Abstract
Dementia is a collection of symptoms affecting a person’s cognition. Dementia is debilitating, and therefore, finding an effective treatment is of utmost importance. Resveratrol, which exhibits neuroprotective effects, has low bioavailability. However, its glucoside polydatin is more bioavailable. Here, the evidence that supports the protective role of polydatin against dementia-related diseases such as Alzheimer’s disease, vascular dementia, alcohol-related dementia, and Lewy body dementias is presented. The beneficial effects of polydatin from a mechanistic perspective are specifically emphasized in this review. Future directions in this area of research are also discussed.
Keywords: Alzheimer's disease, cognitive dysfunction, dementia, neuroprotection, polydatin, Parkinson's disease, resveratrol, vascular dementia
1. INTRODUCTION
Dementia is a group of symptoms affecting memory, thinking, and reasoning skills. It is now no longer being perceived as a normal part of the aging process. Data from the World Health Organization (WHO) indicated that dementia affected 47 million people globally in 2015 [1]. WHO also predicted that the figure will further increase to 75 million in the next 10 years. Dementia has a deleterious impact on aging societies and their families. Thus, finding an effective treatment to halt the progression of dementia is crucial.
Dementia can occur due to a variety of possible diseases including Alzheimer’s disease (AD). It has been demonstrated that the polyphenolic stilbene resveratrol, a potent sirtuin 1 (SIRT1) activator, can exert neuroprotection in several models of AD [2]. A recent clinical trial has shown that resveratrol is relatively safe, and can alter AD biomarker trajectories [3]. Besides, it can also maintain the blood-brain barrier (BBB) integrity in an experimental AD model [4]. Nevertheless, the bioavailability of resveratrol is low due to extensive hepatic metabolism and rapid renal elimination [5]. Thus, the issue of low bioavailability of resveratrol warrants the attention to explore newer compounds such as its analog polydatin.
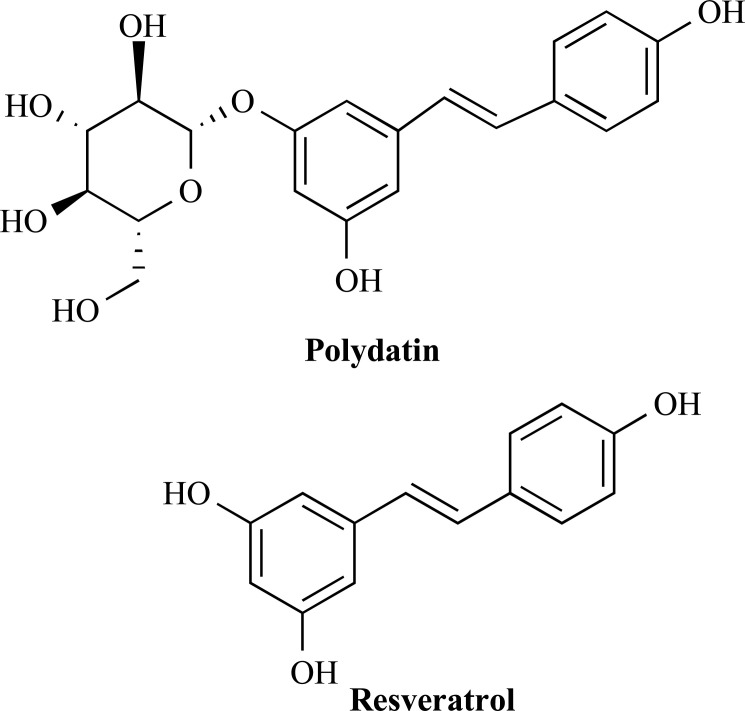
Polydatin, also known as piceid, is a glucoside form of resveratrol. It differs from resveratrol by the substitution of a hydroxyl group for a glucose unit [6]. The chemical structures of polydatin and resveratrol are shown in Fig. 1. Wang et al. (2015) have shown that polydatin and resveratrol could mutually be converted in vivo [7]. Although polydatin can retain the biological properties of resveratrol, it is less susceptible to enzymatic oxidation [8]. In support of this notion, Wang et al. further demonstrated that the concentration of polydatin in rat serum was always higher (~3–5 times) than resveratrol after the animals were orally administered with either polydatin or resveratrol at 200 mg/kg [7].
Fig. (1).
Structures of polydatin and resveratrol.
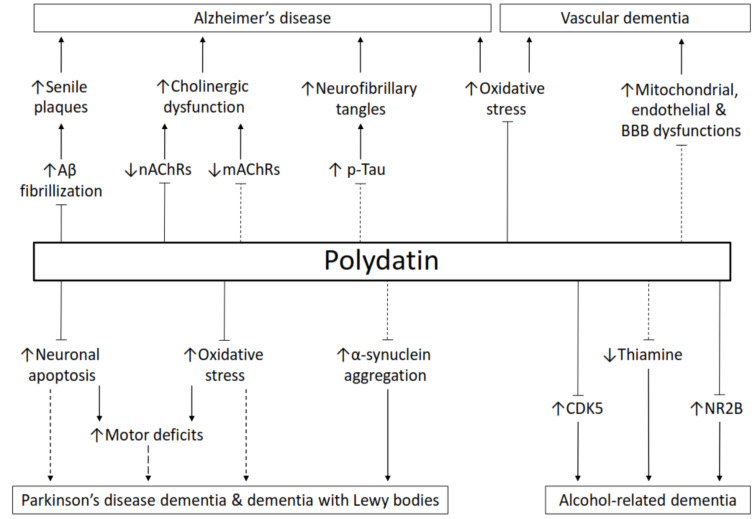
The cellular mechanisms of polydatin in various tissues and organs have been thoroughly reviewed by Du et al. (2013) [9]. The potential protein targets of polydatin have also been identified [10]. It is supposed that polydatin can cross the BBB as it protects brain damage from cerebral ischemia when administered intravenously [11]. Moreover, orally administered polydatin could reduce rotenone-induced neurodegeneration in the substantia nigra [12]. The ability of polydatin to cross the BBB is essential in combating brain disorders. In comparison to its counterpart resveratrol, the literature on the protective role of polydatin against dementia-related diseases is relatively scarce. This paper is aimed to review the protective potential and modes of action of polydatin against dementia-related disorders. Fig. 2 depicts an overview of this paper. The future perspectives of polydatin research in relation to these diseases will also be discussed.
Fig. (2).
The protective role of polydatin against dementia-relating disorders. The lines with an arrowhead indicate facilitation. The lines with a blocked end indicate inhibition. The dashed lines indicate the hypothetical pathways that might be regulated by polydatin. Aβ, amyloid-β; CDK5, cyclin-dependent kinase 5; mAChRs, muscarinic acetylcholine receptors; nAChRs, nicotinic acetylcholine receptors; NR2B, NMDA receptor 2B subunit; p-tau, phosphorylated tau. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2. ALZHEIMER’S DISEASE
AD is a neurodegenerative disorder and accounts for about two-third of the overall cases of dementia [13]. The progression of the disease is accompanied by the gradual impairment of memory and cognitive function. A major hallmark feature of AD is the accumulation of senile plaques in the brain [14]. Senile plaques are aggregates of amyloid-β (Aβ) protein found abundantly in the brain of patients with advanced AD [15]. The aggregation of proteins, in general, can produce higher-order supramolecular structures including fibrillar forms [16]. Aβ fibrils are typical cross β-sheets that are aligned perpendicularly to the axis of the fibril [16]. Thus, discovering agents that can halt or reverse the Aβ fibrillization could potentially treat AD.
