Abstract
Background:
The primary cause of traumatic injury in older adults is fall. Recent reports suggest that cognitive function contributes significantly to fall risk. Therefore, by targeting cognitive function for intervention, we could potentially reduce the incidence of fall and injury.
Primary Objective:
To explore the effectiveness of a 16-week cognitive training (CT) intervention to reduce risk and incidence of fall in community-dwelling older adults at risk for fall.
Outcomes:
Primary outcome is number of falls over a 16-week period (ascertained by fall calendar method). Secondary outcomes include: change fall risk as indicated by improvement in 10M walk and 90-second balance tests.
Design/Methods:
The design is a two-group, randomized controlled trial. Eligible participants are older adults (aged 65–85) residing in the community who are at risk for fall based on physical performance testing. Following completion of 1-week run-in phase, participants are randomly allocated (1:2) to either a group that is assigned to attention control or to the group that receives CT intervention for a total of 16 weeks. Participants are followed for an additional 4 weeks post-intervention. Mann-Whitney U and Student’s t-tests will be used to examine between group differences using intention-to-treat analyses.
Discussion:
Limited evidence supports the potential of CT to improve cognition and gait, but no published study has evaluated whether such an intervention would reduce incidence of fall. The present trial is designed to provide initial answers to this question. CT may also improve functioning important in other activities (e.g. driving), reducing overall risk of injury in elders.
Keywords: falls, injury, aged, balance, gait, executive function
INTRODUCTION
The primary cause of traumatic injury in older adults is fall. It is estimated that 28–40% of community dwelling older adults experience a fall at least once a year, and of those who fall, up to 10% experience a significant injury such as fracture or traumatic brain injury.1 In the United States in 2017, more than 2.97 million nonfatal fall injuries among older adults were treated in emergency departments and more than 873,000 of these patients were hospitalized.2 Similarly, in the European Union, more than 2.3 million older adults sought treatment in the ED annually between 2010–12 and more than 1.4 million of these patients required hospitalization.3 The impact of injury is substantive as older trauma patients are more likely to experience a longer hospital stay4, increased number of complications4,5, higher costs of care6,7 and a higher mortality rate6–8 for any specific injury than their younger counterparts. Studies examining post-fall outcomes in older adults have noted increased use of health care resources9, reduced function and higher rates of disability9–12 and increased risk of nursing home placement.13 Even when no injury occurs, older adults may develop a fear of falling which results in self-imposed activity restriction. This activity restriction often leads to reduced mobility and loss of muscle mass, which, in turn, increases the actual risk of falling.14,15
There are a number of ongoing initiatives aimed at reducing falls in older adults. These programs have focused primarily on physical activity and balance, reducing fear of falling, and home safety.29–31 Although exercise and balance programs have been shown to be effective in reducing fall risk16, initiating or maintaining behavior change related to exercise is known to be difficult. According to statistics from the Older Americans 2016: Key Indicators of Well-Being report, only 12% of older adults meet recommended amounts of regular physical activity.17 Globally, studies report a wide range in the percentage of older adults meeting published guidelines for physical activity (2–83%), with the majority falling in the range of 20–60%.18 Therefore, it would be beneficial to identify and validate new interventions that can be added to our current armamentarium to reduce falls in older adults. One such possible intervention is cognitive training (CT), also called “brain training”.
There is a growing interest among older adults in regards to CT for maintenance or improvement of cognitive function. It has been speculated that performing CT might reduce risk of fall in older adults as higher-level cognitive functions, particularly those involving the frontal lobe, have been implicated as required to safely navigate one’s environment19 and avoid injury,20–22 because of shared neural networks. The CT exercises that may be of most benefit for fall prevention include those that target attention, dual task performance, processing speed and reaction time and executive function (e.g., planning).23,24 A number of small studies have examined the effect of CT on gait parameters in older adults with mixed results regarding efficacy and maintenance.16,25–30 This is likely due to sample populations chosen for intervention (e.g., healthy31, sedentary28 chronic stroke32, fall history30), underpowering of studies30, targeting a single task for intervention27,32 and/or lack of follow up for retention.33 However, significant improvement in gait speed (a predictor of fall risk) has been demonstrated following intervention on both normal walking and walking while performing a cognitive task compared to baseline following a 4-week trial of computerized multi-task CT in sedentary older adults (n=10).28 We are undertaking the present study in order to provide initial data on efficacy of CT to reduce falls and to better understand possible mechanisms by which it may be acting in older adults at risk for fall.
METHODS
Objectives
The primary study objective is to explore the effectiveness of a 16-week cognitive training (CT) intervention to reduce risk and incidence of fall in community-dwelling older adults at risk for fall. Additional objectives of the study are to explore the effectiveness of a CT intervention on cognition and functional outcomes and to examine the feasibility and acceptability of the study protocol and intervention in this population.
Outcomes
Primary Outcome.
At time of allocation, participants will be provided with a Monthly Fall Calendar and instructed in its use. At the end of each day participants are asked to place one of 3 letters in the box for the day: “N” for no fall; “F” for fall; and “I” for fall resulting in an injury), considered the “gold-standard” for falls assessment (See Table 1).34–37 For purposes of this study, a fall was defined as an “unintentional For individuals who experience multiple falls in a single day, they are asked to denote the number (ex. “F-2”). Fall calendars will be collected monthly (See Table 2), and a new calendar provided to the participant during the study period. The time period for primary outcome assessment is falls over the period of 4 months. For any fall resulting in injury, we will request permission from the participant to obtain medical records for any treatment sought at time of injury.
Table 1.
