Abstract
Tissue engineering protocols, such as regenerative endodontic procedures (REPs), comprise biologically based procedures designed to restore normal physiologic function. For REPs, the goal is reconstitution of the pulp-dentin complex by delivering mesenchymal stem cells (MSCs), including the stem cells of the apical papilla (SCAP) into a root canal system. Many patients regain cold sensitivity after REPs, but the mechanism is not understood. We hypothesized that SCAP modulate nociceptive function through a paracrine mechanism that activates cold-sensitive ion channels in neurons. We established a coculture system with human SCAP and rat trigeminal (TG) sensory neurons in order to determine the effect of SCAP co-culture on neuronal responses using whole-cell patch-clamp electrophysiology. TG neurons co-cultured with SCAP demonstrated increased TRPA1-mediated (p < 0.01) and TRPM8-mediated inward current densities (p < 0.01) at 24 h in co-culture. Cold stimulation to SCAP significantly increased ATP release (p < 0.01), and supernatant collected after cold stimulation to SCAP was able to activate cultured TG neurons. Co-culture with SCAP significantly increased sustained ATP-evoked inward current density (p < 0.05). These data suggest that SCAP release trophic factors that act on afferent neurons to enhance cold-sensitive ion channel activity.
Keywords: mesenchymal stem cells, regenerative endodontics, TRPA1, TRPM8, ATP, apical papilla
INTRODUCTION
Tissue engineering procedures are emerging across medicine and dentistry to restore the biological functions of injured tissues. In dentistry, regenerative endodontic procedures (REPs) have become a treatment alternative for immature teeth with open apices diagnosed with pulpal necrosis. These procedures rely heavily on the adequate disinfection of the root canal followed by recruitment of undifferentiated mesenchymal stem cells (MSCs) via evoked bleeding from adjacent apical dental papilla or bone (Lovelace et al., 2011; Chrepa et al., 2015).
Interestingly, positive responses to either cold or electrical stimuli have been reported in approximately half of patients after REPs (Diogenes et al., 2013,2016). In normal healthy teeth, pulpal responses to cold stimuli depend on the inward movement of fluid within dentinal tubules and the subsequent activation of low-threshold A fibers that extend up to 300 μm into dentinal tubules (Narhi et al., 1984; Narhi, 1986; Ahlquist et al., 1994). This hydrodynamic theory of dentinal pain (Brannstrom et al., 1967; Brannstrom and Astrom, 1972; Trowbridge et al., 1980) postulates that the application of either cold or hyperosmotic solutions (Matthews and Vongsavan, 1994) onto the surface of a tooth triggers fluid movement and subsequent neuronal depolarization. However, this theory cannot explain cold sensitivity after REPs as a restorative material blocks neuronal access to coronal dentinal tubules (Diogenes et al., 2016). Thus, there is a gap in knowledge regarding mechanisms of cold transduction after REPs.
It is possible that cold detection may involve the direct activation of thermosensitive ionotropic channels expressed in free nerve ending of sensory neurons. Dental afferent neurons are contained within the trigeminal (TG) nerve and are known to express thermosensitive ion channels such as transient receptor potential ankyrin type 1 (TRPA1) and transient receptor potential melastatin 8 (TRPM8) (Nealen et al., 2003; Story et al., 2003; Kobayashi et al., 2005; Nagata et al., 2005; Kim et al., 2015). These channels are activated by temperatures below 17 °C and 25 °C, respectively and could be involved in the direct transduction of nociceptive signals upon cold stimulus (McKemy et al., 2002; Peier et al., 2002; Story et al., 2003; Kobayashi et al., 2005).
Further, it has been shown that adenosine triphosphate (ATP) can be released from odontoblasts upon cold stimulation (Shibukawa et al., 2015). This is a potentially significant observation since extracellular ATP may serve as a paracrine signaling molecule that activates neurons expressing purinergic receptors. Importantly, TG neurons express purinergic receptors, including the ionotropic channel P2X3 (Cook et al., 1997; Alavi et al., 2001; Renton et al., 2003; Staikopoulos et al., 2007; Kim et al., 2008). Together, the expression of TRPA1 and TRPM8 in TG neurons and the possible temperature-dependent release of ATP from dental pulp cells comprise two alternative hypotheses for cold-detection mechanisms after clinical REPs.
In this study, we evaluated whether a population of human MSCs isolated from the dental apical papilla (stem cells of the apical papilla (SCAP)) increase the activities of TRPA1, TRPM8 and P2X receptors in rat TG neurons. In addition, we determined whether SCAP releases ATP into the extracellular environment after a cold stimulus.
EXPERIMENTAL PROCEDURES
Tooth collection
This study was approved by the IRB of the University of Texas Health Science Center at San Antonio (UTHSCSA). Immature vital mandibular premolar teeth, with at least 2/3 root formation and an open apex, but with an indication for extraction for orthodontic treatment were collected after informed consent. The extracted teeth were immediately placed in ice-cold Hank’s balanced salt solution (HBSS; Sigma Aldrich, St Louis, MO, USA) and used in temperature measurement experiments.
