Abstract
There are knowledge gaps in animal agriculture on how to best mitigate greenhouse gas emissions while maintaining animal productivity. One reason for these gaps is the uncertainties associated with methods used to derive emission rates. This study compared emission rates of methane (CH4) and carbon dioxide (CO2) measured by a commercially available GreenFeed (GF) system with those from (1) a mass flow controller (MFC) that released known quantities of gas over time (i.e., emission rate) and (2) a respiration chamber (RC). The GF and MFC differed by only 1% for CH4 (P = 0.726) and 3% for CO2 (P = 0.013). The difference between the GF and RC was 1% (P = 0.019) for CH4 and 2% for CO2 (P = 0.007). Further investigation revealed that the difference in emission rate for CO2 was due to a small systematic offset error indicating a correction factor could be applied. We conclude that the GF system accurately estimated enteric CH4 and CO2 emission rates of cattle over a short measurement period, but additional factors would need to be considered in determining the 24-hr emission rate of an animal.
Keywords: GreenFeed, methane, carbon dioxide, cattle, feedlot
Introduction
The number of greenhouse gas mitigation studies in animal agriculture has increased significantly in the past 2 decades (Beauchemin et al., 2020). As a result, numerous mitigation strategies have been evaluated for their potential to directly decrease enteric methane (CH4) emissions from ruminants (Hristov et al., 2013; Beauchemin et al., 2020). This research trend has coincided with the development of better tools used to enable more accurate, precise, and temporally complete measurements of enteric CH4 emissions from cattle and sheep (McGinn, 2013). There are now numerous methods available that derive enteric CH4 emission rates of animals by measuring concentration of gas in the breath coupled with a measurement of air flow (Hristov et al., 2018; Garnsworthy et al., 2019).
A recent method to measure enteric emission rate is the commercially available GreenFeed (GF) system (C-Lock Inc., Rapid City, SD). The GF system is an automated head chamber that measures gas concentrations continuously and calculates the animal’s gas emissions when a proximity sensor inside the head chamber detects a visiting animal. Once the animal’s head is in position, the overhead hopper delivers a small predetermined quantity of feed pellets (bait) to encourage the animal to remain in the head chamber for 3 to 7 min. As the animal repeatedly visits the GF unit over time (days or weeks) spot measurements of carbon dioxide (CO2) and CH4 are used to derive an overall mean emission rate for each gas. Details of the GF system are reported by Hammond et al. (2016), Cole et al. (2018), and Hristov et al. (2018).
The GF system has been assessed relative to a variety of methods including respiratory chambers (RC; Hammond et al., 2015; Alemu et al., 2017), which have traditionally been viewed as the “gold standard” of measurement (Garnsworthy et al., 2019). Agreement has been good in some, but not all studies (Hristov et al., 2018). The GF system is difficult to test against other methods because the unit handles a single animal at a time and only for short periods each day, and therefore the individual emission is estimated over a series of visits that occur over several days or weeks. The lack of continuous time series of emission rates within a day makes it very difficult to directly compare the emission rate obtained from the GF system to that obtained using RC. Huhtanen et al. (2019) recognized this limitation and focused on comparing the GF system output against models developed using RC that predict daily enteric emissions.
In our study, a unique approach was used to validate the accuracy of the GF system for estimating CO2 and CH4 emission rates by releasing known concentrations of gases using a mass flow controller (MFC). The comparison was conducted in an open environment and inside an RC. The open environment simulated results having low background concentration similar to a pasture, while those inside the RC represented a barn with potentially higher background concentrations.
Materials and Methods
Experimental design
Emission rates of CH4 and CO2 from the GF system were compared with known emission rates from an MFC in an open environment (outside a respiratory chamber [RC]) and inside a RC. For the inside GF unit, the GF exhaust was vented into the RC volume. For each comparison, 5 increasing release rates were used with 2 replications (days), providing a total of 10 emission rates per comparison (Table 1). For the comparison inside the RC, the 2 systems were operated for each gas release under a steady state. In this configuration, the steady state occurred once the exhaust concentrations from both the GF system and RC were constant.
