Abstract
In this prospective, open‐label, randomized, controlled clinical trial the effects of low‐dose carvedilol, nebivolol, and metoprolol on central arterial pressure and augmentation index (AIx) and its heart rate–corrected value (AIx@75) were assessed. The authors randomized 75 hypertensive patients (18–70 years) to carvedilol 12.5/25 mg, metoprolol 50/100 mg, or nebivolol 2.5/5 mg daily and followed them up for 3 months. Central arterial pressure and AIx were measured with applanation tonometry at baseline and at the end of follow‐up. Analyses were restricted to 60 completers. Central systolic pressure decreased equally in all 3 treatment arms. AIx remained unchanged, while AIx@75 decreased significantly by 5.4%±2.5% in the nebivolol group. According to general linear models, individual change in heart rate was a strong predictor of change in AIx in the carvedilol group (r 2=0.23, P=.03) although no similar association was found in the nebivolol group (r 2=0.09). The impact of β‐blockers with vasodilator effects on pressure augmentation seems to be different with nebivolol having the largest potential of decreasing AIx@75. While AIx changes associated with carvedilol treatment are strongly driven by heart rate changes, those associated with nebivolol treatment seem to be the result of other mechanisms.
In the past decade, increasing attention has been drawn to the unfavorable effects of β‐blockers on central hemodynamics. It is assumed that bradycardia elicited by β‐blockers results in slower development of the forward‐traveling wave and allows the reflected wave—the energy of which is increased due to peripheral vasoconstriction—to arrive during systole. The ensuing interference of the forward‐traveling and reflected waves augments systolic pressure in the large arteries, thereby diminishing the potential of central blood pressure (BP) reduction.1 Indeed, many studies have found that at the same level of peripheral pressure reduction, β‐blockers have a smaller effect on central BP compared with other types of antihypertensive agents.2, 3, 4 According to the Conduit Artery Function Evaluation (CAFE) study, a smaller decrease in central BP may be a plausible mechanism for the reduced effectiveness of β‐blockers in the prevention of cardiovascular events.5
β‐Blockers, however, cannot be considered a homogenous group. Unfavorable hemodynamic effects and clinical outcomes were demonstrated for atenolol, a drug selected for the treatment of hypertension with decreasing frequency.6 Newer β‐blockers, such as carvedilol and nebivolol, may elicit smaller wave reflection as a result of their vasodilatory effects. In older people with isolated systolic hypertension, both atenolol and nebivolol increased augmentation index (AIx), with the latter evoking a smaller rise.7 In younger populations with essential hypertension, heart rate‐corrected AIx (AIx@75) decreased in nebivolol‐treated groups, but not in groups receiving atenolol8 or metoprolol.9 Carvedilol treatment was also associated with smaller central augmented pressure10 and a larger decrease in AIx11 compared with metoprolol treatment. To our knowledge, however, no randomized trials have compared the effect of both vasodilator β‐blockers (carvedilol and nebivolol) with a nonvasodilator β‐blocker (such as metoprolol) on central BP and its determinants.
In the current parallel‐group, randomized, controlled trial, our primary objective was to assess the effects of low‐dose carvedilol, nebivolol, and metoprolol on central arterial pressure, AIx, and AIx@75. We hypothesized that carvedilol and nebivolol would decrease central arterial pressure more and increase AIx and AIx@75 less than metoprolol. As a secondary objective, we studied the effects of the above β‐blockers on forward and reflected pressure wave amplitude, pulse pressure amplification, and carotid‐femoral pulse wave velocity (PWV). We also investigated how changes in heart rate influence changes in AIx for each drug. Owing to their vasodilatory effects, we expected to observe some heart rate–independent effects of carvedilol and nebivolol.
Methods
This was a prospective, open‐label, 1:1:1, parallel‐group, randomized, controlled clinical trial with blinded endpoint evaluation comparing the effects of 3‐month treatment with carvedilol, nebivolol, and metoprolol on central hemodynamics.
Study Population
β‐Blocker‐naïve, treated, or untreated hypertensive patients (18–70 years) with uncontrolled hypertension were recruited at the 1st Department of Medicine, Semmelweis University and from local general practices. Uncontrolled hypertension was defined as seated office systolic and/or diastolic BP ≥140/90 mm Hg calculated from the average of the second and the third readings of 3 consecutive measurements. For patients with diabetes mellitus or chronic kidney disease (estimated glomerular filtration rate ≤60 mL/min/1.73 m2), a cutoff value of 130/80 mm Hg was used. Patients with severe hypertension (BP ≥180/110 mm Hg), atrial fibrillation, or contraindications for β‐blocker use were excluded. The study was approved by the National Institute of Pharmacy and by the ethics committee of the Hungarian Medical Research Council and was registered at EUDRACT (2008‐001509‐40). All patients gave written informed consent prior to participation.
