Abstract
Background
Neuromyelitis Optica Spectrum Disorder (NMOSD) is a chronic autoimmune disease of the central nervous system that causes recurrent attacks of optic neuritis, myelitis, and brainstem symptoms, resulting in severe neurological disability. Preventive treatment with immunosuppressive agents reduces relapse rate and improves long-term prognosis. In recent years, the potential therapeutical effect of new agents has been investigated. Two of these, the anti-interleukin 6 (IL-6) agents tocilizumab and satralizumab, have been studied in active NMOSD.
Objective
To systematically review the current data regarding the efficacy and safety of anti-IL-6 agents in NMOSD.
Results
Fourteen case reports and 5 case series of intravenous tocilizumab have shown beneficial clinical and paraclinical effects compared to commonly used therapies, and another case series of subcutaneous tocilizumab has shown it is as effective as the IV formulation. A phase 2 comparative trial has shown tocilizumab IV to be more effective than azathioprine for relapse prevention. A phase 3 trial of subcutaneous satralizumab versus placebo, has shown a lower risk of relapse in the sartralizumab-treated group, both as add-on therapy to stable immunosuppressant and as monotherapy. Tocilizumab also reduced pain severity in two trials and fatigue scores in one trial, but satralizumab did not significantly improve pain and fatigue. Adverse events with both agents were relatively mild and comparable to placebo and azathioprine.
Conclusion
The anti-Il-6 agents tocilizumab and satralizumab show promising results in active NMOSD.
Further randomized, larger-scale trials are needed to better define the role of these agents in the growing arsenal of NMOSD treatments.
Keywords: NMOSD, ; MOG-antibody disease,; treatment,; efficacy,; safety, ; interleukin-6,; anti-IL-6 receptor; tocilizumab,; satralizumab
1. Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a chronic autoimmune disease of the central nervous system (CNS) that typically manifests with recurrent episodes of optic neuritis (ON), longitudinally extensive transverse myelitis (LETM) and area postrema syndrome, while less typical manifestations include brainstem and cerebellar syndromes, narcolepsy and diencephalic syndromes, and cerebral syndromes [1-5]. The term “neuromyelitis optica” was first used by Devic already in 1894 [6], and the clinical condition has also been described as “optic spinal multiple sclerosis” as there was disagreement, whether it was a subtype of multiple sclerosis (MS) [7, 8]. Only in 2004, with the discovery of an autoantibody directed against the astrocytic aquaporin 4 (AQP4) water channel present in the serum of approximately 80% of patients, has NMO been considered a distinct clinicopathological entity [8-10]. Further advances in diagnostic procedures have enabled to identify a subgroup of patients with a similar clinical phenotype who harbor a different autoantibody, directed against the myelin oligodendrocyte glycoprotein (MOG) [8, 11-13]. While AQP4- and MOG-positive patients share several clinical and radiological features, there are also distinctive clinical, radiological, and socio-epidemiological features between the two groups [14-22], which have led several authors to consider them as separate nosological entities [23-25]. However, according to the international consensus diagnostic criteria of NMOSD, both AQP4- and MOG-positive patients can be classified under the definition of NMOSD when fulfilling the required clinical and radiological characteristics [26].
NMOSD typically follows a relapsing course, characterized by recurrent severe attacks leading to accrual neurological disability [2, 3, 27]. Therefore, immunosuppressive treatment aimed to prevent further attacks is strongly recommended [2, 8, 24, 27-36]. The commonly used medications include oral corticosteroids (CS), azathioprine(AZA), mycophenolate mofetil (MMF), and rituximab (RTX). In the past years, new therapeutic strategies have been developed, aiming at targeting specific components of the immunologic cascade responsible for the inflammatory process of NMOSD. The most novel therapies in this category include monoclonal antibodies against the CD19 B cell antigen, the C5 complement protein, and the interleukin-6 (IL-6) receptor [27, 34-36].
IL-6 is a cytokine involved in the mediation of inflammatory and immune-mediated responses. IL-6 generally exerts a pro-inflammatory effect, especially during chronic inflammatory conditions such as collagen-induced arthritis [37] and murine colitis [38], where it is involved in modulating specific cellular and humoral immune responses, including B lymphocyte differentiation, immunoglobulin secretion and T cell activation [39]. In the central nervous system (CNS), IL-6 levels are increased in experimental autoimmune encephalomyelitis (EAE), an animal model of autoimmune inflammatory disease [40]. Although EAE has for long been considered a model of multiple sclerosis [41, 42], one of the auto-antigens used for its induction is MOG [42-45], and many of its clinical and pathological features, such as the predilection for the optic nerve and spinal cord, are more similar to MOG/NMOSD than multiple sclerosis [14, 44].
Over recent years, there is growing evidence for a role of the IL-6 signaling pathway in the pathogenesis of NMOSD. These include both in vitro evidence of decreased blood-brain barrier function, increased chemokine production and enhanced leukocyte migration [46], prolonged survival of AQP4 antibody-secreting cells and increased AQP4 antibody production by IL-6 [47-49], and in vivo evidence of increased serum and CSF IL-6 levels, particularly during relapses [47, 50-57]. In addition to this data, which supports a possible role of IL-6 in the inflammatory activity associated with relapses of NMOSD, IL-6 may also have a role in the mechanisms involved in pain and fatigue: IL-6 is one of the cytokines involved in the initiation of pain process [58]; blockade of IL-6 reduces pain in animal models [59, 60]; and anti-IL-6 therapy improves fatigue in rheumatoid arthritis (RA) [61], another disease where IL-6 has an important pathophysiologic role [62]. These observations have paved the way for the use of monoclonal antibodies blocking the IL-6 receptor in the treatment of NMOSD.
Tocilizumab is a humanized monoclonal antibody against the IL-6 receptor, which, in its intravenous (IV) formulation, is currently approved for the treatment of RA, giant cell arteritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome [63-66]. Tocilizumab for subcutaneous (SC) administration is approved for RA [67] and giant cell arteritis [66]. Satralizumab (also known as SA237) is another anti-IL-6 monoclonal antibody, which has been designed specifically for the treatment of NMOSD and is administered subcutaneously [68]. This review is aimed to systematically summarize the currently available information regarding the efficacy and safety of these agents in NMOSD.
2. Materials and methods
We searched the current literature concerning anti-IL-6 therapies in NMOSD using Pubmed, Embase (Ovid), and Web of Science databases. Search keywords used include Interleukin-6, il-6, anti-interleukin-6, anti-il-6, tocilizumab, satralizumab, SA237, neuromyelitis optica, neuromyelitis optica spectrum disorder(s), NMOSD, myelin-oligodendrocyte glycoprotein, MOG-antibody-associated disease, MOG-antibody-associated, and MOG-associated. Where possible, subject headings for Interleukin-6, tocilizumab, satralizumab, myelin-oligodendrocyte glycoprotein, and neuromyelitis optica were also included.
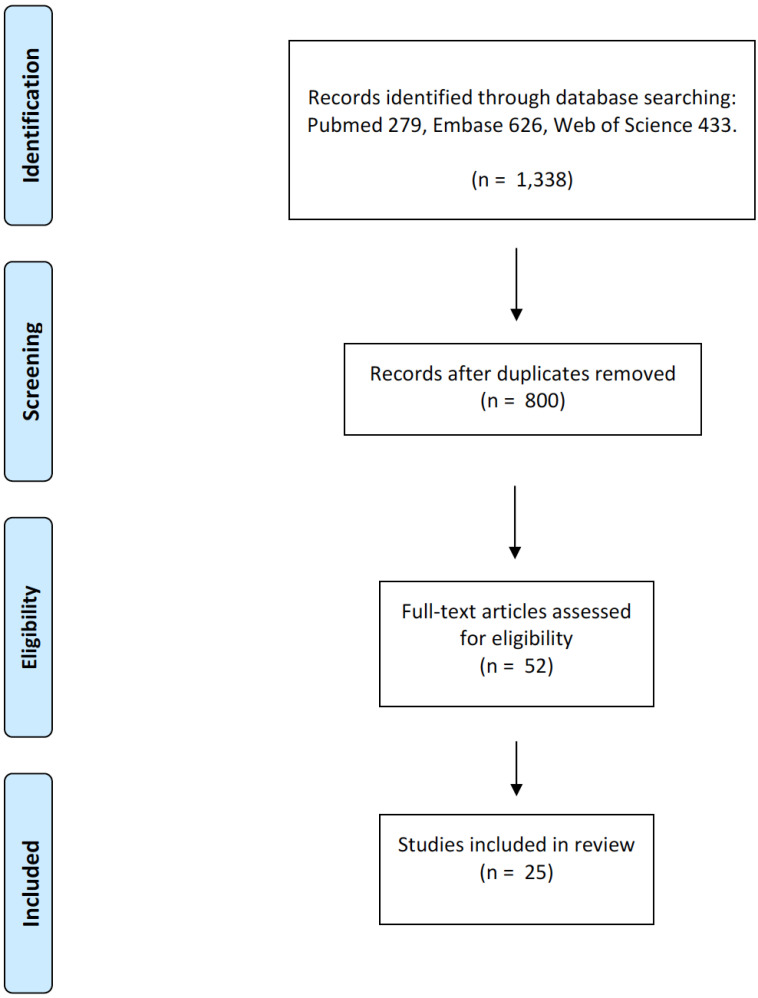
Searches were performed through December 31, 2019, and limited to English language results only. The initial search resulted in 1,338 abstracts. When duplicate citations were removed, the results decreased to 800 abstracts. Of these 800 abstracts, 52 were chosen for full-text screening. Of the 52, 25 references were selected for inclusion.
