Abstract
Background
Parkinson’s disease (PD) has been one of the substantial social, medical concerns and, burdens of the present time. PD is a gradually devastating neurodegenerative disorder of the neurological function marked with α-synucleinopathy affecting numerous regions of the brain-gut axis, as well as the central, enteric, and autonomic nervous system. Its etiology is a widely disputed topic.
Objective
This review emphasizes to find out the correlation among the microbial composition and the observable disturbances in the metabolites of the microbial species and its impact on the immune response, which may have a concrete implication on the occurrence, persistence and, pathophysiology of PD via the gut-brain axis.
Methods
An in-depth research and the database was developed from the available peer-reviewed articles to date (March 2020) utilizing numerous search engines like PubMed, MEDLINE and, other internet sources.
Results
Progressively increasing shreds of evidence have proved the fact that dysbiosis in the gut microbiome plays a central role in many neurological disorders, such as PD. Indeed, a disordered microbiome-gut-brain axis in PD could be focused on gastrointestinal afflictions that manifest primarily several years prior to the diagnosis, authenticating a concept wherein the pathological pathway progresses from the intestine reaching the brain.
Conclusion
The microbiota greatly affects the bidirectional interaction between the brain and the gut via synchronized neurological, immunological, and neuroendocrine mechanisms. It can be concluded that a multitude of factors discussed in this review steadily induce the onset of dysbacteriosis that may exacerbate the etiologic mechanism of Parkinson’s disease.
Keywords: Gut microbiota, parkinson’s disease, gastrointestinal tract, α-synuclein, TLRs, gut-brain axis
1. Introduction
Parkinson’s disease (PD) is a gradually developing neuroinflammatory syndrome and is chronically progressive in nature that affects about 107-108 persons per population of 1, 00,000. The occurrence of PD varies with age from 0.3% at ages 55-65 years to 1% at 85-89 years and gradually increases with the age and could double by 2030 [1, 2]. The key players detected in patients with PD are dopaminergic
neuronal loss and α-synuclein aggregation in the lewy bodies that invade the substantia nigra pars compacta [3]. The experimental evidences suggest that the progression and pathogenesis of PD are associated with inflammation of the immune system, α-synuclein aggregation, and dysfunction of the mitochondria, oxidative stress and alterations within the gut microbiota/microbiome (GM) [4]. The key aspect of GM in the pathogenesis of Parkinson’s disease via the gut-brain axis has emerged as a primary spotlight in these recent years and it has opened up novel opportunities for the intervention of PD and its therapy. In some incredible review studies, the strength of this evidence encouraging and supporting the concept has been extensively explored and debated [5-9]. This review entails light upon the regulation and influence of the gut microbiome on the gut-brain axis via various immunological mechanisms. It was hypothesized that the pathology of α-synuclein extends from the enteric nervous system (ENS) to the central nervous system (CNS), which is correlated with the identification of α‐synuclein tangles in the appendix and colonic tissue before PD begins [10-12]. Recently, pathogenic misfolded α-synuclein accumulations have been shown to migrate in a mouse model from the gut and reaching the brain, confirming Braak's theory of idiopathic PD etiology [13]. Increasing evidences also confirm the immune system participation in the overall outcome of PD. Chronic inflammation in the gastrointestinal tract (GIT) occurs as the function of pathogen entry and other environmental factors, making up a connection with the brain via various mechanisms discussed below that might be susceptible for certain individuals to exacerbate the existing PD condition [14-16]. Houser and Tansey suggested a PD pathogenesis model which was derived from the part of the gut in which an apparent inflammatory stimulus may contribute to minimal-grade inflammation, cause transformation in the composition of microflora, and increase the permeability of the intestine, thereby enabling the bacteria to leak the subsequent inflammatory metabolites. Therefore, leakage and intrusion in the gut system will raise blood-brain barrier (BBB) permeability and aggregation of α-synuclein, resulting in neuroinflammation and neurodegeneration [14, 17]. Changes in the microbial composition can contribute to a transition in the distribution of bacterial mediated metabolites, which may entail low-grade inflammation, a significant cause for the occurrence of the PD [14, 18]. The communication between the brain and intestine is regulated by various pathways. The most obvious route is through the vagus nerve, which originates in the medulla via the dorsal motor nerve and spreads to the viscera via the abdomen. The predominant parasympathetic modulation of basic intestinal functions is provided by the vagus nerve, with the plentiful nerve supply to the small intestine, appendix and the stomach that declines proximally and further distally [14]. Response and stimuli arising within the intestine may activate vagal afferent signalling, a vital element of the reflex of neuroimmune inflammatory process networks leading to the regulation of the tonic peripheral immune system. There is also evidence to show that the vagus nerve can serve as a specific and direct portal through which substances from the intestine will move towards the brain [14, 19, 20]. It has been shown that variations in the composition and distribution of intestinal microflora might change the concentrations of these molecules together with growth factor levels and protein signaling in the brain, thereby increasing the possibility for major functional shifts [21, 22]. The prolonged anti-parkinsonian therapy usually following a few years of dopaminergic therapy, PD patients suffer from dopa-resistant motor symptoms and non motor symptoms. Existing chronic drug therapy serves various side effects such as constipation, neurasthenia, hypotension, episodes of akinetic freezing, hallucinations, and rhinitis. The treatment with the anti-parkinsonian drugs is less directed towards diminishing the cause of PD. Thus, this review emphasizes to understand the various other interrelated mechanisms and targets that exacerbate the etiology of the PD [23, 24].
2. The Gut Microbiota: The basic concepts and its unseen functions
One amongst the greatest domains (250–400 m2) of the antigens, host, and the environmental components throughout the body of a human is the human gastrointestinal tract (GIT). In an average life span, nearly 60 tons of food migrates via human GIT, together with an excess of environmental microbes that present a major danger to the health of the gut. The set of archaea, eukarya, and bacteria colonizing the GIT are considered as the 'Gut microbiota.' Over thousands of years of human evolution, it has co-evolved with the host to establish a mutually beneficial and intimate relationship. It has been reported that the amount of microbes invading the GI tract reaches 1014, which is 10 times [25]. With extremely sophisticated methods of profiling and characterizing ecosystems being established, a task for the microbiota has become regularly evident in the significant number of diseases that may be extra-intestinal and intestinal [30, 31]. Moreover, it was affirmed that the human is the “meta-organism” comprising 10 to 100 times more the bacterial cells than the cells of humans itself, that immunologically and metabolically combine and interact with each other. The Gut microbiota improves the metabolic state, endocrine signalling, and resistance to infections and is affected by gender, age, ethnicity, medication (particularly antibiotics), stress, diet, gastrointestinal infections, smoking, etc. Within every individual, if calculated at various periods, there are substantial differences in the composition of GM. While the definition of healthy microbiota cannot be established these days, and it is not known that microbiota's richness and complexity are measures of its safety [32]. The creation of the microbiota starts in the womb as the eventual birth represents the child's first significant colonization, with results finding substantial variations in Gut Microbiome in vaginally delivered infants relative to those conceived by caesarean (C-section surgery). The microbiota of vaginally born children is defined by the Lactobacillus and Prevotella taxa, whereas the other infants born by C-section are monopolized and inhabited by the species Streptococcus and Staphylococcus. Infants delivered via C-section bear Enterobacter and Kliebsella more often. Furthermore, in children born by C-section [33-36], colonization with Bacteroides and Bifidobacteria is deferred. Such distinctions tend to have impacts that continue in adulthood with vaginally born infants at reduced harm of developing asthma, obesity, and allergies, relative to the other raised by the C-section [33]. Throughout the beginning and up to 3 years of development, the microflora is continually evolving but remains stable thereafter throughout the lifespan [35].
2.1. Feasible Consequences of the GM Composition in the Periphery: Crosstalks between Periphery and Brain
The microbiome benefits the host for several advantages across distinctive physiological functions, such as preserving the stability of the gut and colon or defining the intestinal epithelium, fighting against pathogens, maintaining host immunity, and collecting energy [26-29]. Nevertheless, owing to an abnormal microbial composition and makeup, known as dysbiosis, there is scope for the failure of these pathways and may be a result of the influence of factors such as genetics, environmental factors, diet, stress, neurotransmitters and metabolites [30, 31].
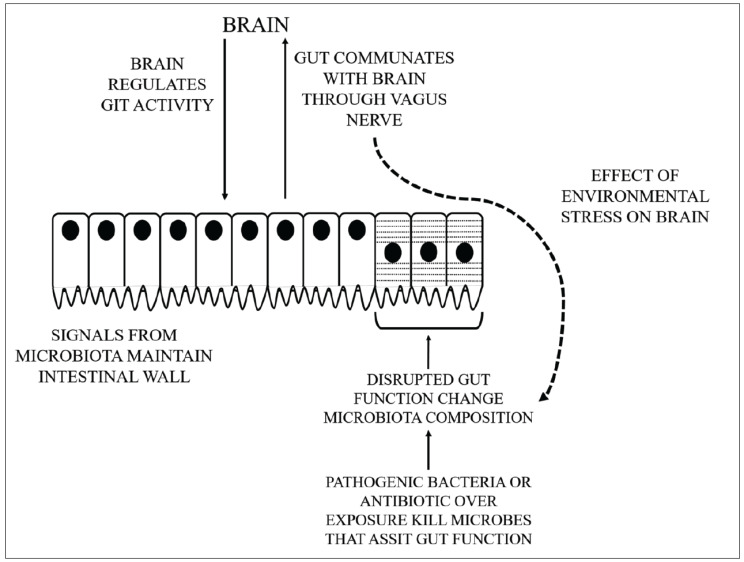
A number of mechanisms may occur that result in the peripheral-central crosstalks in the microbiota-gut-brain-axis, as shown in Fig. 1:
Fig. (1).
The complicated crosstalks between GM and brain. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Generation of various bioactive peptides by the gut microbes that involve secondary bile acids, short-chain fatty acids (SCFAs), neurotransmitters, and gut hormones. SCFAs may reach the circulatory system and it is probable that they are likely to transmit to the brain through this mechanism [37, 38].