Randomization
After screening eligibility for enrollment, patients were randomly assigned to receive one of the following 3 antihypertensive agents for the following 3‐month period: carvedilol 12.5 mg daily, metoprolol succinate 50 mg daily, or nebivolol 2.5 mg daily. For treated patients, trial medication was added on top of their unaltered background medication. Randomization was performed in blocks of 6. Investigators were blinded to randomization; at patient enrollment, they called the study center and received the next available treatment allocation. Patients and investigators responsible for enrollment and treatment were not blinded to treatment assignment; however, investigators analyzing outcome variables were blinded to treatment assignment and to the sequence of measurements.
Protocol
After enrollment, baseline clinical, hemodynamic, and laboratory data were obtained. At 1 month, drug side effects were evaluated and office BP was measured. In patients whose office BP was still in the hypertensive range, the dose of the study drug was doubled. At the end of the 3‐month treatment period, hemodynamic and laboratory measurements were repeated. All hemodynamic measurements were made in the morning, (2–3 hours after taking the morning dose of trial medication).
Hemodynamic Measurements
At each visit, office BP and heart rate were measured in triplicate by trained personnel after a 5‐minute rest in a seated position. Patients then received an ambulatory BP monitoring (ABPM) device (ABPM‐04, TensioMed, Budapest, Hungary)12 that measured BP in regular intervals (every 15 minutes during the day, every 30 minutes at night) for a 24‐hour period. The following morning, after the ABPM device had been taken off, central BP measurements were performed. After 10 minutes of supine rest, 3‐3 sequences of carotid and femoral pulse waves, each containing 10 to 15 pulse contours were recorded with a validated PulsePen tonometer (DiaTecne srl, Milan, Italy)13 by well‐trained personnel. Carotid pulse waves were calibrated with diastolic and mean brachial arterial pressures obtained from an OMRON M4‐I oscillometric device (OMRON Matsusaka Co Ltd, Matsusaka City, Japan) after each sequence of measurement.14 Mean arterial pressure was calculated as diastolic pressure + pulse pressure/3. AIx was defined as the difference between the second and first peak or shoulder of the central arterial waveform, expressed as a percentage of the pulse pressure.15 The location of shoulders was estimated by the PulsePen software using the fourth derivative of the raw pressure curve.16, 17 Pulse pressure amplification (AP) was calculated as brachial pulse pressure/central pulse pressure. Pulse wave travel distance was assessed as the difference between suprasternal notch‐to‐carotid site and suprasternal notch‐to‐femoral site obtained by surface tape measurements. Dividing this path length with the corresponding pulse transit time resulted in PWV values.15 Forward‐traveling and reflected pressure wave amplitudes were determined using the triangulation flow method. Details of the waveform decomposition method have been described elsewhere.18, 19 In all patients, the average of 2 or 3 sets of measurements were used in the calculations, depending on the quality of the recorded pressure curves.
Laboratory Measurements
Blood glucose, lipid profile, uric acid, potassium, calcium, creatinine, albumin, and urinary albumin‐to‐creatinine ratio were determined by standard laboratory methods.
Power Calculation
Considering 10 mm Hg and 10% as the standard deviation of changes in systolic central arterial pressure and AIx, respectively, a study with 20 patients in each treatment arm would be required to detect a 9 mm Hg or a 9% difference between central BP or AIx as being significant with the conventional 5% α error and 80% power. Assuming an approximately 20% dropout rate, we planned to randomize 75 patients.
Data Analysis
Baselines
All analyses were performed using the per‐protocol sample. Baseline clinical, biochemical, and hemodynamic data of the 3 patient groups were compared by one‐way analysis of variance (with Tukey post hoc tests) for continuous and χ2 tests for categorical variables. Post hoc analyses for categorical variables were done by univariate logistic regression with simple contrasts.
Changes Over Time
Mixed models with a random intercept adjusted for baseline differences in covariates were created to test for within‐group changes over time and also to test for between‐group differences. The Tables report baseline values for each group, within‐group changes over time, and the P value for the heterogeneity of these between‐group changes.
Additionally, in the case of AIx and backward‐wave amplitude, the above‐described mixed models were further adjusted for heart rate (centered at a rate of 75 per minute) and for a possible interaction of heart rate and treatment assignment at the second investigation. For graphical representation we calculated estimated marginal means for each treatment group and time point at a heart rate of 75 per minute.
Ancillary Analysis
To describe the association between changes in heart rate and changes in the AIx, general linear models were created with the change of AIx as the outcome and treatment allocation and change in heart rate as covariates including an interaction term into the model. We tested whether the slopes were different from zero and also whether slopes were different between groups.
All analyses were performed by SPSS version 14.0 (SPSS, Inc, Chicago, IL). Data are presented as number (percentage), mean±standard error, or 95% confidence intervals. Two‐sided P values <.05 were considered statistically significant.
