Abstract
Peptides are promising antagonists against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2). To expedite drug discovery, a computational approach is widely employed for the initial screening of anti-SARS-CoV-2 candidates. This study aimed to investigate the potential of peptides from quinoa seed proteins as multi-target antagonists against SARS-CoV-2 spike glycoprotein receptor-binding domain, main protease, and papain-like protease. Five quinoa proteins were hydrolyzed in silico by papain and subtilisin. Among the 1465 peptides generated, seven could interact stably with the key binding residues and catalytic residues of the viral targets, mainly via hydrogen bonds and hydrophobic interactions. The seven peptides were comparable or superior to previously reported anti-SARS-CoV-2 peptides based on docking scores. Key residues in the seven peptides contributing to binding to viral targets were determined by computational alanine scanning. The seven peptides were predicted in silico to be non-toxic and non-allergenic. The peptides ranged between 546.66 and 3974.87 g/mol in molecular mass, besides exhibiting basic and cationic properties (isoelectric points: 8.26–12.10; net charges: 0.1–4.0). Among the seven peptides, VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS was found to bind the largest number of key residues on the targets. In conclusion, seven putative non-toxic, non-allergenic, multi-target anti-SARS-CoV-2 peptides were identified from quinoa seed proteins. The in vitro and in vivo efficacies of the seven peptides against SARS-CoV-2 deserve attention in future bench-top testing.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10989-021-10214-y.
Keywords: Antiviral peptide, Bioinformatics, COVID-19, Quinoa seed protein, SARS-CoV-2
Introduction
The coronavirus disease 2019 (COVID-19) outbreak posed a significant threat to human health worldwide (Huang et al. 2020). The receptor-binding domain (RBD) of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) spike glycoproteins plays a vital role in facilitating entry into the host cell via its interactions with the human angiotensin-converting enzyme 2 (hACE2) (Wang et al. 2020; Yi et al. 2020). Mutations of nine key binding residues of RBD, namely Leu455, Phe456, Ser459, Gln474, Ala475, Phe486, Phe490, Gln493, and Pro499, have been empirically demonstrated to abolish the binding affinity of SARS-CoV-2 spike glycoproteins to hACE2 (Yi et al. 2020). This supports the hypothesis that targeting the key binding residues could block the binding of SARS-CoV-2 spike glycoproteins to hACE2, thus precluding the infections of host cells (Yi et al. 2020). Following the release of the viral genome into the host cytoplasm and the synthesis of viral polyproteins, SARS-CoV-2 main protease (Mpro) and papain-like protease (PLpro) cleave the polyproteins into functional fragments that are crucial for viral replication. It was expected that impeding the action of the two proteases by targeting the catalytic residues of Mpro (His41 and Cys145) and PLpro (Cys111, His272, and Asp286) could suppress SARS-CoV-2 replication and the spread of infection (Ortega et al. 2020; Wlodawer et al. 2020). To date, no human proteases with similar cleavage specificities as Mpro and PLpro are known. Thus inhibitors against SARS-CoV-2 Mpro and PLpro are likely non-toxic to human cells (Zhang et al. 2020). Taken together, the SARS-CoV-2 spike glycoprotein RBD, Mpro, and PLpro make attractive targets for the discovery and development of effective antiviral drugs against COVID-19.
Since the COVID-19 outbreak, many research groups have started searching for potential anti-COVID-19 agents, adopting the in silico approach. Through virtual screening and molecular docking, several natural and artificial inhibitors, as well as repurposed drugs that potentially block the entry of SARS-CoV-2 into human cells, were discovered (Choudhary et al. 2020; Luo et al. 2020; Wong et al. 2020a, 2021). Likewise, computational discoveries of inhibitors of Mpro and PLpro were made (Ortega et al. 2020; Wong et al. 2021; Kandeel et al. 2020).
Food-derived bioactive peptides have drawn attention for their potential applications as functional food ingredients, therapeutics, vaccines, and diagnostic agents (Haggag et al. 2018; Chai et al. 2017; Wong et al. 2020b). Plant seeds are good sources of antiviral peptides (Sonawane and Arya 2018). To the best of our knowledge, there are yet reports of antiviral peptides from quinoa seeds. However, the discovery of bioactive peptides, such as antioxidant, anti-angiotensin-converting-enzyme-I, anti-inflammatory, and anti-dipeptidyl-peptidase-IV peptides, from quinoa seeds are well documented (Aluko and Monu 2003; Nongonierma et al. 2015; Galante et al. 2020; Ren et al. 2017). The release of bioactive peptides from food proteins is often accomplished by enzymatic hydrolysis (Chai et al. 2017; Wong et al. 2020b). Papain and subtilisin could effectively release bioactive peptides from quinoa proteins (Aluko and Monu 2003; Nongonierma et al. 2015). Therefore, the two enzymes were chosen for in silico release of peptides from quinoa seed proteins in this study. To date, several peptide-based compounds, namely N3, VIR251, and SBP1, were demonstrated empirically as antagonists against SARS-CoV-2 Mpro, PLpro, and spike glycoprotein RBD, respectively (VanPatten et al. 2020; Jin et al. 2020; Rut et al. 2020). Compared to phytochemicals, peptides are still underexplored as SARS-CoV-2 inhibitors. Therefore, the objective of this study was to computationally investigate the potential of quinoa-derived peptides as multi-target antagonists against three key SARS-CoV-2 targets: spike glycoprotein RBD, Mpro, and PLpro. The multi-target approach would be efficacious in dealing with the high mutation rates of RNA viruses-associated diseases, as is expected of COVID-19 (Pachetti et al. 2020). In this study, computational alanine scanning was performed to investigate the significance of each residue of the putative multi-target peptides towards the stability of their binding to viral targets. In silico prediction of physicochemical properties, pharmacokinetics, druglikeness, medicinal chemistry, toxicity and allergenicity of the shortlisted multi-target peptides were also carried out to provide hints for their antiviral potential and safety risk.
