) or α-synuclein and ubiquitin levels (factors killed dopaminergic cells), in PD models.
Regarding the protection against diabetes mellitus complications on the central nervous system, Kim et al. reported an increase in new cell proliferation in the dentate gyrus in both normal and diabetic rats after 3 days of intraperitoneal treatments with 100 mg/kg of heat-extracted leaves of M. alba [62]. The increased expression of neuropeptide Y, which relates to the cell division, most likely mediated this effect. Additionally, another study showed that 0.3 g/kg of flavonoids extracted from M. alba in 8 weeks period could chronically recover a severe peripheral nerve injury in diabetic rats. Accordingly, the oral administration of these compounds led to an increase in the myelin sheath area and the myelinated fiber cross-sectional area. The study demonstrated that the extramedullary fiber number, the onion-bulb type myelin destruction, and the degeneration of mitochondria and Schwann cells reduced remarkably [27].
Another effect shown is the effect on catalepsy induced by antidopaminergic agents [23]. Haloperidol (a non-selective D2 dopamine antagonist) and metoclopramide (dopaminergic blocking agent) inhibited dopamine transmission by blocking its receptor in the striatum, causing catalepsy. The authors showed that there was an increase in catalepsy score at 100 mg/kg of M. alba leaves extract after 28 days treatment period. On the other hand, footshock-induced aggression, which was associated with the increase in dopamine level in the brain, was also attenuated with the use of 50, 100 and 200 mg/kg of mulberry leaves extract proved by the increase in latency to fight and the decrease in fighting attacks. These results indicated that M. alba leaves extract might possess antidopaminergic activity.
Hwang et al. [63] suggested that 5 consecutive days of treatment of M. alba fruit extract caused modulation of MAO (monoamine oxidase) in mice brain after physical stress. The study indicated that there was a recovery of the decreased MAO-A level and the increased MAO-B level to their normal levels after 30 minutes of swimming.
Finally, several abnormalities in the brain that caused by S. mansoni infection in the form of a disturbance in the antioxidant system, the attenuation of GABA level and AchE activity returned to normal levels when treated with 200 – 800 mg/kg of 70% methanol extract of M. alba leaves after 10 exposed days. Also, these extracts induced normal brain parenchymal cells from histological view with fewer abnormalities of neuronal architecture compared to the control group [42]. Regarding neuro-amelioration against AD, El-baz et al. [45] realized that there was an increase in the ratio of 8-OHdG/2-dG, pointing out the DNA damage, and a decrease in levels of the expression of DHCR24 and FKBP1B genes which play an essential role in degenerative disorders in AD mice. These expressions turned to the normal levels compared to mice of the control group by consuming 300 mg/kg of M. alba or M. ruba for six weeks. The authors suggested that these effects were associated with the inhibition of reactive oxygen species (ROS) as well as the apoptotic marker (caspases-8), and the promotion of antioxidant enzyme activity (such as GSH).
The mechanism of activities of M. alba fruit is shown in Fig. (2).
Fig. (2).
Mechanism of positive effects of Morusalba fruit in brain functions Bcl-2: B-cell lymphoma 2, CREB: cyclic AMP response element-binding protein, ERK: extracellular-signal-regulated kinase, GSK: glycogen synthase kinase-3β, ROS: reactive oxygen species. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. DISCUSSION
Varied types of mulberry have exhibited their protection of brain functions through in vivo models. M. alba was the most popular tested plant for varied effects. This plant showed the antioxidant effect in the brain, cognitive function improvements, antidepressant effect, sedative effect, antidopaminergic effect, anti-PD-like symptoms effect, MAO modulation, prevention of AD and the complications of central nervous in diabetic mice. Meanwhile, M. nigra was only tested for antioxidant effect, antidepressant effect and memory improvement. M. indica and M. laevigata had a sedative effect at the high doses. Besides, M. indica and M. atropurpurea could inhibit oxidative stress. Also, M. atropurpurea enhanced the memory of aging mice. Especially, ethanol fruit extract of M. rubra could promote the antioxidant defense whereas wine made from this plant had no positive affection on the antioxidant system. Additionally, M. rubra was the sole plant that had the anticonvulsant effect in this review. Almost all these effects were subchronic or chronic with the duration of treatments ranging from 5 days to 12 weeks. Exceptionally, the anticonvulsant effect and antidepressant, anxiolytic and sedative effects were acute which showed their protection against the triggers when pretreating with the plants 30 – 60 minutes before the tests.