Although the mechanistic insights of resveratrol in AD have extensively been reviewed [2, 3, 17-19], the comparative literature of its role with other stilbenes including polydatin is still lacking. Rivière et al. (2007, 2010) compared the inhibitory effect of stilbenes, including polydatin and resveratrol, on the formation of Aβ fibrils in an in vitro cell-free system [6, 20]. Curcumin was selected as a positive control in their studies because this polyphenol has been reported to possess a potent anti-amyloidogenic effect [21]. Among all the stilbene compounds tested, at the equimolar concentration of 10 μM, the effectiveness of polydatin and resveratrol in inhibiting Aβ fibril formation was ~20% greater than that of the curcumin control group [20]. Rivière et al. also found that polydatin is equally effective as resveratrol in inhibiting Aβ fibril formation with the IC50 value ranging from 4.7 to 6.0 μM [6, 20]. Other stilbene derivatives such as astringine, piceatannol, viniferin, and resveratrol diglucoside could also inhibit Aβ polymerization but the effect was weaker than the curcumin control [20]. In a different study using HPLC and electron microscopy, Rivière et al. also demonstrated in another in vitro cell-free study that polydatin could destabilize Aβ oligomers and fibrils, and convert these macromolecules to non-neurotoxic monomers [22]. The authors proposed that the mechanism of this destabilization could be a dynamic interaction between polydatin and Aβ, which could open the hydrophobic zipper, and shift the reversible equilibrium “random coil↔β-sheet” to the disordered structure. Table 1 summarizes the pharmacological effects of polydatin on dementia-related disorders.
Table 1.
A summary of the pharmacological effects of polydatin on dementia-relating illnesses.
| Disease Model | Beneficial Effects | Type of Exposure | Experimental Unit |
Polydatin
Concentrations |
Description of Effects | Refs. |
|---|---|---|---|---|---|---|
| Alzheimer's disease | Prevention of Aβ aggregation | In vitro | Cell-free system | 10 μM | Inhibit Aβ25-35 polymerization | Rivière et al. [6, 20] |
| In vitro | Cell-free system | 10 μM | Destabilize Aβ25-35 fibrils and oligomers | Rivière et al. [22] | ||
| Upregulation of nAChRs | In vitro | SH-SY5Y cells | 0.1 and 0.25 mg/mL for 48 h | Increase α3 and α7 nAChRs, reverse Aβ1-42-induced decrease of α3 and α7 nAChRs | Xiao et al. [26] | |
| Vascular dementia | Protection against learning and memory impairments | In vivo | Adult male Sprague-Dawley rats | 12.5-50 mg/kg/day for 30 days; p.o. | Attenuate cognitive deficits, decrease MDA levels, increase CAT and SOD activities |
Li et al. [33] |
| In vivo | Seven-day-old neonatal Sprague-Dawley rats | 10 mg/kg/day for 10 days; i.p. | Attenuate cognitive deficits, increase BDNF expression |
Sun et al. [34] | ||
| Alcohol-related dementia | Protection against learning and memory impairments | In vivo | Adult male Sprague-Dawley rats | 12.5 and 50 mg/kg/day for 30 days; i.g. | Attenuate cognitive deficits | Zhang et al. [66] |
| In vivo | Rat primary hippocampal neurons | 12.5 and 50 mg/kg/day for 30 days; i.g. | Increase cell viability, decrease CDK5 expression and activity | Zhang et al. [66] | ||
| In vivo | Alcoholic patients | 40 mg (b.i.d.) for 14 days; p.o. | Attenuate cognitive deficits, decrease lipid peroxidation | Pace et al. [68] | ||
| In vivo | Rat prefrontal cortex | 0.1, 0.2, and 0.4% for 10 days; i.g.; The stock concentration was unreported |
Decrease NR2B mRNA expression |
Xu et al. [76] | ||
| Parkinson's disease | Protection against dopaminergic neurodegeneration | In vitro | SH-SY5Y cells | 10-30 µM for 1h prior to dopamine exposure | Reduce lipid peroxidation, inhibit apoptosis, activate MAPKs |
Potdar et al. [8] |
| In vivo | Adult male Sprague-Dawley rats | 20-80 mg/kg/day for 5 weeks; p.o. | Reverse damage in striatal region, increase motor performance |
Chen et al. [12] | ||
| In vivo | Adult male Wistar rats | 25-100 mg/kg/day for 4 weeks; i.g. | Reverse damage in striatal region, increase motor performance, reduce proinflammatory mediators, reduce the activation of NF-κB, increase p-AKT, p-GSK-3β, and Nrf2 levels | Huang et al. [46] | ||
| In vitro | BV-2 cells | 100-400 μM for 1h | Reduce proinflammatory mediators, reduce the activation of NF-κB, increase p-AKT, p-GSK-3β, and Nrf2 levels | Huang et al. [46] | ||
| In vitro | SH-SY5Y cells | Increase cell viability, MMP, and Sirt 1 expression, decrease ROS and DJ1 expression, induce Atg5-mediated autophagy | Bai et al. [47] |
Abbreviations: Aβ, amyloid-β; BDNF, brain-derived neurotrophic factor; CAT, catalase; CDK5, cyclin-dependent kinase 5; GSK-3β, glycogen synthase kinase-3β; MAPKs, mitogen-activated protein kinases; MDA, malondialdehyde; MMP, mitochondrial membrane potential; nAChRs, NF-κB, nuclear factor-κB; nicotinic acetylcholine receptors; NR2B, NMDA receptor 2B subunit; Nrf2, nuclear factor erythroid 2-related factor 2; ROS, reactive oxygen species; Sirt1, sirtuin 1; SOD, superoxide dismutase.
The accumulation of Aβ plaques precedes other pathological changes and causes degeneration of neurons. The degeneration of cholinergic forebrain neurons is regarded as the most prominent among the neurons in AD [23]. A reduction in the number of nicotinic acetylcholine receptors (nAChRs) has also been demonstrated in AD [24]. Treatment with the nAChR agonist such as nicotine can augment attention and cognitive functions in normal humans and animals [25]. Thus, nAChRs may be crucial therapeutic targets for AD treatment. A study by Xiao et al. has shown that Aβ1-42 (5 µM) could downregulate the expression of α3 and α7 nAChRs in the human neuroblastoma SY-SY5Y cell line [26]. On the other hand, polydatin (0.1 mg/mL) could upregulate the expression of α3 and α7 nAChRs, and reverse Aβ1-42-induced downregulation of α3 and α7 nAChRs [26]. Recently, resveratrol has also been demonstrated to inhibit Aβ aggregation and cognitive decline in transgenic AD mice via upregulating the expression of α7 nAChRs in the brain [27]. Although muscarinic acetylcholine receptors (mAChRs) are also affected in AD [24], the role of polydatin and resveratrol in this receptor subtype is unclear.
