Abstract
Study Objectives:
To evaluate the association of preoperative sleep pattern with posthysterectomy pain perception and satisfaction with surgery.
Methods:
This pilot study included women undergoing minimally invasive hysterectomy for benign conditions. Sleep quality, insomnia severity, and insomnia risk were assessed pre- and postoperatively via standard questionnaires. Total sleep time, wake after sleep onset, and sleep efficiency were measured before and after hysterectomy using daily sleep diaries and wrist-worn actigraphy. Pain perception and satisfaction with hysterectomy were assessed postoperatively. Repeated-measures analysis of variance, Pearson’s correlation, and linear regression were used for analysis.
Results:
Twenty women participated; of them 16 had complete data and were analyzed. Total sleep time increased from 384 ± 102 minutes before to 468 ± 96 minutes after surgery (P = .023). Wake after sleep onset, a measure of sleep fragmentation, increased from 26 ± 15.1 minutes before to 52 ± 22.9 minutes after surgery (P = .014). Pearson’s correlation showed preoperative total sleep time was inversely correlated with postoperative pain intensity (r = –.92, P = .01). Preoperative wake after sleep onset was positively correlated with postoperative pain intensity (r = .86, P = .008). Preoperative insomnia severity and insomnia risk were positively associated with postoperative pain and pain behaviors (β = 0.41, P < .05; β = 0.55, P < .01, respectively). Finally, preoperative sleep efficiency was positively associated with overall satisfaction with hysterectomy (β = 0.39, P < .05).
Conclusions:
Sleep duration and fragmentation increase following hysterectomy. Shorter, more fragmented preoperative sleep is associated with greater postoperative pain intensity. Better preoperative sleep was associated with more satisfaction after hysterectomy. Further studies are needed to determine if preoperative sleep interventions such as cognitive behavioral therapy improve pain perception and satisfaction after hysterectomy.
Citation:
Nowakowski S, Levy-Meeks ME, Dawson DB, et al. Association of preoperative sleep pattern with posthysterectomy pain: a pilot study. J Clin Sleep Med. 2020;16(11):1901–1908.
Keywords: actigraphy, hysterectomy, preoperative sleep, postoperative pain, sleep, women
BRIEF SUMMARY
Current Knowledge/Study Rationale: Little is known about sleep as a predictor of posthysterectomy pain. This study aims to evaluate the association of preoperative sleep pattern with posthysterectomy pain and satisfaction with surgery.
Study Impact: This study demonstrated that shorter, more fragmented preoperative sleep is associated with greater pain intensity and pain behaviors following hysterectomy while better preoperative sleep was associated with more satisfaction after hysterectomy. Further studies are needed to determine if preoperative sleep interventions such as cognitive behavioral therapy improve pain perception and satisfaction after hysterectomy.
INTRODUCTION
Hysterectomy is the most frequent non–pregnancy-related major surgery in reproductive-age women in the United States.1 Although there has been a clear trend toward more utilization of minimally invasive approaches,2 pain after hysterectomy continues to represent a significant clinical problem. In fact, persistent postoperative pain after hysterectomy is estimated to affect 5–30% of patients.3 This is particularly important given the current interest in Enhanced Recovery After Surgery and the estimated 0.5–5% incidence of new, persistent opioid use after hysterectomy.4,5 Therefore, there is a need for a better understanding of the factors affecting posthysterectomy pain including new paradigms.
There is evidence suggesting that sleep deprivation impacts pain perception through central brain mechanisms.6–8 A recent study published in the Journal of Neuroscience8 found that sleep deprivation lowers pain threshold in healthy volunteers. Using functional magnetic resonance imaging, the investigators found that sleep deprivation amplifies pain reactivity in the primary somatosensory cortex and induces changes in pain-related brain activity in the thalamus and anterior and middle insula in addition to the somatosensory cortex. This association is usually not limited to the sleep duration but also includes sleep fragmentation or disruption. For instance, in 1 study, individuals with experimentally induced sleep fragmentation over the course of 2 consecutive nights showed a lower threshold for superficial and deep painful stimuli.9 In addition, there is evidence10,11 suggesting that abnormal sleep may affect immune and inflammatory responses,10,11 potentially affecting postoperative inflammatory response and healing.
Sleep disturbances are prevalent among women undergoing hysterectomy.12–14 For example, it was noted that the number of awakenings increased postoperatively for patients undergoing both vaginal and abdominal hysterectomy.14 Sleep and related psychological disturbances are particularly important around hysterectomy compared with other surgeries as the loss of the uterus can pose a major psychological trauma to many women. This likely amplifies the stress, anxiety, and sleep disturbances.
Despite the evidence linking hysterectomy and sleep disturbances, no studies have specifically examined the association of preoperative sleep pattern with postoperative pain after hysterectomy. Therefore, we performed this study to examine the hypothesis that poor preoperative sleep quantity and/or quality is associated with worse postoperative pain perception among women undergoing minimally invasive hysterectomy for benign indications.