The HPA complex activation by stress tends to secrete cortisol. This can affect intestinal motility, integrity and mucus production, owing to such a shift in gut microbiota composition that the immune cells and vagus nerves mediated by the LPS on the gram negative bacteria may influence the brain [39].
Cross-reaction of the bacterial proteins with human antigens to induce the dysfunctional stimuli to the adaptive immunity [40, 41].
Microbes possess microbe-associated molecular patterns that can be identified in the ENS by toll-like receptors. In addition, immune signaling is an essential route by which microbiota-gut-brain signaling takes place. Pro-inflammatory cytokines and chemokine synthesis affected by the intestinal microbiota may send signals to the brain through the circulatory system [40-42].
The vagus nerve is a significant part of the sensory route and the main modes of contact between the intestine and the brain, which is strongly engaged in communicating the microbiota-good-brain axis, regulation of the brain and behavior [43].
The modulation of the barrier integrity in the CNS or peripheral structures due to various pathological events leads to the essential physiological dysregulation and opening of tightly controlled interaction pathways triggering unwanted crosstalks and the movement of cells or noxious substances that sensitize or aggravate established conditions. Internal neuroinflammatory processes may be potentiated by the leakage encouraging peripheral cells to become infiltrated into the brain parenchyma. It may also be precipitated by the neuroinflammation itself. In the regulation of the immune system, peripheral inflammatory markers such as cytokines and chemokines are vital signaling factors that may influence both central and peripheral systems. Through the saturable transport, the chemokines and cytokines are actively transported through the BBB and any changes in blood expression concentrations may have a direct or indirect influence on the function of the CNS. Peripheral inflammation may be a significant contributor to both PD etiology and disease progression [44, 45].
2.2. Role of Gut Microbiota in the Pathogenesis of Parkinson’s Disease
The intricacy of the pathogenic pathways influencing neurodegenerative diseases such as PD is owing to multifactorial shifts taking place at the molecular stage that is affected by environmental and genetic factors. However, the causes and triggers for the basic pathogenesis of PD need to be elucidated. Meanwhile, evolving research rapidly affirm the role of imbalances in the composition of the GM population structures as a pathophysiological component that exacerbates the PD progression [46, 47]. The association between intestinal microflora and enteric neurons has been established as a result of recent findings [55, 43] and their ability for the regulation of the hypothalamus-pituitary-adrenal (HPA) axis, accompanied by further synthesis of mediators involved in optimal functioning of the brain [55-58].
Chronic stress in rotenone models triggers a deregulation of the HPA axis that may ultimately result in dysbacteriosis, marked by a noticeable decline in the microbial counting relating the bifidobacterium family leading to the damage of E.coli. Extended exposure results in intestinal permeability that generates a “leaky intestine,” colitis, and dysosmia by triggering significant neurochemical and neuroanatomical changes [59-63]. An imbalance in the microbiota (dysbacteriosis) of the host may result in the production of cellular degeneration, low- inflammation, and cellular energy imbalance accompanied by enhanced oxidative stress [64]. Overactivity of differentiation clusters can impair immune cell responses at the periphery that will disrupt the integrity of the barriers and its function against the lipopolysaccharides (LPS) of the bacteria as well as other toxins [65-68]. Dysbiosis can trigger various diseases, and one of the specific effects of intestinal flora upon the BBB is shown by the E. faecalis and E. lenta species, which are capable of metabolizing levodopa, the key medication given to patients with PD. It has been demonstrated that the L-dopa does not enter the Brain barrier for the dopamine exudation, culminating in a very narrower path due to these micro-organisms [69]. As per the evidence, tyrosine decarboxylase of microbe has the potential to limit dopamine exudation, hence limiting levodopa's effect during PD [70]. Another study reported that the occurrence of H. pylori infection among all patients of PD was associated with the inhibition of the absorption of the levodopa, leading to increased motor impairment [71]. Likewise, overgrowth of small intestinal bacteria influences about 54-67% of patients with PD [72]. In a research carried out by Fasano et al., all the incidences of overgrowth of the small intestinal microbe in PD were shown to be substantially elevated than in the control subjects, that correlate not only with impairments of motor centers but further weaken the motor fluctuations[73]. Altered composition of the microbiome has also been identified in chemical-induced PD models, such as exposure to rotenone or to MPTP. In samples taken from feces of MPTP-induced Parkinson mice, that were compatible with clinical results, decreased amounts of Firmicutes clostridales and Firmicutes together with elevated values of the Turicibacterales, Enterobacteriales, and Proteobacteria have been found [74]. Equivalently essential in the rotenone-treated model of PD mice, an overall decline in microbial diversity and major alterations in microbiota composition were observed, as shown in Table 1 [5, 62]. A new analysis of Sprague-Dawley rats treated with rotenone has observed an enhanced abundance of Lactobacillus, Sutterella, Bifidobacterium and Turicibacterales and less abundance of the S24-7, Oscillospira and Prevotella in the colon and the small intestine [75]. Using fecal microbiota transplants and α-synuclein overexpression mice, they found that invasion of animals (mice) with the microflora of patients suffering with PD, culminated in a serious phenotypic character relative to the mice obtaining a transplant of microbe from safe subjects [76, 77]. While the causal association between gut microbiota and PD has not been established, it is noteworthy that microbiota is needed for α-synuclein aggregation and PD-typical phenotype. Interestingly, a significant shift in the GM in the prodromal period of the PD, i.e., REM behavior disorder of sleep, indicates the shifts in the GM are not at all secondary action caused by the therapy with the dopamine [78]. In six separate studies [50, 51, 79], Lactobacillaceae sp. were shown to be the more prevalent within the stool of patients with Parkinson, and lower in the other two studies [51, 83]. Mihaila et al. also observed a greater abundance of Lactobacillaceae in PD patient's saliva relative to controls [84]. Erysipelotrichaceae family, a bacterial family, was reported to have increased in 3 separate studies in PD stools [78, 83], while others reported to have lesser or stayed unaltered relative to stable control subjects [53, 81, 83]. It has been documented that the butyrate forming Faecalibacterium [50, 58, 54, 86], Roseburia [58, 82, 83] Dorea [54, 58, 83], and Blautia [58, 81-83] are less prevalent in stools of PD patients.
3. The Gut-Brain Axis and its relevance in PD
The gut microbiota, microbes in the digestive tract of people, contains both pathogenic and commensal organisms. The microbial composition may differ considerably in accordance with the host under physiological conditions as per the mutualistic and climate functions. Gut microbiota influences permeability, intestinal immunity, secretion, motility as well as bidirectional coordination between both these enteric (ENS) and central nervous system (CNS) via processes of neuroendocrine, immunology and neurology. Observations in animal PD models have shown the obvious abnormalities in the network of the nigrostriatal pathway have contributed to distorted colonic pathophysiology, whereas colon inflammatory response induces nigrostriatal equilibrium disruption [86]. Both elemental factors of the brain-gut axis are now accepted as being engaged in PD, however, the deregulated microbiota-gut-brain axis (GBA) may focus on gastrointestinal impairment, the most frequently identified non-motor symptoms in Parkinson. Such GI symptoms involve malnutrition, constipation, impaired gastrointestinal emptying, dysphasia and issues with defecation [87, 88]. Clinical studies have already demonstrated that dysregulation of the gut precedes Parkinson's disease through diagnosing at least a decade before. An early study proved that persistent, inflammation of the gut would speed up the age of occurrence of disease, exacerbate α-synuclein pathology that further triggers neuroinflammation in α-synuclein mutated PD model of mice [89]. Sudo et al. initially suggested the notion of GBA when they found an abnormal output to stress in the mice free from germs. The GBA is a bidirectional interface between these ENS and the CNS that links their mental and cognitive regions of the brain with the distant intestinal processes of the immune systems, endocrine, the GM and the intestinal epithelium. This comprises many parts of the nervous system, which include the brain and spinal cord, the ENS, ANS and the HPA axis. The ANS, including the limbs of the sympathetic and parasympathetic areas, controls the lumen signals(afferent) that are transmitted to the CNS through spinal, enteric, and vagal channels and the signals(efferent) from the CNS to the enteric system and the distant intestinal border [9, 90]. Innervations of the vagus nerve with the whole of the intestinal region through the colonic flexure located left regarded the microflora metabolite transmitter and send the information to the brain. The HPA is the component of our limbic network, engaging in emotional, memory actions and acts as central tension efferent nerve managing reactions to stressors of some type. Systemic pro-inflammatory signals and environmental stress may trigger the limbic system through secreting the hypothalamus corticotrophin releasing factor (CRF) and, in effect, activates the secretion of adrenocorticotropic hormone exudates on stimuli from the pituitary gland and eventually contributes to the releasing action of cortisol from the adrenal gland. The cortisol is a significant hormone for stress implicated in various metabolic and physiological processes, particularly in the brain. The ENS is a multidisciplinary neural meshwork with 2 ganglionated-plexuses, submucosal and myenteric, consisting of enteric glial cells (EGCs) and neurons. EGCs have been proposed for reflecting the ENS alternative of CNS astrocytes, as they are immunohistochemically and morphologically similar to astrocytes. The humoral elements of the GBA are the enteroendocrine pathway, the mucosal immune system and the metabolites produced from the microbiota [9]. Enteroendocrine cells (EECs) release hormones such as hydroxytryptamine (5-HT) and ghrelin that have a wider range of effects on the functions of the brain and gut [91]. The intestinal epithelium creates a controlled barrier between the intestinal lumen and the circulating blood products, regarded as the intestinal epithelium barrier (IEB), and acts to avoid the transport of toxic external toxins to consume and release the numerous nutrients [92]. Among these frameworks of the IEB, the rigid epithelial and close pathways are significant because they connect via adjacent enterocytes just to ascertain the permeability of paracellular cells via the intercellular space laterally and are made up with the transmembrane substances, i.e., occludins and claudins that bound to the cytoskeleton of the actin by proteins of high molecular weight termed as zone occludens [93]. These complexes are beneath the control of the GM as well as its metabolites that serve a crucial role in the reversible communication pathway between the gut and the brain. Each element of the gut-brain axis mentioned above can be influenced individually by the pathology of PD to varying degrees [94].