Methods
In Silico Proteolysis
The sequences of five major storage proteins of the quinoa seed (2S albumin-like, 11S seed storage globulin, 11S globulin seed storage protein 2-like, 13S globulin seed storage protein 1-like, and 13S globulin seed storage protein 2-like) were downloaded from NCBI (https://www.ncbi.nlm.nih.gov) (Clark et al. 2015) on 14 August 2020. These protein sequences were subjected to in silico hydrolysis by papain (EC 3.4.22.2) and subtilisin (EC 3.4.21.62) separately by using the BIOPEP-UWM web-server (Minkiewicz et al. 2019).
Molecular Docking Analyses with HPEPDOCK
The crystal structures of three SARS-CoV-2 targets, namely spike glycoprotein RBD (PDB ID: 6LZG) (Wang et al. 2020), Mpro (PDB ID: 6LU7) (Jin et al. 2020), and PLpro (PDB ID: 6WX4) (Rut et al. 2020), were retrieved from Protein Data Bank (http://www.rcsb.org/pdb) (Berman et al. 2000; Burley et al. 2018) on 20 July, 22 July, and 28 August 2020, respectively. The proteins were prepared for molecular docking using BIOVIA Discovery Studio Visualizer (BIOVIA, Dassault Systèmes, BIOVIA Discovery Studio Visualizer, Version 20.1.0.192, San Diego: Dassault Systèmes, 2020). Co-crystallized water, heteroatom, and ligand were removed, whereas polar hydrogens were added. The bound ligands hACE2, N3, and VIR251 in complex with the crystal structures of spike glycoprotein RBD, Mpro, and PLpro, respectively, were separated and prepared in the same manner as protein preparation. The optimized structures saved in the pdb format were taken for HPEPDOCK analysis (Zhou et al. 2018). The three prepared viral proteins were uploaded as receptor inputs, whereas the prepared hACE2, N3, and VIR251 were uploaded as binding site references for analysis on spike glycoprotein RBD, Mpro, and PLpro, respectively. Quinoa peptides released by in silico papain and subtilisin hydrolyses were entered in the FASTA format as peptide inputs. Reference peptides previously reported to potentially bind with SARS-CoV-2 viral proteins were mostly entered in the FASTA format as peptide inputs, except for reference peptides p18, p28, and VIR250, which were uploaded as in the pdb format. Tables S1–S3 show previously reported peptides that we used as reference peptides to compare with quinoa seed peptides.
The docking scores computed for the quinoa and reference peptides were recorded. Three dimensional (3D) diagram of protein-peptide docking models were visualized by using BIOVIA Discovery Studio Visualizer. The two dimensional (2D) diagram of docked models with lower (more negative) docking scores relative to SBP1, N3 and/or VIR251 were visualized with LigPlot+ v.2.2. (Wallace et al. 1995; Laskowski and Swindells 2011). The molecular interactions, including hydrogen bonds, hydrophobic interactions, salt bridges, and external bonds, between the peptides and viral proteins were checked.
Computational Alanine Scanning
Computational alanine scanning was performed using the BUDE alanine scan server (https://balas.app) (Wood et al. 2020) to identify peptide residues that are critical for binding to the three target viral proteins. The models of selected peptides docked to the viral proteins were uploaded to the server. The approach involves substituting each residue with alanine in turn, followed by computation of the resultant change (∆∆G) in the overall binding energy (Ibarra et al. 2019).
Prediction of Physicochemical Properties, Pharmacokinetics, Druglikeness, Medicinal Chemistry, Toxicity, and Allergenicity
SwissADME (http://www.swissadme.ch) was used to calculate physicochemical descriptors and predict ADME criteria, pharmacokinetics, druglike nature, as well as medicinal chemistry acceptance (Daina et al. 2017). The SMILES format of the peptides were obtained from the NovoPro server (https://www.novoprolabs.com). Further, the molecular mass, isoelectric point, and net charge at neutral pH of selected quinoa peptides were predicted by using an online peptide property calculator (https://pepcalc.com). The potential toxicity of peptides was predicted by using the ToxinPred server (http://crdd.osdd.net/raghava/toxinpred) (Gupta et al. 2013) as previously described (Chai et al. 2019). AllerTOP v.2.0 (http://www.ddg-pharmfac.net/AllerTOP) (Dimitrov et al. 2014) was used for allergenicity prediction.
Results and Discussion
In silico proteolysis of quinoa seed storage proteins by using papain and subtilisin produced 883 and 582 peptide fragments, respectively (Table S4). The peptides can be collectively reduced into 654 unique sequences of 2–33 residues (data not shown). HPEPDOCK analysis of the unique sequences on Mpro, PLpro, and spike glycoprotein RBD returned docking scores ranging from − 36.954 to − 251.813, − 41.689 to − 223.713, and − 36.904 to − 210.374, respectively (Table S5). By selecting for peptides with lower (more negative) docking scores than those computed for N3, VIR251, and/or SBP1, we narrowed down the 654 peptides to 18 (data not shown). N3, VIR251, and SBP1 are peptide-based inhibitors whose inhibition of Mpro, PLpro and spike glycoprotein RBD were demonstrated empirically (VanPatten et al. 2020; Jin et al. 2020; Rut et al. 2020). Next, by selecting for peptides which could bind to at least one key binding residue on the spike glycoprotein RBD, at least one catalytic-dyad residue in Mpro, and at least one catalytic-triad residue in PLpro, we narrowed down the 18 peptides to eight potential trifunctional peptides (Table 1, Tables S6–S8). All of the eight peptides listed in Table 1 could bind more stably to PLpro in comparison with VIR251, as suggested by their lower scores relative to that of VIR251. Furthermore, PHWNIN and ERHHRGGRGRQS could also bind more stably to Mpro when compared to N3. None of the 654 quinoa peptides analyzed had a lower docking score than SBP1 when docked against the spike glycoprotein RBD (Table S5). Considering their predicted binding to key binding/catalytic residues in the three target proteins, the eight peptides are potential trifunctional inhibitory peptides against SARS-CoV-2. Targeting multiple proteins associated with crucial pathways of SARS-CoV-2 infection is more likely to control COVID-19 conditions in contrast to targeting only a single viral protein (Zhou et al. 2020).
Table 1.
