Abstract
Study Objectives:
Restless sleep disorder (RSD) has recently been characterized clinically and polysomnographically in children and differentiated from restless legs syndrome (RLS). Heart rate variability is a reliable method to quantify autonomic changes during sleep. The aim of this study was to characterize heart rate variability in children with RSD, RLS, and individuals without these disorders, with the hypothesis that children with RSD have a shift toward sympathetic predominance during sleep.
Methods:
We analyzed polysomnographic recordings from 32 children who fulfilled RSD diagnostic criteria (19 boys and 13 girls), 32 children with RLS (20 boys and 12 girls), and 33 individuals without disorders (17 boys and 16 girls). Four electrocardiographic epochs were chosen, 1 for each stage, and were analyzed for automatic detection of R waves. Time domain and frequency domain heart rate variability parameters were obtained and analyzed.
Results:
In terms of time domain, only the standard deviation of the average interval between successive R waves during stage N3 was slightly but significantly higher in patients with RSD than in patients with RLS. In terms of frequency domain, in patients with RSD, the very-low-frequency and low-frequency bands were increased (vs patients with RLS and individuals without disorders, respectively), whereas low-frequency/high-frequency ratio tended to be increased in both patients with RSD and with RLS. In rapid eye movement sleep, low-frequency/high-frequency ratio was increased in both patients with RSD and with RLS. The low-frequency/high-frequency ratio increased in patients with RLS during quiet wakefulness preceding sleep.
Conclusions:
Children with RSD have increased sympathetic activation during sleep, particularly N3 and rapid eye movement sleep, compared with individuals without disorders but, as expected, not during wakefulness. Differently, children with RLS have sympathetic activation during relaxed wakefulness preceding sleep and during sleep.
Citation:
DelRosso LM, Bruni O, Ferri R. Heart rate variability during sleep in children and adolescents with restless sleep disorder: a comparison with restless legs syndrome and normal controls. J Clin Sleep Med. 2020;16(11):1883–1890.
Keywords: heart rate variability, restless sleep disorder, restless legs syndrome
BRIEF SUMMARY
Current Knowledge/Study Rationale: Restless sleep disorder has recently been characterized and proposed as a new pediatric diagnosis. Children with restless sleep disorder have significant sleep disruption and daytime symptoms. Because of increased movements at night, it is important to characterize heart rate variability in children with restless sleep disorder in comparison with children with other movement disorders such as restless legs syndrome but in particular in comparison to individuals without disorders.
Study Impact: We hypothesize that children with restless sleep disorder, because of increased movements and possible arousals, will have a shift toward sympathetic predominance during sleep.
INTRODUCTION
Restless sleep disorder (RSD) has been recently identified in children and adolescents presenting with frequent movements during sleep and daytime impairment. The first publication proposing RSD as a primary sleep disorder evaluated children characterized clinically by parental report of frequent nocturnal body movements including repositioning and moving large muscle groups during sleep.1 The parents did not report difficulty falling asleep, symptoms of restless legs syndrome (RLS), or nocturnal awakenings but instead noted that children were “very restless” while sleeping and used descriptive terms such as “he moves like a helicopter,” “moves all night,” “trashes the bed,” or “bedsheets are everywhere.” The parents identified children with RSD as having a different sleep pattern than siblings, and there was evidence of daytime impairment such as excessive sleepiness, decreased school performance, or some hyperactivity.1 Despite these symptoms, evaluation of these children did not show evidence of a sleep disorder, medical condition, psychiatric comorbidity, or medication effect that could potentially explain the nocturnal movement. Parents also stated that the movements occurred almost every night and at any hour of the night. This prompted the second study on RSD, in which video-polysomnography (PSG) demonstrated that children with RSD present with at least 5 body movements per hour of sleep, reduced total sleep time, and increased number of awakenings.2 In comparison to children with RLS, patients with RSD show similar sleep disruption but without the leg movement activation.1 RSD seems to have a prevalence only slightly lower than that of RLS among children referred for disordered sleep to a specialized center.3
The nature of sleep disruption in RSD still needs to be clarified, and the ongoing research is focusing on the possible mechanism(s) underlying the night sleep changes typical of this condition and on their similarities/differences with those of other sleep disorders, such as RLS. Children with RLS present with the urge to move the legs usually in the evening or during periods of rest. The urge is relieved by movement.4 Autonomic system balance is an important physiologic feature of sleep and is modified by the presence of different sleep disorders. For this reason, it now appears important to characterize the features of the autonomic system regulation during sleep also in RSD.