3.1. Interactions of the Gut-Brain Axis Relating to the Emotional and Behavioral Symptoms of PD
The intestinal gut has the tendency to interact in either manner: individually or in relation to the brain. The brain relation (gut-brain axis) is a two-way process that travels from the brain region to the intestinal area, and conversely. It has known for a long time that certain mood shifts, psychiatric problems, impact on the intestinal stage, such as emotions, depression, and lack of appetite, or rise of appetite. The serotonin acts in the intestine, as neurotransmitter in the suppression of sleep, anger, agitation, mood, body temperature, appetite, and vomiting and holds the responsibility of equilibrium within its place (its distinctive amounts in our system are associated with depressed mindset). More than 50 per cent of dopamine production exists here, a neurotransmitter controlling the levels of satisfaction in the human brain among its functions. Its secretion takes place in circumstances with pleasure and enables one to look for the practice of fun jobs. This implies that in some regions in the brain, such as the preferentially located cortex and the nucleus accumbens, sex, food, and different medications are also stimulants for the production and release of dopamine [95].
3.1.1. Well-being
Although 90% of the serotonin, the “prosperity hormone,” is generated and processed there, the mood is rooted in the stomach.
3.1.2. Stress
The brain receives energy and fuel from the bowel in an emergency, and the guts give out indications such as stomach distress.
3.1.3. Memory
The burning of body fat by certain proteins is responsible and accountable for memory, explaining why these obese individuals are far better liable to the problem of dementia.
3.1.4. Sleep
The neurons of the stomach develop benzodiazepines (BZDs) that can calm and trigger sleeping property as we relieve the gut.
3.1.5. Fear
Panic is forcing the brain to alarm and terrify the large intestine. This does not have time to consume liquid enough, thus causes diarrhea
3.1.6. Gluttony
Billions of microbes in the intestine want their own foods to survive, so they are even greedier than us.
There is little research into the relationship between the brain, emotional state, and the microflora.
The findings are rather tentative. At the clinical stage, understanding precisely whether it will have an effect is hard to pin down. These findings point towards the possibility of utilizing probiotics as the alternative therapy to medications that cure anxiety as well as psychiatric issues, because they can actually intensify the results, although that is also provisional. Probiotics or foods high in beneficial bacteria, such as fermented milk and yogurts, showed a good effect on one’s behavior: Lactobacilli and Bifidobacterium are able to produce γ-amino butyric acid, a brain neurotransmitter that controls multiple neurological functions and whose deficiency is linked to depression and anxiety [51].
3.2. Dysregulation of the Gut-Brain Axis, Increased Intestinal Permeability, and α-synucleinopathy in PD
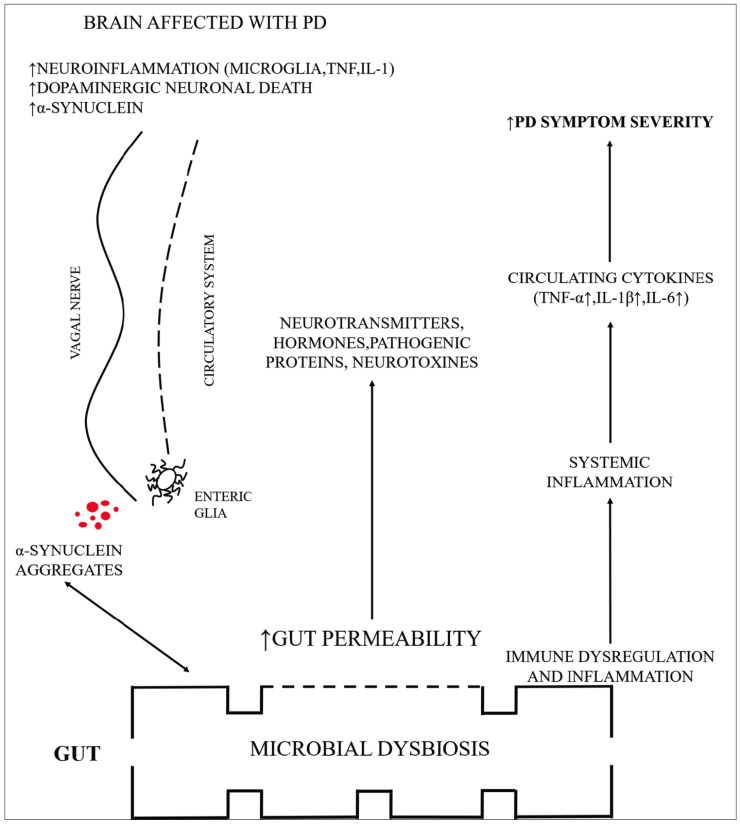
Many people with PD typically suffer from GI dysfunctions, which may include dysphasia, diarrhea, slowing of gastric emptying, constipation, hyper salivation and abnormal bowel habits. Raised permeability of the membrane (mucosal), gut constipation and the relating inflammation of the GIT are conditions directly linked to the makeup of the gastrointestinal microbiota and the metabolites obtained from microbials. Increased permeability of the intestine, a disorder regarded as a leaky intestinal syndrome, was found in the early phases of PD correlating the enteric issues. Elevated permeability of intestine due to an impaired intestinal barrier mechanism, promotes the translocation of microbes that, in effect, can induce inflammation and cause oxidative stress, resulting in ENS synucleinopathy [63]. In addition, elevated values of LPS might damage the BBB's integrity, causing neuroinflammation and SNc damage [96]. The dysbiosis in the gut and overgrowth of the small intestinal raises translocation and intestinal permeability of bacteria, assessing over-response of an immune system and consequent inflammation of the systemic as well as CNS, as summarized in Fig. 2. EBCs are capable of producing various neuroactive compounds, including catecholamines, serotonin, glutamate- amino butyric acid and SCFAs [97-99]. It has been hypothesized that the number of microorganism-produced neurotransmitters, neurohormones, and neuromodulators, however, provides the severe intricacy of this network. It remains uncertain whether these microbial neurosubstances are produced to an acceptable level for host processing to exert some sort of influence, or might be transmitted via systemic circulation to central neurocircuits [97-100].
Fig. (2).
A summarized form of possible mechanisms through which altered microbiota sends signals to the brain affected with PD. . (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The peripheral innervations of the tract are diverse, organized into 3 grades of the plexus activities of the ENS including vaso-regulation, bowel motility and management of permeability and the secretion of other gastroenteropancreatic hormones [101]. Furthermore, compared to what happens in the BBB, there are multiple remnants of astrocytes at the boundary of the intestinal barrier that provide a possible route to contact the nervous system. We have around 100 million neurons in the stomach, which is greater than what the spinal cord contains [102]. The plethora of neurons inside the ENS helps one to sense our gut's inner world as well as its contents. Most of this neural strength is reflected in the intricate everyday cycle of metabolism, through decaying food and nutrient absorption. Expelling waste involves contractions of the mechanical, chemical, and rhythmic muscle to push everything to the bottom. Hence it may regulate the gut actions independently to the brain, equipped with its own senses [103]. Proinflammatory immune function and disorders resulting in elevated amounts of α-synuclein in the intestine and brain have been shown [14, 104]. Overexpression of the α-synuclein will then cause its accumulation [14]. Over-expressed and accumulated α-synuclein, in effect, will induce proinflammatory stimuli within these immune cells, generating a +ve feedback which would facilitate the spreading of α-synuclein to various parts [105, 106]. The pathology of α-synuclein in the periphery could be transmitted to the brain. Although chronic systemic inflammation alone could serve to pathologically altered α-synuclein throughout the CNS, peripheral inflammation may be necessary [107]. Moreover, it has recently been shown that human α-synuclein, inserted into the gastrointestinal tract of the rats, may relocate the vagus nerve dorsally to the motor region of the nucleus within brainstem. The migration was induced by a micro-tubular coordinated transport process in neurons that demonstrated similarly for the oligomeric, monomeric, and other fibrillar types of α-synuclein [20]. This proof analysis indicates improvements in α-synuclein can impact directly the brain through the vagus nerve. α-syn stimulates microglia in the brain that could already be prepared by the systemic immune and α-syn in the CNS, raising the risk of neurodegeneration and expanding the period by which, indicating α-syn inclusions and resulting into neurodegeneration [105, 106, 108, 109]. Heiko Braak et al. suggested a first step staging method for the advancement of the pathology of PD via brain with the DMV participation. Questions were presented about Braak's staging scheme and his research methodology [14]. Nevertheless, despite most of Braak's staging, several reports also offered evidence and research intended to contradict his suggestion have also shown that the plurality of cases with PD(~54-81,7%) sticks entirely and only a slight fraction (~7.1-8.3%) does not exhibit disease in the DMV while it is located somewhere else in brain regions. Hence numerous PD cases exhibit a type of disease that progresses the pathological condition and DMV is severely affected [14].
