Abstract
Study Objectives:
To investigate the prevalence of mildly collapsible upper airways (defined by therapeutic continuous positive airway pressure [CPAP] values ≤ 8 cm H2O) in moderate to severe obstructive sleep apnea patients treated with CPAP and to determine their clinical, functional, and nocturnal polysomnographic characteristics.
Methods:
Eighty-seven patients with moderate to severe obstructive sleep apnea consecutively treated with CPAP were retrospectively investigated. Two nocturnal home sleep portable monitoring studies were performed at baseline and during treatment. Participants were categorized according to therapeutic CPAP values: ≤ 8 cm H2O (group 1), 8–12 cm H2O (group 2), ≥ 12 cm H2O (group 3). Anthropometric, awake respiratory function, symptoms, comorbidities, and nocturnal home sleep portable monitoring studies data were collected.
Results:
Mild upper airway collapsibility (therapeutic CPAP values ≤ 8 cm H2O) was present in 25.3% of patients. They showed more favorable apnea-hypopnea index, oxygen desaturation index, mean nocturnal saturation, sleep time with oxygen saturation < 90%, desaturation nadir, and supine position. Oxygen desaturation index showed a weak association with anatomical collapsibility. Using the receiver operating characteristic curve, the area under the curve for the oxygen desaturation index vs CPAP pressure requirements ≤ 8 cm H2O was low and oxygen desaturation index ≤ 40.8/h showed a sensitivity of 63.3% and a specificity of 69.2% to detect patients with mild collapsibility.
Conclusions:
A quarter of moderate to severe patients under CPAP therapy had mild collapsibility and were likely to also be good candidates for alternative and better tolerated non-CPAP therapies. Baseline anthropometric, clinical, and respiratory function characteristics did not predict mild upper airway collapsibility determined by CPAP pressure requirements ≤ 8 cm H2O.
Citation:
Bosi M, Incerti Parenti S, Fiordelli A, Poletti V, Alessandri-Bonetti A. Upper airway collapsibility in patients with OSA treated with continuous positive airway pressure: a retrospective preliminary study. 2020;16(11):1839–1846.
Keywords: obstructive sleep apnea, continuous positive airway pressure, upper airway collapsibility
BRIEF SUMMARY
Current Knowledge/Study Rationale. Therapeutic continuous positive airway pressure (CPAP) level requirement easily provides information on upper airway collapsibility, a pathophysiological trait that influences the efficacy of treatments for obstructive sleep apnea. This study investigates the prevalence of mild collapsibility in patients treated with CPAP together with their clinical, functional, and nocturnal polysomnographic characteristics.
Study Impact. Mild upper airway collapsibility was shown in 25.3% of moderate to severe obstructive sleep apnea patients treated with CPAP, as determined by CPAP level requirements ≤ 8 cm H2O, thus being likely to respond to alternative and well tolerated non-CPAP therapies; baseline anthropometric, clinical, and respiratory function characteristics were not predictive for mild collapsibility. Such information may help clinicians tailor the therapy to the individual characteristics of each patient, thus optimizing treatment response.
INTRODUCTION
Obstructive sleep apnea (OSA) is a complex disorder with a multifactorial pathogenesis. There is growing interest in the characterization of the anatomic and nonanatomic pathophysiological traits that, alone or in combination, contribute to the development of OSA.1
Four pathophysiological traits have been identified: passive airway collapsibility, upper airway muscle gain, arousal threshold, and loop gain (LG).2–4 The interaction between pathophysiological traits is complex and differs among patients: the increasing understanding of these features and their consideration within an accurate process of differential diagnosis will allow the tailoring of the therapy to the individual characteristics of each patient, thereby optimizing treatment response.
Eckert et al5 have elaborated the PALM scale (pharyngeal critical pressure [Pcrit], arousal threshold, LG, upper airway muscle gain) to assist in weighting the relative contribution of each pathophysiological trait. This classification categorizes patients into 3 subgroups based on the specific mechanisms involved in the pathogenesis of OSA: for example, those who have high collapsibility and will require anatomical treatment such as continuous positive airway pressure (CPAP) (PALM 1) are differentiated from those who may be candidates for non-CPAP therapies such as novel pharmacological agents or oxygen (PALM 3). Current evidence suggests that patients with OSA with mild upper airway collapsibility are likely to be suitable candidates for non-CPAP therapies like oxygen/sedatives targeted to LG or arousal threshold,6,7 and/or reducing Pcrit (ie, upper airway surgeries, positional therapy, mandibular advancement device [MAD], weight loss) administered in an isolated or associated way.8–10
Despite the critical role of upper airway collapsibility in driving OSA pathogenesis and treatment efficacy, gold standard methods for quantifying the degree of upper airway collapsibility require specialized equipment and technically difficult methodologies. For this reason there is a need for accurate, easily accessible, and simplified ways of determine this pathophysiological information.11
Landry et al11 have recently demonstrated that a patient’s therapeutic CPAP level provides useful information on upper airway collapsibility, with values ≤ 8.0 cm H2O being consistent with a high likelihood of mild collapsibility, a key determinant in the therapeutic management of OSA. They did not find strong relationships between Pcrit, age, apnea-hypopnea index (AHI), body mass index, or sex and did not describe in a detailed way the demographic, anthropometric, and polysomnographic findings of patients who had a CPAP value of ≤ 8 cm H2O.
The aim of this study was to investigate the prevalence of mildly collapsible upper airways in patients with moderate to severe OSA treated with CPAP, as well as to determine the clinical, functional, and nocturnal polysomnographic characteristics of participants with mild collapsibility defined by therapeutic CPAP values ≤ 8 cm H2O.
