Abstract
Purpose of Review
Pathophysiology of hypertensive disorders of pregnancy (HDP), especially preeclampsia, has not been fully elucidated. Most trials aimed at the prevention of preeclampsia have failed to show significant benefit and investigation of novel, modifiable risk factors is sorely needed. Sleep disordered breathing (SDB), a group of disorders for which treatments are available, meets these criteria. SDB impacts about a third of all pregnancies and is associated with hypertension in the general non-pregnant population.
Recent Findings
Recent studies have shown a high prevalence of SDB, especially in complicated pregnancies. Several studies have shown that pregnant women with SDB have a higher risk for developing HDP, and these two disorders are associated with similar maternal long-term cardiovascular outcomes. Based on limited animal models of gestational intermittent hypoxia and human studies, SDB and HDP share similar risk factors and some pathophysiological mechanisms. However, there is paucity of studies addressing causality of this association and identifying therapeutic targets for intervention.
Summary
Maternal SDB represents a novel and modifiable risk factor of HDP. Further studies are needed in order to establish the exact mechanisms underlying this association and to identify specific areas for clinical interventions.
Keywords: Sleep disordered breathing, Pregnancy, Hypertensive disorders of pregnancy, Preeclampsia, Placenta
Introduction
Hypertensive disorders of pregnancy (HDP) are a group of disorders comprising preeclampsia, gestational hypertension, eclampsia, and chronic hypertension. Preeclampsia is defined by new-onset hypertension diagnosed after 20 weeks’ gestation or, in some cases, with onset in the post-partum period [1, 2]. The definition of preeclampsia has evolved over time and varies among professional societies’ guidelines; however, it is agreed that preeclampsia is a heterogeneous systemic condition [1, 3]. Preeclampsia occurs in 2–8% of pregnancies and represents one of the most common causes of maternal and perinatal mortality worldwide [1, 4]. While advances in maternal fetal medicine have led to a decrease in eclampsia, mainly through an improvement in antenatal care and preventive interventions [5], the prevalence of preeclampsia has increased over the past three decades. The risk of developing preeclampsia among women giving birth in 2003 in the USA was 6-fold higher when compared with the risk among women giving birth in 1980 [6]. Similar increases were reported in Europe [7]. This is in part due to changes in diagnostic criteria used and, likely, to an increase in diagnostic accuracy. Other contributing factors include an increase in associated risk factors such as maternal obesity and other preexisting morbidity, as well as an increase in maternal age, infertility, and multiple pregnancy [5, 8]. Maternal HDP also heavily impact the cost of health care. Based on a US database study, the mean combined maternal and infant health care cost for deliveries complicated by preeclampsia that occurred between 2010 and 2015 was $41,790, nearly double the cost of hypertension without preeclampsia features ($24,182, p < 0.001), and more than three-fold higher than the cost of an uncomplicated pregnancy ($13,187, p <0.001) [9]. Moreover, history of preeclampsia is an independent risk factor for developing cardiovascular diseases later in life, and this association remains statistically significant after controlling for important confounding factors such age, body mass index (BMI), diabetes, and gestational diabetes [10]. Specifically, history of preeclampsia is a risk factor for chronic hypertension, heart failure [risk ratio 4.19; 95%CI 2.09–8.38)], coronary heart disease [risk ratio 2.50; 95%CI 1.43–4.37)], and stroke [risk ratio 1.81; 95%CI 1.29–2.55)], and overall increases mortality due to cardiovascular diseases [10].
Different strategies have been proposed to improve early identification of pregnant women at risk for hypertensive disorders and to improve maternal and neonatal outcomes. Some of the conducted trials included nutritional interventions and use of medications, such as vitamins C, E, and D, fish oil, folic acid, calcium, garlic supplementation, low-sodium diet, and aspirin. Among all those strategies, the only intervention associated with a significant reduction in the risk of preeclampsia, among selected populations, is aspirin [11]. In a recent Cochrane database systematic review and meta-analysis, aspirin was associated with only a modest reduction of risk for preeclampsia (18%) [12].
Hence, the efficacy and usefulness of available preventive and therapeutic strategies are limited, and the exact pathophysiology and risk factors of hypertensive disorders, especially preeclampsia, have not been fully elucidated [13••].
As HDP are responsible for up to 16% of maternal death in the USA, Europe, and Canada, and 26% of maternal death in Latin America and the Caribbean [5, 14], and for the reasons listed above, the identification of novel modifiable risk factors that can also serve as interventional targets is paramount. Sleep disordered breathing (SDB), a spectrum of disorders characterized by airflow limitation, intermittent hypoxemia, and recurrent arousals, has emerged as a potential target that meets these criteria. SDB has been associated with cardiovascular disease in the general population and may impact some target organs. In this manuscript, we review the evidence linking SDB to HDP, discuss plausible mechanistic pathways linking the two disorders, and appraise available evidence examining the impact of SDB-directed therapy on hemodynamic and hypertensive outcomes.
SDB in Pregnancy Epidemiology
SDB in pregnancy has gained significant interest in the past few decades due to its high prevalence in the pregnant population and the described association with adverse perinatal outcomes. Data has shown that snoring, a condition on the spectrum of SDB, occurs in about a third of all pregnant women by the third trimester [15, 16]. In a large multicenter prospective study, the prevalence of obstructive sleep apnea, a disorder characterized by airflow limitation, recurrent arousals, and intermittent hypoxia, was 8% in low risk primiparous women by mid-pregnancy [17••]. However, this prevalence appears to be significantly higher in high-risk pregnancies, ranging from 15% in pregnancies complicated by obesity [18] to up to 70% in pregnancies complicated by diabetes or growth restriction [19–21]. In pregnancies complicated by HDP, the prevalence of SDB was reported to be at least two times higher than the prevalence among normotensive pregnant women [22–24]. It is noteworthy, however, that there is a significant amount of variability in the definition of SDB in these studies [25], as well as the gestational age at which the condition is identified. Despite these high prevalence rates, SDB continues to be underdiagnosed and under coded, as demonstrated by national and population-based studies in the USA and around the world [26••, 27–29]. These rates likely reflect poor screening by pregnancy healthcare providers [30].
SDB and Hypertensive Disorders of Pregnancy
The association of SDB with hypertension has been well established in the general, non-pregnant population. Obstructive sleep apnea affects nearly half of patients with chronic hypertension [31,32]. The association is only partially explained by shared risk factors such as obesity [33]. In large epidemiological studies, obstructive sleep apnea was shown to increase the risk of both incident and prevalent hypertension [34–36] in a dose-response relationship. REM-related sleep apnea, a subtype of obstructive sleep apnea where obstructive events occur predominantly during REM sleep, is also independently associated with systemic hypertension [37].