3.3. Importance of Perturbed BBB and Gut Permeability Relating to the Role of GM and their Metabolites in PD
Established for regulating the physiological functions within the human body, the CNS is also dynamically mediated by both the physiological and pathological conditions of the body. Such circumstances alter quite often and sometimes constitute modifications made into the GM that directly modify the CNS through the crosstalk, as revealed by numerous studies. The BBB is a dynamic physical barrier comprises mainly of tight and adhere endothelial junctions with limited paracellular permeability that prohibits huge and possibly harmful substances from reaching the brain, thereby regulating molecular traffic between the cerebrospinal fluid and circulatory system. The information may transmit via the BBB by numerous mechanisms between the GI tract and CNS. BBB-permeable substances typically have minimal or no charge with low molecular weight and posses lipid-soluble characteristics. It has been shown by various studies that intestinal metabolic products manifest these features, which allow easy entry to modify brain physiology via the BBB [110-114]. Modulations in the BBB and intestinal permeability, microbial dysbiosis, and intestinal epithelial barrier permeability or dysfunction were reported as essential mechanisms for PD progression. The importance of the BBB in the development of neurodegenerative disorders such as PD is widely recognized, proposing that disturbed blood-brain communication is the vital process that renders neuronal dysfunction. BBB perturbation could further be affected by various factors such as oxidative and psychosocial stress [111]. The GM transforms dietary materials, like micro- and macronutrients, polyphenols, and fibers into a variety of metabolites including trimethylamines, short-chain fatty acids, vitamins and derivatives of amino acids. Such metabolites derived from microbial flora and dietary components have vital signaling and metabolic roles that can modify host homeostasis, particularly brain function and integrity of the BBB. Microbiota taxonomic assessment has defined different linkages for both specific neuroinflammation and commensal bacteria and microbial metabolites in PD patients along with the alteration in population groups of beneficial bacteria (such as genus Prevotella) and pathogenic bacteria (Enterobacteriaceae family). Specific studies demonstrating the influence of microbial metabolites on the BBB permeability and brain are summarized below. In the amino acid catabolism, GM plays a vital role and the amino acid products may significantly affect the equilibrium between the development of inhibitory and excitatory neurotransmitters crucial for the proper functioning of the brain. Lactobacillus and Bifidobacterium species metabolize glutamate, the most abundant excitatory neurotransmitter in the brain to generate the main inhibitory neurotransmitter π- aminobutyric acid (GABA). Short chain fatty acids (SCFAs) promote upper-gut motility and colonic blood flow and the BBB access via the bloodstream and influence the direct function of its integrity. For example, a decrease in BBB permeability is observed in germ-free mice after butyrate colonization along with producer Clostridium by up-regulating tight junctional proteins. It is also possible that modifying SCFA levels may be helpful in avoiding or managing neural loss. The microglial cell maturation controls BBB integrity, neurogenesis, and neuroinflammation, and is modulated by SCFA in the germ-free mice, in which the lowered microglia number, morphology and function can be saved by 4-week SCFA delivery. Vitamin K is produced by Kliebsella pneumoniae, Escherichia coli, Eubacterium and Propionibacterium and has been demonstrated to have a beneficial effect in the modification of the α-synuclein fibrillization linked with PD. Furthermore, systemic immune activation can cause perturbed BBB integrity and response due to variability of the BBB in these models prevents the generalizability of most preclinical researches to human microbiome interactions [115].
4. Toll-like receptor (TLR): Unraveling its role in the Inflammatory signals reaching the brain
Among the multiple Pattern recognition receptors (PRRs) in extracellular and intracellular conditions, TLRs perhaps the most important class of receptors in the mammalians, discovered in 1988 in Drosophila melanogaster [116]. Thirteen TLRs have been now discovered in mammals, including humans, out of which, 10 have been described completely in human cells, corresponding not to the epithelial tissue and immune cells but also to the PNS, CNS, neurons and EGCs. The spread of these immune sensors illustrates neural cells' potential for sustaining immune stimulus and their critical emphasis in maintaining the homeostasis [116, 56, 118, 119]. Besides sustaining gut homeostasis and other important physiological functions of the host, gut flora is the source with a variety of TLR ligand that, under some circumstances, may recognize harmful stimuli and respond by exerting proinflammatory influence such as a cytokine, chemokine production. The production of the cytokines is crucial for the microglial polarization and termed as ‘M1’ state, a classically active state in the brain encouraging the neuroprotection. The response is shifted from M1 to M2 phenotype (alternatively active state) where the destruction can be repaired efficaciously, unless an unregulated or chronic inflammation due to prolonged production of inflammatory mediators leads to the tissue damage [117, 130-132]. Despite the fact that microbial components are potent and efficient TLR ligands, the intestine is extremely insensitive to TLR substrates because epithelium exhibits limited TLRs under its physiological environment [117]. By comparison, modified intestinal microbiota and impaired intestinal epithelial border stimulate TLRs that further cause descending signalling response, inducing inflammation and stress of oxidative factors in PD patients in the brain and gut. The studies that have centered focus upon the functioning of TLRs have displayed their role in transmitting signals to the brain. While the precise cause of the intermittent PD is yet to be identified, rising testimony suggests that α-syn stimulates microglias, causing inflammation, contributing to neuron deterioration. Extracellular fibrillar, α-syn produced by the oligodendrocytes is identified as “PAMP” via microglial TLR Type-2 (as TLR TYPE-1 heterodimer), that further stimulates descending pathways comprising NF-B and MyD88, inducing the formation of Tumour necrosis factor (TNF) and the Interleukin-1 [120, 121] and the localization and time-dependent specific activation of TLRs [122]. TLR4 also tends to interact with α‐synuclein in in-vitro tests, activating microglial cells, including α‐syn acquisition, pro-inflammatory release of cytokine, and stimulation of oxidative stress [123]. These results have been substantiated in a murine model induced by MPTP, wherein the genetic exclusion of TLR-4 transmitting, preserved the mice against deterioration of neurons, demonstrating the chief function of TLR4 in the inflammatory signaling during PD [124]. Current data from laboratories indicates a possible presence of the endogenous inflammatory sensor NLRP-3 that might be augmented by TLR signals. Limited caspase-1 stimulation and secretion of IL-1 along with depletion of dopaminergic neurons were observed in NLRP3-deficient mice after an assault to MPTP [125]. It has been documented that fibrillar α-syn requires TLR2 on a person's monocytes to prepare the inflammatory NLRP3 [126]. Interestingly, it has been shown that NLRP3 inflammatory repression is regulated by dopamine specifically, but is still uncertain if the presence of the inflammatory mediators precedes the degradation of the neurons, or is the consequence of neuronal arrest [127]. Thus, TLR2 and TLR4 have been found to be the most conclusive proof for a function of TLRs in PD. The role of TLR2 and TLR4 in Parkinson's can be paradoximal: their induction in microglia may cause neurotoxicity, yet on the other side, they may be necessary for the clearance of α-syn, which is therefore neuroprotective in nature [128]. Various evidence indicates that epigenetic reprogramming may induce microglial priming, as demonstrated in qualified immune cells. In patients suffering from PD, intestinal membrane function is disrupted, putting them at such a risk of microbial exposure [14], so translocating bacteria or products derived from bacteria, e.g., LPS (a TLR-4 ligand) that causes the systemic inflammation, contributes to more serious degeneration of neurons [129].
The role of TLRs in neuronal disintegration is progressively recognized on the basis of the following scientific evidence:
Specific nervous system cells transmit TLRs;
TLRs triggered by the presence of α-synuclein;
Prolonged recognition of pathogen stimulates inflammatory reaction preceding neural loss;
Suppressing the role of TLRs slows the progression of Parkinson’s disease [130].
According to the study, TLR(type-4) removal has been shown to inhibit the exposure of microglia (phagocytic) with a subsequent aggregation of microglia and intensified dopaminergic neurodegeneration [140], emphasizing the neuroprotective influence of TLR(Type-4) signalling induced by the dispersion of certain synuclein, similar to reported preserved function of TLR-2 against β-amyloid and certain synucleins. Further research based on the identification of the enteric microbiota-derived variables suggests that these variables are responsible for TLR activity and the corresponding signalling turn-out of TLR stimulation should offer new comprehension into its dynamic interaction between the host and microflora in PD and other related neurodeteriorating disorders [12, 133].
Conclusion
It can be concluded that a multitude of factors discussed above steadily induce the onset of dysbacteriosis that may exacerbate the etiologic mechanism of Parkinson’s disease. GM performs a prominent appearance in its growth and equanimity of various body parts, and modifications in their content and configuration are assumed to commence prior to the actual onset of the motor characteristics in the PD. These adjustments may be linked to the fundamental pathogenesis of PD via different pathways, direct and indirect. Currently, various evidence support that the unidentified external pathogen that inevitably contributes to PD, travels within the gastrointestinal tract leading in GM dysfunctioning that slowly destroys the intestinal obstacle to enter the enteric region. The deposition of the α-syn in PD will begin in the enteric system, accelerating to a firm level and ultimately transmitting to the central regions through synapsis cell interaction. Greater knowledge of the impact of GM modifications in the CNS and ENS will help establish the association between gut microflora, inflammation, α-synucleinopathy, neuroprotection, neurodegeneration, neurotoxicity in patients suffering from PDs. Current studies and evidence from mice and clinical theories are mostly associated and the query is still unanswered about the outlook regarding the altered composition of the gut microbiota and PD pathology. One possible theory is that both factors relate to PD pathology:
Variations in the composition of gut microbial proceed the pathology and
Differences in the composition of gut microbial are not exclusively mutual
However, no treatment has yet been developed to fully cure PD, a better comprehension of microbiome and brain function can elucidate light on PD's etiological progression and offer new possibilities for the treatment. A more detailed understanding of such factors may provide possible targeting attention for clinical testing to assess new disease-altering interventions for PD like probiotics, SCFA and GM-altering medications, recombinant ghrelin, and TNF-α antagonist.
Future directions
Pharmaceutical and nutraceutical agents serve as possible modulators for the microflora-GBA and TLRs regulation in PD pathogenesis progression. For the future, numerous novel disease modulating medications provide possible targets for clinical testing to assess their role in PD. These include the Dietary supplements (Sylimarin, DHA), Prebiotics (SCFAs), probiotics (L.rhamnosus), TNF-α inhibitors, GM altering compounds, all of which target potential TLRs and may reduce the neural dysfunctioning, reduce oxidative stress, enhance BDNF expression and maintain gut integrity[134-144]. Apart from this, numerous recent studies emphasize the potential confounding effects of the other directly or indirectly acting medications in research on the GM. A plethora of drugs have been mentioned, such as Proton pump inhibitors (PPIs), metformin (anti-diabetic medication) and (escitaopram) selective serotonin reuptake inhibitors antidepressants have a well-described effect on GM via altering the microbial profiles or by activating the GBA signals [145-150].
