Abstract
Study Objectives:
Exercise capacity is impaired in obstructive sleep apnea (OSA). There are conflicting reports on the effect of continuous positive airway pressure (CPAP) on maximal exercise capacity. The objective of this review was to determine if there is a change in exercise capacity and anaerobic threshold following CPAP treatment in OSA patients.
Methods:
We conducted a systematic review and meta-analyses to summarize the changes in peak rate of oxygen uptake (V̇O2 peak) or maximum rate of oxygen uptake (V̇O2 max) and anaerobic threshold (AT) during cardiopulmonary exercise testing following CPAP intervention in patients with OSA. A systematic literature review was conducted to identify published literature on markers of V̇O2 peak, V̇O2 max, and AT pre- vs post-CPAP using a web-based literature search of PubMed/MEDLINE, Embase, CINAHL, and Cochrane review (CENTRAL) databases. Two independent reviewers screened the articles for data extraction and analysis.
Results:
The total search of all the databases returned 470 relevant citations. Following application of eligibility criteria, 6 studies were included in the final meta-analysis for V̇O2 peak, 2 studies for V̇O2 max, and five studies for AT. The meta-analysis showed a mean net difference in V̇O2 peak between pre- and post-CPAP of 2.69 mL·kg–1·min–1, P = .02, favoring treatment with CPAP. There was no difference in V̇O2 max or AT with CPAP treatment (mean net difference 0.66 mL·kg–1·min–1 [P = .78] and –144.98 mL·min–1 [P = .20] respectively).
Conclusions:
There is a paucity of high-quality studies investigating the effect of CPAP on exercise capacity. Our meta-analysis shows that V̇O2 peak increases following CPAP treatment in patients with OSA, but we did not observe any change in V̇O2 max or AT. Our findings should be considered preliminary and we recommend further randomized controlled trials to confirm our findings and to clarify the peak and maximum rates of oxygen uptake adaptations with CPAP therapy.
Citation:
Fletcher HV, Cho PSP, Loong SL et al. Effect of continuous positive airway pressure on maximal exercise capacity in patients with obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med. 2020;16(11): 1847–1855.
Keywords: continuous positive airway pressure, obstructive sleep apnea, aerobic exercise, physical activity, cardiopulmonary exercise test
BRIEF SUMMARY
Current Knowledge/Study Rationale: Exercise capacity is impaired in obstructive sleep apnea. It is unclear if exercise capacity and anaerobic threshold change in obstructive sleep apnea patients after continuous positive airway pressure treatment. This systematic review and meta-analyses summarized the changes in peak rate of oxygen uptake, maximum rate of oxygen uptake, and anaerobic threshold with continuous positive airway pressure intervention.
Study Impact: A web-based literature search included PubMed/MEDLINE, Embase, CINAHL, and Cochrane review (CENTRAL) databases. The analysis finds a significant change in peak rate of oxygen uptake but does not support a change in maximum rate of oxygen uptake or anaerobic threshold after continuous positive airway pressure treatment. More randomized controlled trials are recommended to confirm our conclusions.
INTRODUCTION
In the UK, it is estimated that 1.5 million adults have obstructive sleep apnea (OSA), with under a third currently diagnosed and being treated.1 OSA increases the risk of developing hypertension,2 cardiovascular disease, obesity, stroke events, and diabetes.3,4 Cardiorespiratory fitness can be measured by assessing volume of oxygen (V̇O2), which is the rate of oxygen uptake during cardiopulmonary exercise testing, with V̇O2 max representing the maximum value under ideal conditions and V̇O2 peak representing the highest value achieved within the constraints of the individual or study conditions. These markers can be used to prognosticate cardiac and respiratory disease in patients with high cardiorespiratory risk.5–7 A V̇O2 peak has been reported in OSA patients8–13.
A recent systematic review and meta-analysis reported lower maximal exercise capacity in OSA patients compared to healthy individuals.14,15 Continuous positive airway pressure (CPAP) is the first-line treatment for significant OSA. Several studies have assessed the effect of CPAP treatment on behavior, metabolism, and energy expenditure.14,17–18 Interest also lies with the objective measures of physical activity and exercise training interventions in addition to CPAP treatment in OSA patients.18–20 Associations between physical activity and OSA patients indicate that patients are neither physically capable nor psychologically motivated to exercise.8,21–24 However, CPAP has been shown to have a favorable effect on physical activity in patients with OSA. A basis for change could be through a change in motivation (based on symptomatic improvement) or a fundamental change in oxidative capacity. Few systematic reviews have investigated the literature comparing pre- and post-CPAP treatment in maximal exercise capacity (V̇O2 peak and V̇O2 max) and AT markers.
To our knowledge, no systematic review or meta-analyses has been conducted to summarize the changes in maximal exercise capacity with CPAP intervention in patients with OSA. Therefore, the main objective of this systematic review and meta-analysis was to determine if there were changes in maximal exercise capacity (V̇O2 max and V̇O2 peak) and anaerobic threshold following CPAP treatment in OSA patients. A secondary aim was to explore if other physiological markers altered the effect of CPAP on maximal exercise capacity and AT.
METHODS
PRISMA
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement and recommendations were followed (www.prisma-statement.org). The study is registered on PROSPERO [ID number 138158] (www.crd.york.ac.uk/prospero/). No public or patient involvement occurred in this study.
Search strategy
A systematic literature review was conducted to identify published literature that investigated maximal exercise capacity and AT in OSA patients before and after CPAP treatment. A web-based literature search included PubMed/MEDLINE, Embase, CINAHL, and Cochrane review (CENTRAL) databases. Search terms were selected to reflect the intervention, population, and outcome parameters to allow for the specific comparison of the intervention to be assessed by using PICO, (P: adults, I: CPAP, C: pre- and post-treatment, O: maximal exercise capacity and AT).