Each heartbeat in the heart rate is regulated by an orchestration between the sympathetic and parasympathetic nervous systems. The variability in heart rate (HRV) provides valuable information on the status of the autonomic nervous system, particularly in several medical and sleep disorders. This information can be derived from the intervals between successive R waves (RR intervals) obtained in the single electrocardiographic lead in PSG. Typically, HRV is measured based on statistics derived from 2 domains. The time domain HRV parameters are statistical measures of the RR time series, whereas the frequency domain parameters are obtained by fast Fourier transform (FFT) or other methods to assess the underlying components of the heart rate at various frequencies. Parasympathetic activity, mostly related to the respiratory cycle, is the major contributor to the HRV high-frequency (HF) band (0.15–0.5 Hz), whereas the low-frequency (LF) band (0.04–0.15 Hz) is a marker of sympathetic and vagal modulation.5
Early studies on heart rate changes during sleep have demonstrated that heart rate decreases during sleep and reaches the lowest values during deep sleep.6 More recent studies have confirmed a predominance of the parasympathetic modulation of HRV with progression to deeper sleep stages7 and a shift toward sympathetic predominance in rapid eye movement (REM) sleep.8 Besides clear changes during sleep stages, HRV has been demonstrated to be affected by sleep disorders such as obstructive sleep apnea,9,10 periodic leg movements during sleep disorder,11 and other conditions associated with increased sleep fragmentation,12 clearly indicating a shift toward sympathetic predominance during sleep in the presence of these sleep disorders.13
For all these reasons, our current study aims to characterize HRV in children with RSD and RLS and individuals without disorders. We hypothesize that children with RSD have a shift toward sympathetic predominance during sleep with an increased LF/HF ratio, similarly to RLS, and higher than that of individuals without disorders.
METHODS
Participants
Thirty-two children who fulfilled RSD diagnostic criteria (19 boys and 13 girls; age range, 5–17 years), 32 children with RLS (20 boys and 12 girls; age range, 5–18 years), and 33 individuals without disorders (17 boys and 16 girls; age range, 5–18 years) from our 3-year database were included. Children with RSD were consecutively recruited, whereas children in the group without disorders were normal children from a prior research study used for comparison. We did a sample size calculation with the aim to be able to disclose a statistically significant difference with α of 0.05 and power of 0.8 for comparisons characterized by a moderate-to-high effect size of 0.65, which indicated the need to include 30 participants per group. Diagnostic criteria used for RSD included the following: (1) the child exhibits a pattern of sleep characterized by restless sleep or motor behaviors involving large muscle groups consisting of frequent repositioning, moving bed sheets, or falling out of bed; (2) the movements are exclusively sleep related, occurring at any time after sleep onset, and persist at intervals through the night; (3) the movements occur almost every night at an index of at least 5 movements per hour of sleep; (4) sleep latency and sleep time are within expected for age; (5) the movements result in a significant complaint by patient or parent of at least one of the following: (i) interference (or parental perception) with nocturnal sleep, (ii) impairment in daytime function (sleepiness or school performance), and (iii) irritability or hyperactivity; and (6) the condition is not better explained by behavioral disorders, medical disorders, or medication effect. Criteria 1–6 must be met.3
Exclusion criteria were as follows: age younger than 5 years, use of medications that altered sleep parameters (eg, antihistamines, antidepressants, antiepileptics), presence of comorbid sleep disorder (eg, obstructive sleep apnea, central sleep apnea, parasomnias, behavioral insomnia), medical or psychiatric conditions known to affect sleep (uncontrolled eczema, asthma, pain, neurodevelopmental disorders, genetic syndrome, neuromuscular disorders), or use of caffeine.
The sample size, even if not very large, was decided based on practical considerations of the real possibilities of recruitment of patients and on the fact that we aimed at disclosing statistical significance for effect sizes of at least 0.8 (considered to be a large effect size), with an α of 0.05 and power of 80%. This yielded the need of a sample size of at least 26 participants in each group. Thus, with the number of participants effectively recruited, we obtained a power of 88.3%.
All children underwent PSG. The study was approved by the local institutional review board.
PSG
PSG was recorded following the American Academy of Sleep Medicine standards14 and included electroencephalogram (2 frontal, 2 central, and 2 occipital channels, referred to as the contralateral mastoid); electrooculogram, electromyogram of the submentalis muscle, electromyogram of the right and left tibialis anterior muscles, respiratory signals, effort signals for thorax and abdomen, oximetry, capnography, and a single lead electrocardiographic, video, and audio recording. Calibrations were performed per routine standard by the sleep technician. Epochs and all sleep parameters were scored by a certified sleep technologist and board-certified sleep physician, according to standard criteria.14
HRV analysis
To study sleep stage–related HRV, for each recording, a series of 4 electrocardiographic epochs were chosen, 1 for each stage: quiet wakefulness preceding sleep; stage N2, stage N3, and stage R. We selected, for each stage, the first artifact-free and arousal-free ten 30-second epochs (300 seconds in total) occurring in the recording. These epochs did not contain body or leg movements. The middle 256 seconds of each epoch were then used for the subsequent analysis. Electrocardiographic signal (sampled at 256 Hz) was analyzed for automatic detection of R waves with a computer program (Hypnolab, SWS Soft, Italy) using a simple threshold plus first and second derivative algorithm. HRV is usually evaluated as 24-hour, short-term (∼5 minutes) or brief, and ultra-short term (<5 minutes). Although FFT is not suitable for the quantification of ultra-short-term HRV, it has been generally used for the evaluation of short-term HRV during sleep because of the possibility to compute it by taking into account the different sleep stages. An epoch length of 256 seconds (approximately 5 minutes) is a widely accepted parameter for studying what is called short-term HRV and is a power of 2 (28 = 256), a requirement for the computation of FFT.15 The raw RR interval time series obtained in this way were then used for HRV analysis in the time domain, with the calculation of the following statistical parameters: (1) mean RR value, standard deviation of all RR intervals; (2) the square root of the mean of the sum of the squares of differences between adjacent RR intervals; (3) number of pairs of adjacent RR intervals differing by more than 50 ms in the entire epoch; and (4) percentage of number of pairs of adjacent RR intervals differing by more than 50 ms in the entire epoch among the total RR intervals).