3. Results
Of the 25 publications included in the review, 19 papers document the efficacy and safety of tocilizumab in NMOSD – 12 case reports [69-80], four retrospective case series of tocilizumab IV [81-84], one retrospective case series of tocilizumab SC [85], one prospective case series of tocilizumab IV [86], and 1 phase 2 clinical trial of tocilizumab IV (TANGO study) [87]. Updated data from 2 case series [83, 86] reporting data on additional patients not previously reported, have been recently published and considered in this review as well [88, 89].
Of the 12 case reports of tocilizumab in NMOSD, 11 are documenting treatment outcomes in AQP4+ patients [69-77, 79, 80], and 1 in seronegative patient [78]. Except for 2 case reports of tocilizumab therapy in pediatric patients [73, 75], all other studies document treatment outcomes in adults. The TANGO study is a randomized comparative trial of tocilizumab versus azathioprine in highly active NMOSD, where the primary endpoint is time to first relapse following the study initiation. The final results of this study have not been published yet, but interim analysis has been presented [87].
Two case reports [90, 91] and two retrospective case series [85, 89] report efficacy and safety data of tocilizumab in MOG-positive patients. Although the two MOG-positive case reports were diagnosed as MOG-associated optic neuritis [90] and MOG-antibody spectrum disorder [91], review of these cases revealed both patients fulfill the diagnostic criteria of NMOSD without AQP4 antibodies, as they had at least 2 of the clinical characteristics as required by the international consensus diagnostic criteria [26]. Therefore, we considered these cases as MOG+ NMOSD.
The safety and efficacy of satralizumab in NMOSD were evaluated in two randomized, double-blind, placebo-control trials. In the SAKuraSky study, sartralizumab was administered as an add-on treatment to baseline immunosuppressive therapies that included oral corticosteroids, azathioprine, and mycophenolate mofetil [92, 93]. In contrast, in the SAKuraStar study, satralizuamb was administered as monotherapy. The latter study is currently still underway, but interim data has been presented [94].
In all cases, both tocilizumab and satralizumab were administered in the context of highly- active disease after several prior immunosuppressive therapies failed to suppress disease activity.
The primary efficacy and safety data of both drugs are summarized in Table 1.
Table 1.
Summary of efficacy and safety data in NMOSD and MOG-associated antibody disease.
| Author (Year of Publication) | Study Design | Number of Participants | Diagnosis | Treatment Protocol | Treatment Duration | Main Efficacy Outcomes | Adverse Events and Safety Outcomes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Araki et al. (2013) | Case report | 1 | AQP4 + NMOSD | TCZ IV 8 mg/kg q4W |
6 months | One minor relapse; improved EDSS from 3.5 to 2; improved pain; no change in brain and spinal MRI findings; reduced AQP4 Ab titers | ↓ systolic BP; Lymphocytopenia; enteritis; URI |
|||||||||||||
| Kieseier et al. (2013) |
Case report | 1 | AQP4 + NMOSD | TCZ IV 8 mg/kg q4W |
16 months | No relapses; improved EDSS from 6 to 2.5; no new or enhanced MRI lesions; reduced intrathecal IL-6 levels | None | |||||||||||||
| Lauenstein et al. (2014) |
Case report | 1 | AQP4 + NMOSD | TCZ IV 8 mg/kg q4W |
1 year | No relapses; improved EDSS from 9 to 2.5; decreased spinal cord abnormal signal on MRI; decreased AQP4 Ab titers | None | |||||||||||||
| Harmel et al. (2014) | Case report | 1 | AQP4 + NMOSD | TCZ IV 8 mg/kg q4W |
1 year | No relapses; mild improvement id EDSS (from 9.0 to 8.0); decreased tumefactive lesion on brain MRI; AQP4 Ab titers persistently high | None | |||||||||||||
| Komai et al. (2016) | Case report | 1 | AQP4 + NMOSD | TCZ IV 8 mg/kg q4W |
120 days | No relapses; improved EDSS from 8.5 to 6; reduced AQP4 Ab titers | None | |||||||||||||
| Marino et al. (2017) | Case report | 1 | AQP4 + NMOSD | TCZ IV 7 mg/kg q4W |
3 years | No relapses | None | |||||||||||||
| Meinl et al. (2018) | Case report | 1 | AQP4 + NMOSD | TCZ IV 8 mg/kg q4W |
6 months | 2 relapses | Grade 2 granulocytopenia associated with bladder and bronchial infections | |||||||||||||
| Mancinelli et al. (2018) |
Case report | 1 | AQP4 + NMOSD | TCZ IV 8 mg/kg q4W |
12 months | No relapses; No new MRI activity | None | |||||||||||||
| Breu et al. (2019) | Case report | 2 | AQP4 + NMOSD | TCZ IV 8 mg/kg q4W |
18-36 months | No relapses; no new MRI lesions; persistently high AQP4 ab titers | None | |||||||||||||
| Capobianco et al. (2019) | Case report | 1 | AQP4 + NMOSD | TCZ IV 8 mg/kg q4W |
36 months *TCZ administered during pregnancy |
No relapses; no MRI activity | None | |||||||||||||
| Siwoski et al. (2019) | Case report | 1 | AQP4 + NMOSD | Not reported | 6 months | No relapses; mild improvement in neurological function | Worsened migraine | |||||||||||||
| Hayward-Koennecke et al. (2019) |
Case report | 1 | MOG-associated optic neuritis | TCZ IV 8 mg/kg q2-6W |
4.5 years | No relapses; stable MRI | None | |||||||||||||
| Novi et al. (2019) | Case report | 1 | MOG-antibody spectrum disorder | TCZ IV 8 mg/kg q4W |
24 months | No relapses; improved spinal MRI lesions | None | |||||||||||||
| Meca-Lallana et al. (2017) | Case report | 1 | Double -Seronegative NMODS | TCZ IV 8 mg/kg q4W |
3 months | No relapses; No new MRI lesions | None | |||||||||||||
| Ayzenberg et al. (2013) |
Case series | 3 | AQP4 + NMOSD | TCZ IV 6mg\kg q4-6W |
18 months (median) |
Median ARR decreased from 3 (range 2.3-3) to 0.6(range 0-1.3); EDSS improved in one patient and remained stable in two; no new or enhancing MRI lesions | UTI (n=1); mild oral mucositis (n=1) |
|||||||||||||
| Araki et al. (2014) | Case series | 7 | AQP4 + NMOSD | TCZ IV 8 mg/kg q4W |
1 year | Mean ARR decreased from 2.9 ±1.1 to 0.4± 0.8; EDSS score decreased from 5.1± 1.7 (range, 3.0–6.5) to 4.1± 1.6 (range, 2.0–6.0); improved pain and fatigue; no new or enhancing MRI lesions; reduced AQP4 Ab titers | URI (n=2); acute enterocolitis (n=2); acute pyelonephritis (n=1); leukocytopenia and/or lymphocytopenia(n=3); anemia (n=2); ↓ systolic BP (n=1) |
|||||||||||||
| Ringelstein et al. (2015) |
Case series | 8 | AQP4 + NMOSD | TCZ IV 6-8 mg/kg q4W |
30.9±15.9 months (mean) | Median ARR decreased from 4 (interquartile range 5-3) to 0.4(interquartile range 0.8-0); Mean EDSS improved from 7.3 (interquartile range 8.4-5.4) to 5.5 (interquartile range 6.5-2.6); Improved MRI, AQP4 Ab titers and pain level | Elevated cholesterol levels(N=6); mild postinfusion nausea (n = 1); transient gastritis (n = 1); transient diarrhea (n = 1); headache (n = 1); fatigue (n = 2); recurrent UTI (n = 3); DVT (N=1); Leukopenia or neutropenia (N=2); mild increase in liver enzymes (N=3) |
|||||||||||||
| Carreon Guarnizo et al. (2019) |
Case series | 5 | AQP4 + NMOSD (N=2); double-seronegative NMOSD (N=3) | TCZ IV 8 mg/kg q4W |
2.3±1years | Reduced mean ARR from 1.8 ± 1.3 to 0.2 ± 0.4; no significant change in EDSS; no new or enhancing MRI lesions | Neutropenia (N=1); Recurrent UTI (N=1) |
|||||||||||||