Another main culprit of AD is hyperphosphorylation of tau protein [14]. This leads to the formation of neurofibrillary tangles, another pathological marker of AD. Resveratrol can alleviate tau hyperphosphorylation [28, 29]. Despite this important finding, the role of polydatin in preventing tau hyperphosphorylation is poorly understood.
3. VASCULAR DEMENTIA
Vascular dementia (VaD) is the second most common cause of dementia, accounting for ~15% of cases [30]. The disease can lower the quality of life (QOL) of patients and their caregivers. VaD is caused by a variety of vascular disorders that affect the brain such as cerebral infarction, cerebral hemorrhage, and subarachnoid hemorrhage [31]. It has also been reported that 25-30% of ischemic stroke survivors develop VaD [32]. The protection of cerebrovascular disease by polydatin has been extensively reviewed by Tang et al. (2019) [11].
VaD model can be induced experimentally by four-vessel occlusion (4-VO) method in which both vertebral arteries and common carotid arteries are occluded. As indicated by the Morris water maze test, rats with 4-VO showed severe cognitive deficits [33]. The 4-VO rats orally treated with polydatin (50 mg/kg) showed significant cognitive improvement on day 4 with the escape latency reduced by half [33]. Biochemical assays indicated that polydatin reduced the level of malondialdehyde and increased the catalase and superoxide dismutase activities [33]. Polydatin could also alleviate oxygen-glucose deprivation-induced injuries in cultured rat’s cortical neurons [33]. Furthermore, Sun et al. (2014) showed that polydatin attenuated cognitive impairment in rats with hypoxic-ischemic brain injury via upregulating brain-derived neurotrophic factor [34]. Overall, these findings suggest that the therapeutic potential of polydatin against VaD is partly due to its neuroprotective and antioxidant effects.
Risk factors for VaD include diabetes, hypertension, and metabolic syndrome. There are various animal models of VaD including the ones that mimic its risk factors [35]. Mechanisms implicated in the pathogenesis of VaD may include mitochondrial, endothelial, and BBB dysfunctions, as well as inflammation [35]. For instance, resveratrol has been reported to improve cognition in several animal models of VaD including the 4-VO, bilateral common carotid artery occlusion, and streptozotocin-induced diabetic models by attenuating inflammation, mitochondrial dysfunction, oxidative stress, and apoptosis [36-41]. Therefore, the protective mechanisms of polydatin on other models of VaD should be further explored.
4. LEWY BODY DEMENTIAS
Dementia is common and occurs in 40-70% of Parkinson’s disease (PD) patients [42]. Parkinson’s disease dementia (PDD) and dementia with Lewy bodies (DLB) are accompanied by motor changes including gait disturbances and parkinsonism. PDD and DLB are classified as Lewy body dementias due to the presence of abnormal protein aggregates known as Lewy bodies [43]. The Lewy bodies, consisting of mainly α-synuclein, are found intracellularly inside the neurons in the brainstem, or within the cortex. PDD refers to the cognitive impairment that develops at least a year after the diagnosis of PD [44]. On the other hand, DLB refers to dementia symptoms that develop sooner, or within one year of the onset of motor symptoms [44].
In addition to Lewy pathology, PD is also characterized by the degeneration of dopaminergic neurons in the substantia nigra pars compacta of the brain [45]. Clinical motor symptoms of PD include bradykinesia, rigidity, tremor, and postural instability. Prolonged exposure to rotenone (a specific complex 1 inhibitor and a common pesticide) has been demonstrated to cause parkinsonism characteristics in rats. Several studies have reported that rotenone selectively damaged the nigrostriatal dopaminergic neurons. For example, a study by Chen et al. (2015) showed a sub-lethal concentration of rotenone (2 mg/kg/day, s.c., 5 weeks) impaired ATP, reduced glutathione, superoxide dismutase, and thioredoxin levels, but increased the level of malondialdehyde in the striatum of rats [12]. Polydatin (40-80 mg/kg), administered orally, could significantly prevent the rotenone-induced biochemical changes and neurodegeneration in the substantia nigra [12]. Similarly, polydatin could also alleviate motor dysfunctions, and exhibit potent antioxidant activity in two other animal models of PD, i.e. 6-hydroxydopamine-treated rats and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice [12].
Treatment with polydatin (25-100 mg/kg/day, i.g.) for 4 weeks also improved motor performance and protected against dopaminergic neurodegeneration of the substantia nigra by suppressing the activation of microglia and the release of pro-inflammatory mediators in a rat model of lipopolysaccharide-induced PD [46]. Polydatin induced similar cellular changes in lipopolysaccharide-treated murine microglia BV-2 cells by regulating the AKT/GSK3β-Nrf2/NF-κB signaling pathway [46]. In another in vitro PD model employing SH-SY5Ycells, the protective effect of polydatin against rotenone-induced toxicity was regulated by Atg5-mediated autophagy [47]. Most studies on the protective role of polydatin in PD have been focused on examining the mechanisms associated with parkinsonian motor deficits. However, cognitive impairment has also been demonstrated in experimental models of PD [48, 49]. Future studies are needed to examine the capability of polydatin in improving cognition in PD models.
Oxidation can destruct vital molecules in cells, which include proteins, lipids, and DNA. It has been demonstrated that the level of oxidation is high in substantia nigra pars compacta compared to other regions of the human brain [50]. Since the substantia nigra is rich in dopaminergic connections, autoxidation of dopamine molecules might also occur in this region. In accordance, dopamine can undergo a non-enzymatic autoxidative reaction to generate reactive oxygen species (ROS) [51]. Excessive formation of ROS can lead to neuronal damage. Moreover, dopamine could induce lipid peroxidation and apoptotic cell death in human neuroblastoma SH-SY5Y cells [8]. However, Potdar et al. (2018) reported that polydatin pretreatment (10-30 µM) for 1 h prior to dopamine exposure could inhibit the dopamine-induced loss of BCL-2 in SH-SY5Y cells [8]. BCL-2 is an anti-apoptotic protein, which plays an important role in neurogenesis [52]. In addition, polydatin could also suppress the activity of caspase-3/7, a biomarker of apoptosis [8]. Both apoptosis and oxidative stress are closely linked to AD and PD pathogenesis [14, 45, 53].
Phytochemicals such as resveratrol and curcumin can induce autophagic degradation of α-synuclein in experimental parkinsonism [54, 55]. Resveratrol also suppresses α-synuclein expression and aggregation, oxidative stress, and neuroinflammation [56, 57]. Most importantly, resveratrol also alleviated cognitive and motor deficits in A53T α-synuclein mouse model of PD in a dose-dependent manner [56]. The α-synuclein degradation potential of resveratrol is most likely to be mediated via SIRT1 as the SIRT1 inhibitor EX527 could antagonize the protective capability of resveratrol [58]. In a cellular model of PD, Wu et al. (2011) reported that both AMP-activated protein kinase and SIRT1 are required for the resveratrol-mediated neuroprotection [59]. A bioinformatic study demonstrated that SIRT1 is a target of microRNA-214 [60]. In agreement with these findings, a different study showed that resveratrol could attenuate the rise in α-synuclein expression in MPTP-induced PD by upregulating the microRNA-214 expression [61]. Despite the strong connection between resveratrol and α-synuclein, the link between polydatin and Lewy pathology has never been established.