Table 1.
Volume and mass of CO2 and CH4 gas released from the MFC (volumetric m3/min output) and its mass rate (g/min)
| Level | m3/min | g/min |
|---|---|---|
| CO2 | ||
| 1 | 0.001 | 1.71 |
| 2 | 0.002 | 3.43 |
| 3 | 0.003 | 5.16 |
| 4 | 0.004 | 6.86 |
| 5 | 0.005 | 8.59 |
| CH4 | ||
| 1 | 0.0001 | 0.07 |
| 2 | 0.0002 | 0.14 |
| 3 | 0.0003 | 0.21 |
| 4 | 0.0005 | 0.29 |
| 5 | 0.0006 | 0.36 |
Calculation of emission rate
The emission of CO2 and CH4 was calculated for the GF system and RC using a mass balance approach. For both GF and RC systems, the recorded background concentration was subtracted from each peak concentration (Figure 1) and the difference converted to concentration (g/m3) and then multiplied by the airflow value and conversion factors to give an emission rate in g/d (Equation 1):
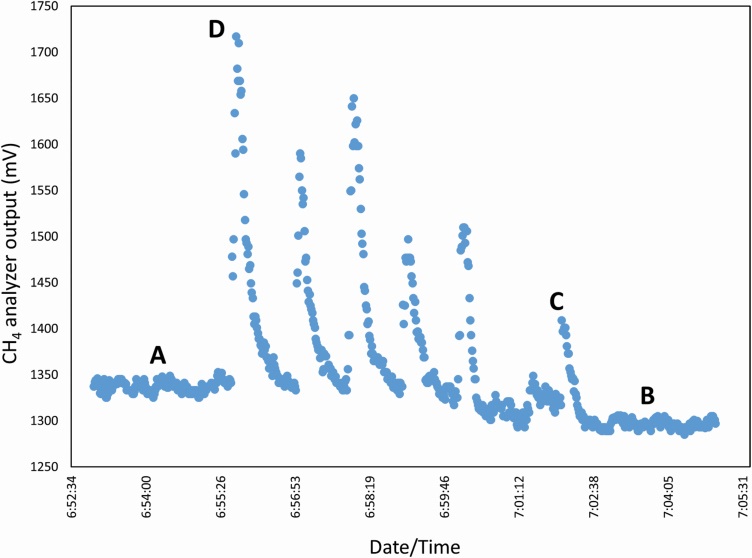
Figure 1.
An example of the GF CH4 gas analyzer output when an animal is using the head chamber (C and D) and when no animal is present (A and B). Each peak represents eructation of different strength (example D vs. C), while the background signal (A and B) is relatively consistent during a visit. The recording was from an unpublished study conducted in a beef feedlot.
| [1] |
where P is air pressure (kPa), MW is molecular weight (g/mol), C is the enhanced mixing ratio (µmol/mol) above-background concentration of either CO2 or CH4, Flow is the air flow through the unit (m3/min), R is the ideal gas constant (kPa m3 K /mol), and T is the temperature in K. As well, a compression factor was used for each gas type (Alicat Scientific Operating Manual, Tucson, AZ).
Calibration of the GreenFeed (GF) system
The GF system detects CH4 and CO2 gas concentrations using a nondispersive near-infrared analyzer (NDIR; Gasmitter D-AGM Plus 1050, Sensor Europe GmbH, Germany). The measurement protocol of the GF system requires calibration of the NDIR using an air mixture to zero the analyzer, and then using a known certified gas concentration to adjust the span of the NDIR. This calibration protocol (when repeated) is used to remove signal “drift” in the NDIR analyzer’s millivolt output over time. The linearity of this relationship (concentration vs. millivolts) for CH4 was verified in our investigation by using an additional span gas standard of 403.6 µmol/mol of CH4. This evaluation confirmed a high precision in the relationship of CH4 concentration to millivolt output of the NDIR (slope = 1.095; R2 =1) and confirms the usefulness of the NDIR technology.