Summary of Outcome Measures and Source of Measurement
| Measure | Source (Instrument/Test[s]) |
|---|---|
| PRIMARY OUTCOME | |
| Falls and Injurious Falls | Self-Report (Prospective Fall Calendar) |
| SECONDARY OUTCOMES | |
| Fall Risk | 10M Walk Test |
| 90 Second Balance Test | |
| Cognition | CANTAB |
| Speed of Processing & Response Time | Simple Response Time |
| Attention | Rapid Visual Information Processing |
| Working Memory | Verbal Recognition Memory |
| Task Shifting | Spatial Working Memory |
| Planning and Decision Making | Intra-Extra Dimensional Set Shift |
| Cambridge Gambling Task | |
| One Touch Stockings of Cambridge | |
| Stop Signal Test | |
| Quantitative Gait and Turning Measures | APDM Inertial Sensors |
| Gait speed (m/s) | Walking trials under testing conditions of: |
| cadence (steps/min) | Usual Pace 4-Meter walking laps × 4 |
| stride length (m) | Usual Pace 7-Meter walking laps × 2 (if space allows) |
| turn duration (s) | Fast Pace 4 or 7-Meter walking laps × 2 (space dependent) |
| turn peak velocity (m/s) | |
| Postural Balance Measures: | APDM Inertial Sensors (ISway): |
| (medial heel-to-medial heel distance = 10 cm) | (Inertial sensor at 5th lumbar level) |
| ISway JERK (m2/s5) | Static Standing 30-second trials under six conditions: |
| ISway PATH (m2/s2) | normal base-firm, eye open and eyes closed; narrow base-firm, eye open and eyes closed; normal base, foam surface, eye open and eyes closed |
| ISway Mean velocity (MV; m/s) | |
| ISway Root mean squared (RMS; m/s2) | |
| Disability | Gill Disability Scale |
Table 2. Study Protocol.
(*denotes data are collected, but is secondary outcome)
| Study period | ||||||||
|---|---|---|---|---|---|---|---|---|
| Enrollment | Allocation | Post-allocation | Exit | |||||
| Time point | −T2 | −T1 | T0 | T1 (4 wks) | T2 (8 wks) | T3 (12 wks) | T4 (16 wks) | T5 (20 wks) |
| Eligibility screening | ||||||||
| Phone screening | X | |||||||
| In-person screening | ||||||||
| 10M walk | X | |||||||
| 90 sec balance | X | |||||||
| Informed consent | X | |||||||
| Run-In Period | ||||||||
| Vision screen | X | |||||||
| Medication review | X | |||||||
| Home safety check | X | |||||||
| Allocation | X | |||||||
| Intervention | ||||||||
| Active Control | XXXXXXX | XXXXXXX | XXXXXXX | XXXXXXX | ||||
| Web-based Cognitive Training | XXXXXXX | XXXXXXX | XXXXXXX | XXXXXXX | ||||
| Assessments | ||||||||
| Baseline | ||||||||
| Demographics | X | |||||||
| Pre-existing conditions | X | |||||||
| Medications | X | |||||||
| Social Support | X | |||||||
| Outcome Variables | ||||||||
| Primary | ||||||||
| Falls/Injurious Falls | X | X | X | X | * | |||
| Secondary | ||||||||
| Fall Risk | ||||||||
| 10M walk | X | X | X | X | ||||
| 90 sec balance | X | X | X | X | ||||
| Cognition | ||||||||
| CANTAB | X | X | X | X | ||||
| Gait/Turning | ||||||||
| APDM | X | X | X | X | ||||
| Postural Sway | ||||||||
| APDM | X | X | X | X | ||||
| Disability | ||||||||
| Gill Disability | X | X | X | X | ||||
| Other Variables | ||||||||
| Short Physical | X | X | X | |||||
| Performance | X | |||||||
| Battery | ||||||||
| Timed Up and Go | X | X | X | X X |
||||
| Exit Interview | ||||||||
Secondary Outcomes.
Of interest as a secondary outcome in this study is reduction in risk of fall at the end of the 16-week intervention (See Tables 1 and 2). This is defined by 1) an increase in the 10M walking speed from baseline38,39 or 2) an increase in the Balance test score from baseline.40 As secondary endpoints, we will measure cognitive outcomes (using the Cambridge Neurological Assessment Battery [CANTAB])41–45, additional gait and postural sway measures (using body-worn inertial sensor system, the APDM Mobility Lab, Portland, OR; See Tables 1 and 2)46–49, and disability (Gill Disability Scale50,51). We will explore if there is retention of benefit of the intervention on fall risk, gait, balance and cognitive outcomes one month following end of the intervention. Feasibility and acceptability of the study protocol and CT intervention will also be evaluated.
Study Design
The design is a two-group randomized controlled trial following a 1-week run-in procedure comparing 16-weeks of a web-based CT intervention to attention control (See Figure 1 and Table 2).
Figure 1. Study flow diagram.
