Abstract
Laboratory diagnostics play an essential role in pandemic preparedness. In January 2020, the first US case of COVID-19 was confirmed in Washington State. At the same time, the Washington State Public Health Laboratory (WA PHL) was in the process of building upon and initiating innovative preparedness activities to strengthen laboratory testing capabilities, operations, and logistics. The response efforts of WA PHL, in conjunction with the Centers for Disease Control and Prevention, to the COVID-19 outbreak in Washington are described herein—from the initial detection of severe acute respiratory syndrome coronavirus 2 through the subsequent 2 months.
Factors that contributed to an effective laboratory response are described, including preparing early to establish testing capacity, instituting dynamic workforce solutions, advancing information management systems, refining laboratory operations, and leveraging laboratory partnerships. We also report on the challenges faced, successful steps taken, and lessons learned by WA PHL to respond to COVID-19.
The actions taken by WA PHL to mount an effective public health response may be useful for US laboratories as they continue to respond to the COVID-19 pandemic and may help inform current and future laboratory pandemic preparedness activities.
The first US case of COVID-19 was confirmed in Washington State and was announced on January 20, 2020, by the US Centers for Disease Control and Prevention (CDC) and the Washington State Department of Health (WA DOH).1 By March 31, all 39 counties in Washington were reporting laboratory-confirmed cases (n = 5771) resulting in 290 deaths.2 At the time, the largest number of confirmed cases (n = 2709) and infections resulting in death (n = 181) were reported from King County, Washington. As severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continued to spread through Washington over the subsequent months, residents remained at risk for exposure, and the availability and capacity of COVID-19 laboratory testing remained paramount to an effective public health response to this pandemic. Diagnostic testing can confirm infection, guide patient care, improve understanding of the spread of SARS-CoV-2, and inform the implementation of evidence-based measures to slow transmission.
In response to reports of COVID-19, CDC established an Incident Management System on January 7, 2020; it activated the Emergency Operations Center in Atlanta, Georgia, on January 20 to provide continuing and enhanced support to the outbreak response. Multidisciplinary teams were deployed by CDC to support state health departments in epidemiological investigations, clinical management, public communications, and laboratory operations. On January 21, Washington State Public Health Laboratory (WA PHL) established an on-site Incident Management Team (IMT) to facilitate response activities and support laboratory logistics and operations. To effectively coordinate with CDC and public health partners, the Association of Public Health Laboratories also established its Incident Command System and activated its Emergency Operations Center on January 22.
CDC developed the 2019-novel coronavirus (2019-nCoV) real-time reverse transcriptase polymerase chain reaction (rRT-PCR) diagnostic panel to detect SARS-CoV-2 from upper- and lower-respiratory specimens, and the US Food and Drug Administration (FDA) issued an Emergency Use Authorization for the test on February 4, 2020.3 WA PHL began testing for SARS-CoV-2 using the CDC assay on February 28, 2020, and communicated with state and federal government leadership, such as the leadership of the Epidemiology and Laboratory Capacity for Infectious Diseases Cooperative Agreement, to secure funding and redirect work to support the response in Washington.
We report here on the pioneering response efforts of WA PHL, together with CDC, to the COVID-19 outbreak in Washington. A timeline of events and laboratory-confirmed cases during this response (through March 31, 2020) is summarized in Figure 1. Factors that contributed to an effective laboratory response included
preparing early to establish testing capacity,
instituting dynamic workforce solutions,
advancing information management systems,
refining laboratory operations, and
leveraging laboratory partnerships.