Ex vivo temperature measurement following simulated ‘cold test” in extracted human teeth
A thermocouple probe (0.4 mm in diameter) connected to an Oakton-360 thermometer (Oakton; Vernon Hills, IL, USA) unit was placed through the apical foramen of each tooth (n = 6) until reaching the pulp chamber roof. The positioning of the thermocouple probe was confirmed radiographically. Next, the teeth were placed in a 37 °C circulating water bath to the level of the cementoenamel junction to simulate the heat sinking properties of the periodontium. A cotton pellet (size 2) saturated with EndoIce™ (Hygenic; Akron, OH, USA) was placed directly onto the occlusal surface of each tooth for either 5 or 10 s. The change in the temperature was recorded for three consecutive tests 5 min apart. Next, in paired design, each tooth was accessed with a dental high speed handpiece fitted with a carbide bur (#1557, Brasseler; Savannah, GA, USA), the coronal pulp was removed with a round diamond bur to approximately 2 mm below the CEJ. Then, to simulate the typical restoration placed in REPs, a piece of collatape (Colla Tape®; Zimmer Dental; Carlsbad, CA, USA) was placed over the tissue, followed by a 3 mm of white MTA (Dentsply), a 3-mm layer of Fuji IX™, GC America, Alsip, IL, USA) and a final restoration with a bonded composite resin Z-100™, 3 M, St Paul, MN. The thermocouple was re-positioned in the coronal most aspect of the pulp space touching the pulp-restoration interphase and the temperature measurements repeated as described above.
SCAP
Previously characterized MSCs, namely stem cells of apical papilla (SCAP), were used in all experiments (Ruparel et al., 2013). Cells were cultured and expanded in media composed of alpha-minimum essential medium (Gibco, Grand Island, NY, USA) supplemented with heat-inactivated 10% fetal bovine, 1 l-glutamine (Gibco), penicillin (100U/mL, Gemini, West Sacramento, CA, USA), and streptomycin (100 mg/mL, Gemini) to 10-cm cell culture dishes. Cells were allowed to expand in culture to 70–80% confluency followed by treatment with 0.05% trypsin (Gibco, Carlsbad, CA, USA). Cell suspension concentration was determined by a TC10 automated cell counter (Bio-Rad, Hercules, CA, USA), and cells between the third and eighth passages were used in all experiments in this study.
Rat primary TG neurons and SCAP co-culture
Adult male Sprague-Dawley rats (weight, 200–250 g each) (Charles River, Wilmington, MA, USA) were used in this study. All animal study protocols were approved by the IACUC of UTHSCSA and conformed to the International Association for the Study of Pain (IASP) and Federal guidelines. Animals were housed for 1 week before the experiments with food and water available ad lib. After isoflurane euthanasia, TG ganglia dissected from male rats were treated with collagenase-dispase (1 mg/mL; Roche), and neuronal cultures were prepared as previously described (Diogenes et al., 2006)
In brief, TG neurons were cultured onto 10-mm laminin-coated coverslips (1 × 103 neurons/coverslip) placed in 24 well plates, and allowed to attach for 2 h. Next, cell culture inserts fitted with 1-μm pore membranes (BD Biosciences, Bedford, MA, USA) containing 1 × 105 SCAP were placed in half of the wells containing the neurons. These inserts allowed for the co-culture of these two cell types and the exchange of soluble factors, while preventing their physical contact. The co-cultures were maintained in basal SCAP culture media (described above) at 37 °C and 5% CO2 for 24 h. Nerve growth factor (NGF) was not added to neuron culture media since NGF has been reported to modulate TRPA1 and TRPM8 (Diogenes et al., 2007).
Patch-clamp electrophysiology
Recordings were made in whole-cell patch-clamp (holding potential (Vh) of —60 mV) configuration at 22–24 °C from the somata of small- to medium-sized neurons (1540 pF). Data were acquired and analyzed using an Axopatch 200B amplifier and pCLAMP10.0 software (Axon Instruments; Union City, CA, USA). Recording data were filtered at 0.5–2.5 kHz and sampled at 210 kHz. Borosilicate pipettes with filaments (Sutter, Novato, CA, USA) were polished to resistances of 34 MΩ in whole-cell pipette solution. Access resistance (Rs) was compensated 40–80% when appropriate up to the value of 7–10 MΩ. Data were rejected when Rs changed >20% during recording, leak currents were >50 pA, or input resistance was <300 MΩ. Standard external solution contained (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 d-glucose, and 10 Hepes, pH 7.4. Standard pipette solution contained (in mM): 140 KCl, 1 MgCl2, 1 CaCl2, 10 EGTA, 10 d-glucose, 10 Hepes, pH 7.3. Mustard oil, menthol and ATP were applied to neurons using a fast, pressure-driven and computer-controlled 5-channel system (MicroData Instrument, Plainfield, NJ, USA).
ATP release quantification
SCAP were placed in thin-walled PCR tubes (Fisher Scientific) at the concentration of 5 × 105 cells/0.5 ml in phenol free Hanks Balanced Salt Solution (HBSS; Gibco, Grand Island, NY, USA) at 37 °C. Next, tubes containing cells were placed in a PCR Techne™ 5PrimeG gradient thermocycler (Techne™; Burlington, NJ, USA) and either maintained at 37 °C for 10 min or exposed to five cycles of 37 °C for 1.75 min followed by 15 °C for 0.25 min. Next, the tubes were immediately centrifuged at 2000 rpm for 2 min, and supernatant aliquots were used for ATP quantification by luminescence using the Cell-titer Glo reagent (Promega, Madison, WI, USA). The pelleted cells were resuspended in media and live cell number determined following trypan blue staining. Further, supernatant was collected and applied to cultured TG neurons for evaluation with patch-clamp electrophysiology.
Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from TG neurons cultured in absence or presence of SCAP in a co-culture system for 1 day using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions (three individual experiments in duplicate, a total of n = 6 for each group). Total RNA (0.1 μg) was converted to complementary cDNA using the High Capacity RNA-to-cDNA kit (Thermo Fisher Scientific) and used TaqMan® RT-PCR reactions (Life Technologies, Carlsbad, CA, USA) using the Taqman® gene expression assays for human TRPA1 (assay # Hs00175798_m1), TRPM8 (assay # Hs01066596_m1), P2X3 (assay# Hs01125554_m1) and the endogenous reference glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (assay# Hs02758991_g1) (Life Technologies). The relative expression of DSPP was determined using the comparative delta-delta cycle threshold method (ΔΔCt) using the TG neuron only group as the calibrator.
Statistics
Data were analyzed by t-test (two groups) or one-way ANOVA with Tukey’s post hoc test depending on the experimental design using GraphPad Prism (GraphPad, San Diego, CA, USA). P < 0.05 was considered significant.
RESULTS
Application of a 5-s cold stimulus onto the occlusal surface of immature premolars decreased the intracoronal temperature from 35.8 ± 1.5 °C to 29 ± 0.5 °C (p < 0.01). However, after the simulated REP restorative procedures, the temperature from 35.8 ± 1.5 °C to 25.8 ± 1.1 °C (p < 0.001) and 16 ± 0.5 °C (p < 0.001) when the cold stimulus was applied for 5 and 10 s, respectively (Fig. 1A).
Fig. 1.
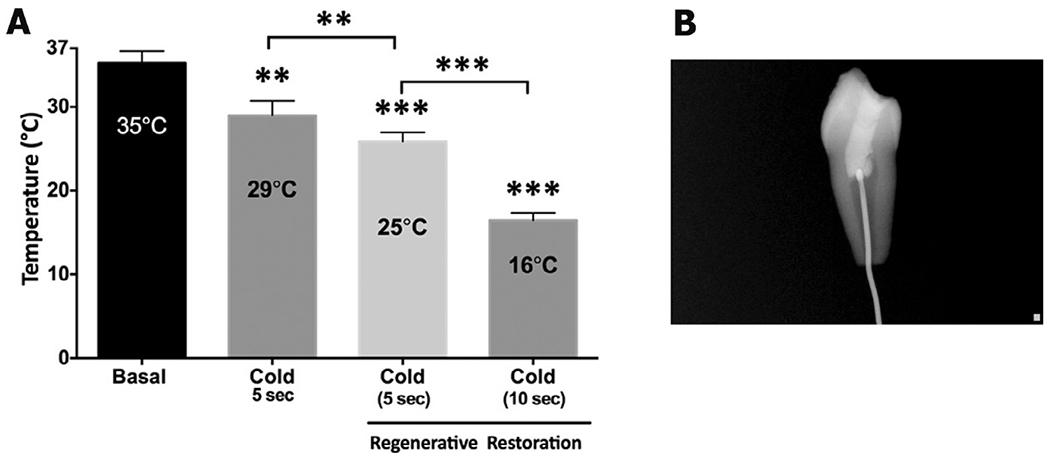
Pulp temperatures after cold stimulation of normal and endodontic-treated teeth. A thermocouple probe was placed through the apical foramen into the pulp chamber of untreated and endodontic-treated teeth. (A) A cotton pellet saturated with EndoIce was applied to occlusal surfaces for 5 or 15 s, and changes in temperature were recorded. (n = 6/group). Data are presented as mean ± std dev of recorded temperature. **p < 0.01 and ***p < 0.001; One-Way ANOVA with Tukey’s post hoc test (B) Placement of the thermocouple probe in the pulp chamber was confirmed radiographically prior to cold stimulation.
Since TRPA1 and TRPM8 are activated by temperatures below 17 °C and 25 °C, respectively (McKemy et al., 2002; Peier et al., 2002; Story et al., 2003), we evaluated the effect of stem cell co-culture on TRPA1-and TRPM8-mediated responses in TG neurons. The TRPA1 agonist mustard oil (25 μM) was applied to TG neurons cultured alone or with SCAP. SCAP coculture significantly increased the magnitude of mustard oil-evoked inward current (Fig. 2A, p < 0.001) and current density in TG neurons (Fig. 2B, p < 0.01, representative traces in Fig. 2C). Similarly, application of the TRPM8 agonist menthol (50 μM) to TG neurons cocultured with SCAP exhibited significantly increased inward currents (Fig. 3A, p < 0.01) and current density (Fig. 3B, p < 0.01), with representative traces illustrated in Fig. 3C. Collectively, this study demonstrates that SCAP releases a soluble factor that significantly increases TRPA1 and TRPM8 responses in TG neurons.
Fig. 2.