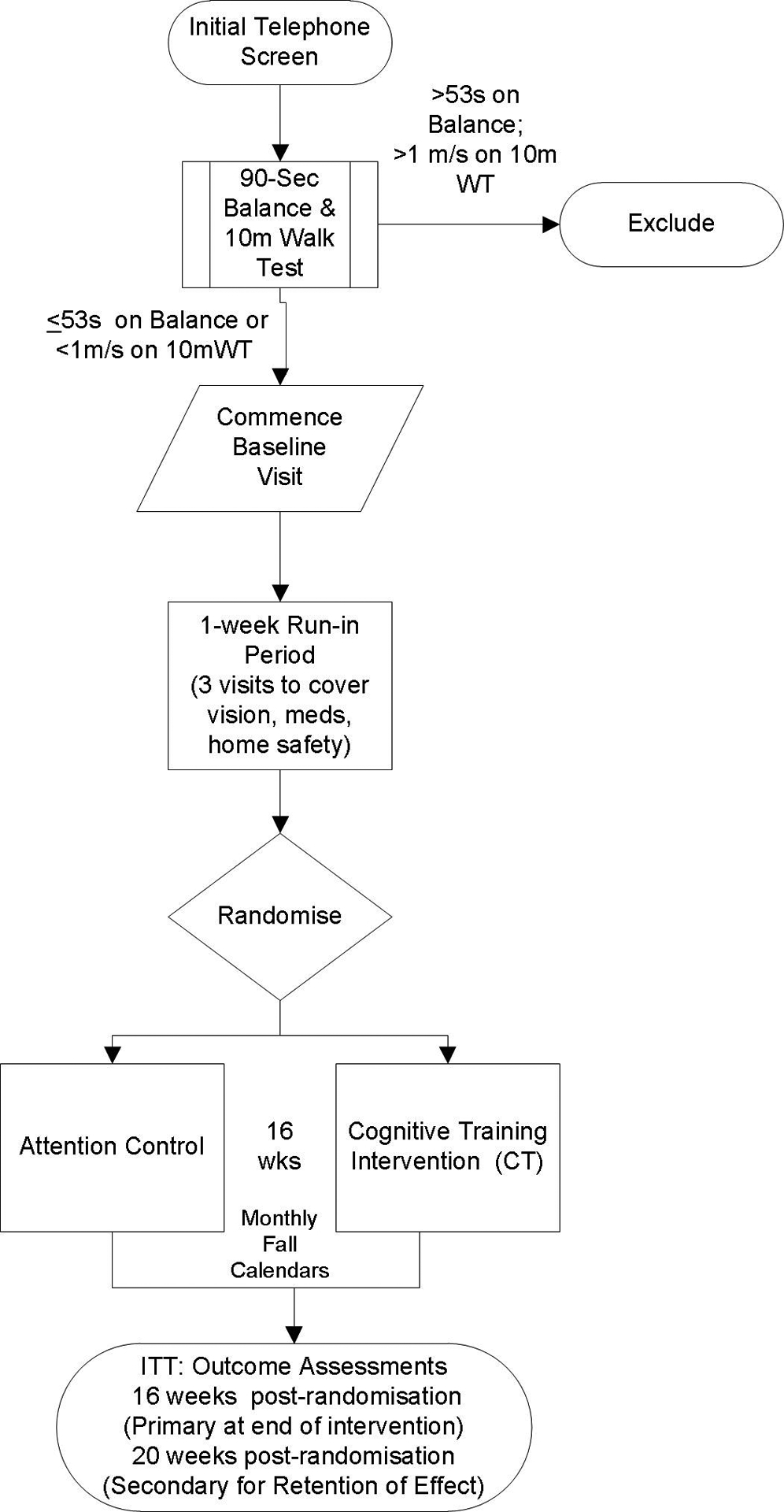
ITT, intention to treat; WT, walk test.
Recruitment and Screening (−T2 and −T1)
We plan to collect complete data on community-dwelling older adult participants (65–85 years of age) without a history of dementia at risk for falls (N=60). Study participants are recruited from the greater Seattle area via advertisement. Interested persons are screened for eligibility in a two-step process, first via the phone, then in-person. If interested participants pass initial eligibility criteria via phone (Table 3), they are scheduled for an in-person visit by a trained clinical investigator to assess fall risk with the 10M Walk52 and 90-second Balance tests (semi-tandem, tandem stance and single leg stance; each timed for 30 sec). In-person screening visits are scheduled on a rolling basis due to study staffing and equipment availability to a maximum of four weekly, with a target enrollment of two participants per week. Persons assessed as at risk for fall (10-meter walk cut off of <1 m/s or Balance test of ≤53 sec)40,52 are eligible for inclusion in the study (See Figure 1 for study diagram).
Table 3.
Eligibility Criteria for FACT2 Study
| Phone Screen: Eligibility Criteria | Phone Screen: Exclusion Criteria |
|---|---|
|
|
In-person Screen: Eligibility Criteria
At risk for fall as assessed by physical performance: 10M Walk test (< 1m/sec) OR 90-second balance test (≤53 seconds)
Baseline Visit (-T1)
If eligible for inclusion after the in-person screening, the research staff member discusses the project in detail with the individual to ascertain interest in further participation and to allow for provision of informed consent. Following informed consent, the baseline visit commences. At this visit, participants are asked about a) demographics (age, gender, race/ethnicity, formal education, income level, insurance status); b) pre-existing conditions (Charlson method53); c) current medications (brown bag method). Questionnaires are administered to participants at baseline to assess: 1) social support (MOS Social Support Survey54) and 2) functional status (Gill Disability Scale55,56). Participants undergo an assessment of cognitive abilities using the Cambridge Neuropsychological Test Automated Battery (CANTAB)42,43,45,60 to provide an external method of assessment to that provided by the CT intervention (See Table 1 for specific tests). Lastly, in-home gait and mobility measures are assessed. Gait, mobility and postural sway data are collected using a body-worn inertial sensor system, the APDM Mobility Lab system (APDM Wearable Technologies, Portland, OR). Use of the APDM system involves “instrumenting” the participant with 6 body worn inertial sensors (2 ankle, 2 wrist, 1 waist belt, 1 upper torso) that contain tri-axial accelerometers, gyroscopes, and magnetometers. Participants are asked to wear walking shoes during the assessments. A gait belt and guarding technique61 is used by a trained investigator during all testing for safety. Data are recorded via wireless transmission from the inertial sensors to a laptop computer and processed with Mobility Lab software. For the instrumented walking assessment, participants are first asked to walk continuous laps with approximately 180-degree turns, at their comfortable (or usual) pace. They complete four 4-meter laps and two 7-meter laps (if space allows) at usual pace. Lastly, participants are asked to walk as quickly as possible, but not so quickly that they lose their balance (2 × 4 or 7-meters; see Table 1 for measures). Participants are able to take rest breaks between activities as needed.
For the postural sway assessment, data are collected with APDM ISway during 6 static standing trials: (1) eyes open/normal base/firm surface (medial heel-to-medial heel distance = 10 cm), (2) eyes open/narrow base/firm surface (feet together) (3) eyes closed/normal base/firm surface, and (4) eyes closed/narrow base/firm surface, (5) eyes open/usual base/foam surface, (6) eyes closed/usual base/foam surface. Participants wear walking shoes and are asked to stand still and keep their arms at their sides during testing. During the eyes open standing conditions, participants are instructed to focus on an “x” placed at eye-level on the wall in front of them. Participants do not use an assistive device, but wear a gait belt and are closely guarded and assisted as needed into the starting position. Measures utilized to assess postural sway are detailed in Table 1. In addition to the gait and balance measures, we collected data to obtain instrumented assessment of mobility: a) the Short Physical Performance Battery (SPPB) and b) the Timed Up and Go (TUG).62 The SPPB includes tests of static balance, gait speed, and sit to stand. For the sit to stand trial, participants are instructed to move from a seated position to a standing position five times as quickly, but as safely as possible. Participants undertake two trials, with the fastest of the two trials used as the measure. A sit to stand time of 17 seconds or more has been associated with high risk for fall.40 For the TUG, a time greater than 14 seconds indicates an individual being at risk for fall.63
Run-in and Allocation (T0)
During the 1-week run-in period, a trained member of the research team meets with the study subject three times over the course of a week (approximately 20 minutes per visit) to perform: 1) vision screen; 2) medication review and 3) home fall safety check. This run-in period allows the study staff to provide hazard reduction interventions per CDC recommendations64 prior to randomization to reduce the risk of these issues introducing bias across groups.