| Araki M. (2019) | Case series | 19 (including previously reported 7 patients) | AQP4 + NMOSD | TCZ IV 8 mg/kg q4W |
Up to 6 years and 8 months; > 1 year in 15 patients | Decreased number of relapses in all patients; 10 patients relapse-free; decreased ARR from 2.2±1.1 to 0.3±0.7 in the 15 patients treated for >1 year; decreased EDSS from 4.5±1.8 to 3.8±1.4; improved pain and fatigue scores; decreased AQP4 Ab titers | Iron-deficiency anemia (N=4) | |||||||||||||
| Author (Year of Publication) | Study Design | Number of Participants | Diagnosis | Treatment Protocol | Treatment Duration | Main Efficacy Outcomes | Adverse Events and Safety Outcomes | |||||||||||||
| Dalla costa et al. (2019) | Case series | 14 | AQP4 + NMOSD (N=10); seronegative NMOSD* (N=4) | TCZ IV 8 mg/kg q4W |
Not reported | Decreased EDSS from 6.5±1.3 to 5.5±0.5; No new or enhancing MRI lesions | Infusion reaction (N=1); Neutropenia (N=2); Several infections (other data not available) |
|||||||||||||
| Ringelstein et al. (2019) | Case series | 45 (including previously reported 8 AQP+ patients) | AQP4 + NMOSD (N=32); seronegative NMOSD (N=7); MOG+ encephalomyelitis (N=6) | TCZ IV 6-8 mg/kg q4W (N=44); TCZ SC (N=1) |
3-100 months | Mean ARR decreased from 1.83 to 0.58; mean EDSS decreased from 5.2 to 4.7 | Not reported | |||||||||||||
| Lotan et al. (2019) | Case series | 12 | AQP4 + NMOSD (N=7); MOG+ NMOSD (N=2); double-seronegative NMOSD (N=3) | TCZ SC 162 mg q1-2W | 31.8±18.8 months (mean) | The median number of relapses decreased from 2.0 (interquartile range = 4.0-0.25) in the year before TCZ to 0 (interquartile range = 1.75-0) in the year after treatment; Overall median ARR decreased from 2.0 (interquartile range = 5.75-1.29) before treatment to 0 (interquartile range= 1.0-0) on treatment; ambulatory status improved in two patients and remained stable in nine; AQP-4 and MOG antibody titers decreased or disappeared in all but one AQP4 seropositive patient; New MRI lesions were detected in 1 out of 10 patients for whom a follow-up brain MRI was available, and in 2 out of 9 patients for whom a follow-up spinal cord MRI was available | UTI(N=4); Elevated cholesterol levels(N=3); Skin abscess (N=2); Pain at the injection site (N=1); Neutropenia (N=1) |
|||||||||||||
| Shi et al. (2019) | Phase 2 comparative trial of TCZ Vs. AZA | 118 (59 in each treatment arm) |
AQP4 + NMOSD (N=103); seronegative NMOSD* (N=15) | TCZ IV 8 mg/kg q4W or AZA 2-3 mg/kg/d |
48 weeks | 91.5% relapse-free in the tocilizumab group Vs. 67.8% in the azathioprine group; Sustained reduction in disability was more likely among patients treated with tocilizumab than patients with azathioprine; Serum levels of anti-AQP4-ab were reduced significantly by 42% with tocilizumab compared to 15% with azathioprine | fatigue, skin rash, leukopenia or elevated liver enzymes in 20 patients (34%) receiving tocilizumab |
|||||||||||||
| Author (Year of Publication) | Study Design | Number of Participants | Diagnosis | Treatment Protocol | Treatment Duration | Main Efficacy Outcomes |
Adverse Events and Safety Outcomes |
|||||||||||||
| Yamamura et al. (2019) | Phase 3 randomized, double-blind placebo-controlled add-on trial | 83 (41 in the sartralizumab group and 42 in the placebo group) |
AQP4 + NMOSD (N=55); seronegative NMOSD* (N=28) | Sartralizumab SC 120 mg q4W | Median 107.4 weeks in the sartralizumab group and 32.5 weeks in the placebo group during the double-blind period | 8 patients (20%) in the sartralizumab group had relapse vs. 18 patients (43%) in the placebo group; 89% and 78% in the sartralizumab group were free of relapses at 48 and 96 weeks vs. 66% and 59% in the placebo group, respectively; ARR during the double-blind period 0.11 in the sartralizumab group vs. 0.32 in the placebo group; No significant change in pain and fatigue |
Infections (N=28); serious infections (N=2); injected-related reaction (N=5); Benign thyroid neoplasm (N=1); Colon adenoma (N=1); Uterine leiomyoma (N=1) |
|||||||||||||
| Traboulsee et al. (2019) | Phase 3 randomized, double-blind placebo-controlled monotherapy trial | 95 (2:1 randomization to sartralizumab or placebo) | AQP4 + NMOSD and seronegative NMOSD* (number of patients in each group not available) | Sartralizumab SC 120 mg q4W | Data not available | sartralizumab monotherapy reduced the risk of relapse by 55% compared with placebo; the proportion of relapse-free patients at week 48 and 96 were 76.1% and 72.1% in the sartralizumab group versus 61.9% and 51.2% in the placebo group, respectively | A similar proportion of adverse events and serious infections in the sartralizumab and placebo group (type of adverse events and infections not available) | |||||||||||||
Legend: AQP4-aquaporin 4; MOG-myelin oligodendrocyte glycoprotein; AZA-azathioprine; TCZ-tocilizumab; BP-blood pressure; URI-upper respiratory tract infection; UTI-urinary tract infection; DVT-deep vein thrombosis. *indicates that MOG-antibody status is not reported.
3.1. Anti-Il-6 Effect on Relapse Rate
Tocilizumab significantly reduced the annual relapse rate (ARR) in all studies. Among the 12 case reports of tocilizumab therapy in NMOSD, one patient had a minor relapse [69], one patient had two relapses [79], and no relapses were reported in the other 10 cases [70-78, 80] (Table 1). A significant reduction in annualized relapse rate (ARR) is documented in 5 case series of tocilizumab in NMOSD: Ayzenberg et al. report median ARR of 3 (range 2.3-3) before tocilizumab and 0.6(range 0-1.3) after treatment in three AQP4+ patients during a median follow up time of 18 months [81]; Araki et al. report mean ARR of 2.9 ±1.1 before tocilizumab and 0.4± 0.8 after treatment in seven AQP4+ patients during a 1-year follow-up [86]; Ringelstein et al. report median ARR of 4 (interquartile range 5-3) before tocilizumab treatment and 0.4 (interquartile range 0.8-0) after treatment in eight AQP4+ patients during a mean follow up of 30.9±15.9 months [83]; Carreon Guarnizo et al. report median ARR of 1.8 ± 1.3 before treatment and 0.2 ± 0.4 after treatment in five NMOSD patients (two AQP4+, three double seronegative) during 2.3±1 years of follow up [82]; Lotan et al. found a similar reduction in ARR with the subcutaneous formulation of tocilizumab [from a median of 2 (interquartile range =4.0-0.25) before treatment to 0 (interquartile range =1.75-0) after treatment] in 12 patients (seven AQP4+, two MOG+ and
three double seronegative) during a mean follow up time of 31.8±18.8 months [85]. Araki M. has recently reported continued results from the original 2014 series, comprising an additional 12 patients who were not reported previously. Out of the 19 patients in this updated series, 15 received tocilizumab for more than a year, in whom the ARR decreased from 2.2± 1.1 in the year before tocilizumab treatment to 0.3±0.7 after treatment [88]. Ringelstein et al. have recently reported treatment outcomes of tocilizumab among 45 patients, comprising eight previously reported AQP4+ NMOSD patients, and an additional 24 AQP4+ NMOSD, seven seronegative NMOSD and six MOG+ encephalomyelitis. The mean ARR for the entire cohort decreased from 1.83 to 0.58 [89] (Table 1).