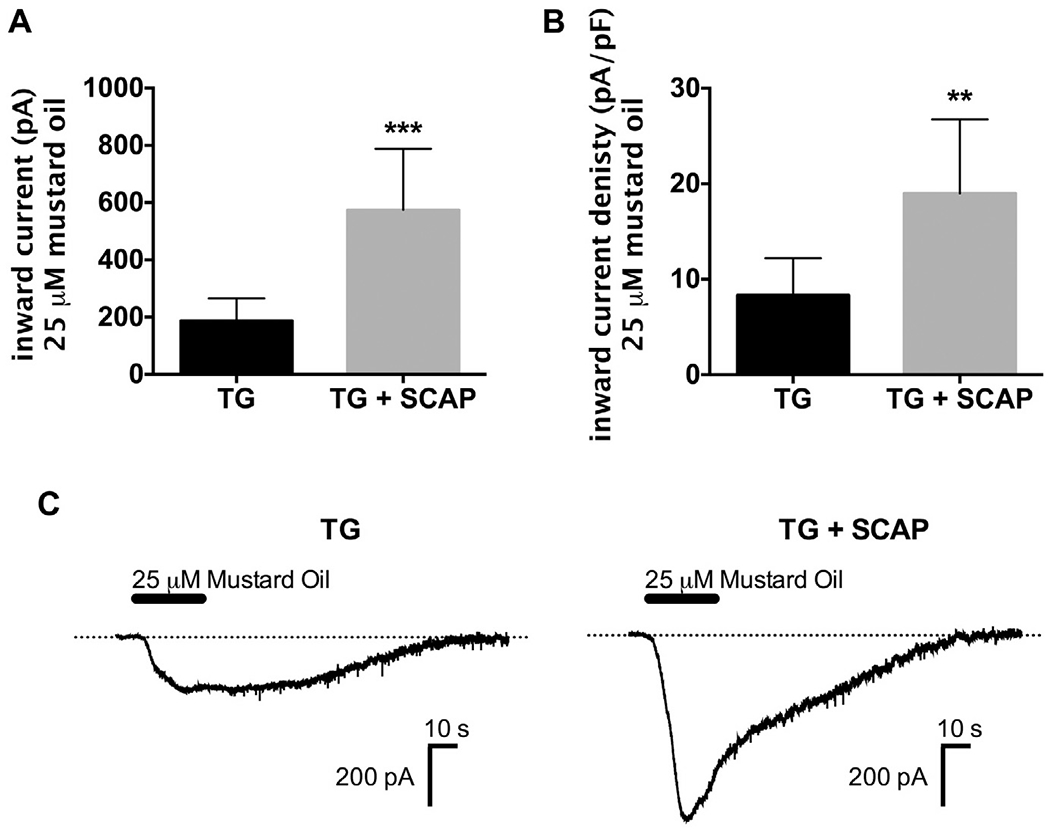
Co-culture with SCAP increased mustard-oil evoked inward currents in TG neurons. (A, B) Mustard oil (25 μM)-evoked inward current (A) and current density (B) were measured from TG neurons cultured for 24 h alone or in co-culture with SCAP (1 × 105 cells) (n = 7–8/group, **p < 0.01, ***p < 0.001). (C) Representative traces of mustard oil-evoked inward currents in TG neurons cultured alone or in co-culture with SCAP. Data were analyzed by unpaired Student’s t-test. Error bars are SEM.
Fig. 3.
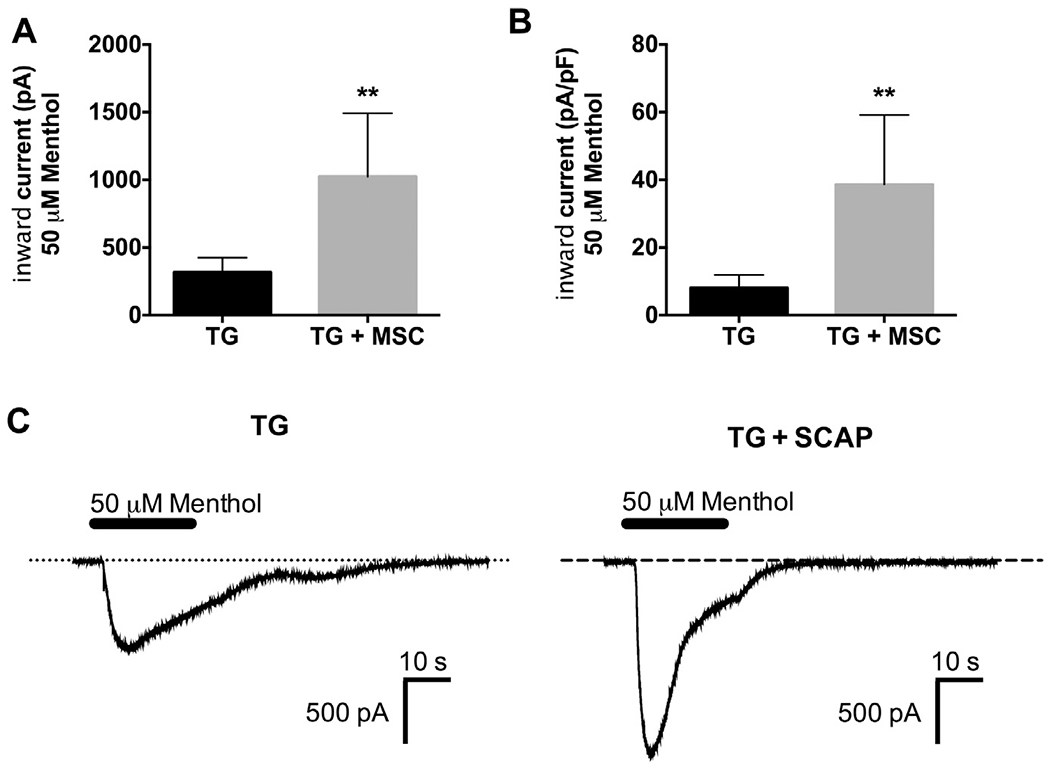
Co-culture with SCAP increased menthol-evoked inward currents in TG neurons. (A, B) Menthol (50 μM)-evoked inward current (A) and current density (B) were measured from TG neurons cultured for 24 h alone or in co-culture with SCAP (1 × 105 cells) (n = 6–8/group, **p < 0.01). (C) Representative traces of menthol-evoked inward currents in TG neurons cultured alone or in co-culture with SCAP. Data were analyzed by unpaired Student’s t-test. Error bars are SEM.