The NDIR analyzer used by the GF system reports concentration of CO2 and CH4 every second. An example for a companion study shows the typical signal from the NDIR analyzer for CH4 (Figure 1). The GF system calculates the emission rate as the average difference in concentration between the peak (e.g., levels C and D occur when the head chamber is occupied by an animal) and background concentrations (e.g., levels A and B occur when no animal visits the head chamber). However, when the NDIR is recording at near limits of its full scale, its sensitivity declines. As stated by Honeycutt et al. (2019), NDIR sensors are assumed to be insensitive for environmental applications because background concentration of many environmentally important gases such as CH4 at 2 µmol/mol are below the NDIR resolution.
In addition to calibrating the NDIR, it is desirable to perform a whole-system evaluation using a release and recovery approach to account for other sources of variability (Alemu et al., 2017), e.g., the airflow from the head chamber can depend upon the condition of the air filter and fan. A minimum airflow of 27 L/s in the exhaust duct is required to ensure the entire breath cloud from the animal eructating in the head chamber is captured by the air stream and then measured by the NDIR analyzer. If the air flow is less than that threshold value, the loss of CO2 and CH4 would under-estimate the emission rate.
Mass flow controller
The MFC was tested to ensure the recorded flow of gases was correct. The accuracy of the MFC set-points was validated using dinitrogen (N2) because N2 is readily available from specialty gas providers at a nominal cost, which meant the run duration could be long and the weight change large to ensure the scale was representative of the emission rate of cattle. The set points on the MFC provided a steady flow of gas at each of the 5 flow rates of N2 with the measurements repeated over 2 d (Table 1). A 20-min interval was used between gas releases. The mass (g) of the N2 released was calculated for each of the 5 release rates. It is reasonable to assume the same accuracy would exist for CO2 and CH4.
Use of a respiration chamber (RC) for comparison
Details of the RC used in the study are given by Vyas et al. (2018). Methane (model Ultramat 5E; Siemens Inc., Karlsruhe, Germany) and CO2 analyzers (model LI-7000, LI-COR Environmental, Lincoln, NE) monitored the concentrations of CH4 and CO2, respectively, in the intake and exhaust air ducts and the emission rate of CH4 and CO2, gases were quantified by measuring the difference between the inlet and exhaust concentrations and airflow through the RC. Calibration of the RC was performed the day previous to the study by releasing a known quantity of CH4 in the chamber using the MFC, and the recovered amount was used to adjust the chamber to 100% recovery. As a side note, the normal operation of the RC is 1 of 4 chambers (Vyas et al., 2018) that are normalized using the same calibration gases.
Following calibration, the GF system was placed inside the RC (4.4 m wide × 3.7 m deep × 3.9 m) allowing simultaneous comparisons as CO2 and CH4 that were injected into the head chamber of the GF unit from outside the RC using the MFC. The recording of GF vs. RC data started once the gas concentrations reached a steady state at the GF head chamber and the RC exhaust. The mass balance (Equation 1) was used to determine the emission rate of the GF and RC systems.
