Abstract
The root cause of non-inherited Alzheimer’s disease (AD) remains unknown despite hundreds of research studies performed to attempt to solve this problem. Since proper prophylaxis remains the best strategy, many scientists have studied the risk factors that may affect AD development. There is robust evidence supporting the hypothesis that cardiovascular diseases (CVD) may contribute to AD progression, as the diseases often coexist. Therefore, a lack of well-defined diagnostic criteria makes studying the relationship between AD and CVD complicated. Additionally, inflammation accompanies the pathogenesis of AD and CVD, and is not only a consequence but also implicated as a significant contributor to the course of the diseases. Of note, АроЕε4 is found to be one of the major risk factors affecting both the cardiovascular and nervous systems. According to genome wide association and epidemiological studies, numerous common risk factors have been associated with the development of AD-related pathology. Furthermore, the risk of developing AD and CVDs appears to be increased by a wide range of conditions and lifestyle factors: hypertension, dyslipidemia, hypercholesterolemia, hyperhomocysteinemia, gut/oral microbiota, physical activity, and diet. This review summarizes the literature and provides possible mechanistic links between CVDs and AD.
Keywords: Alzheimer’s disease, cardiovascular disease, vascular risk factors, dementia, neuroinflammation, АроЕ
1. INTRODUCTION
Dementia is well known as a heterogeneous set of pathologies characterized by similar patterns of progressively worsening cognitive dysfunction and behavioral impairment due to neurodegeneration. It has been estimated that 46.8 million people globally are suffering from dementia. This total is expected to reach about 70 million by the year 2030 and 131 million people by 2050. Currently, the worldwide cost of dementia amounts to US$ 818 billion. Almost 58% of patients live in low- and middle-income countries, but this percentage is likely to exceed 68% by the year 2050 [1].
The greatest portion of dementia patients is diagnosed with Alzheimer’s disease (AD) [2]. Alzheimer’s disease may be divided into two dominant types: late-onset Alzheimer’s disease (LOAD), appearing at the age after age 65; and early-onset Alzheimer’s disease (EOAD), which starts before age 65. It is important to note that familial Alzheimer’s disease only accounts for about 1-2% percent and usually is associated with EOAD [2]. A key problem is the early identification of AD at preclinical stage before the manifestation of the clinical symptoms. This prodromal phase is characterized by Aβ oligomer deposition in the extracellular space, intraneuronal neurofibrillary tangles and neuropil threads formation, hippocampal volume loss, and synaptic and neuronal loss [2-4]. Cognitive decline, impairments of learning, attention, language, memory, visuospatial orientation, behavior, social comportment, praxis, and gnosis are described as typical clinical symptoms as well [2-4]. Unfortunately, the root cause of AD remains unknown, despite hundreds of research studies over the past several decades. Therefore, since proper prophylaxis is doubtless the best strategy, the study of risk factors may help clinicians identify individuals likely to develop AD and to avoid AD altogether or at least gain additional years of productive life before the full impact of the disease’s progression.
Concurrently, cardiovascular diseases (CVD) are the #1 cause of death worldwide, taking an estimated 17.9 million lives yearly, which is about one-third of total deaths [5]. Stroke and heart failure cause nearly 85% of deaths [5]. CVDs are known to be a diverse set of diseases, including, for example, heart failure, atherosclerosis, and cerebrovascular disease [6]. Although various therapies have been developed in recent decades [7-12], sadly, pathological alterations are often irreversible and cannot be cured completely. Therefore, as with AD, the best plan of action is to take preventive measures, which are aimed to lower risk factors influence and promote a healthy life. The main risk factors appear to be tobacco and alcohol usage, unhealthy diets, physical inactivity, and obesity.
Beyond the risks of mortality and physical disability, several studies have linked CVD to an elevated risk of AD. In this review article, the literature is summarized and possible links between CVDs and AD are provided.
2. EARLY-ONSET ALZHEIMER’S DISEASE
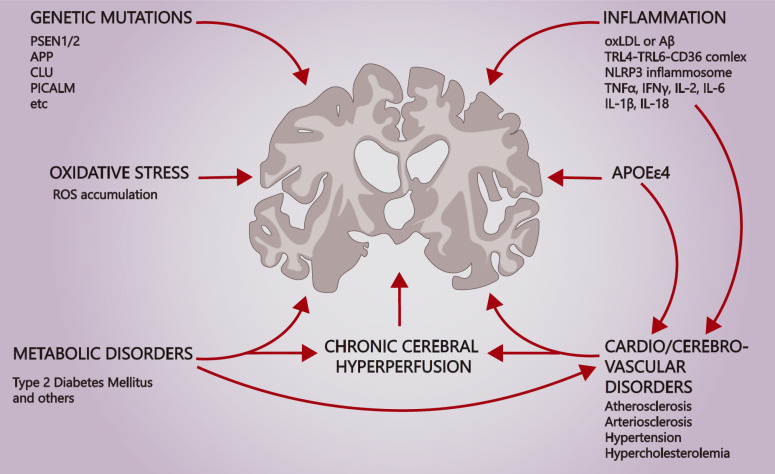
Alzheimer’s disease arises in association with myriad risk factors (Fig. 1). About 5.5% of cases develop as an early-onset Alzheimer’s disease (EOAD), emerging before 65 years of age [13]. Mutations in genes that encode factors involved in processing β-amyloid precursor protein (APP) often are implicated in AD pathogenesis.
Fig. (1).
Map of Alzheimer's disease risk factors. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Early damage to the synapses between the entorhinal cortex-hippocampal network and the molecular layer of the dentate gyrus (perforant pathway) in the AD brain results in short-term memory deficits [14, 15]. In more severe memory loss, there is a disconnection of the cortico-cortical fibers in the frontal, parietal, and temporal cortices [14, 16]. Further degeneration of the connections between Meinert’s basal nucleus and neocortex affects the ascending cholinergic activating system [14, 17]. Despite intensive research over the past 113 years, a comprehensive picture of brain regions affected by Alzheimer's is yet to be obtained, as the disease damages not only a single neuronal population but also multiple neuronal networks of the brain.
Gamma-secretase complex (γ-secretase) is described as membrane integral fermentative complex that is involved in APP processing, and mutations in the gene of its subunit PSEN1/2 may result in EOAD development and Aβ deposition [18-21]. Mutations within PSEN1/2 genes that result in γ-secretase complex destabilization, disruption of sequential APP cleavage, and Aβ42 secretion enhancement have been indicated [22-26]. Published reports reveal more than 200 mutations occurring within the PSEN1 gene that are associated with EOAD [26, 27]. Mutations of PSEN1 may alter the activity of PS1 in a different manner, for example, I202F, F237I, L248R, and V261F substitutions decrease PS1 proteolytic activity, while I143V, F177L, and M233L have almost no effect, while I213L, L226F, and L424V increase it [18]. Mutations in the PSEN2 gene related to EOAD development are rare in comparison, amounting to fewer than 50 mutations [26, 28]. It is important to note that some AD-related mutations in PSEN1/2 genes do not cause a familial form of EOAD [29].
Another important component of amyloidogenesis is APP itself, mutations in which may result in EOAD development [20, 26, 30]. Nowadays, 60 mutations within the APP gene have been described, out of which 27 lead to Alzheimer’s disease development and are known to be pathogenic [31]. Seoul, Swedish, and London mutations are three of the most prominent mutations, occurring at β- and γ-cleavage sites [32, 33]. Seoul mutation is described as a substitution V669L, which, according to in silico analysis, possibly alters normal APP processing due to a tertiary protein fold disruption [32]. Swedish mutation is characterized as double substitution KM670/671NL at the site of β-cleavage, which results in the β-cleavage enhancement, Aβ40 and Aβ42 elevation, and development of a familial form of EOAD [34, 35]. A recent study revealed that Swedish double mutation repair, using the CRISPR/Cas9 genome-editing technique, diminishes Aβ42 secretion, which may be suggested as evidence of Swedish mutation relation to EOAD and may become one of the prospective therapeutic strategies for familial EOAD prevention [36]. London mutation occurs at the γ-cleavage site of APP and leads to the PS1 conformational change and the elevation of the Aβ42/Aβ40 ratio [37, 38]. Another pathological mutation, Flemish, occurs within the inhibitory domain of APP and plays the opposite role and enhances Gamma (y)-secretase activity as well as Aβ production [39]. Although mutations are mostly negative, Icelandic mutation has been described as the only protective mutation in AD, as it diminishes Aβ [40, 41].
3. CVD AND ALZHEIMER’S DISEASE COMMON RISK FACTORS
Studying the relationship between CVD and AD is complicated. The diagnostic criteria of AD usually exclude patients with any clinically significant CVD [42], even though CVD independently can lead to cognitive impairment and the diagnosis of dementia (e.g., mild vascular cognitive impairment, vascular dementia, mixed dementia) [43, 44]. Nevertheless, there is evidence supporting the hypothesis that CVD can contribute to the development of AD [45-48]. Neuropathological studies suggest that the presence of ischemic microvascular lesions is significantly higher in the brains of patients diagnosed with AD. The incidence of neuropathologically confirmed AD cases was 11-fold higher in women who had cerebral infarctions evident at autopsy. A large number of epidemiological studies have shown an association between CVDs and neurodegenerative disorders or dementia-related pathology. However, it remains unexplained how small vessel disease affects the clinical course of AD as well as CVDs [49-51]. It is not clear whether this relationship is the reason that they have common risk factors or whether CVDs are directly or indirectly involved in the pathogenesis leading to AD.
3.1. Hypertension
Chronic hypertension has been suggested as one of the most significant risk factors for AD that can be controlled by pharmaceutical therapies and lifestyle changes [52, 53]. Hypertension adversely affects the structural integrity of cerebral blood vessels, forms atherosclerotic plaques in cerebral arteries, and induces hyperlipidemia [54, 55]. The use of antihypertensive treatments may reduce the risk of dementia [56-59]. A double-blind placebo-controlled study showed that active hypertension treatment reduced the incidence of dementia by 50% compared to the control group [57]. Despite this finding, previous controlled clinical trials failed to show the outcome benefits of these drugs on cognitive performance in AD patients. In summary, the reasons for the reduction in dementia incidence during anti-hypertension treatment remain elusive, and whether there is a drug-specific effect or a general effect of controlling hypertension in this population is not known. The only thing known with confidence is that blood pressure regulation during midlife may postpone the onset of AD.
3.2. Cerebrovascular Disease
As was stated before, vascular neurocognitive disorders (VNCD) share risk factors, such as arterial hypertension, T2DM, smoking, and several symptoms with AD. According to the American Psychiatric Association (APA) recommendations presented in the latest edition of DSM-5 [60], probable vascular neurodegenerative disorder is characterized by either parenchymal injury attributed to CVD or temporal relation to one or more documented cerebrovascular events or both clinical and genetic evidence. The description of a clinical picture of major or mild VNCD patients may include multiple infarctions in conjunction with lesions in the white matter, basal ganglia, thalamus, and gradual or fluctuating decline of cognition. At the same time, DSM-5 authors note that VNCD, as well as AD, may have mixed etiology, and often it is not possible to separate these states from one another. In these cases, APA recommends diagnosing mild or major neurocognitive disorder [60].
Despite the fact that etiology and pathobiology of Alzheimer’s disease are still a subject for discussion, the so-called “two-hit hypothesis” would lighten up briefly in this section. The first “hit” is reduced cellular blood flow and the second is the dysfunction of the blood-brain barrier. Both of these states may be induced by amyloid as well as the other vascular risk factors. Lower oxygen supply to the neurons results sequentially in acidosis, mitochondrial disorders, and oxidative stress. As a consequence, this process leads to elevated ROS levels and increased Aβ42 production as well as tau hyperphosphorylation. The BBB dysfunction leads to impairment of Aβ clearance from cerebrospinal fluid. Both these processes effect Aβ42 accumulation in the brain in a synergic manner resulting in neuronal loss and cognition decline [61-65]. Nevertheless, it must be admitted that there is no compelling evidence showing a causal relationship between AD and vascular disorders [66]. However, it has been shown that several cerebrovascular disorders are associated with significant impacts on AD development and severity [66].
4. METABOLIC DISORDERS
4.1. Dyslipidemia and Hypercholesterolemia
Epidemiological studies have shown that high blood cholesterol levels might contribute to the pathogenesis of AD. One possible mechanism that has been proposed is that high blood cholesterol levels increase the risk of CVD [67]. In animals fed a high cholesterol diet, an elevated level of Aβ depositions was found. Whereas in studies involving humans, high levels of LDL-cholesterol and low levels of HDL-cholesterol have been found [68]. Moreover, the distribution and cholesterol levels in brain cells can affect Aβ metabolism. Thus, it is not a surprise that several clinical studies have shown that statins that are administered to lower blood cholesterol levels may protect against AD as well [69].
In recent years, studies have shown that the homeostasis of lipids in the brain, both cholesterol and sphingolipids (such as gangliosides), are of great importance [70] with respect to the lipid composition of the cell membrane and the formation of lipid rafts associated with the formation and toxicity of Aβ. These results have renewed the impetus to study the multifaceted role of lipids in amyloidogenesis, neuroinflammation, neuroplasticity, and mitochondrial synaptic function [71, 72]. Nevertheless, it remains unanswered whether disturbances of lipid homeostasis observed in AD are the cause or result of the disease [70, 71, 73].
4.2. T2DM and Insulin Resistance
There are several epidemiological studies that show that type 2 diabetes mellitus (T2DM) increases the risk of AD. Diabetes doubled the incidence of dementia, AD, and vascular dementia (VaD), as well as increased mortality. Patients with T2DM were less likely to have β-amyloid and tangles but more likely to have cerebral infarcts. Elderly people with diabetes develop a more extensive vascular pathology, which alone or together with AD-type pathology leads to a higher risk of dementia [74].
However, the links between diabetes and AD go beyond the epidemiological association. The brain of Alzheimer’s patients showed evidence of reduced expression of insulin as well as of neuronal insulin receptors compared with those of age-matched controls [75]. This gradually leads to the breakdown of the entire insulin-signaling pathway, which manifests as insulin resistance, and consequently, brain metabolism and cognitive function, which is one of the best-documented abnormalities in AD. These observations led Dr. de la Monte and her colleagues to suggest that AD is a neuroendocrine disorder resembling type 2 diabetes mellitus [76]. The truth may be more complex, but the so-called “brain diabetes” is called type 3 diabetes in some quarters. Diabetes can exacerbate cognitive deficits and lead to dementia, through the damage caused by systemic blood circulation and microcirculation; however, its devastating role does not end there.
There are insulin receptors in the brain, particularly in the hippocampus, and the primary center of episodic memory formation [75]. Insulin affects the electrochemical and biochemical action of neurons, from neurotransmitters associated with memory and learning to the enzymes that participate in the metabolism of Aβ [75, 77]. Thus, the production of insulin is directly related to neuroinflammation, amyloidogenesis, oxidative stress, and mitochondrial function, thereby constituting a candidate link between the endocrine and the neurodegenerative diseases [77]. Additionally, the brain insulin resistance characterized by the ineffective response of the brain cells to insulin resulting in impairments in synaptic, metabolic, and immune response functions, is a hallmark of AD [78]. However, whether the insulin resistance and AD are linked causally or represent unrelated changes in aging is unclear [78].