METHODS
Study participants
Participants were 20 premenopausal women (>21 years old) scheduled to undergo minimally invasive (laparoscopic and vaginal) hysterectomy for benign gynecologic conditions, such as abnormal uterine bleeding, uterine fibroids, adenomyosis, and endometriosis. Patients were recruited from The University of Texas Medical Branch Women’s Healthcare Clinic. Gynecologists identified and counseled patients and obtained informed written consents prior to enrollment. Exclusion criteria included untreated severe mental health disorder (schizophrenia, psychotic disorder, current substance use disorder, bipolar disorder, suicide attempt, or psychiatric hospitalization in the last 3 years). Of the 20 enrolled women, 4 were not included in analysis due to incomplete data. The study was approved by The University of Texas Medical Branch Institutional Review Board.
Procedures
Following initial screening and consent, eligible women were scheduled for a baseline assessment visit to complete questionnaires on sleep and pain. All participants consented to electronic medical records access. At the baseline session, participants were provided with a daily diary to record sleep and pain, as well as wrist-worn actigraphy to be worn for 7 days prior to surgery. Following hysterectomy, assessment measures (questionnaires, daily diary, and actigraphy) were repeated. The study flow chart is shown in Figure S1 in the supplemental material.
Measures
Sleep
Five instruments were used to evaluate sleep.
Consensus Sleep Diary:
The Consensus Sleep Diary15 was used with some adaptation to measure the daily frequency and severity of sleep disturbances, including 9 core items that query (1) napping, (2) bedtime (time into bed), (3) lights out (time of first attempt to sleep), (4) time to fall asleep, (5) number of awakenings, (6) duration of awakenings, (7) final wake-up time, (8) rise time (time out of bed), (9) a rating of sleep quality, and an optional space for writing comments. Daily pain rating was included in the diary. Participants were given the option to complete the daily sleep diary electronically using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, TN) or paper format. Participants were asked to complete sleep diary questions each morning for 7 days before and 7 days following hysterectomy. Items were averaged over the 7-day period before and following the hysterectomy to create 2 scores for each variable (pre- and postoperative).
Wrist actigraphy:
Wrist actigraphy16 was used as an objective measure of sleep. Women wore an Actiwatch Spectrum Pro (Respironics, Inc., Murrysville, PA) on their nondominant wrist for 7 consecutive days before and 7 days following hysterectomy. Bedtime (time into bed), lights out (time sleep was attempted), wake time (final wake time), and rise time (time out of bed) were recorded. Actigraphy data were collected in 30-second epochs and analyzed with Philips Actiware version 6.0.0 software (Respironics, Inc., Murrysville, PA). Actiware software uses validated algorithms that take into account wrist movement in determining the likelihood of sleep versus wakefulness during each 30-second epoch.
Variables of interest included the following:
Wake after sleep onset (WASO), defined as the number of minutes of wakefulness between actigraphy-defined sleep-onset time and actigraphy-defined final wake time.
Total sleep time (TST), defined as the difference between actigraphy-defined sleep onset and actigraphy-defined final wake time minus actigraphy-defined WASO.
Sleep efficiency, calculated using the formula TST divided by time in bed × 100 (%).
Figure S2 in the supplemental material illustrates examples of individuals with normal and abnormal sleep patterns (actograms) in addition to the Actiwatch device used in this study.
Pittsburgh Sleep Quality Index:
The Pittsburgh Sleep Quality Index17 is a 10-item and 7-component validated survey instrument designed to assess general sleep quality that includes 7 component scores, which are summed to yield 1 global score from 0 to 21. Participants were asked to rate the quality of their sleep overall over the past month, using a 4-point scale (0 = very good and 3 = very bad). Scores ≥5 indicate clinically significant poor sleep quality.
Insomnia Severity Index:
The Insomnia Severity Index18,19 is a 7-item, validated self-report scale that assesses symptoms of insomnia. Items are scored on a 0–4 scale to yield a score of 0–28. Higher scores indicate greater insomnia severity. Scores >10 are consistent with clinical insomnia.
Ford Insomnia Response to Stress Test:
The Ford Insomnia Response to Stress Test20 is a 27-item questionnaire (Likert-scale format) regarding sleep disturbance in response to commonly experienced stressful situations. Items are scored on a 4-point scale (1 = not likely and 4 = very likely). Scores ≤15 points indicate low risk of insomnia, scores of 16–17 points indicate moderate risk of insomnia, and scores ≥18 points indicate a high risk of insomnia. The measure has been previously found to be reliable and valid for measuring insomnia response to stress.20
Pain
Four instruments were used to measure pain.