METHODS
We performed a retrospective study that included patients with OSA consecutively treated with CPAP from January 1, 2019 to September 1, 2019 at the Department of Respiratory and Sleep Medicine, Umberto I Hospital, Lugo, Italy. The study was approved by the Ethics Committee (protocol 33/2020/I.5/285) and was carried out in accordance with the current standards recommended for the reporting of observational studies in epidemiology (STROBE statement; https://strobe-statement.org/index.php?id=strobe-home). Data collection was performed between December 15, 2019 and January 31, 2020 by 1 expert operator (M.B., a physician certified by the Italian Association of Sleep Medicine) and through manual review of medical records.
Participants underwent 2 home sleep portable monitoring (HSPM) studies carried out according to the guidelines of Italian Association of Sleep Medicine and the Italian Association of Hospital Pulmonologists.12 HSPMs were performed under baseline conditions and during treatment with CPAP using Embletta MPR Sleep System (Embla Systems Inc., Broomfield, CO), NoxT3 Sleep Monitor (Nox Medical, Reykjavik, Iceland), and Vital Night Plus (Dr. Fenyves und Gut GMBH, Rangendingen, Germany). The basal HSPM registration set consists of a snoring sensor, an oronasal thermal sensor, a nasal pressure transducer, a thoracic and abdominal effort sensor (with inductive plethysmograph or pneumatic sensor), a pulse oximeter, and a position sensor. In the HSPM after CPAP titration, flow was recorded with an uncalibrated flow meter connected to a differential pressure transducer and mask pressure with a pressure transducer reporting the atmospheric pressure.
Respiratory events were analyzed based on the 2015 standards of the American Academy Sleep Medicine.13 Apnea was defined as a drop in signal of the oronasal thermal sensor by ≥ 90% of pre-event baseline, with a duration ≥ 10 seconds. Apneas were classified as obstructive, mixed, or central based on respiratory efforts. Hypopnea was considered as a ≥ 30% drop in flow for ≥ 10 seconds that was associated with ≥ 4% desaturation, according to the “acceptable” rules of the American Academy Sleep Medicine, 2015.13
The St. Mary’s Hospital Sleep Questionnaire was used to test the duration of sleep, thereby determining the beginning and the end of the period of analysis, removing periods of movement and upright position.14 We calculated the apnea-hypopnea index (AHI) per hour of analysis time, the supine AHI per hour of analysis time, the obstructive apnea-hypopnea index per hour of analysis time, the obstructive apnea index per hour of analysis time, the central apnea index per hour of analysis time, the percentage of obstructive hypopneas in relation to the total obstructive events, the mean apnea duration in seconds, the central-mixed apnea index per hour of analysis time, the oxygen desaturation index (ODI) ≥ 4% per hour of analysis time, the mean nocturnal saturation (mean saturation), sleep time with an oxygen saturation < 90% (T90), nadir oxygen saturation (nadir), analysis time, and supine position time (supine position). OSA was diagnosed according to the recommendations of the International Classification of Sleep Disorders, 3rd ed.15
Titration was carried out by an autotitrating positive airway pressure (APAP) device in order to determine the fixed value of the therapeutic CPAP level using a procedure that is widely practiced even if not thoroughly described in statements or guidelines promoted by scientific associations yet.16–18 However, the procedure adopted was based on key points that have emerged from the literature.19–28 Briefly, the procedure employed in our operative unit contemplates the domiciliary use of an APAP DreamStation (Philips Respironics) or AirSense 10 (ResMed, San Diego, CA) set at 6–20 cm H2O for a minimum of 10 days in order to minimize the variability of sleep position between nights, and to allow sufficient patient adaptation. The data were analyzed starting chronologically from the most recent to find at least 3 nights with use of the device for ≥ 4 hours, with good self-reported sleep quality and with no or limited losses. The therapeutic CPAP value was the average of the P95% (the level of pressure at or below obstructive events are eliminated for 95% of the time) calculated manually from loss-free tracing periods. Once the fixed CPAP pressure had been extrapolated from the built-in software of the APAP device, an AHI < 5 events/h and a mean saturation > 90% at the control nocturnal HSPM indicated control of the upper airways.
The finding of worse values during the nocturnal HSPM entailed the repetition of the nocturnal evaluation to modify the CPAP values until a sufficient control of the upper airways was obtained. Patients who still presented AHI > 5 events/h and mean saturation < 90% with the maximum tolerable pressure were considered under partial control.
The exclusion criteria were as follows: neurological disorders, stimulants or sedatives consumption, alcohol or drug abuse, severe chronic lung disease, and severe and unstable cardiovascular or metabolic conditions.
Participants were categorized into 3 groups according to the therapeutic CPAP value: ≤ 8 cm H2O (group 1, Gr1), between 8 and 12 cm H2O (group 2, Gr2), and ≥ 12 cm H2O (group 3, Gr3). The value of CPAP ≤ 8 cm H2O was considered coherent with a mildly collapsible upper airway.11
Patients were evaluated with ventilatory function tests: flow/volume curve, pulse oximetry in awake rest sitting position, and blood gas analysis in rest awake sitting position. Self-reported sleepiness was investigated with the Epworth Sleepiness Scale. Data regarding symptoms (restless sleep, insomnia, restless sleep with insomnia, periodic leg movements, restless, asymptomaticity), and comorbidities (hypertension, chronic ischemic or arrhythmogenic cardiomyopathy and/or cerebrovasculopathy, and glucose and lipid metabolism alterations, none) were recorded using an unstructured patient interview.