Docking scores computed for eight potential trifunctional peptides and their number of interactions with the target proteins
| Proteases used for hydrolysis | Peptides releaseda | Docking scores based on viral proteinsb | Number of key binding/catalytic residues the peptides interacting withc | ||||
|---|---|---|---|---|---|---|---|
| Mpro | PLpro | RBD | Mpro | PLpro | RBD | ||
| Papain | PNWKIN | − 200.405 | − 200.518 | − 192.529 | 2 | 1 | 3 |
| PHYNN | − 212.036 | − 190.318 | − 172.688 | 2 | 1 | 2 | |
| PHWNIN | − 243.337 | − 210.290 | − 190.799 | 1 | 1 | 2 | |
| Subtilisin | VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS | − 211.578 | − 223.713 | − 207.146 | 2 | 3 | 4 |
| TKHGGRINTL | − 206.586 | − 188.343 | − 169.248 | 2 | 1 | 4 | |
| PKRF | − 182.408 | − 191.522 | − 145.040 | 2 | 2 | 2 | |
| ERHHRGGRGRQS | − 217.103 | − 202.493 | − 207.936 | 1 | 2 | 3 | |
| AIRAMPL | − 188.547 | − 189.679 | − 146.175 | 2 | 1 | 1 | |
aQuinoa peptides derived from each protease treatment were sorted in descending order based on the total number of interactions they were predicted to have with the viral proteins
bValues in bold are lower (more negative) than those computed for N3 (− 215.634) and VIR251 (− 186.078), the inhibitors complexed to the Mpro and PLpro crystal structures, respectively
cKey binding residues of spike glycoprotein RBD (Leu455, Phe456, Ser459, Gln474, Ala475, Phe486, Phe490, Gln493 and Pro499) are those critical for binding between RBD and hACE2. The catalytic-dyad residues in the active site of Mpro are His41 and Cys145. The catalytic-triad residues in the active site of PLpro are Cys111, His272 and Asp286
In this study, we also analyzed the docking scores of several reference peptides compared to the quinoa seed peptides. The docking score of VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS was up to 1.32-fold lower than those of reference peptides QRPR, p28, and LPIY when docked against Mpro (Table S5), and up to 1.53-fold lower than VIR251, p28, VIR250, and p18 when docked against PLpro (Table S5). When docked against the spike glycoprotein RBD, the docking score of VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS was up to 1.67-fold lower than reference peptides PQQQF, PISCR, VPW, and VQVVN (Table S5). Hence, based on the docking score, VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS potentially bind more stably to Mpro, PLpro, and the spike glycoprotein RBD when compared with some anti-COVID-19 peptides reported in the literature.
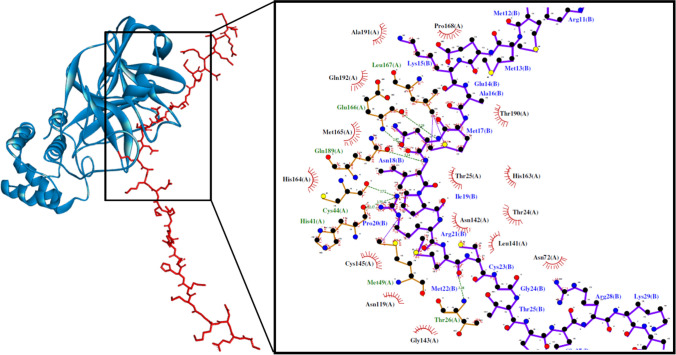
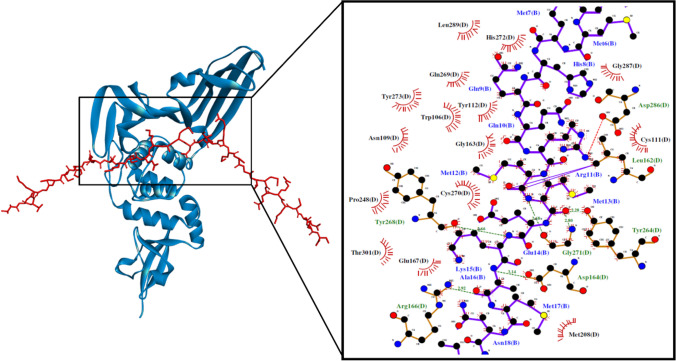
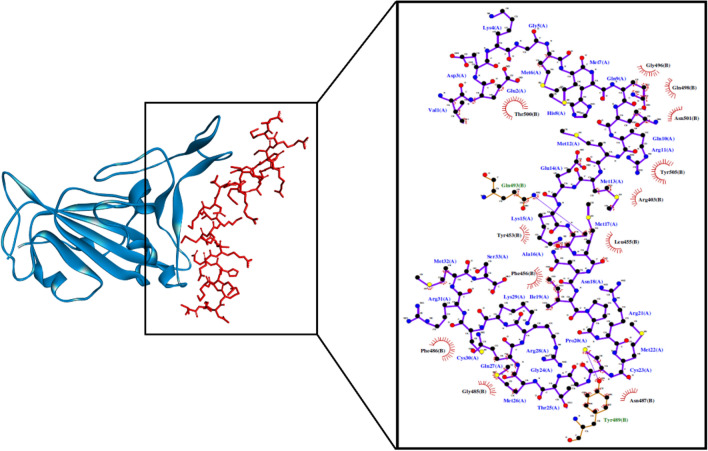
The elucidation of intermolecular interactions between the quinoa peptides and the three viral proteins is necessary to establish the significance of the docking scores obtained. Among the eight potential trifunctional peptides in Table 1, VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS could form the highest number of interactions with three target proteins. It could potentially bind to His41 and Cys145 on Mpro via hydrophobic interaction (Fig. 1). His41 of Mpro plays the important role of deprotonating Cys145, activating the latter for its nucleophilic attack on the peptide bond of SARS-CoV-2 polyproteins. Thus, disruption of the two catalytic-dyad residues could potentially dampen Mpro activity (Ramos-Guzmán et al. 2020). VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS could also form five hydrogen bonds with His41, Glu166, and Gln189, which are in the substrate-binding pocket of Mpro (Jin et al. 2020). Multiple hydrogen bonds formed between the peptide and residues in the Mpro substrate-binding pocket could lock the peptide inside the pocket, inhibiting Mpro activity (Jin et al. 2020). Notably, among all peptides analyzed in this study, VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS is the only peptide that could bind to all three catalytic-triad residues of PLpro (Table S7). It was predicted to bind to Cys111 and His272 on PLpro via hydrophobic interaction and binding to Asp286 via the salt bridge (Fig. 2). Its three catalytic-triad residues mediate the proteolysis of viral polyproteins by PLpro: Cys111 as a nucleophile, His272 as a general acid, with the assistance of Asp286 (Henderson et al. 2020). In light of those mentioned above, the cleavage of SARS-CoV-2 polyproteins by Mpro and PLpro could potentially be inhibited by VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS. Moreover, the peptide was predicted to form hydrophobic interactions with key binding residues Leu455, Phe456, Phe486, and Gln493 on RBD (Fig. 3). The four residues are crucial for hACE2-SARS-CoV-2 complex formation, a prerequisite for viral attachment to the host cell (Yi et al. 2020). Meanwhile, the high affinity between SARS-CoV-2 spike glycoprotein and hACE2 is accounted for by significant hydrophobic attractions between the hydrophobic surfaces of the two proteins at their binding interface (Li et al. 2020). Hence, VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS could potentially compromise the binding of SARS-CoV-2 spike glycoprotein RBD with hACE2.