Table 1.
Distinctions in Bacterial Composition in patients suffering from Parkinson’s (PD) relative to healthy subjects (HS).
| Decrease in Levels | Increase in Levels | Method Adopted | PD | HS | Refs. |
|---|---|---|---|---|---|
|
Prevotella, Puniceicoccaceae family, Blautia, Roseburia, Clostridium XIVa |
Lactobacillaceae, Bifidobacterium, Lactobacilli, Rikenellaceae |
16S rRNA | 64 | 64 | [48] |
| Prevotella copri, Eubacterium bioforme | Firmicutes, Akkermansia muciniphila | shotgun | 193 | 113 | [49] |
| Corpobacillaceae, Lachnospiraceace, Faecalibacterium, | Oxalobacteraceae, Ralstonia | 16S rRNA | 38 | 34 | [50] |
| Clostridium coccoides, prevotell, Clostridrium leptum, Bacteriodes fragilas | Lactobacillus sp. | Targeted qPCR | 45 | 32 | [51] |
| Prevotell sp., Bacteriods sp., Dorea, Faecalibacterium species | Lactobacilli, Oscillospira, Christenesenella, Eubactereace sp., Catabacter, Bifidobacterium | 16S rRNA | 89 | 66 | [52] |
| Clostridales incertae sedis IVsp., Prevotellaceae family |
Lactobacilli, Bradyrhizobiaceae species, Enterobacteriaceae family |
16S rRNA | 72 | 72 | [53] |
| Sediminibacterium sp, Lactobacillus sp. | Aquabacterium, Anaerotruncus, Clostridium XVIII, Sphingomonas, Butyricicoccus species, Anaerotruncus species | 16S rRNA | 45 | 45 | [54] |
Table 2.
Potential modulators of the Microbiome-Gut-Brain Axis targeting toll-like receptors are summarized.
| Modulator | Impact on Microbiome-Gut-Brain Axis | Target | Refs. |
|---|---|---|---|
| Prebiotics | |||
| Galacto oligosaccharides | Villus surface enhancement in the small intestine | TLR1-TLR13 | [110, 134] |
| Fructo oligosaccharides | Raised expression of BDNF in the hippocampus | TLR2, TLR4 | [110, 135] |
| Short-chain fatty acids (SCFA) | Colonic epithelium probity maintenance | TLR4 | [110, 136] |
| Probiotics | |||
| L.rhamnosus | GABA-A receptor modulation | TLR1, TLR2, TLR6 | [110,137] |
| Lactobacillus reuteri | Increased frequency of evacuation and enhanced bowel movement | TLR1, TLR2, TLR6 | [113, 138] |
| Lactobacillus casei shirota | Diminishing bloating of viscera and pain and improvement in the consistency of the stool | TLR1, TLR2, TLR6 | [114, 139] |
| Dietary supplements | |||
| Docosahexaenoic acid (DHA) | Improvement in the neuronal mitochondrial dysfunctioning by diminishing the oxidative stress | TLR2, TLR4 | [111, 140, 141] |
| Panax Notoginseng | Repression of the microglial stimulation and limiting the release of TNF-α and IL-6 release | TLR4 | [142] |
| Sylimarine | Neuroprotective effect and antioxidant (salvaging the free radicals) | TLR4 | [143] |
Acknowledgements
The authors would like to thank Chitkara University for providing the basic facilities for the completion of the current article.
list of Abbreviations
- CNS
Central nervous system
- CRF
Corticotrophin releasing factor
- DAMP
Damage associated molecular patter
- EES
Enteroendocrine cell
- EGC
Enteric glial cells
- ENS
Enteric nervous system
- GBA
Gut brain axis
- GIT
Gastrointestinal tract
- GM
Gut microbiota/microbiome
- HPA
Hypothalamus pituitary adrenal axis
- IEB
Intestinal epithelium barrier
- LPS
Lipopolysaccharides
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NLR2
NOD like receptors-2
- PAMP
Pathogen associated molecular pattern
- PD
Parkinson’s disease
- PNS
Peripheral nervous system
- PRR
Pattern recognition receptors
- SCFA
Short chain fatty acids
- TLR
Toll like receptors
Consent for Publication
Not applicable.
Funding
No grant was accepted from any not-for-profit sector or funding agency for this review.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Radhakrishnan D.M., Goyal V. Parkinson’s disease: A review. Neurol. India. 2018;66(Suppl.):S26–S35. doi: 10.4103/0028-3886.226451. [DOI] [PubMed] [Google Scholar]
- 2.Zeng X.S., Geng W.S., Jia J.J., Chen L., Zhang P.P. Cellular and molecular basis of neurodegeneration in Parkinson disease. Front. Aging Neurosci. 2018;10:109. doi: 10.3389/fnagi.2018.00109. https://dx.doi.org/10.3389%2Ffnagi.2018.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai Bradaric B., Patel A., Schneider J.A., Carvey P.M., Hendey B. Evidence for angiogenesis in Parkinson’s disease, incidental Lewy body disease, and progressive supranuclear palsy. J. Neural Transm. (Vienna) 2012;119(1):59–71. doi: 10.1007/s00702-011-0684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blandini F. Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J. Neuroimmune Pharmacol. 2013;8(1):189–201. doi: 10.1007/s11481-013-9435-y. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Pardo P., Hartog M., Garssen J., Kraneveld A.D. Microbes tickling your tummy: the Importance of the Gut-Brain axis in parkinson’s disease. Curr. Behav. Neurosci. Rep. 2017;4(4):361–368. doi: 10.1007/s40473-017-0129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghammer P. How does parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov. Disord. 2018;33(1):48–57. doi: 10.1002/mds.27138. [DOI] [PubMed] [Google Scholar]
- 7.Gershanik O.S. Does Parkinson’s disease start in the gut? Arq. Neuropsiquiatr. 2018;76(2):67–70. doi: 10.1590/0004-282x20170188. [DOI] [PubMed] [Google Scholar]
- 8.Surmeier D.J., Halliday G.M., Simuni T. Calcium, mitochondrial dysfunction and slowing the progression of Parkinson’s disease. 2017. [DOI] [PMC free article] [PubMed]
- 9.Yang D., Zhao D., Ali Shah S.Z., Wu W., Lai M., Zhang X., Li J., Guan Z., Zhao H., Li W., Gao H., Zhou X., Yang L. The role of the gut microbiota in the pathogenesis of Parkinson’s Disease. Front. Neurol. 2019;10:1155. doi: 10.3389/fneur.2019.01155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braak H., Rüb U., Gai W.P., Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. (Vienna) 2003;110(5):517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 11.Killinger B.A., Madaj Z., Sikora J.W., Rey N., Haas A.J., Vepa Y., Lindqvist D., Chen H., Thomas P.M., Brundin P., Brundin L., Labrie V. The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci. Transl. Med. 2018;10(465):eaar5280. doi: 10.1126/scitranslmed.aar5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shannon K.M., Keshavarzian A., Dodiya H.B., Jakate S., Kordower J.H. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov. Disord. 2012;27(6):716–719. doi: 10.1002/mds.25020. [DOI] [PubMed] [Google Scholar]
- 13.Kim S., Kwon S-H., Kam T-I., Panicker N., Karuppagounder S.S., Lee S., Lee J.H., Kim W.R., Kook M., Foss C.A., Shen C., Lee H., Kulkarni S., Pasricha P.J., Lee G., Pomper M.G., Dawson V.L., Dawson T.M., Ko H.S. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron. 2019;103(4):627–641.e7. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houser M.C., Tansey M.G. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis. 2017;3:3. doi: 10.1038/s41531-016-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klingelhoefer L., Reichmann H. Pathogenesis of Parkinson disease--the gut-brain axis and environmental factors. Nat. Rev. Neurol. 2015;11(11):625–636. doi: 10.1038/nrneurol.2015.197. [DOI] [PubMed] [Google Scholar]
- 16.Abbott R.D., Ross G.W., Petrovitch H., Tanner C.M., Davis D.G., Masaki K.H., Launer L.J., Curb J.D., White L.R. Bowel movement frequency in late-life and incidental Lewy bodies. Mov. Disord. 2007;22(11):1581–1586. doi: 10.1002/mds.21560. [DOI] [PubMed] [Google Scholar]
- 17.Tansey M.G., Romero-Ramos M. Immune system responses in Parkinson’s disease: Early and dynamic. Eur. J. Neurosci. 2019;49(3):364–383. doi: 10.1111/ejn.14290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampson T. The impact of indigenous microbes on Parkinson’s disease. Neurobiol. Dis. 2020;135:104426. doi: 10.1016/j.nbd.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Pavlov V.A., Tracey K.J. Neural circuitry and immunity. Immunol. Res. 2015;63(1-3):38–57. doi: 10.1007/s12026-015-8718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmqvist S., Chutna O., Bousset L., Aldrin-Kirk P., Li W., Björklund T., Wang Z.Y., Roybon L., Melki R., Li J.Y. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128(6):805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 21.Stilling R.M., Dinan T.G., Cryan J.F. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014;13(1):69–86. doi: 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- 22.Borre Y.E., Moloney R.D., Clarke G., Dinan T.G., Cryan J.F. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv. Exp. Med. Biol. 2014;817:373–403. doi: 10.1007/978-1-4939-0897-4_17. [DOI] [PubMed] [Google Scholar]
- 23.Gupta M., Kant K., Sharma R., Kumar A. Evaluation of In Silico anti-parkinson potential of β-asarone. Cent. Nerv. Syst. Agents Med. Chem. 2018;18(2):128–135. doi: 10.2174/1871524918666180416153742. [DOI] [PubMed] [Google Scholar]
- 24.Rascol O., Payoux P., Ory F., Ferreira J.J., Brefel-Courbon C., Montastruc J.L. Limitations of current Parkinson’s disease therapy. Ann. Neurol. 2003;53(Suppl. 3):S3–S12. doi: 10.1002/ana.10513. [DOI] [PubMed] [Google Scholar]