Results
Participants and Recruitment
A flow diagram shows the progress and the number of participants through the phases of the trial (Figure 1). Eighty‐four patients were screened for the study but 9 were not randomized because they did not meet the inclusion criteria or declined to participate. Of the 75 patients randomized and allocated to intervention, 3 patients (2 metoprolol and 1 nebivolol) did not receive the allocated intervention because they favored lifestyle modification over drug treatment after the first hemodynamic measurement. Seven patients were lost to follow‐up (3 metoprolol, 2 nebivolol, and 2 carvedilol), as a result of traveling abroad or declining to return to the second hemodynamic measurement. Five patients discontinued medication because they experienced side effects of dizziness, hypotension or toxicoderma (metoprolol), cold extremities (nebivolol), or erectile dysfunction (carvedilol). Among the 60 patients analyzed, 18 were randomized to metoprolol, 21 to nebivolol, and 21 to carvedilol. The rates of lost to follow‐up were not different between randomized treatment groups (P=.23). Patients lost to follow‐up had significantly lower office diastolic BP (P=.02) and a smaller proportion of current smokers (P=.03). Anthropometric data, cardiovascular risk factors other than current smoking, the use of antihypertensive drugs, and baseline hemodynamic variables other than office diastolic BP were not different between patients analyzed or lost to follow‐up.
Figure 1.
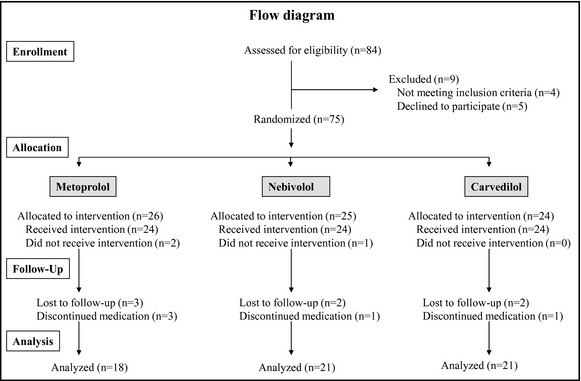
Flow diagram representing the progress of the phases of the study.
Baseline Characteristics of Participants
Anthropometric data, traditional cardiovascular risk factors taken from case history, data regarding established cardiovascular disease, diabetes, renal disease, and use of current medication of the sample analyzed (n=60) are summarized in Table 1. Patients in the 3 treatment groups were of similar age, body mass index, and waist circumference. Patients did not differ regarding their smoking status, family history of cardiovascular disease, and concomitant diseases. The use of diuretics was more frequent among participants receiving metoprolol compared with those receiving nebivolol. Six patients in the metoprolol group, 6 patients in the nebivolol group, and 8 patients in the carvedilol group required a dose increase during their follow‐up visit, respectively.
Table 1.
Baseline Anthropometric Data, Cardiovascular Risk Factors, Established Diseases, and Current Medication of the 3 Treatment Arms Studied
| Metoprolol | Nebivolol | Carvedilol | P Value | |
|---|---|---|---|---|
| No. | 18 | 21 | 21 | |
| Age, y | 49±3 | 49±4 | 45±3 | .63 |
| Male/female | 9/9 | 12/9 | 14/7 | .57 |
| BMI, kg/m2 | 29.6±1.0 | 28.1±0.7 | 29.0±0.8 | .47 |
| Waist circumference, cm | 103±3 | 97±2 | 100±3 | .28 |
| Current smoker | 5 (28) | 4 (19) | 9 (43) | .24 |
| Past smoker | 5 (28) | 3 (14) | 1 (5) | .13 |
| Family history of cardiovascular disease | 7 (39) | 4 (19) | 8 (38) | .30 |
| Established cardiovascular disease | 2 (11) | 0 | 1 (5) | .28 |
| Diabetes | 6 (33) | 8 (38) | 3 (14) | .20 |
| Renal disease | 1 (6) | 0 | 0 | .31 |
| Current medication | ||||
| Treatment‐naïve | 3 (17) | 9 (43) | 9 (43) | .15 |
| ACE inhibitors | 8 (44) | 6 (29) | 3 (14) | .22 |
| Angiotensin receptor blockers | 7 (39) | 3 (14) | 7 (33) | .19 |
| Calcium channel blockers | 7 (39) | 6 (29) | 6 (29) | .93 |
| Diuretics | 12a (67) | 3 (14) | 8 (38) | .04 |
| α‐Blockers | 0 | 0 | 2 (9) | .15 |
| Statins | 6 (33) | 4 (19) | 4 (19) | .49 |
| Oral antidiabetic agents | 6 (33) | 5 (24) | 3 (14) | .37 |
| Insulin | 4 (22) | 3 (14) | 2 (9) | .54 |
Abbreviations: ACE, angiotensin‐converting enzyme; BMI, body mass index. Data are mean±standard error of the mean for continuous variables and number (percentages) for categorical variables. Established cardiovascular disease and family history of cardiovascular disease were defined as history of myocardial infarction, heart failure, stroke, or peripheral arterial disease in the patient studied or in first‐degree relatives before the age of 55 years in men or 65 years in women. a P<.05 vs nebivolol.