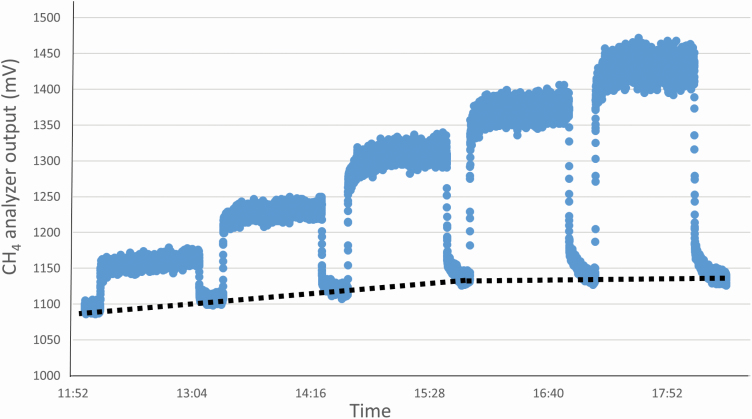
For each measurement, the MFC release rate of CH4 was 0.1, 0.2, 0.3, 0.4, and 0.5 L/min (or 102 to 514 g animal−1 d−1), and for CO2, the 5 rates were 1, 2, 3, 4, and 5 L/min (2,468 to 12,369 g animal−1 d−1). A 20-min interval was used between gas releases. During these rest periods, gas concentration in the RC returned to a background value; however, the background CH4 reading of the GF system did not recover completely (Figure 2).
Figure 2.
Example output for CH4 from the NDIR analyzer used by the GF system during the release of 5 known rates of compressed gases. Note that there is a tendency for the baseline (background) to increase as shown by the dotted line.
Statistical analysis
The CO2 and CH4 emission rates at the 5 repeated release rates (2 days × 5 levels = 10 observations per method) for the GF system were compared to those from the MFC used outside the chamber and to the RC using a paired t-test (PROC T-TEST). Significant differences were assumed to exist at P ≤ 0.05. The differences between methods were further analyzed using accuracy analysis (Allen and Raktoe, 1981) to indicate systematic and random differences. The output of this analysis is the percentage of the difference that is attributed to an offset, regression, and random effects.
Results and Discussion
We compared emission rates (g/d) from the GF system to known emissions from an MFC used outside the chamber, and to an RC by placing the GF system inside the chamber. The unique large dimensions of the RC allowed a direct comparison. By using an MFC rather than an animal to release the gas an exact emission rate was achieved. The comparison made outside the chamber represented an environment with a low background concentration of gas similar to that of a pasture situation, whereas the comparison inside the RC represented a barn environment where background gas concentrations are slightly elevated due to the decline in dispersion, which increases ambient gas concentrations.
GF system comparisons
On average, there was a small, but significant difference in emission rates of CO2 between the GF system and the MFC (outside the chamber) and between GF and RC (inside the chamber; Table 2). The differences in CO2 emission rate were 3% (8.223 vs. 7.946 g/d, P = 0.013) and 2% (8,035 vs. 7,850 g/d, P = 0.007) greater for the GF system for these 2 comparisons, respectively. However, there was no difference (P = 0.726) in CH4 emission rates for GF vs. MFC (1%; 309 vs. 305 g/d, respectively). The difference between the GF vs. RC was also small, albeit significant (1%, 328 vs. 323 g/d, P = 0.019). The small difference in CO2 emission rate between the GF system and the MFC used outside the chamber was due to an offset error, indicating a systematic error and the potential to apply a 3% correction factor. However, the difference in CO2 between the GF system and chamber was attributed to random error that is not conducive to a correction.
Table 2.
Paired t-test and accuracy analysis of carbon oxide and methane for comparison of the GF emissions rates versus the MFC and respiration chamber emission rates (n = 10/treatment)
| Carbon dioxide | Methane | |||
|---|---|---|---|---|
| Item | Outside chamber | Inside chamber | Outside chamber | Inside chamber |
| Emission, g/d | ||||
| GF | 8,223 | 8,035 | 309 | 328 |
| MFC | 7,946 | — | 305 | — |
| Respiration chamber | — | 7,850 | — | 323 |
| Difference, % | 3 | 2 | 1 | 1 |
| P-value | 0.013 | 0.007 | 0.726 | 0.019 |
| Accuracy analysis | ||||
| Offset error, % | 81 | 13 | 3 | 13 |
| Regression error, % | 18 | 13 | 80 | 13 |
| Random error, % | 1 | 74 | 17 | 76 |
Potential influencing factors for the GF system
There are several assumptions that could have contributed to the small differences in emission rates for the GF system. When the GF unit is deployed under normal use, the gas concentration resulting from the animal’s breath/eructation cloud is recorded every second. A fast sampling rate is necessary for the CH4 output signal because it is more erratic than the CO2 signal due to irregular eructation by the animal in contrast to respiration, which accounts for most of the CO2 emission. The data recording must be frequent enough to capture the fluctuating signal, and to distinguish between peak and background concentrations (Figure 1).