- 25.Thursby E., Juge N. Introduction to the human gut microbiota. Biochem. J. 2017;474(11):1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natividad J.M.M., Verdu E.F. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol. Res. 2013;69(1):42–51. doi: 10.1016/j.phrs.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Bäumler A.J., Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535(7610):85–93. doi: 10.1038/nature18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gensollen T., Iyer S.S., Kasper D.L., Blumberg R.S. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D-J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang C., Lin H. Dysbiosis in gastrointestinal disorders. Best Pract. Res. Clin. Gastroenterol. 2016;30(1):3–15. doi: 10.1016/j.bpg.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder B.O., Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016;22(10):1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 32.Castillo-Álvarez F., Marzo-Sola M.E. Role of intestinal microbiota in the development of multiple sclerosis. Neurologia. 2017;32(3):175–184. doi: 10.1016/j.nrl.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Dunn A.B., Jordan S., Baker B.J., Carlson N.S. The maternal infant microbiome: considerations for labor and birth. MCN Am. J. Matern. Child Nurs. 2017;42(6):318–325. doi: 10.1097/NMC.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goedert J.J., Hua X., Yu G., Shi J. Diversity and composition of the adult fecal microbiome associated with history of cesarean birth or appendectomy: Analysis of the American Gut Project. EBioMedicine. 2014;1(2-3):167–172. doi: 10.1016/j.ebiom.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haikal C., Chen Q.Q., Li J.Y. Microbiome changes: an indicator of Parkinson’s disease? Transl. Neurodegener. 2019;8:38. doi: 10.1186/s40035-019-0175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N., Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkar A., Lehto S.M., Harty S., Dinan T.G., Cryan J.F., Burnet P.W.J. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016;39(11):763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long-Smith C., O’Riordan K.J., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Microbiota-Gut-Brain Axis: New therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2020;60:477–502. doi: 10.1146/annurev-pharmtox-010919-023628. [DOI] [PubMed] [Google Scholar]
- 39.Kim Y.K., Shin C. The Microbiota-Gut-Brain axis in neuropsychiatric disorders: pathophysiological mechanisms and novel treatments. Curr. Neuropharmacol. 2018;16(5):559–573. doi: 10.2174/1570159X15666170915141036. https://dx.doi.org/10.2174%2F1570159X15666170915141036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rea K., Dinan T.G., Cryan J.F. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress. 2016;4:23–33. doi: 10.1016/j.ynstr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanuytsel T., van Wanrooy S., Vanheel H., Vanormelingen C., Verschueren S., Houben E., Salim Rasoel S., Tόth J., Holvoet L., Farré R., Van Oudenhove L., Boeckxstaens G., Verbeke K., Tack J. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63(8):1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 42.Alonso C., Guilarte M., Vicario M., Ramos L., Rezzi S., Martínez C., Lobo B., Martin F.P., Pigrau M., González-Castro A.M., Gallart M., Malagelada J.R., Azpiroz F., Kochhar S., Santos J. Acute experimental stress evokes a differential gender-determined increase in human intestinal macromolecular permeability. 2012. [DOI] [PubMed]
- 43.Fülling C., Dinan T.G., Cryan J.F. Gut microbe to brain signaling: what happens in vagus. Neuron. 2019;101(6):998–1002. doi: 10.1016/j.neuron.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Banks W.A. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr. Pharm. Des. 2005;11(8):973–984. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- 45.Fuzzati-Armentero M.T., Cerri S., Blandini F. Peripheral-Central neuroimmune crosstalk in parkinson’s disease: what do patients and animal models tell us? Front. Neurol. 2019;10:232. doi: 10.3389/fneur.2019.00232. https://dx.doi.org/10.3389%2Ffneur.2019.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulak A., Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015;21(37):10609–10620. doi: 10.3748/wjg.v21.i37.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cassani E., Barichella M., Cancello R., Cavanna F., Iorio L., Cereda E., Bolliri C., Zampella M.P., Bianchi F., Cestaro B., Pezzoli G. Increased urinary indoxyl sulfate (indican): new insights into gut dysbiosis in Parkinson’s disease. Parkinsonism Relat. Disord. 2015;21(4):389–393. doi: 10.1016/j.parkreldis.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Aho V.T.E., Pereira P.A.B., Voutilainen S., Paulin L., Pekkonen E., Auvinen P., Scheperjans F. Gut microbiota in Parkinson’s disease: Temporal stability and relations to disease progression. EBioMedicine. 2019;44:691–707. doi: 10.1016/j.ebiom.2019.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barichella M., Severgnini M., Cilia R., Cassani E., Bolliri C., Caronni S., Ferri V., Cancello R., Ceccarani C., Faierman S., Pinelli G., De Bellis G., Zecca L., Cereda E., Consolandi C., Pezzoli G. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 2019;34(3):396–405. doi: 10.1002/mds.27581. [DOI] [PubMed] [Google Scholar]
- 50.Scheperjans F., Aho V., Pereira P.A.B., Koskinen K., Paulin L., Pekkonen E., Haapaniemi E., Kaakkola S., Eerola-Rautio J., Pohja M., Kinnunen E., Murros K., Auvinen P. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015;30(3):350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 51.Qian Y., Yang X., Xu S., Wu C., Song Y., Qin N., Chen S.D., Xiao Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018;70:194–202. doi: 10.1016/j.bbi.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 52.Hasegawa S., Goto S., Tsuji H., Okuno T., Asahara T., Nomoto K., Shibata A., Fujisawa Y., Minato T., Okamoto A., Ohno K., Hirayama M. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS One. 2015;10(11):e0142164. doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keshavarzian A., Green S.J., Engen P.A., Voigt R.M., Naqib A., Forsyth C.B., Mutlu E., Shannon K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015;30(10):1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 54.Petrov V.A., Saltykova I.V., Zhukova I.A., Alifirova V.M., Zhukova N.G., Dorofeeva Y.B., Tyakht A.V., Kovarsky B.A., Alekseev D.G., Kostryukova E.S., Mironova Y.S., Izhboldina O.P., Nikitina M.A., Perevozchikova T.V., Fait E.A., Babenko V.V., Vakhitova M.T., Govorun V.M., Sazonov A.E. Analysis of gut microbiota in patients with parkinson’s disease. Bull. Exp. Biol. Med. 2017;162(6):734–737. doi: 10.1007/s10517-017-3700-7. [DOI] [PubMed] [Google Scholar]
- 55.Barajon I., Serrao G., Arnaboldi F., Opizzi E., Ripamonti G., Balsari A., Rumio C. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J. Histochem. Cytochem. 2009;57(11):1013–1023. doi: 10.1369/jhc.2009.953539. https://dx.doi.org/10.1369%2Fjhc.2009.953539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brun p Giron M.C. Qesari M. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology. 2013;145:1323–1333. doi: 10.1053/j.gastro.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 57.Sudo N. Role of microbiome in regulating the HPA axis and its relevance to allergy. Chem. Immunol. Allergy. 2012;98:163–175. doi: 10.1159/000336510. [DOI] [PubMed] [Google Scholar]
- 58.De V.F., Kovatcheva-Datchary P., Goncalves D. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;2014(156):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 59.Dodiya H.B., Forsyth C.B., Voigt R.M., Engen P.A., Patel J., Shaikh M., Green S.J., Naqib A., Roy A., Kordower J.H., Pahan K., Shannon K.M., Keshavarzian A. Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol. Dis. 2020;135:104352. doi: 10.1016/j.nbd.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 60.Morais L.H., Hara D.B., Bicca M.A., Poli A., Takahashi R.N. Early signs of colonic inflammation, intestinal dysfunction, and olfactory impairments in the rotenone-induced mouse model of Parkinson’s disease. Behav. Pharmacol. 2018;29:199–210. doi: 10.1097/FBP.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 61.Pan-Montojo F., Anichtchik O., Dening Y., Knels L., Pursche S., Jung R., Jackson S., Gille G., Spillantini M.G., Reichmann H., Funk R.H. Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One. 2010;5(1):e8762. doi: 10.1371/journal.pone.0008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perez-Pardo P., Dodiya H.B., Engen P.A., Naqib A., Forsyth C.B., Green S.J., Garssen J., Keshavarzian A., Kraneveld A.D. Gut bacterial composition in a mouse model of Parkinson’s disease. Benef. Microbes. 2018;9(5):799–814. doi: 10.3920/BM2017.0202. [DOI] [PubMed] [Google Scholar]
- 63.Forsyth C.B., Shannon K.M., Kordower J.H., Voigt R.M., Shaikh M., Jaglin J.A., Estes J.D., Dodiya H.B., Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One. 2011;6(12):e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noble E.E., Hsu T.M., Kanoski S.E. Gut to brain dysbiosis: mechanisms linking western diet consumption, the microbiome, and cognitive impairment. Front. Behav. Neurosci. 2017;11:9. doi: 10.3389/fnbeh.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamada N., Seo S.U., Chen G.Y., Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013;13(5):321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 66.Berer K., Krishnamoorthy G. Commensal gut flora and brain autoimmunity: a love or hate affair? Acta Neuropathol. 2012;123(5):639–651. doi: 10.1007/s00401-012-0949-9. [DOI] [PubMed] [Google Scholar]
- 67.Stolp H.B., Dziegielewska K.M., Ek C.J., Potter A.M., Saunders N.R. Long-term changes in blood-brain barrier permeability and white matter following prolonged systemic inflammation in early development in the rat. Eur. J. Neurosci. 2005;22(11):2805–2816. doi: 10.1111/j.1460-9568.2005.04483.x. [DOI] [PubMed] [Google Scholar]