FIGURE 1—
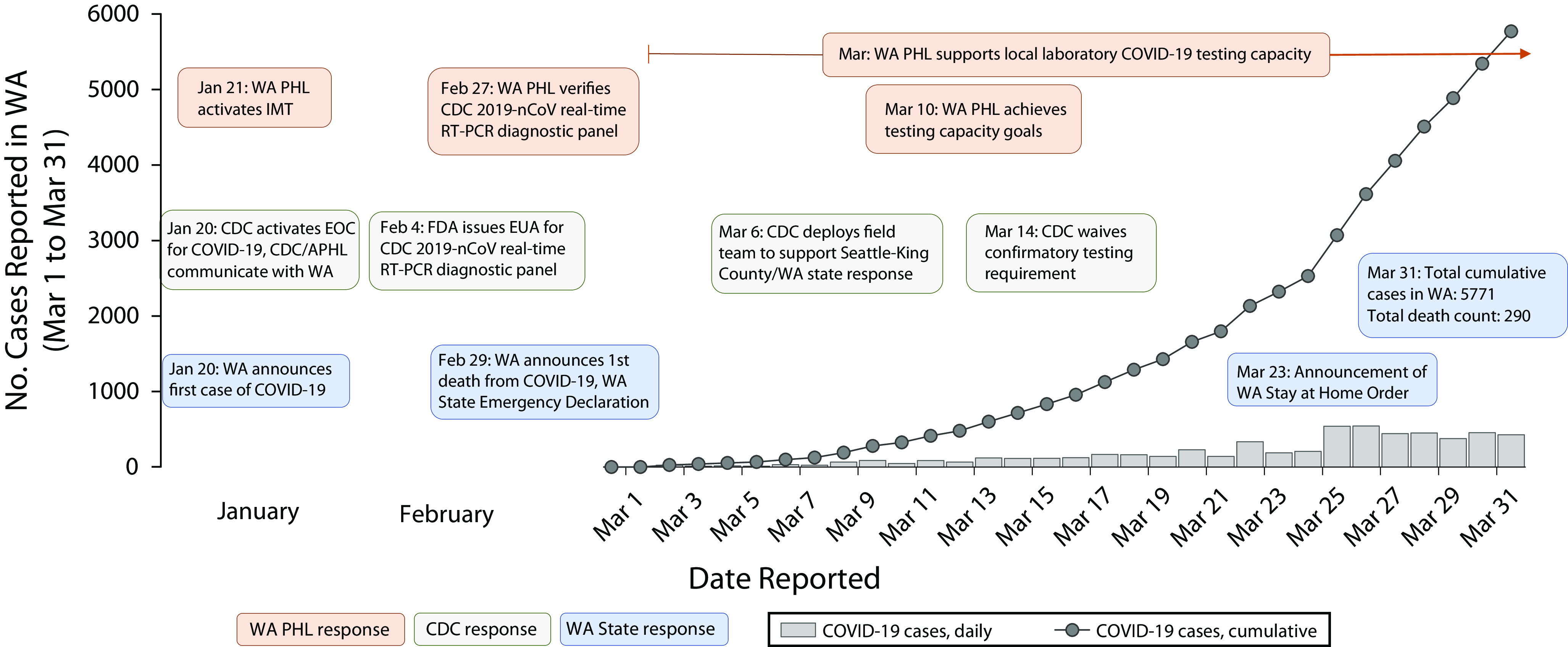
A Timeline of Events and Laboratory-Confirmed COVID-19 Cases During the COVID-19 Response: Washington State; March 1–31, 2020
Note. 2019-nCoV = 2019-novel coronavirus; APHL = Association of Public Health Laboratories; CDC = Centers for Disease Control and Prevention; EUA = Emergency Use Authorization; FDA = US Food and Drug Administration; IMT = Incident Management Team; PHL = Public Health Laboratory; RT-PCR = reverse transcriptase polymerase chain reaction; WA = Washington State. Daily cases (gray bars) and cumulative cases (gray line) are shown. Key events are displayed in text bubbles; WA PHL (orange), CDC (gray), and Washington (blue).
Source. Washington State Department of Health.2
Progress, challenges, and lessons learned in each of these areas are discussed subsequently and summarized in the box on p. 869.
Lessons Learned During the Washington State Public Health Laboratory Response to the COVID-19 Pandemic: Actions That Contributed to an Effective Laboratory Response.
| 1. Initial response preparations and establishing testing capacity |
| • Early and regular assessment of laboratory needs by management and rapid procurement of critical testing supplies, reagents, and equipment |
| • Timely and clear communication among local, state, and federal public health entities regarding onboarding and verification of the diagnostic test |
| 2. Management of a dynamic workforce |
| • Establishment of laboratory-adapted Incident Management Team structure early and approval of continuity of operations plan later in the response to strategically manage staff and maintain productivity |
| • Skilled personnel cross-trained and diverted to the response |
| • Flexibility by management to meet staffing (hiring) and staff (work availability) needs to maintain laboratory operations |
| • Laboratory and epidemiology staff working in proximity, facilitating real-time collaboration between groups |
| 3. Advancing laboratory information management |
| • Development of an internal dashboard tool to share critical, real-time, response-specific information with all involved staff |
| • Development and implementation of an online, barcoded accessioning system to encourage standardization and interoperability of data management |
| • Clear communication and messaging around specimen submission requirements for diagnostic testing to health care providers and submitters statewide |
| 4. Refining laboratory operations and building testing capacity |
| • Employing redundancy in equipment and identifying alternative sources for testing media and reagents in anticipation of supply shortages |
| • Early establishment of clear criteria through standard operating procedures for accepting, rejecting, and redirecting specimens to ensure testing capacity goals were manageable |
| • Waived testing approvals and prioritization processes to ensure continuity of testing and to resolve backlog |
| • Refined laboratory operations to increase testing throughput and maximize use of resources |
| • Staff dedicated to regularly updating inventory needs and 1 full-time operations staff member to maintain supplies (ideally a microbiologist) |
| 5. Leveraging laboratory partnerships |
| • Maximization of local testing capacities (decentralization), rather than relying on the state PHL testing capacity (centralization) |
| • PHL aided local laboratories and hospitals to onboard diagnostic testing by providing test validation materials and technical and regulatory guidance |
| • Engaging in reagent and supply sharing with local laboratory partners as a stopgap measure during times of supply shortages |
| • Working with local and federal partners to ensure fundamental research and public health questions surrounding the response were addressed and public health and policy decision-making were informed by data |
Note. PHL = Public Health Laboratory.