Accurate measurement of air flow is also important, as concentration measurements are combined with air flow to calculate the emission. In the GF unit, a hot-wire anemometer is located within the straight vertical section (inferring laminar air flow) of the exhaust duct at a single point. Using a single-point air flow reading can lead to an offset error in the emission calculation unless great care is taken in locating the average air flow position in the exhaust duct cross-section. In our study, the differences between the emission rate of CH4 and CO2 from the GF and MFC used outside and inside the RC were very small, thus the single point air flow reading is likely not a source of error for the GF system.
The study used the GF system inside a RC to allow a direct comparison of these methods. The exhaust gases from the GF unit were assumed to be well mixed in the RC, and after a time, the system comes to a steady state which allowed the comparison of mass balance of the RC and GF emission rates. The assumption that the GF exhaust air is never recycled through the GF unit creates uncertainty especially under stable atmospheric conditions at night when dispersion is poor or when operating in a poorly ventilated barn where gases may linger around the GF unit. A similar situation exists when using the SF6 tracer method when the normally very low background concentration of SF6 is elevated by the release of the tracer gas (McGinn et al., 2006). To evaluate the potential impact of air stability when the GF system is used outdoors, we used a dispersion model (WINTRAX; Flesch et al., 2005) to evaluate the atmospheric conditions that allow the exhaust gas to re-circulate through the GF system (data not shown). The simulation released CH4 at a height of 3 m representing the exhaust of the GF system and the gas concentration was detected at 0.5 m to represent the height of the GF head chamber. The dispersion model evaluated whether gases released at 3 m are detected at 0.5 m height, thereby influencing the GF concentration measurement. To run this dispersion simulation, we used low and high release rates of CH4 (200 and 400 g/d, respectively) and assumed a worst-case scenario for air dispersion (e.g., low wind speed). The simulated CH4 concentration at 0.5 m was increased by 0.035 and 0.064 µmol/mol for releases of 200 and 400 g/d, respectively. Thus, the increased background concentration at the GF head chamber was small (+1.8% and +3.2%, respectively) relative to the background concentration of 2 µmol/mol. This simulation indicates that the CH4 released from the exhaust of the GF system did not substantially affect the CH4 emission estimate from the GF system.
The NDIR detection limit of 0.5% or 100 µmol/mol at the full scale of 20,000 µmol/mol for the CH4 analyzer used by the GF produces unreliable measurements of background CH4 concentration (~2 µmol/mol). If accurate measurements of background concentration are needed an analyzer other than the NDIR would be needed. However, the uncertainty of background concentrations measured by the GF system has little influence on the difference in average concentration (C) used in Equation 1. For example, the background CH4 concentration measured by the GF system averaged 2.4 µmol/mol in our study while the concentration at the exhaust was 100 to 300 µmol/mol. Honeycutt et al. (2019) state that NDIR analyzers are too insensitive for environmental applications. However, the insensitivity of the NDIR analyzer in the GF system was not a limitation because the high uncertainty of CH4 background concentration was masked by the much greater concentration in the simulated breath/eructation of the animal. The uncertainty of CH4 background concentration on emission rate may be problematic for small ruminants such as sheep, with a tenth of the emission rate of cattle, because the concentration in breath/eructation of the animal is low. In the case of CO2, the NDIR analyzer specifications (www.sensors-nc.com) for the GF unit indicate a high resolution at full scale of 2,000 µmol/mol, a linearity of 1% at full scale (20 µmol/mol), and a resolution at span concentration of 20 µmol/mol. As a result, the NDIR analyzer accurately measures CO2 background concentration of ~400 µmol/mol. For CO2 a very linear relationship for GF emission rates against the MFC release gas existed.