Our review recorded large ranges of used doses across included studies. M. alba extracts showed their activities at the doses ranging from 5 mg/kg to 1 g/kg per day per oral, and at 100 µg/kg – 1000/kg mg via intraperitoneal injection. M. nigra had the used doses between 3 – 100 mg/kg. Similarly, M. atropurpurea, M. indica, and M. laevigata were examined at 100 – 500 mg/kg/day while M. rubra was intraperitoneally injected at 10 mg/kg for anticonvulsant effect, and 20 – 300 mg/kg per oral for antioxidant effect. This raised a challenge for further studies because the doses have not been standardized. It appeared that the authors randomly chose the ranges of doses for testing, although they conducted the same experiments with similar plant characteristics. For the small doses (2 – 10 mg/kg) or very high doses (1 or 10 g/kg), we might need more studies to confirm the efficacy of the extracts with these doses because it is quite hard for the active ingredients in the extract with small doses reaching the active concentrations in the body after they undergo the absorption and metabolism process. Besides, if an extract only showed their effect at very high doses (1 or 10 g/kg), it is impossible to apply these extracts in human studies as there is a need for a large number of plants to obtain the sufficient extracts for chronic interventions. C3G showed its efficacy at 10 mg/kg/day per oral in mice. However, its absorption in the body is quite low; thus, this point must be considered in further studies in humans [64]. Turn back to specific effects, mulberry contains the high concentrations of polyphenol, anthocyanin, phenolic, and flavonoid compounds that might be the source of their antioxidant effect [12, 13, 32, 33]. The results obtained were slightly inconsistent might be due to the variation of the compositions extracted from different species or used solvents. For illustration, most of the extracts of M. alba increased antioxidant enzymes’ levels. However, wine from M. rubra and aqueous leaves extract of M. nigra failed to improve to the antioxidant capacity in mice, whereas methanol leaves extract of M. nigra significantly altered the levels of CAT, SOD and GPx [24, 33, 34]. The content of syringic acid might be a reason. This ingredient, which played a role as a pro-oxidant compound, was the primary phenolic compound in aqueous extract of M. nigra while its concentration was minor in methanol extract of this plant [24, 33]. Instead, the major phenolic acids determined in methanol extract of M. nigra were vanillic acid and chlorogenic acid, and syringic acid only existed with a minor percent [33]. Also, the different efficiency might be attributed to the used doses. Wine from M. rubra in this experiment was only 20 mg/kg whereas M. rubra methanol extract showed the antioxidant effect at 300 mg/kg. However, we can generally see the fact that mulberry had a positive effect on keeping antioxidant enzymes’ activities and the concentration of brain oxygen radicals in balance. This activity might result in other effects such as improving memory [13, 32] or protecting against ischemia [13, 48] and brain cells [27, 44].
Regarding the result of Srikanta et al. [34], they hypothesized that the negative effect of M. nigra on antioxidant capacity might also be due to the poor bioavailability of antioxidant compounds as phenolics, or their inadequate consumption into tissues. In a clinical trial, Goldberg et al. found that the bioavailability of three prevalent phenolics (catechin, quercetin, and resveratrol) was too low compared to experimental concentrations to cause a positive effect in the in vitro studies [65]. However, we found that the main phenolics compounds of mulberry were syringic acid, vanillic acid, chlorogenic acid. The extracts of mulberry also had benefits in the in vivo studies. Therefore, we suggest that the effect of individual compounds on antioxidant enzymes should be investigated in the future to confirm whether they are active compounds. Also, the bioavailability could be studied to predict their realistic efficiency.
The lack of oxygen, glucose, and blood to the brain cell is the main caution of cerebral ischemia [66]. The oxygen-glucose deprivation, therefore, is a popular model to study ischemic cell deaths. Via this model, the pre-treatment with C3G and MG increased the percent of cell viability of PC12 cells and SH-SY5Y cells comparing to the control group [37, 39]. The study found that these positive results in the in vitro experiments promised satisfactory results of protection against ischemia in the in vivo study. In our review, we found that mulberry extracts and their compositions significantly decreased the infarct volume in MCAO mice, asserting their effect on the in vivo studies. Besides, the connection between antioxidant effect and the protection against stroke became quite clear. Free radicals such as H2O2 could cause the death of brain cells [67]. The results of Kang et al. confirmed this by showing that PC12 cells exposure to H2O2 had lower cell viability than the vehicle group [37, 49]. However, pre-treatments of these cells with C3G and GAML enhanced more survival cells showing that free radicals scavenging could protect cell apoptosis [37, 49]. In this case, oxyresveratrol might be a potential candidate for the protection against stroke, as it had a strong antioxidant effect and could inhibit the apoptotic cascades [38, 68]. More important, oxyresveratrol could cross the blood-brain barrier where it could directly show its effects [69].
Besides, the increase in NOX4 expressions could result in ROS generation after cerebral ischemia [70]. Thus, the inhibition of the expressions of this protein by MG in vitro was predicted to prevent excessive ROS expression [39]. MG was also demonstrated to suppress the expression of factors involving the apoptotic cascade, namely poly (ADP-ribose) polymerase, caspase-9, and caspase-3, which could be active in vivo and protect against brain cells death [39]. From our results in this review, there was an actual downgrade of these expressions in MCAO mice implying the activity of MG via the ROS scavenging and the prevention of apoptotic signals. Nevertheless, we must emphasize that MG was intraperitoneally administrated at 0.2 – 5 mg/kg in this study. This is a challenge for its application in the future because it could be hard to reach these concentrations in the body via the oral admistration. We recommend pharmacokinetics studies of MG to predict its effect in clinical in the future.
Regarding the protection against the cognitive deficit, included studies showed that different parts and preparations of mulberry could improve the learning ability and memory impairment. There were studies reported that stress oxidant, hippocampal damage, and GSK-3β activation were associated with downgraded brain functions via neural cell death [71, 72]. Therefore, the hippocampal protection and antioxidant could be a critical factor against these affections. Otherwise, the enhancement of ACh in the brain, which plays a role in encoding and retrieval of spatial memory in the hippocampus, could lead to memory improvement [73]. Our study presented the evidence that was concordant with these theories, as the memory and learning process of mice was improved following by the increase in survival brain cells in several hippocampal areas, the elevation of the acetylcholine levels as well as the suppression of MDA level. These findings reinforced the potential effect of several species of Morus genus with an insight into mechanisms of action.