No relapses were reported in 4 cases of tocilizumab-treated MOG+ NMOSD patients [85, 90, 91].
Information on the total number of relapses during tocilizumab treatment is available in all case reports [69-74, 76-80, 90, 91] and 6 case series [81-83, 85, 86, 88]. Out of 64 NMOSD patients (53 AQP4+, four MOG+, and seven double seronegative) who received tocilizumab in these studies, there were 35 relapses reported in 19 patients (29.7%), while 45 patients (70.3%) were relapse-free. In two case series [84, 89], data on total relapses were not reported. In addition to these studies, interim results of the TANGO randomized clinical trial comprising 59 patients in each of the study treatment arms (of which 50 AQP4+ in the tocilizumab arm and 53 AQP4+ in the azathioprine arm) have shown a significantly higher proportion of relapse-free patients in the tocilizumab- compared to azathioprine-treatment arm during 48 weeks (91.5% Vs. 67.8%, p=0.004; presented at ECTRIMS 2019) [87].
Satralizumab significantly decreased the risk of relapse compared to placebo, both as an add-on and as monotherapy in two randomized, placebo-controlled phase 3 clinical trials. In the SAKuraSky trial, 8 out of 41 patients (20%) in the satralizumab group experienced relapses compared to 18 out of 42 patients (43%) in the placebo group (hazard ratio, 0.38; 95% confidence interval [CI], 0.16 to 0.88; adjusted P=0.02). At 48 weeks, the proportion of relapse-free patients was 89% in the satralizumab group and 66% in the placebo group; at 96 weeks, the proportion of relapse-free patients was 78% in the satralizumab group, and 59% in the placebo group. The annualized relapse rate (AAR) during the double-blind period was 0.11 in the satralizumab group and 0.32 in the placebo group (between-group difference, 0.34; 95% CI, 0.15 to 0.77) [92]. In SAKuraStar, satralizumab monotherapy reduced the risk of relapse by 55% compared with placebo (hazard ratio 0.45; 95% confidence interval 0.23–0.89; p = 0.018), and the proportion of relapse-free patients at week 48 and 96 were 76.1% and 72.1% in the satralizumab group versus 61.9% and 51.2% in the placebo group, respectively [94] (Table 1).
3.2. Tocilizumab Effect on Neurological Disability Status
Six studies report a significant improvement in the neurological status during tocilizumab treatment, as measured by the expanded disability status scale (EDSS) [69, 71, 74, 83, 86, 88], two report mild improvement [72, 80], in 2 the EDSS remained unchanged [81, 82], and in 7 data on EDSS before and after treatment is not available [72, 73, 75-79]. Lotan et al. evaluated the neurological disability by ambulatory status and documented an improved ambulatory status in two patients and a stable state in nine out of 12 patients [85]. A sustained reduction in EDSS is also reported in a significant proportion of tocilizumab-treated patients compared to azathioprine-treated patients in the TANGO study [87] (Table 1).
3.3. Tocilizumab Effect on MRI-related Disease Activity Measures
No radiological disease activity during tocilizumab treatment, as measured by new or enhancing MRI lesions, is reported in 9 studies [69, 70, 73, 76-78, 81, 82, 86, 90], while three others report improvement in lesion volume in the brain [72] and the spinal cord [71, 91]. Ringelstein et al. report no new or enhancing lesions in six patients with spinal cord MRI and three patients with brain MRI available during tocilizumab treatment, while a persisting active spinal cord lesion during the initial treatment period in one patient and an active spinal lesion after 14 months of treatment in another patient are documented [83]. Lotan et at. documented one new asymptomatic brain lesion in 10 available brain MRIs (10%) and two symptomatic spinal cord lesions in 9 available spinal MRIs (22.2%) [85] (Table 1).
3.4. Tocilizumab Effect on AQP4-and MOG-antibody Titers
Decreased AQP4 Ab titers were found in 6 studies [69, 71, 74, 83, 86, 88], while persistently elevated titers are reported in 2 studies [72, 73]. In our study, AQP4 Ab titers during follow up were available for four AQP-4 seropositive patients, of which one patient became seronegative, two patients had lower AQP-4 antibody titers, and one had persistently elevated titers (1:10,000 on cell-based assay) after several years of therapy [85]. AQP4 Ab titers following tocilizumab treatment are not reported in 9 studies [70, 75-82].
Out of ten MOG-positive NMOSD patients who received tocilizumab, two became seronegative following treatment [85]; in one, the MOG Ab titers remained persistently elevated [91], and in seven MOG Ab titers following treatment were not reported [89, 90] (Table 1).
Tocilizumab effect on AQP4- and MOG-antibody status was not correlated with a higher risk of relapse.
3.5. Anti-Il-6 Effect on Pain and Fatigue
The severity of chronic pain during tocilizumab therapy was assessed by a numerical rating scale in 3 clinical trials: Araki et al. report decreased pain levels from 3.0 ± 1.5 before tocilizumab initiation to 1.3 ± 1.3 after six months and 0.9 ± 1.2 after 12 months of treatment in seven patients treated in the 2014 study [86] and from 3.2 ±2.2 to 1.7 ±2.6 in 19 patients in the 2019 study [88]. Ringelstein et al. report decreased pain levels from a median of 6.5 (interquartile range 5.0-7.0) at treatment initiation to 2.5 (interquartile range 0.3-4.5) at last follow up among the eight patients reported in 2015 [83].
In SAKuraSky, satralizumab effect on pain and fatigue was evaluated by the visual analog scale (VAS) pain score and the functional assessment of chronic illness therapy-fatigue (FACIT-F) fatigue score. Compared with placebo, satralizumab did not have a significant effect on pain and fatigue [92].
Araki et al. also evaluated the effect of tocilizumab on general fatigue, reporting decreased numeric levels from 6.1 ± 2.0 at treatment initiation to 3.9 ± 2.1 at six months and 3.0 ± 1.4 at 12 months of treatment in the 2014 series [86], and from 4.4 ± 2.9 to 2.3 ± 1.8 in the 2019 series [88] (Table 1).
3.6. Adverse Events and Safety
No adverse events during tocilizumab treatment are reported in 11 studies [70-78, 90, 91]. Araki et al. report adverse events in the seven-patients series from 2014 [86], but no added information on the additional 12 patients is provided in the 2019 series [88]. Adverse events of tocilizumab in NMOSD are reported in 6 open-label studies [69, 81-83, 85, 86]. These include, in the order of decreasing frequency: lymphopenia, leukopenia and/or neutropenia (N=11); elevated cholesterol levels (N=9); urinary tract infections (UTI; N=9); anemia (N=6); upper respiratory tract infections (URI;N=3);); enterocolitis (N=3); elevated liver enzymes (N=3); reduced systolic blood pressure (N=2); skin abscess (N=2); headache or worsened migraine(N=2); oral mucositis (N=1); pyelonephritis (N=1); gastritis (N=1); nausea (N=1); diarrhea (N=1); fatigue (N=1); deep vein thrombosis (N=1); infusion reactions (N=1); pain at injection site (N=1).
Adverse events In the TANGO trial are reported in 36 patients who received tocilizumab (61%) and in 49 patients receiving azathioprine (83%). Adverse events in the tocilizumab group included infections (URI, N=17, UTI, N=17), anemia (N=16), leukopenia (N=4), or elevated liver enzyme (N=18) [87].
Two death occurred during tocilizumab treatment- in one patient tocilizumab did not likely contribute to death, as the patient was already severely disabled before treatment initiation and died following cervical myelitis after eight months of therapy [85]. The second death is reported in the TANGO trial, but information regarding the causes of death is not yet available(data presented at ECTRIMS 2019) [87].
In SAKuraSky, adverse events are reported in 37 patients (90%) in the satralizumab group and 40 patients (95%) in the placebo group. Adverse events during satralizumab treatment were nasopharyngitis (N=10), upper respiratory tract infection (N=10), headache (N=10), urinary tract infection (N=7), leukopenia (N=6), injection-related reactions (N=5), hypercholesterolemia (N=4), anemia (N=3) and constipation (N=2). No anaphylactic reactions or death have occurred [92]. In SAKuraStar, satralizumab was similarly well-tolerated, and similar proportion adverse events in the satralizumab and placebo groups were reported. Rates of serious infections were similar between groups, and no opportunistic infections, deaths, or anaphylactic reactions were observed [94] (Table 1).
4. Discussion
All the studies included in this systematic review evaluated the effect of anti-IL-6 therapies on relapse prevention in highly active NMOSD. Positive results reported in case reports and small case series were confirmed in a randomized trial of tocilizumab in NMOSD (TANGO, preliminary results) and two worldwide phase 3 clinical trials of satralizumab.