Fig. 1.
The Mpro-VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS docked model presented in 3D (left) and 2D (right) diagrams. The protein and peptide structures are represented in blue and red, respectively. In the 2D diagram, the protein bonds are in orange, whereas that of a peptide is purple. The hydrophobic bonds, external bonds, hydrogen bonds, and salt bridges are displayed in red spoked arcs, purple lines, green and red dashed lines. The projected view displays only the interacting residues at the binding interface (colour figure online)
Fig. 2.
The PLpro-VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS docked model presented in 3D (left) and 2D (right) diagrams. Descriptions for the 2D and 3D diagrams are the same as those in Fig. 1 (colour figure online)
Fig. 3.
The RBD-VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS docked model presented in 3D (left) and 2D (right) diagrams. Descriptions for the 2D and 3D diagrams are the same as those in Fig. 1 (colour figure online)
Based on the in silico alanine scanning experiment on the eight trifunctional peptides (Table S9), the residues that formed a more significant number of interactions with the viral proteins were often the more critical residues for binding and stabilizing the peptide-protein complex. For instance, Arg of AIRAMPL was computed by LigPlot+ to form four salt bridges, three hydrogen bonds, and hydrophobic interactions with PLpro residues; alanine substitution of the residue increased ∆∆G by 20.2079 kJ/mol. Contrarily, those residues predicted by LigPlot+ to have no interactions with the viral proteins had little or no effects on ∆∆G after alanine substitution. Our analysis on spike glycoprotein RBD agrees with Baig et al. (2020) that Phe and His are essential residues for binding to the viral protein. For instance, substituting Phe of PKRF with Ala increased ∆∆G by 23.5656 kJ/mol. Alanine substitution of His of TKHGGRINTL increased ∆∆G by 8.3854 kJ/mol.
Furthermore, substituting Leu of AIRAMPL with Ala increased ∆∆G by 8.5420 kJ/mol. This observation agrees with that of Baig et al. (2020) that a C-terminal Leu likely plays a significant role in stabilizing the interactions between peptides and spike glycoprotein RBD. Information gathered from the in silico alanine scanning experiment could guide future studies to design more effective anti-COVID-19 peptides from the eight candidates reported in this study.
All eight potential trifunctional peptides were basic and cationic (Table 2). As indicated by their isoelectric points (7.99–12.10), the basic properties of the peptides are accounted for by the presence of basic residues. The eight potential trifunctional peptides are comprised of at least 14.3% of basic residues in their sequences. NGAICWGPCPTAFRQIGNCGHFKVRCCKIR is an antiviral peptide derived from mouse beta-defensin-4. The peptide was effective against respiratory viruses, including severe acute respiratory syndrome coronavirus type 1 (SARS-CoV-1) and middle-east respiratory syndrome coronavirus (MERS-CoV) (Zhao et al. 2016). The abundance of basic amino acids in NGAICWGPCPTAFRQIGNCGHFKVRCCKIR was proposed to facilitate late endosomal acidification inhibition, thus precluding viral RNA release into host cells (Zhao et al. 2016).
Table 2.
Predicted physicochemical properties, toxicity, and allergenicity of eight potential trifunctional peptides
| Peptides | Mass (g/mol) | Isoelectric point | Net charge | Toxicity | Allergenicity |
|---|---|---|---|---|---|
| PKRF | 546.66 | 11.52 | 2.0 | Non-toxin | Probable non-allergen |
| PHYNN | 643.65 | 7.99 | 0.1 | Non-toxin | Probable allergen |
| PNWKIN | 770.88 | 10.57 | 1.0 | Non-toxin | Probable non-allergen |
| AIRAMPL | 770.99 | 10.90 | 1.0 | Non-toxin | Probable non-allergen |
| PHWNIN | 779.84 | 8.26 | 0.1 | Non-toxin | Probable non-allergen |
| TKHGGRINTL | 1096.24 | 11.39 | 2.1 | Non-toxin | Probable non-allergen |
| ERHHRGGRGRQS | 1432.51 | 12.10 | 3.2 | Non-toxin | Probable non-allergen |
| VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS | 3974.87 | 10.40 | 4.0 | Non-toxin | Probable non-allergen |
On the other hand, the electrostatic affinity to the negatively-charged viral surface is a possible mechanism by which some cationic antiviral peptides could disrupt the viral envelope (Mahendran et al. 2020; Skalickova et al. 2015). SARS-CoV-2, SARS-CoV-1, and MERS-CoV share similarities in their infection mechanisms (Zhu et al. 2020). Moreover, VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS and NGAICWGPCPTAFRQIGNCGHFKVRCCKIR have comparable physicochemical characteristics in terms of their basic properties and net charges (data not shown). Thus whether the quinoa peptide VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS could also inhibit SARS-CoV-2 by dampening endosomal acidification and disrupting viral envelope, besides acting directly on the three target proteins, is an interesting question to be addressed in future research.