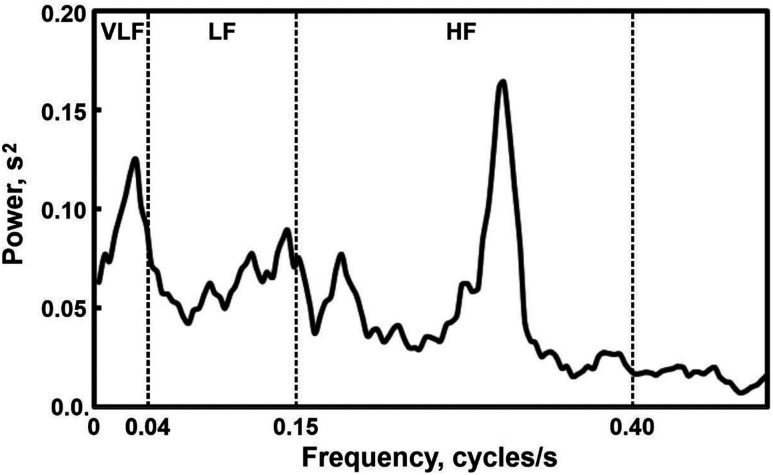
After this step, RR intervals were estimated at each second by linear interpolation of the measured intervals, and the resulting equally spaced 256-value time series were used for HRV analysis in the frequency domain by FFT to estimate power spectral density. The following parameters were thus obtained: (1) very-LF power range (VLF; <0.04 Hz); (2) LF (power in LF range, 0.04–0.15 Hz); (3) HF (power in HF range, 0.15–0.4 Hz); (4) total power (VLF + LF + HF); (5) LF% (LF power in normalized units: LF/[total power – VLF] × 100); (6) HF% (HF power in normalized units: HF/[total power – VLF] × 100); (7) LF/HF ratio. Figure 1 shows, as an example, the power spectrum obtained by FFT of the interpolated RR time series from stage N3 in 1 of the participants included in this study; the boundaries of the different frequency bands considered are marked with vertical dashed lines.
Figure 1. Example of a power spectrum obtained by fast Fourier transform of the interpolated interval between successive electrocardiographic R waves time series from stage N3 in 1 of the participants included in this study.
Both time-domain and frequency-domain analyses were performed following standard guidelines.16
Statistical analysis
The comparison of the sex composition of the groups of participants was carried out by means of the χ2 test. Because of the non-normal distribution of several variables, nonparametric statistics were used. For the comparison between the 3 groups of participants, the Kruskal-Wallis analysis of variance was computed, followed by post hoc comparisons of mean ranks of all pairs of groups, and p values (2-sided significance levels with a Bonferroni adjustment) associated with each comparison were obtained.17
RESULTS
The sex composition of the 3 groups was not statistically different (χ2 = 0.856, not significant). The groups also did not differ for age (Table 1).
Table 1.
Age, sleep architecture, and leg movement activity parameters.
| Parameter | Individuals Without Disorders (n = 33) | RSD (n = 32) | RLS (n = 32) | Kruskal-Wallis ANOVA | Effect Size |
Post Hoc (P <) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQ Range | Median | IQ Range | Median | IQ Range | H(2,95) | P < | Cohen’s d | 1 vs 2 | 2 vs 3 | 1 vs 3 | |
| Age, years | 7.4 | 6–14 | 10.0 | 8–13.5 | 10.0 | 8–14 | 1.324 | NS | 0.049 | |||
| Time in bed, min | 512.5 | 473.5–555 | 508.5 | 480.3–531.8 | 515.0 | 463.8–558.5 | 0.547 | NS | −0.191 | |||
| Sleep period time, min | 474.0 | 450–520.5 | 478.3 | 441.5–496.5 | 484.5 | 443–535.5 | 1.839 | NS | −0.306 | |||
| Total sleep time, min | 457.0 | 422–505 | 422.5 | 375.8–468 | 460.3 | 392.3–517 | 7.503 | .023 | −0.741* | 0.023 | NS | NS |
| Sleep latency, min | 20.0 | 9–38.5 | 24.8 | 11.5–43.3 | 19.0 | 9.5–31.8 | 2.477 | NS | 0.137 | |||
| REM sleep latency, min | 89.5 | 60.5–115 | 143.5 | 107.3–182.5 | 152.8 | 100.3–213.3 | 18.361 | .001 | 0.781* | 0.001 | NS | 0.00043 |
| Stage shifts/h | 9.4 | 7.1–11.7 | 11.5 | 8.2–14.2 | 10.3 | 8.4–13.1 | 4.186 | NS | 0.555* | |||
| Awakenings/h | 2.7 | 1.1–4.7 | 4.2 | 3.4–6.6 | 3.5 | 1.6–5.5 | 9.290 | .0096 | 0.800† | 0.0074 | NS | NS |
| Sleep efficiency, % | 91.8 | 88.4–94.2 | 85.8 | 75–91.6 | 90.4 | 83.8–93.9 | 9.888 | .0071 | −0.820† | 0.005 | NS | NS |
| Stage W, % | 3.8 | 1.5–5.7 | 8.2 | 4–16.7 | 5.0 | 1.7–8.4 | 14.265 | .0008 | 0.853† | 0.0005 | NS | NS |
| Stage N1, % | 4.7 | 3.1–5.8 | 6.0 | 3.5–8.9 | 4.7 | 3.3–6.7 | 4.586 | NS | 0.600* | |||
| Stage N2, % | 47.9 | 44.5–53 | 42.4 | 37.3–53.1 | 45.5 | 38.6–50.3 | 2.411 | NS | −0.322 | |||