The majority of patients treated with both tocilizumab and satralizumab had positive AQP4 antibodies (Table 1). In those studies where both AQP4- positive and AQP4-negative NMOSD cases where included, the treatment effect was better for AQP4-positive patients [82, 85, 87, 92, 94]. A similar trend of better treatment effect in AQP-4 positive patients has been observed for other therapeutic agents, including rituximab [95], eculizumab [96], and inebilizumab [97]. Although this finding may be related to the small number of seronegative patients treated with anti-IL-6 agents, it may also be attributed to the pathophysiological effects of the IL-6 pathway on AQP4 antibody secretion, which may account for the more limited efficacy of IL-6 blockade in seronegative NMOSD. However, in some of the AQP4-positive patients, the Ab titers remained persistently elevated during tocilizumab treatment [72, 73, 85]. This lack of correlation between AQP4 antibody titers and relapse rates, as already reported in other cohorts [98, 99], may indicate that other pathogenic mechanisms are involved and impact disease activity in NMOSD. Moreover, the disparity in treatment effect between seropositive and seronegative patients may again raise the question of whether seronegative NMOSD reflects a separate clinical entity, as suggested by some other clinical and radiological observations [100, 101].
The current data in support of anti-IL-6 therapies for MOG-positive patients is scarce, as only ten patients treated with tocilizumab have been reported [85, 89-91]. Six of these patients are reported together with AQP4+ and seronegative NMOSD patients [89], and a separate analysis of treatment efficacy for the MOG+ patients is not available. Similarly, there is no data about the effectiveness of satralizumab in MOG- antibody-associated disorder (MOGAD).
Other than their effect on disease-activity measures, IL-6 blocking agents have been proposed to have a beneficial therapeutic effect on pain and fatigue. Data regarding the impact of anti-IL-6 agents on these symptoms is available in only three studies, and results are contradictory, as tocilizumab has been reported to reduce both pain and fatigue severity [83, 86, 88], but satralizumab did not have a significant effect on either of these symptoms [92].
Anti-IL-6 therapy in NMOSD is overall well tolerated. The adverse events profile is similar to that reported in RA and includes mainly hematologic abnormalities (i.e., leukopenia, neutropenia, and anemia), infections, and hypercholesterolemia (Table 1). The rate of any adverse events during tocilizumab treatment, including serious adverse events and death, was similar to the rate of such events during azathioprine treatment [87], while the rate of side events during satralizuamb treatment was comparable to placebo [92, 94]. Although localized injection-related side events are more common with subcutaneous administration, they led to discontinuation of therapy in only one tocilizumab-treated patient [85], and none of the satralizumab-treated patients in the SAKuraSky trial [92].
Conclusions
Anti-IL-6 therapies show promising results in NMOSD. While efficacy in preventing relapses has been confirmed in two randomized trials, additional data on the long term safety and efficacy are needed. Also, the possible role of IL-6 blockade in alleviating pain and fatigue needs to be clarified in future studies.
Fig. (1).
Illustrates a flow chart of the papers- selection process for this review. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Acknowledgements
The authors wish to thank Dr. Ilya Kister for reviewing the manuscript.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Rosales D., Kister I. Common and rare manifestations of neuromyelitis optica spectrum disorder. Curr. Allergy Asthma Rep. 2016;16(6):42. doi: 10.1007/s11882-016-0619-4. [DOI] [PubMed] [Google Scholar]
- 2.Weinshenker B.G., Wingerchuk D.M. Neuromyelitis spectrum disorders. Mayo Clin. Proc. 2017;92(4):663–679. doi: 10.1016/j.mayocp.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Wingerchuk D.M., Hogancamp W.F., O’Brien P.C., Weinshenker B.G. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology. 1999;53(5):1107–1114. doi: 10.1212/WNL.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 4.Oh J., Levy M. Neuromyelitis optica: an antibody-mediated disorder of the central nervous system. Neurol. Res. Int. 2012;2012:460825. doi: 10.1155/2012/460825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wingerchuk D.M., Lennon V.A., Lucchinetti C.F., Pittock S.J., Weinshenker B.G. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6(9):805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 6.Devic E. Myélite subaiguë compliquée de névrite optique. Bull. Méd. 1894;8:1033. [Google Scholar]
- 7.Jarius S., Wildemann B. The history of neuromyelitis optica. J. Neuroinflammation. 2013;10(1):8. doi: 10.1186/1742-2094-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad S., Chen J. What you need to know about AQP4, MOG, and NMOSD. Semin. Neurol. 2019;39(6):718–731. doi: 10.1055/s-0039-3399505. [DOI] [PubMed] [Google Scholar]
- 9.Lennon V.A., Wingerchuk D.M., Kryzer T.J., Pittock S.J., Lucchinetti C.F., Fujihara K., Nakashima I., Weinshenker B.G. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 10.Lennon V.A., Kryzer T.J., Pittock S.J., Verkman A.S., Hinson S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005;202(4):473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamvil S.S., Slavin A.J. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol. Neuroimmunol. Neuroinflamm. 2015;2(1):e62. doi: 10.1212/NXI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamid S.H.M., Whittam D., Mutch K., Linaker S., Solomon T., Das K., Bhojak M., Jacob A. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J. Neurol. 2017;264(10):2088–2094. doi: 10.1007/s00415-017-8596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarius S., Ruprecht K., Kleiter I., Borisow N., Asgari N., Pitarokoili K., Pache F., Stich O., Beume L.A., Hümmert M.W., Ringelstein M., Trebst C., Winkelmann A., Schwarz A., Buttmann M., Zimmermann H., Kuchling J., Franciotta D., Capobianco M., Siebert E., Lukas C., Korporal-Kuhnke M., Haas J., Fechner K., Brandt A.U., Schanda K., Aktas O., Paul F., Reindl M., Wildemann B. in cooperation with the Neuromyelitis Optica Study Group (NEMOS). MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J. Neuroinflammation. 2016;13(1):280. doi: 10.1186/s12974-016-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayan R., Simpson A., Fritsche K., Salama S., Pardo S., Mealy M., Paul F., Levy M. MOG antibody disease: A review of MOG antibody seropositive neuromyelitis optica spectrum disorder. Mult. Scler. Relat. Disord. 2018;25:66–72. doi: 10.1016/j.msard.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Sato D.K., Callegaro D., Lana-Peixoto M.A., Waters P.J., de Haidar Jorge F.M., Takahashi T., Nakashima I., Apostolos-Pereira S.L., Talim N., Simm R.F., Lino A.M., Misu T., Leite M.I., Aoki M., Fujihara K. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82(6):474–481. doi: 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber M.S., Derfuss T., Metz I., Brück W. Defining distinct features of anti-MOG antibody associated central nervous system demyelination. Ther. Adv. Neurol. Disorder. 2018;11:1756286418762083. doi: 10.1177/1756286418762083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bensi C., Marrodan M., González A., Chertcoff A., Osa Sanz E., Chaves H., Schteinschnaider A., Correale J., Farez M.F. Brain and spinal cord lesion criteria distinguishes AQP4-positive neuromyelitis optica and MOG-positive disease from multiple sclerosis. Mult. Scler. Relat. Disord. 2018;25:246–250. doi: 10.1016/j.msard.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Chen C., Liu C., Fang L., Zou Y., Ruan H., Wang Y., Cui C., Sun X., Peng L., Qiu W. Different magnetic resonance imaging features between MOG antibody- and AQP4 antibody-mediated disease: A Chinese cohort study. J. Neurol. Sci. 2019;405:116430. doi: 10.1016/j.jns.2019.116430. [DOI] [PubMed] [Google Scholar]
- 19.Kitley J., Waters P., Woodhall M., Leite M.I., Murchison A., George J., Küker W., Chandratre S., Vincent A., Palace J. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol. 2014;71(3):276–283. doi: 10.1001/jamaneurol.2013.5857. [DOI] [PubMed] [Google Scholar]
- 20.Mariano R., Messina S., Kumar K., Kuker W., Leite M.I., Palace J. Comparison of clinical outcomes of transverse myelitis among adults with myelin oligodendrocyte glycoprotein antibody vs aquaporin-4 antibody disease. JAMA Netw. Open. 2019;2(10):e1912732. doi: 10.1001/jamanetworkopen.2019.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramanathan S., Prelog K., Barnes E.H., Tantsis E.M., Reddel S.W., Henderson A.P., Vucic S., Gorman M.P., Benson L.A., Alper G., Riney C.J., Barnett M., Parratt J.D., Hardy T.A., Leventer R.J., Merheb V., Nosadini M., Fung V.S., Brilot F., Dale R.C. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult. Scler. 2016;22(4):470–482. doi: 10.1177/1352458515593406. [DOI] [PubMed] [Google Scholar]
- 22.Sepúlveda M., Armangué T., Sola-Valls N., Arrambide G., Meca-Lallana J.E., Oreja-Guevara C., Mendibe M., Alvarez de Arcaya A., Aladro Y., Casanova B., Olascoaga J., Jiménez-Huete A., Fernández-Fournier M., Ramió-Torrentà L., Cobo-Calvo A., Viñals M., de Andrés C., Meca-Lallana V., Cervelló A., Calles C., Rubio M.B., Ramo-Tello C., Caminero A., Munteis E., Antigüedad A.R., Blanco Y., Villoslada P., Montalban X., Graus F., Saiz A. Neuromyelitis optica spectrum disorders: Comparison according to the phenotype and serostatus. Neurol. Neuroimmunol. Neuroinflamm. 2016;3(3):e225. doi: 10.1212/NXI.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hacohen Y., Palace J. Time to separate MOG-Ab-associated disease from AQP4-Ab-positive neuromyelitis optica spectrum disorder. Neurology. 2018;90(21):947–948. doi: 10.1212/WNL.0000000000005619. [DOI] [PubMed] [Google Scholar]
- 24.Borisow N., Mori M., Kuwabara S., Scheel M., Paul F. Diagnosis and Treatment of NMO Spectrum Disorder and MOG-Encephalomyelitis. Front. Neurol. 2018;9:888. doi: 10.3389/fneur.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarius S., Paul F., Aktas O., Asgari N., Dale R.C., de Seze J., Franciotta D., Fujihara K., Jacob A., Kim H.J., Kleiter I., Kümpfel T., Levy M., Palace J., Ruprecht K., Saiz A., Trebst C., Weinshenker B.G., Wildemann B. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J. Neuroinflammation. 2018;15(1):134. doi: 10.1186/s12974-018-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wingerchuk D.M., Banwell B., Bennett J.L., Cabre P., Carroll W., Chitnis T., de Seze J., Fujihara K., Greenberg B., Jacob A., Jarius S., Lana-Peixoto M., Levy M., Simon J.H., Tenembaum S., Traboulsee A.L., Waters P., Wellik K.E., Weinshenker B.G. International Panel for NMO Diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.2019.