In addition to a direct activation of cold-sensitive TRP channels on neurons, it is possible that cold stimulation triggers the release of ATP from non-neuronal dental pulp cells that could activate nearby free nerve endings. Since SCAP are delivered into the root canal during REPs (Lovelace et al., 2011; Chrepa et al., 2015), we tested this hypothesis by determining whether SCAP release ATP in response to cold. SCAP (5 × 105 cells) were placed in a PCR thermocylcer and either maintained at 37 °C for or exposed to 10 cycles of 37 °C for 1 min followed by 15 °C for 5 s. Tubes were immediately centrifuged and supernatant was collected for ATP quantification. Compared to ATP levels in supernatant from cells held at 37 °C, exposure to the cold stimuli resulted in a significant increase in ATP levels in supernatant (Fig. 4A, p <0.01). Thus, SCAP respond to cold stimulation by releasing ATP. Next, we determined whether soluble factors in the supernatant collected after thermocycling SCAP could activate cultured TG neurons. Supernatant collected from SCAP held at 37 °C was unable to activate cultured TG neurons. However, supernatant collected from cold-stimulated SCAP was able to generate responses in the majority of cultured TG neurons tested (Fig. 4B, C, representative trace in Fig. 4D), suggesting that soluble factors released from cold stimulation to SCAP are able to activate neurons.
Fig. 4.
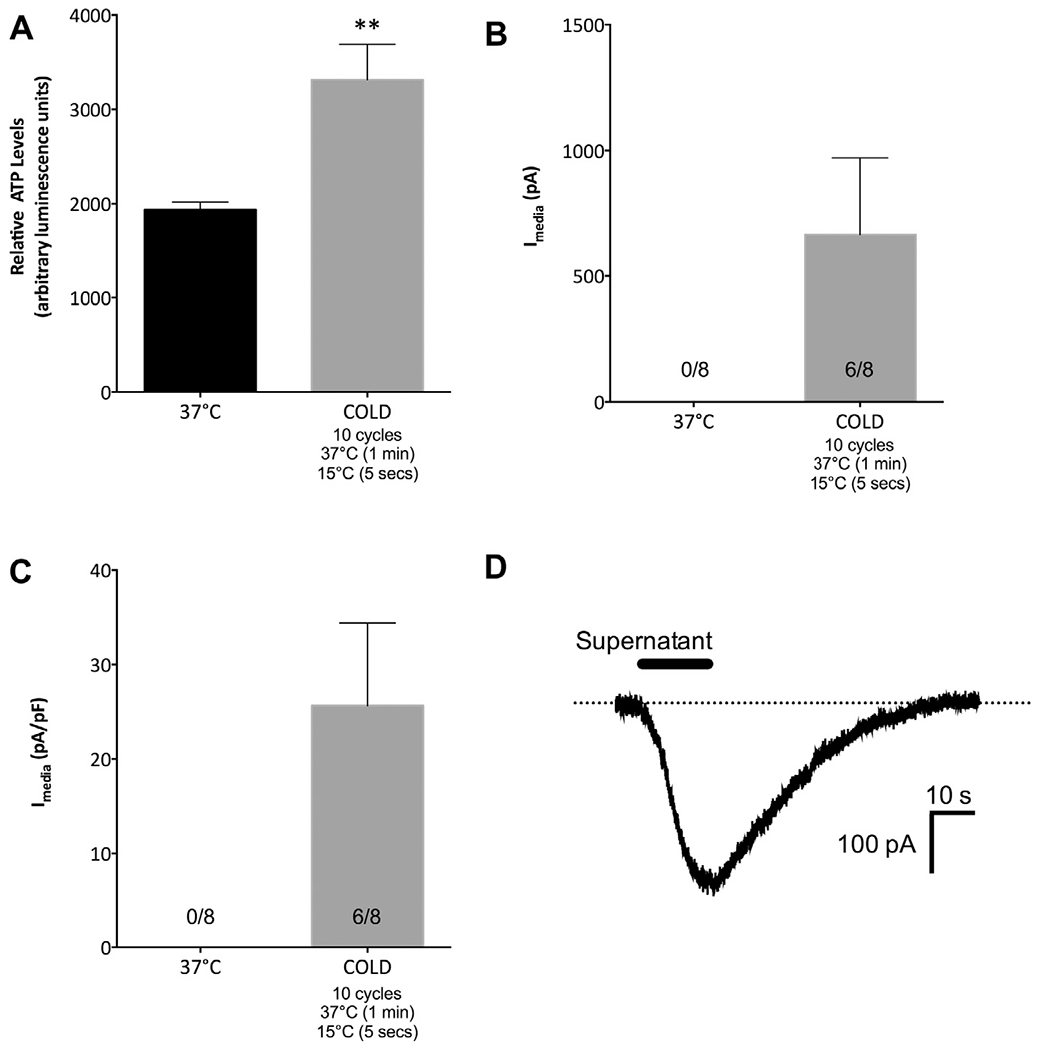
Cold stimulation triggered ATP release from SCAP, and conditioned supernatant activated cultured TG neurons. (A) SCAP (5 × 105 cells) were placed in PCR tubes and maintained at 37 °C for 10 min or exposed to 5 cycles of 37 °C for 1 min and 45 s and 15 °C for 5 s. Tubes were immediately centrifuged at 2000 rpm for 2 min, and supernatant aliquots were used for ATP quantification. Cold stimulation caused an increase in relative ATP levels in conditioned supernatant (n = 7–8/group, **p < 0.01). (B, C) Conditioned supernatant from SCAP maintained at 37 °C was unable to activate acutely (24 h) cultured TG neurons, while conditioned supernatant after cold stimulation to SCAP were able to activate six out of eight neurons tested. (D) Representative trace of inward current generated from conditioned supernatant after cold stimulation to SCAP. Data were analyzed by unpaired Student’s t-test. Error bars are SEM.