4.3. Hyperhomocysteinemia
Moderately high plasma homocysteine levels have been shown to be associated with stroke, VaD, and AD. This risk factor is not associated with other vascular risk factors, nutritional status, or the MTHFR genotype [79]. A meta-analysis of 34 published studies with 9397 subjects demonstrated a significant relationship between plasma total homocysteine levels and the risk for AD [80]. This meta-analysis has provided new insights into the etiology and prevention of AD, revealing the causal link between plasma total homocysteine and the risk for AD. It is noteworthy that homocysteine may affect the AD process through cerebrovascular disease, or directly by increasing β-amyloid deposition in the brain.
5. GENETICS OF AD
Sporadic Alzheimer’s disease (SAD) is widely known as a multifaceted disorder. However, SAD progression is not defined fully yet. Scientists worldwide have engaged in attempts to solve the mystery. Genetic and genomic studies are major strands of research nowadays [81-83]. For instance, the association between APOE allele type and amyloid plaques deposition is well-known and has been corroborated by other studies [84, 85]. A particular finding is that lower odds of SAD development and plaque burden for APOEε2/APOEε2+ allele type has been determined. The odds have been shown to grow in sequence ε2/ε3, ε3/ε3, ε2/ε4, ε3/ε4, ε4/ε4 [84, 86]. Many studies describe APOEε4 as a crucial risk factor exacerbating VaD and SAD progression as well as aggravating the severity of tau-pathology [84, 86-89]. Investigations in silico and in vitro have demonstrated APOEε4, but not APOEε2 and APOEε3, to bind stably to CLEAR motifs, competing with transcriptional factor TFEB [90]. This interaction may lead to the downregulation of pro-autophagic genes, such as MAPLC3B, SQSTM1/P62, LAMP2, in turn, resulting in increased cellular vulnerability in the SAD progression [90]. But in another study, authors showed that several subclusters of neurons and astrocytes of SAD brains were enriched with pro-autophagic gene transcripts [91]. These findings may be explained by the difference of susceptibility of neurons depending on the layer of their disposition, suggesting that deeper layer neurons are less susceptible to the amyloid toxicity [91, 92].
Sex and APOEε4 have been shown to correlate strongly with severity and mortality in SAD patients [93-95]. A family-based analysis of WGS (whole genome sequence) data of 2247 individuals from 605 families revealed the transcription factor ZBTB7C variant as a risk factor for women [93]. BIN1 gene variant has been suggested as possible sex-related gene variants for women, while KAT8 and FERMT2 for men [93]. Another genome-wide association (GWA) study revealed other gene variants that are possibly associated with SAD development and the change in the brain connectivity, including ANTXR2 (which might be involved in extracellular matrix adhesion), CDH18 (neuronal synaptic adhesion), and IGF1 [96].
A strong correlation has been shown between SAD development and the polymorphisms CLU gene, coding clusterin-1, also known as apolipoprotein J, which is involved in Aβ clearance [85, 97-105]. Interestingly, this correlation has been found for SNP rs11136000 (T < C) in Caucasian ethnicity, but not in Asian or Chinese ethnicities, although a previous study showed this relation for both Asian and Caucasian ethnicities [98, 102]. Since SNP rs11136000 (T < C) is located within an intronic region, it seems reasonable to assume that the substitution may, in some way, alter the splicing and expression rate of the gene [100]. Recent transcriptome analyses of the aging brain confirmed that altered pre-mRNA CLU splicing might be associated with SAD development and PTK2B transcription, which is in line with other findings [106, 107]. Nevertheless, the correlation between CLU SNP rs11136000 (T < C) variant and SAD has not been found in several studies performed in Italy, France, and Southern India [105, 108, 109].
Many studies confirm the relation of ABCA7 gene polymorphism and SAD progression [85, 104, 110-112]. ABCA7 gene encodes protein ABCA7 that is involved in lipid transport through the cellular membrane and Aβ clearance via the phagocytic pathway [113]. Research performed on an Icelandic population and later repeated on German, Finnish, Norwegian, and American populations supported the idea of loss-of-function variant ABCA7 and SAD development [110]. ABCA7 SNP rs3764650, which is also located within an intronic region, has been shown to be implicated in SAD [111, 112]. In a study with a French population, ABCA7 SNP rs3764650 was not associated with SAD development [109].
Several studies report a significant relationship between PICALM gene polymorphism and SAD [85, 100, 114-116]. PICALM encodes a protein that is important in Aβ clearance through transcytosis via the blood-brain barrier [114]. PICALM SNP rs3851179G variant has been associated with an elevated risk of SAD development [114]. These results are in accordance with the results of a study of Mediterranean populations from Turkey, Italy, Spain, France, and Lebanon [115]. However, other studies on French and Indian populations do not fit with these data [28, 29].
Different variants of CR1 gene, which encodes the complement component (3b/4b) receptor 1, have been shown to relate to SAD development [85, 104, 109, 117] as well as BIN1 [85, 93, 104, 109, 118], CD33 [85, 104, 112], MS4A6A/MS4A4E [85, 104, 112, 119], TREM2 [85, 120], etc. Epigenetic gene transcription regulation of genes that are associated with SAD development is of utter importance as well. Studies of global histone modifications in the post-mortem AD brains reveal that trimethylated H3K4 (associated with activation of transcription) was increased within immune and stimulus-response genes loci; whereas, it was decreased within the synaptic function and learning genes loci [121]. The relation between H3K27 acetylation within APP, PSEN1, PSEN2, MAPT, CR1 genes loci, and SAD development has been indicated [121, 122]. Besides histone modification, CpG methylation is important as well as other cytosine modifications, which might be positively correlated with aging and neurodegenerative diseases development and progression [123, 124]. Reconciling these varying data may someday help to develop successful therapies against neurodegenerative diseases through epigenome editing [125].
6. THE ROLE OF APOE
ApoE is the 34 KDa protein whose main physiological functions are triglyceride and cholesterol transport and LDL clearance via interaction with LDLRs [126, 127]. ApoE takes part in a variety of processes, including blood-brain barrier (BBB) integrity maintenance and cardiovascular disorders [128-131], immune and inflammation process regulation [127, 132, 133], the pathogenesis of infectious diseases [134], etc. Currently, three isoforms of ApoE (АроЕ2, АроЕ3, АроЕ4) are the most studied. Both ApoE2 and ApoE4 have adverse effects on human health [88, 135]. Isoform ApoE2 has a cysteine residue at position 158 instead of arginine in ApoE3 and ApoE4. This substitution lowers ApoE2 clearance since it is located within the LDL receptor-interacting region and disrupts recognition [126].
ApoE2 isoform has been shown to be protective against AD [86]. ApoE4 has arginine residue at both 158 and 112 positions, while ApoE3 and ApoE2 have cysteine residue at position 112. The arginine residue at position 158 enables it to be recognized by the LDL receptor and to clear triglyceride-rich lipoprotein remnants from the blood [126]. On the other hand, the second substitution at the position 112 alters intramolecular interactions, leading to the disruption of the ApoE tertiary structure. It has been reported that a monomeric state ApoE4 С-terminal domain is relatively unstructured and, in contrast to ApoE3 12-20 and 204-210, residues are present in unfolded state [136]. This leads to an increase in ApoE4 affinity to the lipids and Aβ altering amyloidogenesis kinetics in the brain, Aβ42 oligomers clearance, enhancing its aggregation and fibrillation [88].
АроЕ4-expressing neurons show elevated rates of synaptogenesis and increased Aβ42 secretion [88]. ApoE4 downregulates transcription of the ApoE gene in astrocytes and leads to an increase in the cellular cholesterol content [88]. ApoE4 is found to be not only an AD-related dementia risk factor but also a risk factor in alpha-synucleinopathy, characterized by Lewy bodies deposition and disorders. The exact molecular mechanism remains to be determined [137]. Finally, ApoE gene polymorphism affects a variety of processes and is found to be one of the major risk factors in AD and Lewy body dementia.
7. INFLAMMATION AS A CENTRAL PLAYER IN CVD AND AD
It has long been apparent that the inflammation not only accompanies the pathogenesis of many diseases but also is a consequence of and a significant contributor to the course of these diseases. Recent attention has been directed towards the inflammatory hypothesis of AD, which has stimulated an expansion of the research in neurosciences. Moreover, neuroinflammation appears to have a dual function: a neuroprotective role during an acute-phase response and a detrimental effect under chronic inflammation. Neuroglia plays a pivotal role in inflammation, especially in microglia in patients with neurodegenerative disorders and in macrophages in inflammation-related cardiovascular diseases.
Microglia are resident immune cells of the brain that are derived from erythro-myeloid progenitor cells (EMPs) in the yolk sac [14, 138, 139]. Under normal physiological conditions, microglia are capable of maintaining CNS microenvironment homeostasis, synaptic pruning, and neurodevelopment by releasing brain-derived neurotrophic factor (BDNF) [138, 140]. These cells orchestrate innate immune responses in the CNS by phagocytizing toxic cell debris and controlling infectious pathogens. As with other glia cells, microglia are able to recognize “nonself ” and “altered-self ” cues of pathogens, as well as apoptotic or necrotic cells [140, 141]. These heterogeneous groups of molecules are referred to as pathogen-associated molecular patterns (PAMPs) and apoptotic cell-associated molecular patterns (ACAMPs). Additionally, heat shock proteins (HSP), high-mobility group box chromosomal protein 1 (HMGB-1), and amyloid β (Aβ) fibrils are known as danger-associated molecular patterns (DAMPs) or endogenous danger signals that can be released upon tissue damage or cellular stress [140, 142]. Pattern recognition receptors (PRRs), soluble or membrane-bound, on phagocytic cells sense PAMPs, ACAMPs, and DAMPs expressing “eat me” signals [143]. In response, microglia morphology undergoes a transformation from ramified to amoeboid phenotypes. Consistent with this, activated microglia express PRRs, mainly Toll-like receptors (TLR) and NOD-like receptors (NLRs), triggering multiple innate immune signaling pathways [14, 141, 143, 144]. TLR-4 and TLR-6 receptors are suggested to be co-expressed with cell surface co-receptor (CD36, CD14) [141, 142, 145-147]. These, in turn, initiate NF-κB-dependent signal transduction. Emerging lines of research have shown that abnormal accumulation of oxidized low-density lipoprotein (oxLDL), cholesterol crystals, and fibrillar or soluble amyloid-β facilitate the formation of TLR4-TLR6-CD36 complex, which results in an increase in the production of pro-inflammatory mediators, such as interleukin (IL-1β, IL-2, IL-6, IL-18), interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) chemokines and others [139, 148-152].
Recent insights into Alzheimer's disease indicate that inflammation in the brain of patients with AD occurs in pathologically vulnerable regions, but the specific regions remain unknown [153, 154]. However, there is a hypothesis that a higher level of inflammatory markers is associated with greater brain atrophy. IL-1 is overexpressed in the affected regions of the cerebral cortex of the AD brain, while the number of IL-1 immunoreactive microglia associated with AD plaques increases as well [155, 156].
A further downstream signal cascade leads to the formation of NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome [139, 150, 157]. Similar to the induction of endogenous ligands on TLR4-TLR6-CD36 signaling, these molecules are able to prime and activate the NLRP3 inflammasome [157]. The multi-protein complex processes the pro-inflammatory cytokines into mature form via phosphorylated caspase-1, thereby stimulating the secretion of active IL-1β or IL-18 [141]. Collectively, there are two pathways to activate the inflammasome in atherosclerotic CVD: by directly binding oxLDL with CD36 via TLR or via NLR receptors [146, 152]. There is ample evidence that overexpression of caspase-1 has been observed in the brain lysates of patients with AD or mild cognitive impairment (MCI), which led to the further engendering of IL-1β-driven-neurotoxic effects [158, 159]. According to studies involving APP/PS1/NLRP3−/− and APP/PS1/Casp1−/− mice, amyloid β deposition was reduced due to the shift from Aβ aggregation pathway to Aβ clearance by the phagocytic activity of microglia [151, 158-160]. Therefore, inhibition of caspase-1 and NLR3 inflammasome can be beneficial to attenuate vascular and brain inflammation and may be a viable target for therapeutic interventions.
Atherosclerosis is a lipid storage disease caused by inflammation, contributing to all stages of neurodegenerative diseases, from the initial lesion to the end-stage thrombotic complications. The inflammatory process promotes not only the initiation of atherosclerotic lesions but also its progression to atherosclerotic plaques [161]. It is well-accepted that mild chronic vascular inflammation is a part of the pathophysiology of CVD. Hypertension and atherosclerosis play important roles in understanding the disease’s onset, progression, and pharmacological therapy [162]. The findings of the Whitehall II cohort study (2017) have shown that measures of low-grade systemic inflammation, such as C-reactive protein (CRP) and interleukin-6 (IL-6) in midlife, were strong independent predictors of all-cause of CVD and cardiovascular-related mortality. Furthermore, new evidence from 50 prior cohort studies reveals that CRP and IL-6 could predict future myocardial infarction and stroke [163]. CRP and other systemic inflammatory markers also are associated with the onset of AD. The high levels of CRP can be related to white matter lesions and, thereby, may accelerate the progression of AD [164, 165]. In addition to the aging of the immune system, cerebral atrophy is suggested to be associated with a decrease in the production of anti-inflammatory cytokines, such as IL-6 and IL-8, by the reactive microglia and astrocytes, which was observed in patients with cognitive declines after a stroke [166].
Notably, the imbalance between pro-resolving lipid mediators leads to impaired resolution of inflammation and subsequent generalized increased inflammatory state, such as seen in AD [167-169]. The failure of resolution is propagated by the increased production of specialized pro-resolving mediators (SPMs) derived from n-3 PUFA: DHA-resolvin D series, protectins, maresins, and EPA-resolvin E series [170]. Interestingly, restoring the levels of SPMs (lipoxins and resolvins) can drive the resolution of chronic cardiovascular inflammation [167]. Future investigation is warranted to confirm the use of SPMs as a potential therapeutic target in Alzheimer's disease.
8. POTENTIAL ROLE OF MICROBIOTA AND PERIODONTAL DISEASE
The next potential risk factor involved in pathogenic mechanisms of CVD and AD is the gut and oral microbiota. The gut microbiota (GM) is an ecological community that performs a broad range of regulatory functions within the host organism [147, 171-173]. Of particular relevance, the imbalance of GM may play a secondary role in the pathophysiology of various diseases. It is well-known that atherosclerotic plaques contain bacterial DNA, thereby contributing to the progression of lesions in the vessel wall [174]. In addition, epidemiological studies have suggested that periodontal disease (PD) is strongly associated with increased CVD risk. Concurrently, clinical studies have provided evidence for the influence of periodontal pathogens on CVD pathological mechanisms, such as endothelial dysfunction, oxidative stress, lipid accumulation, vascular remodeling, and atherothrombosis [175, 176].