- 68.Stolp H.B., Johansson P.A., Habgood M.D., Dziegielewska K.M., Saunders N.R., Ek C.J. Effects of neonatal systemic inflammation on blood-brain barrier permeability and behaviour in juvenile and adult rats. Cardiovasc. Psychiatry Neurol. 2011;2011:469046. doi: 10.1155/2011/469046. https://dx.doi.org/10.1155%2F2011%2F469046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maini Rekdal V., Bess E.N., Bisanz J.E., Turnbaugh P.J., Balskus E.P. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;364(6445):eaau6323. doi: 10.1126/science.aau6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Kessel S.P., Frye A.K., El-Gendy A.O., Castejon M., Keshavarzian A., van Dijk G., El Aidy S. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat. Commun. 2019;10(1):310. doi: 10.1038/s41467-019-08294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mridula K.R., Borgohain R., Chandrasekhar Reddy V., Bandaru V. Ch.; Suryaprabha, T. Association of Helicobacter pylori with Parkinson’s Disease. J. Clin. Neurol. 2017;13(2):181–186. doi: 10.3988/jcn.2017.13.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan A.H., Mahadeva S., Thalha A.M., Gibson P.R., Kiew C.K., Yeat C.M., Ng S.W., Ang S.P., Chow S.K., Tan C.T., Yong H.S., Marras C., Fox S.H., Lim S.Y. Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat. Disord. 2014;20(5):535–540. doi: 10.1016/j.parkreldis.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 73.Gabrielli M., Bonazzi P., Scarpellini E., Bendia E., Lauritano E.C., Fasano A., Ceravolo M.G., Capecci M., Rita Bentivoglio A., Provinciali L., Tonali P.A., Gasbarrini A. Prevalence of small intestinal bacterial overgrowth in Parkinson’s disease. Mov. Disord. 2011;26(5):889–892. doi: 10.1002/mds.23566. [DOI] [PubMed] [Google Scholar]
- 74.Lopetuso L.R., Scaldaferri F., Petito V., Gasbarrini A. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 2013;5(1):23. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.von Wrangel C., Schwabe K., John N., Krauss J.K., Alam M. The rotenone-induced rat model of Parkinson’s disease: behavioral and electrophysiological findings. Behav. Brain Res. 2015;279:52–61. doi: 10.1016/j.bbr.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 76.Caggiu E., Paulus K., Galleri G. Homologous HSV1 and alpha-synuclein peptides stimulate a T cell response in Parkinson’s disease. J. Neuroimmonulogy. 2017;310:26–31. doi: 10.1016/j.jneuroim.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 77.Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., Challis C., Schretter C.E., Rocha S., Gradinaru V., Chesselet M.F., Keshavarzian A., Shannon K.M., Krajmalnik-Brown R., Wittung-Stafshede P., Knight R., Mazmanian S.K. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s Disease. Cell. 2016;167(6):1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heintz-Buschart A., Pandey U., Wicke T. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movment sleep behavior disorder. Mov. Disord. 2018;33(1):88–98. doi: 10.1002/mds.27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin C.H., Chen C.C., Chiang H.L., Liou J.M., Chang C.M., Lu T.P., Chuang E.Y., Tai Y.C., Cheng C., Lin H.Y., Wu M.S. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflammation. 2019;16(1):129. doi: 10.1186/s12974-019-1528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hopfner F., Künstner A., Müller S.H., Künzel S., Zeuner K.E., Margraf N.G., Deuschl G., Baines J.F., Kuhlenbäumer G. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res. 2017;1667:41–45. doi: 10.1016/j.brainres.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 81.Li W., Wu X., Hu X., Wang T., Liang S., Duan Y., Jin F., Qin B. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. 2017. [DOI] [PubMed]
- 82.Hill-Burns E.M., Debelius J.W., Morton J.T., Wissemann W.T., Lewis M.R., Wallen Z.D., Peddada S.D., Factor S.A., Molho E., Zabetian C.P., Knight R., Payami H. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017;32(5):739–749. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bedarf J.R., Hildebrand F., Coelho L.P., Sunagawa S., Bahram M., Goeser F., Bork P., Wüllner U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017;9(1):39. doi: 10.1186/s13073-017-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mihaila D., Donegan J., Barns S., LaRocca D., Du Q., Zheng D., Vidal M., Neville C., Uhlig R., Middleton F.A. The oral microbiome of early stage Parkinson’s disease and its relationship with functional measures of motor and non-motor function. PLoS One. 2019;14(6):e0218252. doi: 10.1371/journal.pone.0218252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Unger M.M., Spiegel J., Dillmann K.U., Grundmann D., Philippeit H., Bürmann J., Faßbender K., Schwiertz A., Schäfer K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 86.Garrido-Gil P., Rodriguez-Perez A.I., Dominguez-Meijide A., Guerra M.J., Labandeira-Garcia J.L. Bidirectional neural interaction between central dopaminergic and gut lesions in parkinson’s disease models. Mol. Neurobiol. 2018;55(9):7297–7316. doi: 10.1007/s12035-018-0937-8. [DOI] [PubMed] [Google Scholar]
- 87.Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson’s Disease. Curr. Treat. Options Neurol. 2018;20(12):54. doi: 10.1007/s11940-018-0539-9. [DOI] [PubMed] [Google Scholar]
- 88.Mukherjee A., Biswas A., Das S.K. Gut dysfunction in Parkinson’s disease. World J. Gastroenterol. 2016;22(25):5742–5752. doi: 10.3748/wjg.v22.i25.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kishimoto Y., Zhu W., Hosoda W., Sen J.M., Mattson M.P. Chronic mild gut inflammation accelerates brain neuropathology and motor dysfunction in α-synuclein mutant mice. Neuromolecular Med. 2019;21(3):239–249. doi: 10.1007/s12017-019-08539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng W., He R., Yan Z., Huang Y., Huang W., Cai Z., Su Y., Liu S., Deng Y., Wang Q., Xie H. Regulation of immune-driven pathogenesis in parkinson’s disease by gut microbiota. 2019 doi: 10.1016/j.bbi.2020.01.009. https://dx.doi.org/10.12938%2Fbmfh.19-002 [DOI] [PubMed]
- 91.Gribble F.M., Reimann F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 2016;78:277–299. doi: 10.1146/annurev-physiol-021115-105439. [DOI] [PubMed] [Google Scholar]
- 92.Marchiando A.M., Graham W.V., Turner J.R. Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol. 2010;5:119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- 93.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013;70(4):631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhattarai Y. Microbiota-gut-brain axis: Interaction of gut microbes and their metabolites with host epithelial barriers. Neurogastroenterol. Motil. 2018;30(6):e13366. doi: 10.1111/nmo.13366. [DOI] [PubMed] [Google Scholar]
- 95.Dichter G.S., Damiano C.A., Allen J.A. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J. Neurodev. Disord. 2012;4(1):19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Banks W.A., Erickson M.A. The blood-brain barrier and immune function and dysfunction. Neurobiol. Dis. 2010;37(1):26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 97.Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 98.Foster J.A., McVey Neufeld K.A. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 99.Mazzoli R., Pessione E. The neuro-endocrinological role of microbial glutamate and gaba signaling. Front. Microbiol. 2016;7:1934. doi: 10.3389/fmicb.2016.01934. https://dx.doi.org/10.3389%2Ffmicb.2016.01934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. BioEssays. 2011;33(8):574–581. doi: 10.1002/bies.201100024. [DOI] [PubMed] [Google Scholar]
- 101.Mawe G., Hoffman J. Serotonin signaling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Silver J., Schwab M.E., Popovich P.G. Central nervous system regenerative failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb. Perspect. Biol. 2014;7(3):a020602. doi: 10.1101/cshperspect.a020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lyte M., John Cryan F. Microbial Endocrinology: The Microbiota Gut-Brain Axis in Health and Disease. 1st ed. Abilene, TX, USA: Springer-Verlag New York; 2014. [Google Scholar]
- 104.Kelly L.P., Carvey P.M., Keshavarzian A., Shannon K.M., Shaikh M., Bakay R.A., Kordower J.H. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov. Disord. 2014;29(8):999–1009. doi: 10.1002/mds.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lema Tomé C.M., Tyson T., Rey N.L., Grathwohl S., Britschgi M., Brundin P. Inflammation and α-synuclein’s prion-like behavior in Parkinson’s disease--is there a link? Mol. Neurobiol. 2013;47(2):561–574. doi: 10.1007/s12035-012-8267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Couch Y., Alvarez-Erviti L., Sibson N.R., Wood M.J., Anthony D.C. The acute inflammatory response to intranigral α-synuclein differs significantly from intranigral lipopolysaccharide and is exacerbated by peripheral inflammation. J. Neuroinflammation. 2011;8:166. doi: 10.1186/1742-2094-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sui Y.T., Bullock K.M., Erickson M.A., Zhang J., Banks W.A. Alpha synuclein is transported into and out of the brain by the blood-brain barrier. Peptides. 2014;62:197–202. doi: 10.1016/j.peptides.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cersosimo M.G., Benarroch E.E. Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol. Dis. 2012;46(3):559–564. doi: 10.1016/j.nbd.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 109.Kingsbury A.E., Bandopadhyay R., Silveira-Moriyama L., Ayling H., Kallis C., Sterlacci W., Maeir H., Poewe W., Lees A.J. Brain stem pathology in Parkinson’s disease: an evaluation of the Braak staging model. Mov. Disord. 2010;25(15):2508–2515. doi: 10.1002/mds.23305. [DOI] [PubMed] [Google Scholar]
- 110.Chandra S., Alam M.T., Dey J., Chakrapani P.S. B.; Ray, U.; Srivastava, A.K.; Gandhi, S.; Tripathi, P.P. Healthy Gut, Healthy Brain: The Gut Microbiome in Neurodegenerative Disorders. Curr. Top. Med. Chem. 2020;20(13):1142–1153. doi: 10.2174/1568026620666200413091101. [DOI] [PubMed] [Google Scholar]
- 111.Szőke H., Kovács Z., Bókkon I., Vagedes J., Szabó A.E., Hegyi G., Sterner M.G., Kiss Á., Kapócs G. 2020. [DOI] [PubMed]