The search terms included a combination of terms and medical subject headings (MeSH) and or exploded with in databases. For the treatment, condition, and outcomes, search terms used were “continuous positive airway pressure” OR “APRV Ventilation Mode” OR “Airway Pressure Release Ventilation” OR “Bilevel Continuous Positive Airway Pressure” OR “Biphasic Continuous Positive Airway Pressure” OR “CPAP Ventilation” OR “Continuous Positive Airway Pressure”[MeSH Terms] OR “Continuous Positive Airway Pressure” OR “Nasal Continuous Positive Airway Pressure” OR “nCPAP Ventilation” AND “Sleep Apnea, Obstructive” OR “Apnea, Obstructive Sleep” OR “OSAHS” OR “Obstructive Sleep Apnea” OR “Obstructive Sleep Apnea Syndrome” OR “Sleep Apnea Hypopnea Syndrome” OR “Sleep Apnea Syndrome, Obstructive” OR “Sleep Apnea, Obstructive”[MeSH Terms] OR “Sleep Apnea, Obstructive” OR “Syndrome, Obstructive Sleep Apnea” OR “Syndrome, Sleep Apnea, Obstructive” OR “Syndrome, Upper Airway Resistance, Sleep Apnea” OR “Upper Airway Resistance Sleep Apnea Syndrome” AND “Acute Exercise” OR “Aerobic Exercise” OR “Exercise”[MeSH Terms] OR “Exercise” OR “Exercise Training” OR “Exercise, Aerobic” OR “Exercise, Isometric” OR “Exercise, Physical” OR “Isometric Exercise” OR “Physical Activity” OR “Cardiopulmonary Exercise Testing”.
Terms were searched with all possible combinations using Boolean logical operators. An additional search of bibliographies was conducted to identify any relevant articles. Mendeley Reference Manager software (version 1.19.4; Mendeley Ltd., London, United Kingdom) was used to manage all references.
Eligibility criteria
Inclusion criteria were: published peer reviewed articles conducted in adult humans with a diagnosis of OSA and with an apnea-hypopnea index or respiratory disturbance index score of > 10 events/h on polysomnography. Articles were authors’ original work and only articles published in English were retained. Only articles reporting maximal exercise capacity (V̇O2 max and V̇O2 peak) and AT as means and standard deviations were included. Only articles with clear trial designs were included. Accepted study designs included within group comparison of pre- vs post-CPAP treatment. There was no restriction on the length of the CPAP treatment and no date limits set for the articles. Nonrandomized studies were included.
Articles were excluded if exercise or physical activity interventions were implemented in addition to CPAP treatment. Conference articles were also excluded.
Reviewing procedure and data extraction
Searches of the databases were conducted in December 2018. All obtained references were retained, with data collection being conducted by 2 review authors (HF and PC). The first level of review involved title and abstract screening and removal of duplicated and irrelevant articles and those not published in English. Remaining articles then underwent full test review and assessment against the prespecified eligibility criteria by 2 review team members independently. Disagreements over eligibility of potential articles were resolved through discussion with a third reviewer (KL).
Data extraction was performed for retained articles. Principle summary measures were the differences in V̇O2 max, V̇O2 peak, and AT pre- vs post-CPAP. The following variables were also systematically extracted: (1) author, (2) publication year, (3) participants, (4) apnea-hypopnea index cut-off, (5) sample size, (6) age, (7) duration of CPAP, (8) design, (9) units of measurement, (10) pre-CPAP V̇O2 peak or V̇O2 max/pre-CPAP AT, (11) post-CPAP V̇O2 peak and V̇O2 max/post-CPAP AT (Table 1 and Table 2).
Table 1.
Summary of findings for articles reporting V˙O2 peak.
| Author | Year | AHI Cut-off events/h | Baseline AHI/RDI events/h | Sample Size (n) | Age (years) | Duration of CPAP | Adherence to CPAP (hrs·n–1) | Design | V̇O2 peak or V̇O2 max | Pre (mL·kg–1·min–1) | Post (mL·kg–1·min–1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Taguchi26 | 1997 | Not reported | 62.5 ± 8.6 | 6 | 44.7 ± 13.7 | 7 days | Not reported | Q | V̇O2 max (mL·min–1) | 22.13 ± 4.56* | 25.9 ± 4.8* |
| Ozsarac25 | 2014 | > 30 | 40.7 ± 19.6 | 65 | 48.76 ± 9.2 | 4 weeks | 6.2 ± 0.9 | Q | V̇O2 max (mL·min–1) | 22.5 ± 6.6* | 21.3 ± 5.3* |
| Alonso-Fernández10 | 2006 | > 10 | 43.6 ± 26.6 | 31 | 53 ± 13 | 12 weeks | 6 ± 1 | RCT | V̇O2 peak | 24.53 ± 6.15 | 25.1 ± 8.58 |
| Goel27 | 2015 | > 10 | 35.32 ± 16.99 | 15 | 56.5 ± 9.1 | 4 weeks | Not reported | Q | V̇O2 peak (mL·min–1) | 12.83 ± 3.3* | 17.46 ± 4.44* |
| Lin28 | 2004 | RDI ≥ 30 | 47.3 ± 15.7 | 20 | 43 ± 8 | 2 months | Not reported | Q | V̇O2 peak | 20.41 ± 3.31 | 26.3 ± 4.29 |
| Mortari29 | 2017 | > 30 | 61.7 ± 23.4 | 16 | 52.8 ± 10.8 | 3 months | 6.7 ± 2** | R | V̇O2 peak | 27.81 ± 9.91 | 29.71 ± 7.83 |
| Pendharker30 | 2011 | > 15 | 48.1 ± 33.1 | 15 | 49 ± 6 | 3 months | 5.8 ± 2s | Q | V̇O2 peak | 17 ± 4.2 | 16.6 ± 3.5 |
| Quadri31 | 2017 | > 30 | 45.4 ± 14.9 | 12 | 58 ± 9.7 | 2 months | Not reported | Q | V̇O2 peak | 21.1 ± 3.8 | 23.4 ± 4.3 |
Data are presented as n or mean ± SD, unless reported otherwise. *Original data in articles in mL/min, uncertainty analysis used to change. **Extrapolated from the total hours usage data over 3 months (603 ± 180 written in article). AHI = apnea-hypopnea index, CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea, OSAHS = obstructive sleep apnea hypopnea syndrome, Q = quasi-experimental single group designed study, R = retrospective study, RCT = randomized controlled trial, RDI = respiratory disturbance index, V̇O2 max = peak rate of oxygen uptake, V̇O2 peak = maximum rate of oxygen uptake.