GABA plays a primary role in the transmission in the brain, which mediates the depolarization through K+ and Ca2+ channels, which is vital for the differentiation of brain cells and the development of the brain [74]. The GABAA defects might cause idiopathic epilepsy, which correlated with the mutation in genes of voltage-channels and ligand-gated ion-channels [75]. Hence, GABAergic inhibitory transmission is an essential element in the mechanism of both anxiolytic and anticonvulsant effects [76]. Moreover, the decrease in dopaminergic transmission relates to the enhancement of GABA transmission as well [77]. In our study, we found that mulberry extract increased GABA levels in the brain and might possess antidopaminergic activity through dopamine D2 receptors [23, 58]. These results provided strong evidence of mulberry’s therapeutic potentials relating to GABA receptor and GABA inhibitory transmission. Moreover, flavones are known to bind with the GABAA receptor [77] strongly. In our review, flavonoids could be found in various parts of M. alba (leaves, root, and fruit). From these results, we can see that the GABA receptor and flavones might be clues for the mechanism of anxiolytic and anticonvulsant activities shown in this review, which should be more investigated.
The loss of dopaminergic neurons is well known to be the leading cause of the core motor symptoms in PD patients [78]. In this review, we found that consistent results are showing that ethanol extract of mulberry fruit protected dopaminergic neurons against some neurotoxicity and improved PD-like symptoms in rats [18, 61]. The regulation of ROS generation and some apoptotic signals as Bcl2, Bax protein, or caspase-3 to normal levels indicated that the protection of dopaminergic cells might be related to the modulation of oxidative stress and the apoptosis [61]. These outcomes seem to be incompatible with the antidopaminergic activity of this plant because the dopaminergic blocking could worsen the PD symptoms. That conflict could be attributed to the different used solvents and used parts, as the antidopaminergic activity might result from the presence of flavonoids, tannins, and saponins in mulberry leaves whereas the major compounds of mulberry fruit which improved PD-like behaviors were phenolics, flavonoids and anthocyanins [18, 23, 32]. On top of that, antidopaminergic agents had their adverse reactions in mental health [79]. For example, mulberry treatment enhanced the haloperidol-induced catalepsy as shown in our review [23]. further studies should investigate the compounds in these extracts and their bioactivities to elucidate the active ingredients for each activity to use to suitable intervention and minimize the risk of unexpected effects.
One of our limitations was that we could not compare different species of genus Morus to clarify which species were more beneficial. However, from the general perspective, we realized that many parts from M. alba showed a stronger antioxidant effect than other species. The antioxidant effect of M. nigra depended on used parts and the extracted solvents. Almost all parts of different species of mulberry showed a positive effect on cognitive function improvement, while root bark extract might be responsible for anti-depressive activities. The variable compositions in different extracts could cause a controversial effect, like antidopaminergic action and PD-like behavior improvement. Although there are many studies investigating the effects of mulberry in vivo, there are still no more profound studies investigating the pharmacokinetics of their active ingredients, leading to its limited application in human studies. The doses of these extracts should be considered and standardized to have optimized options for further studies. Finally, we did not include and report the systemic toxicology of mulberry because of our strategy. Other studies should consider this point when conducting their researches with high doses of mulberry extract (1 g or 10 g/kg).
Furthermore, most of the included studies carried out experiments on the whole extracts, but only very few studies identified which compounds were used. In specific cases, MG (a prenylated flavonoid), C3G (an aglycone of anthocyanin) and oxyresveratrol (a stilbenoid) decreased the brain infarct volume. Through these studies, we can only assert that the protection against the stroke caused by these compounds was due to the antioxidant activities and the prevention of the apoptosis suggesting that there would be many compounds that possess this activity. Sanggenon G was the only specific compound that was investigated and showed a useful anti-depressive effect. No active ingredients were used for investigating other activities as an antioxidant effect, PD, and AD-like behavior improvement. It narrows the number of active compounds that further studies should focus on. Instead, we need more studies finding out the active ingredients in specific extracts, making the comparison to determine the most effective candidates. Nevertheless, our study contributes to elucidating the potential activities of the plant-based on evidence, which can promote its application. We recommend further studies to concentrate on investigating compounds from active extracts to provide stronger evidence.
CONCLUSION
Mulberry species proved beneficial to many neurological functions in animal models. M. alba leaves methanol extract; ethyl acetate fraction and n-butanol fraction from methanol extract of M. alba root; and alcohol extract of M. alba root possessed anti-depressant-like effect. The methanol extraction of both M. alba root and M. alba leaves had an anxiolytic effect. Plus, M. laevigata leaves, M. alba methanol extract of leaves and stem bark, as well as its morusin showed the sedative effects at high doses. Varied species exhibited their ability to improve the memory and learning process, including M. alba leaves and fruit, M. nigra leaves, M. atropurpurea fruit. Some specific compounds extracted from mulberry namely MG, oxyresveratrol, and C3G could prevent ischemia in the brain either before or after stroke. Interestingly, M. rubra fruit and morusin increased the onset of convulsive time as well as reduced the convulsive duration. The antioxidant activity is still inconsistent and might be associated with the specific species, solvent for extraction, route of administration, doses, and the main constituents. Anti-Alzheimer disease and anti-Parkinson’s disease activities are intriguing, as the symptoms in mice were improved and the mechanisms were also suggested. However, we need more studies to confirm them. The active ingredients of each species, especially M. alba, should be deeper studied for screening potential candidates for future treatments.