5. ALCOHOL-RELATED DEMENTIA
Alcohol-related cognitive and memory impairments can be developed as a result of excessive and prolonged alcohol consumption [62, 63]. For instance, the hippocampus, a brain structure that is associated with learning and memory formation, is susceptible to ethanol-related toxicity and damage. Studies have shown that hippocampal functions are compromised in animal models [64, 65]. Chronic self-administration of ethanol in rats impaired cognition as quantified using the Morris water maze test [66]. Moreover, acute and chronic alcohol consumption also impairs cognitive skills and abilities in humans [67, 68]. Polydatin could improve cognitive performance in alcoholic patients and rats [66, 68]. Similarly, a study employing Barnes maze task indicated that resveratrol could prevent spatial learning and memory deficits in ethanol-treated mice [69]. In addition, polydatin could also decrease serum lipid peroxidation in alcoholics [68].
Cyclin-dependent kinase 5 (CDK5) is found abundantly in the brain and has been shown to play a crucial role in neuronal function and development [70]. However, dysregulation of CDK5 can result in neurodegeneration [71, 72]. Alcoholic rats also exhibited high levels of CDK5 activity and protein expression [66, 73]. The ethanol might have provoked the brain injury and cognitive impairment by increasing CDK5 activity and protein expression. Polydatin could reduce CDK5 activity and protein expression in the hippocampus of ethanol-treated rats, and ethanol-treated primary hippocampal neuronal cultures [66], suggesting that the neuroprotective ability of polydatin against ethanol-induced neurotoxicity is partly mediated via targeting CDK5.
NMDA receptor 2B subunit (NR2B), a constitutive component of the glutamatergic NMDA receptor, plays an important role in dendritogenesis and synaptic plasticity [74]. However, overexpression of NR2B may lead to excitotoxicity. Studies have shown that chronic alcohol exposure can upregulate the expression of the NR2B gene and protein [75]. Thus, alcohol-induced cognitive impairment is partly mediated by altering the expression of NR2B. It has also been reported that the protective effect of polydatin on learning and memory in alcoholic rats is mediated by suppressing NR2B mRNA expression [76]. Nevertheless, whether polydatin might influence the expression of other NMDA receptor subunits such as NR1, remains to be determined.
Thiamine (vitamin B1) deficiency is associated with neurological deficits exhibited in AD patients [77]. It has been suggested that the deficiency is due to the impairment in the activities of thiamine-dependent enzymes [78]. Chronic alcohol misuse can also lead to thiamine deficiency, which in turn can cause Wernicke-Korsakoff’s syndrome, a neuropsychiatric syndrome characterized by severe cognitive impairment [79]. It is still uncertain if polydatin could regulate the activities and expression of thiamine-dependent enzymes such as thiamine monophosphatase, thiamine diphosphatase, and thiamine pyrophosphokinase.
CONCLUSION AND FUTURE PERSPECTIVES
Polydatin may improve cognitive deficits in many other ways other than the ones discussed in this paper such as attenuating neuronal oxidative damage and inflammation. Neuroinflammation and oxidative stress are widely implicated in dementia. For instance, elevated levels of serum inflammatory cytokines have been observed in AD patients [80]. Chronic systemic inflammation is also associated with an increased risk of cerebral microvasculature disease that leads to the development of VaD [81]. Oxidative stress reflects an imbalance between free radicals and antioxidants in the body. Free radicals produced by oxidative stress are found in several experimental models of diseases including AD and PD [53]. Polydatin could mitigate neuronal cell death in a rat model of permanent middle cerebral artery occlusion via suppressing oxidative stress and neuroinflammation [82]. Studies have shown that polydatin could suppress oxidative stress via inhibiting protein kinase C and activating the nuclear factor erythroid 2-related factor 2 (NRF2)/heme oxygenase-1 (HO-1) pathway [83, 84]. A recent study has demonstrated that polydatin attenuated chemotherapy-induced learning and memory impairment by suppressing inflammation, oxidative stress, and apoptosis in rat hippocampus [85].
There is a growing body of evidence that the inflammatory response is mediated by the activated microglia, the resident immune cells of the central nervous system [86]. Microglia activation is a hallmark of brain pathologies such as neurodegeneration in AD and PD. [87, 88]. It has been demonstrated that polydatin could attenuate traumatic spinal cord injury by inhibiting microglial inflammation via modulation of NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome and inducible nitric oxide synthase pathways [89].
Bone morphogenetic proteins (BMPs), a subfamily of the transforming growth factor-β, play an important role in neuron maturation and brain development. Certain BMPs such as BMP-2, BMP-7, and BMP-9 are expressed in the brain [90-92]. In addition, activation of Wnt/β-catenin signaling has been implicated in AD therapy as it attenuates tau hyperphosphorylation and Aβ production [93-95]. It has been reported that the osteogenic-promoting effect of polydatin could be blocked by inhibiting the Wnt/β-catenin and BMP pathways [96].
The role of Aβ and tau protein in the pathogenesis of AD has extensively been studied [14]. These proteins have been identified as the key target of drug development for the treatment of the disease. However, several world-famous large pharmaceutical companies have been retracting from the drug development project mainly because of great difficulty to demonstrate statistically significant benefit of the drugs in improving prognosis of the disease, i.e., to extend the mechanistically beneficial effects observed in the basic study in animal models to the improvement of dementia symptoms at the stage of clinical application. As a natural compound, polydatin could also exhibit instability and low bioavailability issues that may limit its beneficial action. Therefore, new delivery strategies in overcoming these limitations, and increasing therapeutic efficacy of polydatin should further be explored. For instance, the use of bioenhancers as a drug delivery method may benefit patients by promoting the absorption of poorly bioavailable drugs [97]. Additionally, a recent study has reported that the nano-encapsulated polydatin could preserve the characteristics of the free form [98]. Thus, the encapsulation process may also be another promising strategy for delivering polydatin to its therapeutic sites.