Table 2.
Summary of findings for articles reporting anaerobic threshold.
| Author | Year | Sample Size (n) | Age (years) | Duration of CPAP | Design | AT (L·min–1 or mL·min–1) | Pre (mL·min–1) | Post (mL·min–1) |
|---|---|---|---|---|---|---|---|---|
| Goel27 | 2015 | 15 | 56.5 ± 9.1 | 4 weeks | Q | mL·min–1 | 703 ± 215 | 926 ± 248 |
| Lin28 | 2004 | 20 | 43 ± 8 | 2 months | Q | L·min–1 | 910 ± 140* | 1280 ± 120* |
| Ozsarac25 | 2014 | 65 | 48.76 ± 9.2 | 4 weeks | Q | L·min–1 | 1000 ± 300* | 900 ± 200* |
| Quadri31 | 2017 | 12 | 58 ± 9.7 | 2 months | Q | mL·min–1 | 1229.5 ± 212.9 | 1399.2 ± 372.7 |
| Taguchi26 | 1997 | 6 | 44.7 ± 13.7 | 7 days | Q | mL·min–1 | 985 ± 161 | 1050 ± 139 |
Data are presented as n or mean ± SD, unless reported otherwise. *Original data in articles in mL/min, uncertainty analysis used to change. AT = anaerobic threshold, CPAP = continuous positive airway pressure, Q = quasi-experimental single group designed study, R = retrospective study, RCT = randomized controlled trial.
Statistical analysis
The mean V̇O2 max, V̇O2 peak, and AT were compared pre- and post-CPAP. Where V̇O2 max values were not explicitly reported, the mean and standard deviation values of V̇O2 max or V̇O2 peak were estimated by using uncertainty analysis.22 V̇O2 max and V̇O2 peak were expressed in mL⋅kg–1⋅min–1. AT was expressed in mL·min–1.
Heterogeneity analysis was used to provide an estimation of the variability due to heterogeneity. An I2 index of > 60% reflects increasing heterogeneity.23 Sensitivity analysis was performed for V̇O2 max, V̇O2 peak, and AT using Cochrane Q test.24
Effect sizes were calculated for random effects models using Review Manager (RevMan, Version 5.3.; The Nordic Cochrane Centre, Copenhagen, Denmark) and reported as mean net difference and 95% confidence intervals.24
For subanalysis, tests of normality and Spearman rank tests were performed using Prism (Version 8.3.0 for Windows; GraphPad Software, La Jolla, CA).
RESULTS
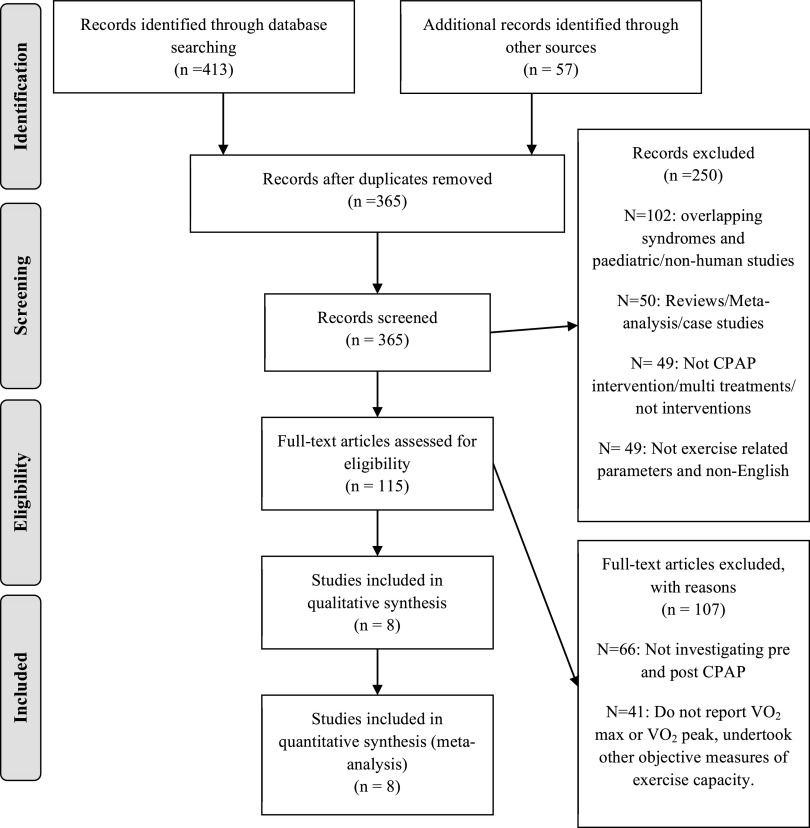
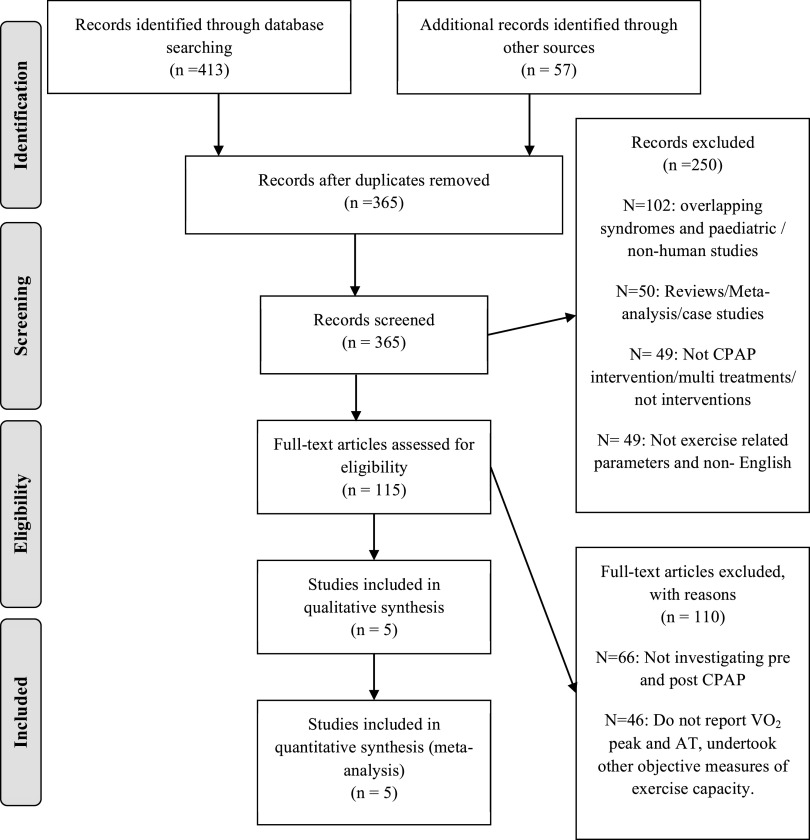
Study selection process is presented in Figure 1, a PRISMA flow diagram. The total search of all the databases provided 470 relevant citations. After the first and second stages of screening, 8 articles remained that reported V̇O2 max or V̇O2 peak pre- and post-CPAP treatment in OSA patients, of which 2 reported V̇O2 max25,26 and 6 reported V̇O2 peak.10,27–31 Five articles met the inclusions criteria for reporting AT pre- and post-CPAP treatment in OSA patients25–28,31 (Figure 2).
Figure 1. PRISMA flow diagram V̇O2 max and V̇O2 peak, for the selection of articles identified and evaluated during the selection process.
CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea, V̇O2 max = maximum rate of oxygen uptake, V̇O2 peak = peak rate of oxygen uptake.
Figure 2. PRISMA flow diagram anaerobic threshold, for the selection of articles identified and evaluated during the selection process.