Table 5.
Phytochemical analysis in studied extracts.
| Refs. | Species | Part Used | Solvent | Phytochemical Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phe | Fla | Ster | Tan | Alk | Sap | Antho | Anthra | CH | Proteins |
Amino Acids |
Terp | Gly | ||||
| [22, 23, 28, 29] | M. alba | Leaves | Methanol | + | + | + | + | + | + | + | + | + | + | + | ||
| [30] | M. alba | Leaves | Methanol/ EASF |
+ | + | + | + | |||||||||
| [29] | M. alba | Leaves | Petroleum ether | + | + | + | + | + | ||||||||
| [29] | M. alba | Leaves | Chloroform | + | + | + | + | + | ||||||||
| [31] | M. alba | Root | Methanol/ EASF |
+ | + | + | ||||||||||
| [12] | M. alba | Fruit | N/A | + | + | |||||||||||
| [32] | M. alba | Fruit | Ethanol | + | + | + | ||||||||||
| [24, 33] | M. nigra | Leaves | Hot water | + | ||||||||||||
| [24, 33] | M. nigra | Leaves | Methanol | + | ||||||||||||
| [29] | M. laevigata | Leaves | Methanol | + | + | + | + | + | + | + | + | + | ||||
| [29] | M. laevigata | Leaves | Chloroform | + | + | + | + | |||||||||
| [29] | M. laevigata | Leaves | Petroleum ether | + | + | + | + | |||||||||
| [34] | M. rubra | Fruit | N/A | + | + | |||||||||||
Alk: alkaloids, Antho: anthocyanins, Anthra: anthraquinones, EASF: ethyl acetate soluble fraction, Fla: flavonoids, Gly: glycosides, N/A: Not applied, Phe: phenolics, Sap: saponins, Ster: steroids, Tan: tannins, Terp: terpenoids.
Acknowledgements
Declared none.
list of ABBREVIATIONS
- 5-HT1A
5-hydroxytryptamine1A
- AchE
Acetylcholinesterase
- AD
Alzheimer diseases
- C3G
Cyanidin-3-O-b-ᴅ-glucopyranoside
- CAT
Catalase
- CMR
Cortex mori radicis
- CREB
Cyclic AMP response element-binding protein
- EASF
Ethyl acetate soluble fraction
- EPM
Elevated plus-maze
- ERK
Extracellular-signal-regulated kinase
- GABA
Gamma-aminobutyric acid
- GAML
GABA in mulberry leaves
- GHL
Global health library
- GPx
Glutathione peroxidase
- GR
Glucocorticoid receptors
- GRd
Glutathione reductase
- GSH
Glutathione
- GST
Glutathione S-transferase
- GSK-3β
Glycogen synthase kinase-3β
- FST
Forced swim test
- HPA
Hypothalamic–pituitary–adrenocortical
- LDH
Lactate dehydrogenase
- MAO
Monoamine oxidase
- MCAO/R
Middle cerebral artery occlusion/reperfusion
- MDA
Malonyldialdehyde
- MG
Mulberrofuran G
- MLE
Mulberry fruit extract
- MLE-AR-14
Mulberry leaf extracted variety AR-14
- MPO
Myeloperoxidase
- MPTP/p
1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine/probenecid
- NO
Nitrite
- NOX
Nicotinamide adenine dinucleotide phosphate oxidase
- NPSH
Non-protein thiol groups
- NYAM
the New York Academy of Medicine Grey Literature Report
- OFT
Open field test
- ROS
Reactive oxygen species
- PC
Protein carbonyl
- PD
Parkinson disease
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SA
Syringic acid
- SIGLE
the System for Information on Grey Literature in Europe
- SOD
Superoxide dismutase
- SYRCLE
The Systematic Review Centre for Laboratory Animal Experimentation
- TAC
Total antioxidant capacity
- TBARS
Thiobarbituric acid reactive substances
- TST
Tail suspension test
- VHL
Virtual health library
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Huang H.P., Ou T.T., Wang C.J. Mulberry (sang shèn zǐ) and its bioactive compounds, the chemoprevention effects and molecular mechanisms in vitro and in vivo. J. Tradit. Complement. Med. 2013;3(1):7–15. doi: 10.4103/2225-4110.106535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y., Xiang L., Wang C., Tang C., He X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS One. 2013;8(7):e71144. doi: 10.1371/journal.pone.0071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim S.H., Choi C.I. Pharmacological Properties of Morus nigra L. (Black Mulberry) as a promising nutraceutical resource. Nutrients. 2019;11(2):11. doi: 10.3390/nu11020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andallu B., Varadacharyulu N.Ch. Antioxidant role of mulberry (Morus indica L. cv. Anantha) leaves in streptozotocin-diabetic rats. Clin. Chim. Acta. 2003;338(1-2):3–10. doi: 10.1016/S0009-8981(03)00322-X. [DOI] [PubMed] [Google Scholar]
- 5.Andallu B., Varadacharyulu N.C. Gluconeogenic substrates and hepatic gluconeogenic enzymes in streptozotocin-diabetic rats: effect of mulberry (Morus indica L.) leaves. J. Med. Food. 2007;10(1):41–48. doi: 10.1089/jmf.2005.034. [DOI] [PubMed] [Google Scholar]
- 6.Lin J-Y., Tang C-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101:140–147. doi: 10.1016/j.foodchem.2006.01.014. [DOI] [Google Scholar]