ESTABLISHING TESTING CAPABILITY AND EARLY PREPARATIONS
Encountering novel pathogens for which no diagnostic test or treatment exists presents a unique set of challenges to public health laboratories. In the case of COVID-19, CDC initially developed and distributed a test that included 3 primer‒probe sets for the detection of viral genetic markers (N1 and N2 to detect 2 regions in the SARS-CoV-2 nucleocapsid [N] gene and N3 for the universal detection of SARS-like coronaviruses) as part of the 2019-nCoV rRT-PCR Diagnostic Panel.3 To perform CDC’s in vitro diagnostic assay under the Emergency Use Authorization and report COVID-19 results, laboratories were required to verify test performance. WA PHL received the CDC test and began the verification process on February 8, 2020; however, N3 reactivity did not match expected results, and this observation was reported back to CDC. After receiving an enforcement discretion from FDA, which gave CDC time to investigate the problem and modify the assay, CDC advised testing laboratories to exclude the N3 primer‒probe set.4 Subsequently, WA PHL verified the performance of the modified assay on February 27, 2020, and identified its first presumptive positive COVID-19 case 2 days later.
In early March 2020, Washington was 1 of 14 states to receive an Epidemiology and Laboratory Capacity grant for COVID-19 epidemiology and laboratory capacity functions. As the outbreak continued to expand in Washington and around the United States, additional COVID-19 testing capacity became available through commercial and academic laboratories. The University of Washington Medicine Clinical Virology Laboratory also began testing for SARS-CoV-2 on March 2, increasing test capacity across Washington.5 To increase its own testing capacity, WA PHL preemptively ordered supplies and reagents to reach the upper limit of its surge-testing capacity and acquired additional testing equipment. The goal was to have a 10- to 14-day inventory of critical testing supplies and reagents, but high demand rapidly affected the national supply chain, including CDC’s International Reagent Resource.6
WA PHL worked with CDC to streamline acquisition of critical supplies from federal partners, the International Reagent Resource, and an extended list of commercial vendors to mitigate supply chain difficulties. The challenges with verification of the initial CDC in vitro diagnostic assay at WA PHL resulted in lost time during its initial response efforts to implement testing.
RESTRUCTURING PUBLIC HEALTH LABORATORY STAFF
WA PHL rapidly reorganized its skilled workforce to increase COVID-19 testing efficiency by establishing a laboratory IMT structure. The IMT was arranged based on key components of COVID-19 testing operations: specimen accessioning, RNA extraction, PCR assay, data management, logistics, facilities, and applications (Figure 2). A liaison between epidemiology and laboratory operations was established to facilitate communication and optimize collaboration between these groups. Furthermore, on March 8, 2 on-site CDC liaisons were added to the IMT structure at WA PHL to assist coordinated federal and state-level response efforts. Each group supervisor provided twice-daily updates on supply and reagent inventory, staffing needs, equipment, and operations to quickly identify and address issues related to the testing process. In addition, the King County Department of Community and Human Services approved initiation of WA PHL’s COVID-19 continuity of operations plan,7 which allowed it to halt or divert nonessential diagnostic, surveillance, and environmental testing, freeing staff with relevant expertise to be assigned to SARS-CoV-2 testing operations.