- 28.Sato D., Callegaro D., Lana-Peixoto M.A., Fujihara K. Brazilian committee for treatment and research in multiple sclerosis. treatment of neuromyelitis optica: an evidence based review. Arq. Neuropsiquiatr. 2012;70(1):59–66. doi: 10.1590/S0004-282X2012000100012. [DOI] [PubMed] [Google Scholar]
- 29.Kessler R.A., Mealy M.A., Levy M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr. Treat. Options Neurol. 2016;18(1):2. doi: 10.1007/s11940-015-0387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimbrough D.J., Fujihara K., Jacob A., Lana-Peixoto M.A., Leite M.I., Levy M., Marignier R., Nakashima I., Palace J., de Seze J., Stuve O., Tenembaum S.N., Traboulsee A., Waubant E., Weinshenker B.G., Wingerchuk D.M. GJCF-CC&BR. Treatment of Neuromyelitis Optica: Review and Recommendations. Mult. Scler. Relat. Disord. 2012;1(4):180–187. doi: 10.1016/j.msard.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stellmann J.P., Krumbholz M., Friede T., Gahlen A., Borisow N., Fischer K., Hellwig K., Pache F., Ruprecht K., Havla J., Kümpfel T., Aktas O., Hartung H.P., Ringelstein M., Geis C., Kleinschnitz C., Berthele A., Hemmer B., Angstwurm K., Young K.L., Schuster S., Stangel M., Lauda F., Tumani H., Mayer C., Zeltner L., Ziemann U., Linker R.A., Schwab M., Marziniak M., Then Bergh F., Hofstadt-van Oy U., Neuhaus O., Zettl U., Faiss J., Wildemann B., Paul F., Jarius S., Trebst C., Kleiter I. NEMOS (Neuromyelitis Optica Study Group). Immunotherapies in neuromyelitis optica spectrum disorder: efficacy and predictors of response. J. Neurol. Neurosurg. Psychiatry. 2017;88(8):639–647. doi: 10.1136/jnnp-2017-315603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres J., Pruitt A., Balcer L., Galetta S., Markowitz C., Dahodwala N. Analysis of the treatment of neuromyelitis optica. J. Neurol. Sci. 2015;351(1-2):31–35. doi: 10.1016/j.jns.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Trebst C., Jarius S., Berthele A., Paul F., Schippling S., Wildemann B., Borisow N., Kleiter I., Aktas O., Kümpfel T. Neuromyelitis Optica Study Group (NEMOS). Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J. Neurol. 2014;261(1):1–16. doi: 10.1007/s00415-013-7169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collongues N., Ayme-Dietrich E., Monassier L., de Seze J. Pharmacotherapy for neuromyelitis optica spectrum disorders: current management and future options. Drugs. 2019;79(2):125–142. doi: 10.1007/s40265-018-1039-7. [DOI] [PubMed] [Google Scholar]
- 35.Paul F., Murphy O., Pardo S., Levy M. Investigational drugs in development to prevent neuromyelitis optica relapses. Expert Opin. Investig. Drugs. 2018;27(3):265–271. doi: 10.1080/13543784.2018.1443077. [DOI] [PubMed] [Google Scholar]
- 36.Kleiter I., Gold R. Present and Future Therapies in Neuromyelitis Optica Spectrum Disorders. Neurotherapeutics. 2016;13(1):70–83. doi: 10.1007/s13311-015-0400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonzi T., Fattori E., Lazzaro D., Costa P., Probert L., Kollias G., De Benedetti F., Poli V., Ciliberto G. Interleukin 6 is required for the development of collagen-induced arthritis. J. Exp. Med. 1998;187(4):461–468. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto M., Yoshizaki K., Kishimoto T., Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J. Immunol. 2000;164(9):4878–4882. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 39.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006;8(Suppl. 2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gijbels K., Van Damme J., Proost P., Put W., Carton H., Billiau A. Interleukin 6 production in the central nervous system during experimental autoimmune encephalomyelitis. Eur. J. Immunol. 1990;20(1):233–235. doi: 10.1002/eji.1830200134. [DOI] [PubMed] [Google Scholar]
- 41.Constantinescu C.S., Farooqi N., O’Brien K., Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011;164(4):1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wekerle H., Kojima K., Lannes-Vieira J., Lassmann H., Linington C. Animal models. Ann. Neurol. 1994;36(Suppl.):S47–S53. doi: 10.1002/ana.410360714. [DOI] [PubMed] [Google Scholar]
- 43.Schluesener H.J., Sobel R.A., Linington C., Weiner H.L. A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J. Immunol. 1987;139(12):4016–4021. [PubMed] [Google Scholar]
- 44.Stefferl A., Brehm U., Linington C. The myelin oligodendrocyte glycoprotein (MOG): a model for antibody-mediated demyelination in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Neural Transm. Suppl. 2000;(58):123–133. doi: 10.1007/978-3-7091-6284-2_10. [DOI] [PubMed] [Google Scholar]
- 45.Sriram S., Steiner I. Experimental allergic encephalomyelitis: a misleading model of multiple sclerosis. Ann. Neurol. 2005;58(6):939–945. doi: 10.1002/ana.20743. [DOI] [PubMed] [Google Scholar]
- 46.Takeshita Y., Obermeier B., Cotleur A.C., Spampinato S.F., Shimizu F., Yamamoto E., Sano Y., Kryzer T.J., Lennon V.A., Kanda T., Ransohoff R.M. Effects of neuromyelitis optica-IgG at the blood-brain barrier in vitro. Neurol. Neuroimmunol. Neuroinflamm. 2016;4(1):e311. doi: 10.1212/NXI.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chihara N., Aranami T., Sato W., Miyazaki Y., Miyake S., Okamoto T., Ogawa M., Toda T., Yamamura T. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc. Natl. Acad. Sci. USA. 2011;108(9):3701–3706. doi: 10.1073/pnas.1017385108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang K.H., Ro L.S., Lyu R.K., Chen C.M. Biomarkers for neuromyelitis optica. Clin. Chim. Acta. 2015;440:64–71. doi: 10.1016/j.cca.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Uzawa A., Mori M., Kuwabara S. Cytokines and chemokines in neuromyelitis optica: pathogenetic and therapeutic implications. Brain Pathol. 2014;24(1):67–73. doi: 10.1111/bpa.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melamed E., Levy M., Waters P.J., Sato D.K., Bennett J.L., John G.R., Hooper D.C., Saiz A., Bar-Or A., Kim H.J., Pandit L., Leite M.I., Asgari N., Kissani N., Hintzen R., Marignier R., Jarius S., Marcelletti J., Smith T.J., Yeaman M.R., Han M.H., Aktas O., Apiwattanakul M., Banwell B., Bichuetti D., Broadley S., Cabre P., Chitnis T., De Seze J., Fujihara K., Greenberg B., Hellwig K., Iorio R., Jarius S., Klawiter E., Kleiter I., Lana-Peixoto M. Nakashima; O’Connor, K.; Palace, J.; Paul, F.; Prayoonwiwat, N.; Ruprecht, K.; Stuve, O.; Tedder, T.; Tenembaum, S.; Garrahan, J.P.; Aires, B.; van Herle, K.; van Pelt, D.; Villoslada, P.; Waubant, E.; Weinshenker, B.; Wingerchuk, D.; Würfel, J.; Zamvil, S. Update on biomarkers in neuromyelitis optica. Neurol. Neuroimmunol. Neuroinflamm. 2015;2(4):e134. doi: 10.1212/NXI.0000000000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uzawa A., Mori M., Arai K., Sato Y., Hayakawa S., Masuda S., Taniguchi J., Kuwabara S. Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult. Scler. 2010;16(12):1443–1452. doi: 10.1177/1352458510379247. [DOI] [PubMed] [Google Scholar]
- 52.Içöz S., Tüzün E., Kürtüncü M., Durmuş H., Mutlu M., Eraksoy M., Akman-Demir G. Enhanced IL-6 production in aquaporin-4 antibody positive neuromyelitis optica patients. Int. J. Neurosci. 2010;120(1):71–75. doi: 10.3109/00207450903428970. [DOI] [PubMed] [Google Scholar]