Following successful completion of the run-in period, participants are randomly allocated (1:2) to either a group that is the attention control (n=20) or to the group that receives cognitive training (CT) intervention for a total of 16 weeks (n=40). Participants are not to be informed that they were assigned to “attention control” or “cognitive training” conditions; rather, individuals in both groups are told that the aim of the study is to determine whether participation in “computer learning activities” reduces the risk of falls and improves functional ability. Sealed envelopes, prepared in advance, assign the participants to one of the two groups. The randomization schedule is prepared by the consulting statistician using block randomization (blocks of 6) prior to the research team’s initial visit to interested parties. This approach ensures that consent is obtained prior to disclosure of group assignment and persons consent to participate in the study, regardless of the group they are assigned to. The envelope is not opened until completion of the run-in procedure. The principal investigator, co-investigators and outcome assessors are blinded to allocation until the end of the trial.
Intervention
Cognitive training, also known as “brain training”, involves scheduled completion of specific tests of executive function, visuospatial orientation and perceptual speed. The CT is completed using a web-based computer interface (Cognifit, Inc.), with task difficulty tailored to the participant’s abilities. We will use the Cognifit research interface as it allows tailoring of the intervention to specific tasks selected by the research team. This software system has been tested for reliability and validity. The software system has been used in a range of populations for research purposes including older adults in addition to commercial use.60,65–67
The individual completes a 14-task baseline testing session, after which the program identifies individualized, tailored training goals for the intervention tasks. Individualized feedback is provided to the user regarding progress towards goals during each session. Our intervention includes 48 training sessions over 16 weeks (recommended 3 sessions per week; each session lasts approximately 20 minutes and covers 3 different cognitive tasks tailored to individual baseline ability and progress to date). Training sessions can be completed on either PC or Mac platform and use a user identified login/password allowing secure access at the individual’s home or a community setting per user accessibility and preference. The training allows the user to pick up from the last session to promote completion of the intervention. Because of their linkage to fall and injury prevention, we selected the following specific cognitive tasks to target in the intervention:
Reaction Time (the ability to perceive and process a stimulus and respond)
Processing Speed (the ability to fluently perform easy/over-learned tasks)
Awareness (the ability to evaluate one’s own cognitive functioning, realization, perception or knowledge)
Divided Attention (the ability to execute more than one task at a time)
Inhibition (the ability to ignore irrelevant information while performing a task)
Planning (the ability to anticipate and develop the best way to execute a task)
Shifting (the ability to redirect attention from one channel of information to another)
Updating (the ability to respond in a flexible and adaptive manner to keep up with environmental changes)
We track sessions completed on a weekly basis and provide reminders as needed. Discontinuation of intervention is made on participant request.
Attention Control
Participants assigned to the attention control condition are provided with programmatic activities that are designed to control for nonspecific treatment effects (computer use, interaction with study staff). Participants will engage in an equal number of sessions (3 sessions/week for 16 weeks) watching preselected healthy aging-related video content on the computer (e.g. NIHSenior Health videos on talking with your provider, taking medications safely, and making the most of a medical visit, how to exercise safely).Participants will be asked to briefly note any information gained from each video on a personal discussion board provided to them within the content module. We track sessions completed on a weekly basis and provide reminders as needed. Discontinuation of intervention is made on participant request.
8-, 16- and 20-week Assessments
A member of the research team blinded to intervention/control group assignment conducts participant outcome assessments at the 8-, 16- and 20-week time periods (see Table 2). These assessments occur in same setting and under similar conditions to baseline testing (e.g., same shoes are to be worn). Following the 20-week assessment, an exit interview is completed in order to gain insight into the protocol’s acceptability including participant perceptions of the CT intervention. Participant interviews use a semi-structured interview protocol as a guide to explore their experiences, overall perceived benefits, challenges and attitudes towards the study protocol. All interview sessions are digitally recorded.
ANALYSIS
All planned statistical analyses will be performed using intention-to-treat. The primary time point of interest in this analysis is the 16-week post-randomization outcome assessment; however, means, standard deviations and distributions will be used to describe the outcomes of interest at baseline, midpoint of active intervention phase and 4 weeks post completion of intervention (week 20 post-randomization). A value of p<0.05 will be considered statistically significant. Should there be significant differences between groups on baseline demographic variables despite randomization, we will examine the relationship between the variable and the outcome using sensitivity analyses.
The primary outcome of interest in the proposed RCT is reduction number of falls and injurious falls (See Table 1) at the end of the intervention (16 weeks). We are also interested in the retention of effect 1-month post-intervention. Number of falls and injurious falls will be determined from fall calendar data. Between group differences will be assessed using Mann Whitney U test. Of secondary interest as outcome in this study is reduction in risk of fall which is determined by an increase in the 10M walking speed from baseline and an increase in the Balance test score from baseline. Between group differences will be assessed using Student’s t-test. In exploratory analyses, we will examine the change in risk of fall over time using linear mixed models for longitudinal data. As secondary endpoints, we will measure cognitive outcomes (CANTAB), functional outcomes (walking Gill Disability, inertial sensor gait and turn measures, and ISway measures; See Table 1). Outcomes will be compared between groups using either t-tests or Mann-Whitney U tests as appropriate. In exploratory analyses, we will examine the change in outcome measures over time using linear mixed models for longitudinal data.