- 7.Zadernowski R., Naczk M., Nesterowicz J. Phenolic acid profiles in some small berries. J. Agric. Food Chem. 2005;53(6):2118–2124. doi: 10.1021/jf040411p. [DOI] [PubMed] [Google Scholar]
- 8.Kim A.J., Park S. Mulberry extract supplements ameliorate the inflammation-related hematological parameters in carrageenan-induced arthritic rats. J. Med. Food. 2006;9(3):431–435. doi: 10.1089/jmf.2006.9.431. [DOI] [PubMed] [Google Scholar]
- 9.Barman T.K., Chatterjee G.K., Nag Chaudhuri A.K., Pal S.P. Some pharmacological actions of the extract of Morus indica Linn. roots. Indian J. Pharmacol. 1980;12:237–241. [Google Scholar]
- 10.Zhang H., Ma Z.F., Luo X., Li X. Effects of Mulberry fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants. 2018;7(5):69. doi: 10.3390/antiox7050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olfa R., Manel B., Sami F., Mohamed A. Phytochemicals from mulberry extract (Morus sp.): Antioxidant and neuroprotective potentials. J. Appl. Pharm. 2017;7:217–222. [Google Scholar]
- 12.Wattanathorn J., Muchimapura S., Thukhammee W., Tong-un T., Wannanon P., Phunchago N., Kaewkaen P., Chantes T., Kaewruang W., Pimpasalee S., Wongareonwanakij S. Mulberry fruits protects against age-related cognitive decline. Am. J. Appl. Sci. 2012;9:1503–1511. doi: 10.3844/ajassp.2012.1503.1511. [DOI] [Google Scholar]
- 13.Wattanathorn J., Phunchago N., Muchimapura S., Thukhum-Mee W., Chaisiwamongkol K., Kaewrueng W., Wongareonwanakij S. Mulberry fruit mitigates alcohol neurotoxicity and memory impairment induced by chronic alcohol intake. Am. J. Appl. Sci. 2012;9:484–491. doi: 10.3844/ajassp.2012.484.491. [DOI] [Google Scholar]
- 14.Lee S., Kim D.H., Lee J.H., Ko E.S., Oh W.B., Seo Y.T., Jang Y.P., Ryu J.H., Jung J.W. Involvement of histaminergic system in the anxiolytic-like activities of Morus alba leaves in mice. Biol. Pharm. Bull. 2013;36(11):1692–1699. doi: 10.1248/bpb.b13-00126. [DOI] [PubMed] [Google Scholar]
- 15.Prince M., Guerchet M., Prina M. World Alzheimer Report. 2013 https://www.alz.co.uk/research/world-report-2013
- 16.Dorsey E.R., Constantinescu R., Thompson J.P., Biglan K.M., Holloway R.G., Kieburtz K., Marshall F.J., Ravina B.M., Schifitto G., Siderowf A., Tanner C.M. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 17.Rafii M.S., Aisen P.S. Recent developments in Alzheimer’s disease therapeutics. BMC Med. 2009;7:7. doi: 10.1186/1741-7015-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu P.S., Moon M., Choi J.G., Oh M.S. Mulberry fruit ameliorates Parkinson’s-disease-related pathology by reducing α-synuclein and ubiquitin levels in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/ probenecid model. J. Nutr. Biochem. 2017;39:15–21. doi: 10.1016/j.jnutbio.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Qiao A., Wang Y., Zhang W., He X. Neuroprotection of brain-targeted bioactive dietary artoindonesianin O (AIO) from mulberry on rat neurons as a novel intervention for Alzheimer’s disease. J. Agric. Food Chem. 2015;63(14):3687–3693. doi: 10.1021/acs.jafc.5b00396. [DOI] [PubMed] [Google Scholar]
- 20.Kuk E.B., Jo A.R., Oh S.I., Sohn H.S., Seong S.H., Roy A., Choi J.S., Jung H.A. Anti-Alzheimer’s disease activity of compounds from the root bark of Morus alba L. Arch. Pharm. Res. 2017;40(3):338–349. doi: 10.1007/s12272-017-0891-4. [DOI] [PubMed] [Google Scholar]
- 21.Hooijmans C.R., Rovers M.M., de Vries R.B.M., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nade V.S., Kawale L.A., Yadav A.V. Protective effect of Morus alba leaves on haloperidol-induced orofacial dyskinesia and oxidative stress. Pharm. Biol. 2010;48(1):17–22. doi: 10.3109/13880200903029357. [DOI] [PubMed] [Google Scholar]
- 23.Yadav A.V., Nade V.S. Anti-dopaminergic effect of the methanolic extract of Morus alba L. leaves. Indian J. Pharmacol. 2008;40(5):221–226. doi: 10.4103/0253-7613.44154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalmagro A.P., Camargo A., Zeni A.L.B. Morus nigra and its major phenolic, syringic acid, have antidepressant-like and neuroprotective effects in mice. Metab. Brain Dis. 2017;32(6):1963–1973. doi: 10.1007/s11011-017-0089-y. [DOI] [PubMed] [Google Scholar]
- 25.Kaewkaen P., Wattanathorn J., Thong-Un T., Muchimapura S., Wannanond P., Thukhummee W., Kaewrueng W., Wongareonwanakij S., Kraipoch S., Meesilp P. Mulberry fruits extract mitigate vascular dementia. Am. J. Appl. Sci. 2012;9:1789–1795. doi: 10.3844/ajassp.2012.1789.1795. [DOI] [Google Scholar]