Statistical analysis
Data were presented using descriptive statistics: median values (interquartile range, IQR) for continuous variables, and percentages for categorical variables. For continuous variables the differences between the groups were analyzed using the Kruskal-Wallis analysis of variance test, with post-hoc analysis being performed when necessary. For categorical variables, the percentages were compared using the chi-squared test and Fisher’s exact test. The nonparametric Spearman test was used to evaluate the correlation between therapeutic CPAP level and HSPM baseline parameters, and the poligraphic variables statistically significant were used for stepwise multiple regression analysis. Stepwise multiple regression is a method analyzing multiple variables and simultaneously removing those that are not significant, leaving as a final result only the variables that explain the distribution of the dependent variable. We used variables from stepwise multiple regression analysis to calculate receiver operating characteristic curves and the best cut-off values that identified patients with mild collapsibility. The level of statistical significance was set at P < .05. All statistical analyses were conducted with IBM SPSS version 20.0.
RESULTS
Ninety-four consecutive patients with OSA were treated with CPAP in the analyzed period. Study results were obtained from 87 patients with moderate to severe OSA. Data and results relative to the whole sample (including 7 patients with mild OSA) are reported in the supplemental material.
According to the therapeutic CPAP value, participants were categorized as follows: Gr1 (n = 22), Gr2 (n = 33), and Gr3 (n = 32). Anthropometric, awake respiratory function, and self-reported sleepiness data are presented in Table 1. No between group differences were found for these variables. Sex distribution did not differ among the groups (Pearson’s chi-squared 0.845, P = .655).
Table 1.
Anthropometric, respiratory function, and self-reported sleepiness data.
| Total Sample (n = 87) | Group 1 (n = 22) | Group 2 (n = 33) | Group 3 (n = 32) | Kruskal-Wallis Test Chi-squared Test | |
| Age (years) | 60.0 (54.0–67.0) | 59.5 (52.0–69.5) | 60.0 (56.0–65.5) | 60.0 (49.25–66.0) | P = .820 |
| Sex F (%) | 28.7 | 27.3 | 24.2 | 34.4 | P = .655 |
| Weight (kg) | 95.0 (85.0–115.0) | 90.0 (78.5–106.0) | 94.5 (87.25–109.0) | 107.5 (89.0–124.0) | P = .105 |
| Height (cm) | 170.0 (163.5–178.5) | 169.0 (160.0–177.75) | 171.0 (165.0–178.75) | 170.0 (165.0–180) | P = .512 |
| BMI | 34.05 (29.075–38.825) | 32.35 (26.05–37.125) | 31.9 (29.225–36.425) | 35.4 (31.675–40.925) | P = .109 |
| Neck circumference (cm) | 44.0 (41.0–47.0) | 42.0 (39.0–45.0) | 44.5 (42.25–47.0) | 45.5 (42.0–48.0) | P = .056 |
| FVC (L) | 89.5 (80.25–99.45) | 90.5 (83.5–109.5) | 90.0 (81.0–103.0) | 89.0 (77.5–96.5) | P = .739 |
| FEV1 (L) | 89.0 (74.25–100.0) | 91.0 (75.0–108.0) | 89.0 (70.5–103.0) | 87.0 (72.5–98.5) | P = .725 |
| Blood pH | 7.4 (7.39–7.42) | 7.4 (7.39–7.4125) | 7.4 (7.3825–7.4275) | 7.4 (7.39–7.43) | P = .758 |
| PaCO2 (mm Hg) | 38.1 (35.575–42.0) | 37.95 (35.675–41.9) | 38.6 (35.65–41.9) | 34.6 (38.4–42.3) | P = .893 |
| PaO2 (mm Hg) | 70.0 (63.4–74.0) | 70.5 (61.9–78.2) | 70.4 (62.85–72.8) | 70.0 (66.6–74.0) | P = .820 |
| BE (mEq/l) | –0.8 (–2.0–0.725) | –1.2 (–2.55–0.925) | –0.4 (–1.7–2.3) | –0.8 (–1.7–0.35) | P = .359 |
| Pulsoximetry (%) | 97.0 (95.5–98) | 97.0 (96.0–98.0) | 97.0 (95.0–98.0) | 97.0 (95.0–98.0) | P = .953 |
| ESS | 6.5 (3.0–9.0) | 4.5 (1.75–9.0) | 7.0 (5.0–9.0) | 6.0 (3.0–9.0) | P = .283 |
Data are shown as median (interquartile range). n = number of patients, BE = base excess, BMI = body mass index, ESS = Epworth Sleepiness Scale, FEV1 = forced expiratory volume in the first second, FVC = forced vital capacity, PaCO2 = partial pressure of carbon dioxide, PaO2 = partial pressure of oxygen.
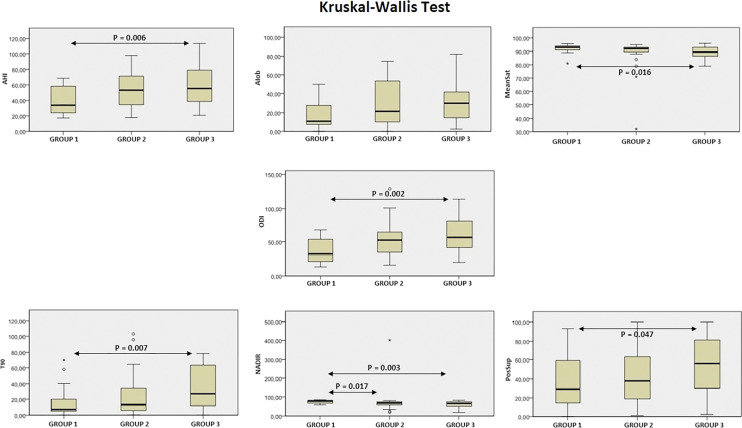
Nocturnal HSPM parameters and indexes under baseline conditions were reported in Table 2. Statistically significant Gr1–Gr3 differences were found for AHI (P =.006), the percentage of obstructive hypopneas in relation to the total obstructive events (P = .028), ODI (P = .002), mean saturation (P = .016), T90 (P = .007), nadir (P = .003) and supine position (P = .047), while a statistically significant Gr1–Gr2 difference was detected only for nadir (P = .017) (Table 2, Figure 1).