SDB has been associated with HDP in numerous studies. Large cross-sectional studies have linked self-reported snoring with HDP [15, 16, 38] even after adjusting for multiple confounders such as age BMI history of chronic hypertension, history of diabetes mellitus, and in some studies history of any renal disease, fertility treatment, and other confounders [15]. More recently, multiple studies of various designs have shown obstructive sleep apnea to be an independent risk factor for HDP [17••, 18, 27–29, 39].
As the vast majority of pregnant women with SDB have obesity, and obesity is a risk factor for HDP, it is obvious that obesity may act as a confounder. Maternal obesity increases the risk for gestational hypertension [OR: 4.67 (95%CI 3.07–7.09)] and preeclampsia [OR: 2.49 (95%CI 1.29–4.78)], as shown by a large European population based study [40]. Maternal obesity is also an independent risk factor for histological placental abnormalities, seen in preeclampsia, such as lack of vascular remodeling of the uterine spiral arteries [41, 42].
Prospective studies have consistently shown an increase in the risk of HDP in women with SDB, independently of age and BMI. The NuMOM2B study enrolled primiparous women from multiple centers in the US and demonstrated that SDB, defined as a respiratory event index ≥5 events per hour identified in early (6–15 weeks) or mid pregnancy (22–31 weeks), was associated with a two-fold increased risk of HDP, after adjusting for age and BMI. In another smaller study examining the association of obstructive sleep apnea with HDP in a cohort of women with obesity, SDB, defined similarly, was associated with 3.4-fold increase in the development of preeclampsia, even after adjusting for age, BMI, and diabetes. These data suggest that SDB is a risk factor independently of BMI. Larger epidemiological studies have adjusted for age and obesity and multiple other potential confounders [26••, 27]. Importantly, however, the epidemiological studies that have demonstrated this association have adjusted for a diagnosis of obesity based on an International Classification of Disease-9 code, rather than BMI, due to lack of the availability of the latter. As the ICD-9 code may not be fully reliable in reflecting the extent of chronic conditions in a given cohort [43], the use of obesity code in these models may have resulted in underrepresentation of this diagnosis. On the other hand, smaller studies that have matched for BMI did not demonstrate an increased risk of SDB among women with HDP compared with BMI matched normotensive women examined within 4 weeks of the gestational age of cases [41•]. The authors of this study do acknowledge, however, that the study was significantly underpowered due to the fact that the prevalence of SDB was much higher in controls than previously anticipated. Separating the effect of obesity from that of obstructive sleep apnea will likely be difficult as in most cohorts, obesity is one of the main determinants of obstructive sleep apnea [17, 45, 46]. However, the bulk of current data seem to point to SDB being a risk factor above and beyond the risk imparted by obesity.
Biological Plausibility
Pathophysiology of HDP
Pathophysiology of HDP, especially preeclampsia, has not been fully elucidated for many reasons. From a clinical stand-point, preeclampsia is a heterogeneous condition. Diagnostic criteria continue to vary among definitions and worldwide, and this condition is only described in humans, limiting animal studies [13••]. Despite these limitations, preeclampsia is considered to be the result of a complex interaction between the placenta, maternal intrinsic factors such as preexisting morbidity, and extrinsic factors to which pregnant women may be exposed [13••, 41]. One of the first preeclampsia models, or two-stage placental model, did not explain why some forms of HDP present in the third trimester and did not include the role of any maternal risk factors for preeclampsia. This model is now thought to be more complex, with different maternal factors contributing to preeclampsia, either early in pregnancy, affecting placental development, or late in gestation, when maternal conditions can be associated with an impairment of placental functions, after a normal formation of the placenta [41].
Multiple mechanisms are responsible for the interaction between maternal factors and placenta in the development of preeclampsia, including increase in oxidative stress and inflammation, abnormal maternal-fetal immunotolerance, and an imbalance between anti-angiogenic factors, along with alterations in the maternal renin-angiotensin system and sympathetic nervous system [41,47]. Similar mechanisms have been described in SDB (see Fig. 1).
Fig. 1.
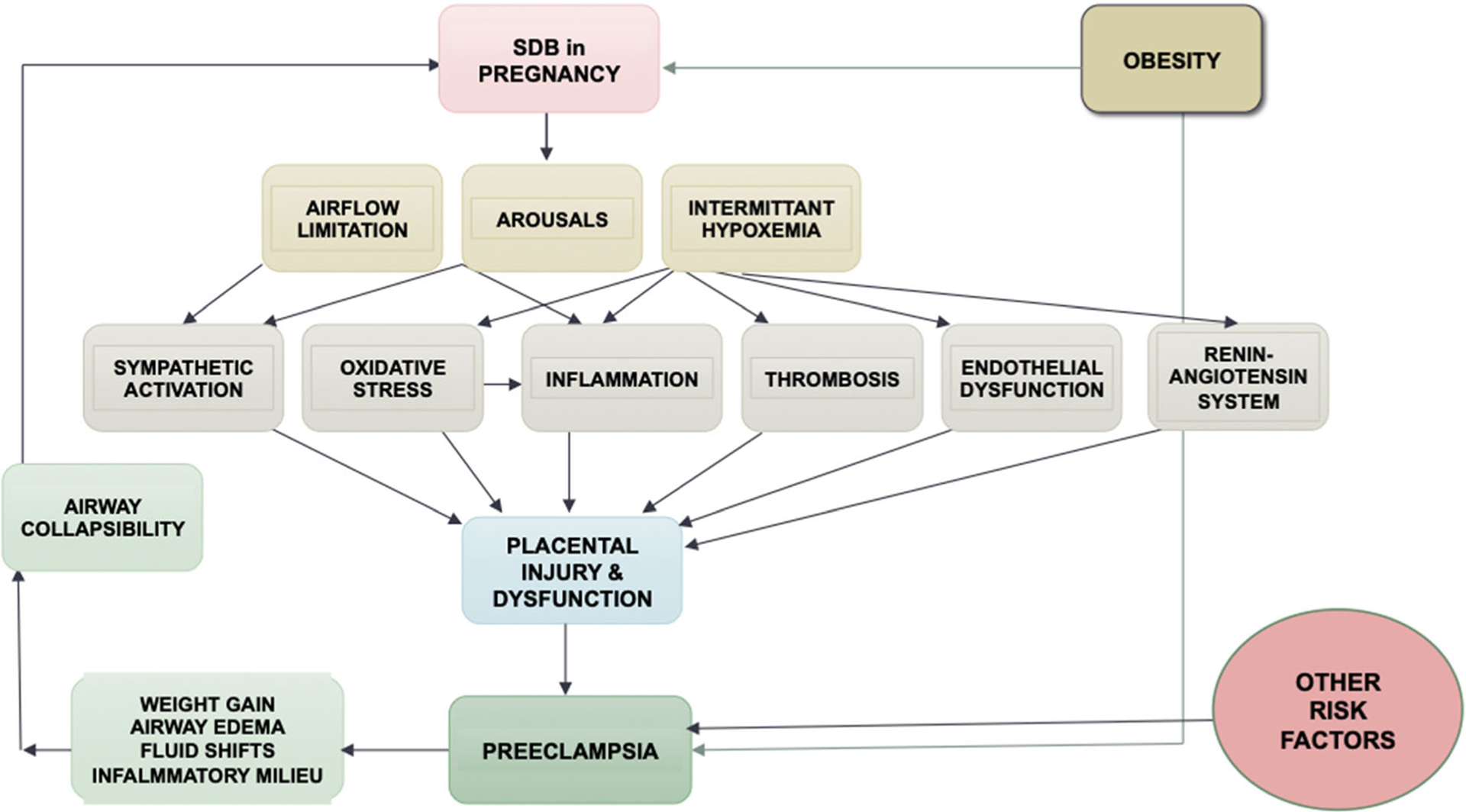
A conceptual model of the association of SDB and hypertensive disorders of pregnancy. SDB sleep disordered breathing
Pathobiology of the Association of SDB and HDP
A few potential mechanisms have been hypothesized to explain the increased risk for HDP among pregnant women with SDB [48–51]. However, few published studies have directly tested the hypothesized pathways linking SDB to HDP. Next, we will review the extant literature.