Since SCAP release ATP in response to cold, we determined the effect of SCAP co-culture with TG neurons on responses to ATP-mediated inward currents through ionotropic P2X channels. P2X channels expressed in TG ganglia include the P2X3 homotrimer, the P2X2 homotrimer and P2X2/3 heterotrimer (Staikopoulos et al., 2007). We utilized the current kinetics of ATP responses in cultured TG neurons to evaluate changes in transient ATP-evoked inward current density (P2X3 receptors) and changes in sustained ATP-evoked inward current density (P2X2 and/or P2X2/3 receptors). SCAP co-culture with TG neurons had no effect on transient current density in cultured TG neurons (Fig. 5A, representative traces in Fig. 5B), but SCAP co-culture caused a significant increase in ATP-evoked sustained current density (Fig. 5C, p < 0.05, representative traces in Fig. 5D). Thus, co-culture with SCAP resulted in an enhancement of ATP-evoked sustained current in cultured TG neurons, possibly via an enhancement of responses mediated through P2X2 and P2X2/3 receptors.
Fig. 5.
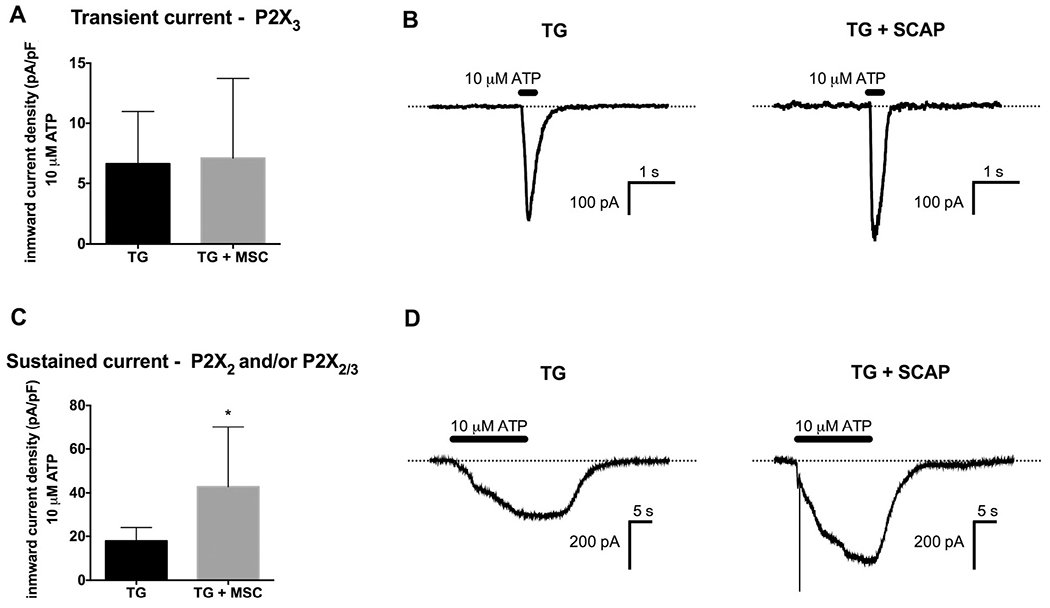
Co-culture with SCAP increased sustained ATP-evoked inward currents in TG neurons. (A) Transient ATP (10 μM)-evoked inward current density was measured from TG neurons cultured for 24 h alone or in co-culture with SCAP (1 × 105 cells) (n = 5–7/group). (B) Representative traces of transient ATP-evoked inward currents in TG neurons cultured alone or in co-culture with SCAP. (C) Sustained ATP (10 μM)-evoked inward current density was measured from TG neurons cultured for 24 h alone or in co-culture with SCAP (1 × 105 cells) (n = 8–10/group, *p < 0.05). (D) Representative traces of sustained ATP-evoked inward currents in TG neurons cultured alone or in co-culture with SCAP.
Lastly, quantitative gene expression analysis was performed to investigate whether the increase in TRPA1, TRPM8 and P2X3 activities were due to upregulation in the expression of these ionotropic channels. Co-culture with SCAP for 1 day did not result in a significant change in the gene expression of TRPA1, TRPM8 and P2X3 transcripts in TG neurons (Fig. 6).
Fig. 6.
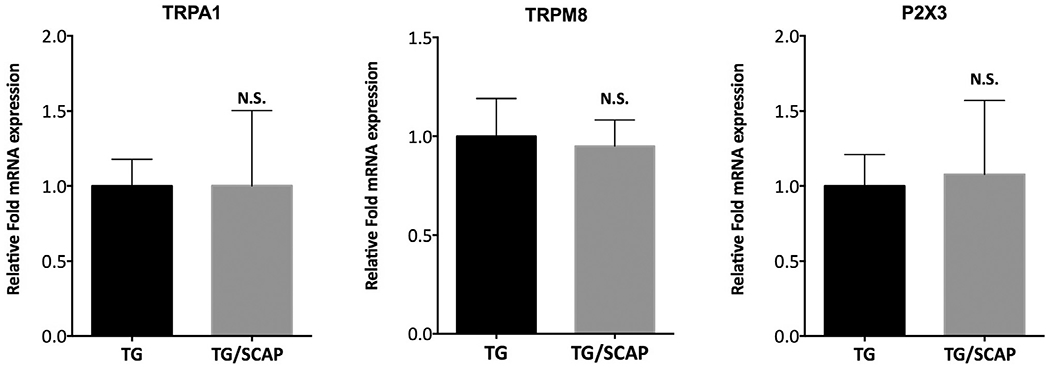
Co-culture with SCAP for 1 day does not increase the gene expression of TRPA1, TRPM8 and P2X3 in trigeminal ganglia (TG) neurons. Data are presented as mean and standard deviation of fold increase in mRNA expression for each target (sample n = 6/group). Data were analyzed by unpaired Studenťs t-test. N.S. = not statistically significant.