Table 2.
Respiratory indexes during sleep.
| Total sample (n = 87) | Group 1 (n = 22) | Group 2 (n = 33) | Group 3 (n = 32) | Kruskal-Wallis Test | |
| Time analysis (s) | 430.0 (381.5–475.5) | 449.0 (415.95–487.0) | 419.5 (372.0–462.7) | 433.0 (353.25–472.5) | P = .142 |
| AHI (n events/h) | 49.3 (32.8–67.0) | 33.75 (24.0–58.4) | 53.2 (34.5–71.25) | 55.35 (38.68–79.03) | P = .008* G1–G3 .006* |
| AHIsup (n events/h) | 60.5 (40.5–74.95) | 51.8 (26.45–71.9) | 64.9 (43.75–75.85) | 56.1 (45.73–79.8) | P = .250 |
| AHIob (n events/h) | 40.7 (26.8–62.9) | 32.8 (20.0–53.2) | 45.5 (29.8–71.0) | 40.45 (33.5–72.4) | P = .094 |
| AIob (n events/h) | 22.0 (9.1–40.95) | 10.9 (7.58–27.3) | 21.6 (10.2–53.6) | 29.75 (14.7–41.7) | P = .046* |
| AIcent (n events/h) | 0.3 (0.0–1.1) | 0.35 (0.75–0.92) | 0.4 (0.0–1.7) | 0.0 (0.0–1.0) | P = .306 |
| Hob/AHob% | 42.1 (18.2–63.1) | 57.05 (30.7–79.7) | 42.3 (18.2–63.0) | 27.33 (13.34–61.2) | P = .033* G1–G3 P = .028* |
| Apnea duration (s) | 22.0 (18.0–28.0) | 20.5 (17.7–24.7) | 23.0 (18.0–29.0) | 24.0 (18.0–28.7) | P = .699 |
| ODI (n/h) | 50.2 (31.5–66.2) | 32.2 (21.65–53.6) | 52.5 (34.55–64.5) | 56.75 (41.3–81.2) | P = .003* G1–G3 P = .002* |
| Mean saturation (%) | 92.0 (89.0–93.0) | 93.0 (91.0–94.0) | 92.0 (89.5–93.0) | 89.4 (86.4–93.0) | P = .021* G3–G1 P = .016* |
| T90 (%) | 14.8 (6.2–39.1) | 7.25 (4.93–20.0) | 13.3 (6.0–34.2) | 27.0 (11.9–63.4) | P = .009* G1–G3 P = .007* |
| Nadir (%) | 69.0 (57.0–78.0) | 78.0 (66.75–83.0) | 68.0 (57.25–75.0) | 66.0 (50.0–75.0) | P = .002* G3–G1 P = .003* G2–G1 P = .017* |
| Supine position (%) | 40.0 (22.0–68.0) | 29.3 (15.25–59.0) | 38.0 (19.0–63.0) | 56.0 (30.25–81.3) | P = .049* G1–G3 P = .047* |
Data are shown as median (interquartile range). n = number of patients, AHI = apnea-hypopnea index, AHIsup = apnea-hypopnea index in supine position, AHIob = obstructive apnea-hypopnea index, AIob = obstructive apnea index, AIcent = central apnea index, Hob/AHob% = percentage of obstructive hypopneas/total obstructive events, Nadir = nadir oxygen saturation, ODI = oxygen desaturation index ≥ 4%, T90 = time with oxygen saturation lower than 90%. *Statistically significant values.
Figure 1. Kruskal-Wallis tests for respiratory indexes.
AHI = apnea-hypopnea index, AIob = obstructive apnea index, Nadir = nadir oxygen saturation, ODI = oxygen desaturation index ≥ 4%, PosSup = supine position, T90 = time with oxygen saturation lower than 90%.
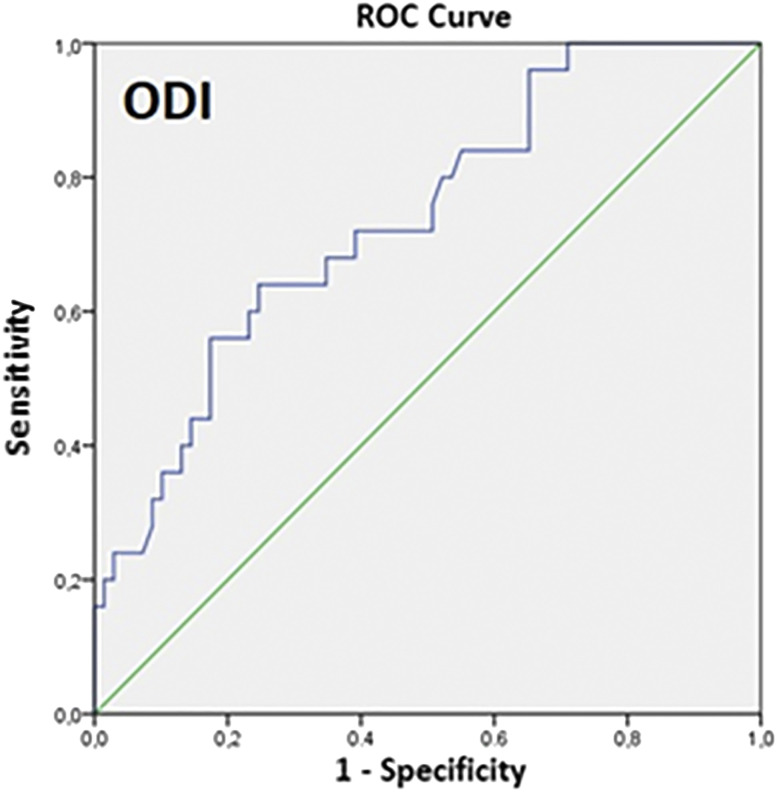
A statistically significant correlation was found between therapeutic CPAP level and AHI (r = .366, P = .000), obstructive apnea-hypopnea index (r = .249, P = .025), obstructive apnea index (r = .270, P = .015), ODI (r = .399, P = .000), mean saturation (r = .303, P = .005), T90 (r = .354, P = .001), and nadir (r = .337, P = .005). A stepwise multiple regression analysis was performed using all of these parameters (AHI, obstructive apnea-hypopnea index, obstructive apnea index, ODI, mean saturation, T90, desaturative nadir) as independent variables of the therapeutic CPAP level. Only ODI maintained a statistical significance, but the degree of correlation was low (intercept 8.818, P = .000; ODI standardized coefficient beta = 0.417, P = .000; R = .417; R2 = .173). The relationship between ODI and anatomical collapsibility (defined by CPAP values) was further analyzed with the receiver operating characteristic curve (Figure 2): the area under the curve was .726 (P = .002); ODI ≤ 40.8/h showed a sensitivity of 63.3% and a specificity of 69.2% to detect patients with CPAP values ≤ 8 cm H2O (Youden’s index = .328).