- 53.Wei Y., Chang H., Li X., Wang H., Du L., Zhou H., Xu W., Ma Y., Yin L., Zhang X. Cytokines and tissue damage biomarkers in first-onset neuromyelitis optica spectrum disorders: significance of interleukin-6. Neuroimmunomodulation. 2018;25(4):215–224. doi: 10.1159/000494976. [DOI] [PubMed] [Google Scholar]
- 54.Uzawa A., Mori M., Masuda H., Ohtani R., Uchida T., Sawai S., Kuwabara S. Interleukin-6 analysis of 572 consecutive CSF samples from neurological disorders: A special focus on neuromyelitis optica. Clin. Chim. Acta. 2017;469:144–149. doi: 10.1016/j.cca.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Uzawa A., Mori M., Sawai S., Masuda S., Muto M., Uchida T., Ito S., Nomura F., Kuwabara S. Cerebrospinal fluid interleukin-6 and glial fibrillary acidic protein levels are increased during initial neuromyelitis optica attacks. Clin. Chim. Acta. 2013;421:181–183. doi: 10.1016/j.cca.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 56.Wang H., Wang K., Zhong X., Dai Y., Qiu W., Wu A., Hu X. Notable increased cerebrospinal fluid levels of soluble interleukin-6 receptors in neuromyelitis optica. Neuroimmunomodulation. 2012;19(5):304–308. doi: 10.1159/000339302. [DOI] [PubMed] [Google Scholar]
- 57.Uzawa A., Mori M., Ito M., Uchida T., Hayakawa S., Masuda S., Kuwabara S. Markedly increased CSF interleukin-6 levels in neuromyelitis optica, but not in multiple sclerosis. J. Neurol. 2009;256(12):2082–2084. doi: 10.1007/s00415-009-5274-4. [DOI] [PubMed] [Google Scholar]
- 58.Vallejo R., Tilley D.M., Vogel L., Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10(3):167–184. doi: 10.1111/j.1533-2500.2010.00367.x. [DOI] [PubMed] [Google Scholar]
- 59.Arruda J.L., Sweitzer S., Rutkowski M.D., DeLeo J.A. Intrathecal anti-IL-6 antibody and IgG attenuates peripheral nerve injury-induced mechanical allodynia in the rat: possible immune modulation in neuropathic pain. Brain Res. 2000;879(1-2):216–225. doi: 10.1016/S0006-8993(00)02807-9. [DOI] [PubMed] [Google Scholar]
- 60.Serizawa K., Tomizawa-Shinohara H., Yasuno H., Yogo K., Matsumoto Y. Anti-IL-6 Receptor antibody inhibits spontaneous pain at the pre-onset of experimental autoimmune encephalomyelitis in mice. Front. Neurol. 2019;10:341. doi: 10.3389/fneur.2019.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strand V., Burmester G.R., Ogale S., Devenport J., John A., Emery P. Improvements in health-related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24-week randomized controlled RADIATE study. Rheumatology (Oxford) 2012;51(10):1860–1869. doi: 10.1093/rheumatology/kes131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srirangan S., Choy E.H. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2010;2(5):247–256. doi: 10.1177/1759720X10378372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brunner H.I., Ruperto N., Zuber Z., Keane C., Harari O., Kenwright A., Lu P., Cuttica R., Keltsev V., Xavier R.M., Calvo I., Nikishina I., Rubio-Pérez N., Alexeeva E., Chasnyk V., Horneff G., Opoka-Winiarska V., Quartier P., Silva C.A., Silverman E., Spindler A., Baildam E., Gámir M.L., Martin A., Rietschel C., Siri D., Smolewska E., Lovell D., Martini A., De Benedetti F. Paediatric Rheumatology International Trials Organisation PRINTO; Pediatric Rheumatology Collaborative Study Group (PRCSG). Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomised, double-blind withdrawal trial. Ann. Rheum. Dis. 2015;74(6):1110–1117. doi: 10.1136/annrheumdis-2014-205351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hashizume M., Tan S.L., Takano J., Ohsawa K., Hasada I., Hanasaki A., Ito I., Mihara M., Nishida K. Tocilizumab, a humanized anti-IL-6R antibody, as an emerging therapeutic option for rheumatoid arthritis: molecular and cellular mechanistic insights. Int. Rev. Immunol. 2015;34(3):265–279. doi: 10.3109/08830185.2014.938325. [DOI] [PubMed] [Google Scholar]
- 65.Le R.Q., Li L., Yuan W., Shord S.S., Nie L., Habtemariam B.A., Przepiorka D., Farrell A.T., Pazdur R. FDA Approval Summary: Tocilizumab for treatment of chimeric antigen receptor t cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23(8):943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stone J.H., Tuckwell K., Dimonaco S., Klearman M., Aringer M., Blockmans D., Brouwer E., Cid M.C., Dasgupta B., Rech J., Salvarani C., Schett G., Schulze-Koops H., Spiera R., Unizony S.H., Collinson N. Trial of tocilizumab in giant-cell arteritis. N. Engl. J. Med. 2017;377(4):317–328. doi: 10.1056/NEJMoa1613849. [DOI] [PubMed] [Google Scholar]
- 67.Biggioggero M., Crotti C., Becciolini A., Favalli E.G. Tocilizumab in the treatment of rheumatoid arthritis: an evidence-based review and patient selection. Drug Des. Devel. Ther. 2018;13:57–70. doi: 10.2147/DDDT.S150580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reichert J.M. Antibodies to watch in 2017. MAbs. 2017;9(2):167–181. doi: 10.1080/19420862.2016.1269580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Araki M., Aranami T., Matsuoka T., Nakamura M., Miyake S., Yamamura T. Clinical improvement in a patient with neuromyelitis optica following therapy with the anti-IL-6 receptor monoclonal antibody tocilizumab. Mod. Rheumatol. 2013;23(4):827–831. doi: 10.3109/s10165-012-0715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kieseier B.C., Stüve O., Dehmel T., Goebels N., Leussink V.I., Mausberg A.K., Ringelstein M., Turowski B., Aktas O., Antoch G., Hartung H.P. Disease amelioration with tocilizumab in a treatment-resistant patient with neuromyelitis optica: implication for cellular immune responses. JAMA Neurol. 2013;70(3):390–393. doi: 10.1001/jamaneurol.2013.668. [DOI] [PubMed] [Google Scholar]
- 71.Lauenstein A.S. Treating neuromyelitis optica with the interleukin-6 receptor antagonist tocilizumab. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2013-202939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harmel J., Ringelstein M., Ingwersen J., Mathys C., Goebels N., Hartung H.P., Jarius S., Aktas O. Interferon-β-related tumefactive brain lesion in a Caucasian patient with neuromyelitis optica and clinical stabilization with tocilizumab. BMC Neurol. 2014;14:247. doi: 10.1186/s12883-014-0247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Breu M., Glatter S., Höftberger R., Freilinger M., Kircher K., Kasprian G., Seidl R., Kornek B. Two cases of pediatric aqp4-antibody positive neuromyelitis optica spectrum disorder successfully treated with tocilizumab. Neuropediatrics. 2019;50(3):193–196. doi: 10.1055/s-0039-1684004. [DOI] [PubMed] [Google Scholar]
- 74.Komai T., Shoda H., Yamaguchi K., Sakurai K., Shibuya M., Kubo K., Takahashi T., Fujio K., Yamamoto K. Neuromyelitis optica spectrum disorder complicated with Sjogren syndrome successfully treated with tocilizumab: A case report. Mod. Rheumatol. 2016;26(2):294–296. doi: 10.3109/14397595.2013.861333. [DOI] [PubMed] [Google Scholar]
- 75.Marino A., Narula S., Lerman M.A. First pediatric patient with neuromyelitis optica and sjögren syndrome successfully treated with tocilizumab. Pediatr. Neurol. 2017;73:e5–e6. doi: 10.1016/j.pediatrneurol.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 76.Capobianco M. 2019.