The linearity of the NDIR analyzer should be checked periodically using at least 3 calibration points (C-Lock recommends 2 points thereby assuming linearity) representing the expected output range from the study. If the calibration shows linearity throughout the range of measured concentrations, then despite the uncertainty of background concentration, the concentration in the animal’s breath can be derived accurately.
Our study is a short snapshot of the GF system and neglects the final step in determining the 24-hr emission rate of an animal. Velazco et al. (2016) report that additional errors occur in estimating the daily emission rate from individual animals over a 24-hr period. According to their study, to minimize the risk of biasing the estimation of CH4 emission using the GF system, regular visits over the day by animals are needed to estimate the diel cycle of enteric emissions.
Conclusions
The wide use of the GF system by researchers to estimate enteric CH4 from ruminants has been, in part, due to its “turn-key” approach that requires little training of personnel and animals. Its main application has been to document much-needed enteric CH4 emissions for greenhouse gas inventory and mitigation research. We have shown that the performance of the GF system was as good as that of a MFC and RC for CO2 and CH4, where differences were ≤3%. A recommended change in the calibration protocol is the addition of a third calibration point for the gas analyzer. The small difference in CO2 emission rates between systems was attributed to systematic errors that could be accounted for using a 3% offset. We conclude that the GF system has the potential to accurately measure emission rates from cattle when used in an open environment or well-ventilated barn.
Acknowledgments
Funding was from Agriculture and Agri-Food Canada. Thank you to Scott Zimmerman (C-Lock Inc.) for his valuable input into an earlier draft of the manuscript.
Glossary
Abbreviations
- CH4
methane
- CO2
carbon dioxide
- GF
GreenFeed
- MFC
mass flow controller
- N2
dinitrogen
- NDIR
non-dispersive near-infrared analyzer
- RC
respiratory chamber.
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature cited
- Alemu, A. W., Vyas D., Manafiazar G., Basarab J. A., and Beauchemin K. A.. . 2017. Enteric methane emissions from low-and high-residual feed intake beef heifers measured using GreenFeed and respiration chamber techniques. J. Anim. Sci. 95:3727–3737. doi: 10.2527/jas.2017.1501 [DOI] [PubMed] [Google Scholar]
- Allen, O. B., and Raktoe B. L.. . 1981. Accuracy analysis with special reference to the prediction of grassland yield. Biom. J. 23:371–388. doi: 10.1002/bimj.4710230404 [DOI] [Google Scholar]
- Beauchemin, K. A., Ungerfeld E. M., Eckard R., and Wang M.. . 2020. REVIEW: fifty years of research on rumen methanogenesis - lessons learned and future challenges for mitigation. Anim. 14(S1):s2–s16. doi: 10.1017/S1751731119003100 [DOI] [PubMed] [Google Scholar]
- Cole, N. A., Parker D. B., Todd R. W., Leytem A. B., Dungan R. S., Hales K. E., Ivey S. L., and Jennings J.. . 2018. Use of new technologies to evaluate the environmental footprint of feedlot systems. Transl. Anim. Sci. 2:89–100. doi: 10.1093/tas/txx001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch, T. K., Wilson J. D., Harper L. A., and Crenna B. P.. . 2005. Estimating gas emission from a farm using an inverse-dispersion technique. Atmos. Environ. 39:4863–4874. doi: 10.1016/j.atmosenv.2005.04.032 [DOI] [Google Scholar]