While polydatin could be an important nutraceutical agent to treat dementia-related illnesses, the literature on its protective effects is considerably lacking in comparison to resveratrol. The molecular mechanisms underlying the effects of polydatin in the treatment of dementia remain poorly characterized. Thus, many more studies are required to elucidate the protective mechanisms of polydatin for cognitive impairment before it could be considered for further clinical development in treating dementia.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.World Health Organization 2017 https://www.who.int/mental_health/neurology/dementia/action_plan_2017_2025/en/
- 2.Ahmed T., Javed S., Javed S., Tariq A., Šamec D., Tejada S., Nabavi S.F., Braidy N., Nabavi S.M. Resveratrol and Alzheimer’s disease: Mechanistic insights. Mol. Neurobiol. 2017;54(4):2622–2635. doi: 10.1007/s12035-016-9839-9. [DOI] [PubMed] [Google Scholar]
- 3.Sawda C., Moussa C., Turner R.S. Resveratrol for Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2017;1403(1):142–149. doi: 10.1111/nyas.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao H.F., Li N., Wang Q., Cheng X.J., Li X.M., Liu T.T. Resveratrol decreases the insoluble Aβ1-42 level in hippocampus and protects the integrity of the blood-brain barrier in AD rats. Neuroscience. 2015;310:641–649. doi: 10.1016/j.neuroscience.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Gambini J., Inglés M., Olaso G., Lopez-Grueso R., Bonet-Costa V., Gimeno-Mallench L., Mas-Bargues C., Abdelaziz K.M., Gomez-Cabrera M.C., Vina J., Borras C. Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell. Longev. 2015;2015:837042. doi: 10.1155/2015/837042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivière C., Papastamoulis Y., Fortin P.Y., Delchier N., Andriamanarivo S., Waffo-Teguo P., Kapche G.D., Amira-Guebalia H., Delaunay J.C., Mérillon J.M., Richard T., Monti J.P. New stilbene dimers against amyloid fibril formation. Bioorg. Med. Chem. Lett. 2010;20(11):3441–3443. doi: 10.1016/j.bmcl.2009.09.074. [DOI] [PubMed] [Google Scholar]
- 7.Wang H.L., Gao J.P., Han Y.L., Xu X., Wu R., Gao Y., Cui X.H. Comparative studies of polydatin and resveratrol on mutual transformation and antioxidative effect in vivo. Phytomedicine. 2015;22(5):553–559. doi: 10.1016/j.phymed.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Potdar S., Parmar M.S., Ray S.D., Cavanaugh J.E. Protective effects of the resveratrol analog piceid in dopaminergic SH-SY5Y cells. Arch. Toxicol. 2018;92(2):669–677. doi: 10.1007/s00204-017-2073-z. [DOI] [PubMed] [Google Scholar]
- 9.Du Q.H., Peng C., Zhang H. Polydatin: a review of pharmacology and pharmacokinetics. Pharm. Biol. 2013;51(11):1347–1354. doi: 10.3109/13880209.2013.792849. [DOI] [PubMed] [Google Scholar]
- 10.Pan B., Ren Y., Liu L. Uncovering the action mechanism of polydatin via network pharmacological target prediction. RSC Advances. 2018;8(34):18851–18858. doi: 10.1039/C8RA03124J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang K.S., Tan J.S. The protective mechanisms of polydatin in cerebral ischemia. Eur. J. Pharmacol. 2019;842:133–138. doi: 10.1016/j.ejphar.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y., Zhang D.Q., Liao Z., Wang B., Gong S., Wang C., Zhang M.Z., Wang G.H., Cai H., Liao F.F., Xu J.P. Anti-oxidant polydatin (piceid) protects against substantia nigral motor degeneration in multiple rodent models of Parkinson’s disease. Mol. Neurodegener. 2015;10(1):4. doi: 10.1186/1750-1326-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiest K.M., Roberts J.I., Maxwell C.J., Hogan D.B., Smith E.E., Frolkis A., Cohen A., Kirk A., Pearson D., Pringsheim T., Venegas-Torres A., Jetté N. The prevalence and incidence of dementia due to Alzheimer’s disease: A systematic review and meta-analysis. Can. J. Neurol. Sci. 2016;43(Suppl. 1):S51–S82. doi: 10.1017/cjn.2016.36. [DOI] [PubMed] [Google Scholar]
- 14.Tang K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019;233:116695. doi: 10.1016/j.lfs.2019.116695. [DOI] [PubMed] [Google Scholar]
- 15.Baumann B., Woehrer A., Ricken G., Augustin M., Mitter C., Pircher M., Kovacs G.G., Hitzenberger C.K. Visualization of neuritic plaques in Alzheimer’s disease by polarization-sensitive optical coherence microscopy. Sci. Rep. 2017;7:43477. doi: 10.1038/srep43477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dasgupta M., Kishore N. Selective inhibition of aggregation/fibrillation of bovine serum albumin by osmolytes: Mechanistic and energetics insights. PLoS One. 2017;12(2):e0172208. doi: 10.1371/journal.pone.0172208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drygalski K., Fereniec E., Koryciński K., Chomentowski A., Kiełczewska A., Odrzygóźdź C., Modzelewska B. Resveratrol and Alzheimer’s disease. From molecular pathophysiology to clinical trials. Exp. Gerontol. 2018;113:36–47. doi: 10.1016/j.exger.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Gomes B.A.Q., Silva J.P.B., Romeiro C.F.R., Dos Santos S.M., Rodrigues C.A., Gonçalves P.R., Sakai J.T., Mendes P.F.S., Varela E.L.P., Monteiro M.C. Neuroprotective mechanisms of resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018;2018:8152373. doi: 10.1155/2018/8152373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kou X., Chen N. Resveratrol as a Natural autophagy regulator for prevention and treatment of Alzheimer’s Disease. Nutrients. 2017;9(9):E927. doi: 10.3390/nu9090927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivière C., Richard T., Quentin L., Krisa S., Mérillon J.M., Monti J.P. Inhibitory activity of stilbenes on Alzheimer’s beta-amyloid fibrils in vitro. Bioorg. Med. Chem. 2007;15(2):1160–1167. doi: 10.1016/j.bmc.2006.09.069. [DOI] [PubMed] [Google Scholar]
- 21.Ono K., Hasegawa K., Naiki H., Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J. Neurosci. Res. 2004;75(6):742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 22.Rivière C., Delaunay J.C., Immel F., Cullin C., Monti J.P. The polyphenol piceid destabilizes preformed amyloid fibrils and oligomers in vitro: hypothesis on possible molecular mechanisms. Neurochem. Res. 2009;34(6):1120–1128. doi: 10.1007/s11064-008-9883-6. [DOI] [PubMed] [Google Scholar]
- 23.Bohnen N.I., Kaufer D.I., Ivanco L.S., Lopresti B., Koeppe R.A., Davis J.G., Mathis C.A., Moore R.Y., DeKosky S.T. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch. Neurol. 2003;60(12):1745–1748. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- 24.Lombardo S., Maskos U. Role of the nicotinic acetylcholine receptor in Alzheimer's disease pathology and treatment. 2015. [DOI] [PubMed]