FIGURE 2—
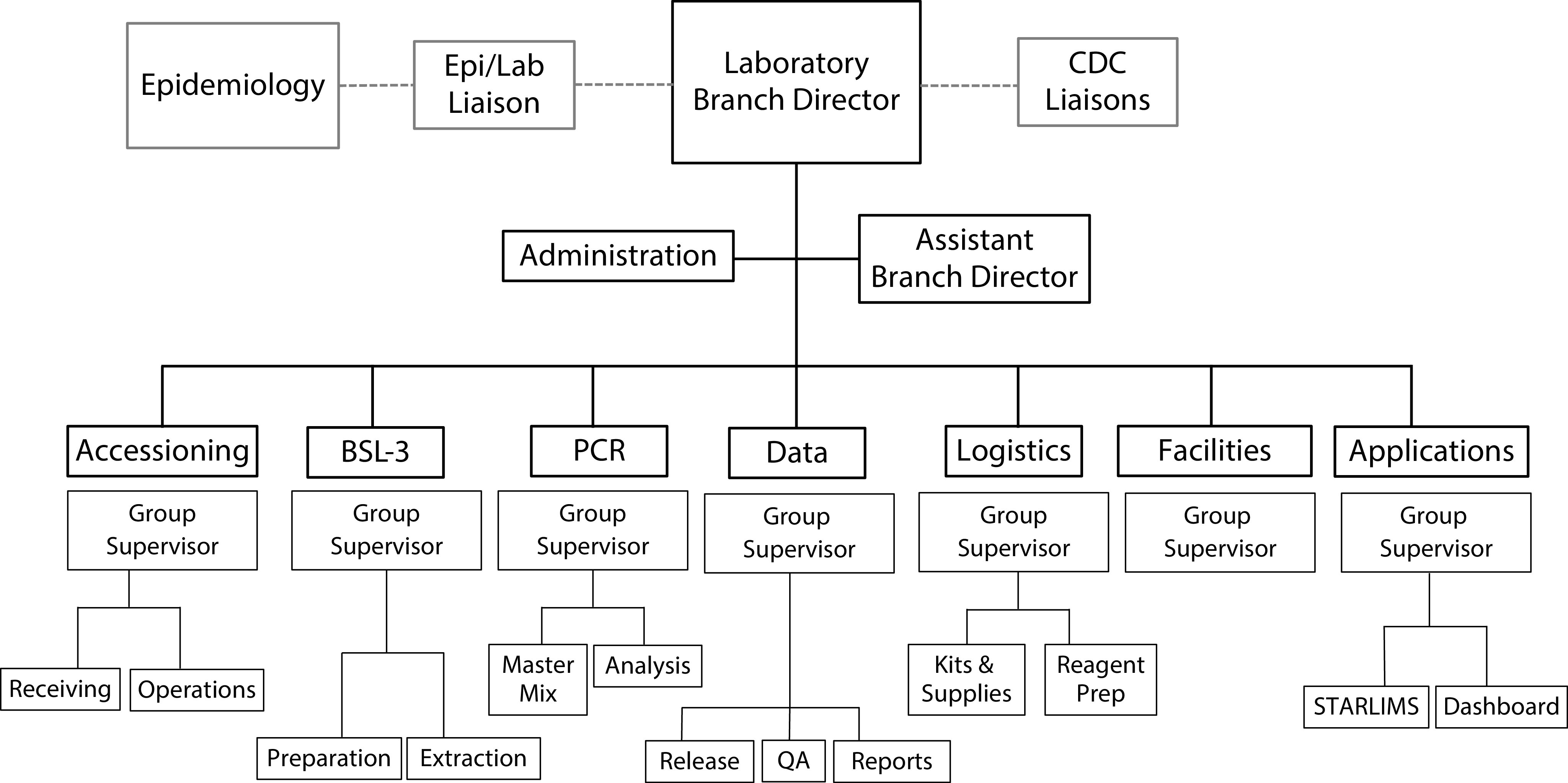
Washington State Public Health Laboratory Adapted Incident Management Team Structure for the COVID-19 Response
Note. BSL = biosafety level; CDC = Centers for Disease Control and Prevention; Epi/Lab = epidemiology/laboratory; PCR = polymerase chain reaction; QA = quality assurance.
Maintaining continuity of testing relied on dynamic restructuring of the WA PHL workforce. Biodefense laboratory staff in the BioWatch program8 were redirected to the COVID-19 response, and other staff members were reassigned to data-entry positions to help with accessioning when specimen receipt volumes intensified. Initially, emergency activation status allowed the laboratory to hire 6 additional nonpermanent staff, including 4 microbiologists, to help achieve surge-testing capacity goals. Federal assistance supported continued onboarding of additional staff members during 2020 to help relieve staff and prevent burnout. Existing trained personnel were diverted to the COVID-19 response, and staff working volunteer-based overtime hours allowed the laboratory to expand testing to 17 hours a day, 7 days per week. Following Seattle, Washington, school closures on March 12, work shifts were adapted to provide flexibility for staff who were parents to young children. A collaboration between WA PHL and University of Washington facilitated hiring of students from the School of Public Health to assist in epidemiology and accessioning roles. WA PHL epidemiologists and laboratory scientists worked in the same physical location, and this facilitated close and timely collaboration between these 2 groups. Overall, early establishment of an IMT structure enabled WA PHL to efficiently coordinate and maintain laboratory testing operations while providing the flexibility to adapt workforce needs to meet challenges faced during the outbreak.