Figure 2. Receiver operating characteristic curve for oxygen desaturation index (ODI) vs CPAP value ≤ 8 cm H2O.
Symptoms and comorbidities are reported in Table 3. No symptoms and no comorbidities were reported by 35.6% and 26.6% of the patients, respectively. At the HSPM performed after CPAP titration, the median of analyzed time was 403.5 (IQR 339.5–452.8) minutes, AHI was 1.8 events/h (IQR 0.8–4.4), and mean saturation was 93.5% (IQR 92.4–95.0). The Kruskal-Wallis test showed a statistically significant difference for AHI between Gr1 (median 0.9, IQR 0.6–3.1) and Gr3 (median 3.0, IQR 1.0–7.3) (P = .028).
Table 3.
Symptoms and comorbidities.
| Total Sample (n=87) | Group 1 (n = 22) | Group 2 (n = 33) | Group 3 (n = 32) | Chi-squared Test | |
| Symptoms | |||||
| 1 - Not refreshing sleep | 18.4% | 13.6% | 18.2% | 21.9% | P = .744 |
| 2 - Insomnia | 17.2% | 18.2% | 15.2% | 18.8% | P = .240 |
| 1 and 2 | 12.6% | 4.5% | 18.2% | 12.5% | P = .068 |
| No symptom | 35.6% | 27.3% | 36.4% | 40.6% | P = .598 |
| Comorbidities | |||||
| 1 - Hypertension | 46.0% | 45.5% | 48.5% | 43.8% | P = .927 |
| 2 - Cerebrocardiopathy | 2.3% | 0.0% | 6.1% | 0.0% | P = .183 |
| 3 - Diabetes/dyslipidemia | 3.4% | 4.5% | 0.0% | 6.2% | P = .365 |
| 1-2-3 | 21.8% | 18.2% | 30.3% | 15.6% | P = .593 |
| No comorbidity | 26.6% | 31.8% | 15.2% | 34.4% | P = .171 |
A partial control of the upper airways under the maximum tolerated pressure was obtained in 6 patients of Gr2 (AHI [events/h]: 5.5, 5.6, 8.0, 8.6, 10.3, 14.3) as well as in 10 patients of Gr3 (AHI [events/h]: 5.5, 6.2, 7.2, 7.3, 8.0, 8.3, 11.6, 18.7, 22.5, 23.2).
For the overall sample the median CPAP value was 11.0 (IQR 8.0–12.0) cm H2O. Results were comparable between the sample of 87 patients and the sample of 94 patients. Particularly, for the association between ODI and anatomical collapsibility evaluated by analyzing the receiver operating characteristic curve, the area under the curve was low (0.738, P = .000). An ODI ≤ 35.1/h had a sensitivity of 64% and a specificity of 75% for detecting patients with CPAP value ≤ 8 cm H2O.
DISCUSSION
The major finding of the present study is that 25.3% of patients with moderate to severe OSA treated with CPAP based on the clinical severity of the disorder showed pressure requirements ≤ 8 cm H2O, a value that has already been described as sensitive (89%) and specific (84%) for detecting a mildly collapsible upper airway.11
A growing body of literature has shown that patients with OSA with mildly collapsible upper airways are likely to be suitable candidates for non-CPAP therapies.6–10
An accurate patient selection is essential to optimize MAD outcomes, avoid unnecessary costs and waste of time, and reduce undesired side effects.29–31 It has been demonstrated that mandibular advancement reduces pharyngeal collapsibility in a dose-dependent manner without major concurrent effects on pharyngeal muscle responsiveness or effectiveness.32–34 These findings imply that MADs mainly act through improvement in pharyngeal anatomy. Accordingly, Edwards et al6 found that MADs determine a reduction in AHI mediated by improvements in upper airway collapsibility under passive and active conditions, without significant effects on muscle function, LG, or arousal threshold. A more favorable treatment response has been observed in those patients with a mild anatomic collapsibility and a lower LG at baseline.6 These results are consistent with another study by Bamagoos et al35 confirming that the efficacy of MADs is greater with moderate pharyngeal collapsibility, lower LG, higher arousal threshold, and lower response to arousal at baseline.