Moreover, there is a close correlation between levels of circulating triglycerides (TG), high-density lipoprotein (HDL), cholesterol crystals, and GM [177, 178]. The GM of individuals who suffer from CVDs produces more pro-inflammatory molecules and, consequently, enhances chronic inflammation [179]. The inflammatory state can be aggravated by the impaired intestinal barrier function, resulting in bacterial translocation and, by the presence of microbial by-products, in the systemic circulation. These factors seem to contribute to further progression of CVD to heart failure and atherosclerosis [174, 176, 180]. Some studies have revealed that both glycemic control and plasma lipid profiles are improved in combination with prebiotics administration in CVD patients [174].
The interaction between the central nervous system (CNS) homeostasis and GM is called the “microbiota-gut-brain axis” [181]. Accumulating evidence indicates that there is bidirectional communication affecting both the immune and neuronal systems [172, 182, 183]. The BBB and intestinal mucosa are more permeable during an inflammatory state or microbiota dysbiosis. Under these conditions, β-amyloid and lipopolysaccharides (LPSs) can pass easily through protective barriers via LPS/amyloid426 triggered cytokines [147, 173]. LPS binds to TLR4-MD2 by CD14 and mediates the LPS-induced inflammatory cascade [182]. The release of inflammatory mediators into the intestinal tract may exacerbate the neuroinflammation and disease-specific pathogenic mechanisms, accelerating neuronal impartment. LPS and other microbiome-derived neurotoxins, produced by intestinal bacteria (e.g., beta-N-methylamino-L-alanine (BMAA)), are proposed to be involved in Aβ misfolding [184]. GM in AD patients can induce the activation of NLRP3 inflammasome [172, 182, 185].
A few studies have shown that periodontitis is associated not only with CVD but also with an increase in cognitive decline in AD due to poor oral health [186, 187]. Recent findings indicate that the infection Porphyromonas gingivalis and its LPS are closely associated with the development of a sporadic form of AD [188-190]. Besides, Treponema denticola, Tannerella forsythia were also identified in the brain of AD patients, which reflects a link between chronic periodontitis and cognitive impairment [188, 191].
The novel antimicrobial hypothesis has been given to possible physiological roles of Aβ as an antimicrobial peptide (AMP), utilizing fibrillation to protect the host from a broad spectrum of infectious agents [189]. According to past clinical trials, there was a higher infection rate among study participants during anti-Aβ therapies. As a response to infections in the brain, the production antimicrobial activity of Aβ enhances [189, 190]. In particular, the various spirochetal infections are considered as a causative factor of AD [148, 192-194]. A recent study demonstrated that Aβ plaques colocalize with Borrelia burgdorferi antigens and bacterial DNA [195].
9. OXIDATIVE STRESS
Mitochondria are known to be the most vulnerable cellular organelles and the primary reactive oxygen species (ROS) source inside the cell. Oxidative stress appears to be one of the risk factors affecting neurodegenerative disorders’ pathogenesis. While there is no scientific consensus on the root cause of SAD, mitochondrial disorders play a crucial role in both amyloid theory and mitochondrial one [196]. For example, cellular respiration disruption due to the dysfunction of α-ketoglutarate dehydrogenase complex, pyruvate dehydrogenase complex, and cytochrome-C oxidase is suggested to be a contributing factor [197]. Cellular respiration disruption inexorably leads to depleted adenosine triphosphate (ATP) synthesis and increased ROS production, as well as mitochondria depolarization and breakdown.
It should be noted that noncoding regions are quite rare in mitochondrial genome regions, increasing the odds of ROS-induced mutagenesis in coding regions [198]. 4977bp mtDNA deletion is reported to be another important indicator of the AD progression, leading to a loss of genes that encode electron transport chain (ETC) complexes subunits and mitochondrial transfer ribonucleic acid (tRNA), which causes mitochondrial dysfunction and ROS accumulation [199-202].
These factors are implicated in demolishing mitochondrial functioning and depolarization during AD, which s supported by studies reporting increased mitophagy levels in AD [40, 203, 204]. In addition, mitochondrial over-proliferation and fragmentation have been observed, likely as a result of upregulated DRP1 expression and its interaction with Aβ and p-Tau [205, 206]. The condition may be exacerbated by diminished autophagy due to autophagy apparatus dysfunction caused by an inherited mutation within the PSEN1 gene [207, 208].
Nucleotide oxidation seems to be another important cellular homeostasis disorder that is associated not only with AD but also with Parkinson’s and Huntington’s diseases [209, 210]. The abundance of 8-oxoG inside the cell results in G-to-T transversion mutations due to its ability to form stable bonds with cytosine as well as with adenine [198, 211]. It is important to note, because of the mitochondrial deficiency and the elevated cellular ROS levels, that nuclear DNA oxidation may occur. For instance, P62 promotor oxidative damage downregulates expression of p62 leading to autophagy disruption, in turn resulting in an AD-like phenotype in mice [212, 213].
10. DIET AND PHYSICAL ACTIVITY
There are several studies that link physical activity and diet with CVD and AD. The Mediterranean diet (MedDiet) and MIND diet (a combination of the MedDiet and DASH (Dietary Approaches to Stop Hypertension)) seem to be protective. Research using MedDiet has shown, in Cox models, that individuals with middle tertile diet adherence have 2-14% lower risk of AD; while those belonging to the highest tertile diet adherence have 32% to 40% reduced risk of AD [214]. The authors of the MEDIS study, published in 2017 (n=3,128), reported that pro-inflammatory dietary habits were correlated with hypercholesterolemia and T2DM. Logically, the anti-inflammatory potential of these diets should improve health status [215, 216].
The recent results of the Health and Retirement Study (n=5,907) presented a new approach. The impact of neuroprotective dietary approaches (MedDiet and MIND) on cognitive health has been shown in elderly individuals. Sticking to the MedDiet and MIND diet is independently associated with better cognitive function and lower risk of cognitive impairment [217].
The data indicate there is undeniable value in using n-3 long-chain polyunsaturated fatty acids (DHA-docosahexaenoic acid and EPA-eicosapentaenoic acid) in both CVD and AD. The omega-3 consumption reduces plasma triglycerides, resting heart rate, blood pressure, and chronic inflammation, and may improve myocardial filling and efficiency, as well as vascular function [169, 218]. The n-3 LC-PUFA supplementation might regulate cytokines expression and, consequently, the production of EPA and DHA-derived specialized pro-resolving mediators (SPMs) [170, 219]. Based on MMSE scores, omega-3 fatty acids intake (EPA and DHA) may decrease the rate of cognitive decline [220].
However, a meta-analysis conducted by Wu et al. [221] revealed that omega-3 supplementation does not lower the risk of AD, while the consumption of fatty fish reduces AD risk by 36%. The same results were obtained in a study analyzing the association of omega-3 fatty acid with the risk of fatal or nonfatal coronary heart disease [222]. The combined evidence suggests that the claimed benefits of omega-3 supplementation remain elusive. Some possible reasons for ambiguous results include a varied time of supplementation, genetic differences (APOE ɛ4 or non-APOE ɛ4 allele carries), differences in the amount of omega-3 (from 200 mg to over 2 g/day), use of different sources of omega-3 (plant oils enriched only with EPA and DHA precursors), and the severity of the disease [170].
Moreover, the growing body of evidence points to benefits obtained by combined dietary interventions (n-3 LC-PUFA, vitamin B complex, vitamin D3, resveratrol, and curcumin) is a more effective way to enhance Aβ clearance and counteract inflammation in AD than one-time use of nutrients [170]. One of the potential combined dietary interventions to prevent AD, mentioned by Luchsinger et al. [223], is to decrease the homocysteine levels through supplementation with folic acid and vitamins B6 and B12. However, van Dijk et al. [216] have shown that in elderly individuals with hyperhomocysteinemia, the administration of vitamin B12 and folic acid did not significantly affect the endothelial function or mild systemic inflammation.
In addition, there is an inverse dose-response relationship between physical activity, CVD, and mortality risk. Three-quarters of deaths due to CVDs could be prevented with adequate changes in lifestyle, including increased daily physical activity [224]. Physical activity plays an important role both in primary and secondary prevention of CVD by reducing the impact of the disease, delaying progression, and preventing recurrence. Similarly, physical health prevents dementia due to AD and slows the progression of the disease. Aerobic physical activity seems to improve endothelial function by stimulating the production of nitric oxide, mitigates vascular inflammation, and stimulates vascular regeneration [224]. Interestingly, compared with people without physical activity, people with some physical activity had a 25-38% lower risk of AD, while people with higher physical activity obtained a reduced risk for AD by 33-48% [214].
CONCLUSION
The numerous studies of AD over the past several decades have found aspects of the pathophysiology, including trigger events, to be opaque and elusive. A major contributing factor is that the diagnostic criteria of AD usually exclude patients with any significant CVD, according to recommendations of the American Psychological Association. Conversely, CVD can independently lead to cognitive impairment and dementia. Nevertheless, there is strong evidence supporting the conjecture that CVD contributes, at least in part, to the progression of AD. Recent findings show the important relationship between these two diseases and lifestyle factors, such as diet, physical activity, blood pressure, blood cholesterol levels, homocysteine level, and diabetes. There is no doubt that there is a relationship between AD and CVD not only because they share common risk factors but also because they have common molecular mechanisms of emergence. Understanding the etiology and mechanisms of one of these diseases may lead to develop novel therapeutic strategies that may be applicable to both AD and CVD. Finally, it is concluded that intense research over the past several decades has provided us with invaluable information on different processes and AD progression. There are many risk factors that have a robust or moderate correlation with the AD. Nevertheless, this information remains insufficient for the invention and development of successful therapeutic strategies; therefore, there are many vital questions that remain unanswered.
LIST OF ABBREVIATIONS
- ABCA7
ATP-Binding Cassette sub-family A member 7
- AD
Alzheimer’s Disease
- APA
American Psychological Association
- APOE
Apolipoprotein E
- APP
Amyloid Precursor Protein
- Aβ
Amyloid β
- BBB
Blood-Brain Barrier
- BIN1
Bridging Integrator 1
- BMAA
Beta-N-Methylamino-L-Alanine
- CD
Cluster of Differentiation
- CDH18
Cadherin 18
- CLEAR
Coordinated Lysosomal Expression and Regulation
- CLU
Clusterin
- CNS
Central Nervous System
- CR1
Complement Receptor Type 1
- CRP
C-reactive Protein
- CVD
Cardiovascular Disease
- DASH
Dietary Approaches to Stop Hypertension
- DHA
Docosahexaenoic Acid
- DRP1
Dynamin-1-like Protein
- DSM-5
Diagnostic and Statistical Manual of mental disorders, fifth edition
- EOAD
Early Onset Alzheimer’s Disease
- EPA
Eicosapentaenoic Acid
- ETC
Electron Transport Chain
- FERMT2
Fermitin Family Member 2
- GM
Gut Microbiota
- GWA study
Genome-Wide Association study
- IGF1
Insulin-like Growth Factor 1
- IL
Interleukin
- KAT8
Lysine (K) Acetyltransferase 8
- LC-PUFAs
Long Chain Polyunsaturated Fatty Acids
- LDL
Low Density Lipoproteins
- LPS
Lipopolysaccharide
- MD2
Myeloid Differentiation Factor 2
- MedDiet
Mediterranean Diet
- MMSE
Mini-Mental State Exam
- MS4A6A/MS4A4E
Membrane spanning 4-domains A6A/E
- MTHFR
Methylene tetrahydrofolate Reductase
- NLR
NOD-like Receptors
- NLRP3
NLR-family Pyrin Domain Containing 3
- P62
Sequestosome 1
- PD
Periodontal Disease
- PICALM
Phosphatidylinositol Binding Clathrin Assembly Protein
- PS1
Presenilin 1
- PSEN1/2
Presenilin 1/2
- PTK2B
Protein Tyrosine Kinase 2 beta
- PUFA
Polyunsaturated Fatty Acids
- ROS
Reactive Oxygen Species
- SAD
Sporadic Alzheimer’s Disease
- SNP
Single-Nucleotide Polymorphisms
- T2DM
Type 2 Diabetes Mellitus
- TFEB
Transcription Factor EB
- TLR
Toll-like Receptor
- TREM2
Triggering Receptor Expressed on Myeloid cells 2
- VaD
Vascular Dementia
- VNCD
Vascular Neurocognitive Disorders
- WGS
Whole Genome Sequence
- ZBTB7C
Zinc Finger and BTB Domain Containing 7C
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This study was supported by GALLY International Research Institute, San Antonio, TX, USA. This work also supported by Russian Academic Excellence project "5-100” for the Sechenov University, Moscow, Russia.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
- 1. https://www.alz.co.uk/
- 2.Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;2020:2020. [Google Scholar]