| Stage N3, % | 22.3 | 19.2–26 | 23.3 | 19.4–26 | 23.9 | 19.7–31.1 | 1.637 | NS | −0.028 | |||
| Stage R, % | 21.7 | 17.2–26.4 | 14.6 | 8.6–19.3 | 17.4 | 11.9–20.6 | 20.764 | .000001 | −1.216† | 0.000034 | NS | 0.0039 |
| PLMS index, n/h | 1.1 | 0.3–2.1 | 0.0 | 0–0.7 | 2.2 | 1.1–6.3 | 27.534 | .000001 | −0.229 | 0.007 | 0.000001 | NS |
| Total LMS index, n/h | 12.2 | 9–16.5 | 0.0 | 0–12.4 | 19.2 | 13.5–26.9 | 34.810 | .000001 | −0.809† | 0.0039 | 0.000001 | 0.024 |
*Medium effect size. †Large effect size. ANOVA = analysis of variance, IQ = interquartile, LMS = leg movements during sleep, NS = not significant, PLMS = periodic leg movements during sleep.
Table 1 also reports the comparison of the PSG parameters found in the 3 groups. Patients with RSD tended to have less total sleep time and lower sleep efficiency than individuals without disorders; REM latency and number of awakenings were significantly higher than in individuals without disorders, together with a decreased percentage of REM sleep and lower leg movement activity parameters. The latter parameters were the only parameters significantly different between patients with RSD and RLS; they were significantly higher in participants with RLS.
Table 2 shows the comparison between the time-domain HRV parameters obtained in the 3 groups of participants; only the standard deviation of the average RR interval during stage N3 tended to be higher in patients with RSD than in patients with RLS, with an associated medium effect size.
Table 2.
Time domain HRV parameters.
| Parameter | Individuals Without Disorders (n = 33) | RSD (n = 32) | RLS (n = 32) | Kruskal-Wallis ANOVA | Effect Size |
Post Hoc (P <) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQ Range | Median | IQ Range | Median | IQ Range | H(2,95) | P < | Cohen’s d | 1 vs 2 | 2 vs 3 | 1 vs 3 | |
| Stage W | ||||||||||||
| Mean RR, ms | 736.8 | 634.3–14 | 732.0 | 632.5–14 | 776.2 | 699.5–14 | 2.936 | NS | −0.443 | |||
| SDNN | 50.5 | 38.6–558.5 | 48.7 | 38–558.5 | 50.5 | 45–558.5 | 0.766 | NS | −0.193 | |||
| RMSSD | 47.6 | 36.3–535.5 | 40.6 | 31.2–535.5 | 41.6 | 27.5–535.5 | 1.634 | NS | −0.300 | |||
| Stage N2 | ||||||||||||
| Mean RR, ms | 770.1 | 658.1–517 | 777.0 | 688.9–517 | 830.7 | 734.7–517 | 3.014 | NS | −0.358 | |||
| SDNN | 53.6 | 37.8–31.8 | 48.8 | 36.4–31.8 | 53.2 | 36.3–31.8 | 0.197 | NS | 0.137 | |||
| RMSSD | 56.1 | 34.3–213.3 | 49.4 | 35.6–213.3 | 50.5 | 33.1–213.3 | 0.893 | NS | 0.016 | |||
| Stage N3 | ||||||||||||
| Mean RR, ms | 805.4 | 662.2–13.1 | 782.1 | 709.4–13.1 | 823.2 | 744.5–13.1 | 1.883 | NS | −0.346 | |||
| SDNN | 44.6 | 26.9–5.5 | 54.4 | 36.2–5.5 | 38.4 | 30.7–5.5 | 6.846 | .033 | 0.592* | NS | 0.05 | NS |
| RMSSD | 50.0 | 31.2–93.9 | 54.4 | 36.8–93.9 | 40.4 | 30.7–93.9 | 4.039 | NS | 0.294 | |||
| Stage R | ||||||||||||
| Mean RR, ms | 813.1 | 671.7–8.4 | 764.0 | 686–8.4 | 772.4 | 742.1–8.4 | 2.137 | NS | −0.357 | |||
| SDNN | 61.2 | 32.4–6.7 | 59.1 | 37.3–6.7 | 51.3 | 38.4–6.7 | 0.872 | NS | 0.031 | |||
| RMSSD | 43.8 | 31–50.3 | 44.0 | 23.6–50.3 | 38.7 | 31.3–50.3 | 1.306 | NS | −0.286 | |||
NN50 (number of interval differences of successive RR intervals greater than 50 ms) and pNN50 (ratio of NN50 by the total number of RR intervals) have been omitted in this table because they are highly correlated with RMSSD. *Medium effect size. ANOVA = analysis of variance, IQ = interquartile, NS = not significant, RMSSD = square root of the mean squared differences of successive RR intervals, RR = interval between successive electrocardiographic R waves, SDNN = standard deviation of the average RR interval.