Brachial systolic, mean, and diastolic BPs decreased significantly in all treatment groups, measured either as office BP or as BP taken during hemodynamic data collection. There was no between‐group difference in the degree of BP decrease. Confirming a decrease in BP, 24‐hour BP monitoring was successful in 45 patients (16 in the metoprolol, 18 in the nebivolol, and 11 in the carvedilol groups). In the carvedilol group, however (probably because of the relatively low number of patients), the drop in BP was not statistically significant according to the ABPM measurements. As expected, all drugs reduced heart rate to the same extent in each treatment groups (Table 2).
Table 2.
Brachial and Carotid Blood Pressures, Heart Rate, and Pulse Wave Velocity Before and After β‐Blocker Treatment
| Metoprolol | Nebivolol | Carvedilol | P Value | |
|---|---|---|---|---|
| Office measurements | ||||
| SBP, mm Hg | 150±3 | 151±2 | 147±1 | .56 |
| ΔSBP, mm Hg | −22 (−27 to −17) | −21 (−26 to −16) | −14 (−19 to −10) | |
| DBP, mm Hg | 92±3 | 88±2 | 92±2 | .46 |
| ΔDBP, mm Hg | −15 (−19 to −10) | −12 (−16 to −8) | −11 (−15 to −7) | |
| Heart rate, beats per min | 84±3 | 82±10 | 84±8 | .42 |
| ΔHeart rate, beats per min | −14 (−18 to −10) | −10 (−14 to −6) | −11 (−14 to −7) | |
| ABPM (n=45) | ||||
| 24 h SBP, mm Hg | 136±4 | 139±3 | 136±5 | .40 |
| Δ24‐h SBP (mm Hg) | −9 (−16 to −2) | −8 (−15 to −2) | −2 (−10 to 6) | |
| Δ24‐h DBP, mm Hg | 81±2 | 79±3 | 85±3 | .15 |
| Δ24‐h DBP, mm Hg | −8 (−12 to −4) | −7 (−10 to −3) | −2 (−7 to 3) | |
| Hemodynamic measurements | ||||
| Heart rate, beats per min | 74±2 | 74±2 | 77±3 | .34 |
| ΔHeart rate, beats per min | −7 (−11 to −3) | −7 (−11 to −3) | −10 (−14 to −7) | |
| SBP brachial, mm Hg | 135±4 | 142±3 | 140±3 | .27 |
| ΔSBP brachial, mm Hg | −7 (−14 to −1) | −14 (−20 to −8) | −9 (−15 to −3) | |
| DBP brachial, mm Hg | 79±2 | 80±2 | 84±3 | .48 |
| ΔDBP brachial, mm Hg | −7 (−10 to −3) | −8 (−12 to −5) | −5 (−9 to −2) | |
| SBP carotid, mm Hg | 121±3 | 128±3 | 124±3 | .23 |
| ΔSBP carotid, mm Hg | −6 (−11 to −1) | −12 (−16 to −7) | −7 (−11 to −2) | |
| DBP carotid, mm Hg | 80±2 | 80±2 | 84±3 | .43 |
| ΔDBP carotid, mm Hg | −7 (−10 to −3) | −8 (−12 to −5) | −6 (−9 to −2) | |
| PWV, m/s | 9.6±1.0 | 9.5±0.7 | 8.9±0.3 | .92 |
| ΔPWV, m/s | −0.8 (−2.4 to 0.7) | −0.6 (−2.1 to 0.8) | −0.4 (−1.8 to 1.0) | |
| AIx, % | 15.5±3.5 | 14.4±4.2 | 8.5±4.2 | .47 |
| ΔAIx, % | 0.4 (−4.7 to 5.4) | −3.5 (−8.2 to 1.2) | −0.3 (−5.0 to 4.4) | |
| AP | 1.33±0.02 | 1.33±0.03 | 1.41±0.03 | .72 |
| ΔAP | −0.02 (−0.08 to 0.04) | −0.05 (−0.10 to 0.005) | −0.05 (−0.11 to 0.002) | |
| Pf, mm Hg | 31.6±2.2 | 37.2±2.4 | 33.8±2.3 | .95 |
| ΔPf, mm Hg | −0.7 (−4.8 to 3.4) | −1.4 (−5.2 to 2.5) | −1.5 (−5.4 to 2.3) | |
| Pb, mm Hg | 17.6±1.1 | 20.1±1.2 | 18.0±1.3 | .20 |
| ΔPb, mm Hg | 1.0 (−1.0 to 3.0) | −1.5 (−3.3 to 0.4) | −0.2 (−2.1 to 1.6) | |
Abbreviations: ABPM, ambulatory blood pressure monitoring; AIx, augmentation index; AP, pulse pressure amplification; DBP, diastolic blood pressure; Pb, reflected wave amplitude; Pf, forward travelling wave amplitude; PWV, pulse wave velocity; SBP, systolic blood pressure. Data are displayed as baseline mean±standard error of the mean. Changes are displayed as change from baseline (95% confidence interval). P value represents the level of difference between drug effect (P for heterogeneity).