- 3.Long J.M., Holtzman D.M. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179(2):312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack C.R., Jr, Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Holtzman D.M., Jagust W., Jessen F., Karlawish J., Liu E., Molinuevo J.L., Montine T., Phelps C., Rankin K.P., Rowe C.C., Scheltens P., Siemers E., Snyder H.M., Sperling R. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1
- 6. https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 7.Canter R.G., Penney J., Tsai L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature. 2016;539(7628):187–196. doi: 10.1038/nature20412. [DOI] [PubMed] [Google Scholar]
- 8.Weller J., Budson A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000 Res. 2018;7:7. doi: 10.12688/f1000research.14506.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry D., Sperling R., Katz R., Berry D., Dilts D., Hanna D., Salloway S., Trojanowski J.Q., Bountra C., Krams M., Luthman J., Potkin S., Gribkoff V., Temple R., Wang Y., Carrillo M.C., Stephenson D., Snyder H., Liu E., Ware T., McKew J., Fields F.O., Bain L.J., Bens C. Building a roadmap for developing combination therapies for Alzheimer’s disease. Expert Rev. Neurother. 2015;15(3):327–333. doi: 10.1586/14737175.2015.996551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L.K., Chao S.P., Hu C.J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020;27(1):18. doi: 10.1186/s12929-019-0609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panza F., Solfrizzi V., Seripa D., Imbimbo B.P., Lozupone M., Santamato A., Tortelli R., Galizia I., Prete C., Daniele A., Pilotto A., Greco A., Logroscino G. Tau-based therapeutics for Alzheimer’s disease: active and passive immunotherapy. Immunotherapy. 2016;8(9):1119–1134. doi: 10.2217/imt-2016-0019. [DOI] [PubMed] [Google Scholar]
- 12.Lalut J., Payan H., Davis A., Lecoutey C., Legay R., Sopkova-de Oliveira Santos J., Claeysen S., Dallemagne P., Rochais C. Rational design of novel benzisoxazole derivatives with acetylcholinesterase inhibitory and serotoninergic 5-HT4 receptors activities for the treatment of Alzheimer’s disease. Sci. Rep. 2020;10(1):3014. doi: 10.1038/s41598-020-59805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu X.C., Tan L., Wang H.F., Jiang T., Cao L., Wang C., Wang J., Tan C.C., Meng X.F., Yu J.T. Rate of early onset Alzheimer’s disease: a systematic review and meta-analysis. Ann. Transl. Med. 2015;3(3):38. doi: 10.3978/j.issn.2305-5839.2015.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Przedborski S., Masliah E., Cosentino M. Neuroimmune pharmacology. 2nd ed. Springer International Publishing; 2017. [Google Scholar]
- 15.Grady C.L., Furey M.L., Pietrini P., Horwitz B., Rapoport S.I. Altered brain functional connectivity and impaired short-term memory in Alzheimer’s disease. Brain. 2001;124(Pt 4):739–756. doi: 10.1093/brain/124.4.739. [DOI] [PubMed] [Google Scholar]
- 16.Anderson, M., The Neuropsychology of Cortical Dementias. New York: Springer Publishing Company; 2015. p. 481. [Google Scholar]
- 17.Kumbhare D., Palys V., Toms J., Wickramasinghe C.S., Amarasinghe K., Manic M., Hughes E., Holloway K.L. Nucleus basalis of meynert stimulation for dementia: theoretical and technical considerations. Front. Neurosci. 2018;12(614):614. doi: 10.3389/fnins.2018.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai X.C., Yan C., Yang G., Lu P., Ma D., Sun L., Zhou R., Scheres S.H.W., Shi Y. An atomic structure of human γ-secretase. Nature. 2015;525(7568):212–217. doi: 10.1038/nature14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y.E., Cho H., Kim H.J., Na D.L., Seo S.W., Ki C.S. PSEN1 variants in Korean patients with clinically suspicious early-onset familial Alzheimer’s disease. Sci. Rep. 2020;10(1):3480. doi: 10.1038/s41598-020-59829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanoiselée H.M., Nicolas G., Wallon D., Rovelet-Lecrux A., Lacour M., Rousseau S., Richard A.C., Pasquier F., Rollin-Sillaire A., Martinaud O., Quillard-Muraine M., de la Sayette V., Boutoleau-Bretonniere C., Etcharry-Bouyx F., Chauviré V., Sarazin M., le Ber I., Epelbaum S., Jonveaux T., Rouaud O., Ceccaldi M., Félician O., Godefroy O., Formaglio M., Croisile B., Auriacombe S., Chamard L., Vincent J.L., Sauvée M., Marelli-Tosi C., Gabelle A., Ozsancak C., Pariente J., Paquet C., Hannequin D., Campion D. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med. 2017;14(3):e1002270. doi: 10.1371/journal.pmed.1002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu P., Bai X.C., Ma D., Xie T., Yan C., Sun L., Yang G., Zhao Y., Zhou R., Scheres S.H.W., Shi Y. Three-dimensional structure of human γ-secretase. Nature. 2014;512(7513):166–170. doi: 10.1038/nature13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szaruga M., Munteanu B., Lismont S., Veugelen S., Horré K., Mercken M., Saido T.C., Ryan N.S., De Vos T., Savvides S.N., Gallardo R., Schymkowitz J., Rousseau F., Fox N.C., Hopf C., De Strooper B., Chávez-Gutiérrez L. Alzheimer’s-Causing mutations shift Aβ length by destabilizing γ-Secretase-Aβn interactions. Cell. 2017;170(3):443–456.e14. doi: 10.1016/j.cell.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe M.S., Yankner B.A. Sorting out presenilins in Alzheimer’s Disease. Cell. 2016;166(1):13–15. doi: 10.1016/j.cell.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 24.Giau V.V., Bagyinszky E., Yang Y.S., Youn Y.C., An S.S.A., Kim S.Y. Genetic analyses of early-onset Alzheimer’s disease using next generation sequencing. Sci. Rep. 2019;9(1):8368. doi: 10.1038/s41598-019-44848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arber C., Toombs J., Lovejoy C., Ryan N.S., Paterson R.W., Willumsen N., Gkanatsiou E., Portelius E., Blennow K., Heslegrave A., Schott J.M., Hardy J., Lashley T., Fox N.C., Zetterberg H., Wray S. Familial Alzheimer’s disease patient-derived neurons reveal distinct mutation-specific effects on amyloid beta. Mol. Psychiatry. 2019;••• doi: 10.1038/s41380-019-0410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giau V.V., Bagyinszky E., Youn Y.C., An S.S.A., Kim S. APP, PSEN1, and PSEN2 mutations in asian patients with early-onset Alzheimer Disease. Int. J. Mol. Sci. 2019;20(19):E4757. doi: 10.3390/ijms20194757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. https://www.alzforum.org/mutations/psen-1
- 28. https://www.alzforum.org/mutations/psen-2
- 29.Nicolas G., Wallon D., Charbonnier C., Quenez O., Rousseau S., Richard A.C., Rovelet-Lecrux A., Coutant S., Le Guennec K., Bacq D., Garnier J.G., Olaso R., Boland A., Meyer V., Deleuze J.F., Munter H.M., Bourque G., Auld D., Montpetit A., Lathrop M., Guyant-Maréchal L., Martinaud O., Pariente J., Rollin-Sillaire A., Pasquier F., Le Ber I., Sarazin M., Croisile B., Boutoleau-Bretonnière C., Thomas-Antérion C., Paquet C., Sauvée M., Moreaud O., Gabelle A., Sellal F., Ceccaldi M., Chamard L., Blanc F., Frebourg T., Campion D., Hannequin D. Screening of dementia genes by whole-exome sequencing in early-onset Alzheimer disease: input and lessons. Eur. J. Hum. Genet. 2016;24(5):710–716. doi: 10.1038/ejhg.2015.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter S., Brayne C. Understanding the roles of mutations in the amyloid precursor protein in Alzheimer disease. Mol. Psychiatry. 2018;23(1):81–93. doi: 10.1038/mp.2017.218. [DOI] [PubMed] [Google Scholar]
- 31. https://www.alzforum.org/mutations/app
- 32.Bagyinszky E., Kang M.J., Van Giau V., Shim K., Pyun J.M., Suh J., An S.S.A., Kim S. Novel amyloid precursor protein mutation, Val669Leu (“Seoul APP”), in a Korean patient with early-onset Alzheimer’s disease. Neurobiol. Aging. 2019;84:236.e1–236.e7. doi: 10.1016/j.neurobiolaging.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 33.Thordardottir S., Graff C. Findings from the swedish study on familial alzheimer’s disease including the APP swedish double mutation. J. Alzheimers Dis. 2018;64(s1):S491–S496. doi: 10.3233/JAD-179922. [DOI] [PubMed] [Google Scholar]
- 34.Mullan M., Crawford F., Axelman K., Houlden H., Lilius L., Winblad B., Lannfelt L. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat. Genet. 1992;1(5):345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- 35.Nilsberth C., Westlind-Danielsson A., Eckman C.B., Condron M.M., Axelman K., Forsell C., Stenh C., Luthman J., Teplow D.B., Younkin S.G., Näslund J., Lannfelt L. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat. Neurosci. 2001;4(9):887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- 36.György B., Lööv C., Zaborowski M.P., Takeda S., Kleinstiver B.P., Commins C., Kastanenka K., Mu D., Volak A., Giedraitis V., Lannfelt L., Maguire C.A., Joung J.K., Hyman B.T., Breakefield X.O., Ingelsson M. CRISPR/Cas9 mediated disruption of the swedish app allele as a therapeutic approach for early-onset Alzheimer’s Disease. Mol. Ther. Nucleic Acids. 2018;11:429–440. doi: 10.1016/j.omtn.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herl L., Thomas A.V., Lill C.M., Banks M., Deng A., Jones P.B., Spoelgen R., Hyman B.T., Berezovska O. Mutations in amyloid precursor protein affect its interactions with presenilin/gamma-secretase. Mol. Cell. Neurosci. 2009;41(2):166–174. doi: 10.1016/j.mcn.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu T.H., Yan Y., Kang Y., Jiang Y., Melcher K., Xu H.E. Alzheimer’s disease-associated mutations increase amyloid precursor protein resistance to γ-secretase cleavage and the Aβ42/Aβ40 ratio. Cell Discov. 2016;2:16026. doi: 10.1038/celldisc.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian Y., Bassit B., Chau D., Li Y.M. An APP inhibitory domain containing the Flemish mutation residue modulates gamma-secretase activity for Abeta production. Nat. Struct. Mol. Biol. 2010;17(2):151–158. doi: 10.1038/nsmb.1743. [DOI] [PubMed] [Google Scholar]
- 40.Maloney J.A., Bainbridge T., Gustafson A., Zhang S., Kyauk R., Steiner P., van der Brug M., Liu Y., Ernst J.A., Watts R.J., Atwal J.K. Molecular mechanisms of Alzheimer disease protection by the A673T allele of amyloid precursor protein. J. Biol. Chem. 2014;289(45):30990–31000. doi: 10.1074/jbc.M114.589069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jonsson T., Atwal J.K., Steinberg S., Snaedal J., Jonsson P.V., Bjornsson S., Stefansson H., Sulem P., Gudbjartsson D., Maloney J., Hoyte K., Gustafson A., Liu Y., Lu Y., Bhangale T., Graham R.R., Huttenlocher J., Bjornsdottir G., Andreassen O.A., Jönsson E.G., Palotie A., Behrens T.W., Magnusson O.T., Kong A., Thorsteinsdottir U., Watts R.J., Stefansson K. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488(7409):96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 42.Román G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H., Amaducci L., Orgogozo J.M., Brun A., Hofman A. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/WNL.43.2.250. [DOI] [PubMed] [Google Scholar]
- 43.Skrobot O.A., O’Brien J., Black S., Chen C., DeCarli C., Erkinjuntti T., Ford G.A., Kalaria R.N., Pantoni L., Pasquier F., Roman G.C., Wallin A., Sachdev P., Skoog I., Ben-Shlomo Y., Passmore A.P., Love S., Kehoe P.G. The vascular impairment of cognition classification consensus study. Alzheimers Dement. 2017;13(6):624–633. doi: 10.1016/j.jalz.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Skrobot O.A., Black S.E., Chen C., DeCarli C., Erkinjuntti T., Ford G.A., Kalaria R.N., O’Brien J., Pantoni L., Pasquier F., Roman G.C., Wallin A., Sachdev P., Skoog I., Ben-Shlomo Y., Passmore A.P., Love S., Kehoe P.G. Progress toward standardized diagnosis of vascular cognitive impairment: Guidelines from the vascular impairment of cognition classification consensus study. Alzheimers Dement. 2018;14(3):280–292. doi: 10.1016/j.jalz.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Krishnamurthi R.V., Feigin V.L., Forouzanfar M.H., Mensah G.A., Connor M., Bennett D.A., Moran A.E., Sacco R.L., Anderson L.M., Truelsen T., O’Donnell M., Venketasubramanian N., Barker-Collo S., Lawes C.M., Wang W., Shinohara Y., Witt E., Ezzati M., Naghavi M., Murray C. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob. Health. 2013;1(5):e259–e281. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S., Shafe A.C., Cowie M.R. UK stroke incidence, mortality and cardiovascular risk management 1999-2008: time-trend analysis from the General Practice Research Database. BMJ Open. 2011;1(2):e000269. doi: 10.1136/bmjopen-2011-000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Connor M.D., Walker R., Modi G., Warlow C.P. Burden of stroke in black populations in sub-Saharan Africa. Lancet Neurol. 2007;6(3):269–278. doi: 10.1016/S1474-4422(07)70002-9. [DOI] [PubMed] [Google Scholar]
- 48.Stampfer M.J. Cardiovascular disease and Alzheimer’s disease: common links. J. Intern. Med. 2006;260(3):211–223. doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 49.White L., Petrovitch H., Hardman J., Nelson J., Davis D.G., Ross G.W., Masaki K., Launer L., Markesbery W.R. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann. N. Y. Acad. Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 50.Rothwell P.M., Coull A.J., Silver L.E., Fairhead J.F., Giles M.F., Lovelock C.E., Redgrave J.N., Bull L.M., Welch S.J., Cuthbertson F.C., Binney L.E., Gutnikov S.A., Anslow P., Banning A.P., Mant D., Mehta Z. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet. 2005;366(9499):1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 51.Snowdon D.A., Greiner L.H., Mortimer J.A., Riley K.P., Greiner P.A., Markesbery W.R. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277(10):813–817. doi: 10.1001/jama.1997.03540340047031. [DOI] [PubMed] [Google Scholar]
- 52.Qiu C., Winblad B., Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 53.Cipollini V., Troili F., Giubilei F. Emerging biomarkers in vascular cognitive impairment and dementia: from pathophysiological pathways to clinical application. Int. J. Mol. Sci. 2019;20(11):E2812. doi: 10.3390/ijms20112812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iadecola C., Davisson R.L. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7(6):476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skoog I., Gustafson D. Update on hypertension and Alzheimer’s disease. Neurol. Res. 2006;28(6):605–611. doi: 10.1179/016164106X130506. [DOI] [PubMed] [Google Scholar]