SDB is associated with elevated inflammation in non-pregnant populations [52], and work from our group demonstrated that SDB may exacerbate GDM-associated inflammation in pregnancy, with elevated circulating pro-inflammatory cytokines IL-6, IL-8, and TNF-α [53]. Induction of gestational intermittent hypoxia in mice resulted in higher plasma levels of TNF-α [54••], and late gestation intermittent hypoxia led to epigenetic changes to genes involved in inflammation [55]. There is evidence that pro-inflammatory cytokines (e.g., Il-6) are elevated in pregnant women with preeclampsia [56, 57]. No studies, to our knowledge, have examined maternal inflammation as a pathway to HDP among women with SDB in pregnancy.
Evidence on the association between SDB and oxidative stress is sparse, and findings have been mixed. Khan et al. found that obstructive sleep apnea in pregnancy was associated with lower circulating oxidative stress markers [58], which may be explained by protective effects of estradiol against oxidative stress in response to intermittent hypoxia [59]. In contrast, Koken et al. [60] found that lipoperoxidation was increased in pregnant women who snored. Consistent with Koken, using a mouse model of intermittent gestational hypoxia, Badran et al. found that hypoxia resulted in higher levels of oxidative stress in placental tissue [54]. In women with preeclampsia, a recent meta-analysis found that oxidative stress was increased and anti-oxidant capacity reduced relative to pregnant controls [61]. Discrepancies in data on oxidative stress in SDB in pregnancy may be due to differences in markers used, as well as differences in site of measurement (circulating versus tissue), and possibly due to timing of exposure during pregnancy, given exponential increases in sex steroid hormones with pregnancy progression (see below section). Mechanistic studies examining oxidative stress as a pathway to HDP in women with SDB have not been conducted, and more research is needed to determine the association between SDB and oxidative stress in pregnancy, given the previously mixed findings.
In healthy pregnancies, sympathetic activation increases and parasympathetic activation decreases across gestation. Pulse transit time, i.e., the length of time for a pulse to travel from the left ventricle to a peripheral arterial site, measures change in arterial stiffness caused by sympathetic activation. Link and colleagues [45] found that women with SDB (snoring or obstructive sleep apnea) had higher pulse transit time compared with women without SDB, indicating potential hyper-activation of the SNS in pregnancy in women with SDB. Preeclampsia is also a state of sympathetic hyper-activation [62]. Consistent with other mechanistic studies, no research has tested SNS as a pathway linking SDB to HDP; however, there are studies underway examining this particular pathway.
Physiologic vascular adaptations of pregnancy include a decrease in vascular resistance and uterine spiral arteries remodeling to accommodate increased blood volume without resulting in gestational hypertension [63–65]. Pregnancies affected by hypertensive disorders are characterized by poor adaptation within maternal vasculature, including endothelial dysfunction, lack of spiral arteries remodeling, higher inflammation, increased uterine vascular resistance, and decreased blood flow and oxygen delivery from the placenta to the fetus [64, 65]. Evidence from animal models demonstrates that prenatal intermittent hypoxia results in the release of soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sEng), which inhibits vasodilation [54••, 66]. In addition, animal models show that prenatal intermitted hypoxia impairs vasoreactivity at the level of the uterine arteries without, however, affecting spiral artery remodeling [54••]. Impaired endothelial vasoreactivity has been observed in women with preeclampsia [67], suggesting that poor maternal vascular adaptations in pregnancy in women with SDB may serve as a pathway to HDP; however, no studies have longitudinally examined whether abnormal vascular reactivity predicts HDP in women with SDB.
Finally, the renin-angiotensin system, integral in the regulation of blood pressure and fluid and sodium balance and involved in placental trophoblast invasion and placental blood flow, has been proposed as a mechanism explaining increased risk for HDP in women with SDB in pregnancy. In preeclampsia, the circulating RAS systems is suppressed, while the utero-placental system is upregulated [68], yet the RAS has not been studied in pregnancies affected by SDB.
Taken together, a review of the literature demonstrates the paucity of studies examining pathways to HDP in pregnancies affected by SDB.
Pregnancy-Specific Nuances to Pathobiological Mechanisms
Though, at first glance, the mechanisms linking the two disorders appear to be similar to those linking SDB and cardiovascular outcomes outside pregnancy, there are some nuances that need to be taken into account in the pregnant population. These nuances justify the need to mechanistically examine the association of SDB with pregnancy-specific outcomes, rather than extrapolate knowledge from the non-pregnant population. One of the main differences is the timeframe from exposure to SDB to the occurrence of the adverse outcome. While most of the research linking SDB to adverse cardiovascular outcomes span decades, cardiovascular outcomes in pregnancy occur within the course of the gestation period. This suggests the possibility that some accelerated mechanism occurs in this unique population. Furthermore, the cardiovascular system undergoes some profound hemodynamic changes that are unique to pregnancy, where systemic vascular resistance decreases and cardiac output increases significantly. More so, pregnancy is a dynamic state; thus, factors and mechanisms that apply in early pregnancy may not necessarily apply in late gestation. Sex steroid hormone levels, for instance, exponentially increase during the course of pregnancy. Animal studies have shown a significant effect of sex steroid hormones, such as estradiol, on downstream mechanistic pathways of intermittent hypoxia [69]. Estradiol administration to ovariectomized rats exposed to intermittent hypoxia was found to prevent the effects of chronic intermittent hypoxia on cardiovascular measures such as systolic and diastolic blood pressure and heart rate [59] and resulted in a significant reduction in NADPH oxidase activity in the brain and the adrenals [59]. Similar findings were identified in vascular tissues [70]. On the other hand, ovariectomy in mice exposed to intermittent hypoxia showed an increase in mRNA expression of key pro-inflammatory cytokines in the heart compared with sham mice exposed to normoxia [71]. Estrogens may also interfere with molecular downstream effects of sympathetic activation [72]. Other unique differences in this population that impact pathobiological pathways include the presence of a fetoplacental unit, which may act as a potential target “organ,” but may also act as a biologically active organ that may impact perinatal outcomes such as hypertensive disorders of pregnancy.