To better understand feasibility of the study, we will estimate the proportion of older adults found eligible for inclusion who actually agree to participate in such a study. Further, we will compare the characteristics of those who are willing to participate with those who are not. We will also evaluate numbers of study who complete the study protocol and note any differences in participant characteristics in those who drop out. We will also note the numbers of older adults who express interest in the study but are not eligible. To evaluate acceptability of the study procedures and intervention, transcripts of interview sessions will be digitally recorded and transcribed verbatim for descriptive content analysis.68
Sample Size
The primary outcome of interest in the study is reduction in falls. From national and our own past studies, we expect between 10–20% of the sample to experience a fall during a given month. Simply looking at the cross-section of the 60 cases, the power is low (<.45); however, based on monthly intervals using pooled cross-sectional approach we will have more observations. Power will be .68 to .88 if the probability of a fall is .10 and between .84 and .99 if the probability of a fall is .20. The MDC for gait speed in community-dwelling older adults has been reported to be 0.10 seconds.38
Data Monitoring
Because this is a single-site trial that has been classified as minimal risk, the PI, Dr. Thompson, has the responsibility for oversight of participant safety and data quality. The PI has primary responsibility for submitting any and all necessary reports to funder and the University of Washington’s IRB. All data obtained from this study will be used for research purposes only and will comply with Federal HIPAA regulations. Ongoing quality control procedures will be implemented for data collection, storage and processing. All adverse events occurring during the course of the study will be collected, documented and reported to the PI. The occurrence of any adverse event will be assessed weekly at study meetings. Study personnel will review the event forms from the previous week for events that were reported as new. The investigators will follow all adverse events (AEs) until the point of a satisfactory resolution.
The relationship of AEs to study participation will be determined by the PI according to the University of Washington IRB’s Adverse Event Reporting Policy: Not related; Possibly Related to the Research Intervention; Related. Study-related adverse events that result in medical problems, breach or possible breach of confidentiality or privacy or inappropriate access of PHI will be reported within 24 hours per policy. Other events or problems will be reported within 10 business days of becoming aware of the issue per policy. Within 24 hours after a reportable AE, SAE or unanticipated problem has been reported by the participant, it will be graded by the PI, and then will be submitted by the PI to the IRB. The Institutional Official(s) will review the event and discuss the report per university policy (above). After IRB review and acknowledgement, the PI will further review, and the PI will forward a copy of the reportable AE, SAE or unanticipated problem and IRB acknowledgement letter to the funder through the University’s Office of Sponsored Programs.
In accordance with the institution’s policy, an AE is reportable as it meets all of the following criteria: 1) is unexpected; 2) is related and/or possibly related, and 3) is serious and/or suggests that the research places participants or others at a greater risk of physical or psychological harm than was previously known or recognized. Additionally, per the institution policy, all participant deaths, protocol deviations, complaints about the research, and breaches of confidentiality are reportable events. An example of an AE would be new onset wrist pain and tenderness (symptom) related to increased computer use. The steps to be taken for this AE would include: 1) recommending rest of the affected extremity for 24–48 hours; 2) if symptoms did not subside with rest alone, the participant would be withdrawn from the study and referred to their primary care provider for further treatment. Reporting would follow procedures noted above. An example of an SAE would be a fall with injury of a participant during active study procedures (e.g., when undergoing gait analysis with the study team). This would be viewed as a serious event, and would require the study team member to assess the injury and determine if EMS needs to be called to immediately evaluate and manage the participant’s injury or if the participant should be referred to the nearest Emergency Department or their Primary Care Provider for further evaluation. All injuries will be coded for severity using the Abbreviated Injury Scoring System (rates injuries from 1 (minor) to 6 (non-survivable)) and the participant followed until resolution of the injury. The circumstances surrounding such an event would be participant to a root-cause analysis by the PI in collaboration with the study team to assess what study procedures may have contributed to the SAE and what, if anything, needs to be modified in the protocol to prevent a future occurrence. The PI would follow the reporting procedures noted above. An example of an unanticipated problem would be the participant becoming wheelchair dependent during the study period. As this is an exclusion criterion, we would remove the participant from the study. There is no advanced plan for interim assessment for this study.
Ethics Approval and Clinical Trials Registration
This study has been approved by the Human Subjects Division of the University of Washington (IRB # 50219). All data will be managed to maintain participant confidentiality and maintain blinding until unmasking using the REDCap System.69 Following completing of the exit interview, participants will be offered access to whichever intervention they were not randomized to receive free of charge. The trial is registered at clinicaltrials.gov as NCT03190460.
DISCUSSION
This project is focused on implementing and evaluating the feasibility of the CT intervention for reducing falls in at-risk older adults which could prove to be a significant addition to the current fall risk reduction armamentarium. If proven successful, the intervention can provide a powerful, scalable tool for fall prevention in older adults given that Internet usage has more than tripled to 67%, with more than 75% of users 65 years of age and older going online at least daily.70 The proposal integrates novel information technology solutions in support of fall prevention for both intervention and outcome assessment. The quantitative mobility measures are likely to be more sensitive for detecting change as well as providing a better understanding of biomechanical mechanisms of action. Finally, by maintaining or improving cognitive function, the intervention may be applicable to other areas of injury prevention in older adults, such as driving.
Acknowledgements:
The authors would like to acknowledge the contributions of the Harborview Injury Prevention and Research Center for critique of the study design as it was a work in progress. We acknowledge the input of Dr. Evelyn Shatil to prior studies that was helpful to this work. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the Institute of Translational Health Sciences. REDCap is a secure, web-based application designed to support research studies. REDCap at ITHS is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR002319.
Funding:
National Institute of Nursing Research R21NR01554.
Footnotes
Competing Interests: None.
Ethics Approval: Human Subjects Division of the University of Washington.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Hilaire J. Thompson, Biobehavioral Nursing and Health Informatics, School of Nursing, The University of Washington, Core Faculty, Harborview Injury Prevention and Research Center, Seattle, WA USA.