In this study, the eight potential trifunctional peptides ranged between 4 and 33 residues in length and between 546.66 and 3974.87 g/mol in molecular mass (Table 2). Overall, these peptide lengths fall within the range of 2–39 residues reported for FDA-approved peptide drugs (including both marketed drugs and clinical candidates) as reviewed by Santos et al. (2016). The same authors revealed that a majority of the peptide drugs have molecular masses of up to 1800 g/mol (Santos et al. 2016). Hence, with the exception of VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS, the other seven trifunctional peptides in this study also fit the molecular mass range of of the aforementioned FDA-approved peptide drugs. However, molecular mass alone should not render VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS (3974.87 g/mol) unworthy of further consideration; the peptide is still clearly smaller than two FDA-approved antiviral peptide drugs (tifuvirtide, 5037 g/mol; enfuvirtide, 4565 g/mol) (Santos et al. 2016; National Center for Biotechnology Information 2021a, 2021b). In general, in terms of peptide length and molecular mass, the eight potential trifunctional peptides do not differ considerably from currently known peptide drugs.
All the eight peptides listed in Table 2 were predicted to be non-toxic. Only antiviral peptides that could impair viral infection without being toxic to human cells can be considered useful and desired therapeutic agents (Mulder et al. 2013). Our results also agree with Gupta et al. (2013) that non-toxic peptides are often comprised of Ala, Arg, Gln, Ile, Leu, Lys, Met, Thr, and Val. For instance, 64% of the residues of VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS are composed of the residues above. Except for PHYNN, the seven other potential trifunctional peptides are probable non-allergenic (Table 2). These seven non-allergenic peptides are of interest because of the predicted low risks of sensitization and allergic reactions. Allergic inflammation may reduce the production of innate interferons and delay antiviral responses in the host cells (Edwards et al. 2017). Hence, at least based on in silico analysis, the antiviral effects of the seven non-allergenic peptides are less likely to be compromised by allergenicity when compared with PHYNN. Furthermore, the ability of the seven shortlisted peptides to target key proteins that are involved in pre- and post-cell-entry viral activities implies their prophylactic and therapeutic potential.
The usefulness of the SwissADME web tool in predicting the physicochemical properties, lipophilicity, druglikeness and pharmacokinetics of hypotensive dipeptides derived from flaxseeds was reported by Ji et al. (2020). In this study, we also used SwissADME for a similar analysis (Table 3). Predictors of the potential of a drug to be orally bioavailable include the ratio of sp3 hybridized carbons over the total carbon count of the molecule (Fraction Csp3), number of rotatable bonds, number of hydrogen bond acceptors (HBA), number of hydrogen bond donors (HBD) and topological polar surface area (TPSA) (Santos et al. 2016). According to Santos et al. (2016), a majority of the FDA-approved peptide drugs that are orally available have up to 20 rotatable bonds, with fraction Csp3 of up to 0.55. Among the eight potential trifunctional peptides, only PHYNN, with 21 rotatable bonds and fraction Csp3 of 0.43 (Table 3), could be taken as generally matching the two criteria. Meanwhile, except for ERHHRGGRGRQS and VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS, the other six trifunctional peptides were found to have between 8 and 18 HBA and 9–19 HBD. These six peptides have lipophilicity (log Po/w) of − 0.36 to − 4.62 (Table 3). Thus the six peptides may predictably be orally available as most orally available peptide drugs have up to 50 HBA and up to 25 HBD, in addition to lipophilicity between − 5 and 8 (Santos et al. 2016). Furthermore, except for TKHGGRINTL, ERHHRGGRGRQS and VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS, the other five trifunctional peptides have TPSA between 224.55 and 325.48 Å2. This points to the chance of these five peptides being orally available as majority of orally available peptide drugs have TPSA of up to 400 Å2 (Santos et al. 2016). To sum up, based on the six descriptors above, PHYNN is the most likely to be orally available.
Table 3.
Physicochemical properties, lipophilicity, water solubility, pharmacokinetics, druglikeness and medicinal chemistry friendliness of the eight potential trifunctional peptides as analyzed by using SwissADME
| Parameters | Peptides | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PKRF | PHYNN | PNWKIN | AIRAMPL | PHWNIN | TKHGGRINTL | ERHHRGGRGRQS | VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS | ||
| Physicochemical properties | Number of heavy atoms | 39 | 46 | 55 | 53 | 56 | 77 | 101 | 267 |
| Number of aromatic heavy atoms | 6 | 11 | 9 | 0 | 14 | 5 | 10 | 5 | |
| Fraction Csp3 | 0.58 | 0.43 | 0.56 | 0.76 | 0.47 | 0.67 | 0.56 | 0.70 | |
| Number of rotatable bonds | 21 | 21 | 28 | 30 | 26 | 47 | 65 | 180 | |
| Number of H-bond acceptors | 8 | 11 | 11 | 10 | 11 | 18 | 24 | 55 | |
| Number of H-bond donors | 9 | 10 | 11 | 10 | 11 | 19 | 30 | 58 | |