- 26.Lim D.W., Kim Y.T., Park J-H., Baek N-I., Han D. Antidepressant-like effects of the ethyl acetate soluble fraction of the root bark of Morus alba on the immobility behavior of rats in the forced swim test. Molecules. 2014;19(6):7981–7989. doi: 10.3390/molecules19067981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song-Tao M., Dong-lian L., Jing-jing D., Yan-juan P. Protective effect of mulberry flavonoids on sciatic nerve in alloxan-induced diabetic rats. Braz. J. Pharm. Sci. 2014;50:765–771. doi: 10.1590/S1984-82502014000400012. [DOI] [Google Scholar]
- 28.Yadav A.V., Kawale L.A., Nade V.S. Effect of Morus alba L. (mulberry) leaves on anxiety in mice. Indian J. Pharmacol. 2008;40(1):32–36. doi: 10.4103/0253-7613.40487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aditya Rao S., Ramesh C., Kuppast I.J., Mahmood R., Prabhakar B. CNS Depressant activity in two species of Mulberry. J. Pharm. Res. 2012;5:4879–4880. [Google Scholar]
- 30.Nade V.S., Kawale L.A. Targeting serotonergic pathway for anti-amnesic activity by Morus alba L. Int. J. Pharm. Sci. Drug Res. 2015;7:27–32. [Google Scholar]
- 31.Nade V.S., Kawale L.A., Naik R.A., Yadav A.V. Adaptogenic effect of Morus alba on chronic foot shock-induced stress in rats. Indian J. Pharmacol. 2009;41(6):246–251. doi: 10.4103/0253-7613.59921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaewkaen P., Tong-Un T., Wattanathorn J., Muchimapura S., Kaewrueng W., Wongcharoenwanakit S. Mulberry fruit extract protects against memory impairment and hippocampal damage in animal model of vascular dementia. Evid. Based Complement. Alternat. Med. 2012;2012:263520. doi: 10.1155/2012/263520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turgut N.H., Mert D.G., Kara H., Eğilmez H.R., Arslanbas E., Tepe B., Güngör H., Yilmaz N., Tuncel N.B. Effect of black mulberry (Morus nigra) extract treatment on cognitive impairment and oxidative stress status of D-galactose-induced aging mice. Pharm. Biol. 2016;54(6):1052–1064. doi: 10.3109/13880209.2015.1101476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srikanta A.H., Kumar A., Sukhdeo S.V., Peddha M.S., Govindaswamy V. The antioxidant effect of mulberry and jamun fruit wines by ameliorating oxidative stress in streptozotocin-induced diabetic Wistar rats. Food Funct. 2016;7(10):4422–4431. doi: 10.1039/C6FO00372A. [DOI] [PubMed] [Google Scholar]
- 35.Lim D.W., Baek N.I., Kim Y.T., Lee C., Kim I.H., Han D. Enhanced anti-immobility effects of Sanggenon G isolated from the root bark of Morus alba combined with the α2-antagonist yohimbine in the rat forced swim test. J. Nat. Med. 2016;70(3):679–682. doi: 10.1007/s11418-016-0975-3. [DOI] [PubMed] [Google Scholar]
- 36.Lim D.W., Jung J.W., Park J.H., Baek N.I., Kim Y.T., Kim I.H., Han D. Antidepressant-like effects of sanggenon g, isolated from the root bark of Morus alba, in Rats: involvement of the serotonergic system. Biol. Pharm. Bull. 2015;38(11):1772–1778. doi: 10.1248/bpb.b15-00471. [DOI] [PubMed] [Google Scholar]
- 37.Kang T.H., Hur J.Y., Kim H.B., Ryu J.H., Kim S.Y. Neuroprotective effects of the cyanidin-3-O-β-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci. Lett. 2006;391(3):122–126. doi: 10.1016/j.neulet.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 38.Andrabi S.A., Spina M.G., Lorenz P., Ebmeyer U., Wolf G., Horn T.F.W. Oxyresveratrol (trans-2,3 ',4,5 '-tetrahydroxystilbene) is neuroprotective and inhibits the apoptotic cell death in transient cerebral ischemia. Brain Res. 2004;1017:98–107. doi: 10.1016/j.brainres.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 39.Hong S., Kwon J., Kim D-W., Lee H.J., Lee D., Mar W., Mulberrofuran G. Mulberrofuran G protects ischemic injury-induced cell death via Inhibition of NOX4-mediated ROS generation and er stress. Phytother. Res. 2017;31(2):321–329. doi: 10.1002/ptr.5754. [DOI] [PubMed] [Google Scholar]
- 40.Sheeba Saji S., Abhishek D.R.R., Venkatesh K.R. Anti-Oxidant activity of various leaf extracts of mulberry species in rotenone induced oxidative stress model of rat. J. Chem. Pharm. Sci. 2016;9:2732–2736. [Google Scholar]
- 41.Nade V.S., Yadav A.V. Anti-stress effect of ethyl acetate soluble fraction of Morus alba in chronic restraint stress. Pharm. Biol. 2010;48(9):1038–1046. doi: 10.3109/13880200903473741. [DOI] [PubMed] [Google Scholar]
- 42.Bauomy A.A. The potential role of Morus alba leaves extract on the brain of mice infected with Schistosoma mansoni. CNS Neurol. Disord. Drug Targets. 2014;13(9):1513–1519. doi: 10.2174/1871527313666140806120717. [DOI] [PubMed] [Google Scholar]