Frequency-domain HRV parameters are reported in Table 3. In this case, during quiet wakefulness preceding sleep, only patients with RLS showed a LF/HF ratio higher than that of individuals without disorders, whereas during sleep stage N2, no differences were detected among the 3 groups. During sleep stage N3, the VLF band tended to be higher in patients with RSD compared with both individuals without disorders and children with RLS, with a medium effect size; the LF band power was higher in patients with RSD compared with individuals without disorders, with a medium-to-large effect size, similar to the effect size obtained for LF/HF ratio (somewhat higher in RSD than in individuals without disorders), which, however, did not reach statistical significance. During stage R, only the LF/HF ratio was found to be higher in both RSD and RLS, with a moderate effect size.
Table 3.
Frequency domain HRV parameters.
| Parameter | Individuals Without Disorders (n = 33) | RSD (n = 32) | RLS (n = 32) | Kruskal-Wallis ANOVA | Effect Size |
Post Hoc (P <) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQ Range | Median | IQ Range | Median | IQ Range | H(2,95) | P < | Cohen’s d | 1 vs 2 | 2 vs 3 | 1 vs 3 | |
| Stage W | ||||||||||||
| VLF, beats/s2 | 0.089 | 0.065–0.119 | 0.078 | 0.063–0.107 | 0.089 | 0.068–0.14 | 1.315 | NS | 0.000 | |||
| LF, beats/s2 | 0.121 | 0.080–0.208 | 0.113 | 0.094–0.152 | 0.139 | 0.100–0.181 | 0.515 | NS | −0.111 | |||
| HF, beats/s2 | 0.181 | 0.128–0.299 | 0.143 | 0.115–0.228 | 0.150 | 0.102–0.272 | 1.375 | NS | −0.316 | |||
| Total, beats/s2 | 0.424 | 0.277–0.616 | 0.373 | 0.301–0.477 | 0.387 | 0.310–0.545 | 0.630 | NS | −0.230 | |||
| LF/HF | 0.615 | 0.513–0.769 | 0.841 | 0.591–1.037 | 0.890 | 0.649–1.141 | 8.034 | .018 | 0.442 | NS | NS | 0.017 |
| Stage N2 | ||||||||||||
| VLF, beats/s2 | 0.110 | 0.088–0.159 | 0.121 | 0.084–0.215 | 0.148 | 0.086–0.194 | 0.401 | NS | 0.143 | |||
| LF, beats/s2 | 0.223 | 0.124–0.289 | 0.188 | 0.117–0.343 | 0.184 | 0.122–0.305 | 0.631 | NS | 0.175 | |||
| HF, beats/s2 | 0.069 | 0.058–0.098 | 0.075 | 0.055–0.113 | 0.079 | 0.051–0.099 | 0.406 | NS | −0.060 | |||
| Total, beats/s2 | 0.423 | 0.299–0.549 | 0.385 | 0.277–0.678 | 0.438 | 0.268–0.607 | 0.044 | NS | 0.084 | |||
| LF/HF | 0.587 | 0.424–0.674 | 0.700 | 0.520–0.920 | 0.693 | 0.516–0.924 | 5.542 | NS | 0.279 | |||
| Stage N3 | ||||||||||||
| VLF, beats/s2 | 0.054 | 0.039–0.075 | 0.065 | 0.055–0.080 | 0.051 | 0.040–0.061 | 7.286 | .026 | 0.602* | NS | 0.028 | NS |
| LF, beats/s2 | 0.091 | 0.056–0.111 | 0.137 | 0.072–0.184 | 0.081 | 0.059–0.129 | 7.848 | .02 | 0.789* | 0.021 | NS | NS |
| HF, beats/s2 | 0.177 | 0.103–0.217 | 0.191 | 0.143–0.331 | 0.144 | 0.111–0.259 | 4.110 | NS | 0.373 | |||
| Total, beats/s2 | 0.326 | 0.209–0.392 | 0.437 | 0.284–0.618 | 0.297 | 0.234–0.420 | 6.622 | .0365 | 0.603* | NS | NS | NS |
| LF/HF | 0.495 | 0.407–0.570 | 0.567 | 0.434–0.909 | 0.555 | 0.446–0.734 | 5.859 | NS (.053) | 0.792* | |||
| Stage R | ||||||||||||
| VLF, beats/s2 | 0.093 | 0.053–0.126 | 0.102 | 0.077–0.157 | 0.099 | 0.075–0.148 | 2.004 | NS | 0.325 | |||
| LF, beats/s2 | 0.142 | 0.071–0.178 | 0.137 | 0.077–0.182 | 0.130 | 0.075–0.161 | 0.331 | NS | 0.056 | |||
| HF, beats/s2 | 0.182 | 0.109–0.287 | 0.151 | 0.086–0.248 | 0.168 | 0.115–0.199 | 1.159 | NS | −0.319 | |||
| Total, beats/s2 | 0.436 | 0.240–0.571 | 0.447 | 0.233–0.575 | 0.389 | 0.289–0.461 | 0.471 | NS | −0.111 | |||
| LF/HF | 0.570 | 0.486–0.751 | 0.834 | 0.587–1.049 | 0.787 | 0.557–1.054 | 8.936 | .011 | 0.589* | 0.02 | NS | 0.044 |
*Medium effect range. abs. = absolute power, ANOVA = analysis of variance, HF = high-frequency band, IQ = interquartile, LF = low-frequency band, NS = not significant, VLF = very-low-frequency band.