- 25.Shimohama S., Kihara T. Nicotinic receptor-mediated protection against beta-amyloid neurotoxicity. Biol. Psychiatry. 2001;49(3):233–239. doi: 10.1016/S0006-3223(00)01100-8. [DOI] [PubMed] [Google Scholar]
- 26.Xiao H.T., Qi X.L., Liang Y., Lin C.Y., Wang X., Guan Z.Z., Hao X.Y. Membrane permeability-guided identification of neuroprotective components from Polygonum cuspidatun. Pharm. Biol. 2014;52(3):356–361. doi: 10.3109/13880209.2013.837078. [DOI] [PubMed] [Google Scholar]
- 27.Cao K., Dong Y.T., Xiang J., Xu Y., Li Y., Song H., Yu W.F., Qi X.L., Guan Z.Z. The neuroprotective effects of SIRT1 in mice carrying the APP/PS1 double-transgenic mutation and in SH-SY5Y cells over-expressing human APP670/671 may involve elevated levels of α7 nicotinic acetylcholine receptors. Aging (Albany NY) 2020;12(2):1792–1807. doi: 10.18632/aging.102713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jhang K.A., Park J.S., Kim H.S., Chong Y.H. Resveratrol ameliorates tau hyperphosphorylation at Ser396 site and oxidative damage in rat hippocampal slices exposed to vanadate: Implication of ERK1/2 and GSK-3beta signaling cascades. J. Agric. Food Chem. 2017;65(44):9626–9634. doi: 10.1021/acs.jafc.7b03252. [DOI] [PubMed] [Google Scholar]
- 29.Schweiger S., Matthes F., Posey K., Kickstein E., Weber S., Hettich M.M., Pfurtscheller S., Ehninger D., Schneider R., Krauß S. Resveratrol induces dephosphorylation of Tau by interfering with the MID1-PP2A complex. Sci. Rep. 2017;7(1):13753. doi: 10.1038/s41598-017-12974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Brien J.T., Thomas A. Vascular dementia. Lancet. 2015;386(10004):1698–1706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- 31.Smith E.E. Clinical presentations and epidemiology of vascular dementia. Clin. Sci. (Lond.) 2017;131(11):1059–1068. doi: 10.1042/CS20160607. [DOI] [PubMed] [Google Scholar]
- 32.Kalaria R.N., Akinyemi R., Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim. Biophys. Acta. 2016;1862(5):915–925. doi: 10.1016/j.bbadis.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R.P., Wang Z.Z., Sun M.X., Hou X.L., Sun Y., Deng Z.F., Xiao K. Polydatin protects learning and memory impairments in a rat model of vascular dementia. Phytomedicine. 2012;19(8-9):677–681. doi: 10.1016/j.phymed.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Sun J., Qu Y., He H., Fan X., Qin Y., Mao W., Xu L. Protective effect of polydatin on learning and memory impairments in neonatal rats with hypoxic-ischemic brain injury by up-regulating brain-derived neurotrophic factor. Mol. Med. Rep. 2014;10(6):3047–3051. doi: 10.3892/mmr.2014.2577. [DOI] [PubMed] [Google Scholar]
- 35.Venkat P., Chopp M., Chen J. Models and mechanisms of vascular dementia. Exp. Neurol. 2015;272:97–108. doi: 10.1016/j.expneurol.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gocmez S.S., Şahin T.D., Yazir Y., Duruksu G., Eraldemir F.C., Polat S., Utkan T. Resveratrol prevents cognitive deficits by attenuating oxidative damage and inflammation in rat model of streptozotocin diabetes induced vascular dementia. Physiol. Behav. 2019;201:198–207. doi: 10.1016/j.physbeh.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Ma X., Sun Z., Liu Y., Jia Y., Zhang B., Zhang J. Resveratrol improves cognition and reduces oxidative stress in rats with vascular dementia. Neural Regen. Res. 2013;8(22):2050–2059. doi: 10.3969/j.issn.1673-5374.2013.22.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen D., Tian X., Sang W., Song R. Effect of melatonin and resveratrol against memory impairment and hippocampal damage in a rat model of vascular dementia. Neuroimmunomodulation. 2016;23(5-6):318–331. doi: 10.1159/000454681. [DOI] [PubMed] [Google Scholar]
- 39.Sun Z.K., Ma X.R., Jia Y.J., Liu Y.R., Zhang J.W., Zhang B.A. Effects of resveratrol on apoptosis in a rat model of vascular dementia. Exp. Ther. Med. 2014;7(4):843–848. doi: 10.3892/etm.2014.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yadav A., Sunkaria A., Singhal N., Sandhir R. Resveratrol loaded solid lipid nanoparticles attenuate mitochondrial oxidative stress in vascular dementia by activating Nrf2/HO-1 pathway. Neurochem. Int. 2018;112:239–254. doi: 10.1016/j.neuint.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y., Li Y., Wang Y., Wang G., Mao L., Zhang D., Wang J. Effects of resveratrol on learning and memory in rats with vascular dementia. Mol. Med. Rep. 2019;20(5):4587–4593. doi: 10.3892/mmr.2019.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aarsland D., Andersen K., Larsen J.P., Lolk A., Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch. Neurol. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 43.Gomperts S.N. Lewy body dementias: dementia with Lewy bodies and Parkinson disease dementia. Continuum (Minneap. Minn.) 2016;22(2):435–463. doi: 10.1212/CON.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKeith I.G., Boeve B.F., Dickson D.W., Halliday G., Taylor J.P., Weintraub D., Aarsland D., Galvin J., Attems J., Ballard C.G., Bayston A., Beach T.G., Blanc F., Bohnen N., Bonanni L., Bras J., Brundin P., Burn D., Chen-Plotkin A., Duda J.E., El-Agnaf O., Feldman H., Ferman T.J., Ffytche D., Fujishiro H., Galasko D., Goldman J.G., Gomperts S.N., Graff-Radford N.R., Honig L.S., Iranzo A., Kantarci K., Kaufer D., Kukull W., Lee V.M.Y., Leverenz J.B., Lewis S., Lippa C., Lunde A., Masellis M., Masliah E., McLean P., Mollenhauer B., Montine T.J., Moreno E., Mori E., Murray M., O’Brien J.T., Orimo S., Postuma R.B., Ramaswamy S., Ross O.A., Salmon D.P., Singleton A., Taylor A., Thomas A., Tiraboschi P., Toledo J.B., Trojanowski J.Q., Tsuang D., Walker Z., Yamada M., Kosaka K. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang K.S. Protective effect of arachidonic acid and linoleic acid on 1-methyl-4-phenylpyridinium-induced toxicity in PC12 cells. Lipids Health Dis. 2014;13:197. doi: 10.1186/1476-511X-13-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang B., Liu J., Meng T., Li Y., He D., Ran X., Chen G., Guo W., Kan X., Fu S., Wang W., Liu D. Polydatin prevents lipopolysaccharide (LPS)-induced Parkinson’s disease via regulation of the AKT/GSK3β-Nrf2/NF-κB signaling axis. Front. Immunol. 2018;9:2527. doi: 10.3389/fimmu.2018.02527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bai H., Ding Y., Li X., Kong D., Xin C., Yang X., Zhang C., Rong Z., Yao C., Lu S., Ji L., Li L., Huang W. Polydatin protects SH-SY5Y in models of Parkinson’s disease by promoting Atg5-mediated but parkin-independent autophagy. Neurochem. Int. 2020;134:104671. doi: 10.1016/j.neuint.2020.104671. [DOI] [PubMed] [Google Scholar]