Patient-Reported Outcomes Measurement Information System Pain Intensity–Short Form 3a:
This instrument was used to measure pain severity. The Patient-Reported Outcomes Measurement Information System (PROMIS) is a set of standardized validated tools developed by the National Institutes of Health to evaluate health outcomes. The PROMIS pain-intensity tool includes a small set of pain-severity items that cover average pain (1 item), worst pain (1 item), and pain right now (1 item).21–23 Response options ranging from 1 to 5 for each item (1 = no pain and 5 = very severe pain) are calculated into t-scores. These 3 items cover a broad range on the pain-intensity continuum comparable to a numeric pain-intensity rating scale.
PROMIS Pain Behavior–Short Form 7a:
This is a short, 7-question survey that measures self-reported external manifestations of pain behaviors that typically indicate to others that an individual is experiencing pain. These actions or reactions can be verbal or nonverbal; involuntary or deliberate. They include observable displays (sighing, crying), pain-severity behaviors (resting, guarding, facial expressions, and asking for help), and verbal reports of pain. The Pain Behavior Short Form is universal rather than disease specific. It assesses pain behavior over the past 7 days. Response options ranging from 1 to 6 for each item (1 = have no pain and 6 = always have pain) are calculated into t-scores.
Short Form–36:
The Short Form–36 is a widely used health-related quality-of-life questionnaire. Originally, the Short Form–36 was developed by the Medical Outcome Study by the RAND Corporation. It contains 36 items designed to measure 8 health concepts, including physical functioning, role limitations due to physical problems, bodily pain, general health perception, vitality, social functioning, role limitations due to emotional problems, and mental health. Scores can by calculated by each domain individually, and raw scores are then combined and translated to a summary score ranging from 0 to 100. A higher score indicates better health status in each domain. The measure was found to be reliable and responsive in measuring symptoms for many health conditions, including endometriosis.24
Short Form McGill Pain Questionnaire:
The Short Form McGill Pain Questionnaire25 assesses pain using 15 descriptors (11 sensory and 4 affective). Participants rated pain intensity from 0 (none) to 3 (severe). Three pain scores were determined based on the sums of intensity ratings for sensory, affective, and total descriptors. Higher scores indicate greater affective, sensory, and total/global pain.
Hysterectomy satisfaction
One instrument was used to evaluate hysterectomy satisfaction.
Treatment Satisfaction Scale:
This scale is a 10-question measure adapted from the Consumer Report’s survey26 that was provided to patients following hysterectomy. It measures participants’ perceptions of the treatment’s effectiveness, benefits, and influence on quality of life (eg, improvements in work productivity, mood). Questions are measured on a 5-point Likert scale (1 = not at all, and 5 = very much). The questionnaire was adapted by adding “hysterectomy” to several questions to increase clarity and specificity. A higher score indicated greater overall satisfaction with the hysterectomy.
Outcome variables and covariates
Primary outcome variables were defined as PROMIS pain intensity for pain and actigraphy TST and WASO for sleep. All other outcome measures are considered secondary. We used these secondary measures to further support the findings, avoid any spurious results, and make sure there is a consistent pattern. Potential covariates included demographic and clinical variables. Demographics (age, education, race/ethnicity), medical history, and current medications were obtained from the patient’s electronic medical record and verified with participants. Depression was measured using the Center for Epidemiological Studies–Depression Scale27; anxiety was measured using the State Trait Anxiety Inventory,28 and fatigue was measured using the Multidimensional Fatigue Inventory.29 Associations between potential covariates and primary study outcomes (sleep and pain) were evaluated using Pearson’s and Spearman correlations. Covariates were selected based on the potential clinical significance, and associations with the outcomes were evaluated for inclusion in the regression with a P < .10 threshold. Age, body mass index, depression, anxiety, and fatigue were considered as potential covariates but did not meet the P < .10 criterion. Thus, they were not included in subsequent linear regression models of sleep and pain.
Statistical analysis
We first checked data for any missing variables. We then performed descriptive statistics for demographic and clinical characteristics of the study population. Repeated-measures analysis of variance was performed to compare preoperative with postoperative sleep variables. Prior to analysis of variance, the data were found to have met assumptions for independence, normality, and sphericity. We also conducted a series of paired-sample t tests and found results did not differ. We used Pearson’s correlation and simple linear regression to examine the association of preoperative sleep with both postoperative pain and satisfaction with hysterectomy. This pilot observational study has a power of >97% to detect a large effect size (correlation coefficient, r = .8) of the primary outcome (PROMIS posthysterectomy pain) with a significance level of .05. Models were 2-sided at α = 0.05. Covariates were not included in the analysis. We used SPSS Statistics version 24 (IBM Corporation, Armonk, NY) and SigmaPlot version 12 (Systat, Inc., San Jose, CA).