For all these reasons, mechanisms underlying the association of SDB with HDP would need to be focused on in this population, and extrapolation of potential therapeutic targets from the general, non-pregnant population may not be reliable.
SDB and the Placenta
The placenta is a key organ in the development of preeclampsia. Hence, establishing alterations in placental function or morphology in relation to SDB may help strengthen the hypothesis of causality between SDB and preeclampsia (see Table 1). Data linking SDB to placental function remains limited. Retrospective data from our laboratory showed that women with SDB have a higher degree of imbalance in angiogenic and anti-angiogenic markers compared with women without the diagnosis. In a study examining circulating placental growth factor (PlGF) and soluble fms-like tyrosine 1 (s-flt-1), markers secreted by the placenta, we demonstrated a higher ratio of s-flt1/PLGF in women with SDB compared with those without, alterations that were in the same order of magnitude as women who later developed preeclampsia in other studies [73, 79••]. A case report also described a woman with severe SDB and preeclampsia who had elevated s-flt-1 levels; following initiation of continuous positive airway pressure (CPAP), the gold standard therapy for sleep apnea, both clinical and biological markers, improved. Systolic blood pressure dropped, and so did urinary protein and s-flt-1 levels. The patient was then able to remain pregnant for 30 additional days [80]. Similarly, we have demonstrated that pregnancy-associated plasma protein-A, a first trimester biomarker known to regulate trophoblast invasion into the decidua [81], was altered in a similar direction in women with sleep apnea [79••] as it was in women who later developed preeclampsia [82].
Table 1.
Sir Bradford Hill criteria and their application to SDB and preeclampsia [17••, 26••, 27, 54••, 73, 74••, 75–78]
| Pros | Cons | |
|---|---|---|
| Strength | Two-three-fold increased risk | |
| Consistency | Association across study designs and populations | |
| Specificity | Some markers associated with OSA but not snoring | Minimal data |
| Temporality | SDB at 6–15 weeks associated with later development of PEC | Unclear if SDB preceded biological changes associated with PEC and not just clinical changes since PEC is usually determined in early pregnancy |
| Biological gradient | Severity of SDB associated with higher risk of PEC | |
| Biological plausibility | Explained by shared mechanisms and placental involvement | |
| Coherence | Minimal laboratory-based data exist | |
| Experiment | Recent experimental data of gestational intermittent hypoxia demonstrate changes in mechanistic pathways linked to PEC | |
| Analogy | High altitude model of chronic hypoxia | Mechanisms for chronic vs. intermittent hypoxia may be different |
| Reversibility | Data mainly on hemodynamic improvement- ongoing trials pending |
PEC: preeclampsia; SDB: sleep disordered breathing; OSA: obstructive sleep apnea
Further, we demonstrated that both sleep apnea and snoring were associated with a significantly elevated risk for fetal normoblastemia, a marker of chronic hypoxia, when compared with controls [aOR 19.07 (97.5% CI 5.1–95.6) vs. 7.8 (CI 2.5–34.1)] [74••]. In the same study, there was an association between both sleep apnea and snoring with placental expression of carbonic anhydrase IX, a marker of tissue hypoxia, compared with controls [aOR 7.8 (CI 1.9–52.7) vs. 3.3 (CI 1.07–11.3)]. Neither of these findings appeared to be impacted by covariates such as BMI, hypertension, or diabetes mellitus. Finally, using an animal model of obstructive sleep apnea, placentas of dams exposed to intermittent gestational hypertension displayed higher placental hypoxia, oxidative stress, and cell death relative to unexposed dams [54••]. Thus, emerging evidence indicates an effect of SDB on placental physiology, which may serve as a pathway to HDP.
The placenta was also recently implicated by sleep apnea in a metabolic context. In a study of 53 pregnant women, placental weight positively correlated with the apnea hypopnea index, even after controlling for maternal BMI. In addition, leptin expression was 1.8-fold higher in placentas of women with OSA compared with controls when only lean women were examined [83]. Though the findings from this recent study may not directly support the hypothesis of the placenta being a target organ in the association of SDB and hypertensive disorders of pregnancy, it points to the placenta as an organ that is sensitive to the intermittent hypoxia and the sympathetic activation that are typical of SDB.
Other Potential Explanations of the Association
Conversely, preeclampsia may lead to the development of SDB. Extracellular fluid volume is increased in preeclampsia [84] due to vascular leaks and reductions in colloid oncotic pressure [85]. Colloid oncotic pressures drop during the course of pregnancy by about 10% [86]. This drop is more pronounced in women with preeclampsia and may be explained by renal albumin losses and impaired hepatic albumin synthesis [87]. As colloid oncotic pressures drop, interstitial edema due to extravascular fluid volume expansion may develop in various tissues, including the upper airway. In a study examining upper airway dimensions in pregnant and non-pregnant women, 75% of all women with preeclampsia reported snoring, compared with 28% of pregnant women without the diagnosis. Upper airway dimensions measured using acoustic reflections showed that women with preeclampsia had narrowing in the upper airway in both the upright and the supine position [88].
Furthermore, it is plausible that systemic involvement of the inflammatory system in preeclampsia [89] may be associated with airway inflammation, especially since a similar inflammatory profile is observed in preeclampsia and SDB, with elevated levels of interleukin-6 (IL-6) and tumor necrosis factor-a [90, 91]. Though this pro-inflammatory profile may well be a consequence of SDB, it may also lead to SDB by causing upper airway edema and decreased upper airway patency. Thus, though many factors support causality between SDB and HDP, a reverse directionality is also plausible. It is also possible that the two disorders coexist as they share numerous risk factors.