ADVANCING INFORMATION MANAGEMENT SYSTEMS
COVID-19 specimens were received in larger numbers than WA PHL had previously managed, straining existing laboratory information management systems. The applications group, working in information technology, developed and operationalized an internal digital dashboard early in the response. It provides a visual summary of the current status and trends of COVID-19 testing information to monitor progress and impact in real time. The dashboard is used to share information throughout WA PHL including in-house specimen inventory in queue for testing, specimen status in the testing workflow, and specimen test results (positive, negative, inconclusive). The dashboard facilitated improved laboratory–epidemiology communication and allowed the laboratory to quickly adapt its specimen prioritization strategy as needed.
During normal operations, WA PHL manually accessioned specimens submitted to the laboratory; however, high demand for COVID-19 testing resulted in a bottleneck when specimen information was entered into the Laboratory Information Management System. To mitigate the bottleneck, WA PHL established a partnership with Microsoft to develop and implement an online, custom-built, barcoded electronic test-ordering system to streamline its workflow. The system allowed submitters to fill out test requisition forms online before submitting specimens to the laboratory for testing, and required fields ensured that submitters provided essential information. When printed, the form generated a Quick Response code capturing all the information entered by the submitting facility. Upon receipt at the laboratory, the Quick Response code was scanned and information was autotranscribed into the Laboratory Information Management System. This reduced errors in manually transcribed submission forms and allowed for accessioning of specimens in seconds rather than minutes. When the electronic test ordering system went live at WA PHL on April 10, 2020, 25 health care facilities across the state began using the online portal to complete and submit test requisition forms electronically.
Another challenge occurred when specimens lacking essential, associated information were submitted for testing, which required additional, time-consuming case-finding efforts. As specific patient information pertaining to symptoms and previous travel was initially required to inform testing priorities (e.g., approval of person under investigation by epidemiology staff), personnel were required to contact specimen submitters for this information to proceed with testing. The WA DOH and PHL worked with local jurisdictions across Washington to create messaging around specimen submission requirements for COVID-19 testing to health care providers and submitters statewide. A quality assurance team under the data group in the IMT structure was established to provide educational outreach to submitters regarding appropriate labeling of specimens and requisition forms. Clear communication to specimen submitters and innovation to improve existing information management systems were central to WA PHL’s ability to accommodate the unprecedented specimen processing demands during the outbreak.
REFINING OPERATIONS AND BUILDING TESTING CAPACITY
Enhancing testing capacity for timely diagnosis of COVID-19 was essential to the response efforts in Washington. Redundancy in approved COVID-19 testing platforms was employed to ensure continuity of testing in anticipation of supply and reagent shortages. To comply with quality management systems employed by WA PHL, standard operating procedures, risk assessments, and plans to verify test performance were compiled before delivery of new equipment. WA PHL maximized their testing workflow by developing a COVID-19–specific specimen processing standard operating procedure that established accept, reject, and redirect criteria; specified instructions for testing COVID-19 specimens in the order of receipt; and waived epidemiology-based testing approvals to test specimens meeting person-under-investigation criteria to ensure continuity of testing and to resolve backlogs. Initially, to increase throughput of testing, the sample layout on each rRT-PCR plate was reoriented when using alternative extraction platforms. This ensured the maximum number of samples were tested on each plate per run. In subsequent months, additional high-throughput real-time PCR instruments and fully automated sample-to-result systems were purchased.
A major hurdle in maximizing testing capacity was the shortage of testing supplies, including swabs, transport media, and RNA extraction reagents. Two CDC liaisons were stationed at WA PHL to directly communicate laboratory needs related to supply shortages and provide guidance on amendments under the Emergency Use Authorization. Because of the scarcity of supplies, CDC wrote and shared a standard operating procedure for in-house preparation of viral transport media and provided swabs and media to mitigate shortages. WA PHL utilized Laboratory Response Network partners, such as the nearby Madigan Army Medical Center, for stopgap reagent sharing to mitigate impact of supply shortages. The FDA also released alternative recommendations including a list of example products and different distributors for testing supplies that were otherwise limited in availability.9
WA PHL was able to achieve its surge capacity goal of testing 400 specimens per day by March 10 (Figure 1). By the end of 2020, the laboratory could perform 1500 tests per day, 7 days per week. WA PHL and University of Washington Medicine Clinical Virology Laboratory provided CDC with suggestions for improving testing throughput, including verification of a test using a single viral target and development of a multiplex PCR assay. Refining laboratory operations and implementing redundancy in testing processes provided flexibility when kits and reagents were limited. In retrospect, WA PHL realized that having a dedicated microbiologist for procurement of testing supplies with an understanding of the COVID-19 testing process would be helpful to identify multiple vendors and acquire analogous supplies to those recommended by the FDA that could be limited in availability in a rapid-response situation.