RESULTS
Characteristics of study participants
Demographic and operative characteristics of study participants are presented in Table 1. Participants were, on average, 41 ± 7.3 years old with a body mass index of 30 ± 5.5 kg/m2.
Table 1.
Demographic and operative characteristics of the study population.
| Values | |
|---|---|
| Age, mean (SD), years | 41 (7.3) |
| Race/ethnicity, n (%) | |
| White | 12 (75) |
| Black | 3 (18.8) |
| Other | 1 (6.2) |
| Hispanic/Latina | 4 (25) |
| Education, n (%) | |
| High school/some college/vocational | 8 (50) |
| College graduate or higher | 8 (50) |
| Marital status, n (%) | |
| Single | 4 (25) |
| Married | 8 (50) |
| Divorced | 3 (18.8) |
| Committed relationship | 1 (6.2) |
| BMI, mean (SD), kg/m2 | 30 (5.5) |
| Nicotine use, n (%) | 3 (18.8) |
| Gravidity, mean (SD), n | 4 (2.1) |
| Parity, mean (SD), n | 2 (1.5) |
| Prior abdominal surgery,a n (%) | 14 (87.5) |
| Preoperative diagnosis, n (%) | |
| Pelvic pain | 14 (87.5) |
| Menorrhagia/menometrorrhagia | 11 (68.8) |
| Abnormal uterine bleeding | 9 (56) |
| Stress incontinence | 5 (31.3) |
| Uterine fibroids | 4 (25) |
| Ovarian cysts | 2 (12.5) |
| Endometriosis | 1 (6.3) |
| Dyspareunia | 1 (6.3) |
| Polycystic ovarian syndrome | 1 (6.3) |
| Hysterectomy type, n (%) | |
| Total laparoscopic | 9 (56.3) |
| Total laparoscopic, robotic assisted | 4 (25) |
| Vaginal | 2 (12.5) |
| Laparoscopic assisted total vaginal | 1 (6.3) |
| Oophorectomy performed, n (%) | 7 (43.8) |
| Estimated blood loss, mean (SD), mL | 101 (111.4) |
| Uterine weight, mean (SD), g | 187 (99) |
| Postoperative complications,b n (%) | 1 (6.25) |
| Length of stay, mean (SD), days | 0.6 (1) |
Sample size: n = 16, BMI = body mass index.
Previous abdominal surgeries include tubal ligation, endometriosis surgery, appendectomy, cesarean section, lysis of abdominall adhesions, hysteroscopy, cholecystectomy, myomectomy, and salpingostomy.
Postoperative complications include failure to void, hemoperitoneum, acute blood loss anemia, bacterial vaginosis, profuse vaginal bleeding, cuff dehiscence, hypertensive urgency, urinary tract infection, and surgical menopause symptoms.
The sleep diaries were collected for a range of 7–14 days (mean = 9 days) preoperatively and 7–21 days (mean = 16 days) postoperatively. Actigraphy was recorded during a range of 5–26 days (mean = 9 days) preoperatively and 5–18 days (mean = 12 days) postoperatively. Range of days for sleep diary and actigraphy was based around perioperative gynecology clinic appointments. Both sleep diary and actigraphy data were truncated to 7 days immediately prior to and following the hysterectomy. Questionnaires (eg, Pittsburgh Sleep Quality Index, PROMIS) were administered electronically via REDCap survey and completed in a range of 2–23 days (mean =10 days) prior to and in a range of 9–26 days (mean = 16 days) following hysterectomy.
In terms of sleep characteristics, we found that TST increased after hysterectomy, as did sleep fragmentation and napping time (Table 2 and Figure S3). Mean actigraphy-assessed TST increased from 6.4 hours before to 7.8 hours after hysterectomy. Mean WASO, a measure of sleep fragmentation, also increased from 26 minutes before to 52 minutes after hysterectomy. Mean self-reported time spent napping increased from 15 minutes before to 67 minutes after hysterectomy as reported by daily sleep diary. Pain characteristics are also presented in Table 2. As expected, average daily pain ratings increased from 2 (out of 10) before to 4 after hysterectomy and physical functioning decreased from an average score of 68 before to 49 after hysterectomy (P = .031).
Table 2.