- 48.Haga H., Matsuo K., Yabuki Y., Zhang C., Han F., Fukunaga K. Enhancement of ATP production ameliorates motor and cognitive impairments in a mouse model of MPTP-induced Parkinson’s disease. Neurochem. Int. 2019;129:104492. doi: 10.1016/j.neuint.2019.104492. [DOI] [PubMed] [Google Scholar]
- 49.Ma Y., Zhan M., OuYang L., Li Y., Chen S., Wu J., Chen J., Luo C., Lei W. The effects of unilateral 6-OHDA lesion in medial forebrain bundle on the motor, cognitive dysfunctions and vulnerability of different striatal interneuron types in rats. Behav. Brain Res. 2014;266:37–45. doi: 10.1016/j.bbr.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 50.Floor E., Wetzel M.G. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J. Neurochem. 1998;70(1):268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- 51.Umek N., Geršak B., Vintar N., Šoštarič M., Mavri J. Dopamine autoxidation is controlled by acidic pH. Front. Mol. Neurosci. 2018;11:467. doi: 10.3389/fnmol.2018.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Opferman J.T., Kothari A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2018;25(1):37–45. doi: 10.1038/cdd.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang T., Sun Q., Chen S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016;147:1–19. doi: 10.1016/j.pneurobio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Limanaqi F., Biagioni F., Busceti C.L., Ryskalin L., Polzella M., Frati A., Fornai F. Phytochemicals bridging autophagy induction and alpha-synuclein degradation in parkinsonism. Int. J. Mol. Sci. 2019;20(13):3274. doi: 10.3390/ijms20133274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gautam S., Karmakar S., Batra R., Sharma P., Pradhan P., Singh J., Kundu B., Chowdhury P.K. Polyphenols in combination with β-cyclodextrin can inhibit and disaggregate α-synuclein amyloids under cell mimicking conditions: A promising therapeutic alternative. Biochim. Biophys. Acta. Proteins Proteomics. 2017;1865(5):589–603. doi: 10.1016/j.bbapap.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L.F., Yu X.L., Ji M., Liu S.Y., Wu X.L., Wang Y.J., Liu R.T. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson’s disease. Food Funct. 2018;9(12):6414–6426. doi: 10.1039/C8FO00964C. [DOI] [PubMed] [Google Scholar]
- 57.Liu Q., Zhu D., Jiang P., Tang X., Lang Q., Yu Q., Zhang S., Che Y., Feng X. Resveratrol synergizes with low doses of L-DOPA to improve MPTP-induced Parkinson disease in mice. Behav. Brain Res. 2019;367:10–18. doi: 10.1016/j.bbr.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 58.Guo Y.J., Dong S.Y., Cui X.X., Feng Y., Liu T., Yin M., Kuo S.H., Tan E.K., Zhao W.J., Wu Y.C. Resveratrol alleviates MPTP-induced motor impairments and pathological changes by autophagic degradation of α-synuclein via SIRT1-deacetylated LC3. Mol. Nutr. Food Res. 2016;60(10):2161–2175. doi: 10.1002/mnfr.201600111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Y., Li X., Zhu J.X., Xie W., Le W., Fan Z., Jankovic J., Pan T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals. 2011;19(3):163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pittenger S.T., Schaal V.L., Moore D., Guda R.S., Koul S., Yelamanchili S.V., Bevins R.A., Pendyala G. MicroRNA cluster miR199a/214 are differentially expressed in female and male rats following nicotine self-administration. Sci. Rep. 2018;8(1):17464. doi: 10.1038/s41598-018-35747-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z.H., Zhang J.L., Duan Y.L., Zhang Q.S., Li G.F., Zheng D.L. MicroRNA-214 participates in the neuroprotective effect of Resveratrol via inhibiting α-synuclein expression in MPTP-induced Parkinson’s disease mouse. Biomed. Pharmacother. 2015;74:252–256. doi: 10.1016/j.biopha.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 62.Cheng C., Huang C.L., Tsai C.J., Chou P.H., Lin C.C., Chang C.K. Alcohol-related dementia: A systemic review of epidemiological studies. Psychosomatics. 2017;58(4):331–342. doi: 10.1016/j.psym.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 63.Sachdeva A., Chandra M., Choudhary M., Dayal P., Anand K.S. Alcohol-related dementia and neurocognitive impairment: A review study. Int. J. High Risk Behav. Addict. 2016;5(3):e27976. doi: 10.5812/ijhrba.27976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerace E., Landucci E., Totti A., Bani D., Guasti D., Baronti R., Moroni F., Mannaioni G., Pellegrini-Giampietro D.E. Ethanol toxicity during brain development: Alterations of excitatory synaptic transmission in immature organotypic hippocampal slice cultures. Alcohol. Clin. Exp. Res. 2016;40(4):706–716. doi: 10.1111/acer.13006. [DOI] [PubMed] [Google Scholar]
- 65.Mandal C., Park K.S., Jung K.H., Chai Y.G. Ethanol-related alterations in gene expression patterns in the developing murine hippocampus. Acta Biochim. Biophys. Sin. (Shanghai) 2015;47(8):581–587. doi: 10.1093/abbs/gmv050. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y., Li S., Wang W., Xu C., Liang S., Liu M., Hao W., Zhang R. Beneficial effects of polydatin on learning and memory in rats with chronic ethanol exposure. Int. J. Clin. Exp. Pathol. 2015;8(9):11116–11123. [PMC free article] [PubMed] [Google Scholar]
- 67.Silva J.B.S., Cristino E.D., Almeida N.L., Medeiros P.C.B., Santos N.A.D. Effects of acute alcohol ingestion on eye movements and cognition: A double-blind, placebo-controlled study. PLoS One. 2017;12(10):e0186061. doi: 10.1371/journal.pone.0186061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pace M.C., Passavanti M.B., Aurilio C., Sansone P., Aurilio R.D.E., Maria S., Lama S., Federico A., Ravagnan G., Caraglia M., Stiuso P. Polydatin administration improves serum biochemical parameters and oxidative stress markers during chronic alcoholism: a pilot study. In Vivo. 2015;29(3):405–408. [PubMed] [Google Scholar]
- 69.Ranney A., Petro M.S. Resveratrol protects spatial learning in middle-aged C57BL/6 mice from effects of ethanol. Behav. Pharmacol. 2009;20(4):330–336. doi: 10.1097/FBP.0b013e32832f0193. [DOI] [PubMed] [Google Scholar]
- 70.Kawauchi T. Cdk5 regulates multiple cellular events in neural development, function and disease. Dev. Growth Differ. 2014;56(5):335–348. doi: 10.1111/dgd.12138. [DOI] [PubMed] [Google Scholar]
- 71.Gupta K.K., Singh S.K. Cdk5: A main culprit in neurodegeneration. Int. J. Neurosci. 2019;129(12):1192–1197. doi: 10.1080/00207454.2019.1645142. [DOI] [PubMed] [Google Scholar]