LEVERAGING LABORATORY PARTNERSHIPS
Many of the US public health laboratory systems and networks in place today were created in the wake of past public health emergency events (e.g., anthrax attacks [2001], Hurricane Katrina [2005], the threat of pandemic influenza [2017–2018]). The sentinel clinical laboratory network in Washington, part of the Laboratory Response Network, facilitates partnerships with local, state, and federal laboratories to recognize and respond to emerging public health threats. The Clinical Laboratory Advisory Council, which serves as an advisory group to the Washington DOH, was also established more than 25 years ago with the vision to develop public–private partnership among the laboratory community. WA PHL’s response to COVID-19 has further highlighted how partnerships within public health laboratory systems and beyond enhanced the collective laboratory response in Washington.
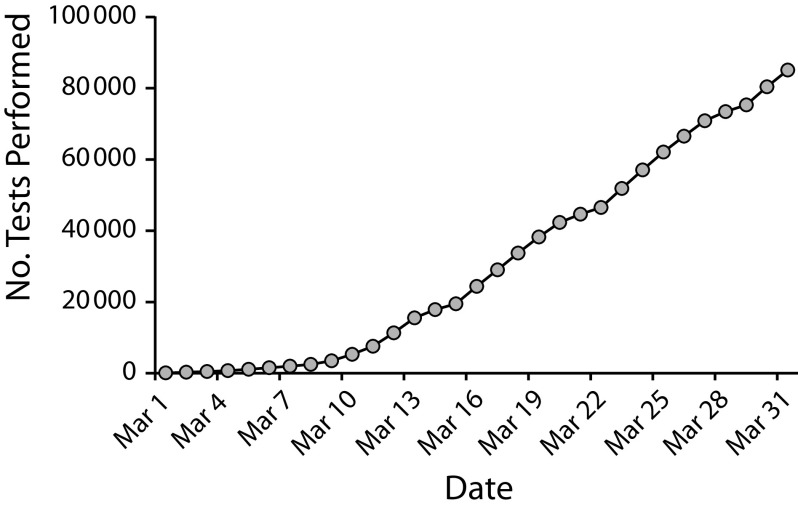
Anticipating a high demand for COVID-19 testing, WA PHL management prioritized building capacity by establishing testing partners in the state. As a state PHL with a longstanding history of spearheading laboratory network initiatives10 and maintaining a robust laboratory quality assurance program,11 WA PHL was and continues to be a resource for academic, clinical, and commercial laboratories requesting technical and regulatory guidance, reagents, and resources for COVID-19 testing. By March 31, WA PHL in partnership with the DOH’s State Laboratory Quality Assurance Program had assisted 15 local laboratories and hospitals to establish COVID-19 testing by providing test validation materials. The WA PHL also provided technical guidance on validation requirements and regulatory guidance in compliance with regulations set forth by the Centers for Medicare and Medicaid Services through the Clinical Laboratory Improvement Amendments and FDA Emergency Use Authorization regulatory requirements. Communication between the state laboratory and local testing sites occurred via the WA DOH Laboratory Quality Assurance channel, where WA PHL acted as the resource for all local testing inquiries. The Association of Public Health Laboratories also supported the response by providing member laboratories assistance with quality testing, reporting, technical matters, and communications. Collective testing capacity of local and national laboratories contributed to the considerable number of COVID-19 tests completed in Washington during the outbreak (Figure 3).2
FIGURE 3—
Cumulative COVID-19 Tests Performed by Local, State, and National Laboratories Supporting Washington Testing Capacity: March 1–31, 2020)
Note. As of March 31, 2020, 15 local medical laboratories, comprising 3 nonprofit, 6 hospital, and 6 commercial laboratories, were supported by the Washington State Public Health Laboratory to onboard COVID-19 testing.