Primary Outcomes
Carotid systolic and diastolic BPs fell significantly in each treatment groups, without any statistical difference between the drugs. None of the drugs had any significant effect on AIx. AIx@75, however, was reduced by 5.42%±2.5% (P=.034) in the nebivolol group. Carvedilol and metoprolol did not change AIx@75 significantly (Table 2 and Figure 2).
Figure 2.
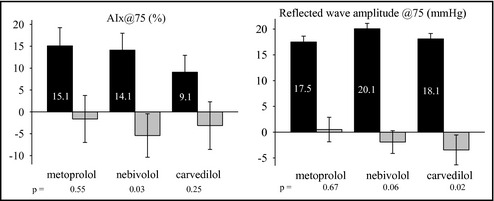
Estimated augmentation index (AIx) and reflected wave amplitude centered at 75 beats per minute heart rate. Estimation was based on mixed model analysis. Black columns represent baseline data±standard error of the mean and gray columns represent change from baseline, with error bars indicating 95% confidence interval.
Secondary Outcomes
Reflected wave amplitude remained unchanged in all study groups. Reflected wave amplitude corrected for heart rate of 75 per minute, however, decreased in the carvedilol group (by 3.4±1.4 mm Hg, P=.02) and showed a decreasing tendency in the nebivolol group (by 2.0±1.0 mm Hg, P=.057), but no change was observed in patients taking metoprolol (Figure 2). Forward‐traveling wave amplitude did not change throughout the study. There was a significant interaction between heart rate and taking carvedilol on reflected wave amplitude (P=.026). Neither pulse pressure amplification nor PWV changed significantly throughout the study (Table 2).
Ancillary Analyses
Determinants of changes in AIx were studied in simple and multiple linear regression models. There was a significant association between individual changes in heart rate and changes in AIx within the carvedilol group, but no such association was observed in the metoprolol or nebivolol groups (P for heterogeneity=.03). The slope of the relationship in the nebivolol group was significantly different from that in the carvedilol (P=.01) or metoprolol (P=.048) groups. (Figure 3).
Figure 3.
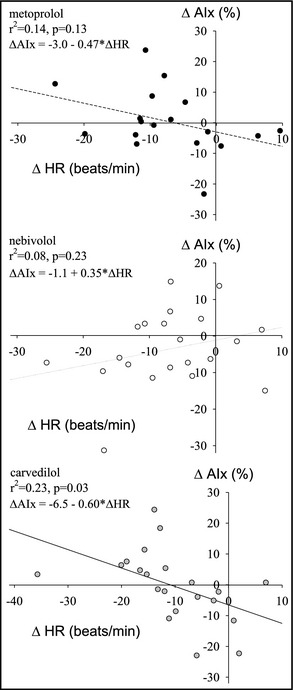
Relationship between changes in heart rate (HR) and changes in augmentation index (AIx) for metoprolol (upper panel), nebivolol (middle panel), and carvedilol (lower panel).
Safety Measurements
Laboratory data collected at the onset and at the end of the study are presented in Table 3. Blood glucose, lipid parameters, creatinine, electrolytes, uric acid, albumin, and urinary albumin‐to‐creatinine ratio did not differ between treatment groups at either study visit. There was a slight but statistically significant rise in plasma potassium levels in patients taking carvedilol. Other parameters measured did not change during the study.
Table 3.