Sleep and pain characteristics of the study population.
| Prehysterectomy | Posthysterectomy | Pa | |
|---|---|---|---|
| Pittsburgh Sleep Quality Indexb | 9 (3.4) | 8 (2.7) | .756 |
| Insomnia Severity Indexb | 11 (4.9) | 7 (7.2) | .068 |
| Sleep diary (self-reported),c minutes | |||
| Sleep-onset latencyb | 38 (30.3) | 33 (37.5) | .439 |
| Wake after sleep onsetb | 26 (15.1) | 52 (22.9) | .014 |
| Total sleep timed | 390 (60) | 420 (72) | .073 |
| Naptime duration | 15 (11.8) | 67 (49.1) | .023 |
| Sleep quality (0 = worst, 10 = best)d | 5 (1.8) | 6 (1.9) | .588 |
| Actigraphy (objective)c | |||
| Sleep-onset latency,b minutes | 42 (41.3) | 36 (21.8) | .293 |
| Wake after sleep onset,b minutes | 52 (25.4) | 72 (20.6) | .060 |
| Total sleep time,d minutes | 384 (102) | 468 (96) | .023 |
| Sleep efficiency,d % | 75 (18.2) | 78 (6.8) | .324 |
| PROMIS Pain Intensity–3A | 56 (6.2) | 51 (6.8) | .059 |
| PROMIS Pain Behavior–7A | 61 (6.1) | 62 (6.1) | .188 |
| Short Form–36 Physical Functioninge | 68 (22.4) | 49 (24.7) | .031 |
| Daily pain diary ratingc | |||
| Average pain (0: none, 10: most severe) | 2.2 (1.7) | 3.8 (2.0) | .050 |
| Worst pain (0: none, 10: most severe) | 3.7 (2.8) | 5.0 (2.6) | .057 |
| Short Form McGill Pain Questionnaire | |||
| Affective pain | 9.4 (4.2) | 7.2 (3.3) | .366 |
| Sensory pain | 28.3 (6.7) | 18.3 (6.7) | .018 |
| Total score | 37.7 (10.1) | 25.5 (9.5) | .046 |
Sample size = 16. Values are presented as means (SDs). PROMIS = Patient-Reported Outcomes Measurement Information System.
Within-subjects (pre/post hysterectomy) differences were assessed using repeated-measures analysis of variance.
Lower score/value indicates better sleep for (1) Pittsburgh Sleep Quality Index, (2) Insomnia Severity Index, (3) sleep-onset latency, and wake after sleep onset (sleep diary/actigraphy).
Daily diary ratings and actigraphy data were averaged over 7 days immediately prior to hysterectomy for prehysterectomy values and 7 days immediately following hysterectomy for posthysterectomy values.
Higher value indicates better sleep for (1) total sleep time (sleep diary/actigraphy), (2) sleep quality (sleep diary), (3) sleep efficiency (actigraphy).
Higher pain score indicates greater pain for all measures except for Short Form–36 Physical Functioning, where a high score indicates greater physical functioning.
Association of preoperative sleep and posthysterectomy pain
To examine the association of preoperative sleep pattern with posthysterectomy pain, we performed Pearson’s correlation and linear regression with findings presented in Table 3 and Figure 1.
Table 3.
Association (linear regression) between preoperative sleep and postoperative pain.
| PROMIS Pain Intensity, β (SE) | PROMIS Pain Behavior, β (SE) | Pain Diary Worst Pain, β (SE) | |
|---|---|---|---|
| Sleep diary (self-reported) | |||
| WASO (minutes) | 0.16 (5.3) | 0.01 (8.8) | 0.48 (2.1) |
| TST (minutes) | 0.02 (5.8) | 0.91 (2.7)* | 0.01 (2.8) |
| Actigraphy (objective) | |||
| WASO (minutes) | 0.76 (2.1)** | 0.48 (3.2)* | 0.44 (2.2) |
| TST (minutes) | 0.01 (4.2) | 0.01 (4.5) | 0.93 (0.2)* |
| Insomnia Severity Index score | 0.01 (7.4) | 0.41 (4.9)* | 0.01 (2.9) |
| Insomnia risk: FIRST score | 0.44 (6.4) | 0.55 (4.3)** | 0.08 (2.9) |
*P < .05, **P < .01. FIRST = Ford Insomnia Response to Stress Test; PROMIS = Patient-Reported Outcomes Measurement Information System; TST = total sleep time; WASO = wake after sleep onset; β = regression coefficient (represents the slope of the linear relation of the predictor variable and the outcome variable).
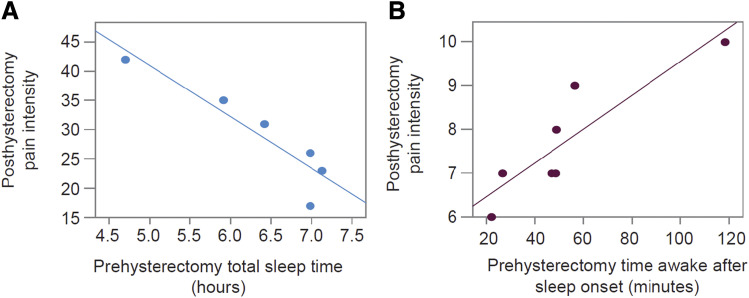
Figure 1. Correlation between sleep measures and pain intensity.