- 56.Verghese J., Lipton R.B., Hall C.B., Kuslansky G., Katz M.J. Low blood pressure and the risk of dementia in very old individuals. Neurology. 2003;61(12):1667–1672. doi: 10.1212/01.WNL.0000098934.18300.BE. [DOI] [PubMed] [Google Scholar]
- 57.Forette F., Seux M.L., Staessen J.A., Thijs L., Birkenhäger W.H., Babarskiene M.R., Babeanu S., Bossini A., Gil-Extremera B., Girerd X., Laks T., Lilov E., Moisseyev V., Tuomilehto J., Vanhanen H., Webster J., Yodfat Y., Fagard R. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352(9137):1347–1351. doi: 10.1016/S0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 58.Yasar S., Xia J., Yao W., Furberg C.D., Xue Q.L., Mercado C.I., Fitzpatrick A.L., Fried L.P., Kawas C.H., Sink K.M., Williamson J.D., DeKosky S.T., Carlson M.C. Antihypertensive drugs decrease risk of Alzheimer disease: ginkgo evaluation of memory study. Neurology. 2013;81(10):896–903. doi: 10.1212/WNL.0b013e3182a35228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murray M.D., Hendrie H.C., Lane K.A., Zheng M., Ambuehl R., Li S., Unverzagt F.W., Callahan C.M., Gao S. Antihypertensive medication and dementia risk in older adult african americans with hypertension: a prospective cohort study. J. Gen. Intern. Med. 2018;33(4):455–462. doi: 10.1007/s11606-017-4281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C: American Psychiatric Association; 2013. p. 947. [Google Scholar]
- 61.Winkler E.A., Sagare A.P., Zlokovic B.V. The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain Pathol. 2014;24(4):371–386. doi: 10.1111/bpa.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santos C.Y., Snyder P.J., Wu W.C., Zhang M., Echeverria A., Alber J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimers Dement. (Amst.) 2017;7:69–87. doi: 10.1016/j.dadm.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takeda S., Rakugi H., Morishita R. Roles of vascular risk factors in the pathogenesis of dementia. Hypertens. Res. 2020;43(3):162–167. doi: 10.1038/s41440-019-0357-9. [DOI] [PubMed] [Google Scholar]
- 64.Love S., Miners J.S. Cerebrovascular disease in ageing and Alzheimer’s disease. Acta Neuropathol. 2016;131(5):645–658. doi: 10.1007/s00401-015-1522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kisler K., Nelson A.R., Montagne A., Zlokovic B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017;18(7):419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y., Braidy N., Poljak A., Chan D.K.Y., Sachdev P. Cerebral small vessel disease and the risk of Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2018;47:41–48. doi: 10.1016/j.arr.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 67.Bogers R.P., Bemelmans W.J., Hoogenveen R.T., Boshuizen H.C., Woodward M., Knekt P., van Dam R.M., Hu F.B., Visscher T.L., Menotti A., Thorpe R.J., Jr, Jamrozik K., Calling S., Strand B.H., Shipley M.J. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch. Intern. Med. 2007;167(16):1720–1728. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- 68.Reitz C., Tang M.X., Schupf N., Manly J.J., Mayeux R., Luchsinger J.A. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch. Neurol. 2010;67(12):1491–1497. doi: 10.1001/archneurol.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El Gaamouch F., Jing P., Xia J., Cai D. Alzheimer’s Disease risk genes and lipid regulators. J. Alzheimers Dis. 2016;53(1):15–29. doi: 10.3233/JAD-160169. [DOI] [PubMed] [Google Scholar]
- 70.Hatzifilippou E., Koutsouraki E., Costa V.G., Baloyannis S.J. Antibodies against gangliosides in patients with dementia. Am. J. Alzheimers Dis. Other Demen. 2014;29(8):660–666. doi: 10.1177/1533317514534953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koutsouraki E., Hatzifilippou E., Michmizos D., Banaki T., Costa V., Baloyannis S. The probable auto-antigenic role of lipids (anti-ganglioside antibodies) in the pathogenesis of Alzheimer’s disease. J. Alzheimers Dis. 2014;42(Suppl. 3):S163–S166. doi: 10.3233/JAD-132633. [DOI] [PubMed] [Google Scholar]
- 72.Gattaz W.F., Forlenza O.V., Talib L.L., Barbosa N.R., Bottino C.M. Platelet phospholipase A(2) activity in Alzheimer’s disease and mild cognitive impairment. J. Neural Transm. (Vienna) 2004;111(5):591–601. doi: 10.1007/s00702-004-0142-y. [DOI] [PubMed] [Google Scholar]
- 73.Hatzifilippou E., Koutsouraki E., Banaki T., Traka M., Costa V.G., Baloyannis S.J. Antibodies against GM1 in demented patients. Am. J. Alzheimers Dis. Other Demen. 2008;23(3):274–279. doi: 10.1177/1533317508317816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahtiluoto S., Polvikoski T., Peltonen M., Solomon A., Tuomilehto J., Winblad B., Sulkava R., Kivipelto M. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75(13):1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- 75.Luchsinger J.A., Tang M.X., Shea S., Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63(7):1187–1192. doi: 10.1212/01.WNL.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 76.de la Monte S.M., Tong M., Daiello L.A., Ott B.R. Early-Stage Alzheimer’s Disease Is Associated with simultaneous systemic and central nervous system dysregulation of insulin-linked metabolic pathways. J. Alzheimers Dis. 2019;68(2):657–668. doi: 10.3233/JAD-180906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ribe E.M., Lovestone S. Insulin signalling in Alzheimer’s disease and diabetes: from epidemiology to molecular links. J. Intern. Med. 2016;280(5):430–442. doi: 10.1111/joim.12534. [DOI] [PubMed] [Google Scholar]
- 78.Arnold S.E., Arvanitakis Z., Macauley-Rambach S.L., Koenig A.M., Wang H.Y., Ahima R.S., Craft S., Gandy S., Buettner C., Stoeckel L.E., Holtzman D.M., Nathan D.M. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat. Rev. Neurol. 2018;14(3):168–181. doi: 10.1038/nrneurol.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rai V. Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism and Alzheimer Disease Risk: a Meta-Analysis. Mol. Neurobiol. 2017;54(2):1173–1186. doi: 10.1007/s12035-016-9722-8. [DOI] [PubMed] [Google Scholar]
- 80.Hu Q., Teng W., Li J., Hao F., Wang N. Homocysteine and Alzheimer’s Disease: Evidence for a causal link from mendelian randomization. J. Alzheimers Dis. 2016;52(2):747–756. doi: 10.3233/JAD-150977. [DOI] [PubMed] [Google Scholar]
- 81.Van Cauwenberghe C., Van Broeckhoven C., Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet. Med. 2016;18(5):421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carmona S., Hardy J., Guerreiro R. The genetic landscape of Alzheimer disease. Handb. Clin. Neurol. 2018;148:395–408. doi: 10.1016/B978-0-444-64076-5.00026-0. [DOI] [PubMed] [Google Scholar]
- 83.Verheijen J., Sleegers K. Understanding Alzheimer Disease at the interface between genetics and transcriptomics. Trends Genet. 2018;34(6):434–447. doi: 10.1016/j.tig.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 84.Castellano J.M., Kim J., Stewart F.R., Jiang H., DeMattos R.B., Patterson B.W., Fagan A.M., Morris J.C., Mawuenyega K.G., Cruchaga C., Goate A.M., Bales K.R., Paul S.M., Bateman R.J., Holtzman D.M. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 2011;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kunkle B.W., Grenier-Boley B., Sims R., Bis J.C., Damotte V., Naj A.C., Boland A., Vronskaya M., van der Lee S.J., Amlie-Wolf A., Bellenguez C., Frizatti A., Chouraki V., Martin E.R., Sleegers K., Badarinarayan N., Jakobsdottir J., Hamilton-Nelson K.L., Moreno-Grau S., Olaso R., Raybould R., Chen Y., Kuzma A.B., Hiltunen M., Morgan T., Ahmad S., Vardarajan B.N., Epelbaum J., Hoffmann P., Boada M., Beecham G.W., Garnier J.G., Harold D., Fitzpatrick A.L., Valladares O., Moutet M.L., Gerrish A., Smith A.V., Qu L., Bacq D., Denning N., Jian X., Zhao Y., Del Zompo M., Fox N.C., Choi S.H., Mateo I., Hughes J.T., Adams H.H., Malamon J., Sanchez-Garcia F., Patel Y., Brody J.A., Dombroski B.A., Naranjo M.C.D., Daniilidou M., Eiriksdottir G., Mukherjee S., Wallon D., Uphill J., Aspelund T., Cantwell L.B., Garzia F., Galimberti D., Hofer E., Butkiewicz M., Fin B., Scarpini E., Sarnowski C., Bush W.S., Meslage S., Kornhuber J., White C.C., Song Y., Barber R.C., Engelborghs S., Sordon S., Voijnovic D., Adams P.M., Vandenberghe R., Mayhaus M., Cupples L.A., Albert M.S., De Deyn P.P., Gu W., Himali J.J., Beekly D., Squassina A., Hartmann A.M., Orellana A., Blacker D., Rodriguez-Rodriguez E., Lovestone S., Garcia M.E., Doody R.S., Munoz-Fernadez C., Sussams R., Lin H., Fairchild T.J., Benito Y.A., Holmes C., Karamujic-Comic H., Frosch M.P., Thonberg H., Maier W., Roshchupkin G., Ghetti B., Giedraitis V., Kawalia A., Li S., Huebinger R.M., Kilander L., Moebus S., Hernandez I., Kamboh M.I., Brundin R., Turton J., Yang Q., Katz M.J., Concari L., Lord J., Beiser A.S., Keene C.D., Helisalmi S., Kloszewska I., Kukull W.A., Koivisto A.M., Lynch A., Tarraga L., Larson E.B., Haapasalo A., Lawlor B., Mosley T.H., Lipton R.B., Solfrizzi V., Gill M., Longstreth W.T., Jr, Montine T.J., Frisardi V., Diez-Fairen M., Rivadeneira F., Petersen R.C., Deramecourt V., Alvarez I., Salani F., Ciaramella A., Boerwinkle E., Reiman E.M., Fievet N., Rotter J.I., Reisch J.S., Hanon O., Cupidi C., Andre Uitterlinden A.G., Royall D.R., Dufouil C., Maletta R.G., de Rojas I., Sano M., Brice A., Cecchetti R., George-Hyslop P.S., Ritchie K., Tsolaki M., Tsuang D.W., Dubois B., Craig D., Wu C.K., Soininen H., Avramidou D., Albin R.L., Fratiglioni L., Germanou A., Apostolova L.G., Keller L., Koutroumani M., Arnold S.E., Panza F., Gkatzima O., Asthana S., Hannequin D., Whitehead P., Atwood C.S., Caffarra P., Hampel H., Quintela I., Carracedo A., Lannfelt L., Rubinsztein D.C., Barnes L.L., Pasquier F., Frolich L., Barral S., McGuinness B., Beach T.G., Johnston J.A., Becker J.T., Passmore P., Bigio E.H., Schott J.M., Bird T.D., Warren J.D., Boeve B.F., Lupton M.K., Bowen J.D., Proitsi P., Boxer A., Powell J.F., Burke J.R., Kauwe J.S.K., Burns J.M., Mancuso M., Buxbaum J.D., Bonuccelli U., Cairns N.J., McQuillin A., Cao C., Livingston G., Carlson C.S., Bass N.J., Carlsson C.M., Hardy J., Carney R.M., Bras J., Carrasquillo M.M., Guerreiro R., Allen M., Chui H.C., Fisher E., Masullo C., Crocco E.A., DeCarli C., Bisceglio G., Dick M., Ma L., Duara R., Graff-Radford N.R., Evans D.A., Hodges A., Faber K.M., Scherer M., Fallon K.B., Riemenschneider M., Fardo D.W., Heun R., Farlow M.R., Kolsch H., Ferris S., Leber M., Foroud T.M., Heuser I., Galasko D.R., Giegling I., Gearing M., Hull M., Geschwind D.H., Gilbert J.R., Morris J., Green R.C., Mayo K., Growdon J.H., Feulner T., Hamilton R.L., Harrell L.E., Drichel D., Honig L.S., Cushion T.D., Huentelman M.J., Hollingworth P., Hulette C.M., Hyman B.T., Marshall R., Jarvik G.P., Meggy A., Abner E., Menzies G.E., Jin L.W., Leonenko G., Real L.M., Jun G.R., Baldwin C.T., Grozeva D., Karydas A., Russo G., Kaye J.A., Kim R., Jessen F., Kowall N.W., Vellas B., Kramer J.H., Vardy E., LaFerla F.M., Jockel K.H., Lah J.J., Dichgans M., Leverenz J.B., Mann D., Levey A.I., Pickering-Brown S., Lieberman A.P., Klopp N., Lunetta K.L., Wichmann H.E., Lyketsos C.G., Morgan K., Marson D.C., Brown K., Martiniuk F., Medway C., Mash D.C., Nothen M.M., Masliah E., Hooper N.M., McCormick W.C., Daniele A., McCurry S.M., Bayer A., McDavid A.N., Gallacher J., McKee A.C., van den Bussche H., Mesulam M., Brayne C., Miller B.L., Riedel-Heller S., Miller C.A., Miller J.W., Al-Chalabi A., Morris J.C., Shaw C.E., Myers A.J., Wiltfang J., O’Bryant S., Olichney J.M., Alvarez V., Parisi J.E., Singleton A.B., Paulson H.L., Collinge J., Perry W.R., Mead S., Peskind E., Cribbs D.H., Rossor M., Pierce A., Ryan N.S., Poon W.W., Nacmias B., Potter H., Sorbi S., Quinn J.F., Sacchinelli E., Raj A., Spalletta G., Raskind M., Caltagirone C., Bossu P., Orfei M.D., Reisberg B., Clarke R., Reitz C., Smith A.D., Ringman J.M., Warden D., Roberson E.D., Wilcock G., Rogaeva E., Bruni A.C., Rosen H.J., Gallo M., Rosenberg R.N., Ben-Shlomo Y., Sager M.A., Mecocci P., Saykin A.J., Pastor P., Cuccaro M.L., Vance J.M., Schneider J.A., Schneider L.S., Slifer S., Seeley W.W., Smith A.G., Sonnen J.A., Spina S., Stern R.A., Swerdlow R.H., Tang M., Tanzi R.E., Trojanowski J.Q., Troncoso J.C., Van Deerlin V.M., Van Eldik L.J., Vinters H.V., Vonsattel J.P., Weintraub S., Welsh-Bohmer K.A., Wilhelmsen K.C., Williamson J., Wingo T.S., Woltjer R.L., Wright C.B., Yu C.E., Yu L., Saba Y., Pilotto A., Bullido M.J., Peters O., Crane P.K., Bennett D., Bosco P., Coto E., Boccardi V., De Jager P.L., Lleo A., Warner N., Lopez O.L., Ingelsson M., Deloukas P., Cruchaga C., Graff C., Gwilliam R., Fornage M., Goate A.M., Sanchez-Juan P., Kehoe P.G., Amin N., Ertekin-Taner N., Berr C., Debette S., Love S., Launer L.J., Younkin S.G., Dartigues J.F., Corcoran C., Ikram M.A., Dickson D.W., Nicolas G., Campion D., Tschanz J., Schmidt H., Hakonarson H., Clarimon J., Munger R., Schmidt R., Farrer L.A., Van Broeckhoven C.M. C. O. D.; DeStefano, A. L.; Jones, L.; Haines, J. L.; Deleuze, J. F.; Owen, M. J.; Gudnason, V.; Mayeux, R.; Escott-Price, V.; Psaty, B. M.; Ramirez, A.; Wang, L. S.; Ruiz, A.; van Duijn, C. M.; Holmans, P. A.; Seshadri, S.; Williams, J.; Amouyel, P.; Schellenberg, G. D.; Lambert, J. C.; Pericak-Vance, M. A. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat. Genet. 2019;51(3):414–430. doi: 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reiman E.M., Arboleda-Velasquez J.F., Quiroz Y.T., Huentelman M.J., Beach T.G., Caselli R.J., Chen Y., Su Y., Myers A.J., Hardy J., Paul Vonsattel J., Younkin S.G., Bennett D.A., De Jager P.L., Larson E.B., Crane P.K., Keene C.D., Kamboh M.I., Kofler J.K., Duque L., Gilbert J.R., Gwirtsman H.E., Buxbaum J.D., Dickson D.W., Frosch M.P., Ghetti B.F., Lunetta K.L., Wang L.S., Hyman B.T., Kukull W.A., Foroud T., Haines J.L., Mayeux R.P., Pericak-Vance M.A., Schneider J.A., Trojanowski J.Q., Farrer L.A., Schellenberg G.D., Beecham G.W., Montine T.J., Jun G.R. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat. Commun. 2020;11(1):667. doi: 10.1038/s41467-019-14279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Therriault J., Benedet A.L., Pascoal T.A., Mathotaarachchi S., Savard M., Chamoun M., Thomas E., Kang M.S., Lussier F., Tissot C., Soucy J.P., Massarweh G., Rej S., Saha-Chaudhuri P., Poirier J., Gauthier S., Rosa-Neto P. APOEε4 potentiates the relationship between amyloid-β and tau pathologies. Mol. Psychiatry. 2020;••• doi: 10.1038/s41380-020-0688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin Y.T., Seo J., Gao F., Feldman H.M., Wen H.L., Penney J., Cam H.P., Gjoneska E., Raja W.K., Cheng J., Rueda R., Kritskiy O., Abdurrob F., Peng Z., Milo B., Yu C.J., Elmsaouri S., Dey D., Ko T., Yankner B.A., Tsai L.H. APOE4 causes widespread molecular and cellular alterations associated with alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron. 2018;98(6):1141–1154.e7. doi: 10.1016/j.neuron.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yin Y.W., Li J.C., Wang J.Z., Li B.H., Pi Y., Yang Q.W., Fang C.Q., Gao C.Y., Zhang L.L. Association between apolipoprotein E gene polymorphism and the risk of vascular dementia: a meta-analysis. Neurosci. Lett. 2012;514(1):6–11. doi: 10.1016/j.neulet.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 90.Parcon P.A., Balasubramaniam M., Ayyadevara S., Jones R.A., Liu L., Shmookler Reis R.J., Barger S.W., Mrak R.E., Griffin W.S.T. Apolipoprotein E4 inhibits autophagy gene products through direct, specific binding to clear motifs. Alzheimers Dement. 2018;14(2):230–242. doi: 10.1016/j.jalz.2017.07.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grubman A., Chew G., Ouyang J.F., Sun G., Choo X.Y., McLean C., Simmons R.K., Buckberry S., Vargas-Landin D.B., Poppe D., Pflueger J., Lister R., Rackham O.J.L., Petretto E., Polo J.M. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 2019;22(12):2087–2097. doi: 10.1038/s41593-019-0539-4. [DOI] [PubMed] [Google Scholar]
- 92.Romito-DiGiacomo R.R., Menegay H., Cicero S.A., Herrup K. Effects of Alzheimer’s disease on different cortical layers: the role of intrinsic differences in Abeta susceptibility. J. Neurosci. 2007;27(32):8496–8504. doi: 10.1523/JNEUROSCI.1008-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prokopenko D., Hecker J., Kirchner R., Chapman B.A., Hoffman O., Mullin K., Hide W., Bertram L., Laird N., DeMeo D.L., Lange C., Tanzi R.E. Identification of Novel Alzheimer’s disease loci using sex-specific family-based association analysis of whole-genome sequence data. Sci. Rep. 2020;10(1):5029. doi: 10.1038/s41598-020-61883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Podcasy J.L., Epperson C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016;18(4):437–446. doi: 10.31887/DCNS.2016.18.4/cepperson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arnold M., Nho K., Kueider-Paisley A., Massaro T., Huynh K., Brauner B. MahmoudianDehkordi, S.; Louie, G.; Moseley, M.A.; Thompson, J.W.; John-Williams, L.S.; Tenenbaum, J.D.; Blach, C.; Chang, R.; Brinton, R.D.; Baillie, R.; Han, X.; Trojanowski, J.Q.; Shaw, L.M.; Martins, R.; Weiner, M.W.; Trushina, E.; Toledo, J.B.; Meikle, P.J.; Bennett, D.A.; Krumsiek, J.; Doraiswamy, P.M.; Saykin, A.J.; Kaddurah-Daouk, R.; Kastenmüller, G. Sex and APOE ε4 genotype modify the Alzheimer’s disease serum metabolome. Nat. Commun. 2020;11(1):1148. doi: 10.1038/s41467-020-14959-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elsheikh S.S.M., Chimusa E.R., Mulder N.J., Crimi A. Genome-wide association study of brain connectivity changes for Alzheimer’s Disease. Sci. Rep. 2020;10(1):1433. doi: 10.1038/s41598-020-58291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nelson A.R., Sagare A.P., Zlokovic B.V. Role of clusterin in the brain vascular clearance of amyloid-β. Proc. Natl. Acad. Sci. USA. 2017;114(33):8681–8682. doi: 10.1073/pnas.1711357114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han Z., Qu J., Zhao J., Zou X. Analyzing 74,248 samples confirms the association between CLU rs11136000 polymorphism and Alzheimer’s Disease in caucasian but not chinese population. Sci. Rep. 2018;8(1):11062. doi: 10.1038/s41598-018-29450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guerreiro R.J., Beck J., Gibbs J.R., Santana I., Rossor M.N., Schott J.M., Nalls M.A., Ribeiro H., Santiago B., Fox N.C., Oliveira C., Collinge J., Mead S., Singleton A., Hardy J. Genetic variability in CLU and its association with Alzheimer’s disease. PLoS One. 2010;5(3):e9510. doi: 10.1371/journal.pone.0009510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harold D., Abraham R., Hollingworth P., Sims R., Gerrish A., Hamshere M.L., Pahwa J.S., Moskvina V., Dowzell K., Williams A., Jones N., Thomas C., Stretton A., Morgan A.R., Lovestone S., Powell J., Proitsi P., Lupton M.K., Brayne C., Rubinsztein D.C., Gill M., Lawlor B., Lynch A., Morgan K., Brown K.S., Passmore P.A., Craig D., McGuinness B., Todd S., Holmes C., Mann D., Smith A.D., Love S., Kehoe P.G., Hardy J., Mead S., Fox N., Rossor M., Collinge J., Maier W., Jessen F., Schürmann B., Heun R., van den Bussche H., Heuser I., Kornhuber J., Wiltfang J., Dichgans M., Frölich L., Hampel H., Hüll M., Rujescu D., Goate A.M., Kauwe J.S., Cruchaga C., Nowotny P., Morris J.C., Mayo K., Sleegers K., Bettens K., Engelborghs S., De Deyn P.P., Van Broeckhoven C., Livingston G., Bass N.J., Gurling H., McQuillin A., Gwilliam R., Deloukas P., Al-Chalabi A., Shaw C.E., Tsolaki M., Singleton A.B., Guerreiro R., Mühleisen T.W., Nöthen M.M., Moebus S., Jöckel K.H., Klopp N., Wichmann H.E., Carrasquillo M.M., Pankratz V.S., Younkin S.G., Holmans P.A., O’Donovan M., Owen M.J., Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009;41(10):1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lambert J.C., Heath S., Even G., Campion D., Sleegers K., Hiltunen M., Combarros O., Zelenika D., Bullido M.J., Tavernier B., Letenneur L., Bettens K., Berr C., Pasquier F., Fiévet N., Barberger-Gateau P., Engelborghs S., De Deyn P., Mateo I., Franck A., Helisalmi S., Porcellini E., Hanon O., de Pancorbo M.M., Lendon C., Dufouil C., Jaillard C., Leveillard T., Alvarez V., Bosco P., Mancuso M., Panza F., Nacmias B., Bossù P., Piccardi P., Annoni G., Seripa D., Galimberti D., Hannequin D., Licastro F., Soininen H., Ritchie K., Blanché H., Dartigues J.F., Tzourio C., Gut I., Van Broeckhoven C., Alpérovitch A., Lathrop M., Amouyel P. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Genet. 2009;41(10):1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 102.Liu G., Wang H., Liu J., Li J., Li H., Ma G., Jiang Y., Chen Z., Zhao B., Li K. The CLU gene rs11136000 variant is significantly associated with Alzheimer’s disease in Caucasian and Asian populations. Neuromolecular Med. 2014;16(1):52–60. doi: 10.1007/s12017-013-8250-1. [DOI] [PubMed] [Google Scholar]
- 103.Foster E.M., Dangla-Valls A., Lovestone S., Ribe E.M., Buckley N.J. Clusterin in Alzheimer’s disease: Mechanisms, genetics, and lessons from other pathologies. Front. Neurosci. 2019;13:164. doi: 10.3389/fnins.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Almeida J.F.F., Dos Santos L.R., Trancozo M., de Paula F. Updated Meta-Analysis of BIN1, CR1, MS4A6A, CLU, and ABCA7 variants in Alzheimer’s Disease. J. Mol. Neurosci. 2018;64(3):471–477. doi: 10.1007/s12031-018-1045-y. [DOI] [PubMed] [Google Scholar]
- 105.Shankarappa B.M., Kota L.N., Purushottam M., Nagpal K., Mukherjee O., Viswanath B., Varghese M., Bharath S., Jain S. Effect of CLU and PICALM polymorphisms on AD risk: A study from south India. Asian J. Psychiatr. 2017;27:7–11. doi: 10.1016/j.ajp.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 106.Raj T., Li Y.I., Wong G., Humphrey J., Wang M., Ramdhani S., Wang Y.C., Ng B., Gupta I., Haroutunian V., Schadt E.E., Young-Pearse T., Mostafavi S., Zhang B., Sklar P., Bennett D.A., De Jager P.L. Integrative transcriptome analyses of the aging brain implicate altered splicing in Alzheimer’s disease susceptibility. Nat. Genet. 2018;50(11):1584–1592. doi: 10.1038/s41588-018-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Padhy B., Hayat B., Nanda G.G., Mohanty P.P., Alone D.P. Pseudoexfoliation and Alzheimer’s associated CLU risk variant, rs2279590, lies within an enhancer element and regulates CLU, EPHX2 and PTK2B gene expression. Hum. Mol. Genet. 2017;26(22):4519–4529. doi: 10.1093/hmg/ddx329. [DOI] [PubMed] [Google Scholar]
- 108.Seripa D., Panza F., Paroni G., D’Onofrio G., Bisceglia P., Gravina C., Urbano M., Lozupone M., Solfrizzi V., Bizzarro A., Boccardi V., Piccininni C., Daniele A., Logroscino G., Mecocci P., Masullo C., Greco A. Role of CLU, PICALM, and TNK1 genotypes in aging with and without Alzheimer’s Disease. Mol. Neurobiol. 2018;55(5):4333–4344. doi: 10.1007/s12035-017-0547-x. [DOI] [PubMed] [Google Scholar]
- 109.Vivot A., Glymour M.M., Tzourio C., Amouyel P., Chêne G., Dufouil C. Association of Alzheimer’s related genotypes with cognitive decline in multiple domains: results from the Three-City Dijon study. Mol. Psychiatry. 2015;20(10):1173–1178. doi: 10.1038/mp.2015.62. [DOI] [PubMed] [Google Scholar]
- 110.Steinberg S., Stefansson H., Jonsson T., Johannsdottir H., Ingason A., Helgason H., Sulem P., Magnusson O.T., Gudjonsson S.A., Unnsteinsdottir U., Kong A., Helisalmi S., Soininen H., Lah J.J., Aarsland D., Fladby T., Ulstein I.D., Djurovic S., Sando S.B., White L.R., Knudsen G.P., Westlye L.T., Selbæk G., Giegling I., Hampel H., Hiltunen M., Levey A.I., Andreassen O.A., Rujescu D., Jonsson P.V., Bjornsson S., Snaedal J., Stefansson K. Loss-of-function variants in ABCA7 confer risk of Alzheimer’s disease. Nat. Genet. 2015;47(5):445–447. doi: 10.1038/ng.3246. [DOI] [PubMed] [Google Scholar]
- 111.Vacher M., Porter T., Villemagne V.L., Milicic L., Peretti M., Fowler C., Martins R., Rainey-Smith S., Ames D., Masters C.L., Rowe C.C., Doecke J.D., Laws S.M. Validation of a priori candidate Alzheimer’s disease SNPs with brain amyloid-beta deposition. Sci. Rep. 2019;9(1):17069. doi: 10.1038/s41598-019-53604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hollingworth P., Harold D., Sims R., Gerrish A., Lambert J.C., Carrasquillo M.M., Abraham R., Hamshere M.L., Pahwa J.S., Moskvina V., Dowzell K., Jones N., Stretton A., Thomas C., Richards A., Ivanov D., Widdowson C., Chapman J., Lovestone S., Powell J., Proitsi P., Lupton M.K., Brayne C., Rubinsztein D.C., Gill M., Lawlor B., Lynch A., Brown K.S., Passmore P.A., Craig D., McGuinness B., Todd S., Holmes C., Mann D., Smith A.D., Beaumont H., Warden D., Wilcock G., Love S., Kehoe P.G., Hooper N.M., Vardy E.R., Hardy J., Mead S., Fox N.C., Rossor M., Collinge J., Maier W., Jessen F., Rüther E., Schürmann B., Heun R., Kölsch H., van den Bussche H., Heuser I., Kornhuber J., Wiltfang J., Dichgans M., Frölich L., Hampel H., Gallacher J., Hüll M., Rujescu D., Giegling I., Goate A.M., Kauwe J.S., Cruchaga C., Nowotny P., Morris J.C., Mayo K., Sleegers K., Bettens K., Engelborghs S., De Deyn P.P., Van Broeckhoven C., Livingston G., Bass N.J., Gurling H., McQuillin A., Gwilliam R., Deloukas P., Al-Chalabi A., Shaw C.E., Tsolaki M., Singleton A.B., Guerreiro R., Mühleisen T.W., Nöthen M.M., Moebus S., Jöckel K.H., Klopp N., Wichmann H.E., Pankratz V.S., Sando S.B., Aasly J.O., Barcikowska M., Wszolek Z.K., Dickson D.W., Graff-Radford N.R., Petersen R.C., van Duijn C.M., Breteler M.M., Ikram M.A., DeStefano A.L., Fitzpatrick A.L., Lopez O., Launer L.J., Seshadri S., Berr C., Campion D., Epelbaum J., Dartigues J.F., Tzourio C., Alpérovitch A., Lathrop M., Feulner T.M., Friedrich P., Riehle C., Krawczak M., Schreiber S., Mayhaus M., Nicolhaus S., Wagenpfeil S., Steinberg S., Stefansson H., Stefansson K., Snaedal J., Björnsson S., Jonsson P.V., Chouraki V., Genier-Boley B., Hiltunen M., Soininen H., Combarros O., Zelenika D., Delepine M., Bullido M.J., Pasquier F., Mateo I., Frank-Garcia A., Porcellini E., Hanon O., Coto E., Alvarez V., Bosco P., Siciliano G., Mancuso M., Panza F., Solfrizzi V., Nacmias B., Sorbi S., Bossù P., Piccardi P., Arosio B., Annoni G., Seripa D., Pilotto A., Scarpini E., Galimberti D., Brice A., Hannequin D., Licastro F., Jones L., Holmans P.A., Jonsson T., Riemenschneider M., Morgan K., Younkin S.G., Owen M.J., O’Donovan M., Amouyel P., Williams J. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat. Genet. 2011;43(5):429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fu Y., Hsiao J.H., Paxinos G., Halliday G.M., Kim W.S. ABCA7 Mediates Phagocytic clearance of Amyloid-β in the brain. J. Alzheimers Dis. 2016;54(2):569–584. doi: 10.3233/JAD-160456. [DOI] [PubMed] [Google Scholar]
- 114.Zhao Z., Sagare A.P., Ma Q., Halliday M.R., Kong P., Kisler K., Winkler E.A., Ramanathan A., Kanekiyo T., Bu G., Owens N.C., Rege S.V., Si G., Ahuja A., Zhu D., Miller C.A., Schneider J.A., Maeda M., Maeda T., Sugawara T., Ichida J.K., Zlokovic B.V. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat. Neurosci. 2015;18(7):978–987. doi: 10.1038/nn.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Masri I., Salami A., El Shamieh S., Bissar-Tadmouri N. 2019. [DOI] [PubMed]