Impact of Therapy in SDB on Hypertensive Pregnancy Outcomes
Few studies have examined the impact of therapy for SDB on hemodynamics and HDP. Though the vast majority of studies are based on small experimental data, existing data are promising. In a laboratory-based application of positive airway pressure in women with severe preeclampsia, nocturnal blood pressure significantly improved in all stages of sleep while women were on positive airway pressure [92]. In another randomized controlled trial, the same investigators demonstrated a significant improvement in nocturnal cardiac output in women with preeclampsia randomized to positive airway pressure [93]. A small randomized controlled trial that examined blood pressure measurements in pregnant women with SDB assigned to positive airway pressure in early gestation showed significantly lower blood pressure measurements around delivery [94]. However, adequately powered data evaluating the impact of SDB-directed interventions on the development of HDP or meaningful outcomes in hypertensive women is currently lacking. An ongoing multicenter randomized controlled trial is examining the impact of positive airway pressure initiated in early gestation for obstructive sleep apnea on hypertensive disorders of pregnancy (NCT #03487185). Minimal data have examined the use of a mandibular advancement device on blood pressure outcomes. In a small, randomized controlled study, neither CPAP nor a mandibular advancement device had a significant impact on morning blood pressure or inflammatory markers [95]. Similarly, there is paucity of data regarding the use of aspirin, as a preventive measure for preeclampsia, in women with SDB. SDB is not currently an indication for the use of low dose aspirin though the potential impact of chronic intermittent hypoxemia on vascular responses in the non-pregnant population may be modulated by thromboxane inhibition and COX pathways [96]. Conversely, though obesity is currently a clinical indication for prophylaxis with low-dose aspirin (81 mg/day) [11], severe obesity may impact the effect of low dose aspirin dosing on thromboxane inhibition [97] and doses possibly modified. Therefore, further studies are needed to investigate efficacy of aspirin or other preventive mechanisms in reducing the risk of preeclampsia among specific high-risk pregnant populations, in the presence of relatively common morbidity, such as obesity and SDB.
Conclusion
Hypertensive disorders of pregnancy represent one of the major obstetric morbidity worldwide. Maternal SDB may represent a novel and modifiable risk factor in the pathophysiology of HDP; however, much remains to be studied (see Table 2). Further studies are needed to investigate underlying mechanisms of this association, identify targets for intervention, and examine the impact of interventional strategies on HDP and its consequences.
Table 2.
Areas for future research in the association of SDB and HDP
| Preeclampsia perspective | Sleep perspective |
|---|---|
| Role of SDB in development and treatment of preeclampsia: | Explore association of non-SDB sleep disturbances with HDP |
| Understand mechanistic pathways linking SDB to preeclampsia | Understand sleep postpartum and relationship between pregnancy outcomes and long-term sleep |
| Identify mechanistic potential therapeutic targets | Explore alternative definitions of SDB in pregnancy such as outcome-based definitions or alternative event definitions |
| Assess impact of SDB therapy on maternal outcomes in large studies and identify optimal timing to initiate therapy | |
| Understand if sleep disturbances mediate or exacerbate perinatal outcomes of preeclampsia | |
| Role of SDB in long-term cardiovascular outcome among women with history of SDB and HDP. | |
| Bidirectional relationship of SDB and preeclampsia |
SDB sleep disordered breathing, HDP hypertensive disorders of pregnancy
Acknowledgments
We would like to thank Ms. Beth Hott for her assistance in preparing the manuscript for submission.
Funding Information
GB is funded by National Heart, Lung, and Blood Institute R01HL130702 and National Institute of Child Health and Human Development R01HD078515 of the National Institutes of Health. MHB is funded by P20 GM103652 from the National Institute of General Medical Sciences, National Institutes of Health.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol 2019;133(1):e1–e25. doi: 10.1097/aog.0000000000003018. [DOI] [PubMed] [Google Scholar]
- 2.Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206(6):470–5. 10.1016/j.ajog.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Khan N, Andrade W, De Castro H, Wright A, Wright D, Nicolaides KH. Impact of new definitions of pre-eclampsia on incidence and performance of first-trimester screening. Ultrasound Obstet Gynecol. 2020;55(1):50–7. 10.1002/uog.21867. [DOI] [PubMed] [Google Scholar]
- 4.Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol. 2013;25(2): 124–32. 10.1097/GCO.0b013e32835e0ef5. [DOI] [PubMed] [Google Scholar]
- 5.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of preeclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391–403. 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ. 2013;347:f6564. 10.1136/bmj.f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlstrom BL, Engh ME, Bukholm G, Oian P. Changes in the prevalence of pre-eclampsia in Akershus County and the rest of Norway during the past 35 years. Acta Obstet Gynecol Scand. 2006;85(8):916–21. 10.1080/00016340500442449. [DOI] [PubMed] [Google Scholar]
- 8.Poon LC, Rolnik DL, Tan MY, Delgado JL, Tsokaki T, Akolekar R, et al. ASPRE trial: incidence of preterm pre-eclampsia in patients fulfilling ACOG and NICE criteria according to risk by FMF algorithm. Ultrasound Obstet Gynecol. 2018;51(6):738–42. 10.1002/uog.19019. [DOI] [PubMed] [Google Scholar]
- 9.Hao J, Hassen D, Hao Q, Graham J, Paglia MJ, Brown J, et al. Maternal and infant health care costs related to preeclampsia. Obstet Gynecol. 2019;134(6):1227–33. 10.1097/aog.0000000000003581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2). 10.1161/circoutcomes.116.003497. [DOI] [PubMed] [Google Scholar]
- 11.ACOG Committee Opinion No. 743: low-dose aspirin use during pregnancy. Obstet Gynecol 2018;132(1):e44–e52. doi: 10.1097/aog.0000000000002708. [DOI] [PubMed] [Google Scholar]
- 12.Duley L, Meher S, Hunter KE, Seidler AL, Askie LM. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2019;2019(10). 10.1002/14651858.CD004659.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.••.Maric-Bilkan C, Abrahams VM, Arteaga SS, Bourjeily G, Conrad KP, Catov JM, et al. Research recommendations from the National Institutes of Health workshop on predicting, preventing, and treating preeclampsia. Hypertension. 2019;73(4):757–66. 10.1161/hypertensionaha.118.11644 [DOI] [PMC free article] [PubMed] [Google Scholar]; These recommendations, provided by a large multidisciplinary workshop, highlight the main research gaps and provide guidance to future clinical and translational research on pathophysiology, identification of novel biomarkers, outcome and management of preeclampsia.
- 14.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–74. 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 15.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36(4):849–55. 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien LM, Bullough AS, Owusu JT, Tremblay KA, Brincat CA, Chames MC, et al. Snoring during pregnancy and delivery outcomes: a cohort study. Sleep. 2013;36(11):1625–32. 10.5665/sleep.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.••.Facco FL, Parker CB, Reddy UM, Silver RM, Koch MA, Louis JM, et al. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol. 2017;129(1):31–41. 10.1097/aog.0000000000001805 [DOI] [PMC free article] [PubMed] [Google Scholar]; This is one of the first prospective studies assessing prevalence of obstructive sleep apnea among a large cohort of low-risk primiparous women and its association with hypertensive disorders of pregnancy and gestational diabetes. It is a multicenter study, involving eight different clinical sites and with obstructive sleep apnea screening performed by an independent and blinded central reading center, limiting potential bias.