AT = anaerobic threshold, CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea.
Description of studies
The characteristics of the articles are presented in Table 1 and Table 2. The sample size of all the included studies was 175 patients, of whom 18.9% were female. The mean age ranged from 43 to 58 years. Baseline apnea-hypopnea index or respiratory disturbance index ranged between 35 and 62 events/h, signifying a cohort of patients with severe OSA.
The total sample sizes of the studies retained for the meta-analyses of V̇O2 max,10,27–31 V̇O2 peak,25,26 and AT25–28,31 were 71, 104, and 119 patients, respectively.
Analysis of publication bias
Overall the quality of the evidence of CPAP improving V̇O2 max, V̇O2 peak, and AT in patients with OSA was graded as high and the risk of bias as unclear (Table 3). The quality of the one RCT study was low risk of bias.10 All of the quasi-experimental, single-group–designed studies were considered as having very low/unclear quality.25–31
Table 3.
Results of the quality analysis of the included studies.
| Quality Criteria | Alonso-Fernández10 | Goel27 | Lin28 | Mortari29 | Ozsarac25 | Pendharker30 | Quadri31 | Taguchi26 |
|---|---|---|---|---|---|---|---|---|
| Sample randomization | Yes | No | No | No | No | No | No | No |
| Comparison between treatments | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Blinding of participants | Yes | No | No | No | No | No | No | No |
| Description of measurement | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Statistical analysis | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Defined inclusion/exclusion criteria | Yes | No | Yes | Yes | Unclear–exclusion only | Yes | Yes | No |
| Report of follow-up | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Unclear |
| Risk of bias* | Low | High | High | Unclear | High | Unclear | Unclear | High |
*Risk of bias assessment: Yes (high) = 0–4, unclear = 5–6, No (low) = 7.
Effect of CPAP on V̇O2 max and V̇O2 peak
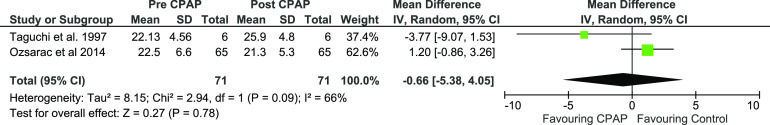
The mean V̇O2 max net difference between pre- and post-CPAP in patients with OSA was –0.66 mL·kg–1·min–1 (P < .78) not reaching statistical significance. Mean V̇O2 max, pre-CPAP, and post-CPAP expressed a 95% CI of –5.38 to –4.05 (Figure 3, Figure S1 in the supplemental material, and Table 1).
Figure 3. Forest plot for mean V̇̇O2 max in mL·kg–1·min–1 pre- and post-CPAP treatment in OSA patients.
CPAP = continuous positive airway pressure, IV = random-effect model, SD = standard deviation.
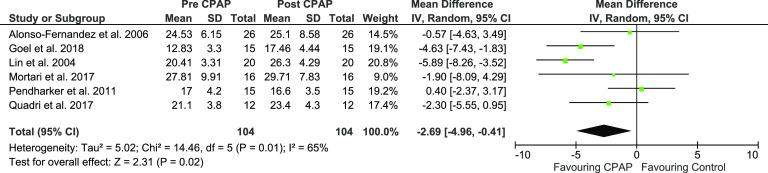
The mean V̇O2 peak net difference between pre- and post-CPAP in patients with OSA was –2.69 mL·kg–1·min–1 (P < .02) favoring treatment with CPAP and reaching statistical significance. Mean V̇O2 peak, pre-CPAP and post-CPAP expressed a 95% CI of –4.96 to –0.41 (Figure 4, Figure S2 in the supplemental material, and Table 1).