- 77.Mancinelli C. 2018. [Google Scholar]
- 78.2017.
- 79.Meinl I., Kuempfel T. Early relapse after rituximab initiation in a patient with neuromyelitis optica spectrum disease and side effects to subsequent tozilizumab therapy: need for further treatment options? 2018. [Google Scholar]
- 80.Siwoski M-V., Lynch S.G. 2019. [Google Scholar]
- 81.Ayzenberg I., Kleiter I., Schröder A., Hellwig K., Chan A., Yamamura T., Gold R. Interleukin 6 receptor blockade in patients with neuromyelitis optica nonresponsive to anti-CD20 therapy. JAMA Neurol. 2013;70(3):394–397. doi: 10.1001/jamaneurol.2013.1246. [DOI] [PubMed] [Google Scholar]
- 82.Carreón G.E., Hernández C.R., Castillo T., T., Meca L.V., Arocas C.V., Iniesta M.F., Olascoaga U.J., Meca L.J.E. Experience with tocilizumab in patients with neuromyelitis optica spectrum disorders. 2019.
- 83.Ringelstein M., Ayzenberg I., Harmel J., Lauenstein A.S., Lensch E., Stögbauer F., Hellwig K., Ellrichmann G., Stettner M., Chan A., Hartung H.P., Kieseier B., Gold R., Aktas O., Kleiter I. Long-term therapy with interleukin 6 receptor blockade in highly active neuromyelitis optica spectrum disorder. JAMA Neurol. 2015;72(7):756–763. doi: 10.1001/jamaneurol.2015.0533. [DOI] [PubMed] [Google Scholar]
- 84.Dalla C.G. 2019.
- 85.Lotan I., Charlson R.W., Ryerson L.Z., Levy M., Kister I. Effectiveness of subcutaneous tocilizumab in neuromyelitis optica spectrum disorders. Mult. Scler. Relat. Disord. 2019;39:101920. doi: 10.1016/j.msard.2019.101920. [DOI] [PubMed] [Google Scholar]
- 86.Araki M., Matsuoka T., Miyamoto K., Kusunoki S., Okamoto T., Murata M., Miyake S., Aranami T., Yamamura T. Efficacy of the anti-IL-6 receptor antibody tocilizumab in neuromyelitis optica: a pilot study. Neurology. 2014;82(15):1302–1306. doi: 10.1212/WNL.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi F-D. 2019.
- 88.Araki M. Blockade of IL-6 signaling in neuromyelitis optica. Neurochem. Int. 2019;130:104315. doi: 10.1016/j.neuint.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 89.Ringelstein M. 2019. [Google Scholar]
- 90.Hayward-Koennecke H., Reindl M., Martin R., Schippling S. Tocilizumab treatment in severe recurrent anti-MOG-associated optic neuritis. Neurology. 2019;92(16):765–767. doi: 10.1212/WNL.0000000000007312. [DOI] [PubMed] [Google Scholar]
- 91.Novi G., Gastaldi M., Franciotta D., Pesce G., Benedetti L., Uccelli A. Tocilizumab in MOG-antibody spectrum disorder: a case report. Mult. Scler. Relat. Disord. 2019;27:312–314. doi: 10.1016/j.msard.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 92.Yamamura T., Kleiter I., Fujihara K., Palace J., Greenberg B., Zakrzewska-Pniewska B., Patti F., Tsai C.P., Saiz A., Yamazaki H., Kawata Y., Wright P., De Seze J. Trial of satralizumab in neuromyelitis optica spectrum disorder. N. Engl. J. Med. 2019;381(22):2114–2124. doi: 10.1056/NEJMoa1901747. [DOI] [PubMed] [Google Scholar]
- 93.Yamamura T. Efficacy of satralizumab (SA237) in subgroups of patients in SAkuraSky: a Phase III double-blind, placebo-controlled, add-on study in patients with neuromyelitis optica spectrum disorder (NMOSD) (S43.008). Neurology. 2019;92(15) Suppl.:S43.008 [Google Scholar]
- 94.Traboulsee A. 2019.
- 95.Damato V., Evoli A., Iorio R. Efficacy and safety of rituximab therapy in neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. JAMA Neurol. 2016;73(11):1342–1348. doi: 10.1001/jamaneurol.2016.1637. [DOI] [PubMed] [Google Scholar]
- 96.Pittock S.J., Berthele A., Fujihara K., Kim H.J., Levy M., Palace J., Nakashima I., Terzi M., Totolyan N., Viswanathan S., Wang K.C., Pace A., Fujita K.P., Armstrong R., Wingerchuk D.M. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N. Engl. J. Med. 2019;381(7):614–625. doi: 10.1056/NEJMoa1900866. [DOI] [PubMed] [Google Scholar]
- 97.Cree B.A.C., Bennett J.L., Kim H.J., Weinshenker B.G., Pittock S.J., Wingerchuk D.M., Fujihara K., Paul F., Cutter G.R., Marignier R., Green A.J., Aktas O., Hartung H.P., Lublin F.D., Drappa J., Barron G., Madani S., Ratchford J.N., She D., Cimbora D., Katz E. N-MOmentum study investigators. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394(10206):1352–1363. doi: 10.1016/S0140-6736(19)31817-3. [DOI] [PubMed] [Google Scholar]
- 98.Kessler R.A., Mealy M.A., Jimenez-Arango J.A., Quan C., Paul F., López R., Hopkins S., Levy M. Anti-aquaporin-4 titer is not predictive of disease course in neuromyelitis optica spectrum disorder: A multicenter cohort study. Mult. Scler. Relat. Disord. 2017;17:198–201. doi: 10.1016/j.msard.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 99.Akaishi T., Takahashi T., Nakashima I., Abe M., Ishii T., Aoki M., Fujihara K. Repeated follow-up of AQP4-IgG titer by cell-based assay in neuromyelitis optica spectrum disorders (NMOSD). J. Neurol. Sci. 2020;410:116671. doi: 10.1016/j.jns.2020.116671. [DOI] [PubMed] [Google Scholar]
- 100.Jarius S., Ruprecht K., Wildemann B., Kuempfel T., Ringelstein M., Geis C., Kleiter I., Kleinschnitz C., Berthele A., Brettschneider J., Hellwig K., Hemmer B., Linker R.A., Lauda F., Mayer C.A., Tumani H., Melms A., Trebst C., Stangel M., Marziniak M., Hoffmann F., Schippling S., Faiss J.H., Neuhaus O., Ettrich B., Zentner C., Guthke K., Hofstadt-van Oy U., Reuss R., Pellkofer H., Ziemann U., Kern P., Wandinger K.P., Bergh F.T., Boettcher T., Langel S., Liebetrau M., Rommer P.S., Niehaus S., Münch C., Winkelmann A., Zettl U. U.K.; Metz, I.; Veauthier, C.; Sieb, J.P.; Wilke, C.; Hartung, H.P.; Aktas, O.; Paul, F. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: A multicentre study of 175 patients. J. Neuroinflammation. 2012;9:14. doi: 10.1186/1742-2094-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kıyat-Atamer A., Ekizoğlu E., Tüzün E., Kürtüncü M., Shugaiv E., Akman-Demir G., Eraksoy M. Long-term MRI findings in neuromyelitis optica: seropositive versus seronegative patients. Eur. J. Neurol. 2013;20(5):781–787. doi: 10.1111/ene.12058. [DOI] [PubMed] [Google Scholar]