- 18.Louis J, Auckley D, Miladinovic B, Shepherd A, Mencin P, Kumar D, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120(5):1085–92. 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pamidi S, Marc I, Simoneau G, Lavigne L, Olha A, Benedetti A, et al. Maternal sleep-disordered breathing and the risk of delivering small for gestational age infants: a prospective cohort study. Thorax. 2016;71(8):719–25. 10.1136/thoraxjnl-2015-208038. [DOI] [PubMed] [Google Scholar]
- 20.Fung AM, Wilson DL, Lappas M, Howard M, Barnes M, O’Donoghue F, et al. Effects of maternal obstructive sleep apnoea on fetal growth: a prospective cohort study. PLoS One. 2013;8(7): e68057. 10.1371/journal.pone.0068057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reutrakul S, Zaidi N, Wroblewski K, Kay HH, Ismail M, Ehrmann DA, et al. Interactions between pregnancy, obstructive sleep apnea, and gestational diabetes mellitus. J Clin Endocrinol Metab. 2013;98(10):4195–202. 10.1210/jc.2013-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien LM, Bullough AS, Chames MC, Shelgikar AV, Armitage R, Guilleminualt C, et al. Hypertension, snoring, and obstructive sleep apnoea during pregnancy: a cohort study. BJOG. 2014;121(13):1685–93. 10.1111/1471-0528.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Champagne K, Schwartzman K, Opatrny L, Barriga P, Morin L, Mallozzi A, et al. Obstructive sleep apnoea and its association with gestational hypertension. Eur Respir J. 2009;33(3):559–65. 10.1183/09031936.00122607. [DOI] [PubMed] [Google Scholar]
- 24.Reid J, Skomro R, Cotton D, Ward H, Olatunbosun F, Gjevre J, et al. Pregnant women with gestational hypertension may have a high frequency of sleep disordered breathing. Sleep. 2011; 34(8): 1033–8. 10.5665/SLEEP.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oyieng’o DO, Kirwa K, Tong I, Martin S, Antonio Rojas-Suarez J, Bourjeily G. Restless legs symptoms and pregnancy and neonatal outcomes. Clin Ther. 2016;38(2):256–64. 10.1016/j.clinthera.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.••.Louis JM, Mogos MF, Salemi JL, Redline S, Salihu HM. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998–2009. Sleep. 2014;37(5): 843–9. 10.5665/sleep.3644 [DOI] [PMC free article] [PubMed] [Google Scholar]; This large population study estimates the prevalence of obstructive sleep apnea in pregnancy and demonstrates the association between this condition and adverse maternal and infant outcomes, by using a U.S. database including more than 55 millions of subjects.
- 27.Bourjeily G, Danilack VA, Bublitz MH, Lipkind H, Muri J, Caldwell D, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;38:50–7. 10.1016/j.sleep.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bin YS, Cistulli PA, Ford JB. Population-based study of sleep apnea in pregnancy and maternal and infant outcomes. J Clin Sleep Med. 2016;12(6):871–7. 10.5664/jcsm.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206(2):136 e1–5. 10.1016/j.ajog.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Bourjeily G, Raker C, Paglia MJ, Ankner G, O’Connor K. Patient and provider perceptions of sleep disordered breathing assessment during prenatal care: a survey-based observational study. Ther Adv Respir Dis. 2012;6(4):211–9. 10.1177/1753465812444958. [DOI] [PubMed] [Google Scholar]
- 31.Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58(5):811–7. 10.1161/hypertensionaha.111.179788. [DOI] [PubMed] [Google Scholar]
- 32.Drager LF, Genta PR, Pedrosa RP, Nerbass FB, Gonzaga CC, Krieger EM, et al. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105(8):1135–9. 10.1016/j.amjcard.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Min HJ, Cho YJ, Kim CH, Kim DH, Kim HY, Choi JI, et al. Clinical features of obstructive sleep apnea that determine its high prevalence in resistant hypertension. Yonsei Med J. 2015;56(5): 1258–65. 10.3349/ymj.2015.56.5.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. Jama. 2012;307(20):2169–76. 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study JAMA. 2000;283(14):1829–36. [DOI] [PubMed] [Google Scholar]
- 36.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84. [DOI] [PubMed] [Google Scholar]
- 37.Mokhlesi B, Finn LA, Hagen EW, Young T, Hla KM, Van Cauter E, et al. Obstructive sleep apnea during REM sleep and hypertension. Results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190(10):1158–67. 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franklin KA, Holmgren PA, Jonsson F, Poromaa N, Stenlund H, Svanborg E. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest. 2000;117(1):137–41. [DOI] [PubMed] [Google Scholar]
- 39.Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010;202(3):261 e1–5. 10.1016/j.ajog.2009.42.867. [DOI] [PubMed] [Google Scholar]
- 40.Gaillard R, Steegers EA, Hofman A, Jaddoe VW. Associations of maternal obesity with blood pressure and the risks of gestational hypertensive disorders. The Generation R Study J Hypertens. 2011; 29(5):937–44. 10.1097/HJH.0b013e328345500c. [DOI] [PubMed] [Google Scholar]
- 41.Staff AC. The two-stage placental model of preeclampsia: an update. J Reprod Immunol. 2019;134–135:1–10. 10.1016/j.jri.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Avagliano L, Bulfamante GP, Morabito A, Marconi AM. Abnormal spiral artery remodelling in the decidual segment during pregnancy: from histology to clinical correlation. J Clin Pathol. 2011;64(12): 1064–8. 10.1136/jclinpath-2011-200092. [DOI] [PubMed] [Google Scholar]
- 43.Garvin JH, Redd A, Bolton D, Graham P, Roche D, Groeneveld P, et al. Exploration of ICD-9-CM coding of chronic disease within the Elixhauser Comorbidity Measure in patients with chronic heart failure. Perspect Health Inf Manag. 2013;10:1b. [PMC free article] [PubMed] [Google Scholar]
- 44.• Wilson DL, Walker SP, Fung AM, Pell G, O’Donoghue FJ, Barnes M, et al. Sleep-disordered breathing in hypertensive disorders of pregnancy: a BMI-matched study. J Sleep Res. 2018. 10.1111/jsr.12656 [DOI] [PubMed] [Google Scholar]; This prospective cross-sectional study demonstrates the importance of maternal obesity as confounding factor in the association between sleep-disordered breathing, diagnosed by objective methods, and gestational hypertensive disorders. Study design of future research on this topic should aim to quantify the role of obesity in the pathophysiology of the association between sleep-disordered breathing and hypertensive disorders.