In addition to testing and support, WA PHL worked with local and federal partners to ensure that fundamental research and public health questions around SARS-CoV-2 testing and COVID-19 were addressed and public health and policy decision-making were informed by data. For example, WA PHL collaborated with the Fred Hutchinson Cancer Research Center to provide de-identified aliquots of SARS-CoV-2–positive specimens to inform genomic epidemiology and improve understanding of the evolution of the virus as the pandemic progressed.12 WA PHL also worked with the Seattle and King County DOH and the CDC to demonstrate asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term skilled nursing facility in King County.13,14
CONCLUSIONS
The current COVID-19 pandemic marks the third emergence of a novel coronavirus in the 21st century, following severe acute respiratory syndrome (SARS) in 2002 and Middle East respiratory syndrome (MERS) in 2012,15 and highlights the continual global public health threat posed by respiratory viruses. It has been reported that the confirmed cases of COVID-19 in the United States do not accurately reflect the total burden of the pandemic.16 It is probable that SARS-CoV-2 infection in the population of Washington during this time outnumbered the laboratory-confirmed cases reported herein, as testing was limited and guidance for meeting person-under-investigation and testing criteria was more heavily focused on symptomatic individuals. Insufficient testing, such as undiagnosed asymptomatic infections, as well as imperfect test accuracy, have been shown to contribute to this difference.16 Thus, health care, social, and economic impacts of the COVID-19 pandemic have resulted in unprecedented challenges to our public health systems in responding to and controlling the outbreak.
In the United States, state and local public health departments and laboratories are central to the effective management of major health crises. This work describes challenges faced, successful steps taken, and lessons learned by WA PHL to respond to COVID-19 (see box on p. 869). Here, we reported WA PHL response efforts from initial detection of SARS-CoV-2 in the United States on January 20, 2020, through the subsequent 2 months of the outbreak in Washington, to inform other US laboratories mounting their own responses to COVID-19, as well as future laboratory pandemic preparedness activities. To conclude, we summarize the gaps and needs informed by the operational-level experience of WA PHL in response to the COVID-19 outbreak in Washington and offer possible approaches to advance the systems underlying and supporting the public health laboratory’s core functions.17
The COVID-19 response in Washington required timely communication and standardized information sharing among laboratories, health care practitioners, and public health officials at local, state, and federal levels. The time required initially to verify the rRT-PCR diagnostic assay stalled efforts early in the response and resulted in testing delays. Aspects of information management and reporting systems requiring manual inputs placed a burden on public health staff and data managers to input, share, and analyze critical information and data specific to the COVID-19 outbreak. In addition, interoperability to achieve integrated data management among clinical, private, and public health partners was required. Improved information exchange among these partners and clear messaging for specimen submitters could greatly reduce time spent on retrospectively rectifying submitter information discrepancies, which was unsustainable for WA DOH staff during the COVID-19 outbreak.
WA PHL implemented short-term changes to their Laboratory Information Management System and public health information management systems to adapt to the outbreak. The laboratory partnered with Microsoft to develop standardized test requisition forms with barcode accessioning to reduce accessioning time and error. WA PHL also developed an internal digital dashboard for timely communication of critical information to all laboratory staff involved in the response. CDC staff stationed onsite at WA PHL improved interagency communication within the context of the outbreak; however, it also highlighted the need for long-term, sustainable solutions for improving communication between state and federal public health partners. Implementation of modern, standardized, and integrated laboratory information management systems and broader health information systems could mitigate a future need for short-term, stop-gap solutions like those described previously.
Shortages in essential reagents, supplies, and personal protective equipment to perform COVID-19 testing continues to be one of the greatest challenges to laboratory response during this pandemic. Federally managed public information sources, such as supply availability, may reduce time spent on inquiries. This could be accomplished via an online dashboard offering a list of vendors for all approved supplies and alternatives, as well as real-time updates of their availability during an outbreak response event. This approach could be expanded to communicate standardized federal (CDC and FDA) guidance documents, funding sources available to support laboratory and workforce infrastructure, and other key information, providing timely notifications to partners when updates are made during a response.
Lessons learned by WA PHL highlight elements it found to be critical to an effective public health response such as (1) timely, consistent communication between public health partners at local, state, and federal levels; (2) modern, standardized, and integrated health information management systems; and (3) adequate resources to effectively begin diagnostic testing and build surge capacity. Federal oversight of critical supplies and the ability for states to request and obtain supplies managed by the Strategic National Stockpile18 may be helpful during shortages. Adapting and streamlining laboratory testing is not only vital during response surge testing but it also builds process efficiency during nonoutbreak responses as well as preparedness for future public health emergencies.