Figure 4. Forest plot for mean V̇̇O2 peak in mL·kg–1·min–1 pre- and post-CPAP treatment in OSA patients.
CPAP = continuous positive airway pressure, IV = random-effect model, SD = standard deviation.
Effect of CPAP on anaerobic threshold
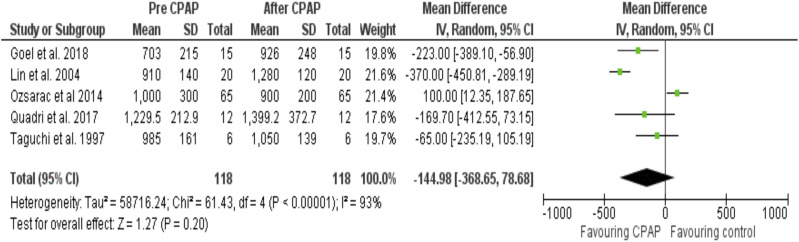
Mean net difference (95% CI) AT before and after CPAP treatment was –144.98 (–368.65 to –78.68) mL·min–1 (P < .20) (Figure 5, Figure S3 in the supplemental material, and Table 2).
Figure 5. Forest plot for mean AT in mL·min–1 pre- and post-CPAP treatment in OSA patients.
CPAP = continuous positive airway pressure, IV = random-effect model, MD = mean difference, SD = standard deviation.
Subanalysis
Table 4 shows the Spearman correlation coefficients between baseline patient characteristics and change in V̇O2 max and V̇O2 peak (ΔV̇O2 max and peak) with CPAP within the included studies. There was a significant inverse relationship between the ΔV̇O2 max and peak and baseline body mass index (BMI) (r = –.785, P = .027). There was no significant correlation between ΔV̇O2 max and peak and age or pre-CPAP V̇O2 max and peak (Table 4). There was no statistically significant association between the change in AT and baseline BMI.
Table 4.
Spearman correlation coefficients between change in exercise capacity following CPAP therapy and baseline patient characteristics.
| Change in V̇O2 max and peak vs Baseline BMI | Change in V̇O2 max and peak vs Age | Change in V̇O2 max and peak vs Baseline V̇O2 max and peak | Change in AT vs Baseline BMI | |
|---|---|---|---|---|
| Spearman r | –.78 | –.09 | –.42 | –.50 |
| P-Value (two-tailed) | .02 | .84 | .29 | .45 |
AT = anaerobic threshold, BMI = body mass index, V̇O2 max = maximum volume of oxygen uptake, V̇O2 peak = peak rate of oxygen uptake.
Sensitivity analysis
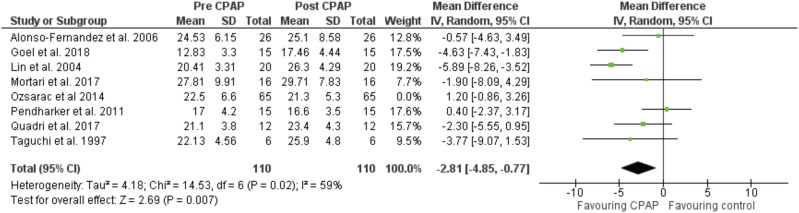
The heterogeneity I2 index (Cochrane Q test) was 66% between the studies reporting V̇O2 max (Figure 3) and 65% in the V̇O2 peak articles (Figure 4). The heterogeneity I2 index between the studies reporting AT was 93% (Figure 5). Further removal of one article (Ozsarac et al, 2014) improved heterogeneity to 59% (Figure 6), suggesting that this article skewed the heterogeneity of the data analysis, although this cannot be concluded due to the small sample of articles included in this systematic review.
Figure 6. Forest plot for mean V̇O2 max & peak in mL·kg–1·min–1 pre- and post-CPAP treatment in OSA patients with 1 author removed to show the change in heterogeneity.
CPAP = continuous positive airway pressure, IV = random-effect model, SD = standard deviation.
DISCUSSION
Our systematic review was conducted to assess the effect of CPAP therapy on V̇O2 max, V̇O2 peak, and AT in OSA patients. The meta-analysis showed a statistical increase in V̇O2 peak with CPAP therapy, but there was no change in V̇O2 max. There was no effect on AT with CPAP therapy.
A beneficial effect of CPAP on V̇O2 is plausible since V̇O2 peak is dependent on levels of oxygen delivered to vital organs and is an indirect assessment of cardiac output.32 CPAP is known to improve left ventricular function, cardiac index, and thus arterial oxygen content, constituting the rationale for benefit in patients with heart failure.33,34 Furthermore, CPAP treatment has been shown to significantly reduce cardiovascular risk or stroke in patients with severe OSA, and assessment of V̇O2 has been linked to cardiovascular risk profiling.4,35 Others suggest that CPAP may rebalance the autonomic nervous system leading to greater exercise performance.31 It has also been postulated that CPAP could improve V̇O2 by affecting a decrease in the production of reactive oxygen species such as interleukin-8, tumor necrosis factor α, and interleukin-6, resulting in improvements in cardiovascular, ventilatory, and musculoskeletal systems.27
Our finding of the improvement in V̇O2 peak but not V̇O2 max may be explained by true differential effect from CPAP or by methodological limitation due to the lack of data on V̇O2 max. V̇O2 max is defined as the true V̇O2 maximum value, but its measurement can prove challenging for some patients due to their inability to complete the protocol (for example, due to fatigue, joint pain, lack of fitness). V̇O2 peak, by contrast, is considered a “system-limited” peak oxygen uptake,36 defined as the maximum observed value within the capability of the patient and accounting for any such constraints. It is possible that CPAP therapy facilitates an improvement in V̇O2 peak by improving a patient’s systemic limitations, without affecting the true V̇O2 max. To alter an individual’s V̇O2 max requires exercise training that is conducted at the right intensities, duration, and type of training,37 and CPAP alone may not result in a change in oxidative capacity. Alternatively, we are cognizant that there was very limited data on V̇O2 max available for inclusion in our meta-analysis. Only 2 studies reported V̇O2 max, of which 1 study dominated the weighting in the analysis.25 This study found no change in V̇O2 max despite reported adequate CPAP adherence, but the adherence levels were self-reported by patients and this is recognized to be prone to overestimation by as much as 50%.38 We therefore caution making definitive conclusions on the effect of CPAP on V̇O2 max until further data is available.