- 116.Xu W., Wang H.F., Tan L., Tan M.S., Tan C.C., Zhu X.C., Miao D., Yu W.J., Jiang T., Tan L., Yu J.T. The impact of PICALM genetic variations on reserve capacity of posterior cingulate in AD continuum. Sci. Rep. 2016;6:24480. doi: 10.1038/srep24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brouwers N., Van Cauwenberghe C., Engelborghs S., Lambert J.C., Bettens K., Le Bastard N., Pasquier F., Montoya A.G., Peeters K., Mattheijssens M., Vandenberghe R., Deyn P.P., Cruts M., Amouyel P., Sleegers K., Van Broeckhoven C. Alzheimer risk associated with a copy number variation in the complement receptor 1 increasing C3b/C4b binding sites. Mol. Psychiatry. 2012;17(2):223–233. doi: 10.1038/mp.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Franzmeier N., Rubinski A., Neitzel J., Ewers M. The BIN1 rs744373 SNP is associated with increased tau-PET levels and impaired memory. Nat. Commun. 2019;10(1):1766. doi: 10.1038/s41467-019-09564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deming Y., Filipello F., Cignarella F., Cantoni C., Hsu S., Mikesell R., Li Z., Del-Aguila J.L., Dube U., Farias F.G., Bradley J., Budde J., Ibanez L., Fernandez M.V., Blennow K., Zetterberg H., Heslegrave A., Johansson P.M., Svensson J., Nellgård B., Lleo A., Alcolea D., Clarimon J., Rami L., Molinuevo J.L., Suárez-Calvet M., Morenas-Rodríguez E., Kleinberger G., Ewers M., Harari O., Haass C., Brett T.J., Benitez B.A., Karch C.M., Piccio L., Cruchaga C. The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer’s disease risk. Sci. Transl. Med. 2019;11(505):eaau2291. doi: 10.1126/scitranslmed.aau2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kleinberger G., Yamanishi Y., Suárez-Calvet M., Czirr E., Lohmann E., Cuyvers E., Struyfs H., Pettkus N., Wenninger-Weinzierl A., Mazaheri F., Tahirovic S., Lleó A., Alcolea D., Fortea J., Willem M., Lammich S., Molinuevo J.L., Sánchez-Valle R., Antonell A., Ramirez A., Heneka M.T., Sleegers K., van der Zee J., Martin J.J., Engelborghs S., Demirtas-Tatlidede A., Zetterberg H., Van Broeckhoven C., Gurvit H., Wyss-Coray T., Hardy J., Colonna M., Haass C. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 2014;6(243):243ra86. doi: 10.1126/scitranslmed.3009093. [DOI] [PubMed] [Google Scholar]
- 121.Narayan P., Dragunow M. Alzheimer’s Disease and histone code alterations. Adv. Exp. Med. Biol. 2017;978:321–336. doi: 10.1007/978-3-319-53889-1_17. [DOI] [PubMed] [Google Scholar]
- 122.Marzi S.J., Leung S.K., Ribarska T., Hannon E., Smith A.R., Pishva E., Poschmann J., Moore K., Troakes C., Al-Sarraj S., Beck S., Newman S., Lunnon K., Schalkwyk L.C., Mill J. A histone acetylome-wide association study of Alzheimer’s disease identifies disease-associated H3K27ac differences in the entorhinal cortex. Nat. Neurosci. 2018;21(11):1618–1627. doi: 10.1038/s41593-018-0253-7. [DOI] [PubMed] [Google Scholar]
- 123.Bennett D.A., Yu L., Yang J., Srivastava G.P., Aubin C., De Jager P.L. Epigenomics of Alzheimer’s disease. Transl. Res. 2015;165(1):200–220. doi: 10.1016/j.trsl.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fetahu I.S., Ma D., Rabidou K., Argueta C., Smith M., Liu H., Wu F., Shi Y.G. Epigenetic signatures of methylated DNA cytosine in Alzheimer’s disease. Sci. Adv. 2019;5(8):eaaw2880. doi: 10.1126/sciadv.aaw2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu X.S., Jaenisch R. Editing the Epigenome to tackle brain disorders. Trends Neurosci. 2019;42(12):861–870. doi: 10.1016/j.tins.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 126.Frieden C., Wang H., Ho C.M.W. A mechanism for lipid binding to apoE and the role of intrinsically disordered regions coupled to domain-domain interactions. Proc. Natl. Acad. Sci. USA. 2017;114(24):6292–6297. doi: 10.1073/pnas.1705080114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Theendakara V., Peters-Libeu C.A., Bredesen D.E., Rao R.V. Transcriptional effects of apoe4: relevance to Alzheimer’s disease. Mol. Neurobiol. 2018;55(6):5243–5254. doi: 10.1007/s12035-017-0757-2. [DOI] [PubMed] [Google Scholar]
- 128.Main B.S., Villapol S., Sloley S.S., Barton D.J., Parsadanian M., Agbaegbu C., Stefos K., McCann M.S., Washington P.M., Rodriguez O.C., Burns M.P. Apolipoprotein E4 impairs spontaneous blood brain barrier repair following traumatic brain injury. Mol. Neurodegener. 2018;13(1):17. doi: 10.1186/s13024-018-0249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Halliday M.R., Rege S.V., Ma Q., Zhao Z., Miller C.A., Winkler E.A., Zlokovic B.V. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2016;36(1):216–227. doi: 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bell R.D., Winkler E.A., Singh I., Sagare A.P., Deane R., Wu Z., Holtzman D.M., Betsholtz C., Armulik A., Sallstrom J., Berk B.C., Zlokovic B.V. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485(7399):512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Altenburg M., Johnson L., Wilder J., Maeda N. Apolipoprotein E4 in macrophages enhances atherogenesis in a low density lipoprotein receptor-dependent manner. J. Biol. Chem. 2007;282(11):7817–7824. doi: 10.1074/jbc.M610712200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brown C.M., Choi E., Xu Q., Vitek M.P., Colton C.A. The APOE4 genotype alters the response of microglia and macrophages to 17beta-estradiol. Neurobiol. Aging. 2008;29(12):1783–1794. doi: 10.1016/j.neurobiolaging.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vitek M.P., Brown C.M., Colton C.A. APOE genotype-specific differences in the innate immune response. Neurobiol. Aging. 2009;30(9):1350–1360. doi: 10.1016/j.neurobiolaging.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tudorache I.F., Trusca V.G., Gafencu A.V., Apolipoprotein E., Apolipoprotein E. A Multifunctional protein with implications in various pathologies as a result of its structural features. Comput. Struct. Biotechnol. J. 2017;15:359–365. doi: 10.1016/j.csbj.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mahley R.W., Rall S.C.J. 2001.
- 136.Chetty P.S., Mayne L., Lund-Katz S., Englander S.W., Phillips M.C. Helical structure, stability, and dynamics in human apolipoprotein E3 and E4 by hydrogen exchange and mass spectrometry. Proc. Natl. Acad. Sci. USA. 2017;114(5):968–973. doi: 10.1073/pnas.1617523114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhao N., Attrebi O.N., Ren Y., Qiao W., Sonustun B., Martens Y.A., Meneses A.D., Li F., Shue F., Zheng J., Van Ingelgom A.J., Davis M.D., Kurti A., Knight J.A., Linares C., Chen Y., Delenclos M., Liu C.C., Fryer J.D., Asmann Y.W., McLean P.J., Dickson D.W., Ross O.A., Bu G. APOE4 exacerbates α-synuclein pathology and related toxicity independent of amyloid. 2020. [DOI] [PMC free article] [PubMed]
- 138.Sevenich L. Brain-Resident microglia and blood-borne macrophages orchestrate central nervous system inflammation in neurodegenerative disorders and brain cancer. Front. Immunol. 2018;9:697. doi: 10.3389/fimmu.2018.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Labzin L.I., Heneka M.T., Latz E. Innate immunity and neurodegeneration. Annu. Rev. Med. 2018;69:437–449. doi: 10.1146/annurev-med-050715-104343. [DOI] [PubMed] [Google Scholar]
- 140.Hernangómez M., Carrillo-Salinas F.J., Mecha M., Correa F., Mestre L., Loría F., Feliú A., Docagne F., Guaza C. Brain innate immunity in the regulation of neuroinflammation: therapeutic strategies by modulating CD200-CD200R interaction involve the cannabinoid system. Curr. Pharm. Des. 2014;20(29):4707–4722. doi: 10.2174/1381612820666140130202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Heneka M.T., Kummer M.P., Latz E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014;14(7):463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- 142.Gong T., Liu L., Jiang W., Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020;20(2):95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 143.Amarante-Mendes G.P., Adjemian S., Branco L.M., Zanetti L.C., Weinlich R., Bortoluci K.R. Pattern recognition receptors and the host cell death molecular machinery. Front. Immunol. 2018;9:2379. doi: 10.3389/fimmu.2018.02379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kinney J.W., Bemiller S.M., Murtishaw A.S., Leisgang A.M., Salazar A.M., Lamb B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. (N. Y.) 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stewart C.R., Stuart L.M., Wilkinson K., van Gils J.M., Deng J., Halle A., Rayner K.J., Boyer L., Zhong R., Frazier W.A., Lacy-Hulbert A., El Khoury J., Golenbock D.T., Moore K.J. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010;11(2):155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sheedy F.J., Grebe A., Rayner K.J., Kalantari P., Ramkhelawon B., Carpenter S.B., Becker C.E., Ediriweera H.N., Mullick A.E., Golenbock D.T., Stuart L.M., Latz E., Fitzgerald K.A., Moore K.J. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013;14(8):812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jiang C., Li G., Huang P., Liu Z., Zhao B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimers Dis. 2017;58(1):1–15. doi: 10.3233/JAD-161141. [DOI] [PubMed] [Google Scholar]
- 148.Ashraf G.M., Tarasov V.V., Makhmutova A., Chubarev V.N., Avila-Rodriguez M., Bachurin S.O., Aliev G. The possibility of an infectious etiology of Alzheimer Disease. Mol. Neurobiol. 2019;56(6):4479–4491. doi: 10.1007/s12035-018-1388-y. [DOI] [PubMed] [Google Scholar]
- 149.Whittington R.A., Planel E., Terrando N. Impaired resolution of inflammation in Alzheimer’s Disease: A Review. Front. Immunol. 2017;8:1464. doi: 10.3389/fimmu.2017.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heneka M.T., Kummer M.P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., Griep A., Axt D., Remus A., Tzeng T.C., Gelpi E., Halle A., Korte M., Latz E., Golenbock D.T. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Karasawa T., Takahashi M. Role of NLRP3 Inflammasomes in atherosclerosis. J. Atheroscler. Thromb. 2017;24(5):443–451. doi: 10.5551/jat.RV17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jefferson A.L., Massaro J.M., Wolf P.A., Seshadri S., Au R., Vasan R.S., Larson M.G., Meigs J.B., Keaney J.F., Jr, Lipinska I., Kathiresan S., Benjamin E.J., DeCarli C. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology. 2007;68(13):1032–1038. doi: 10.1212/01.wnl.0000257815.20548.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Perry G. Alzheimer’s disease: Advances for a New Century. Vol. 3. Amsterdam: IOS Press; 2013. p. 670. [Google Scholar]
- 155.Shaftel S.S., Griffin W.S., O’Banion M.K. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J. Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I.R., McGeer P.L., O’Banion M.K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F.L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21(3):383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Elliott E.I., Sutterwala F.S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 2015;265(1):35–52. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zigmond M.J., Rowland L.P., Coyle J.T. Neurobiology of brain disorders: biological basis of neurological and psychiatric disorders. Amsterdam: Elsevier AP; 2015. [Google Scholar]
- 159.Venegas C., Heneka M.T. Inflammasome-mediated innate immunity in Alzheimer’s disease. FASEB J. 2019;33(12):13075–13084. doi: 10.1096/fj.201900439. [DOI] [PubMed] [Google Scholar]
- 160.Tejera D., Mercan D., Sanchez-Caro J.M., Hanan M., Greenberg D., Soreq H., Latz E., Golenbock D., Heneka M.T. Systemic inflammation impairs microglial Aβ clearance through NLRP3 inflammasome. EMBO J. 2019;38(17):e101064. doi: 10.15252/embj.2018101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Libby P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006;83(2):456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 162.Siti H.N., Kamisah Y., Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul. Pharmacol. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 163.Singh-Manoux A., Shipley M.J., Bell J.A., Canonico M., Elbaz A., Kivimäki M. Association between inflammatory biomarkers and all-cause, cardiovascular and cancer-related mortality. CMAJ. 2017;189(10):E384–E390. doi: 10.1503/cmaj.160313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yaffe K., Kanaya A., Lindquist K., Simonsick E.M., Harris T., Shorr R.I., Tylavsky F.A., Newman A.B. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292(18):2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 165.van Dijk E.J., Prins N.D., Vermeer S.E., Vrooman H.A., Hofman A., Koudstaal P.J., Breteler M.M. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation. 2005;112(6):900–905. doi: 10.1161/CIRCULATIONAHA.104.506337. [DOI] [PubMed] [Google Scholar]
- 166.Chen A., Oakley A.E., Monteiro M., Tuomela K., Allan L.M., Mukaetova-Ladinska E.B., O’Brien J.T., Kalaria R.N. Multiplex analyte assays to characterize different dementias: brain inflammatory cytokines in poststroke and other dementias. Neurobiol. Aging. 2016;38:56–67. doi: 10.1016/j.neurobiolaging.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pirault J., Bäck M. Lipoxin and resolvin receptors transducing the resolution of inflammation in cardiovascular disease. Front. Pharmacol. 2018;9:1273. doi: 10.3389/fphar.2018.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fraga V.G., Carvalho M.D.G., Caramelli P., de Sousa L.P., Gomes K.B. Resolution of inflammation, n-3 fatty acid supplementation and Alzheimer disease: A narrative review. J. Neuroimmunol. 2017;310:111–119. doi: 10.1016/j.jneuroim.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 169.Zhong X., Guo L., Zhang L., Li Y., He R., Cheng G. Inflammatory potential of diet and risk of cardiovascular disease or mortality: A meta-analysis. Sci. Rep. 2017;7(1):6367. doi: 10.1038/s41598-017-06455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Szczechowiak K., Diniz B.S., Leszek J. Diet and Alzheimer’s dementia - Nutritional approach to modulate inflammation. Pharmacol. Biochem. Behav. 2019;184:172743. doi: 10.1016/j.pbb.2019.172743. [DOI] [PubMed] [Google Scholar]
- 171.de J R De-Paula V.; Forlenza, A.S.; Forlenza, O.V. Relevance of gutmicrobiota in cognition, behaviour and Alzheimer’s disease. Pharmacol. Res. 2018;136:29–34. doi: 10.1016/j.phrs.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 172.Shen H., Guan Q., Zhang X., Yuan C., Tan Z., Zhai L., Hao Y., Gu Y., Han C. New mechanism of neuroinflammation in Alzheimer’s disease: The activation of NLRP3 inflammasome mediated by gut microbiota. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2020;100:109884. doi: 10.1016/j.pnpbp.2020.109884. [DOI] [PubMed] [Google Scholar]