Upper airway surgery (uvulopalatopharyngoplasty, transpalatal advancement pharyngoplasty) decreased pharyngeal collapsibility between 8.1 and 9.2 cm H2O.9,36–38 In accordance with these findings, it has been speculated that a mild collapsibility can also be a useful indicator of good response to upper airway surgeries.35,39 This is only a hypothesis and needs to be tested directly.
It is likely that the extent of Pcrit recovery obtained with weight loss8 or positional therapy10 can fall within the range of pressure consistent with a good therapeutic response for patients with mild collapsibility. These too are hypotheses not directly tested.
The notion that therapeutic CPAP level can be used as an easily and routinely accessible method to identify patients with high probability of mildly collapsible airways is known. Although this needs to be confirmed by a prospective study, this simple information has the potential to allow personalized therapy of OSA by assisting clinicians to better select the patients most suitable for individual non-CPAP therapies.
The main finding of this study is that 25.3% of patients with OSA treated with CPAP as first therapeutic option were highly likely to have a mildly collapsible upper airway and, therefore, could have been good candidates for alternative and well tolerated non-CPAP therapies. The therapeutic response depends on the basal Pcrit value and how much it falls in response to the treatment,35,39 and literature data, certainly not definitive, are consistent with the hypothesis that a mild collapsibility is correctable by Pcrit recovery obtainable with non-CPAP therapies.8–10,36–38 To our knowledge, a short-term therapeutic step-down from CPAP has not been an aspect considered in the management of patients with OSA16, 40–41, 43; however, in our opinion, the fact that 1 patient out of 4 of those treated with CPAP could change for better tolerated therapies is a relevant piece of information.
The second main finding of the present study is that mild upper airway collapsibility, as determined by CPAP pressure requirements ≤ 8 cm H2O, cannot be predicted using anthropometric, clinical (self-reported sleepiness, symptoms, comorbidities), and respiratory function data. Baseline nocturnal HSPM parameters were more favorable in Gr1, with statistical significance among group differences being observed for AHI, the percentage of obstructive hypopneas in relation to the total obstructive events, ODI, mean saturation, T90, desaturation nadir, and supine position. The stepwise multiple regression analysis showed that only ODI was associated with anatomical collapsibility, as defined by CPAP values. Using the receiver operating characteristic curve, area under the curve for ODI versus CPAP pressure requirements ≤ 8 cm H2O was moderate and values of ODI ≤ 40.8/h provided the best value of Youden’s index (Y test = .328; sensitivity 63.3% and specificity 69.2%) to detect a mildly collapsible upper airway. Because of the low sensitivity and specificity, this ODI cut-off is not clinically helpful at differentiating between mildly and severely collapsible airways. Overall, we did not find HSPM parameters alternative to CPAP therapeutic values in order to detect pharyngeal collapsibility.
To our knowledge, no detailed data were available on the relationship between the degree of upper airway collapsibility and HSPM/polysomnographic parameters. Eckert et al5 described AHI and REM/non-REM AHI ratio in 3 different subgroups. Data scattering around the mean value was comparable among the groups, confirming that it is difficult to detect patients with mild collapsibility using HSPM/polysomnographic parameters. Landry et al11 found that CPAP pressure requirements ≤ 8 cm H2O were coherent with a mild collapsibility but did not provide nocturnal HSPM/polysomnographic parameters.
There is growing search for simplified tools to evaluate physiological traits.44–46 Considering these data it is possible to speculate that an acute CPAP challenge test (simple, accessible, and relatively inexpensive) should be built and could be an important supplementary tool in the management of patients with OSA before choosing long-term therapeutic options.
Results were comparable between the sample of 87 patients with moderate to severe OSA and the whole sample of 94 patients.
Study limitations
Polysomnography is the gold standard for the evaluation of OSA severity, allowing an accurate definition of the indexes per hour of sleep. HSPM can underestimate the severity of the disease because it is based on the estimated total sleep time. However, HSPM has already been widely used throughout literature, as well as in everyday clinical practice.18 The adoption of an accurate sleep questionnaire together with the removal of periods in upright position should have minimized this bias.39
The way in which therapeutic CPAP level is determined is likely to be a critical component contributing to the strength of the predictive relationship between CPAP level and Pcrit.
Landry at al11 used a laboratory titration but also hypothesized that, because the most recent APAP devices use sensitive measurement of flow-limited breathing to titrate pressure, a therapeutic CPAP level determined from APAP devices (conventionally defined by the 90/95th percentile pressure statistic) is also likely to predict upper airway collapsibility. The APAP devices we used (DreamStation or AirSense 10) have algorithms aimed at correcting for snoring and inspiratory flow limitation. This indicates that the probability that this study’s results are similar to those of the reference study is high. Future studies are required for the definitive confirmation.
Our group of patients was selected and representative of moderate to severe OSA with short-term adherence to CPAP. It is our standard to use CPAP as first option in moderate to severe OSA and non-CPAP therapies in mild OSA. For this reason, a very small number of mild patients (7) were treated with CPAP. We included the data from these 7 patients in the supplemental material, but we preferred not to confound the data of the main paper. It is conceivable that a large percentage of patients with mild OSA have a mild collapsibility, but the main aim of this study was to verify how many patients destined to be treated with CPAP as first option may easily be treated with alternative therapies after short-term stepdown from CPAP.
A larger sample size could increase the significance of the findings; our data need to be confirmed by a prospective study.
CONCLUSIONS
Quantitative data on the pathophysiological traits of OSA are currently acquired in highly specialized sleep centers using challenging, time-consuming, and expensive techniques that cannot be utilized for routine assessment. Finding alternative, accessible tools for the clinic or polysomnographic studies is, therefore, mandatory.