- Garnsworthy, P. C., Difford G. F., Bell M. J., Bayat A. R., Huhtanen P., Kuhla B., Lassen J., Peiren N., Pszczola M., Sorg D., . et al. 2019. Comparison of methods to measure methane for use in genetic evaluation of dairy cattle. Animals 9:837. doi: 10.3390/ani9100837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, K. J., Humphries D. J., Crompton L. A., Green C., and Reynolds C. K.. . 2015. Methane measurements from cattle: estimates from short-term measurements using a GreenFeed compared with measurements obtained using respiration chambers or sulphur hexafluoride tracer. Anim. Feed Sci. Technol. 203:41–52. doi: 10.1016/j.anifeedsci.2015.02.008 [DOI] [Google Scholar]
- Hammond, K. J., Waghorn G. C., and Hegarty R. S.. . 2016. The GreenFeed system for measurement of enteric methane emission from cattle. Anim. Prod. Sci. 56(3):181–189. doi: 10.1071/AN15631 [DOI] [Google Scholar]
- Honeycutt, W. T., Ley M. T., and Materer N. F.. . 2019. Precision and limits of detection for selected commercially available, low-cost carbon dioxide and methane gas sensor. Sensors 19:3157. doi: 10.3390/s19143157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov, A. N., Kebreab E., Niu M., Oh J., Bannink A., Bayat A. R., Boland T. M., Brito A. F., Casper D. P., Crompton L. A., . et al. 2018. Symposium review: uncertainties in enteric methane inventories, measurement techniques, and prediction models. J. Dairy Sci. 101:6655–6674. doi: 10.3168/jds.2017-13536 [DOI] [PubMed] [Google Scholar]
- Hristov, A. N., Oh J., Lee C., Meinen R., Montes F., Ott T., Firkins J., Rotz A., Dell C., Adesogan A., . et al. 2013. Mitigation of greenhouse gas emissions in livestock production: a review of technical options for non-CO2 emissions. In: Gerber P. J., Henderson B. and Makkar H. P. S. editors. FAO Animal production and health paper No. 177. FAO, Rome, Italy; p. 1–226. Retrieved on 10 July 2019 from www.fao.org/3/i3288e/i3288e.pdf [Google Scholar]
- Huhtanen, P., Ramin M., and Hristov A. N.. . 2019. Enteric methane emission can be reliably measured by the GreenFeed monitoring unit. Livest. Sci. 222:31–40. doi: 10.1016/j.livsci.2019.01.017 [DOI] [Google Scholar]
- McGinn, S. M., 2013. Developments in micrometeorological techniques for methane emissions. Animal. 7(s2):386–393. doi: 10.1017/S1751731113000657 [DOI] [PubMed] [Google Scholar]
- McGinn, S. M., Beauchemin K. A., Iwaasa A. D., and McAllister T. A.. . 2006. Assessment of the sulfur hexafluoride (SF6) tracer technique for measuring enteric methane emissions from cattle. J. Environ. Qual. 35:1686–1691. doi: 10.2134/jeq2006.0054 [DOI] [PubMed] [Google Scholar]
- McGinn, S. M., Flesch T. K., Harper L. A., and Beauchemin K. A.. . 2006. An approach for measuring methane emissions from whole farms. J. Environ. Qual. 35:14–20. doi: 10.2134/jeq2005.0250 [DOI] [PubMed] [Google Scholar]
- Velazco, J. I., Mayer D. G., Zimmerman S., and Hegarty R. S.. . 2016. Use of short-term breath measures to estimate daily methane production by cattle. Animal 10:25–33. doi: 10.1017/S1751731115001603 [DOI] [PubMed] [Google Scholar]
- Vyas, D., Alemu A. W., McGinn S. M., Duval S. M., Kindermann M., and Beauchemin K. A.. . 2018. The combined effects of supplementing monensin and 3-nitrooxypropanol on methane emissions, growth rate, and feed conversion efficiency in beef cattle fed high-forage and high-grain diets. J. Anim. Sci. 96:2923–2938. doi: 10.1093/jas/sky174 [DOI] [PMC free article] [PubMed] [Google Scholar]