- 173.Li Z., Zhu H., Zhang L., Qin C. The intestinal microbiome and Alzheimer’s disease: A review. Animal Model Exp Med. 2018;1(3):180–188. doi: 10.1002/ame2.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ott S.J., El Mokhtari N.E., Musfeldt M., Hellmig S., Freitag S., Rehman A., Kühbacher T., Nikolaus S., Namsolleck P., Blaut M., Hampe J., Sahly H., Reinecke A., Haake N., Günther R., Krüger D., Lins M., Herrmann G., Fölsch U.R., Simon R., Schreiber S. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation. 2006;113(7):929–937. doi: 10.1161/CIRCULATIONAHA.105.579979. [DOI] [PubMed] [Google Scholar]
- 175.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. Links between atherosclerotic and periodontal disease. Exp. Mol. Pathol. 2016;100(1):220–235. doi: 10.1016/j.yexmp.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 176.Koren O., Spor A., Felin J., Fåk F., Stombaugh J., Tremaroli V., Behre C.J., Knight R., Fagerberg B., Ley R.E., Bäckhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA. 2011;108(Suppl. 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Allayee H., Hazen S.L. Contribution of gut bacteria to lipid Levels: another metabolic role for microbes? Circ. Res. 2015;117(9):750–754. doi: 10.1161/CIRCRESAHA.115.307409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yu Y., Raka F., Adeli K. The role of the gut microbiota in lipid and lipoprotein metabolism. J. Clin. Med. 2019;8(12):E2227. doi: 10.3390/jcm8122227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Galkina E., Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tang W.H., Kitai T., Hazen S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017;120(7):1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kowalski K., Mulak A. Brain-gut-microbiota axis in alzheimer’s disease. J. Neurogastroenterol. Motil. 2019;25(1):48–60. doi: 10.5056/jnm18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cerovic M., Forloni G., Balducci C. Neuroinflammation and the Gut microbiota: possible alternative therapeutic targets to counteract Alzheimer’s Disease? Front. Aging Neurosci. 2019;11:284. doi: 10.3389/fnagi.2019.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Giau V.V., Wu S.Y., Jamerlan A., An S.S.A., Kim S.Y., Hulme J. Gut microbiota and their neuroinflammatory implications in Alzheimer’s Disease. Nutrients. 2018;10(11):E1765. doi: 10.3390/nu10111765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lukiw W.J. Gastrointestinal (GI) Tract microbiome-derived neurotoxins-potent neuro-inflammatory signals from the gi tract via the systemic circulation into the brain. Front. Cell. Infect. Microbiol. 2020;10:22. doi: 10.3389/fcimb.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Luca M., Di Mauro M., Di Mauro M., Luca A. Gut microbiota in alzheimer’s disease, depression, and type 2 diabetes mellitus: the role of oxidative stress. Oxid. Med. Cell. Longev. 2019;2019:4730539. doi: 10.1155/2019/4730539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ide M., Harris M., Stevens A., Sussams R., Hopkins V., Culliford D., Fuller J., Ibbett P., Raybould R., Thomas R., Puenter U., Teeling J., Perry V.H., Holmes C. Periodontitis and cognitive decline in Alzheimer’s Disease. PLoS One. 2016;11(3):e0151081. doi: 10.1371/journal.pone.0151081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Aguayo S., Schuh C.M.A.P., Vicente B., Aguayo L.G. Association between Alzheimer’s Disease and oral and gut microbiota: are pore forming proteins the missing link? J. Alzheimers Dis. 2018;65(1):29–46. doi: 10.3233/JAD-180319. [DOI] [PubMed] [Google Scholar]
- 188.Singhrao S.K., Olsen I. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J. Oral Microbiol. 2019;11(1):1563405. doi: 10.1080/20002297.2018.1563405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pastore A., Raimondi F., Rajendran L., Temussi P.A. Why does the Aβ peptide of Alzheimer share structural similarity with antimicrobial peptides? Commun. Biol. 2020;3(1):135. doi: 10.1038/s42003-020-0865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gosztyla M.L., Brothers H.M., Robinson S.R. Alzheimer’s amyloid-β is an antimicrobial peptide: a review of the evidence. J. Alzheimers Dis. 2018;62(4):1495–1506. doi: 10.3233/JAD-171133. [DOI] [PubMed] [Google Scholar]
- 191.Leblhuber F., Huemer J., Steiner K., Gostner J.M., Fuchs D. Knock-on effect of periodontitis to the pathogenesis of Alzheimer’s disease? Wien. Klin. Wochenschr. 2020;132(17-18):493–498. doi: 10.1007/s00508-020-01638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Itzhaki R.F., Golde T.E., Heneka M.T., Readhead B. Do infections have a role in the pathogenesis of Alzheimer disease? Nat. Rev. Neurol. 2020;16(4):193–197. doi: 10.1038/s41582-020-0323-9. [DOI] [PubMed] [Google Scholar]
- 193.Giridharan V.V., Masud F., Petronilho F., Dal-Pizzol F., Barichello T. Infection-induced systemic inflammation is a potential driver of Alzheimer’s disease progression. Front. Aging Neurosci. 2019;11:122. doi: 10.3389/fnagi.2019.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ghaisas S., Maher J., Kanthasamy A. Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 2016;158:52–62. doi: 10.1016/j.pharmthera.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Miklossy J. Bacterial amyloid and DNA are important constituents of senile plaques: further evidence of the spirochetal and biofilm nature of senile plaques. J. Alzheimers Dis. 2016;53(4):1459–1473. doi: 10.3233/JAD-160451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Swerdlow R.H. Mitochondria and mitochondrial cascades in Alzheimer’s Disease. J. Alzheimers Dis. 2018;62(3):1403–1416. doi: 10.3233/JAD-170585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhu X., Smith M.A., Perry G., Aliev G. Mitochondrial failures in Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 2004;19(6):345–352. doi: 10.1177/153331750401900611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Santos R.X., Correia S.C., Zhu X., Lee H.G., Petersen R.B., Nunomura A., Smith M.A., Perry G., Moreira P.I. Nuclear and mitochondrial DNA oxidation in Alzheimer’s disease. Free Radic. Res. 2012;46(4):565–576. doi: 10.3109/10715762.2011.648188. [DOI] [PubMed] [Google Scholar]
- 199.Phillips N.R., Simpkins J.W., Roby R.K. Mitochondrial DNA deletions in Alzheimer’s brains: a review. Alzheimers Dement. 2014;10(3):393–400. doi: 10.1016/j.jalz.2013.04.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Aliev G., Seyidova D., Neal M.L., Shi J., Lamb B.T., Siedlak S.L., Vinters H.V., Head E., Perry G., Lamanna J.C., Friedland R.P., Cotman C.W. Atherosclerotic lesions and mitochondria DNA deletions in brain microvessels as a central target for the development of human AD and AD-like pathology in aged transgenic mice. Ann. N. Y. Acad. Sci. 2002;977:45–64. doi: 10.1111/j.1749-6632.2002.tb04798.x. [DOI] [PubMed] [Google Scholar]
- 201.Lezza A.M., Boffoli D., Scacco S., Cantatore P., Gadaleta M.N. Correlation between mitochondrial DNA 4977-bp deletion and respiratory chain enzyme activities in aging human skeletal muscles. Biochem. Biophys. Res. Commun. 1994;205(1):772–779. doi: 10.1006/bbrc.1994.2732. [DOI] [PubMed] [Google Scholar]
- 202.Jou M.J., Peng T.I., Wu H.Y., Wei Y.H. Enhanced generation of mitochondrial reactive oxygen species in cybrids containing 4977-bp mitochondrial DNA deletion. Ann. N. Y. Acad. Sci. 2005;1042:221–228. doi: 10.1196/annals.1338.024. [DOI] [PubMed] [Google Scholar]
- 203.Moreira P.I., Siedlak S.L., Wang X., Santos M.S., Oliveira C.R., Tabaton M., Nunomura A., Szweda L.I., Aliev G., Smith M.A., Zhu X., Perry G. Autophagocytosis of mitochondria is prominent in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2007;66(6):525–532. doi: 10.1097/01.jnen.0000240476.73532.b0. [DOI] [PubMed] [Google Scholar]
- 204.Moreira P.I., Siedlak S.L., Wang X., Santos M.S., Oliveira C.R., Tabaton M., Nunomura A., Szweda L.I., Aliev G., Smith M.A., Zhu X., Perry G. Increased autophagic degradation of mitochondria in Alzheimer disease. Autophagy. 2007;3(6):614–615. doi: 10.4161/auto.4872. [DOI] [PubMed] [Google Scholar]
- 205.Manczak M., Reddy P.H. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum. Mol. Genet. 2012;21(11):2538–2547. doi: 10.1093/hmg/dds072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Manczak M., Calkins M.J., Reddy P.H. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum. Mol. Genet. 2011;20(13):2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Martín-Maestro P., Gargini R.A., Sproul A., García E., Antón L.C., Noggle S., Arancio O., Avila J., García-Escudero V. Mitophagy failure in fibroblasts and ipsc-derived neurons of Alzheimer’s Disease-associated presenilin 1 Mutation. Front. Mol. Neurosci. 2017;10:291. doi: 10.3389/fnmol.2017.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Martín-Maestro P., Gargini R., Perry G., Avila J., García-Escudero V. PARK2 enhancement is able to compensate mitophagy alterations found in sporadic Alzheimer’s disease. Hum. Mol. Genet. 2016;25(4):792–806. doi: 10.1093/hmg/ddv616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Moreira P.I., Nunomura A., Nakamura M., Takeda A., Shenk J.C., Aliev G., Smith M.A., Perry G. Nucleic acid oxidation in Alzheimer disease. Free Radic. Biol. Med. 2008;44(8):1493–1505. doi: 10.1016/j.freeradbiomed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 210.Nunomura A., Perry G., Hirai K., Aliev G., Takeda A., Chiba S., Smith M.A. Neuronal RNA oxidation in Alzheimer’s disease and Down’s syndrome. Ann. N. Y. Acad. Sci. 1999;893:362–364. doi: 10.1111/j.1749-6632.1999.tb07855.x. [DOI] [PubMed] [Google Scholar]
- 211.Leon J., Sakumi K., Castillo E., Sheng Z., Oka S., Nakabeppu Y. 8-Oxoguanine accumulation in mitochondrial DNA causes mitochondrial dysfunction and impairs neuritogenesis in cultured adult mouse cortical neurons under oxidative conditions. Sci. Rep. 2016;6:22086. doi: 10.1038/srep22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ramesh Babu J., Lamar Seibenhener M., Peng J., Strom A.L., Kemppainen R., Cox N., Zhu H., Wooten M.C., Diaz-Meco M.T., Moscat J., Wooten M.W. Genetic inactivation of p62 leads to accumulation of hyperphosphorylated tau and neurodegeneration. J. Neurochem. 2008;106(1):107–120. doi: 10.1111/j.1471-4159.2008.05340.x. [DOI] [PubMed] [Google Scholar]
- 213.Du Y., Wooten M.C., Gearing M., Wooten M.W. Age-associated oxidative damage to the p62 promoter: implications for Alzheimer disease. Free Radic. Biol. Med. 2009;46(4):492–501. doi: 10.1016/j.freeradbiomed.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Scarmeas N., Luchsinger J.A., Schupf N., Brickman A.M., Cosentino S., Tang M.X., Stern Y. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302(6):627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tyrovolas S., Haro J.M., Foscolou A., Tyrovola D., Mariolis A., Bountziouka V., Piscopo S., Valacchi G., Anastasiou F., Gotsis E., Metallinos G., Papairakleous N., Polychronopoulos E., Matalas A.L., Lionis C., Zeimbekis A., Tur J.A., Sidossis L.S., Panagiotakos D.B. Anti-Inflammatory nutrition and successful ageing in elderly individuals: the multinational MEDIS Study. Gerontology. 2018;64(1):3–10. doi: 10.1159/000479065. [DOI] [PubMed] [Google Scholar]
- 216.van Dijk S.C., Enneman A.W., Swart K.M., van Wijngaarden J.P., Ham A.C., de Jonge R., Blom H.J., Feskens E.J., Geleijnse J.M., van Schoor N.M., Dhonukshe-Rutten R.A., de Jongh R.T., Lips P., de Groot L.C., Uitterlinden A.G., van den Meiracker T.H., Mattace-Raso F.U., van der Velde N., Smulders Y.M. Effect of vitamin B12 and folic acid supplementation on biomarkers of endothelial function and inflammation among elderly individuals with hyperhomocysteinemia. Vasc. Med. 2016;21(2):91–98. doi: 10.1177/1358863X15622281. [DOI] [PubMed] [Google Scholar]
- 217.McEvoy C.T., Guyer H., Langa K.M., Yaffe K. Neuroprotective diets are associated with better cognitive function: the health and retirement study. J. Am. Geriatr. Soc. 2017;65(8):1857–1862. doi: 10.1111/jgs.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mozaffarian D., Wu J.H. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011;58(20):2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 219.Fiala M., Kooij G., Wagner K., Hammock B., Pellegrini M. Modulation of innate immunity of patients with Alzheimer’s disease by omega-3 fatty acids. FASEB J. 2017;31(8):3229–3239. doi: 10.1096/fj.201700065R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang X.W., Hou W.S., Li M., Tang Z.Y. Omega-3 fatty acids and risk of cognitive decline in the elderly: a meta-analysis of randomized controlled trials. Aging Clin. Exp. Res. 2016;28(1):165–166. doi: 10.1007/s40520-015-0381-9. [DOI] [PubMed] [Google Scholar]
- 221.Wu S., Ding Y., Wu F., Li R., Hou J., Mao P. Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: a meta-analysis. Neurosci. Biobehav. Rev. 2015;48:1–9. doi: 10.1016/j.neubiorev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 222.Aung T., Halsey J., Kromhout D., Gerstein H.C., Marchioli R., Tavazzi L., Geleijnse J.M., Rauch B., Ness A., Galan P., Chew E.Y., Bosch J., Collins R., Lewington S., Armitage J., Clarke R. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3(3):225–234. doi: 10.1001/jamacardio.2017.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Luchsinger J.A., Mayeux R. Cardiovascular risk factors and Alzheimer’s disease. Curr. Atheroscler. Rep. 2004;6(4):261–266. doi: 10.1007/s11883-004-0056-z. [DOI] [PubMed] [Google Scholar]
- 224.Alves A.J., Viana J.L., Cavalcante S.L., Oliveira N.L., Duarte J.A., Mota J., Oliveira J., Ribeiro F. Physical activity in primary and secondary prevention of cardiovascular disease: Overview updated. World J. Cardiol. 2016;8(10):575–583. doi: 10.4330/wjc.v8.i10.575. [DOI] [PMC free article] [PubMed] [Google Scholar]