(A) Pearson's correlation between preoperative sleep duration (sleep diary total sleep time) and postoperative pain intensity (PROMIS Pain Intensity form 3A) showing an inverse relation (r = −.92, P = .01). (B) Pearson’s correlation between preoperative sleep fragmentation (actigraphy WASO) and postoperative pain intensity (PROMIS Pain Intensity form 3A) showing a positive relation (r = .86, P = .008). PROMIS = Patient-Reported Outcomes Measurement Information System; WASO = wake after sleep onset.
TST
Preoperative TST reported in the daily sleep diary was inversely correlated with pain behaviors following hysterectomy (r = −.92, P = .01; Figure 1A). Greater actigraphy-assessed TST prior to hysterectomy was associated with a lower average daily rating of worst pain following hysterectomy.
WASO
WASO is a measure of sleep fragmentation. Preoperative actigraphy-assessed WASO positively correlated with pain intensity and pain behaviors (r = .86, P = 0.008; Figure 1B).
Insomnia Severity Index
Greater preoperative insomnia severity was associated with greater postoperative pain behaviors (β = 0.41, P < .05).
Ford Insomnia Response to Stress Test
Preoperative insomnia risk as a response to stress was positively associated with postoperative pain behaviors (β = 0.55, P < .01).
Preoperative sleep and satisfaction with hysterectomy
To examine the association of preoperative sleep pattern with satisfaction with hysterectomy, we performed linear regression modeling with findings presented in Table 4.
Table 4.
Association (linear regression) between preoperative sleep and satisfaction with hysterectomy.
| Satisfied with Procedure, β (SE) | Helpful in Alleviating Symptoms, β (SE) | Helpful in Treating Pain, β (SE) | Overall Treatment Satisfaction, β (SE) | |
|---|---|---|---|---|
| Sleep diary (self-reported) | ||||
| Naps (minutes) | 0.82 (0.24)** | 0.71 (0.35)* | 0.71 (0.33)* | 0.1 (9.2) |
| Actigraphy (objective) | ||||
| Sleep-onset latency | 0.08 (0.78) | 0.13 (0.75) | 0.19 (0.52) | 0.53 (2.47)** |
| Sleep efficiency | 0.05 (0.80) | 0.1 (0.76) | 0.14 (0.54) | 0.39 (6.2)* |
*P < .05, **P < .01. β = regression coefficient (represents the slope of the linear relation of the predictor variable and the outcome variable).
Napping
Greater preoperative report of napping was positively associated with increased satisfaction with hysterectomy (β = 0.82, P < .01).
Sleep-onset latency
Shorter preoperative actigraphy-assessed sleep-onset latency (time to fall asleep) was associated with the higher overall satisfaction with hysterectomy (β = 0.53, P < .01).
Sleep efficiency
Preoperative actigraphy-assessed sleep efficiency (TST divided by time in bed) was positively associated with overall satisfaction with hysterectomy (β = 0.39, P < .05).
Additional results
Three participants reported taking narcotics (acetaminophen with hydrocodone), and none reported taking sedative hypnotics perioperatively.
DISCUSSION
The results of this pilot study show that better preoperative sleep is associated with less postoperative pain and higher satisfaction with surgery after minimally invasive hysterectomy for benign conditions.
It is important to appreciate the neuroscience of the sleep–pain connection. In the Krause et al8 study cited earlier, the authors also found that the relation between pain and sleep is bidirectional. Therefore, it is possible that poor sleep, pain, and stress form a vicious cycle around surgery. Preoperative stress may lead to sleep deprivation, which may lower pain threshold increasing pain perception after surgery, which, in turn, further exaggerates sleep deprivation and so on. Breaking this vicious cycle may become critical for effective pain control. Unfortunately, it appears that opiates, commonly used postoperatively, may be counterproductive as morphine seems to disturb the normal sleep pattern.30 Specifically, it appears that opiates disrupt the most restorative stages of sleep, including slow wave (stage 3 or deep) sleep and rapid eye movement sleep.31 Therefore, there may be a need for an alternative sleep-friendly pain-management approach, particularly in cases with poor sleep.
The findings of our pilot observational study may have potential clinical and research implications. However, before making any conclusions or recommendations, larger interventional studies including randomized controlled trials are required. This is particularly timely given the current trend toward outpatient surgeries32 and the recently introduced Enhanced Recovery After Surgery protocols showing substantial reduction in opioid use without compromising pain control.33 The inclusion of sleep assessment into the multidisciplinary approach in these protocols may warrant consideration.