| Molar refractivity | 149.93 | 160.79 | 202.96 | 206.63 | 202.46 | 273.98 | 350.66 | 1001.83 | |
| TPSA (Å2) | 224.55 | 300.82 | 322.82 | 316.33 | 325.48 | 525.37 | 789.00 | 1944.79 | |
| Lipophilicity | Consensus log Po/w | − 0.80 | − 3.14 | − 1.60 | − 0.36 | − 1.50 | − 4.62 | − 8.94 | − 12.55 |
| Water solubility | Log S (ESOL) | 0.33 | 0.79 | − 0.45 | − 0.59 | − 0.71 | 1.14 | 3.98 | 2.50 |
| Class (ESOL) | Highly soluble | Highly soluble | Very soluble | Very soluble | Very soluble | Highly soluble | Highly soluble | Highly soluble | |
| Pharmacokinetics | GI absorption | Low | Low | Low | Low | Low | Low | Low | Low |
| P-gp substrate | No | No | Yes | Yes | Yes | Yes | Yes | Yes | |
| CYP1A2 inhibitor | No | No | No | No | No | No | No | No | |
| CYP2C19 inhibitor | No | No | No | No | No | No | No | No | |
| CYP2C9 inhibitor | No | No | No | No | No | No | No | No | |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | No | |
| CYP3A4 inhibitor | No | No | No | No | No | No | No | No | |
| Druglikeness | Lipinski’s rule (number of violations) | No (3) | No (3) | No (3) | No (3) | No (3) | No (3) | No (3) | No (3) |
| Bioavailability score | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | |
| Medicinal chemistry | Leadlikeness (number of violations) | No (2) | No (2) | No (2) | No (2) | No (2) | No (2) | No (2) | No (2) |
| Synthetic accessibility | 4.87 | 5.37 | 6.52 | 7.16 | 6.49 | 9.34 | 10.00 | 10.00 | |
Fraction Csp3 ratio of sp3 hybridized carbons over the total carbon count of the molecule, H-bond hydrogen bond, TPSA topological polar surface area, log Po/w partition coefficient between n-octanol and water, ESOL estimated solubility, GI gastrointestinal, P-gp permeability glycroprotein, CYP1A2 cytochrome P450 1A2, CYP2C19 cytochrome P450 2C19, CYP2C9 cytochrome P450 2C9, CYP2D6 cytochrome P450 2D6, CYP3A4 cytochrome P450 3A4
The eight trifunctional peptides may not be bioavailable following oral ingestion due to poor GI absorption. However, there is chance that PKRF and PHYNN may still be taken up by lung cells, the site of SARS-CoV-2 invasion, if these peptides could be effectively introduced into the body non-orally. For PKRF and PHYNN to bind to the SARS-CoV-2 spike glycoproteins when the virus is extracellular, the uptake of the peptides into the human cells is not crucial. But for the two peptides to bind to the intracellular targets Mpro and PLpro, their uptake into the human cells is a prerequisite. On the other hand, the eight trifunctional peptides in this study were predictably not inhibitors of all five cytochrome P450 (CYP) isozymes (CYP1A2, CYP2C19, CYP2C9, CYP2D6 and CYP3A4) (Table 3). The five CYP isozymes play key roles in Phase I biotransformation and their inhibition by a drug may lead to bioaccumulation and subsequent toxicity (Sychev et al. 2018). Thus, this observation may further support the non-toxicity of the eight trifunctional peptides as predicted by ToxinPred (Table 2). Concerning druglikeness and leadlikeness, the eight trifunctional peptides are not favorable. However, as emphasized by Santos et al. (2016), many orally bioavailable peptide drugs, marketed and in clinical trials, do not conform to the existing criteria for oral bioavailability that is applicable to small molecule drugs. For example, tifuvirtide and enfuvirtide, two FDA-approved antiviral peptide drugs, fail to comply with the Lipinski’s rule-of-five (Santos et al. 2016). Meanwhile, Mishra and Dey (2019) reported that peptides that violated the Lipinski’s rule-of-five could exhibit drug-like properties, including penetration of cellular membranes, in experimental studies in vitro. Udenigwe (2014) noted that physical experiments are indispensable for validating the outcomes of in silico discovery of food-derived bioactive peptides. Thus, to sum up, wet-lab experiments should be conducted in the future to verify the bioavailability, toxicity, allergenicity, and druglikeness of the eight potential trifunctional peptides obtained in this study, besides confirming their inhibitory effects on the three SARS-CoV-2 targets Mpro, PLpro and spike glycoproteins.
Conclusion
Overall, from quinoa seed proteins, this study has computationally identified seven non-toxic and likely non-allergenic peptides that could serve as trifunctional inhibitory peptides against SARS-CoV-2. They were predicted to bind to critical binding residues of the spike glycoprotein and critical catalytic residues in the active sites of Mpro and PLpro. Hence, they could potentially block the entry of SARS-CoV-2 into the host cell and suppress viral replication. The seven peptides also have desirable physicochemical properties typical of other known antiviral peptides. Among the seven, VEDKGMMHQQRMMEKAMNIPRMCGTMQRKCRMS that could form the highest number of interactions with key residues in the viral proteins. Thus, this peptide might be the most promising candidate for the future development of peptide-based therapeutics or prophylactics to combat COVID-19.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
FCW and DTK were involved in designing the experiments. TTC, DTK and JHO carried out the acquisition, analysis, and the interpretation of the data. JHO, TTC and FCW prepared the manuscript. All the authors approved the manuscript.
Data Availability
Data of the study are included in the supplementary information files.