Ellen L. McGough, Department of Rehabilitation Medicine, School of Medicine, The University of Washington, Seattle, WA, USA.
George Demiris, School of Nursing and Perelman School of Medicine, The University of Pennsylvania, Philadelphia, PA, USA.
REFERENCES
- 1.Yamada M, Ichihashi N. Predicting the probability of falls in community-dwelling elderly individuals using the trail-walking test. Environmental health and preventive medicine 2010;15(6):386–91. doi: 10.1007/s12199-010-0154-1 [published Online First: 2011/03/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Control NCfIPa. Web-based Injury Statistics Query and Reporting System (WISQARS) Atlanta, GA: Centers for Disease Control and Prevention; 2019. [Available from: https://webappa/cdc.gov/sasweb/ncipc/nfirates.html accessed 5/12/2019. [Google Scholar]
- 3.Turner S, Kisser R, Rogmans W. Falls among older adults in the EU-28: Key facts from available statistics. In: Promotion EEAfIPaS, ed. Amsterdam, Netherlands: EuroSafe, 2015. [Google Scholar]
- 4.McKevitt EC, Calvert E, Ng A, et al. Geriatric trauma: resource use and patient outcomes. Canadian journal of surgery Journal canadien de chirurgie 2003;46(3):211–5. [published Online First: 2003/06/19] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang DN, Rivara FP, Nathens A, et al. Complication rates among trauma centers. Journal of the American College of Surgeons 2009;209(5):595–602. doi: 10.1016/j.jamcollsurg.2009.08.003 [published Online First: 2009/10/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice D, MacKenzie EJ, Associates. Cost of Injury in the United States: A Report to Congress: Institute for Health & Aging, University of California San Francisco, CA Injury Prevention Center, Johns Hopkins University, Baltimore, MD, 1989. [Google Scholar]
- 7.Weir S, Salkever DS, Rivara FP, et al. One-year treatment costs of trauma care in the USA. Expert review of pharmacoeconomics & outcomes research 2010;10(2):187–97. doi: 10.1586/erp.10.8 [published Online First: 2010/04/14] [DOI] [PubMed] [Google Scholar]
- 8.Perdue PW, Watts DD, Kaufmann CR, et al. Differences in mortality between elderly and younger adult trauma patients: geriatric status increases risk of delayed death. The Journal of trauma 1998;45(4):805–10. [published Online First: 1998/10/23] [DOI] [PubMed] [Google Scholar]
- 9.Kiel DP, O’Sullivan P, Teno JM, et al. Health care utilization and functional status in the aged following a fall. Medical care 1991;29(3):221–8. [published Online First: 1991/03/01] [DOI] [PubMed] [Google Scholar]
- 10.Rubenstein LZ, Josephson KR. Falls and their prevention in elderly people: what does the evidence show? The Medical clinics of North America 2006;90(5):807–24. doi: 10.1016/j.mcna.2006.05.013 [published Online First: 2006/09/12] [DOI] [PubMed] [Google Scholar]
- 11.Stel VS, Smit JH, Pluijm SM, et al. Consequences of falling in older men and women and risk factors for health service use and functional decline. Age and ageing 2004;33(1):58–65. doi: 10.1093/ageing/afh028 [published Online First: 2003/12/30] [DOI] [PubMed] [Google Scholar]
- 12.Tinetti ME, Williams CS. The effect of falls and fall injuries on functioning in community-dwelling older persons. The journals of gerontology Series A, Biological sciences and medical sciences 1998;53(2):M112–9. doi: 10.1093/gerona/53a.2.m112 [published Online First: 1998/04/01] [DOI] [PubMed] [Google Scholar]
- 13.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. The New England journal of medicine 1997;337(18):1279–84. doi: 10.1056/nejm199710303371806 [published Online First: 1997/11/05] [DOI] [PubMed] [Google Scholar]
- 14.Tinetti ME, Richman D, Powell L. Falls efficacy as a measure of fear of falling. Journal of gerontology 1990;45(6):P239–43. [published Online First: 1990/11/01] [DOI] [PubMed] [Google Scholar]
- 15.Vellas BJ, Wayne SJ, Romero LJ, et al. Fear of falling and restriction of mobility in elderly fallers. Age and ageing 1997;26(3):189–93. doi: 10.1093/ageing/26.3.189 [published Online First: 1997/05/01] [DOI] [PubMed] [Google Scholar]
- 16.Silsupadol P, Shumway-Cook A, Lugade V, et al. Effects of single-task versus dual-task training on balance performance in older adults: a double-blind, randomized controlled trial. Archives of physical medicine and rehabilitation 2009;90(3):381–7. doi: 10.1016/j.apmr.2008.09.559 [published Online First: 2009/03/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Statistics FIFoA-R. Older Americals 2016: Key Indicators of Well-Being. Washington, DC, 2016. [Google Scholar]
- 18.Sun F, Norman IJ, While AE. Physical activity in older people: a systematic review. BMC public health 2013;13:449. doi: 10.1186/1471-2458-13-449 [published Online First: 2013/05/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman T, Mirelman A, Giladi N, et al. Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. The journals of gerontology Series A, Biological sciences and medical sciences 2010;65(10):1086–92. doi: 10.1093/gerona/glq077 [published Online First: 2010/05/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anstey KJ, von Sanden C, Luszcz MA. An 8-year prospective study of the relationship between cognitive performance and falling in very old adults. Journal of the American Geriatrics Society 2006;54(8):1169–76. doi: 10.1111/j.1532-5415.2006.00813.x [published Online First: 2006/08/18] [DOI] [PubMed] [Google Scholar]
- 21.Delbaere K, Close JC, Heim J, et al. A multifactorial approach to understanding fall risk in older people. Journal of the American Geriatrics Society 2010;58(9):1679–85. doi: 10.1111/j.1532-5415.2010.03017.x [published Online First: 2010/09/25] [DOI] [PubMed] [Google Scholar]