Early and ongoing COVID-19 response efforts by WA PHL paved the way for other US laboratories to mount similar responses. Moreover, creation of a comprehensive response framework relies on building and maintaining strong partnerships. WA PHL acted as a key central partner to many public, private, and commercial laboratories in Washington, providing support and guidance for onboarding testing and strengthening local testing capacity. Actions taken by WA PHL during the COVID-19 pandemic may be useful toward developing a national system of public health surveillance and response. Lessons learned will be valuable as we work together as a nation to continue responding to this pandemic.
ACKNOWLEDGMENTS
We thank Margaret A. Honein, PhD, and Thomas A. Clark, MD, at the Centers for Disease Control and Prevention for their leadership during our field team’s deployment to Seattle, Washington, for the COVID-19 response. We also thank the Washington State Public Health Laboratories’ SARS-CoV-2 testing team and the Association of Public Health Laboratories for their contributions supporting the COVID-19 response.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
HUMAN PARTICIPANT PROTECTION
This study did not involve any experiment or survey that requires human participation; therefore, it is exempted from institutional review board approval.
Footnotes
REFERENCES
- 1.Holshue ML, DeBolt C, Lindquist S et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Washington State Department of Health. Novel Coronavirus Outbreak (COVID-19) 2019. Available at: https://www.doh.wa.gov/Emergencies/NovelCoronavirusOutbreak2020COVID19/DataDashboard. Accessed July 24, 2020.
- 3.Centers for Disease Control and Prevention. 2019-nCoV Real Time RT-PCR Diagnostic Panel instructions for use. 2020. Available at: https://www.fda.gov/media/134922/download. Accessed July 24, 2020.
- 4.Centers for Disease Control and Prevention. Revision to test instructions. CDC 2019 Novel Coronavirus (nCoV) Real-Time RT-PCR Diagnostic Panel (EUA200001) Available at: https://www.aphl.org/Materials/Signed_CDC_Letter_to_PHLs-N3_Removal_Instructions_26Feb2020.pdf. Accessed July 24, 2020.
- 5.Greninger AL, Jerome KR. The first quarter of SARS-CoV-2 testing: The University of Washington Medicine experience. J Clin Microbiol. 2020;58(8):e01416–e01420. doi: 10.1128/JCM.01416-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. International Reagent Resource. 2020. Available at: https://www.internationalreagentresource.org. Accessed July 24, 2020.
- 7.Association of Public Health Laboratories; Guidelines for the Public Health Laboratory Continuity of Operations Plan (COOP) 2011. Available at: https://www.aphl.org/programs/preparedness/Documents/PHPR_2011Feb_PHL-Continuity-of-Operations-Guidelines.pdf. Accessed July 24, 2020.
- 8.Biowatch and Public Health Surveillance. Evaluating Systems for the Early Detection of Biological Threats: Abbreviated Version. Institute of Medicine (US) and National Research Council (US) Committee on Effectiveness of National Biosurveillance Systems: Biowatch and the Public Health System; Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 9.US Food and Drug Administration. FAQs on diagnostic testing for SARS-CoV-2. Available at: https://www.fda.gov/medical-devices/emergency-situations-medical-devices/faqs-diagnostic-testing-sars-cov-2#troubleobtainingviraltransport. Accessed July 24, 2020.
- 10.Astles JR, White VA, Williams LO. Origins and development of the National Laboratory System for public health testing. Public Health Rep. 2010;125(2):18–30. doi: 10.1177/00333549101250S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Washington State Department of Health. Laboratory Quality Assurance. 2020. Available at: https://www.doh.wa.gov/LicensesPermitsandCertificates/FacilitiesNewReneworUpdate/LaboratoryQualityAssurance. Accessed July 24, 2020.
- 12.Bedford T, Greninger AL, Roychoudhury P et al. Cryptic transmission of SARS-CoV-2 in Washington State. Science. 2020;370(6516):571–575. doi: 10.1126/science.abc0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimball A, Hatfield KM, Arons M et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington. MMWR Morb Mortal Wkly Rep. 2020;69(13):377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arons MM, Hatfield KM, Reddy SC et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munster VJ, Koopmans M, van Doremalen N et al. A novel coronavirus emerging in China—key questions for impact assessment. N Engl J Med. 2020;382(8):692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 16.Wu SL, Mertens AN, Crider YS et al. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat Commun. 2020;11(1):4507. doi: 10.1038/s41467-020-18272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Association of Public Health Laboratories. The core functions of public health laboratories. 2014. Available at: https://www.aphl.org/aboutAPHL/publications/Documents/APHLCoreFunctionsandCapabilities_2014.pdf. Accessed July 24, 2020.
- 18. Office of the Assistant Secretary for Preparedness and Response. Strategic National Stockpile. Available at: https://www.phe.gov/about/sns/Pages/default.aspx. Accessed July 24, 2020.