Declarations
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aluko RE, Monu E. Functional and bioactive properties of quinoa seed protein hydrolysates. J Food Sci. 2003;68(4):1254–1258. doi: 10.1111/j.1365-2621.2003.tb09635.x. [DOI] [Google Scholar]
- Baig MS, Alagumuthu M, Rajpoot S, Saqib U. Identification of a potential peptide inhibitor of SARS-CoV-2 targeting its entry into the host cells. Drugs R&D. 2020;20(3):161–169. doi: 10.1007/s40268-020-00312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The protein data bank. Nucleic Acids Res. 2000;28(1):235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley SK, Berman HM, Bhikadiya C, Bi C, Chen L, Di Costanzo L, Christie C, Dalenberg K, Duarte JM, Dutta S, Feng Z, Ghosh S, Goodsell DS, Green RK, Guranović V, Guzenko D, Hudson BP, Kalro T, Liang Y, Lowe R, Namkoong H, Peisach E, Periskova I, Prlić A, Randle C, Rose A, Rose P, Sala R, Sekharan M, Shao C, Tan L, Tao Y-P, Valasatava Y, Voigt M, Westbrook J, Woo J, Yang H, Young J, Zhuravleva M, Zardecki C. RCSB protein data bank: biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2018;47(D1):D464–D474. doi: 10.1093/nar/gky1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai T-T, Law Y-C, Wong F-C, Kim S-K. Enzyme-assisted discovery of antioxidant peptides from edible marine invertebrates: a review. Mar Drugs. 2017;15(2):42. doi: 10.3390/md15020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai T-T, Soo Z-Y, Hsu K-C, Li J-C, Abd Manan F, Wong F-C. Antioxidant activity of semen cassiae protein hydrolysate: thermal and gastrointestinal stability, peptide identification, and in silico analysis. Mod Food Sci Technol. 2019;35(9):38–48. [Google Scholar]
- Choudhary S, Malik YS, Tomar S. Identification of SARS-CoV-2 cell entry inhibitors by drug repurposing using in silico structure-based virtual screening approach. Front Immunol. 2020 doi: 10.3389/fimmu.2020.01664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2015;44(D1):D67–D72. doi: 10.1093/nar/gkv1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7(1):42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov I, Bangov I, Flower DR, Doytchinova I. AllerTOP vol 2–a server for in silico prediction of allergens. J Mol Model. 2014;20(6):2278. doi: 10.1007/s00894-014-2278-5. [DOI] [PubMed] [Google Scholar]
- Edwards MR, Strong K, Cameron A, Walton RP, Jackson DJ, Johnston SL. Viral infections in allergy and immunology: How allergic inflammation influences viral infections and illness. J Allergy Clin Immunol. 2017;140(4):909–920. doi: 10.1016/j.jaci.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galante M, De Flaviis R, Boeris V, Spelzini D. Effects of the enzymatic hydrolysis treatment on functional and antioxidant properties of quinoa protein acid-induced gels. LWT. 2020;118:108845. doi: 10.1016/j.lwt.2019.108845. [DOI] [Google Scholar]
- Gupta S, Kapoor P, Chaudhary K, Gautam A, Kumar R, Open Source Drug Discovery C. Raghava GPS. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE. 2013;8(9):e73957. doi: 10.1371/journal.pone.0073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggag YA, Donia AA, Osman MA, El-Gizawy SA. Peptides as drug candidates: limitations and recent development perspectives. Biomed J Sci Tech Res. 2018;8(4):6659–6662. [Google Scholar]
- Henderson JA, Verma N, Harris RC, Liu R, Shen J. Assessment of proton-coupled conformational dynamics of SARS and MERS coronavirus papain-like proteases: implication for designing broad-spectrum antiviral inhibitors. J Chem Phys. 2020;153(11):115101. doi: 10.1063/5.0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra AA, Bartlett GJ, Hegedüs Z, Dutt S, Hobor F, Horner KA, Hetherington K, Spence K, Nelson A, Edwards TA, Woolfson DN, Sessions RB, Wilson AJ. Predicting and experimentally validating hot-spot residues at protein-protein interfaces. ACS Chem Biol. 2019;14(10):2252–2263. doi: 10.1021/acschembio.9b00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Xu M, Udenigwe CC, Agyei D (2020) Physicochemical characterisation, molecular docking, and drug-likeness evaluation of hypotensive peptides encrypted in flaxseed proteome. Curr Res Food Sci 3:41–50. 10.1016/j.crfs.2020.03.001 [DOI] [PMC free article] [PubMed]
- Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, Zhang B, Li X, Zhang L, Peng C, Duan Y, Yu J, Wang L, Yang K, Liu F, Jiang R, Yang X, You T, Liu X, Yang X, Bai F, Liu H, Liu X, Guddat LW, Xu W, Xiao G, Qin C, Shi Z, Jiang H, Rao Z, Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582(7811):289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- Kandeel M, Abdelrahman AHM, Oh-Hashi K, Ibrahim A, Venugopala KN, Morsy MA, Ibrahim MAA. Repurposing of FDA-approved antivirals, antibiotics, anthelmintics, antioxidants, and cell protectives against SARS-CoV-2 papain-like protease. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1784291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model. 2011;51(10):2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- Li J, Ma X, Guo S, Hou C, Shi L, Zhang H, Zheng B, Liao C, Yang L, Ye L, He X. A hydrophobic-interaction-based mechanism triggers docking between the SARS-CoV-2 spike and angiotensin-converting enzyme 2. Glob Chall. 2020;4(12):2000067. doi: 10.1002/gch2.202000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Su K, Zhang X. Potential of plant proteins digested in silico by gastrointestinal enzymes as nutritional supplement for COVID-19 patients. Plant Foods Hum Nutr. 2020 doi: 10.1007/s11130-020-00850-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendran ASK, Lim YS, Fang C-M, Loh H-S, Le CF. The potential of antiviral peptides as COVID-19 therapeutics. Front Pharmacol. 2020 doi: 10.3389/fphar.2020.575444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkiewicz P, Iwaniak A, Darewicz M. BIOPEP-UWM database of bioactive peptides: current opportunities. Int J Mol Sci. 2019;20(23):5978. doi: 10.3390/ijms20235978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Dey S. Molecular docking studies of a cyclic octapeptide-cyclosaplin from sandalwood. Biomolecules. 2019;9(11):740. doi: 10.3390/biom9110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder K, Lima LA, Miranda V, Dias S, Franco O. Current scenario of peptide-based drugs: the key roles of cationic antitumor and antiviral peptides. Front Microbiol. 2013 doi: 10.3389/fmicb.2013.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nongonierma AB, Le Maux S, Dubrulle C, Barre C, FitzGerald RJ. Quinoa (Chenopodium quinoa Willd.) protein hydrolysates with in vitro dipeptidyl peptidase IV (DPP-IV) inhibitory and antioxidant properties. J Cereal Sci. 2015;65:112–118. doi: 10.1016/j.jcs.2015.07.004. [DOI] [Google Scholar]