There is evidence that patients undergoing hysterectomy experience more anxiety and depressive symptoms compared with other non-hysterectomy gynecologic surgeries.34 In cases of concomitant bilateral oophorectomy, hormonal changes may additionally impact mood, anxiety, and sleep as the relation between menopause and sleep disorders is well established.35,36
This study has some strengths. First, the examination of sleep before hysterectomy as a potential predictor for postoperative pain is novel. In addition, the multidisciplinary nature of the study integrates gynecology, pain, and sleep domains. Furthermore, sleep was evaluated both by self-report and actigraphy. In addition, we adopted standardized, validated tools to evaluate sleep, pain, and satisfaction including the Consensus Sleep Diary, actigraphy, Pittsburgh Sleep Quality Index, PROMIS pain forms, and Treatment Satisfaction Scale. We also followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement guidelines.37 To minimize heterogeneity, we included only minimally invasive hysterectomies for benign indications, as malignancy is known to impact sleep and pain in a variety of ways.38 Similarly, open cases are usually associated with a different pain pattern. Therefore, we excluded cancer and open cases to minimize potential confounders.
The findings of this study may have been impacted by some limitations. The number of participants is small, which may affect certain aspects of the statistical analysis including regression analysis. Therefore, we did not perform subgroup analyses, such as stratifying by the surgical indication, prior surgeries, length of surgery, or other covariates. However, this study was designed as a pilot observational study to prove the feasibility of future large-scale studies that require greater funding. These large studies can also explore potential confounding, mediating, and moderating variables. Another limitation is the short-term time frame in which sleep was assessed, both before and after surgery. Therefore, it is important to take into account the potential immediate effects of surgery on sleep disruption when analyzing the results of this study. Longer preoperative sleep and pain assessment can provide insights on the baseline sleep and pain prior to potential stress-induced disruptions. Similarly, longer postoperative sleep and pain assessments can uncover possible residual sleep and/or pain disruptions beyond the immediate postoperative period. In addition, we used a large number of sleep- and pain-assessment tools in this study. Our goal was to evaluate the same parameter in several ways to avoid spurious results. In retrospect, this may have caused extra work for patients and may have contributed to the lower number of cases with complete data. Last, the descriptive nature of the study precludes causal inferences; hence, randomized controlled trials to evaluate the impact of sleep-directed interventions on postoperative pain are necessary for further understanding of these relationships.
In conclusion, this study shows that preoperative sleep correlates with posthysterectomy pain and satisfaction. Larger studies and randomized controlled trials are needed to examine the effects of sleep interventions, such as cognitive behavioral therapy on pain perception. Additional studies can also examine open cases and further examine the impact of sleep on other operative outcomes, such as length of hospital stay and opioid use. Mechanistic studies can further elucidate the neuroscience behind the association of preoperative sleep and postoperative pain.
DISCLOSURE STATEMENT
All authors participated in the work and have seen and approved the manuscript. Work for this study was performed at the University of Texas Medical Branch at Galveston. This study was funded, in part, by National Institutes of Health (NIH) grant 1R01HD094380 to Mostafa A. Borahay; NIH grants K23NR014008, R01NR018342, and CTSA UL1TR001439 to Sara Nowakowski; South Central Mental Illness Research, Education, and Clinical Center; and Center of Innovations in Quality, Effectiveness and Safety (CIN 13-413).he authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ABBREVIATIONS
- PROMIS
Patient-Reported Outcomes Measurement Information System
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110(5):1091–1095. 10.1097/01.AOG.0000285997.38553.4b [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Jennings K, Borahay MA, et al. Trends in the national distribution of laparoscopic hysterectomies from 2003 to 2010. J Minim Invasive Gynecol. 2014;21(4):656–661. 10.1016/j.jmig.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niraj G, Rowbotham DJ. Persistent postoperative pain: where are we now? Br J Anaesth. 2011;107(1):25–29. 10.1093/bja/aer116 [DOI] [PubMed] [Google Scholar]
- 4.Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As-Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol. 2018;219(5):486. 10.1016/j.ajog.2018.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. 10.1001/jamasurg.2017.0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrimpf M, Liegl G, Boeckle M, Leitner A, Geisler P, Pieh C. The effect of sleep deprivation on pain perception in healthy subjects: a meta-analysis. Sleep Med. 2015;16(11):1313–1320. 10.1016/j.sleep.2015.07.022 [DOI] [PubMed] [Google Scholar]