Safety Parameters Before and 3 Months After β‐Blocker Treatment
| Metoprolol | Nebivolol | Carvedilol | P Value | |
|---|---|---|---|---|
| Glucose, mg/dL | 119±12 | 136±15 | 104±5 | .45 |
| ΔGlucose, mg/dL | 1 (−13 to 14) | −6 (−18 to 6) | 5 (−7 to 17) | |
| Cholesterol, mg/dL | 192±11 | 187±8 | 191±11 | .79 |
| ΔCholesterol, mg/dL | 1 (−16 to 18) | −7 (−22 to 8) | −5 (−20 to 10) | |
| Triglycerides, mg/dL | 137±19 | 176±31 | 209±52 | .70 |
| ΔTriglycerides, mg/dL | 27 (−64 to 119) | −25 (−104 to 55) | 1 (−81 to 82) | |
| HDL cholesterol, mg/dL | 52±4 | 53±5 | 49±2 | .81 |
| ΔHDL cholesterol, mg/dL | −2 (−6 to 2) | −3 (−7 to 0.4) | −3 (−7 to 1) | |
| LDL cholesterol, mg/dL | 121±9 | 115±8 | 121±13 | .90 |
| ΔLDL cholesterol, mg/dL | −1 (−16 to 13) | −5 (−18 to 7) | −5 (−19 to 0) | |
| Uric acid, mg/dL | 5.9±0.4 | 5.3±0.3 | 5.8±0.3 | .17 |
| ΔUric acid, mg/dL | −0.1 (−0.7 to 0.5) | 0.5 (−0.1 to 1.1) | −0.2 (−0.8 to 0.4) | |
| Creatinine, mg/dL | 1.1±0.1 | 0.9±0.0 | 0.9±0.0 | .09 |
| ΔCreatinine, mg/dL | 0.0 (−0.09 to 0.01) | 0.0 (−0.01 to 0.07) | 0.0 (−0.06 to 0.03) | |
| Potassium, mEq/L | 4.4±0.1 | 4.5±0.1 | 4.2±0.1 | .02 |
| ΔPotassium, mEq/L | −0.1 (−0.2 to 0.1) | −0.1 (−0.2 to 0.1) | 0.2 (0.1–0.4) | |
| Calcium, mg/dL | 9.5±0.2 | 9.4±0.1 | 9.3±0.1 | .55 |
| ΔCalcium, mg/dL | 0.3 (−0.2 to 0.8) | 0.0 (−0.5 to 0.4) | 0.1 (−0.3 to 0.6) | |
| Albumin, g/dL | 4.6±0.1 | 4.7±0.1 | 4.7±0.1 | .91 |
| ΔAlbumin, g/dL | −0.1 (−0.3 to 0.1) | −0.1 (−0.2 to 0.1) | −0.1 (−0.2 to 0.1) | |
| UACR, mg/mmola | 0.6 (0.3−1.4) | 0.4 (0.2−2.2) | 0.2 (0.1−0.8) | .54 |
| ΔUACR, mg/mmol | −9.6 (−19.9 to 0.7) | −2.2 (−12.2 to 7.8) | 0.5 (−9.4 to 10.3) |
Abbreviations: HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; UACR, urinary albumin to creatinine ratio. Data are displayed as baseline mean±standard error of the mean or in case of evidence against normal distribution amedian (interquartile range). Changes (Δ) are displayed as change from baseline (95% confidence interval). P value represents the level of difference between drug effect (P for heterogeneity).
Discussion
The aim of the current clinical trial was to evaluate the effect of 3‐month treatment with metoprolol, nebivolol, or low‐dose carvedilol on central hemodynamics in patients with uncontrolled hypertension. In the present study, we found similar decreases of central BP irrespective of treatment arm, while no significant alterations in AIx or PWV were found in either of the groups. In contrast to no changes in the carvedilol and the metoprolol groups, heart rate‐adjusted AIx significantly and reflected wave amplitude nonsignificantly decreased in the nebivolol group. Individual changes in AIx within the nebivolol‐treated group seemed to be independent of changes in heart rate. Carvedilol appears to have the largest potential for decreasing reflected wave amplitude; this effect, however, is strongly dependent on current heart rate.
Our results are in agreement with those of previous studies demonstrating similar decreases in central systolic pressure when comparing different β‐blockers.7, 8 We could not find any significant differences between the central BP‐lowering effects of carvedilol, nebivolol, and metoprolol. We must acknowledge, however, that nebivolol decreased central systolic pressure 6 mm Hg more compared with metoprolol, which was not statistically significant but may be considered biologically significant. PWV did not decrease in any of the groups, probably because of the relatively small decrease in BP and the lack of a direct effect of the drugs on central arteries. Indeed, whenever a fall in PWV during β‐blocker treatment was demonstrated, it seemed to be the consequence of a larger drop in BP and not the reduction of arterial stiffness.7, 8
Tendencies in AIx changes seen in other studies7, 8, 9 suggested that the favorable hemodynamic effect of nebivolol cannot be attributed exclusively to heart rate reduction but could have arisen from its peripheral vascular effects. By demonstrating the lack of a relationship between the fall in AIx and heart rate reduction and a tendency toward a decrease in reflected wave amplitude, our study provides some direct evidence to this hypothesis. A recent study of prehypertensive patients found a significant increase in the production of endogenous nitrovasodilator nitric oxide among nebivolol‐treated participants, assessed by urinary nitrate/nitrite excretion.20 Although we did not perform a direct measurement of nitric oxide production, such increment in nitric oxide formation could contribute to the central hemodynamic effects observed in the nebivolol arm.
As compared with nebivolol, the central hemodynamic effects of the combined α‐ and β‐blocker carvedilol are much less explored.10, 11 In our study, no changes were detected in the carvedilol group in AIx or AIx@75; however, individual changes in heart rate and AIx within the carvedilol group showed a negative association. It seems, therefore, that a stronger β‐receptor antagonism and subsequent fall in heart rate and rise in AIx may override the α‐receptor antagonism‐associated peripheral vasodilation and fall in AIx. One plausible explanation for this finding may be the relatively low dose of carvedilol applied in our study. Carvedilol demonstrates dose‐dependent β‐adrenoceptor antagonist activity from relatively low doses, while it shows evidence of α‐adrenoceptor antagonist activity only at the dose of 25 mg,21 many of our patients did not receive.