DISCUSSION
This is the first study that shows HRV features in children with RSD in comparison to children with RLS and individuals without disorders. The spectral analysis of HRV is a reliable method to quantify autonomic changes during sleep and has been correlated with early risk of cardiovascular morbidity. Our study shows that HRV in children with RSD does not have statistically significant differences in time-domain parameters compared with individuals without disorders; similarly, children with RLS only showed a statistically significant difference in standard deviation of all RR intervals during stage N3. This is different from the adult data in which time-domain parameters have been reported to be affected in patients with RLS during wakefulness.13 In these patients, time-domain HRV measures were significantly decreased during wakefulness, insinuating a sympathetic predominance that is expected in RLS because it is a disorder manifested at bedtime but during wakefulness.13 The fact that little differences were found between patients and controls in time-domain parameters or in the absolute power of HRV bands and they were limited to HRV band power percentage and LF/HF ratio might indicate that the changes in HRV are relatively mild, even if significant. However, it must be noted that this difference between time- and frequency-domain measures in disclosing changes between patients and controls was previously observed in a variety of studies and conditions, for example, in children with Down’s syndrome,18 partial epilepsy,19 and narcolepsy.12
Frequency-domain measures showed a statistically significant difference in children with RLS during wakefulness compared with individuals without disorders, showing increased LF/HF ratio and confirming a sympathetic activation during wakefulness preceding sleep in RLS, which was not seen in RSD. Studies in adults have demonstrated increased sympathetic activation by HRV analysis in patients with RLS during wakefulness.20 An interesting observation in our children with RLS is the low median number of periodic limbs movements of sleep. Our previous research has shown that depending on age, children with RLS may not have elevated periodic limbs movements of sleep but may have elevated isolated leg movements (>15) as shown in the results.21 As we reported previously, children with RSD do not have difficulty with sleep onset or discomfort at bedtime; therefore, this new study confirms that sympathetic activation is not present during wakefulness but instead during sleep in RSD, as shown by the pattern of restlessness with large body movements seen once the child falls asleep.1
In terms of HRV and sleep stages, Herzig et al22 demonstrated that N3 is the most reproducible sleep stage to measure HRV with the lowest LF power during N3 in normal adults. In the current study, both children with RLS and RSD showed a tendency toward a sympathetic predominance during N3; however, because of similar findings in all sleep stages, particularly statistically significant differences during REM sleep, we can reliably state that children with RSD tend to have increased sympathetic predominance during the whole sleep time, with the LF band significantly increased during N3 and REM. It may be argued that REM sleep typically has a sympathetic predominance and that the increase in sympathetic activity during REM sleep may be secondary to increased REM in the early morning hours when cortisol is also increased. However, our study still shows a statistically significant increase in sympathetic predominance when comparing REM sleep between children with RSD and children with RLS with individuals without disorders.
Studies evaluating normal HRV during sleep in children are sparse. Herzig et al23 showed in a population of children younger than in our study that in children 2–6 years old, HRV parameters did not increase with age during sleep. On the contrary, Finley and Nugent24 studied 61 healthy participants from 1 month until 24 years of age during wakefulness, quiet sleep, and active sleep and showed that HRV changed with age with a gradual increase in parasympathetic modulation in the first 6 years of life, with a following gradual decrease. Finally, Walter et al25 recently reported that in individuals without disorders, age is associated with increasing parasympathetic and sympathetic activity (LF power) during both wakefulness and sleep during the developmental period. Thus, similarly to many other sleep features, HRV does show age-related changes during the developmental period that still need to be detailed in more refined studies. These age-related changes may be caused by the interaction between development, environment, and genetics. For example, it is well known that the MEIS1 gene variant significantly influences HRV in RLS in adults.26 Further studies that evaluate these interactions during development and aging are needed.
Our study demonstrates that children with RSD have early changes in HRV as described previously. These findings have pathophysiologic implications but most importantly contribute to highlight the importance of early recognition of RSD in children. Childhood represents a window of opportunity for early identification of potential contributors to cardiovascular complications later on in life. The contribution of sleep disorders to increased cardiovascular risk is known in adults,27–30 but we have a golden opportunity to prevent these consequences with early diagnosis and treatment during childhood.