The identification of patients with mildly collapsible airways through CPAP therapeutic values offers physiopathologic qualitative information that is very important in the therapeutic management of OSA. We found that a quarter of patients who had been treated with CPAP were likely to have a mildly collapsible upper airway, thus being good candidates for alternative and well tolerated non-CPAP therapies. Anthropometric, clinical respiratory function, and HSPM parameters did not predict mild upper airway collapsibility, as determined by CPAP pressure requirements ≤ 8 cm H2O.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ABBREVIATIONS
- AHI
apnea-hypopnea index
- APAP
autotitrating positive airway pressure
- CPAP
continuous positive airway pressure
- Gr1
Group 1
- Gr2
Group 2
- Gr3
Group 3
- HSPM
home sleep portable monitoring
- IQR
interquartile range
- LG
loop gain
- MAD
mandibular advancement device
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- Pcrit
pharyngeal critical pressure
- T90
sleep time with an oxygen saturation < 90%
REFERENCES
- 1.Bosi M, De Vito A, Kotecha B, et al. Phenotyping the pathophysiology of obstructive sleep apnea using polygraphy/polysomnography: a review of the literature. Sleep Breath. 2018;22(3):579–592. 10.1007/s11325-017-1613-3 [DOI] [PubMed] [Google Scholar]
- 2.Wellman A, Eckert DJ, Jordan AS, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol. 2011;110(6):1627–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wellman A, Edwards BA, Sands SA, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol. 2013;114(7):911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owens RL, Edwards BA, Eckert DJ, et al. An integrative model of physiological traits can be used to predict obstructive sleep apnea and response to non positive airway pressure therapy. Sleep. 2015;38(6):961–970. 10.5665/sleep.4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. 10.1164/rccm.201303-0448OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards BA, Andara C, Landry S, et al. Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2016;194(11):1413–1422. 10.1164/rccm.201601-0099OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards BA, Sands SA, Owens RL, et al. The combination of supplemental oxygen and a hypnotic markedly improves obstructive sleep apnea in patients with a mild to moderate upper airway collapsibility. Sleep. 2016;39(11):1973–1983. 10.5665/sleep.6226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz AR, Gold AR, Schubert N, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144(3 pt 1):494–498. 10.1164/ajrccm/144.3_Pt_1.494 [DOI] [PubMed] [Google Scholar]
- 9.Schwartz AR, Schubert N, Rothman W, et al. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1992;145(3):527–532. 10.1164/ajrccm/145.3.527 [DOI] [PubMed] [Google Scholar]
- 10.Joosten SA, Edwards BA, Wellman A, et al. The effect of body position on physiological factors that contribute to obstructive sleep apnea. Sleep. 2015;38(9):1469–1478. 10.5665/sleep.4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landry SA, Joosten SA, Eckert DJ, et al. Therapeutic CPAP level predicts upper airway collapsibility in patients with obstructive sleep apnea. Sleep. 2017;40(6):zsx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braghiroli A, Marrone O, Sanna A, et al. Linee guida di procedura diagnostica nella sindrome delle apnee ostruttive nel sonno dell’adulto. Commissione paritetica Associazione Italiana Pneumologi Ospedalieri (AIPO) e Associazione Italiana Medicina del Sonno (AIMS). Rass Patol App Respir. 2001;16:278–280. [Google Scholar]
- 13.Berry RB, Brooks R, Gamaldo CE, et al; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.2. Darien, IL: American Academy of Sleep Medicine; 2015. [Google Scholar]
- 14.Ellis BW, Johns MW, Lancaster R, Raptopoulos P, Angelopoulos N, Priest RG. The St. Mary’s Hospital sleep questionnaire: a study of reliability. Sleep. 1981;4(1):93–97. 10.1093/sleep/4.1.93 [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 16.Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2019;15(2):335–343. 10.5664/jcsm.7640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgenthaler TI, Aurora RN, Brown T, et al.; Standards of Practice Committee of the AASM ; American Academy of Sleep Medicine . Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep. 2008;31(1):141–147. 10.1093/sleep/31.1.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fietze I, Penzel T, Alonderis A, et al.; COST Action B26 Group . Management of obstructive sleep apnea in Europe. Sleep Med. 2011;12(2):190–197. 10.1016/j.sleep.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 19.Berry RB, Hill G, Thompson L, McLaurin V. Portable monitoring and autotitration versus polysomnography for the diagnosis and treatment of sleep apnea. Sleep. 2008;31(10):1423–1431. [PMC free article] [PubMed] [Google Scholar]
- 20.Cross MD, Vennelle M, Engleman HM, et al. Comparison of CPAP titration at home or the sleep laboratory in the sleep apnea hypopnea syndrome. Sleep. 2006;29(11):1451–1455. 10.1093/sleep/29.11.1451 [DOI] [PubMed] [Google Scholar]
- 21.Kuna ST, Gurubhagavatula I, Maislin G, et al. Noninferiority of functional outcome in ambulatory management of obstructive sleep apnea. Am J Respir Crit Care Med. 2011;183(9):1238–1244. 10.1164/rccm.201011-1770OC [DOI] [PubMed] [Google Scholar]
- 22.McArdle N, Singh B, Murphy M, et al. Continuous positive airway pressure titration for obstructive sleep apnoea: automatic versus manual titration. Thorax. 2010;65(7):606–611. 10.1136/thx.2009.116756 [DOI] [PubMed] [Google Scholar]
- 23.Mulgrew AT, Fox N, Ayas NT, Ryan CF. Diagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation study. Ann Intern Med. 2007;146(3):157–166. 10.7326/0003-4819-146-3-200702060-00004 [DOI] [PubMed] [Google Scholar]