DISCUSSION
Relatively few tissue engineering studies have focused on the interaction between stem cells and neurons. This is likely to be a critical area of research as prior studies demonstrate that stem cells such as SCAP have significant effects on promoting axonal sprouting of TG neurons (de Almeida et al., 2014). These studies suggest that stem cell-neuronal interactions are likely to be crucial in regaining afferent and efferent functions of the peripheral nervous system in engineered tissues (de Almeida et al., 2014).
Here, we have focused on stem cell-neuronal interactions in the transduction of cold stimuli. Prior clinical studies report that about 50% of REP treated teeth regain responsiveness to cold stimuli (Diogenes et al., 2013). This is consistent with a recent human histological study reporting the presence of sensory neurons in the newly formed tissues following a REP in a child (Meschi et al., 2016). These responses cannot be explained by the classic hydrodynamic theory since restorations placed after REP block re-innervation of coronal dentinal tubules. Thus, the mechanism of cold detection after REPs is unknown.
We tested two alternative hypotheses for cold transduction in re-engineered dental pulp. First, it is possible that cold directly activates neuronal TRP channels. In support of this possibility, an ex vivo model system demonstrated that a clinical cold test applied to teeth restored after a REP reached intracoronal temperatures sufficient to activate TRPM8 (Peier et al., 2002) and possibly TRPA1. TRPM8 is expressed in free nerve endings in human dental pulp and in small-sized TG neurons (Tominaga and Caterina, 2004; Alvarado et al., 2007). TRPA1 is expressed in a subpopulation of TG neurons and is up-regulated by pro-inflammatory mediators such as NGF (Diogenes et al., 2007). Therefore, activation of free nerve-endings containing the TRPM8 and TRPA1 ion channels could mediate cold detection after REPs. In addition to reaching activation temperatures in a restored tooth, it is possible that the SCAP regulate TRP channels in TG neurons. This possibility was confirmed by co-culture of SCAP with TG neurons followed by patch-clamp analysis of IMO and IMenthol (Figs. 2 and 3). The mechanism for this SCAP-mediated increase in both TRPA1 and TRPM8 activities is not fully understood, and requires further investigation. However, it does not appear to be through the transcriptional increase in the expression of these channels (Fig. 6). Thus, SCAP modulate the activity and/or expression of TRPA1 and M8 in TG neurons.
The second alternative hypothesis for cold transduction is a temperature-dependent release of a soluble factor from non-neurons. A recent study showed that odontoblasts release ATP in response to cold (Shibukawa et al., 2015) and here, we evaluated whether SCAP also release ATP in response to cold. Our findings indicate that undifferentiated SCAP cells release ATP in response to cold stimuli applied at clinically meaningful temperatures. However, SCAP are likely releasing other factors that can act on neurons. Interestingly, conditioned supernatant collected after thermocycling SCAP to 15 °C was able to activate cultured TG neurons (Fig. 2B), however the kinetics of the responses from the conditioned supernatant (Fig. 2C) were distinct from the kinetics of the P2X receptor-mediated responses (Fig. 3B, D). These findings are consistent with the hypothesis that the conditioned supernatant contains several factors that regulate TG afferent activity.
With respect to effects of stem cell co-culture on P2X receptor activity, stem cell co-culture appeared to enhance only P2X2/3-mediated current density, while P2X3-mediated current density remained unchanged. Both P2X2 and P2X3 have been shown to be expressed in nerve fibers in human dental pulp (Alavi et al., 2001), and based on our studies it appears that heteromultimeric P2X receptor currents are slightly enhanced by co-culture with SCAP (Fig. 3C). Due to the rapid inactivation of these P2X receptor-mediated inward currents, it is possible that ATP-mediated responses are more important in initiating, rather than maintaining, the neuronal response to cold. It is important to note that TG neurons also express G-protein-coupled P2Y receptors (Burnstock, 2007), and amplification of the ATP stimulus through signal transduction pathways coupled to P2Y receptors has the potential to play a large modulatory role in neuronal excitability. Together, these data indicate that SCAP may regulate TG neuronal responsiveness to cold stimuli by at least two distinct mechanisms.
Acknowledgment
The authors deny any conflicts of interest. This work was supported by start-up funds from the University of Texas Health Science Center at San Antonio.