Our meta-analysis showed that V̇O2 peak increased by 2.69 mL·kg–1·min–1 with CPAP therapy. There is a paucity of data on the minimum clinically important difference (MCID) of V̇O2, particularly in patients with OSA, but values ranging between 1.5 and 2.0 mL·kg–1·min–1 have been reported in other groups of patients using either anchor-based, distribution-based, or arbitrarily defined methods.39,40 Others have defined “responders” to interventions based on detectable differences in V̇O2 greater than the technical error of measurement, which may range from 1.5 to 1.8 mL·kg–1·min–1.41,42
The subgroup analysis within this review found a relationship between baseline BMI and ΔV̇O2 max and V̇O2 peak, with higher baseline BMI being associated with smaller changes in V̇O2 max and V̇O2 peak with CPAP treatment. Patients with higher BMI levels are more likely to have additional comorbidities that could interfere with the ability to alter V̇O2 max and V̇O2 peak. A higher BMI is associated with significantly increased risk of cardiovascular morbidity and mortality compared to normal BMI.42 Unfortunately, not all of the studies in our systematic review reported the baseline BMI or weight data, thus the resultant sample size was low. Further studies are needed to investigate this observation. Age and pre-CPAP V̇O2 max and V̇O2 peak levels did not affect the ΔV̇O2 max and V̇O2 peak with CPAP.
Our meta-analysis did not find a statistically significant improvement in AT with CPAP therapy. AT represents the change from aerobic to anaerobic metabolism during exercise and is utilized to determine and observe exercise rehabilitation or programs within a variety of diseases, such as COPD.43 In the clinical setting, AT can provide an index of the functional state of patients.44 Studies have shown that exercise at AT is associated with steady state blood lactate response; however, though it was not clarified whether exercise at a power output above the calculated AT also resulted in constant blood lactate levels.42,43 AT was not the primary outcome in any of the studies included in this meta-analysis. Additionally, AT is a derived measurement from V̇O2 data, and the techniques in measurement of the marker could be disputed. We found no relationship between baseline measures and ΔAT with CPAP.
Several of the studies within this systematic review included younger patients, with the mean age ranging from 43- to 58-years-old. This could suggest a slightly atypical OSA cohort; however, we do recognize that OSA patient cohorts can vary in demographics. The majority were male patients, consistent with OSA prevalence data. Our cohort of patients had a high baseline apnea-hypopnea index representative of severe OSA. It is possible that the effect of CPAP on exercise capacity may differ depending on disease severity, and therefore our findings should not be generalized to patients with milder disease.
The quality analyses of the included articles within our systematic review identified a “high” risk of bias, and the funnel plots demonstrated a publication bias by non-inverse funnel shaped plot (Figure S1, Figure S2, and Figure S3). Furthermore, an unavoidable concern is that only one study was a randomized controlled trial.10 Only two other studies compared treatments types.27 Common limitations across all studies were the small sample sizes, which limit the generalization of the results.28,31–32 These findings highlight the current dearth of high-quality studies in this field and emphasize the need for more randomized controlled trials.
Our study has some limitations. We reported AT in mL·kg–1 rather than mL·kg–1·min–1; this was due to the lack of weight data within some of the studies, and unfortunately this prevents direct comparison or the ability to fully assess oxidative capacity. CPAP adherence was reported in only 4 out of 8 studies (Table 1). Of those, CPAP usage ranged from 5.8 hours per night to 10 hours per night. CPAP adherence of 4 hours or more is usually considered the minimum satisfactory level in clinical practice, although some authors suggest that less can potentially still have clinical benefits.45 Two studies with a negative treatment effect did not report CPAP adherence data, and it is possible that insufficient CPAP usage affected those results. Finally, the duration of CPAP therapy across the studies was not uniform, ranging from 7 days to 3 months. Seven days of CPAP is sufficiently long to correct OSA and the associated arousals and hypoxia events.46 Indeed, we observed a positive treatment effect on V̇O2 in the study that conducted 7 days of CPAP26; however, it is possible that studies of longer duration encounter different changes in motivation or oxidative capacity, which we were not able to account for and thus could have influenced the results.
CONCLUSIONS
We have identified a paucity of high-quality studies investigating the effect of CPAP therapy on exercise capacity in patients with OSA. The results of our meta-analysis showed that V̇O2 peak significantly increases following CPAP treatment in patients with OSA. There was no significant change in V̇O2 max or AT with CPAP. We recommend further randomized controlled trials to confirm our findings and to clarify the V̇O2 adaptations with CPAP therapy.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors declare no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
Authors’ contributions: conception and design: HF, PC, SL, and KL; participants screening: HF; study recruitment: HF; data analysis: HF, SL, and LEP; interpretation of data: HF, PC, SL, LEP, SB, and KL; drafting manuscript: HF, PC, SL, LEP, ASP, SB, and KL; revised manuscript: HF, PC, SL, LEP, ASP, SB, and KL.