- 22.Montero-Odasso M, Verghese J, Beauchet O, et al. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. Journal of the American Geriatrics Society 2012;60(11):2127–36. doi: 10.1111/j.1532-5415.2012.04209.x [published Online First: 2012/11/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirelman A, Herman T, Brozgol M, et al. Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PloS one 2012;7(6):e40297. doi: 10.1371/journal.pone.0040297 [published Online First: 2012/07/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segev-Jacubovski O, Herman T, Yogev-Seligmann G, et al. The interplay between gait, falls and cognition: can cognitive therapy reduce fall risk? Expert review of neurotherapeutics 2011;11(7):1057–75. doi: 10.1586/ern.11.69 [published Online First: 2011/07/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiyamizu M, Morioka S, Shomoto K, et al. Effects of dual task balance training on dual task performance in elderly people: a randomized controlled trial. Clinical rehabilitation 2012;26(1):58–67. doi: 10.1177/0269215510394222 [published Online First: 2011/03/23] [DOI] [PubMed] [Google Scholar]
- 26.Mirelman A, Maidan I, Herman T, et al. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease? The journals of gerontology Series A, Biological sciences and medical sciences 2011;66(2):234–40. doi: 10.1093/gerona/glq201 [published Online First: 2010/11/26] [DOI] [PubMed] [Google Scholar]
- 27.Schwenk M, Zieschang T, Oster P, et al. Dual-task performances can be improved in patients with dementia: a randomized controlled trial. Neurology 2010;74(24):1961–8. doi: 10.1212/WNL.0b013e3181e39696 [published Online First: 2010/05/07] [DOI] [PubMed] [Google Scholar]
- 28.Verghese J, Mahoney J, Ambrose AF, et al. Effect of cognitive remediation on gait in sedentary seniors. The journals of gerontology Series A, Biological sciences and medical sciences 2010;65(12):1338–43. doi: 10.1093/gerona/glq127 [published Online First: 2010/07/21] [DOI] [PubMed] [Google Scholar]
- 29.Yogev-Seligmann G, Giladi N, Brozgol M, et al. A training program to improve gait while dual tasking in patients with Parkinson’s disease: a pilot study. Archives of physical medicine and rehabilitation 2012;93(1):176–81. doi: 10.1016/j.apmr.2011.06.005 [published Online First: 2011/08/19] [DOI] [PubMed] [Google Scholar]
- 30.You JH, Shetty A, Jones T, et al. Effects of dual-task cognitive-gait intervention on memory and gait dynamics in older adults with a history of falls: a preliminary investigation. NeuroRehabilitation 2009;24(2):193–8. doi: 10.3233/nre-2009-0468 [published Online First: 2009/04/03] [DOI] [PubMed] [Google Scholar]
- 31.Li KZ, Roudaia E, Lussier M, et al. Benefits of cognitive dual-task training on balance performance in healthy older adults. The journals of gerontology Series A, Biological sciences and medical sciences 2010;65(12):1344–52. doi: 10.1093/gerona/glq151 [published Online First: 2010/09/15] [DOI] [PubMed] [Google Scholar]
- 32.Yang YR, Wang RY, Chen YC, et al. Dual-task exercise improves walking ability in chronic stroke: a randomized controlled trial. Archives of physical medicine and rehabilitation 2007;88(10):1236–40. doi: 10.1016/j.apmr.2007.06.762 [published Online First: 2007/10/03] [DOI] [PubMed] [Google Scholar]
- 33.Yang YR, Lee YY, Cheng SJ, et al. Relationships between gait and dynamic balance in early Parkinson’s disease. Gait & posture 2008;27(4):611–5. doi: 10.1016/j.gaitpost.2007.08.003 [published Online First: 2007/09/25] [DOI] [PubMed] [Google Scholar]
- 34.Buchner DM, Hornbrook MC, Kutner NG, et al. Development of the common data base for the FICSIT trials. Journal of the American Geriatrics Society 1993;41(3):297–308. [published Online First: 1993/03/01] [DOI] [PubMed] [Google Scholar]
- 35.Hannan MT, Gagnon MM, Aneja J, et al. Optimizing the tracking of falls in studies of older participants: comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston Study. American journal of epidemiology 2010;171(9):1031–6. doi: 10.1093/aje/kwq024 [published Online First: 2010/04/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. The New England journal of medicine 1994;331(13):821–7. doi: 10.1056/nejm199409293311301 [published Online First: 1994/09/29] [DOI] [PubMed] [Google Scholar]
- 37.Peel N Validating recall of falls by older people. Accident; analysis and prevention 2000;32(3):371–2. [published Online First: 2000/04/25] [DOI] [PubMed] [Google Scholar]
- 38.Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-Meter Walk Test for measurements of gait speed in healthy, older adults. Journal of geriatric physical therapy (2001) 2013;36(1):24–30. doi: 10.1519/JPT.0b013e318248e20d [published Online First: 2012/03/15] [DOI] [PubMed] [Google Scholar]
- 39.Quach L, Galica AM, Jones RN, et al. The nonlinear relationship between gait speed and falls: the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston Study. Journal of the American Geriatrics Society 2011;59(6):1069–73. doi: 10.1111/j.1532-5415.2011.03408.x [published Online First: 2011/06/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. Journal of the American Geriatrics Society 2009;57(2):251–9. doi: 10.1111/j.1532-5415.2008.02126.x [published Online First: 2009/02/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bird CM, Papadopoulou K, Ricciardelli P, et al. Monitoring cognitive changes: psychometric properties of six cognitive tests. The British journal of clinical psychology 2004;43(Pt 2):197–210. doi: 10.1348/014466504323088051 [published Online First: 2004/06/01] [DOI] [PubMed] [Google Scholar]
- 42.Louis WJ, Mander AG, Dawson M, et al. Use of computerized neuropsychological tests (CANTAB) to assess cognitive effects of antihypertensive drugs in the elderly. Cambridge Neuropsychological Test Automated Battery. Journal of hypertension 1999;17(12 Pt 2):1813–9. [published Online First: 2000/03/07] [DOI] [PubMed] [Google Scholar]
- 43.Robbins TW, James M, Owen AM, et al. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia (Basel, Switzerland) 1994;5(5):266–81. [published Online First: 1994/09/01] [DOI] [PubMed] [Google Scholar]
- 44.Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. Journal of the Royal Society of Medicine 1992;85(7):399–402. [published Online First: 1992/07/01] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson EE, Maylor EA, Rae G, et al. Cognitive function in healthy older European adults: the ZENITH study. European journal of clinical nutrition 2005;59 Suppl 2:S26–30. doi: 10.1038/sj.ejcn.1602294 [published Online First: 2005/10/29] [DOI] [PubMed] [Google Scholar]