A recent study demonstrated that heart rate is an important pressure‐independent determinant of not only AIx but of reflected wave amplitude as well.19 In the carvedilol‐treated group, adjustment of reflected wave amplitude for heart rate revealed an interaction between these parameters, indicating that at higher and lower heart rates, carvedilol acts with different efficiency. At higher heart rates, carvedilol profoundly decreases reflected wave amplitude, while at lower heart rates, its impact on reflected wave amplitude is minimal. Theoretically, the inverse of heart rate may be considered as an index of sympathovagal balance.22 Higher heart rates representing higher sympathetic outflow to the heart and to vessels may provide an ideal setting for α‐receptor antagonism. Lower heart rates and lower sympathetic outflow, however, may not yield much ground to the α‐receptor–associated effects of carvedilol.
The pharmacologic mechanism of action of carvedilol is thought to be different from that of nebivolol. In contrast to nebivolol, carvedilol‐treated patients do not exhibit an elevation in their plasma nitrate concentration but show an increase in their antioxidant capacity.23
To our knowledge, no clinical study has aimed to investigate the pharmacologic mechanisms, hemodynamic effects, and clinical responses to both newer β‐blockers within the same study. Therefore, we can only speculate that the similar beneficial cardiac effects of nebivolol and carvedilol (eg, reduction of left ventricular hypertrophy9, 24) may represent similar results related to different effects on central hemodynamics.
Limitations
The current study involved only 60 patients and was therefore relatively underpowered to detect subtle changes in hemodynamic parameters or differences between the 3 treatment arms. The 3‐month duration of the clinical trial was also relatively short, considering that β‐blocker effects may differ when studied over a short‐ or long‐term treatment period.9 It must also be acknowledged that the dose of the drugs applied was lower than recommended in large clinical trials or national heart failure guidelines. However, we could observe a significant reduction in heart rate even with these relatively low doses, and the BP of our patients with mild to moderate hypertension in the 3 treatment groups decreased equally and to the normal range. A recent study with carvedilol also suggested that even daily doses >10 mg—the minimal dose that all of our patients received—may have beneficial effects on cardiovascular survival,25 indicating that clinically significant hemodynamic effects may be observed well below the maximal dose recommended. Because of the inclusion of patients already treated with antihypertensive drugs, we cannot exclude a potential confounding effect due to unbalanced randomization. Nevertheless, the frequency of the use of other drug classes—apart from diuretics, a drug class with neutral or minimal effect on central hemodynamics—did not differ between the groups, so we hypothesize that this potential confounding effect appeared equally in the 3 groups of patients. The relatively large difference between patients' office BP and pressure taken during hemodynamic measurements may suggest some misclassification of patients as having uncontrolled hypertension during a single screening visit. This difference, however, may have also arisen from different conditions applied during the 2 measurements. The nonblinded design represents another limitation of the study.
Conclusions
In the current clinical trial we demonstrated that despite the statistically indifferent effect on central arterial pressure, nebivolol has a larger effect on AIx than carvedilol or metoprolol. Our study expands earlier observations by showing that the effects of nebivolol on AIx are independent of the heart rate–decreasing effect of the drug. The impact of carvedilol on AIx or reflected wave amplitude, on the other hand, seems to exhibit strong heart rate dependency. According to these findings, β‐blockers with vasodilatory effects cannot be considered as a homogenous group regarding their effects on central hemodynamics.
Disclosures
This work was supported by research grants from the Hungarian Society of Hypertension and Hungarian Kidney Foundation and by an unrestricted educational grant from EGIS Pharmaceutical Company. AG Tabák is supported by TÁMOP 4.2.4.A/1‐11‐1‐2012‐0001 National Excellence Program—research fellowship co‐financed by the European Union and the European Social Fund. P. Salvi is consultant for DiaTecne srl (Milan, Italy). The remaining authors report no conflicts of interest.
J Clin Hypertens (Greenwich). 2013;15:910–917. DOI: 10.1111/jch.12210. ©2013 Wiley Periodicals, Inc.