ABBREVIATIONS
- AT
anaerobic threshold
- BMI
body mass index
- CPAP
continuous positive airway pressure therapy
- OSA
obstructive sleep apnea
- V̇O2 max
maximal rate of oxygen uptake
- V̇O2 peak
peak rate of oxygen uptake
- V̇O2
volume of oxygen
REFERENCES
- 1. Toolkit for Commissioning and Planning Local NHS Services in the UK Obstructive Sleep Apnoea (OSA). www.blf.org.uk/OSA.
- 2.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. 10.1056/NEJM200005113421901 [DOI] [PubMed] [Google Scholar]
- 3.Rejón-Parrilla JC, Garau M, Sussex J. Obstructive Sleep Apnoea Health Economics Report. OHE Consulting, Office of Health Ecomonics: London, UK; 2014. [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]
- 5.Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison. Am Heart J. 2004;147(2):354–360. 10.1016/j.ahj.2003.07.014 [DOI] [PubMed] [Google Scholar]
- 6.Myers J, Gullestad L, Vagelos R, Do D, Bellin D, Ross H, Fowler MB. Cardiopulmonary exercise testing and prognosis in severe heart failure: 14 mL/kg/min revisited. Am Heart J. 2000;139(1):78–84. 10.1016/S0002-8703(00)90312-0 [DOI] [PubMed] [Google Scholar]
- 7.Older P, Hall A, Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest. 1999;116(2):355–362. 10.1378/chest.116.2.355 [DOI] [PubMed] [Google Scholar]
- 8.Vanhecke TE, Franklin BA, Zalesin KC, et al. Cardiorespiratory fitness and obstructive sleep apnea syndrome in morbidly obese patients. Chest. 2008;134(3):539–545. 10.1378/chest.08-0567 [DOI] [PubMed] [Google Scholar]
- 9.Lin CC, Hsieh WY, Chou CS, Liaw SF. Cardiopulmonary exercise testing in obstructive sleep apnea syndrome. Respir Physiol Neurobiol. 2006;150(1):27–34. 10.1016/j.resp.2005.01.008 [DOI] [PubMed] [Google Scholar]
- 10.Alonso-Fernández A, García-Río F, et al. Obstructive sleep apnoea-hypoapnoea syndrome reversibly depresses cardiac response to exercise. Eur Heart J. 2006;27(2):207–215. 10.1093/eurheartj/ehi621 [DOI] [PubMed] [Google Scholar]
- 11.Ucok K, Aycicek A, Sezer M, et al. Aerobic and anaerobic exercise capacities in obstructive sleep apnea and associations with subcutaneous fat distributions. Lung. 2008;187(1):29–36. 10.1007/s00408-008-9128-0 [DOI] [PubMed] [Google Scholar]
- 12.Nanas S, Sakellariou D, Kapsimalakou S, et al. Heart rate recovery and oxygen kinetics after exercise in obstructive sleep apnea syndrome. Clin Cardiol. 2010;33(1):46–51. 10.1002/clc.20707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanuxem D, Badier M, Guillot C, Delpierre S, Jahjah F, Vanuxem P. Impairment of muscle energy metabolism in patients with sleep apnoea syndrome. Respir Med. 1997;91(9):551–557. 10.1016/s0954-6111(97)90089-5 [DOI] [PubMed] [Google Scholar]
- 14.Mendelson M, Marillier M, Bailly S, et al. Maximal exercise capacity in patients with obstructive sleep apnoea syndrome: a systematic review and meta-analysis. Eur Respir J. 2018;51(6):1702697. 10.1183/13993003.02697-2017 [DOI] [PubMed] [Google Scholar]
- 15.Alameri H, Al-Kabab Y, BaHammam A. Submaximal exercise in patients with severe obstructive sleep apnea. Sleep Breath. 2009;14(2):145–151. [DOI] [PubMed] [Google Scholar]
- 16.Berger M, Kline CE, Cepeda FX, et al. Does obstructive sleep apnea affect exercise capacity and the hemodynamic response to exercise? An individual patient data and aggregate meta-analysis. Sleep Med Rev. 2019;45:42–53. 10.1016/j.smrv.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 17.Shechter A. Effects of continuous positive airway pressure on energy balance regulation: a systematic review. Eur Respir J. 2016;48(6):1640–1657. 10.1183/13993003.00689-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Offenwert E, Vrijsen B, Belge C, Troosters T, Buyse B, Testelmans D. Physical activity and exercise in obstructive sleep apnea. Acta Clin Belg. 2019;74(2):92–101. 10.1080/17843286.2018.1467587 [DOI] [PubMed] [Google Scholar]
- 19.Simpson L, McArdle N, Eastwood PR, et al. Physical inactivity is associated with moderate-severe obstructive sleep apnea. J Clin Sleep Med. 2015;11(10):1091–1099. 10.5664/jcsm.5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsaux CFM, Celis-Morales C, Hoonhout J, et al. Objectively measured physical activity in European adults: Cross-sectional findings from the food4me study. PLoS One. 2016;11(3):e0150902. 10.1371/journal.pone.0150902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong S, Dimsdale JE. Physical activity and perception of energy and fatigue in obstructive sleep apnea. Med Sci Sports Exerc. 2003;35(7):1088–1092. 10.1249/01.MSS.0000074566.94791.24 [DOI] [PubMed] [Google Scholar]
- 22.Taylor JR. An Introduction to Error Analysis: The Study of Uncertainties in Physical Measurements. 2nd ed. Herndon, VA: University Science Books; 1997. [Google Scholar]
- 23.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Review Manager (RevMan) . 2014. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman; accessed July 15, 2020.