- 43.Shih P-H., Chan Y-C., Liao J-W., Wang M-F., Yen G-C. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. J. Nutr. Biochem. 2010;21(7):598–605. doi: 10.1016/j.jnutbio.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Rebai O., Belkhir M., Boujelben A., Fattouch S., Amri M. Morus alba leaf extract mediates neuroprotection against glyphosate-induced toxicity and biochemical alterations in the brain. Environ. Sci. Pollut. Res. Int. 2017;24(10):9605–9613. doi: 10.1007/s11356-017-8584-6. [DOI] [PubMed] [Google Scholar]
- 45.El-Baz K.F., Aly H., Khalil W., Booles H. Neuroameliorative effects of berry extracts in Alzheimer induced rats. Int. J. Pharma Bio Sci. 2016;7 doi: 10.22376/ijpbs.2016.7.4.b548-558. [DOI] [Google Scholar]
- 46.Choi J.H., Kim D.I., Park S.H., Kim J.M., Kim C.M., Lee H.S., Ryu K.S. Effects of mulberry (Morus alba L.) leaf extract on oxygen radicals and their scavenger enzymes in brain of SD rats. Korean J. Biol. Sci. 2000;10:570–576. [Google Scholar]
- 47.Choi J.H., Kim D.I., Park S.H., Kim J.M., Baek Y.H., Lee H.S., Ryu K.S. Effects of mulberry (Morus alba L.) Leaf extract on oxidative stress and membrane fluidity in brain of SD rats. Korean J. Biol. Sci. 2000;10:354–361. [Google Scholar]
- 48.Sheeba S.S., Abhishek D., Raghubir R., Venkatesh Kumar R. Neuroprotective profile of mulberry leaf extract in focal cerebral ischemia model in rats. I.J.R.P.B. 2016;4:116–125. [Google Scholar]
- 49.Kang T.H., Oh H.R., Jung S.M., Ryu J.H., Park M.W., Park Y.K., Kim S.Y. Enhancement of neuroprotection of mulberry leaves (Morus alba L.) prepared by the anaerobic treatment against ischemic damage. Biol. Pharm. Bull. 2006;29(2):270–274. doi: 10.1248/bpb.29.270. [DOI] [PubMed] [Google Scholar]
- 50.Kaewkaen P., Tong-Un T., Wattanathorn J., Muchimapura S., Kaewrueng W., Wongcharoenwanakit S. Effects of mulberry fruit powder in animal model of stroke. Am. J. Agric. Biol. Sci. 2012;7:322–329. doi: 10.3844/ajabssp.2012.322.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamtaj O.R., Behnam M., Mohammadifar M., Sayed A.T., Seyed M.T., Taghizadeh M. The effect of alcoholic extract of Morus Alba leaves on learning and spatial memory in male rats. Qom Univ. Med. Sci. J. 2016;10:1–8. [Google Scholar]
- 52.Kim H.G., Oh M.S. Memory-enhancing effect of Mori Fructus via induction of nerve growth factor. Br. J. Nutr. 2013;110(1):86–94. doi: 10.1017/S0007114512004710. [DOI] [PubMed] [Google Scholar]
- 53.Kim H.G., Park G., Lim S., Park H., Choi J.G., Jeong H.U., Kang M.S., Lee M.K., Oh M.S. Mori Fructus improves cognitive and neuronal dysfunction induced by beta-amyloid toxicity through the GSK-3β pathway in vitro and in vivo. J. Ethnopharmacol. 2015;171:196–204. doi: 10.1016/j.jep.2015.05.054. [DOI] [PubMed] [Google Scholar]
- 54.Kim H.G., Jeong H.U., Park G., Kim H., Lim Y., Oh M.S. Mori folium and Mori Fructus mixture attenuates high-fat diet-induced cognitive deficits in mice. Evid.-Based Complement. Altern. Med; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sattayasai J., Tiamkao S., Puapairoj P. Biphasic effects of Morus alba leaves green tea extract on mice in chronic forced swimming model. Phytother. Res. 2008;22(4):487–492. doi: 10.1002/ptr.2346. [DOI] [PubMed] [Google Scholar]
- 56.Lee M-S., Park W-S., Kim Y.H., Kwon S-H., Jang Y-J., Han D., Morita K., Her S. Antidepressant-like effects of Cortex Mori Radicis extract via bidirectional phosphorylation of glucocorticoid receptors in the hippocampus. Behav. Brain Res. 2013;236(1):56–61. doi: 10.1016/j.bbr.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 57.Ye M., Ke Y., Liu B., Yuan Y., Wang F., Bu S., Zhang Y. Root bark of Morus alba ameliorates the depressive-like behaviors in diabetic rats. Neurosci. Lett. 2017;637:136–141. doi: 10.1016/j.neulet.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 58.Gupta G., Dua K., Kazmi I., Anwar F. Anticonvulsant activity of Morusin isolated from Morus alba: Modulation of GABA receptor. Biomed. Aging Pathol. 2014;4:29–32. doi: 10.1016/j.biomag.2013.10.005. [DOI] [Google Scholar]
- 59.Khan M.A., Rahman A.A., Nahar L., Islam M.B., Alam A.H.M.K. In vivo analgesic and CNS depressant activities of antioxidative stem bark fraction of Morus alba L. Dhaka Univ. J. Pharm. Sci. 2015;13:225–227. doi: 10.3329/dujps.v13i2.21905. [DOI] [Google Scholar]
- 60.Filiz T.S.P., Abdulkadir T., Ayşe K., Bayram M., Yıldırım A.U., Recep S., Hakan G., Ferhan E., Hüseyin P. Effects of Cornus mas L. and Morus rubra L. extracts on penicillin-induced epileptiform activity: an electrophysiological and biochemical study. Acta Neurobiol. Exp. (Warsz.) 2017;77(1):45–56. doi: 10.21307/ane-2017-035. [DOI] [PubMed] [Google Scholar]