- 24.Planès C, D’Ortho MP, Foucher A, et al. Efficacy and cost of home-initiated auto-nCPAP versus conventional nCPAP. Sleep. 2003;26(2):156–160. 10.1093/sleep/26.2.156 [DOI] [PubMed] [Google Scholar]
- 25.Rosen CL, Auckley D, Benca R, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012;35(6):757–767. 10.5665/sleep.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chai-Coetzer CL, Antic NA, Rowland LS, et al. Primary care vs specialist sleep center management of obstructive sleep apnea and daytime sleepiness and quality of life: a randomized trial. JAMA. 2013;309(10):997–1004. 10.1001/jama.2013.1823 [DOI] [PubMed] [Google Scholar]
- 27.Hui DS, Ng SS, To KW, et al. A randomized controlled trial of an ambulatory approach versus the hospital-based approach in managing suspected obstructive sleep apnea syndrome. Sci Rep. 2017;7(45901):45901. 10.1038/srep45901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui DS, Ng SS, Tam WWS. Home-based approach noninferior to hospital-based approach in managing patients with suspected obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2018;197(9):1233–1234. 10.1164/rccm.201711-2185LE [DOI] [PubMed] [Google Scholar]
- 29.Alessandri-Bonetti G, D’Antò V, Stipa C, Rongo R, Incerti-Parenti S, Michelotti A. Dentoskeletal effects of oral appliance wear in obstructive sleep apnoea and snoring patients. Eur J Orthod. 2017;39(5):482–488. [DOI] [PubMed] [Google Scholar]
- 30.Incerti Parenti S, Bortolotti F, Alessandri-Bonetti G. Oral appliances for obstructive sleep apnea. J World Fed Orthod. 2019;8(1):3–8. 10.1016/j.ejwf.2019.01.001 [DOI] [Google Scholar]
- 31.Alessandri-Bonetti G, Ippolito DR, Bartolucci ML, D’Antò V, Incerti-Parenti S. Cephalometric predictors of treatment outcome with mandibular advancement devices in adult patients with obstructive sleep apnea: a systematic review. Korean J Orthod. 2015;45(6):308–321. 10.4041/kjod.2015.45.6.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bamagoos AA, Cistulli PA, Sutherland K, et al. Dose-dependent effects of mandibular advancement on upper airway collapsibility and muscle function in obstructive sleep apnea. Sleep. 2019;42(6):zsz049. 10.1093/sleep/zsz049 [DOI] [PubMed] [Google Scholar]
- 33. doi: 10.1007/s11325-019-01930-3. Bamagoos AA, Eckert DJ, Sutherland K, Ngiam J, Cistulli PA. Dose-dependent effects of mandibular advancement on optimal positive airway pressure requirements in obstructive sleep apnoea. [published online ahead of print, 2019 Aug 29] Sleep Breath . 2019. [DOI] [PubMed] [Google Scholar]
- 34.Bartolucci ML, Bortolotti F, Raffaelli E, D’Antò V, Michelotti A, Alessandri Bonetti G. The effectiveness of different mandibular advancement amounts in OSA patients: a systematic review and meta-regression analysis. Sleep Breath. 2016;20(3):911–919. 10.1007/s11325-015-1307-7 [DOI] [PubMed] [Google Scholar]
- 35.Bamagoos AA, Cistulli PA, Sutherland K, et al. Polysomnographic endotyping to select patients with obstructive sleep apnea for oral appliances. Ann Am Thorac Soc. 2019;16(11):1422–1431. 10.1513/AnnalsATS.201903-190OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodson BT. Changes in airway characteristics after transpalatal advancement pharyngoplasty compared to uvulopalatopharyngoplasty (UPPP). Sleep. 199619(suppl 10):S291–S293. 10.1093/sleep/19.suppl_10.S291 [DOI] [PubMed] [Google Scholar]
- 37.Woodson BT. Retropalatal airway characteristics in uvulopalatopharyngoplasty compared with transpalatal advancement pharyngoplasty. Laryngoscope. 1997;107(6):735–740. 10.1097/00005537-199706000-00006 [DOI] [PubMed] [Google Scholar]
- 38.Tucker Woodson B. Acute effects of palatopharyngoplasty on airway collapsibility. Otolaryngol Head Neck Surg. 1999;121(1):82–86. 10.1016/S0194-5998(99)70130-0 [DOI] [PubMed] [Google Scholar]
- 39.Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest. 1996;110(4):1077–1088. 10.1378/chest.110.4.1077 [DOI] [PubMed] [Google Scholar]
- 40.Randerath WJ, Verbraecken J, Andreas S, et al.; European Respiratory Society task force on non-CPAP therapies in sleep apnoea . Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J. 2011;37(5):1000–1028. 10.1183/09031936.00099710 [DOI] [PubMed] [Google Scholar]
- 41.Epstein LJ, Kristo D, Strollo PJ Jr, et al. ; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine . Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. 10.5664/jcsm.27497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kushida CA, Chediak A, Berry RB, et al.; Positive Airway Pressure Titration Task Force ; American Academy of Sleep Medicine . Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. 10.5664/jcsm.27133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleetham J, Ayas N, Bradley D, et al.; Canadian Thoracic Society Sleep Disordered Breathing Committee . Canadian Thoracic Society 2011 guideline update: diagnosis and treatment of sleep disordered breathing. Can Respir J. 2011;18(1):25–47. 10.1155/2011/506189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards BA, Eckert DJ, McSharry DG, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190(11):1293–1300. 10.1164/rccm.201404-0718OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Ye J, Han D, et al. Physiology-based modeling may predict surgical treatment outcome for obstructive sleep apnea. J Clin Sleep Med. 2017;13(9):1029–1037. 10.5664/jcsm.6716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osman AM, Tong BK, Landry SA, et al. An assessment of a simple clinical technique to estimate pharyngeal collapsibility in people with OSA. Sleep. 2020:zsaa067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.