- 46.McGough EL, Hsu LY, Thompson HJ, et al. Concurrent validity of postural sway measures in older adults with cognitive impairment. Physical & Occupational Therapy in Geriatrics 2018;36(4):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mancini M, Horak FB. Potential of APDM mobility lab for the monitoring of the progression of Parkinson’s disease. Expert review of medical devices 2016;13(5):455–62. doi: 10.1586/17434440.2016.1153421 [published Online First: 2016/02/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mancini M, King L, Salarian A, et al. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. Journal of bioengineering & biomedical science 2011;Suppl 1:007. doi: 10.4172/2155-9538.s1-007 [published Online First: 2011/12/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mancini M, Salarian A, Carlson-Kuhta P, et al. ISway: a sensitive, valid and reliable measure of postural control. Journal of neuroengineering and rehabilitation 2012;9:59. doi: 10.1186/1743-0003-9-59 [published Online First: 2012/08/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gill TM, Gahbauer EA. Evaluating disability over discrete periods of time. The journals of gerontology Series A, Biological sciences and medical sciences 2008;63(6):588–94. doi: 10.1093/gerona/63.6.588 [published Online First: 2008/06/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. Jama 2004;291(13):1596–602. doi: 10.1001/jama.291.13.1596 [published Online First: 2004/04/08] [DOI] [PubMed] [Google Scholar]
- 52.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society 2005;53(10):1675–80. doi: 10.1111/j.1532-5415.2005.53501.x [published Online First: 2005/09/27] [DOI] [PubMed] [Google Scholar]
- 53.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases 1987;40(5):373–83. [published Online First: 1987/01/01] [DOI] [PubMed] [Google Scholar]
- 54.Sherbourne CD, Stewart AL. The MOS social support survey. Social science & medicine (1982) 1991;32(6):705–14. [published Online First: 1991/01/01] [DOI] [PubMed] [Google Scholar]
- 55.Gill TM, Allore HG, Gahbauer EA, et al. Change in disability after hospitalization or restricted activity in older persons. Jama 2010;304(17):1919–28. doi: 10.1001/jama.2010.1568 [published Online First: 2010/11/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gill TM, Baker DI, Gottschalk M, et al. A program to prevent functional decline in physically frail, elderly persons who live at home. The New England journal of medicine 2002;347(14):1068–74. doi: 10.1056/NEJMoa020423 [published Online First: 2002/10/04] [DOI] [PubMed] [Google Scholar]
- 57.Kuslansky G, Buschke H, Katz M, et al. Screening for Alzheimer’s disease: the memory impairment screen versus the conventional three-word memory test. Journal of the American Geriatrics Society 2002;50(6):1086–91. [published Online First: 2002/07/12] [DOI] [PubMed] [Google Scholar]
- 58.Lipton RB, Katz MJ, Kuslansky G, et al. Screening for dementia by telephone using the memory impairment screen. Journal of the American Geriatrics Society 2003;51(10):1382–90. [published Online First: 2003/09/27] [DOI] [PubMed] [Google Scholar]
- 59.Boot WR, Charness N, Czaja SJ, et al. Computer proficiency questionnaire: assessing low and high computer proficient seniors. The Gerontologist 2015;55(3):404–11. doi: 10.1093/geront/gnt117 [published Online First: 2013/10/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haimov I, Hanuka E, Horowitz Y. Chronic insomnia and cognitive functioning among older adults. Behavioral sleep medicine 2008;6(1):32–54. doi: 10.1080/15402000701796080 [published Online First: 2008/04/17] [DOI] [PubMed] [Google Scholar]
- 61.Schmitz TJ, O’Sullivan SB. Examination of Coordination and Balance. In: O’Sullivan S, Schmitz T, Fulk G, eds. Physical Rehabilitation: Assessment & Treatment. 6th ed. Philadelphia, PA: F.A. Davis; 2014:206–50. [Google Scholar]
- 62.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society 1991;39(2):142–8. [published Online First: 1991/02/01] [DOI] [PubMed] [Google Scholar]
- 63.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Physical therapy 2000;80(9):896–903. [published Online First: 2000/08/29] [PubMed] [Google Scholar]
- 64.Stevens JA, Sogolow ED. Preventing Falls: What Works? A CDC Compendium of Effective Community-Based Interventions from Around the World. Atlanta, GA, 2008. [Google Scholar]
- 65.Korczyn AD, Peretz C, Aharonson V, et al. Computer based cognitive training with CogniFit improved cognitive performance above the effect of classic computer games: prospective, randomized, double blind intervention study in the elderly. . Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2007;3(3):S171. [Google Scholar]
- 66.Thompson HJ, Demiris G, Rue T, et al. A Holistic approach to assess older adults’ wellness using e-health technologies. Telemedicine journal and e-health : the official journal of the American Telemedicine Association 2011;17(10):794–800. doi: 10.1089/tmj.2011.0059 [published Online First: 2011/10/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peretz C, Korczyn AD, Shatil E, et al. Computer-based, personalized cognitive training versus classical computer games: a randomized double-blind prospective trial of cognitive stimulation. Neuroepidemiology 2011;36(2):91–9. doi: 10.1159/000323950 [published Online First: 2011/02/12] [DOI] [PubMed] [Google Scholar]
- 68.Sandelowski M Whatever happened to qualitative description? Research in nursing & health 2000;23(4):334–40. [published Online First: 2000/08/15] [DOI] [PubMed] [Google Scholar]
- 69.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010 [published Online First: 2008/10/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson M, Perrin A. Tech Adoption Climbs Among Older Adults. In: Center PR, ed., 2017. [Google Scholar]