- 61.Kim H.G., Ju M.S., Shim J.S., Kim M.C., Lee S-H., Huh Y., Kim S.Y., Oh M.S. Mulberry fruit protects dopaminergic neurons in toxin-induced Parkinson’s disease models. Br. J. Nutr. 2010;104(1):8–16. doi: 10.1017/S0007114510000218. [DOI] [PubMed] [Google Scholar]
- 62.Kim H., Jang M.H., Shin M.C., Chang H.K., Lee T.H., Lim B.V., Jung C.Y., Lee C.Y., Kim E.H., Kim C.J. Folium mori increases cell proliferation and neuropeptide Y expression in dentate gyrus of streptozotocin-induced diabetic rats. Biol. Pharm. Bull. 2003;26(4):434–437. doi: 10.1248/bpb.26.434. [DOI] [PubMed] [Google Scholar]
- 63.Hwang K.H., Kim Y.K. Promoting effect and recovery activity from physical stress of the fruit of Morus alba. Biofactors. 2004;21(1-4):267–271. doi: 10.1002/biof.552210152. [DOI] [PubMed] [Google Scholar]
- 64.Hassimotto N.M., Genovese M.I., Lajolo F.M. Absorption and metabolism of cyanidin-3-glucoside and cyanidin-3-rutinoside extracted from wild mulberry (Morus nigra L.) in rats. Nutr. Res. 2008;28(3):198–207. doi: 10.1016/j.nutres.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 65.Goldberg D.M., Yan J., Soleas G.J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 2003;36(1):79–87. doi: 10.1016/S0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 66.Janardhan V., Qureshi A.I. Mechanisms of ischemic brain injury. Curr. Cardiol. Rep. 2004;6(2):117–123. doi: 10.1007/s11886-004-0009-8. [DOI] [PubMed] [Google Scholar]
- 67.Chandra J., Samali A., Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000;29(3-4):323–333. doi: 10.1016/S0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 68.Lorenz P., Roychowdhury S., Engelmann M., Wolf G., Horn T.F. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: effect on nitrosative and oxidative stress derived from microglial cells. Nitric oxide. Biol. Chem. 2003;9:64–76. doi: 10.1016/j.niox.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Breuer C., Wolf G., Andrabi S.A., Lorenz P., Horn T.F. Blood-brain barrier permeability to the neuroprotectant oxyresveratrol. Neurosci. Lett. 2006;393(2-3):113–118. doi: 10.1016/j.neulet.2005.09.081. [DOI] [PubMed] [Google Scholar]
- 70.Chen H., Song Y.S., Chan P.H. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J. Cereb. Blood Flow Metab. 2009;29(7):1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodrich-Hunsaker N.J., Hopkins R.O. Spatial memory deficits in a virtual radial arm maze in amnesic participants with hippocampal damage. Behav. Neurosci. 2010;124(3):405–413. doi: 10.1037/a0019193. [DOI] [PubMed] [Google Scholar]
- 72.Liang J., Liu L., Xing D. Photobiomodulation by low-power laser irradiation attenuates Aβ-induced cell apoptosis through the Akt/GSK3β/β-catenin pathway. Free Radic. Biol. Med. 2012;53(7):1459–1467. doi: 10.1016/j.freeradbiomed.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Rogers J.L., Kesner R.P. Cholinergic modulation of the hippocampus during encoding and retrieval. Neurobiol. Learn. Mem. 2003;80(3):332–342. doi: 10.1016/S1074-7427(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 74.Galanopoulou A.S. GABA(A) receptors in normal development and seizures: friends or foes? Curr. Neuropharmacol. 2008;6(1):1–20. doi: 10.2174/157015908783769653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wallace R.H., Marini C., Petrou S., Harkin L.A., Bowser D.N., Panchal R.G., Williams D.A., Sutherland G.R., Mulley J.C., Scheffer I.E., Berkovic S.F. Mutant GABA(A) receptor γ2-subunit in childhood absence epilepsy and febrile seizures. Nat. Genet. 2001;28(1):49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 76.Roy-Byrne P.P. The GABA-benzodiazepine receptor complex: structure, function, and role in anxiety. J. Clin. Psychiatry. 2005;66(Suppl. 2):14–20. [PubMed] [Google Scholar]
- 77.Dawson T.M., Snyder S.H. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J. Neurosci. 1994;14(9):5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Surmeier D.J., Sulzer D. The pathology road map in Parkinson disease. Prion. 2013;7(1):85–91. doi: 10.4161/pri.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Veselinović T., Vernaleken I., Cumming P., Henning U., Winkler L., Kaleta P., Paulzen M., Luckhaus C., Gründer G. Antidopaminergic medication in healthy subjects provokes subjective and objective mental impairments tightly correlated with perturbation of biogenic monoamine metabolism and prolactin secretion. Neuropsychiatr. Dis. Treat. 2018;14:1125–1138. doi: 10.2147/NDT.S148557. [DOI] [PMC free article] [PubMed] [Google Scholar]