- 7.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10(5):357–369. 10.1016/j.smrv.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 8.Krause AJ, Prather AA, Wager TD, Lindquist MA, Walker MP. The pain of sleep loss: a brain characterization in humans. J Neurosci. 2019;39(12):2291–2300. 10.1523/JNEUROSCI.2408-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iacovides S, George K, Kamerman P, Baker FC. Sleep fragmentation hypersensitizes healthy young women to deep and superficial experimental pain. J Pain. 2017;18(7):844–854. 10.1016/j.jpain.2017.02.436 [DOI] [PubMed] [Google Scholar]
- 10.Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66(1):143–172. 10.1146/annurev-psych-010213-115205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowakowski S, Matthews KA, von Känel R, Hall MH, Thurston RC. Sleep characteristics and inflammatory biomarkers among midlife women. Sleep. 2018;41(5):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kjølhede P, Langström P, Nilsson P, Wodlin NB, Nilsson L. The impact of quality of sleep on recovery from fast-track abdominal hysterectomy. J Clin Sleep Med. 2012;8(4):395–402. 10.5664/jcsm.2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gögenur I, Bisgaard T, Burgdorf S, van Someren E, Rosenberg J. Disturbances in the circadian pattern of activity and sleep after laparoscopic versus open abdominal surgery. Surg Endosc. 2009;23(5):1026–1031. 10.1007/s00464-008-0112-9 [DOI] [PubMed] [Google Scholar]
- 14.Kim KH, Lee KA. Sleep and fatigue symptoms in women before and 6 weeks after hysterectomy. J Obstet Gynecol Neonatal Nurs. 2009;38(3):344–352. 10.1111/j.1552-6909.2009.01029.x [DOI] [PubMed] [Google Scholar]
- 15.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. 10.5665/sleep.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. 10.1093/sleep/26.3.342 [DOI] [PubMed] [Google Scholar]
- 17.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 18.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 19.Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14(6):429–441. 10.1002/pon.860 [DOI] [PubMed] [Google Scholar]
- 20.Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27(2):285–291. 10.1093/sleep/27.2.285 [DOI] [PubMed] [Google Scholar]
- 21.Revicki DA, Chen WH, Harnam N, et al. Development and psychometric analysis of the PROMIS pain behavior item bank. Pain. 2009;146(1):158–169. 10.1016/j.pain.2009.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook KF, Dunn W, Griffith JW, et al. Pain assessment using the NIH Toolbox. Neurology. 2013;80(11, Suppl 3):S49–S53. 10.1212/WNL.0b013e3182872e80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone AA, Broderick JE, Junghaenel DU, Schneider S, Schwartz JE. PROMIS fatigue, pain intensity, pain interference, pain behavior, physical function, depression, anxiety, and anger scales demonstrate ecological validity. J Clin Epidemiol. 2016;74:194–206. 10.1016/j.jclinepi.2015.08.029 [DOI] [PubMed] [Google Scholar]
- 24.Stull DE, Wasiak R, Kreif N, Raluy M, Colligs A, Seitz C, Gerlinger C. Validation of the SF-36 in patients with endometriosis. Qual Life Res. 2014;23(1):103–117. 10.1007/s11136-013-0442-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–197. 10.1016/0304-3959(87)91074-8 [DOI] [PubMed] [Google Scholar]
- 26.Seligman ME. The effectiveness of psychotherapy: the Consumer Reports study. Am Psychol 1995;50(12):965–974. 10.1037/0003-066X.50.12.965 [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- 28.Spielberger CD. State-Trait Anxiety Inventory: Bibliography. Consulting Psychologists Press: Palo Alto, CA; 1989. [Google Scholar]
- 29.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. 10.1016/0022-3999(94)00125-O [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Yue XF, Qu WM, et al. Morphine inhibits sleep-promoting neurons in the ventrolateral preoptic area via mu receptors and induces wakefulness in rats. Neuropsychopharmacology. 2013;38(5):791–801. 10.1038/npp.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore JT, Kelz MB. Opiates, sleep, and pain: the adenosinergic link. Anesthesiology. 2009;111(6):1175–1176. 10.1097/ALN.0b013e3181bdfa2e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borahay MA, Patel PR, Kilic CH, Kilic GS. Outpatient robotic hysterectomy: clinical outcomes and financial analysis of initial experience. Int J Med Robot Comp. 2014;10(2):244–250. [DOI] [PubMed] [Google Scholar]
- 33.Bergstrom JE, Scott ME, Alimi Y, et al. Narcotics reduction, quality and safety in gynecologic oncology surgery in the first year of enhanced recovery after surgery protocol implementation. Gynecol Oncol. 2018;149(3):554–559. 10.1016/j.ygyno.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 34.Chaudhary S, Bhattacharyya TK. Psychiatric effects of hysterectomy. Med J Armed Forces India. 1995;51(1):27–30. 10.1016/S0377-1237(17)30914-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho NY, Kim S, Nowakowski S, Shin C, Suh S. Sleep disturbance in women who undergo surgical menopause compared with women who experience natural menopause. Menopause. 2019;26(4):357–364. [DOI] [PubMed] [Google Scholar]
- 36.Nowakowski S, Meliska CJ, Martinez LF, Parry BL. Sleep and menopause. Curr Neurol Neurosci Rep. 2009;9(2):165–172. 10.1007/s11910-009-0025-6 [DOI] [PubMed] [Google Scholar]
- 37.Vandenbroucke JP, von Elm E, Altman DG, et al. ; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. 10.1016/j.ijsu.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 38.Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Soc Sci Med. 2002;54(9):1309–1321. 10.1016/S0277-9536(01)00043-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.