- National Center for Biotechnology Information (2021) PubChem compound summary for CID 16130644, Tifuvirtide. https://pubchem.ncbi.nlm.nih.gov/compound/Tifuvirtide. Accessed 22 March 2021
- National Center for Biotechnology Information (2021) PubChem compound summary for CID 24847866, Enfuvirtide. https://pubchem.ncbi.nlm.nih.gov/compound/Enfuvirtide. Accessed 22 March 2021
- Ortega JT, Serrano ML, Jastrzebska B. Class A G protein-coupled receptor antagonist famotidine as a therapeutic alternative against SARS-CoV2: an in silico analysis. Biomolecules. 2020;10(6):954. doi: 10.3390/biom10060954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachetti M, Marini B, Benedetti F, Giudici F, Mauro E, Storici P, Masciovecchio C, Angeletti S, Ciccozzi M, Gallo RC, Zella D, Ippodrino R. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J Transl Med. 2020;18(1):179. doi: 10.1186/s12967-020-02344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Guzmán CA, Ruiz-Pernía JJ, Tuñón I. Unraveling the SARS-CoV-2 main protease mechanism using multiscale methods. ACS Catal. 2020;10(21):12544–12554. doi: 10.1021/acscatal.0c03420. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhu Y, Shi Z, Li J. Detection of lunasin in quinoa (Chenopodium quinoa Willd.) and the in vitro evaluation of its antioxidant and anti-inflammatory activities. J Sci Food Agric. 2017;97(12):4110–4116. doi: 10.1002/jsfa.8278. [DOI] [PubMed] [Google Scholar]
- Rut W, Lv Z, Zmudzinski M, Patchett S, Nayak D, Snipas SJ, El Oualid F, Huang TT, Bekes M, Drag M, Olsen SK. Activity profiling and structures of inhibitor-bound SARS-CoV-2-PLpro protease provides a framework for anti-COVID-19 drug design. BioRxiv. 2020 doi: 10.1101/2020.04.29.068890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos GB, Ganesan A, Emery FS. Oral administration of peptide-based drugs: beyond Lipinski's rule. ChemMedChem. 2016;11(20):2245–2251. doi: 10.1002/cmdc.201600288. [DOI] [PubMed] [Google Scholar]
- Skalickova S, Heger Z, Krejcova L, Pekarik V, Bastl K, Janda J, Kostolansky F, Vareckova E, Zitka O, Adam V, Kizek R. Perspective of use of antiviral peptides against influenza virus. Viruses. 2015;7(10):5428–5442. doi: 10.3390/v7102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane SK, Arya SS. Plant seed proteins: chemistry, technology and applications. Curr Res Nutr Food Sci. 2018;6(2):461–469. doi: 10.12944/CRNFSJ.6.2.20. [DOI] [Google Scholar]
- Sychev DA, Ashraf GM, Svistunov AA, Maksimov ML, Tarasov VV, Chubarev VN, Otdelenov VA, NjP D, Barreto GE, Aliev G. The cytochrome P450 isoenzyme and some new opportunities for the prediction of negative drug interaction in vivo. Drug Des Devel Ther. 2018;12:1147–1156. doi: 10.2147/DDDT.S149069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenigwe CC. Bioinformatics approaches, prospects and challenges of food bioactive peptide research. Trends Food Sci Technol. 2014;36(2):137–143. doi: 10.1016/j.tifs.2014.02.004. [DOI] [Google Scholar]
- VanPatten S, He M, Altiti A, Cheng KF, Ghanem MH, Al-Abed Y. Evidence supporting the use of peptides and peptidomimetics as potential SARS-CoV-2 (COVID-19) therapeutics. Future Med Chem. 2020;12(18):1647–1656. doi: 10.4155/fmc-2020-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace AC, Laskowski RA, Thornton JM. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng Des Sel. 1995;8(2):127–134. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Lu G, Qiao C, Hu Y, Yuen K-Y, Wang Q, Zhou H, Yan J, Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894–904. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodawer A, Dauter Z, Shabalin IG, Gilski M, Brzezinski D, Kowiel M, Minor W, Rupp B, Jaskolski M. Ligand-centered assessment of SARS-CoV-2 drug target models in the protein data bank. FEBS J. 2020 doi: 10.1111/febs.15366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F-C, Xiao J, Wang S, Ee K-Y, Chai T-T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci Technol. 2020;99:44–57. doi: 10.1016/j.tifs.2020.02.012. [DOI] [Google Scholar]
- Wong F-C, Ong J-H, Chai T-T. Identification of putative cell-entry-inhibitory peptides against SARS-CoV-2 from edible insects: an in silico study. eFood. 2020;1(5):357–368. doi: 10.2991/efood.k.200918.002. [DOI] [Google Scholar]
- Wong F-C, Ong J-H, Chai T-T. SARS-CoV-2 spike protein-, main protease- and papain-like-protease-targeting peptides from seed proteins following gastrointestinal digestion: an in silico study. Phytomed Plus. 2021;1(1):100016. doi: 10.1016/j.phyplu.2020.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CW, Ibarra AA, Bartlett GJ, Wilson AJ, Woolfson DN, Sessions RB. BAlaS: fast, interactive and accessible computational alanine-scanning using BudeAlaScan. Bioinformatics. 2020;36(9):2917–2919. doi: 10.1093/bioinformatics/btaa026. [DOI] [PubMed] [Google Scholar]
- Yi C, Sun X, Ye J, Ding L, Liu M, Yang Z, Lu X, Zhang Y, Ma L, Gu W, Qu A, Xu J, Shi Z, Ling Z, Sun B. Key residues of the receptor binding motif in the spike protein of SARS-CoV-2 that interact with ACE2 and neutralizing antibodies. Cell Mol Immunol. 2020;17(6):621–630. doi: 10.1038/s41423-020-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lin D, Sun X, Curth U, Drosten C, Sauerhering L, Becker S, Rox K, Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368(6489):409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Zhou J, Zhang K, Chu H, Liu D, Poon VKM, Chan CCS, Leung HC, Fai N, Lin YP, Zhang AJX, Jin DY, Yuen KY, Zheng BJ. A novel peptide with potent and broad-spectrum antiviral activities against multiple respiratory viruses. Sci Rep. 2016 doi: 10.1038/srep22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Jin B, Li H, Huang SY. HPEPDOCK: a web server for blind peptide-protein docking based on a hierarchical algorithm. Nucleic Acids Res. 2018;46(W1):W443–w450. doi: 10.1093/nar/gky357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6(1):14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Lian X, Su X, Wu W, Marraro GA, Zeng Y. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res. 2020;21(1):224. doi: 10.1186/s12931-020-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data of the study are included in the supplementary information files.