Abbreviations:
- EGTA
ethylene glycol-bis(β-aminoethyl ether)-N,N,N′, N′-tetraacetic acid
- Hepes
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- MSCs
mesenchymal stem cells
- NGF
nerve growth factor
- REPs
regenerative endodontic procedures
- SCAP
stem cells of the apical papilla
- TG
trigeminal
- TRPA1
transient receptor potential ankyrin type 1
- TRPM8
transient receptor potential melastatin 8
REFERENCES
- Ahlquist M, Franzen O, Coffey J, Pashley D (1994) Dental pain evoked by hydrostatic pressures applied to exposed dentin in man: a test of the hydrodynamic theory of dentin sensitivity. J Endod 20:130–134. [DOI] [PubMed] [Google Scholar]
- Alavi AM, Dubyak GR, Burnstock G (2001) Immunohistochemical evidence for ATP receptors in human dental pulp. J Dent Res 80:476–483. [DOI] [PubMed] [Google Scholar]
- Alvarado LT, Perry GM, Hargreaves KM, Henry MA (2007) TRPM8 Axonal expression is decreased in painful human teeth with irreversible pulpitis and cold hyperalgesia. J Endod 33:1167–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannstrom M, Astrom A (1972) The hydrodynamics of the dentine; its possible relationship to dentinal pain. Int Dent J 22:219–227. [PubMed] [Google Scholar]
- Brannstrom M, Linden LA, Astrom A (1967) The hydrodynamics of the dental tubule and of pulp fluid. A discussion of its significance in relation to dentinal sensitivity. Caries Res 1:310–317. [DOI] [PubMed] [Google Scholar]
- Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797. [DOI] [PubMed] [Google Scholar]
- Chrepa V, Henry MA, Daniel BJ, Diogenes A (2015) Delivery of apical mesenchymal stem cells into root canals of mature teeth. J Dent Res 94:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW (1997) Distinct ATP receptors on pain-sensing and stretch sensing neurons. Nature 387:505–508. [DOI] [PubMed] [Google Scholar]
- de Almeida JF, Chen P, Henry MA, Diogenes A (2014) Stem cells of the apical papilla regulate trigeminal neurite outgrowth and targeting through a BDNF-dependent mechanism. Tissue Eng Part A 20:3089–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogenes A, Ruparel NB, Shiloah Y, Hargreaves KM (2016) Regenerative endodontics: a way forward. J Am Dent Assoc (1939) 147:372–380. [DOI] [PubMed] [Google Scholar]
- Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, Hargreaves KM (2006) Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci 26:8126–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogenes A, Akopian AN, Hargreaves KM (2007) NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res 86:550–555. [DOI] [PubMed] [Google Scholar]
- Diogenes A, Henry MA, Teixeira FB, Hargreaves KM (2013) An update on clinical regenerative endodontics. Endod Top 28:2–23. [Google Scholar]
- Kim YS, Paik SK, Cho YS, Shin HS, Bae JY, Moritani M, Yoshida A, Ahn DK, Valtschanoff J, Hwang SJ, Moon C, Bae YC (2008) Expression of P2X3 receptor in the trigeminal sensory nuclei of the rat. J Comp Neurol 506:627–639. [DOI] [PubMed] [Google Scholar]
- Kim YS, Kim TH, McKemy DD, Bae YC (2015) Expression of vesicular glutamate transporters in transient receptor potential melastatin 8 (TRPM8)-positive dental afferents in the mouse. Neuroscience 303:378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K (2005) Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol 493:596–606. [DOI] [PubMed] [Google Scholar]
- Lovelace TW, Henry MA, Hargreaves KM, Diogenes A (2011) Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod 37:133–138. [DOI] [PubMed] [Google Scholar]
- Matthews B, Vongsavan N (1994) Interactions between neural and hydrodynamic mechanisms in dentine and pulp. Arch Oral Biol 39 (Suppl):87S-95S. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416:52–58. [DOI] [PubMed] [Google Scholar]
- Meschi N, Hilkens P, Lambrichts I, Van den Eynde K, Mavridou A, Strijbos O, De Ketelaere M, Van Gorp G, Lambrechts P (2016) Regenerative endodontic procedure of an infected immature permanent human tooth: an immunohistological study. Clin Oral Invest 20:807–814. [DOI] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, Garcia-Anoveros J (2005) Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25:4052–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narhi MV (1986) Responses of pulp nerves to external irritation. Proc Finn Dent Soc 82:190–198. [PubMed] [Google Scholar]
- Narhi M, Hirvonen T, Huopaniemi T (1984) The function of intradental nerves in relation to the sensations induced by dental stimulation. Acupunct Electrother Res 9:107–113. [DOI] [PubMed] [Google Scholar]
- Nealen ML, Gold MS, Thut PD, Caterina MJ (2003) TRPM8 mRNA is expressed in a subset of cold-responsive trigeminal neurons from rat. J Neurophysiol 90:515–520. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A (2002) A TRP channel that senses cold stimuli and menthol. Cell 108:705–715. [DOI] [PubMed] [Google Scholar]
- Renton T, Yiangou Y, Baecker PA, Ford AP, Anand P (2003) Capsaicin receptor VR1 and ATP purinoceptor P2X3 in painful and nonpainful human tooth pulp. J Orofac Pain 17:245–250. [PubMed] [Google Scholar]
- Ruparel NB, de Almeida JF, Henry MA, Diogenes A (2013) Characterization of a stem cell of apical papilla cell line: effect of passage on cellular phenotype. J Endod 39:357–363. [DOI] [PubMed] [Google Scholar]
- Shibukawa Y, Sato M, Kimura M, Sobhan U, Shimada M, Nishiyama A, Kawaguchi A, Soya M, Kuroda H, Katakura A, Ichinohe T, Tazaki M (2015) Odontoblasts as sensory receptors: transient receptor potential channels, pannexin-1, and ionotropic ATP receptors mediate intercellular odontoblast-neuron signal transduction. Pflugers Arch 467:843–863. [DOI] [PubMed] [Google Scholar]
- Staikopoulos V, Sessle BJ, Furness JB, Jennings EA (2007) Localization of P2X2 and P2X3 receptors in rat trigeminal ganglion neurons. Neuroscience 144:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–829. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ (2004) Thermosensation and pain. J Neurobiol 61:3–12. [DOI] [PubMed] [Google Scholar]
- Trowbridge HO, Franks M, Korostoff E, Emling R (1980) Sensory response to thermal stimulation in human teeth. J Endod 6:405–412. [DOI] [PubMed] [Google Scholar]