Limitations of our study include a single-center experience and analysis of only the short-term HRV 256-second epochs (long-term 24-hour HRV, a rare, but important, type of study in the literature on children might be the target of a near-future analysis). Moreover, we only analyzed a single epoch for each sleep stage, and this choice was made because of different reasons as detailed. It has been long known that HRV exhibits a strong circadian modulation, with greater variability during nighttime sleep.31,32 Differently from heart rate, which shows a general declining trend during sleep, HRV exhibits a state-dependent increase through the night, with increased variability over successive REM cycles33; for this reason, we chose to analyze epochs from the first cycle only. Thus, when analyzing HRV during sleep, there are at least 2 main physiologic factors influencing it: sleep stage and sleep cycle (circadian phase). Averaging several epochs (even of the same sleep stage) over different REM cycles would have probably blurred the data and masked eventual small differences between the groups. By taking a single epoch for each stage at its first occurrence at least partially reduced such an intrasleep variability by also taking into account sleep stages. In addition, children with RSD often have >10 movements/hr,2 and this makes it difficult to find several artifact- and movement-free epochs; this is true also for children with RLS that have periodic limbs movements in sleep.21 Finally, a replication of these results on an independent database is now warranted.
In conclusion, our original hypothesis was confirmed by the results of this study, demonstrating that children with RSD have increased sympathetic activation during sleep, particularly N3 and REM, compared with individuals without disorders but, as expected, not during wakefulness. Differently, children with RLS have sympathetic activation during relaxed wakefulness preceding sleep and during sleep. Our study also supports the other clinical and PSG findings indicating that RSD is a disorder that disrupts sleep in children and allows speculation that it can potentially have cardiovascular consequences.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This study was partially funded by Italian Ministry of Health RC Grant 2751598 (R.F.). The authors report no conflicts of interest.
ABBREVIATIONS
- FFT
fast Fourier transform
- HF
high frequency
- HRV
heart rate variability
- LF
low frequency
- PLMS
periodic limbs movements of sleep
- PSG
polysomnography
- REM
rapid eye movement
- RLS
restless legs syndrome
- RR intervals
intervals between successive R waves
- RSD
restless sleep disorder
REFERENCES
- 1.DelRosso LM, Bruni O, Ferri R. Restless sleep disorder in children: a pilot study on a tentative new diagnostic category. Sleep. 2018;41(8). 10.1093/sleep/zsy102 [DOI] [PubMed] [Google Scholar]
- 2.DelRosso LM, Jackson CV, Trotter K, Bruni O, Ferri R. Video-polysomnographic characterization of sleep movements in children with restless sleep disorder. Sleep. 2019;42(4):zsy269. 10.1093/sleep/zsy269 [DOI] [PubMed] [Google Scholar]
- 3.DelRosso LM, Ferri R. The prevalence of restless sleep disorder among a clinical sample of children and adolescents referred to a sleep centre. J Sleep Res. 2019;28(6):e12870. 10.1111/jsr.12870 [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 5.Ferri R, Parrino L, Smerieri A, Terzano MG, Elia M, Musumeci SA, Pettinato S. Cyclic alternating pattern and spectral analysis of heart rate variability during normal sleep. J Sleep Res. 2000;9(1):13–18. 10.1046/j.1365-2869.2000.00190.x [DOI] [PubMed] [Google Scholar]
- 6.Snyder F, Hobson JA, Morrison DF, Goldfrank F. Changes in respiration, heart rate, and systolic blood pressure in human sleep. J Appl Physiol. 1964;19(3):417–422. 10.1152/jappl.1964.19.3.417 [DOI] [PubMed] [Google Scholar]
- 7.Boudreau P, Yeh WH, Dumont GA, Boivin DB. Circadian variation of heart rate variability across sleep stages. Sleep. 2013;36(12):1919–1928. 10.5665/sleep.3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glos M, Fietze I, Blau A, Baumann G, Penzel T. Cardiac autonomic modulation and sleepiness: physiological consequences of sleep deprivation due to 40 h of prolonged wakefulness. Physiol Behav. 2014;125:45–53. 10.1016/j.physbeh.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 9.Kwok KL, Yung TC, Ng DK, Chan CH, Lau WF, Fu YM. Heart rate variability in childhood obstructive sleep apnea. Pediatr Pulmonol. 2011;46(3):205–210. 10.1002/ppul.21268 [DOI] [PubMed] [Google Scholar]
- 10.Nisbet LC, Yiallourou SR, Nixon GM, et al. Nocturnal autonomic function in preschool children with sleep-disordered breathing. Sleep Med. 2013;14(12):1310–1316. 10.1016/j.sleep.2013.07.010 [DOI] [PubMed] [Google Scholar]