- 72.Shah K., Rossie S. Tale of the good and the bad Cdk5: Remodeling of the actin cytoskeleton in the brain. Mol. Neurobiol. 2018;55(4):3426–3438. doi: 10.1007/s12035-017-0525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Camp M.C., Mayfield R.D., McCracken M., McCracken L., Alcantara A.A. Neuroadaptations of Cdk5 in cholinergic interneurons of the nucleus accumbens and prefrontal cortex of inbred alcohol-preferring rats following voluntary alcohol drinking. Alcohol. Clin. Exp. Res. 2006;30(8):1322–1335. doi: 10.1111/j.1530-0277.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- 74.Abarzúa S., Ampuero E., van Zundert B. Superoxide generation via the NR2B-NMDAR/RasGRF1/NOX2 pathway promotes dendritogenesis. J. Cell. Physiol. 2019;234(12):22985–22995. doi: 10.1002/jcp.28859. [DOI] [PubMed] [Google Scholar]
- 75.Chandrasekar R. Alcohol and NMDA receptor: current research and future direction. Front. Mol. Neurosci. 2013;6:14. doi: 10.3389/fnmol.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu C.Y., Li S., Chen L., Hou F.J., Zhng R.L. Effect of polydatin on learning and memory and expression of NR2B in the prefrontal cortex of rats with chronic alcoholism. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2011;27(2):220–235. [PubMed] [Google Scholar]
- 77.Gibson G.E., Hirsch J.A., Fonzetti P., Jordan B.D., Cirio R.T., Elder J. Vitamin B1 (thiamine) and dementia. Ann. N. Y. Acad. Sci. 2016;1367(1):21–30. doi: 10.1111/nyas.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pan X., Sang S., Fei G., Jin L., Liu H., Wang Z., Wang H., Zhong C. Enhanced activities of blood thiamine diphosphatase and monophosphatase in Alzheimer’s disease. PLoS One. 2017;12(1):e0167273. doi: 10.1371/journal.pone.0167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kasse E., Oudman E., Olivier M., Wijnia J.W., Postma A. Subtle object location perception deficits in Korsakoff’s syndrome. J. Clin. Exp. Neuropsychol. 2019;41(9):881–887. doi: 10.1080/13803395.2019.1640864. [DOI] [PubMed] [Google Scholar]
- 80.Mason A., Holmes C., Edwards C.J. Inflammation and dementia: Using rheumatoid arthritis as a model to develop treatments? Autoimmun. Rev. 2018;17(9):919–925. doi: 10.1016/j.autrev.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 81.McColl B.W., Allan S.M., Rothwell N.J. Systemic infection, inflammation and acute ischemic stroke. Neuroscience. 2009;158(3):1049–1061. doi: 10.1016/j.neuroscience.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 82.Shah F.A., Kury L.A., Li T., Zeb A., Koh P.O., Liu F., Zhou Q., Hussain I., Khan A.U., Jiang Y., Li S. Polydatin attenuates neuronal loss via reducing neuroinflammation and oxidative stress in rat MCAO models. Front. Pharmacol. 2019;10:663. doi: 10.3389/fphar.2019.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lv R., Du L., Zhang L., Zhang Z. Polydatin attenuates spinal cord injury in rats by inhibiting oxidative stress and microglia apoptosis via Nrf2/HO-1 pathway. Life Sci. 2019;217:119–127. doi: 10.1016/j.lfs.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 84.Qiao H., Chen H., Dong Y., Ma H., Zhao G., Tang F., Li Z. Polydatin attenuates H2O2-induced oxidative stress via PKC pathway. Oxid. Med. Cell. Longev. 2016;2016:5139458. doi: 10.1155/2016/5139458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tong Y., Wang K., Sheng S., Cui J. Polydatin ameliorates chemotherapy-induced cognitive impairment (chemobrain) by inhibiting oxidative stress, inflammatory response, and apoptosis in rats. Biosci. Biotechnol. Biochem. 2020;84(6):1201–1210. doi: 10.1080/09168451.2020.1722057. [DOI] [PubMed] [Google Scholar]
- 86.Dheen S.T., Kaur C., Ling E.A. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007;14(11):1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- 87.Hansen D.V., Hanson J.E., Sheng M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018;217(2):459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ho M.S. Microglia in Parkinson’s Disease. Adv. Exp. Med. Biol. 2019;1175:335–353. doi: 10.1007/978-981-13-9913-8_13. [DOI] [PubMed] [Google Scholar]
- 89.Lv R., Du L., Liu X., Zhou F., Zhang Z., Zhang L. Polydatin alleviates traumatic spinal cord injury by reducing microglial inflammation via regulation of iNOS and NLRP3 inflammasome pathway. Int. Immunopharmacol. 2019;70:28–36. doi: 10.1016/j.intimp.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 90.Kusakawa Y., Mikawa S., Sato K. BMP7 expression in the adult rat brain. IBRO Rep. 2017;3:72–86. doi: 10.1016/j.ibror.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lauzon M.A., Drevelle O., Faucheux N. Peptides derived from the knuckle epitope of BMP-9 induce the cholinergic differentiation and inactivate GSk3beta in human SH-SY5Y neuroblastoma cells. Sci. Rep. 2017;7(1):4695. doi: 10.1038/s41598-017-04835-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sato T., Mikawa S., Sato K. BMP2 expression in the adult rat brain. J. Comp. Neurol. 2010;518(22):4513–4530. doi: 10.1002/cne.22469. [DOI] [PubMed] [Google Scholar]
- 93.Elliott C., Rojo A.I., Ribe E., Broadstock M., Xia W., Morin P., Semenov M., Baillie G., Cuadrado A., Al-Shawi R., Ballard C.G., Simons P., Killick R. A role for APP in Wnt signalling links synapse loss with β-amyloid production. Transl. Psychiatry. 2018;8(1):179. doi: 10.1038/s41398-018-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jia L., Piña-Crespo J., Li Y. Restoring Wnt/β-catenin signaling is a promising therapeutic strategy for Alzheimer’s disease. Mol. Brain. 2019;12(1):104. doi: 10.1186/s13041-019-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tapia-Rojas C., Inestrosa N.C. Loss of canonical Wnt signaling is involved in the pathogenesis of Alzheimer’s disease. Neural Regen. Res. 2018;13(10):1705–1710. doi: 10.4103/1673-5374.238606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen X.J., Shen Y.S., He M.C., Yang F., Yang P., Pang F.X., He W., Cao Y.M., Wei Q.S. Polydatin promotes the osteogenic differentiation of human bone mesenchymal stem cells by activating the BMP2-Wnt/β-catenin signaling pathway. Biomed. Pharmacother. 2019;112:108746. doi: 10.1016/j.biopha.2019.108746. [DOI] [PubMed] [Google Scholar]
- 97.Peterson B., Weyers M., Steenekamp J.H., Steyn J.D., Gouws C., Hamman J.H. Drug bioavailability enhancing agents of natural origin (bioenhancers) that modulate drug membrane permeation and pre-systemic metabolism. Pharmaceutics. 2019;11(1):E33. doi: 10.3390/pharmaceutics11010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Basta-Kaim A., Ślusarczyk J., Szczepanowicz K., Warszyński P., Leśkiewicz M., Regulska M., Trojan E., Lasoń W. Protective effects of polydatin in free and nanocapsulated form on changes caused by lipopolysaccharide in hippocampal organotypic cultures. Pharmacol. Rep. 2019;71(4):603–613. doi: 10.1016/j.pharep.2019.02.017. [DOI] [PubMed] [Google Scholar]