- 45.Link BN, Eid C, Bublitz MH, Pengo MF, Salameh M, Ludwig KS, et al. Pulse transit time in pregnancy: a new way to diagnose and classify sleep disordered breathing? Sleep. 2019;42(5). 10.1093/sleep/zsz022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bourjeily G, Chambers A, Salameh M, Bublitz MH, Kaur A, Coppa A, et al. Anthropometric measures and prediction of maternal sleep-disordered breathing. J Clin Sleep Med. 2019;15(6):849–56. 10.5664/jcsm.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- 48.Jerath R, Barnes VA, Fadel HE. Mechanism of development of preeclampsia linking breathing disorders to endothelial dysfunction. Med Hypotheses. 2009;73(2):163–6. 10.1016/j.mehy.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Fung AM, Wilson DL, Barnes M, Walker SP. Obstructive sleep apnea and pregnancy: the effect on perinatal outcomes. J Perinatol. 2012;32(6):399–406. 10.1038/jp.2012.14. [DOI] [PubMed] [Google Scholar]
- 50.Izci-Balserak B, Pien GW. Sleep-disordered breathing and pregnancy: potential mechanisms and evidence for maternal and fetal morbidity. Curr Opin Pulm Med. 2010;16(6):574–82. 10.1097/MCP.0b013e32833f0d55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oyiengo D, Louis M, Hott B, Bourjeily G. Sleep disorders in pregnancy. Clin Chest Med. 2014;35(3):571–87. 10.1016/j.ccm.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep. 2007;30(1):29–34. 10.1093/sleep/30.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bublitz MH, Carpenter M, Amin S, Okun ML, Millman R, De La Monte SM, et al. The role of inflammation in the association between gestational diabetes and obstructive sleep apnea: a pilot study. Obstet Med. 2018;11(4):186–91. 10.1177/1753495x18780095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.••.Badran M, Yassin BA, Lin DTS, Kobor MS, Ayas N, Laher I. Gestational intermittent hypoxia induces endothelial dysfunction, reduces perivascular adiponectin and causes epigenetic changes in adult male offspring. J Physiol. 2019;597(22):5349–64. 10.1113/jp277936 [DOI] [PubMed] [Google Scholar]; This represents a comprehensive animal model demonstrating the effects of gestational intermittent hypoxia on maternal uterine function, placenta, fetal weight and placental oxidative stress and angiogenic and anti-angiogenetic factors.
- 55.Khalyfa A, Cortese R, Qiao Z, Ye H, Bao R, Andrade J, et al. Late gestational intermittent hypoxia induces metabolic and epigenetic changes in male adult offspring mice. J Physiol. 2017;595(8):2551–68. 10.1113/jp273570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ouyang YQ, Li SJ, Zhang Q, Cai HB, Chen HP Interactions between inflammatory and oxidative stress in preeclampsia. Hypertens Pregnancy. 2009;28(1):56–62. 10.1080/10641950802233064. [DOI] [PubMed] [Google Scholar]
- 57.Sakai M, Tsuda H, Tanebe K, Sasaki Y, Saito S. Interleukin-12 secretion by peripheral blood mononuclear cells is decreased in normal pregnant subjects and increased in preeclamptic patients. Am J Reprod Immunol. 2002;47(2):91–7. 10.1034/j.1600-0897.2002.1o020.x. [DOI] [PubMed] [Google Scholar]
- 58.Khan N, Lambert-Messerlian G, Monteiro JF, Hodosy J, Tothova L, Celec P, et al. Oxidative and carbonyl stress in pregnant women with obstructive sleep apnea. Sleep Breath. 2018;22(1):233–40. 10.1007/s11325-017-1475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laouafa S, Ribon-Demars A, Marcouiller F, Roussel D, Bairam A, Pialoux V, et al. Estradiol protects against cardiorespiratory dysfunctions and oxidative stress in intermittent hypoxia. Sleep. 2017;40(8). 10.1093/sleep/zsx104, 10.1093/sleep/zsx104. [DOI] [PubMed] [Google Scholar]
- 60.Koken G, Sahin FK, Cosar E, Saylan F, Yilmaz N, Altuntas I, et al. Oxidative stress markers in pregnant women who snore and fetal outcome: a case control study. Acta Obstet Gynecol Scand. 2007;86(11):1317–21. 10.1080/00016340701662183. [DOI] [PubMed] [Google Scholar]
- 61.Taravati A, Tohidi F. Comprehensive analysis of oxidative stress markers and antioxidants status in preeclampsia. Taiwan J Obstet Gynecol. 2018;57(6):779–90. 10.1016/j.tjog.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia - a state of sympathetic overactivity. N Engl J Med. 1996;335(20):1480–5. 10.1056/nejm199611143352002. [DOI] [PubMed] [Google Scholar]
- 63.Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232(1): R27–44. 10.1530/joe-16-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Possomato-Vieira JS, Khalil RA. Mechanisms of endothelial dysfunction in hypertensive pregnancy and preeclampsia. Adv Pharmacol. 2016;77:361–431. 10.1016/bs.apha.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mari G, Hanif F. Intrauterine growth restriction: how to manage and when to deliver. Clin Obstet Gynecol. 2007;50(2):497–509. 10.1097/GRF.0b013e31804c96a9. [DOI] [PubMed] [Google Scholar]
- 66.Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1085–93. 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nova A, Sibai BM, Barton JR, Mercer BM, Mitchell MD. Maternal plasma level of endothelin is increased in preeclampsia. Am J Obstet Gynecol. 1991;165(3):724–7. 10.1016/0002-9378(91)90317-k. [DOI] [PubMed] [Google Scholar]
- 68.Spaan JJ, Bowyer L, Lazzaro VA, McCrohon J, Brown MA. Maternal hemodynamics influence fetal hemodynamics in normal and hypertensive pregnancy. Pregnancy Hypertens. 2013;3(1):10–5. 10.1016/j.preghy.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 69.Boukari R, Laouafa S, Ribon-Demars A, Bairam A, Joseph V. Ovarian steroids act as respiratory stimulant and antioxidant against the causes and consequences of sleep-apnea in women. Respir Physiol Neurobiol. 2017;239:46–54. 10.1016/j.resp.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 70.Ribon-Demars A, Pialoux V, Boreau A, Marcouiller F, Lariviere R, Bairam A, et al. Protective roles of estradiol against vascular oxidative stress in ovariectomized female rats exposed to normoxia or intermittent hypoxia. Acta Physiol (Oxf). 2019;225(2):e13159. 10.1111/apha.13159. [DOI] [PubMed] [Google Scholar]
- 71.Torres M, Palomer X, Montserrat JM, Vazquez-Carrera M, Farre R. Effect of ovariectomy on inflammation induced by intermittent hypoxia in a mouse model of sleep apnea. Respir Physiol Neurobiol. 2014;202:71–4. 10.1016/j.resp.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 72.Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol. 1999;56(1): 31–8. 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- 73.Maynard SE, Crawford SL, Bathgate S, Yan J, Robidoux L, Moore M, et al. Gestational angiogenic biomarker patterns in high risk preeclampsia groups. Am J Obstet Gynecol. 2013;209(1):53 e1–9. 10.1016/j.ajog.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 74.••.Ravishankar S, Bourjeily G, Lambert-Messerlian G, He M, De Paepe ME, Gundogan F. Evidence of placental hypoxia in maternal sleep disordered breathing. Pediatr Dev Pathol. 2015;18(5):380–6. 10.2350/15-06-1647-OA.1 [DOI] [PubMed] [Google Scholar]; This study provides original and relevant information about placental histopathology findings and immunohistochemical markers among pregnancies complicated by maternal obstructive sleep apnea. Compared to controls, those pregnancies presented higher levels of markers of fetoplacental hypoxia.