- 11.Manconi M, Ferri R, Zucconi M, Clemens S, Rundo F, Oldani A, Ferini-Strambi L. Effects of acute dopamine-agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Med. 2011;12(1):47–55. 10.1016/j.sleep.2010.03.019 [DOI] [PubMed] [Google Scholar]
- 12.Antelmi E, Plazzi G, Pizza F, Vandi S, Aricò D, Ferri R. Impact of acute administration of sodium oxybate on heart rate variability in children with type 1 narcolepsy. Sleep Med. 2018;47:1–6. 10.1016/j.sleep.2018.03.020 [DOI] [PubMed] [Google Scholar]
- 13.Sforza E, Pichot V, Cervena K, Barthélémy JC, Roche F. Cardiac variability and heart-rate increment as a marker of sleep fragmentation in patients with a sleep disorder: a preliminary study. Sleep. 2007;30(1):43–51. 10.1093/sleep/30.1.43 [DOI] [PubMed] [Google Scholar]
- 14.Berry RB, Brooks R, Gamaldo CE, et al. ; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.3. Darien, IL: American Academy of Sleep Medicine; 2016. [Google Scholar]
- 15.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. 10.3389/fpubh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. 10.1161/01.CIR.93.5.1043 [DOI] [PubMed] [Google Scholar]
- 17.Siegel S, Castellan NJ. Nonparametric Statistics for the Behavioral Sciences. 2nd ed. New York: McGraw-Hill; 1988. [Google Scholar]
- 18.Ferri R, Curzi-Dascalova L, Del Gracco S, Elia M, Musumeci SA, Pettinato S. Heart rate variability and apnea during sleep in Down’s syndrome. J Sleep Res. 1998;7(4):282–287. 10.1046/j.1365-2869.1998.00111.x [DOI] [PubMed] [Google Scholar]
- 19.Ferri R, Curzi-Dascalova L, Arzimanoglou A, et al. Heart rate variability during sleep in children with partial epilepsy. J Sleep Res. 2002;11(2):153–160. 10.1046/j.1365-2869.2002.00283.x [DOI] [PubMed] [Google Scholar]
- 20.Izzi F, Placidi F, Romigi A, et al. Is autonomic nervous system involved in restless legs syndrome during wakefulness? Sleep Med. 2014;15(11):1392–1397. 10.1016/j.sleep.2014.06.022 [DOI] [PubMed] [Google Scholar]
- 21.Ferri R, DelRosso LM, Aricò D, et al. Leg movement activity during sleep in school-age children and adolescents: a detailed study in normal controls and participants with restless legs syndrome and narcolepsy type 1. Sleep. 2018;41(4). 10.1093/sleep/zsy010 [DOI] [PubMed] [Google Scholar]
- 22.Herzig D, Eser P, Omlin X, Riener R, Wilhelm M, Achermann P. Reproducibility of heart rate variability is parameter and sleep stage dependent. Front Physiol. 2018;8:1100. 10.3389/fphys.2017.01100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzig D, Eser P, Radtke T, et al. Relation of heart rate and its variability during sleep with age, physical activity, and body composition in young children. Front Physiol. 2017;8:109. 10.3389/fphys.2017.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finley JP, Nugent ST. Heart rate variability in infants, children and young adults. J Auton Nerv Syst. 1995;51(2):103–108. 10.1016/0165-1838(94)00117-3 [DOI] [PubMed] [Google Scholar]
- 25.Walter LM, Tamanyan K, Weichard AJ, Davey MJ, Nixon GM, Horne RSC. Sleep disordered breathing in children disrupts the maturation of autonomic control of heart rate and its association with cerebral oxygenation. J Physiol. 2019;597(3):819–830. 10.1113/JP276933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thireau J, Farah C, Molinari N, et al. MEIS1 variant as a determinant of autonomic imbalance in Restless Legs Syndrome. Sci Rep. 2017;7(1):46620. 10.1038/srep46620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. 10.1016/S0140-6736(08)61622-0 [DOI] [PubMed] [Google Scholar]
- 28.Tobaldini E, Costantino G, Solbiati M, et al. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev. 2017;74(Pt B):321–329. 10.1016/j.neubiorev.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 29.Covassin N, Singh P. Sleep duration and cardiovascular disease risk: epidemiologic and experimental evidence. Sleep Med Clin. 2016;11(1):81–89. 10.1016/j.jsmc.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzaga C, Bertolami A, Bertolami M, Amodeo C, Calhoun D. Obstructive sleep apnea, hypertension and cardiovascular diseases. J Hum Hypertens. 2015;29(12):705–712. 10.1038/jhh.2015.15 [DOI] [PubMed] [Google Scholar]
- 31.Jensen-Urstad K, Storck N, Bouvier F, Ericson M, Lindblad LE, Jensen-Urstad M. Heart rate variability in healthy subjects is related to age and gender. Acta Physiol Scand. 1997;160(3):235–241. 10.1046/j.1365-201X.1997.00142.x [DOI] [PubMed] [Google Scholar]
- 32.Malpas SC, Purdie GL. Circadian variation of heart rate variability. Cardiovasc Res. 1990;24(3):210–213. 10.1093/cvr/24.3.210 [DOI] [PubMed] [Google Scholar]
- 33.Cajochen C, Pischke J, Aeschbach D, Borbély AA. Heart rate dynamics during human sleep. Physiol Behav. 1994;55(4):769–774. 10.1016/0031-9384(94)90058-2 [DOI] [PubMed] [Google Scholar]