References
- 1. Wilkinson IB, MacCallum H, Flint L, et al. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morgan T, Lauri J, Bertram D, Anderson A. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens. 2004;7:118–123. [DOI] [PubMed] [Google Scholar]
- 3. Mackenzie IS, McEniery CM, Dhakam Z, et al. Comparison of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated systolic hypertension. Hypertension. 2009;54:409–413. [DOI] [PubMed] [Google Scholar]
- 4. London GM, Asmar RG, O'Rourke MF, Safar ME. Reason Project Investigators. Mechanism(s) of selective systolic blood pressure reduction after a low‐dose combination of perindopril/indapamide in hypertensive subjects: comparison with atenolol. J Am Coll Cardiol. 2004;43:92–99. [DOI] [PubMed] [Google Scholar]
- 5. Williams B, Lacy PS, Thom SM, et al. CAFE Investigators; Anglo‐Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee . Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. [DOI] [PubMed] [Google Scholar]
- 6. Agabiti‐Rosei E, Porteri E, Rizzoni D. Arterial stiffness, hypertension, and rational use of nebivolol. Vasc Health Risk Manag. 2009;5:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhakam Z, Yasmin, McEniery CM, et al. A comparison of atenolol and nebivolol in isolated systolic hypertension. J Hypertens. 2008;26:351–356. [DOI] [PubMed] [Google Scholar]
- 8. Mahmud A, Feely J. Β‐blockers reduce aortic stiffness in hypertension but nebivolol, not atenolol, reduces wave reflection. Am J Hypertens. 2008;21:663–667. [DOI] [PubMed] [Google Scholar]
- 9. Kampus P, Serg M, Kals J, et al. Differential effects of nebivolol and metoprolol on central aortic pressure and left ventricular wall thickness. Hypertension. 2011;57:1122–1128. [DOI] [PubMed] [Google Scholar]
- 10. Jabbour A, Macdonald PS, Keogh AM, et al. Differences between β‐blockers in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized crossover trial. J Am Coll Cardiol. 2010;55:1780–1787. [DOI] [PubMed] [Google Scholar]
- 11. Shah NK, Smith SM, Nichols WW, et al. Carvedilol reduces aortic wave reflection and improves left ventricular/vascular coupling: a comparison with atenolol (CENTRAL Study). J Clin Hypertens (Greenwich). 2011;13:917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barna I, Keszei A, Dunai A. Evaluation of Meditech ABPM‐04 ambulatory blood pressure measuring device according to the British Hypertension Society protocol. Blood Press Monit. 1998;3:363–368. [PubMed] [Google Scholar]
- 13. Salvi P, Lio G, Labat C, et al. Validation of a new non‐invasive portable tonometer for determining arterial pressure wave and pulse wave velocity: the PulsePen device. J Hypertens. 2004;22:2285–2293. [DOI] [PubMed] [Google Scholar]
- 14. El AM, Topouchian J, Asmar R. Evaluation of two devices for self‐measurement of blood pressure according to the international protocol: the Omron M5‐I and the Omron 705IT. Blood Press Monit. 2003;8:127–133. [DOI] [PubMed] [Google Scholar]
- 15. Laurent S, Cockcroft J, Van BL, et al. European Network for Non‐invasive Investigation of Large Arteries: expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 16. Takazawa K, Tanaka N, Takeda K, et al. Underestimation of vasodilator effects of nitroglycerin by upper limb blood pressure. Hypertension. 1995;26:520–523. [DOI] [PubMed] [Google Scholar]
- 17. Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: relationship to pressure wave forms. Circulation. 1980;62:105–116. [DOI] [PubMed] [Google Scholar]
- 18. Westerhof BE, Guelen I, Westerhof N, et al. Quantification of wave reflection in the human aorta from pressure alone: a proof of principle. Hypertension. 2006;48:595–601. [DOI] [PubMed] [Google Scholar]
- 19. Liao CF, Cheng HM, Sung SH, et al. Determinants of pressure wave reflection: characterization by the transit time‐independent reflected wave amplitude. J Hum Hypertens. 2011;25:665–671. [DOI] [PubMed] [Google Scholar]
- 20. Davis JT, Pasha DN, Khandrika S, et al. Central hemodynamics in prehypertension: effect of the β‐adrenergic antagonist nebivolol. J Clin Hypertens (Greenwich). 2013;15:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tham TC, Guy S, McDermott BJ, et al. The dose dependency of the alpha‐ and β‐adrenoceptor antagonist activity of carvedilol in man. Br J Clin Pharmacol. 1995;40:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldberger JJ. Sympathovagal balance: how should we measure it? Am J Physiol. 1999;276:H1273–H1280. [DOI] [PubMed] [Google Scholar]
- 23. Zepeda RJ, Castillo R, Rodrigo R, et al. Effect of carvedilol and nebivolol on oxidative stress‐related parameters and endothelial function in patients with essential hypertension. Basic Clin Pharmacol Toxicol. 2012;111:309–316. [DOI] [PubMed] [Google Scholar]
- 24. Xiaozhen H, Yun Z, Mei Z, Yu S. Effect of carvedilol on coronary flow reserve in patients with hypertensive left‐ventricular hypertrophy. Blood Press. 2010;19:40–47. [DOI] [PubMed] [Google Scholar]
- 25. Nishiyama K, Tsutamoto T, Yamaji M, et al. Dose‐dependent prognostic effect of carvedilol in patients with chronic heart failure–special reference to transcardiac gradient of norepinephrine. Circ J. 2009;73:2270–2275. [DOI] [PubMed] [Google Scholar]