- 112.Gao K., Mu C.L., Farzi A., Zhu W.Y. Tryptophan metabolism: a link between the gut microbiota and brain. Adv. Nutr. 2020;11(3):709–723. doi: 10.1093/advances/nmz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martin C.R., Osadchiy V., Kalani A., Mayer E.A. The brain-gut-microbiome axis. Cell. Mol. Gastroenterol. Hepatol. 2018;6(2):133–148. doi: 10.1016/j.jcmgh.2018.04.003. https://dx.doi.org/10.1016%2Fj.jcmgh.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ma Q., Xing C., Long W., Wang H.Y., Liu Q., Wang R.F. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J. Neuroinflammation. 2019;16(1):53. doi: 10.1186/s12974-019-1434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parker A., Fonseca S., Carding S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2020;11(2):135–157. doi: 10.1080/19490976.2019.1638722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Caputi V., Giron M.C. Microbiome-gut-brain axis and toll-like receptors in Parkinson’s disease. Int. J. Mol. Sci. 2018;19(6):1689. doi: 10.3390/ijms19061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kubinak J.L., Round J.L. Toll-like receptors promote mutually beneficial commensal-host interactions. PLoS Pathog. 2012;8(7):e1002785. doi: 10.1371/journal.ppat.1002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brun P., Gobbo S., Caputi V., Spagnol L., Schirato G., Pasqualin M., Levorato E., Palù G., Giron M.C., Castagliuolo I. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Mol. Cell. Neurosci. 2015;68:24–35. doi: 10.1016/j.mcn.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 119.Okun E., Griffioen K.J., Mattson M.P. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34(5):269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim C., Ho D.H., Suk J.E., You S., Michael S., Kang J., Joong Lee S., Masliah E., Hwang D., Lee H.J., Lee S.J. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Daniele S.G., Béraud D., Davenport C., Cheng K., Yin H., Maguire-Zeiss K.A. Activation of MyD88-dependent TLR1/2 signaling by misfolded α-synuclein, a protein linked to neurodegenerative disorders. Sci. Signal. 2015;8(376):ra45. doi: 10.1126/scisignal.2005965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Béraud D., Maguire-Zeiss K.A. Misfolded α-synuclein and Toll-like receptors: therapeutic targets for Parkinson’s disease. Parkinsonism Relat. Disord. 2012;18(Suppl. 1):S17–S20. doi: 10.1016/S1353-8020(11)70008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fellner L., Irschick R., Schanda K., Reindl M., Klimaschewski L., Poewe W., Wenning G.K., Stefanova N. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia. 2013;61(3):349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Noelker C., Morel L., Lescot T., Osterloh A., Alvarez-Fischer D., Breloer M., Henze C., Depboylu C., Skrzydelski D., Michel P.P., Dodel R.C., Lu L., Hirsch E.C., Hunot S., Hartmann A. Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci. Rep. 2013;3:1393. doi: 10.1038/srep01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yan Y., Jiang W., Liu L., Wang X., Ding C., Tian Z., Zhou R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160(1-2):62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 126.Codolo G., Plotegher N., Pozzobon T., Brucale M., Tessari I., Bubacco L., de Bernard M. Triggering of inflammasome by aggregated α-synuclein, an inflammatory response in synucleinopathies. PLoS One. 2013;8(1):e55375. doi: 10.1371/journal.pone.0055375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Labzin L.I., Heneka M.T., Latz E. Innate immunity and neurodegeneration. Annu. Rev. Med. 2018;69:437–449. doi: 10.1146/annurev-med-050715-104343. [DOI] [PubMed] [Google Scholar]
- 128.Rietdijk C.D., van Wezel R.J., Garssen J., Kraneveld A.D. Neuronal toll-like receptors and neuro-immunity in Parkinson’s disease, Alzheimer’s disease and stroke. Neuroimmunol. Neuroinflamm. 2016;3:27–37. doi: 10.20517/2347-8659.2015.28. [DOI] [Google Scholar]
- 129.Drouin-Ouellet J., Cicchetti F. Inflammation and neurodegeneration: the story ‘retolled’. Trends Pharmacol. Sci. 2012;33(10):542–551. doi: 10.1016/j.tips.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 130.Diamond B., Huerta P.T., Tracey K., Volpe B.T. It takes guts to grow a brain: Increasing evidence of the important role of the intestinal microflora in neuro- and immune-modulatory functions during development and adulthood. BioEssays. 2011;33(8):588–591. doi: 10.1002/bies.201100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Diaz Heijtz R., Wang S., Anuar F., Qian Y., Björkholm B., Samuelsson A., Hibberd M.L., Forssberg H., Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Galland L. The gut microbiome and the brain. J. Med. Food. 2014;17(12):1261–1272. doi: 10.1089/jmf.2014.7000. https://dx.doi.org/10.1089%2Fjmf.2014.7000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stefanova N., Fellner L., Reindl M., Masliah E., Poewe W., Wenning G.K. Toll-like receptor 4 promotes α-synuclein clearance and survival of nigral dopaminergic neurons. Am. J. Pathol. 2011;179(2):954–963. doi: 10.1016/j.ajpath.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alizadeh A., Akbari P., Difilippo E., Schols H.A., Ulfman L.H., Schoterman M.H., Garssen J., Fink-Gremmels J., Braber S. The piglet as a model for studying dietary components in infant diets: effects of galacto-oligosaccharides on intestinal functions. Br. J. Nutr. 2016;115(4):605–618. doi: 10.1017/S0007114515004997. [DOI] [PubMed] [Google Scholar]
- 135.Savignac H.M., Corona G., Mills H., Chen L., Spencer J.P., Tzortzis G., Burnet P.W. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-D-aspartate receptor subunits and D-serine. Neurochem. Int. 2013;63(8):756–764. doi: 10.1016/j.neuint.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rivière A., Selak M., Lantin D., Leroy F., De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. https://dx.doi.org/10.1073%2Fpnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ojetti V., Ianiro G., Tortora A., D’Angelo G., Di Rienzo T.A., Bibbò S., Migneco A., Gasbarrini A. The effect of Lactobacillus reuteri supplementation in adults with chronic functional constipation: a randomized, double-blind, placebo-controlled trial. J. Gastrointestin. Liver Dis. 2014;23(4):387–391. doi: 10.15403/jgld.2014.1121.234.elr. [DOI] [PubMed] [Google Scholar]
- 139.Cassani E., Privitera G., Pezzoli G., Pusani C., Madio C., Iorio L., Barichella M. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol. Dietol. 2011;57(2):117–121. [PubMed] [Google Scholar]
- 140.Hwang D.H., Kim J.A., Lee J.Y. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur. J. Pharmacol. 2016;785:24–35. doi: 10.1016/j.ejphar.2016.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tang R., Lin Y.M., Liu H.X., Wang E.S. Neuroprotective effect of docosahexaenoic acid in rat traumatic brain injury model via regulation of TLR4/NF-Kappa B signaling pathway. Int. J. Biochem. Cell Biol. 2018;99:64–71. doi: 10.1016/j.biocel.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 142.Beamer C.A., Shepherd D.M. Inhibition of TLR ligand- and interferon gamma-induced murine microglial activation by Panax notoginseng. J. Neuroimmune Pharmacol. 2012;7(2):465–476. doi: 10.1007/s11481-011-9333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Haddadi R., Nayebi A.M., Eyvari B.S. Silymarin prevents apoptosis through inhibiting the Bax/caspase-3 expression and suppresses toll like receptor-4 pathway in the SNc of 6-OHDA intoxicated rats. Biomed. Pharmacother. 2018;104:127–136. doi: 10.1016/j.biopha.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 144.Forslund K., Hildebrand F., Nielsen T., Falony G., Le Chatelier E., Sunagawa S., Prifti E., Vieira-Silva S., Gudmundsdottir V., Pedersen H.K., Arumugam M., Kristiansen K., Voigt A.Y., Vestergaard H., Hercog R., Costea P.I., Kultima J.R., Li J., Jørgensen T., Levenez F., Dore J., Nielsen H.B., Brunak S., Raes J., Hansen T., Wang J., Ehrlich S.D., Bork P., Pedersen O. MetaHIT consortium. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Imhann F., Bonder M.J., Vich Vila A., Fu J., Mujagic Z., Vork L., Tigchelaar E.F., Jankipersadsing S.A., Cenit M.C., Harmsen H.J., Dijkstra G., Franke L., Xavier R.J., Jonkers D., Wijmenga C., Weersma R.K., Zhernakova A. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65(5):740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Freedberg D.E., Toussaint N.C., Chen S.P., Ratner A.J., Whittier S., Wang T.C., Wang H.H., Abrams J.A. Proton pump inhibitors alter specific taxa in the human gastrointestinal microbiome: a crossover trial. Gastroenterology. 2015;149(4):883–5.e9. doi: 10.1053/j.gastro.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jackson M.A., Goodrich J.K., Maxan M.E., Freedberg D.E., Abrams J.A., Poole A.C., Sutter J.L., Welter D., Ley R.E., Bell J.T., Spector T.D., Steves C.J. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749–756. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maier L., Pruteanu M., Kuhn M., Zeller G., Telzerow A., Anderson E.E., Brochado A.R., Fernandez K.C., Dose H., Mori H., Patil K.R., Bork P., Typas A. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cussotto S., Strain C.R., Fouhy F., Strain R.G., Peterson V.L., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology (Berl.) 2019;236(5):1671–1685. doi: 10.1007/s00213-018-5006-5. [DOI] [PubMed] [Google Scholar]
- 150.Davey K.J., Cotter P.D., O’Sullivan O., Crispie F., Dinan T.G., Cryan J.F., O’Mahony S.M. Antipsychotics and the gut microbiome: olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl. Psychiatry. 2013;3:e309. doi: 10.1038/tp.2013.83. https://dx.doi.org/10.1038%2Ftp.2013.83 [DOI] [PMC free article] [PubMed] [Google Scholar]