- 75.Salameh M, Lee J, Palomaki G, Eklund E, Curran P, Suarez JAR, et al. Snoring and markers of fetal and placental wellbeing. Clin Chim Acta. 2018;485:139–43. 10.1016/j.cca.2018.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumtepe Y, Dundar O, Cetinkaya K, Ingec M. Preeclampsia and eclampsia incidence in the eastern anatolia region of Turkey: the effects of high altitude. J Turk Ger Gynecol Assoc. 2011;12(1):26–30. 10.5152/jtgga.2011.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller S, Tudor C, Nyima, Thorsten VR, Sonam, Droyoung, et al. Maternal and neonatal outcomes of hospital vaginal deliveries in Tibet. Int J Gynaecol Obstet. 2007;98(3):217–21. 10.1016/j.ijgo.2007.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hanson CW III, Thaler ER. Intubation and upper airway management. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, Senior RM, Pack AI, editors. Fishman’s pulmonary diseases and disorders. Fourth ed. New York: McGraw-Hill Medical; 2008. [Google Scholar]
- 79.••.Bourjeily G, Curran P, Butterfield K, Maredia H, Carpenter M, Lambert-Messerlian G. Placenta-secreted circulating markers in pregnant women with obstructive sleep apnea. J Perinat Med. 2015;43(1):81–7. 10.1515/jpm-2014-0052 [DOI] [PubMed] [Google Scholar]; This is one of the first studies demonstrating that pregnancies complicated by obstructive sleep apnea present an abnormal pattern of angiogenic biomarkers, with lower levels of plasma protein-A and higher soluble vascular endothelial growth factor receptor 1 to placental growth factor ratio compared to controls, after adjusting for multiple confounding factors.
- 80.Whitehead C, Tong S, Wilson D, Howard M, Walker SP. Treatment of early-onset preeclampsia with continuous positive airway pressure. Obstet Gynecol. 2015;125(5):1106–9. 10.1097/AOG.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 81.Anderson UD, Olsson MG, Kristensen KH, Akerstrom B, Hansson SR. Review: biochemical markers to predict preeclampsia. Placenta. 2012;33 Suppl:S42–7. 10.1016/j.placenta.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 82.Audibert F, Boucoiran I, An N, Aleksandrov N, Delvin E, Bujold E, et al. Screening for preeclampsia using first-trimester serum markers and uterine artery Doppler in nulliparous women. Am J Obstet Gynecol. 2010;203(4):383 e1–8. 10.1016/j.ajog.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 83.Kidron D, Bar-Lev Y, Tsarfaty I, Many A, Tauman R. The effect of maternal obstructive sleep apnea on the placenta. Sleep. 2019;42(6). 10.1093/sleep/zsz072. [DOI] [PubMed] [Google Scholar]
- 84.Brown MA, Zammit VC, Mitar DM. Extracellular fluid volumes in pregnancy-induced hypertension. J Hypertens. 1992;10(1):61–8. 10.1097/00004872-199201000-00010. [DOI] [PubMed] [Google Scholar]
- 85.Brown MA. The physiology of pre-eclampsia. Clin Exp Pharmacol Physiol. 1995;22(11):781–91. 10.1111/j.1440-1681.1995.tb01937.x. [DOI] [PubMed] [Google Scholar]
- 86.Oian P, Maltau JM. Transcapillary forces in normal pregnant women. Acta Med Scand Suppl. 1985;693:19–22. [DOI] [PubMed] [Google Scholar]
- 87.Bourjeily G, Miller M. Obstetric disorders in the ICU. Clin Chest Med. 2009;30(1):89–102. 10.1016/j.ccm.2008.88.004. [DOI] [PubMed] [Google Scholar]
- 88.Izci B, Riha RL, Martin SE, Vennelle M, Liston WA, Dundas KC, et al. The upper airway in pregnancy and pre-eclampsia. Am J Respir Crit Care Med. 2003;167(2):137–40. 10.1164/rccm.200206-590OC. [DOI] [PubMed] [Google Scholar]
- 89.Rambaldi MP, Weiner E, Mecacci F, Bar J, Petraglia F. Immunomodulation and preeclampsia. Best Pract Res Clin Obstet Gynaecol. 2019;60:87–96. 10.1016/j.bpobgyn.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 90.Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW Jr, Wallace K, et al. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond). 2016;130(6): 409–19. 10.1042/cs20150702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bauters FA, Hertegonne KB, De Buyzere ML, Joos GF, Chirinos JA, Rietzschel ER. Phenotype and risk burden of sleep apnea: a population-based cohort study. Hypertension. 2019;74(4):1052–62. 10.1161/hypertensionaha.119.13452. [DOI] [PubMed] [Google Scholar]
- 92.Edwards N, Blyton DM, Kirjavainen T, Kesby GJ, Sullivan CE. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med. 2000;162(1):252–7. [DOI] [PubMed] [Google Scholar]
- 93.Blyton D, Sullivan C, Edwards N. Reduced nocturnal cardiac output associated with preeclampsia is minimized with the use of nocturnal nasal CPAP Sleep. 2004;27(1):79–84. [DOI] [PubMed] [Google Scholar]
- 94.Poyares D, Guilleminault C, Hachul H, Fujita L, Takaoka S, Tufik et al. Pre-eclampsia and nasal CPAP: part 2. Hypertension during pregnancy, chronic snoring, and early nasal CPAP intervention. Sleep Med. 2007;9(1):15–21. 10.1016/j.sleep.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 95.Reid J, Taylor-Gjevre R, Gjevre J, Skomro R, Fenton M, Olatunbosun F, et al. Can gestational hypertension be modified by treating nocturnal airflow limitation? J Clin Sleep Med. 2013;9(4):311–7. 10.5664/jcsm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beaudin AE, Pun M, Yang C, Nicholl DD, Steinback CD, Slater DM, et al. Cyclooxygenases 1 and 2 differentially regulate blood pressure and cerebrovascular responses to acute and chronic intermittent hypoxia: implications for sleep apnea. J Am Heart Assoc. 2014;3(3):e000875. 10.1161/jaha.114.000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Finneran MM, Gonzalez-Brown VM, Smith DD, Landon MB, Rood KM. Obesity and laboratory aspirin resistance in high-risk pregnant women treated with low-dose aspirin. Am J Obstet Gynecol. 2019;220(4):385. e1–6. 10.1016/j.ajog.2019.01.222. [DOI] [PubMed] [Google Scholar]