- 25.Ozsarac I, Bayram N, Uyar M, Kosovali D, Gundogdu N, Filiz A. Effects of positive airway pressure therapy on exercise parameters in obstructive sleep apnea. Ann Saudi Med. 2014;34(4):302–307. 10.5144/0256-4947.2014.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taguchi O, Hida W, Okabe S, et al. Improvement of exercise performance with short term nasal continour postivie airway pressure in patients with obstructive sleep apnea. J Exp Med. 1997;183:45–53. [DOI] [PubMed] [Google Scholar]
- 27.Goel AK, Talwar D, Jain SK. Evaluation of short-term use of nocturnal nasal continuous positive airway pressure for a clinical profile and exercise capacity in adult patients with obstructive sleep apnea-hypopnea syndrome. Lung India. 2015;32(3):225–232. 10.4103/0970-2113.156226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin C-C, Lin C-K, Wu K-M, Chou C-S. Effect of treatment by nasal CPAP on cardiopulmonary exercise test in obstructive sleep apnea syndrome. Lung. 2004;182(4):199–212. 10.1007/s00408-004-2502-7 [DOI] [PubMed] [Google Scholar]
- 29.Mortari D, Schmidt R, Watte G, et al. Effects of CPAP Treatment on Exercise Performance in Patients with Obstructive Sleep Apnea Syndrome. SM J Pulm Med. 2017;3(1):1026. [Google Scholar]
- 30.Pendharkar SR, Tsai WH, Eves ND, Ford GT, Davidson WJ. CPAP increases exercise tolerance in obese subjects with obstructive sleep apnea. Respir Med. 2011;105(10):1565–1571. 10.1016/j.rmed.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 31.Quadri F, Boni E, Pini L, et al. Exercise tolerance in obstructive sleep apnea-hypopnea (OSAH), before and after CPAP treatment: Effects of autonomic dysfunction improvement. Respir Physiol Neurobiol. 2017;236:51–56. 10.1016/j.resp.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 32.Lang CC, Karlin P, Haythe J, Tsao L, Mancini DM. Ease of noninvasive measurement of cardiac output coupled with peak VO2 determination at rest and during exercise in patients with heart failure. Am J Cardiol. 2007;99(3):404–405. 10.1016/j.amjcard.2006.08.047 [DOI] [PubMed] [Google Scholar]
- 33.Genovese J, Huberfeld S, Tarasiuk A, Moskowitz M, Scharf SM. Effects of CPAP on cardiac output in pigs with pacing-induced congestive heart failure. Am J Respir Crit Care Med. 1995;152(6):1847–1853. 10.1164/ajrccm.152.6.8520745 [DOI] [PubMed] [Google Scholar]
- 34.Baratz DM, Westbrook PR, Shah PK, Mohsenifar Z. Effect of nasal continuous positive airway pressure on cardiac output and oxygen delivery in patients with congestive heart failure. Chest. 1992;102(5):1397–1401. 10.1378/chest.102.5.1397 [DOI] [PubMed] [Google Scholar]
- 35.Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nuñez N, Caballero-Martinez I, Catalan-Serra P, Almeida-Gonzalez CV. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. Am J Respir Crit Care Med. 2014;189(12):1544–1550. 10.1164/rccm.201311-2012OC [DOI] [PubMed] [Google Scholar]
- 36.Day JR, Rossiter HB, Coats EM, Skasick A, Whipp BJ. The maximally attainable VO2 during exercise in humans: the peak vs. maximum issue. J Appl Physiol. 2003;95(5):1901–1907. 10.1152/japplphysiol.00024.2003 [DOI] [PubMed] [Google Scholar]
- 37.Scribbans TD, Vecsey S, Hankinson PB, Foster WS, Gurd BJ. The effect of training intensity on VO2max in young healthy adults: a meta-regression and meta-analysis. Int J Exerc Sci. 2016;9(2):230–247. [DOI] [PMC free article] [PubMed]
- 38.Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45(1):43. 10.1186/s40463-016-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkinson TJ, Watson EL, Xenophontos S, Gould DW, Smith AC. The “Minimum Clinically Important Difference” in Frequently Reported Objective Physical Function Tests After a 12-Week Renal Rehabilitation Exercise Intervention in Nondialysis Chronic Kidney Disease. Am J Phys Med Rehabil. 2019;98(6):431–437. 10.1097/PHM.0000000000001080 [DOI] [PubMed] [Google Scholar]
- 40.Kothmann E, Batterham AM, Owen SJ, et als. Effect of short-term exercise training on aerobic fitness in patients with abdominal aortic aneurysms: a pilot study. Br J Anaesth. 2009;103(4):505–510. 10.1093/bja/aep205 [DOI] [PubMed] [Google Scholar]
- 41.Williams CJ, Gurd BJ, Bonafiglia JT, et al. A multi-center comparison of VO2peak trainability between interval training and moderate intensity continuous training. Front Physiol. 2019;10(FEB):19. 10.3389/fphys.2019.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan SS, Ning H, Wilkins JT, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3(4):280–287. 10.1001/jamacardio.2018.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belman MJ, Epstein LJ, Doornbos D, Elashoff JD, Koerner SK, Mohsenifar Z. Noninvasive determinations of the anaerobic threshold. Reliability and validity in patients with COPD. Chest. 1992;102(4):1028–1034. 10.1378/chest.102.4.1028 [DOI] [PubMed] [Google Scholar]
- 44.Ohuchi H, Nakajima T, Kawade M, Matsuda M, Kamiya T. Measurement and validity of the ventilatory threshold in patients with congenital heart disease. Pediatr Cardiol. 1996;17(1):7–14. 10.1007/BF02505805 [DOI] [PubMed] [Google Scholar]
- 45.Billings ME, Lévy P, Ayas N. Suboptimal CPAP adherence: half a loaf is better than no bread at all. Eur Respir J. 2020;55(3):2000144. 10.1183/13993003.00144-2020 [DOI] [PubMed] [Google Scholar]
- 46.Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure vs placebo continuous positive airway pressure on sleep quality in obstructive sleep apnea. Chest. 1999;116(6):1545–1549. 10.1378/chest.116.6.1545 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.